Effect of Resveratrol on Markers of Oxidative Stress and Sirtuin 1 in Elderly Adults with Type 2 Diabetes
Abstract
1. Introduction
2. Results
2.1. Clinical Parameters (BMI and Blood Pressure)
2.2. Biochemical Parameters
2.3. OS Markers
2.4. SIRT1 Concentration
2.5. Adverse Events
3. Discussion
4. Materials and Methods
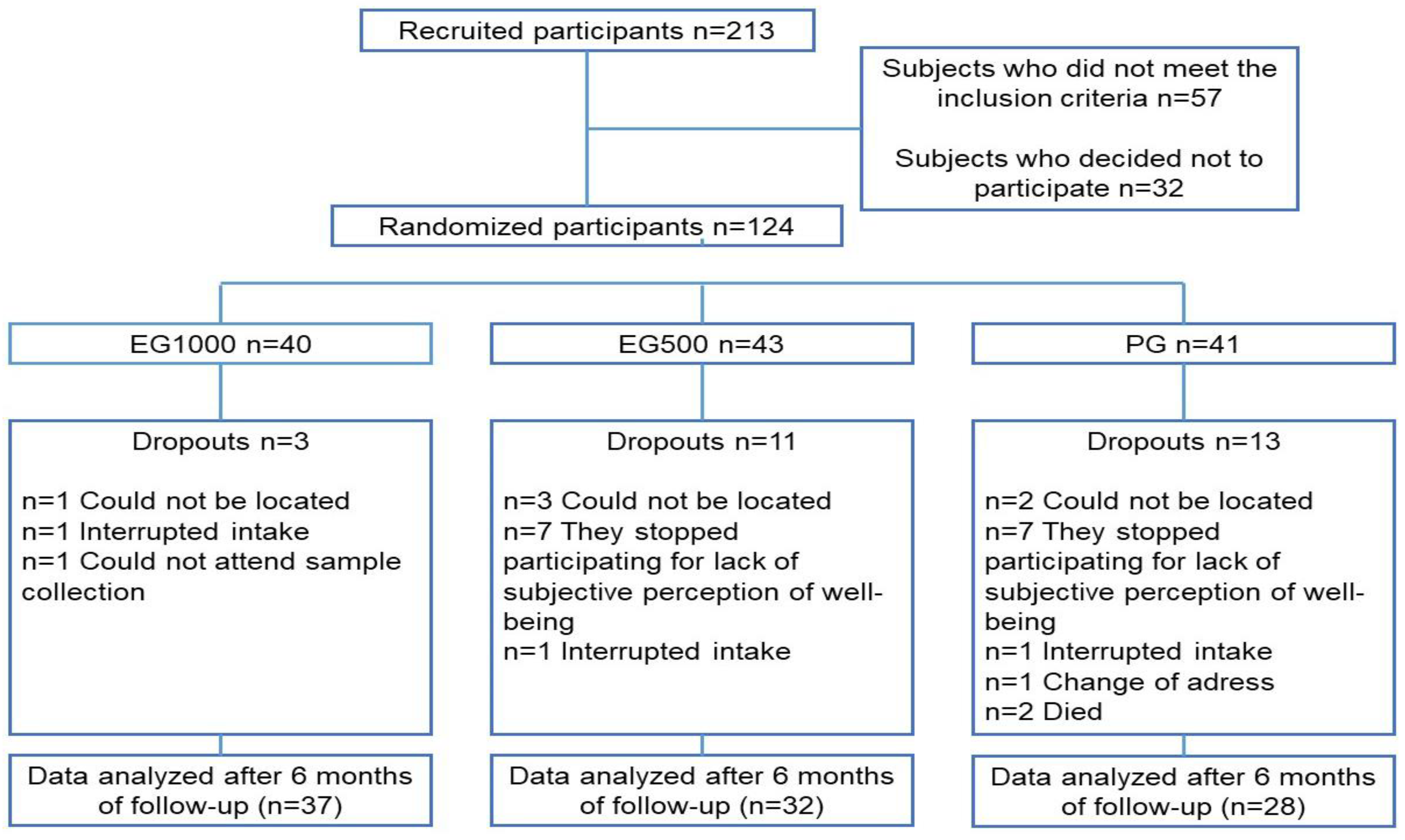
4.1. Population and Study Design
4.2. Measurement of Clinical Parameters (Weight, Height, BMI and Blood Pressure)
4.3. Measurement of Biochemical Parameters
4.4. Measurement of OS Markers
4.4.1. Lipoperoxidation (LPO)
4.4.2. 8-Isoprostane (8-Iso)
4.4.3. Superoxide Dismutase (SOD)
4.4.4. Glutathione Peroxidase (GPx)
4.4.5. Catalase (Cat)
4.4.6. SOD/GPx Ratio
4.4.7. Total Antioxidant Capacity (TAC)
4.4.8. GAP Calculation
4.4.9. Calculation of Oxidative Stress Score (OSS)
4.4.10. OS Index (OSI)
4.5. Measurement of SIRT1
4.6. Statistical Analysis
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- American Diabetes Association. Classification and diagnosis of diabetes: Standards of medical care in diabetes-2018. Diabetes Care 2018, 41 (Suppl. S1), S13–S27. [Google Scholar] [CrossRef] [PubMed]
- Sun, H.; Saeedi, P.; Karuranga, S.; Pinkepank, M.; Ogurtsova, K.; Duncan, B.B.; Stein, C.; Basit, A.; Chan, J.C.N.; Mbanya, J.C.; et al. IDF Diabetes Atlas: Global, regional and country-level diabetes prevalence estimates for 2021 and projections for 2045. Diabetes Res. Clin. Pract. 2022, 183, 109119. [Google Scholar] [CrossRef] [PubMed]
- Deshpande, A.D.; Harris-Hayes, M.; Schootman, M. Epidemiology of diabetes and diabetes-related complications. Phys. Ther. 2008, 88, 1254–1264. [Google Scholar] [CrossRef] [PubMed]
- Yaribeygi, H.; Sathyapalan, T.; Atkin, S.L.; Sahebkar, A. Molecular mechanisms linking oxidative stress and diabetes mellitus. Oxidative Med. Cell. Longev. 2020, 2020, 8609213. [Google Scholar] [CrossRef] [PubMed]
- Pang, L.; Lian, X.; Liu, H.; Zhang, Y.; Li, Q.; Cai, Y.; Ma, H.; Yu, X. Understanding diabetic neuropathy: Focus on oxidative stress. Oxidative Med. Cell. Longev. 2020, 2020, 9524635. [Google Scholar] [CrossRef] [PubMed]
- Liguori, I.; Russo, G.; Curcio, F.; Bulli, G.; Aran, L.; Della-Morte, D. Oxidative stress, aging, and diseases. Clin. Interv. Aging 2018, 13, 757–772. [Google Scholar] [CrossRef]
- Venkataraman, K.; Khurana, S.; Tai, T.C. Oxidative stress in aging—Matters of the heart and mind. Int. J. Mol. Sci. 2013, 14, 17897–17925. [Google Scholar] [CrossRef]
- Pannu, N.; Bhatnagar, A. Resveratrol: From enhanced biosynthesis and bioavailability to multitargeting chronic diseases. Biomed. Pharmacother. 2019, 109, 2237–2251. [Google Scholar] [CrossRef]
- Diaz-Gerevini, G.T.; Repossi, G.; Dain, A.; Tarres, M.C.; Das, U.N.; Eynard, A.R. Beneficial action of resveratrol: How and why? Nutrition 2016, 32, 174–178. [Google Scholar] [CrossRef]
- Koushki, M.; Amiri-Dashatan, N.; Ahmadi, N.; Abbaszadeh, H.A.; Rezaei-Tavirani, M. Resveratrol: A miraculous natural compound for diseases treatment. Food Sci. Nutr. 2018, 6, 2473–2490. [Google Scholar] [CrossRef]
- Meng, X.; Zhou, J.; Zhao, C.N.; Gan, R.Y.; Li, H.B. Health benefits and molecular mechanisms of resveratrol: A narrative review. Foods 2020, 9, 340. [Google Scholar] [CrossRef] [PubMed]
- Galiniak, S.; Aebisher, D.; Bartusik-Aebisher, D. Health benefits of resveratrol administration. Acta Biochim. Pol. 2019, 66, 13–21. [Google Scholar] [CrossRef]
- Colica, C.; Milanović, M.; Milić, N.; Aiello, V.; De Lorenzo, A.; Abenavoli, L. A Systematic review on natural antioxidant properties of resveratrol. Nat. Prod. Commun. 2018, 13, 1195–1203. [Google Scholar] [CrossRef]
- Rauf, A.; Imran, M.; Suleria, H.A.R.; Ahmad, B.; Petersf, D.G.; Mubarak, M.S. A comprehensive review of the health perspectives of resveratrol. Food Funct. 2017, 8, 4284–4305. [Google Scholar] [CrossRef] [PubMed]
- Öztürka, E.; Karaboğa, A.K.A.; Yerer, M.B.; Bishayee, A. Resveratrol and diabetes: A critical review of clinical studies. Biomed. Pharmacother. 2017, 95, 230–234. [Google Scholar] [CrossRef]
- Watroba, M.; Szukiewicz, D. The role of sirtuins in aging and age-related diseases. Adv. Med. Sci. 2016, 61, 52–62. [Google Scholar] [CrossRef]
- Kitada, M.; Ogura, Y.; Monno, I.; Koya, D. Sirtuins and type 2 diabetes: Role in inflammation, oxidative stress, and mitochondrial function. Front. Endocrinol. 2019, 10, 187. [Google Scholar] [CrossRef]
- Goh, K.P.; Lee, H.Y.; Lau, D.P.; Supaat, W.; Chan, Y.H.; Yung, A.F. Effects of resveratrol in patients with type 2 diabetes mellitus on skeletal muscle SIRT1 expression and energy expenditure. Int. J. Sport Nutr. Exerc. Metab. 2014, 24, 2–13. [Google Scholar] [CrossRef]
- Samuel, V.P.; Gupta, G.; Dahiya, R.; Jain, D.A.; Mishra, A.; Dua, K. Current update on preclinical and clinical studies of resveratrol, a naturally occurring phenolic compound. Crit. Rev. Eukaryot. Gene Expr. 2019, 29, 529–537. [Google Scholar] [CrossRef] [PubMed]
- Park, E.J.; Pezzuto, J.M. The pharmacology of resveratrol in animals and humans. Biochim. Biophys. Acta 2015, 1852, 1071–1113. [Google Scholar] [CrossRef] [PubMed]
- Oyenihi, O.R.; Oyenihi, A.B.; Adeyanju, A.A.; Oguntibeju, O.O. Antidiabetic effects of resveratrol: The way forward in its clinical utility. J. Diabetes Res. 2016, 2016, 9737483. [Google Scholar] [CrossRef] [PubMed]
- Singh, A.P.; Singh, R.; Singh, S.V.; Rai, V.; Kaschula, C.H.; Maiti, P.; Gupta, S.C. Health benefits of resveratrol: Evidence from clinical studies. Med. Res. Rev. 2019, 39, 1851–1891. [Google Scholar] [CrossRef] [PubMed]
- García-Martínez, B.I.; Ruiz-Ramos, M.; Pedraza-Chaverri, J.; Santiago-Osorio, E.; Mendoza-Núñez, V.M. Hypoglycemic effect of resveratrol: A systematic review and meta-analysis. Antioxidants 2021, 10, 69. [Google Scholar] [CrossRef]
- Ahmed, T.; Javed, S.; Javed, S.; Tariq, A.; Šamec, D.; Tejada, S.; Nabavi, S.F.; Braidy, N.; Nabavi, S.M. Resveratrol and Alzheimer’s disease: Mechanistic insights. Mol. Neurobiol. 2017, 54, 2622–2635. [Google Scholar] [CrossRef]
- Berman, A.Y.; Motechin, R.A.; Wiesenfeld, M.Y.; Holz, M.K. The therapeutic potential of resveratrol: A review of clinical trials. NPJ Precis. Oncol. 2017, 1, 35. [Google Scholar] [CrossRef] [PubMed]
- Tonetto, I.F.A.; Baptista, M.H.B.; Gomides, D.S.; Pace, A.E. Quality of life of people with diabetes mellitus. Rev. Esc. Enferm. USP 2019, 53, e03424. [Google Scholar] [CrossRef]
- Dehdashtian, E.; Hossein, M.P.; Hemati, K.; Mehrzadi, S.; Hosseinzadeh, A. Therapeutic application of nutraceuticals in diabetic nephropathy: Current evidence and future implications. Diabetes Metab. Res. Rev. 2020, 36, e3336. [Google Scholar] [CrossRef]
- Theodotou, M.; Fokianos, K.; Mouzouridou, A.; Konstantinou, C.; Aristotelous, A.; Prodromou, D.; Chrysokou, A. The effect of resveratrol on hypertension: A clinical trial. Exp. Ther. Med. 2017, 13, 295–301. [Google Scholar] [CrossRef]
- Cao, X.; Luo, T.; Tang, Z. Resveratrol prevents Angll-induced hypertension via AMPK activation and RhoA/ROCK suppression in mice. Hypertens Res. 2014, 37, 803–810. [Google Scholar] [CrossRef]
- Timmers, S.; Konings, E.; Bilet, N.; Houtkooeper, R.; Van Der Weijer, T.; Goossens, G.H.; Hoeks, J.; Van Der Kriekeen, S.; Ryu, D.; Kersten, S.; et al. Calorie restriction-like effects of 30 days of resveratrol (resVidaTM) supplementation on energy metabolism and metabolic profile in obese humans. Cell Metab. 2011, 14, 612–622. [Google Scholar] [CrossRef]
- Méndez-del Villar, M.; González-Ortiz, M.; Martínez-Abundis, E.; Pérez-Rubio, K.; Lizárraga-Valdez, R. Effect of resveratrol administration on metabolic syndrome, insulin sensitivity, and insulin secretion. Metab. Syndr. Relat. Disord. 2014, 12, 497–501. [Google Scholar] [CrossRef] [PubMed]
- Gowd, V.; Kang, Q.; Wang, Q.; Wang, Q.; Chen, F.; Cheng, K.W. Resveratrol: Evidence for its nephroprotective effect in diabetic nephropathy. Adv. Nutr. 2020, 11, 1555–1568. [Google Scholar] [CrossRef] [PubMed]
- Mahjabeen, W.; Khan, D.A.; Mirza, S.A. Role of resveratrol supplementation in regulation of glucose hemostasis, inflammation and oxidative stress in patients with diabetes mellitus type 2: A randomized, placebo-controlled trial. Complement. Ther. Med. 2022, 6, 102819. [Google Scholar] [CrossRef] [PubMed]
- Abdollahi, S.; Salehi-Abargouei, A.; Toupchian, O.; Sheikhha, M.H.; Fallahzadeh, H.; Rahmanian, M.; Tabatabaie, M.; Mozaffari-Khosravi, H. The effect of resveratrol supplementation on cardio-metabolic risk factors in patients with type 2 diabetes: A randomized, double-blind controlled trial. Phytother. Res. 2019, 33, 3153–3162. [Google Scholar] [CrossRef] [PubMed]
- Javid, A.Z.; Hormoznejad, R.; Yousefimanesh, H.; Zakerkish, M.; Haghighi-zadeh, M.H.; Dehghan, P.; Ravanbakhsh, M. The impact of resveratrol supplementation on blood glucose, insulin, insulin resistance, triglyceride, and periodontal markers in type 2 diabetic patients with chronic periodontitis. Phytother. Res. 2016, 31, 108–114. [Google Scholar] [CrossRef]
- Bhatt, J.K.; Thomas, S.; Nanjan, M.J. Resveratrol supplementation improves glycemic control in type 2 diabetes mellitus. Nutr. Res. 2012, 32, 537–541. [Google Scholar] [CrossRef]
- Hoseini, A.; Namazi, G.; Farrokhian, A.; Reiner, Z.; Aghadavod, E.; Bahmani, F.; Asemi, Z. The effects of resveratrol on metabolic status in patients with type 2 diabetes mellitus and coronary heart disease. Food Funct. 2019, 10, 6042–6051. [Google Scholar] [CrossRef]
- Khodabandehloo, H.; Seyyedebrahimi, S.; Esfahani, E.N.; Razi, F.; Meshkani, R. Resveratrol supplementation decreases blood glucose without changing the circulating CD14+ CD16+ monocytes and inflammatory cytokines in patients with type 2 diabetes: A randomized, double-blind, placebo-controlled study. Nutr. Res. 2018, 54, 40–51. [Google Scholar] [CrossRef]
- Hausenblas, H.A.; Schoulda, J.A.; Smoliga, J.M. Resveratrol treatment as an adjunct to pharmacological management in type 2 diabetes mellitus—Systematic review and meta-analysis. Mol. Nutr. Food Res. 2015, 59, 147–159. [Google Scholar] [CrossRef]
- Liu, K.; Zhou, R.; Wang, B.; Mi, M.T. Effect of resveratrol on glucose control and insulin sensitivity: A meta-analysis of 11 randomized controlled trials. Am. J. Clin. Nutr. 2014, 99, 1510–1519. [Google Scholar] [CrossRef]
- De Ligt, M.; Bruls, Y.; Hansen, J.; Habets, M.F.; Havekes, B.; Nascimento, E.B.M.; Moonen-Kornips, E.; Schaart, G.; Schrauwen-Hinderling, V.B.; Lichtenbelt, W.V.M.; et al. Resveratrol improves ex vivo mitochondrial function but does not insulin sensitivity or brown adipose tissue in first degree relatives of patients with type 2 diabetes. Mol. Metab. 2018, 12, 39–47. [Google Scholar] [CrossRef] [PubMed]
- Timmers, S.; De Ligt, M.; Phielix, E.; Van de Weijer, T.; Hansen, J.; Moonen-Kornips, E.; Schaart, G.; Kunz, I.; Hesselink, M.K.C.; Schrauwen-Hinderling, V.B.; et al. Resveratrol as add-on therapy in subjects with well-controlled type 2 diabetes: A randomized controlled trial. Diabetes Care 2016, 19, 2211–2217. [Google Scholar] [CrossRef] [PubMed]
- Thazhath, S.S.; Wu, T.; Bound, M.J.; Checklin, H.L.; Standfield, S.; Jones, K.L.; Horowitz, M.; Rayner, C.K. Administration of resveratrol for 5 wk has no effect on glucagon-like peptide 1 secretion, gastric emptying, or glycemic control in type 2 diabetes: A randomized controlled trial. Am. J. Clin. Nutr. 2016, 103, 66–70. [Google Scholar] [CrossRef] [PubMed]
- Bo, S.; Ponzo, V.; Ciccone, G.; Evangelista, A.; Saba, F.; Goitre, I.; Procopio, M.; Pagano, G.F.; Cassader, M.; Gambino, R. Six months of resveratrol supplementation has no measurable effect in type 2 diabetic patients. A randomized, double blind, placebo-controlled trial. Pharmacol. Res. 2016, 111, 896–905. [Google Scholar] [CrossRef]
- Jeyaraman, M.M.; Al-Yousif, N.S.; Mann, A.S.; Dolinsky, V.W.; Rabbani, R.; Zarychanski, R.; Abou-Setta, A.M. Resveratrol for adults with type 2 diabetes mellitus. Cochrane Database Syst. Rev. 2020, 2020, CD011919. [Google Scholar] [CrossRef]
- García-Martínez, B.I.; Ruiz-Ramos, M.; Pedraza-Chaverri, J.; Santiago-Osorio, E.; Mendoza-Núñez, V.M. Influence of age and dose on the effect of resveratrol for glycemic control in type 2 diabetes mellitus: Systematic review and meta- analysis. Molecules 2022, 27, 5232. [Google Scholar] [CrossRef]
- Zhang, C.; Luo, J.; Yu, B.; Chen, J.; Chen, D. Effect of resveratrol on lipid metabolism in muscle and adipose tissues: A reevaluation in a pig model. J. Funct. Foods. 2015, 14, 590–595. [Google Scholar] [CrossRef]
- Cao, X.; Liao, W.; Xia, H.; Wang, S.; Sun, G. The effect of resveratrol on blood lipid profile: A dose-response Meta-analysis of randomized controlled trials. Nutrients 2022, 14, 3755. [Google Scholar] [CrossRef]
- Lee, C.C.; Adler, A.I.; Sandhu, M.S.; Sharp, S.J.; Forouhi, N.G.; Erqou, S.; Luben, R.; Bingham, S.; Khaw, K.T.; Wareham, N.J. Association of C-reactive protein with type 2 diabetes: Prospective analysis and meta-analysis. Diabetologia 2009, 52, 1040–1047. [Google Scholar] [CrossRef]
- Oguntibeju, O.O. Type 2 diabetes mellitus, oxidative stress and inflammation: Examining the links. Int. J. Physiol. Pathophysiol. Pharmacol. 2019, 11, 45–63. [Google Scholar]
- Meng, T.; Xiao, D.; Muhammed, A.; Deng, J.; Chen, L.; He, J. Anti-inflammatory action mechanisms of resveratrol. Molecules 2021, 26, 229. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.-X.; Li, C.-X.; Kakar, M.U.; Khan, M.S.; Wu, P.-F.; Amir, R.M.; Dai, D.-F.; Naveed, M.; Li, Q.-Y.; Saeed, M.; et al. Resveratrol (RV): A pharmacology review and call for further research. Biomed. Pharmacother. 2021, 143, 112164. [Google Scholar] [CrossRef] [PubMed]
- Khattar, S.; Khan, S.A.; Zaidi, S.A.A.; Darvishikolour, M.; Farooq, U.; Naseef, P.P.; Kurunian, M.S.; Khan, M.Z.; Shamin, A.; Khan, M.M.U.; et al. Resveratrol from dietary supplement to a drug candidate: An assessment of potential. Pharmaceuticals 2022, 15, 957. [Google Scholar] [CrossRef] [PubMed]
- Salehi, B.; Mishra, A.P.; Nigam, M.; Sener, B.; Kilic, M.; Sharifi-Rad, M.; Fokou, P.V.T.; Martins, N.; Sharifi-Rad, J. Resveratrol: A double-edged sword in health benefits. Biomedicines 2018, 6, 91. [Google Scholar] [CrossRef]
- Sattarinezhad, A.; Roozbeh, J.; Yeganeh, S.; Omrani, G.; Shams, M. Resveratrol reduces albuminuria in diabetic nephropathy: A randomized double-blind placebo-controlled clinical trial. Diabetes Metab. 2019, 45, 53–59. [Google Scholar] [CrossRef]
- Collodel, G.; Moretti, E.; Noto, D.; Corsaro, R.; Signorini, C. Oxidation of polyunsaturated fatty acids a promising area of research in infertility. Antioxidants 2022, 11, 1002. [Google Scholar] [CrossRef]
- Sadi, G.; Konat, D. Resveratrol regulates oxidative biomarkers and antioxidant enzymes in the brain of streptozotocin-induced diabetic rats. Pharm. Biol. 2016, 5, 1156–1163. [Google Scholar] [CrossRef]
- Gu, T.; Wang, N.; Wu, T.; Ge, Q.; Chen, L. Antioxidative stress mechanisms behind Resveratrol: A multidimensional analysis. J. Food Qual. 2021, 2021, 5571733. [Google Scholar] [CrossRef]
- Menshchikova, E.B.; Zenkov, N.K.; Tkachev, V.O.; Lemza, A.E.; Kandalintseva, N.N. Protective effect of ARE-Inducing phenol antioxidant TS-13 in chronic inflammation. Bull. Exp. Biol. Med. 2013, 155, 330–334. [Google Scholar] [CrossRef]
- Kou, X.; Kirberger, M.; Yang, Y.; Chen, N. Natural products for cancer prevention associated with Nrf2-ARE pathway. Food Sci. Hum. Wellness 2013, 2, 22–28. [Google Scholar] [CrossRef]
- Seyyedebrahimi, S.; Khodabandehloo, H.; Esfahani, E.N.; Meshkani, R. The effects of resveratrol on markers of oxidative stress in patients with type 2 diabetes: A randomized, double-blind, placebo-controlled clinical trial. Acta Diabetol. 2018, 55, 341–353. [Google Scholar] [CrossRef] [PubMed]
- Sánchez-Rodríguez, M.A.; Santiago-Osorio, E.; Vargas, L.A.; Mendoza-Núñez, V.M. Propuesta de un constructo para evaluar integralmente el estrés oxidativo. Bioquimia 2004, 29, 81–90. [Google Scholar]
- DiNicolantonio, J.J.; McCarty, M.F.; O’Keefe, J.H. Nutraceutical activation of Sirt1: A review. Open Heart 2022, 9, e002171. [Google Scholar] [CrossRef] [PubMed]
- Grzeczka, A.; Kordowitzki, P. Resveratrol and SIRT1: Antiaging cornerstones for oocytes? Nutrients 2022, 14, 510. [Google Scholar] [CrossRef]
- Song, J.; Yang, B.; Jia, X.; Li, M.; Tan, W.; Ma, S.; Shi, X.; Feng, L. Distinctive roles of sirtuins on diabetes, protective or detrimental? Front. Endocrinol. 2018, 9, 724. [Google Scholar] [CrossRef]
- Houtkooper, R.H.; Pirinen, E.; Auwerx, J. Sirtuins as regulators of metabolism and healthspan. Nat. Rev. Mol. Cell Biol. 2016, 13, 225–238. [Google Scholar] [CrossRef]
- Xu, J.; Kitada, M.; Koya, D. The impact of mitochondrial quality control by sirtuins on the treatment of type 2 diabetes and diabetic kidney disease. Biochim. Biophys. Acta Mol. Basis Dis. 2020, 1866, 165756. [Google Scholar] [CrossRef]
- DiNicolantonio, J.J.; McCarty, M.F.; Assanga, S.I.; Lujan, L.L.; O’Keefe, J.H. Ferulic acid and berberine, via SIRT1 and AMPK, may act as cell cleasing promoters of healthy longevity. Open Heart 2022, 9, e001801. [Google Scholar] [CrossRef]
- Khan, M.A.; Chen, H.; Wan, X.; Tania, M.; Xu, A.; Chen, F.; Zhang, D. Regulatori effects of resveratrol on antioxidant enzymes: A mechanism of growth inhibition and apoptosis induction in cancer cells. Mol. Cells. 2013, 35, 219–225. [Google Scholar] [CrossRef]
- Olmos, Y.; Sánchez-Gómez, F.J.; Wild, B.; García-Quintans, N.; Cabezudo, S.; Lamas, S.; Monsalve, M. SirT1 regulation of antioxidant genes is dependent on the formation of a FOXO3a/PGC-1α complex. Antioxid. Redox Signal. 2013, 19, 1507–1521. [Google Scholar] [CrossRef]
- Hwang, J.; Yao, H.; Caito, S.; Sundar, I.K.; Rahman, I. Redox regulation of SIRT1 in inflammation and cellular senescence. Free. Radic. Biol. Med. 2013, 61, 95–110. [Google Scholar] [CrossRef] [PubMed]
- Chachay, V.S.; Kirkpatrick, C.M.; Hickman, I.J.; Ferguson, M.; Prins, J.B.; Martin, J.H. Resveratrol–pills to replace a healthy diet? Br. J. Clin. Pharmacol. 2011, 72, 27–38. [Google Scholar] [CrossRef] [PubMed]
- Ramírez-Garza, S.L.; Laveriano-Santos, E.P.; Marhuenda-Muñoz, M.; Storniolo, C.E.; Tresserra-Rimbau, A.; Vallverdú-Queralt, A.; Lamuela-Raventós, R.M. Health effects of resveratrol: Results from human intervention trials. Nutrients 2018, 10, 1892. [Google Scholar] [CrossRef] [PubMed]
- de Salud, S. Norma Oficial Mexicana NOM-030-SSA2-1999, Para la Prevención, Tratamiento y Control de la Hipertensión Arterial; Secretaría de Salud: Mexico City, Mexico, 1999. [Google Scholar]
- Jentzsch, A.M.; Bachmann, H.; Fürst, P.; Biesalski, H.K. Improved analysis of malondialdehyde in human body fluids. Free. Radic. Biol. Med. 1996, 20, 251–256. [Google Scholar] [CrossRef]
- McCord, J.M.; Fridovich, I. Superoxide dismutase an enzyme function for erythrocuprein. J. Biol. Chem. 1969, 244, 6049–6055. [Google Scholar] [CrossRef]
- Plagia, D.E.; Valentine, W.N. Studies on the quantitative characterization of erytrocyte of glutathione peroxidase. J. Lab. Clin. Med. 1967, 70, 158–169. [Google Scholar]
- Aebi, H. Catalase in vitro. Methods Enzymol. 1984, 105, 121–126. [Google Scholar] [CrossRef]
- Miller, N.J.; Rice, E.C.; Davies, M.J. Total antioxidant status in plasma and body fluids. Methods Enzymol. 1994, 234, 279–293. [Google Scholar] [CrossRef]

| Parameter | EG1000 (n = 37) | EG500 (n = 32) | Placebo (n = 28) | |||
|---|---|---|---|---|---|---|
| Baseline | Six months | Baseline | Six months | Baseline | Six months | |
| Age (years) | 66 ± 6 | 63 ± 7 | 64 ± 5 | |||
| BMI (kg/m2) | 27.6 ± 4.4 | 27.7 ± 4.2 | 27.8 ± 3.6 | 27.9 ± 4.0 | 28.3 ± 3.4 | 27.9 ± 3.0 |
| SBP (mmHg) | 127 ± 16 | 124 ± 12 | 128 ± 17 | 127 ± 14 | 127 ± 10 | 123 ± 10 |
| DBP (mmHg) | 84 ± 6 | 82 ± 9 | 84 ± 13 | 86 ± 8 | 82 ± 7 | 82 ± 9 |
| Parameters | EG1000 (n = 37) | EG500 (n = 32) | Placebo (n = 28) | |||
|---|---|---|---|---|---|---|
| Baseline | Six months | Baseline | Six months | Baseline | Six months | |
| Hemoglobin (g/dL) | 14 ± 1 | 14 ± 2 | 14 ± 1 | 15 ± 2 | 14 ± 2 | 14 ± 2 |
| Hematocrit (%) | 45 ± 4 | 45 ± 4 | 45 ± 6 | 46 ± 5 | 45 ± 6 | 46 ± 5 |
| Glucose (mg/dL) | 169 ± 73 | 186 ± 83 | 185 ± 65 | 203 ± 77 | 189 ± 74 | 184 ± 67 |
| Urea (mg/dL) | 33 ± 8 | 36 ± 14 | 33 ± 14 | 33 ± 15 | 36 ± 11 | 38 ± 17 |
| Creatinine (mg/dL) | 0.92 ± 0.2 | 0.92 ± 0.2 | 0.96 ± 0.2 | 1.0 ± 0.33 | 0.95 ± 0.2 | 1.01 ± 0.3 |
| Uric acid (mg/dL) | 4.5 ± 1.6 | 4.0 ± 1.3 | 4.4 ± 1.8 | 3.8 ± 0.9 | 5.1 ± 1.9 | 4.6 ± 1.4 |
| Total cholesterol (mg/dL) | 200 ± 44 | 202 ± 69 | 196 ± 41 | 211 ± 70 | 198 ± 42 | 210 ± 47 |
| Triglycerides (md/dL) | 170 ± 69 | 147 ±46 * | 219 ± 115 | 221 ± 122 | 197 ± 85 | 205 ± 62 |
| HDL-cholesterol (mg/dL) | 61 ± 26 | 56 ± 16 | 54 ± 16 | 53 ± 15 | 54 ± 17 | 52 ± 11 |
| Albumin (g/dL) | 4.3 ± 0.3 | 4.8 ± 0.6 | 4.4 ± 0.4 | 4.9 ± 0.5 | 4.3 ± 0.3 | 4.8 ± 0.5 |
| CRP (mg/dL) | 0.23 ± 0.3 | 0.22 ± 0.4 | 0.22 ± 0.2 | 0.33 ± 0.3 | 0.37 ± 0.4 | 0.48 ± 0.5 * |
| HbA1c (%) | 7.9 ± 2.0 | 7.8 ± 1.8 | 8.0 ± 2.0 | 8.1 ± 1.5 | 7.7 ± 2.2 | 8.0 ± 1.7 |
| Parameters | EG1000 (n = 37) | EG500 (n = 32) | Placebo (n = 28) | |||
|---|---|---|---|---|---|---|
| Baseline | Six months | Baseline | Six months | Baseline | Six months | |
| LPO (µmol/L) | 0.230 ± 0.08 | 0.212 ± 0.04 | 0.236 ± 0.09 | 0.229 ± 0.06 | 0.219 ± 0.07 | 0.282 ± 0.07 * |
| 8-Iso (pg/mL) | 60 ± 25 | 45 ± 27 | 56 ± 29 | 53 ± 38 | 61 ± 24 | 76 ± 35 † |
| GPx (UI/L) | 7181 ± 3794 | 8362 ± 2910 | 8219 ± 3616 | 8926 ± 3010 | 7635 ± 2988 | 7329 ± 3049 |
| Cat (×104 UI/gHb) | 3.0 ± 1 | 2.9 ± 1 | 2.9 ± 1 | 2.7 ± 1 | 2.9 ± 1 | 2.3 ± 1 |
| TAC (µmol/L) | 1003 ± 247 | 1225 ± 249 * | 1016 ± 216 | 1014 ± 269 | 1004 ± 202 | 993 ± 217 |
| SOD (Ul/L) | 167 ± 15 | 173 ± 12 | 172 ± 16 | 178 ± 10 | 171 ± 15 | 167 ± 10 † |
| SOD/GPx ratio | 0.030 ± 0.01 | 0.023 ± 0.01 | 0.027 ± 0.01 | 0.026 ± 0.01 | 0.026 ± 0.01 | 0.023 ± 0.01 |
| GAP | 283 ± 242 | 434 ± 234 ‡ | 288 ± 221 | 202 ± 284 | 276 ± 175 | 205 ± 229 |
| OSS | 2.5 ± 1 | 1.3 ± 1 | 2.0 ± 1 | 1.7 ± 1 | 1.9 ± 1 | 2.8 ± 1 * |
| EG1000 (n = 37) | EG500 (n = 32) | Placebo (n = 28) | ||||
|---|---|---|---|---|---|---|
| Baseline | Six months | Baseline | Six months | Baseline | Six months | |
| Without OS | 5 (13%) | 13 (35%) * | 3 (10%) | 9 (28%) * | 6 (21%) | 2 (7%) * |
| Mild OS | 14 (38%) | 18 (49%) | 19 (59%) | 15 (47%) | 12 (43%) | 8 (29%) * |
| Moderate OS | 10 (27%) | 5 (13%) * | 9 (28%) | 8 (25%) | 8 (29%) | 15 (54%) * |
| Severe OS | 8 (22%) | 1 (3%) * | 1 (3%) | 0 (0%) | 2 (7%) | 3 (10%) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
García-Martínez, B.I.; Ruiz-Ramos, M.; Pedraza-Chaverri, J.; Santiago-Osorio, E.; Mendoza-Núñez, V.M. Effect of Resveratrol on Markers of Oxidative Stress and Sirtuin 1 in Elderly Adults with Type 2 Diabetes. Int. J. Mol. Sci. 2023, 24, 7422. https://doi.org/10.3390/ijms24087422
García-Martínez BI, Ruiz-Ramos M, Pedraza-Chaverri J, Santiago-Osorio E, Mendoza-Núñez VM. Effect of Resveratrol on Markers of Oxidative Stress and Sirtuin 1 in Elderly Adults with Type 2 Diabetes. International Journal of Molecular Sciences. 2023; 24(8):7422. https://doi.org/10.3390/ijms24087422
Chicago/Turabian StyleGarcía-Martínez, Beatriz Isabel, Mirna Ruiz-Ramos, José Pedraza-Chaverri, Edelmiro Santiago-Osorio, and Víctor Manuel Mendoza-Núñez. 2023. "Effect of Resveratrol on Markers of Oxidative Stress and Sirtuin 1 in Elderly Adults with Type 2 Diabetes" International Journal of Molecular Sciences 24, no. 8: 7422. https://doi.org/10.3390/ijms24087422
APA StyleGarcía-Martínez, B. I., Ruiz-Ramos, M., Pedraza-Chaverri, J., Santiago-Osorio, E., & Mendoza-Núñez, V. M. (2023). Effect of Resveratrol on Markers of Oxidative Stress and Sirtuin 1 in Elderly Adults with Type 2 Diabetes. International Journal of Molecular Sciences, 24(8), 7422. https://doi.org/10.3390/ijms24087422







