Resveratrol for the Management of Human Health: How Far Have We Come? A Systematic Review of Resveratrol Clinical Trials to Highlight Gaps and Opportunities
Abstract
1. Introduction
2. Methods
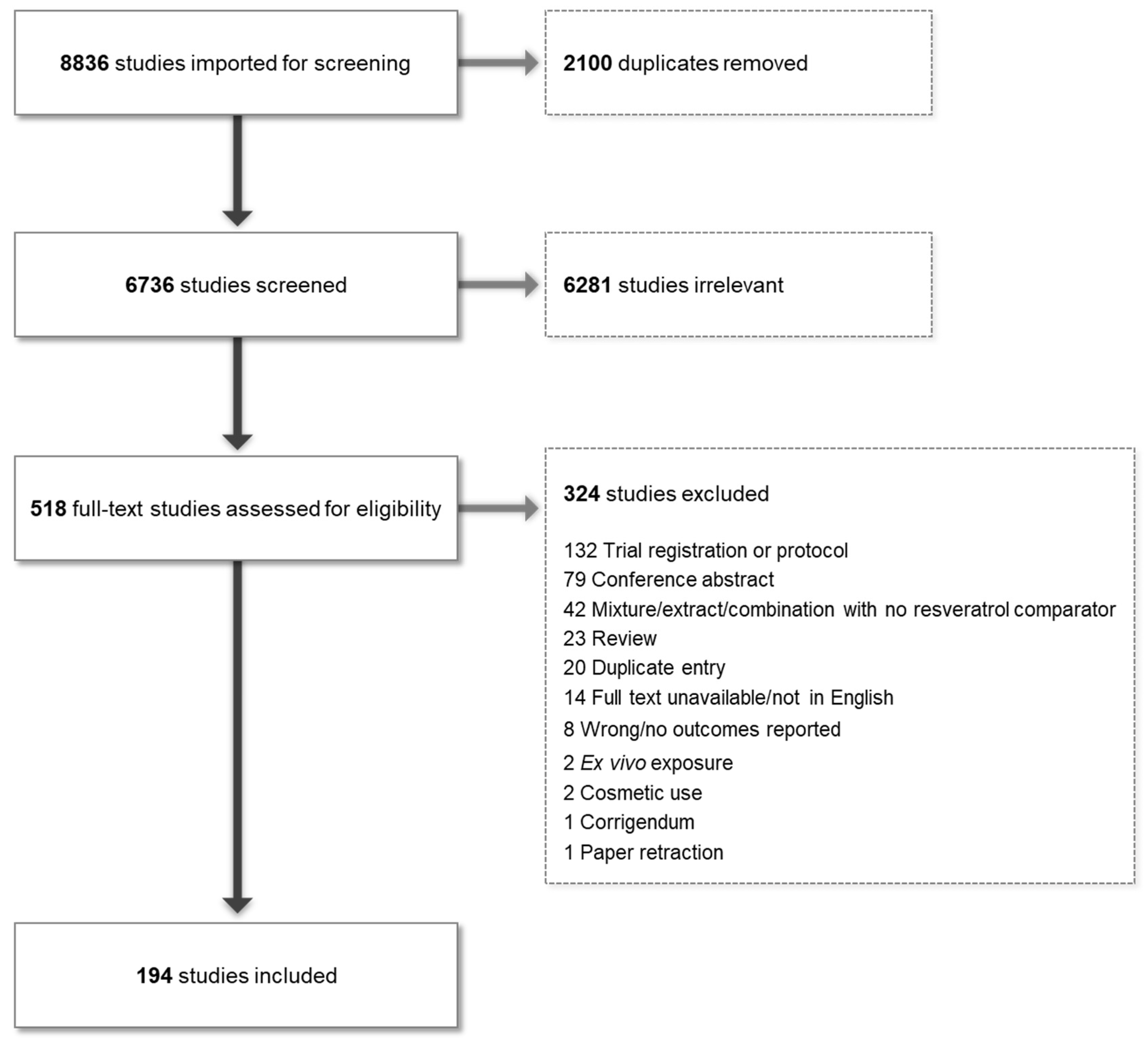
2.1. Database Screening and Inclusion/Exclusion Criteria
2.2. Data Extraction
2.3. Quality Assessment
3. Results
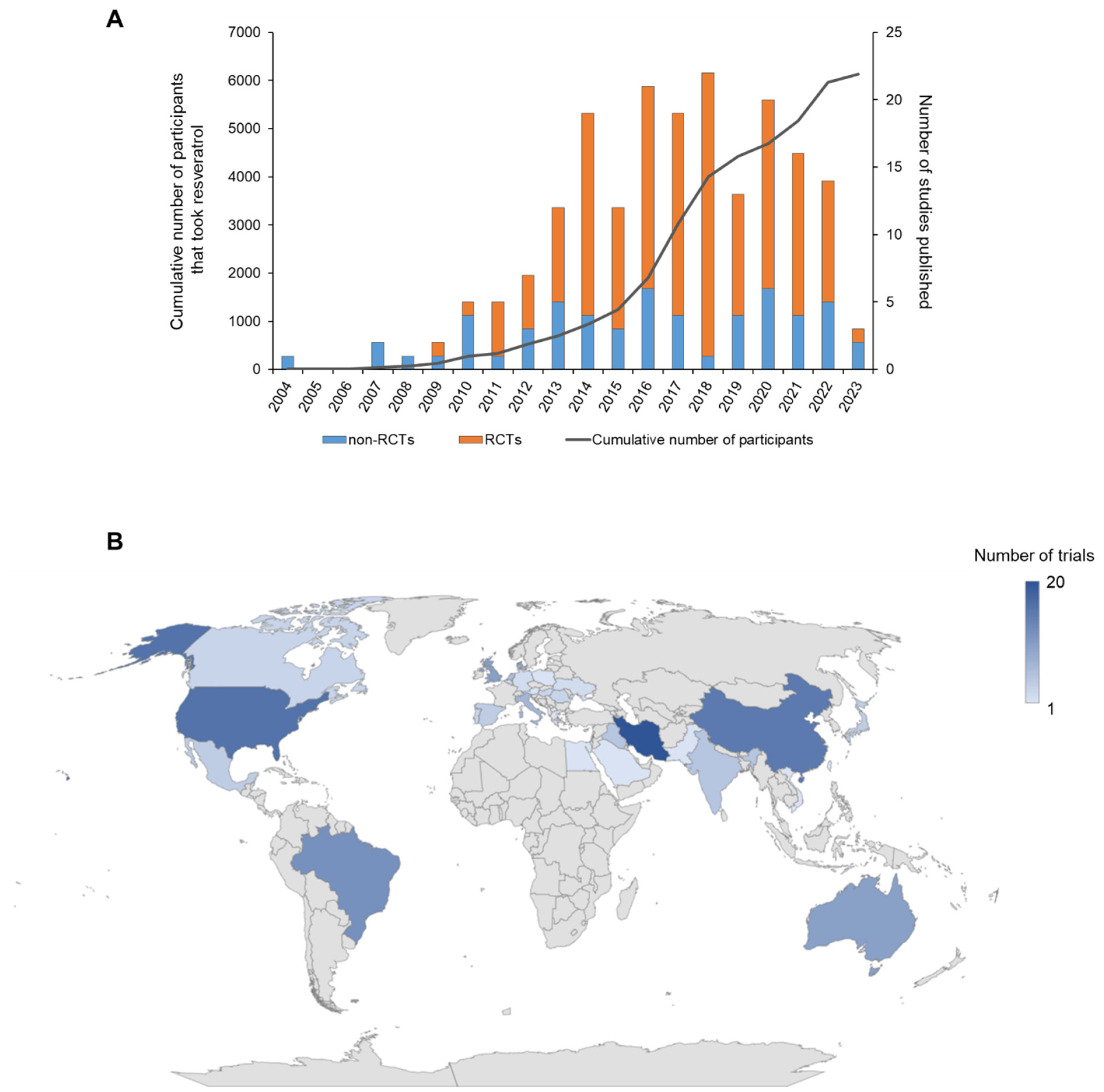
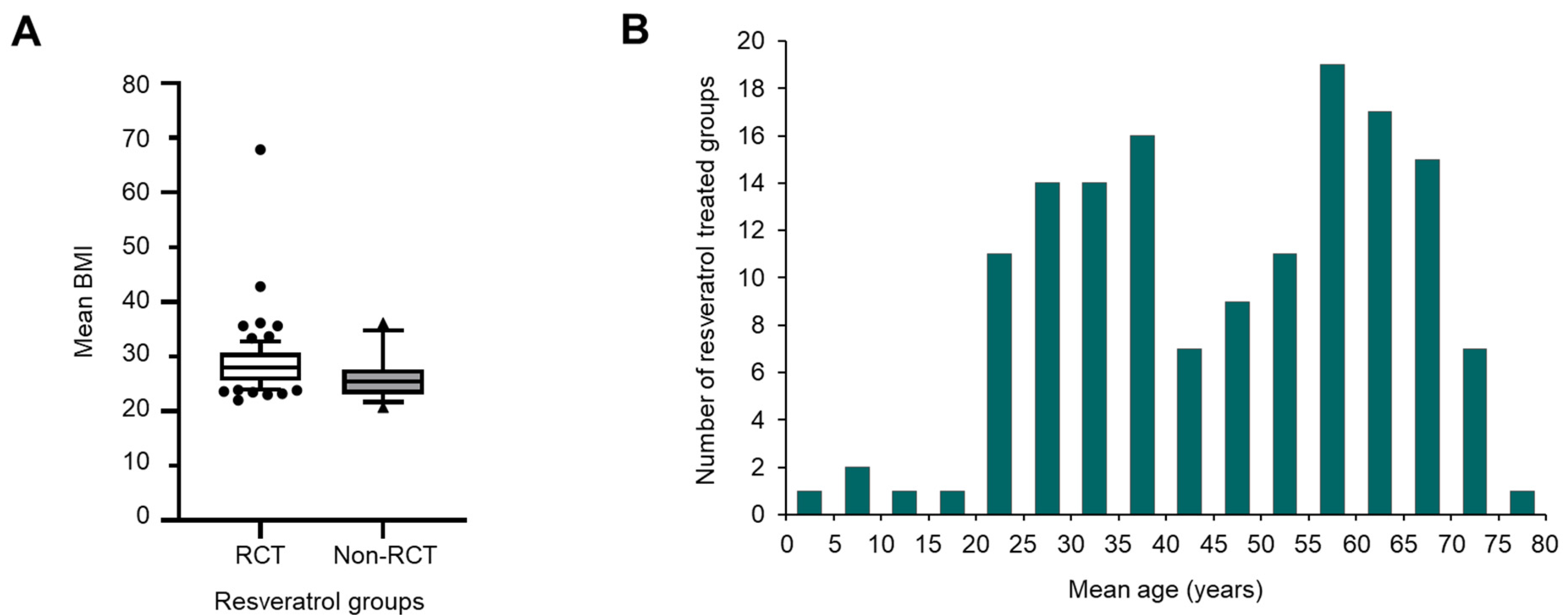
3.1. Number and Geographical Location of RSV Trials
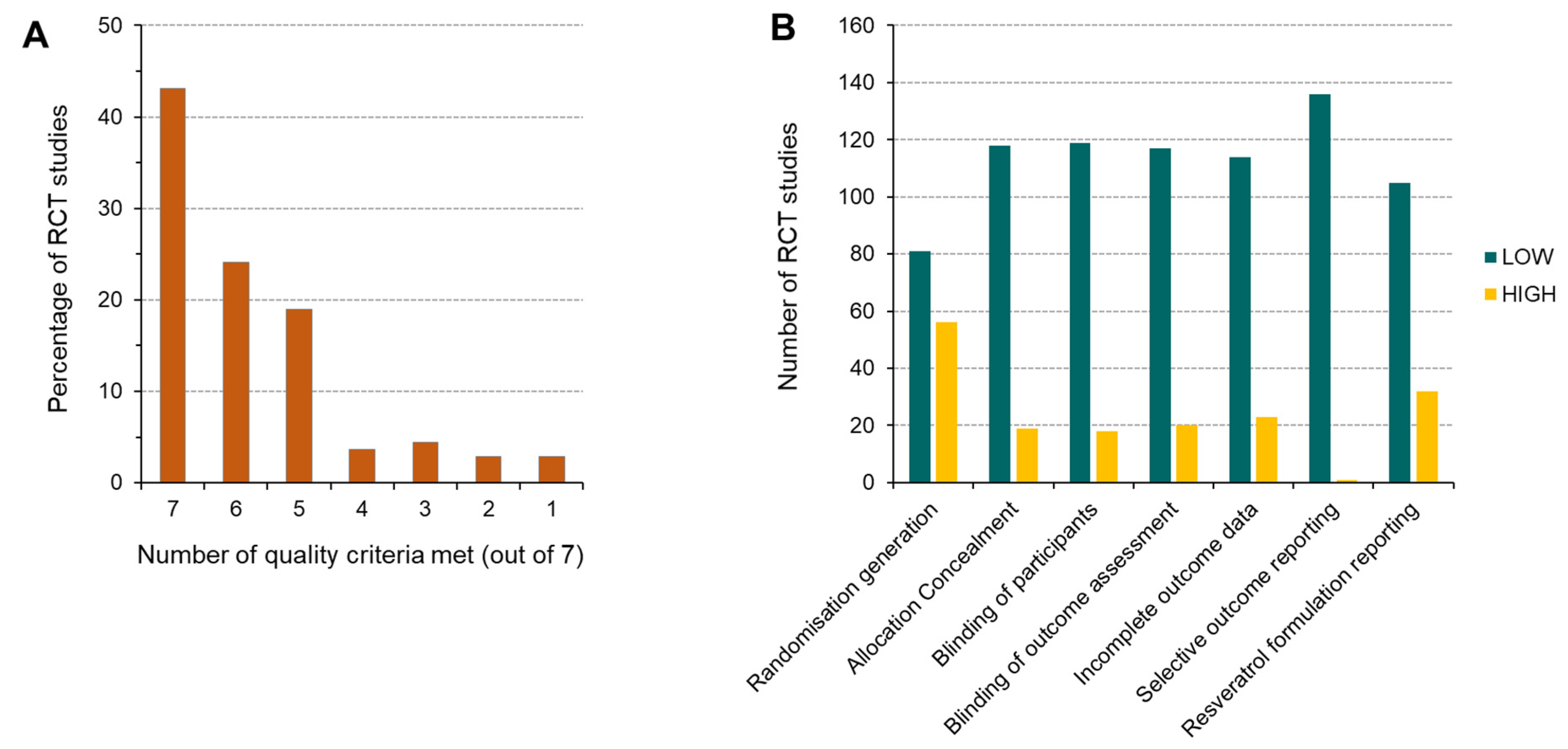
3.2. Quality Assessment—Risk of Bias
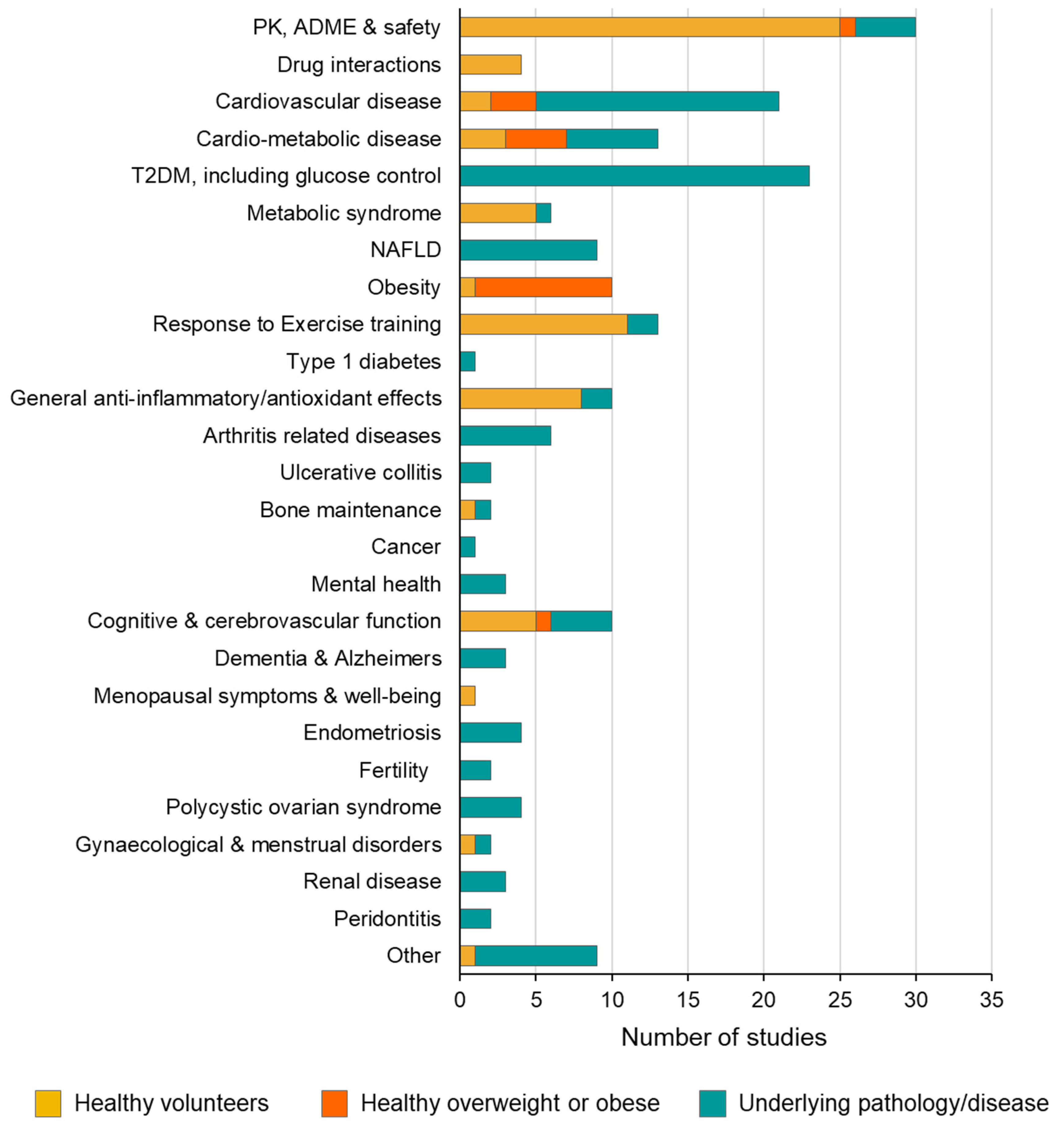
3.3. Clinical Indication and Trial Participants
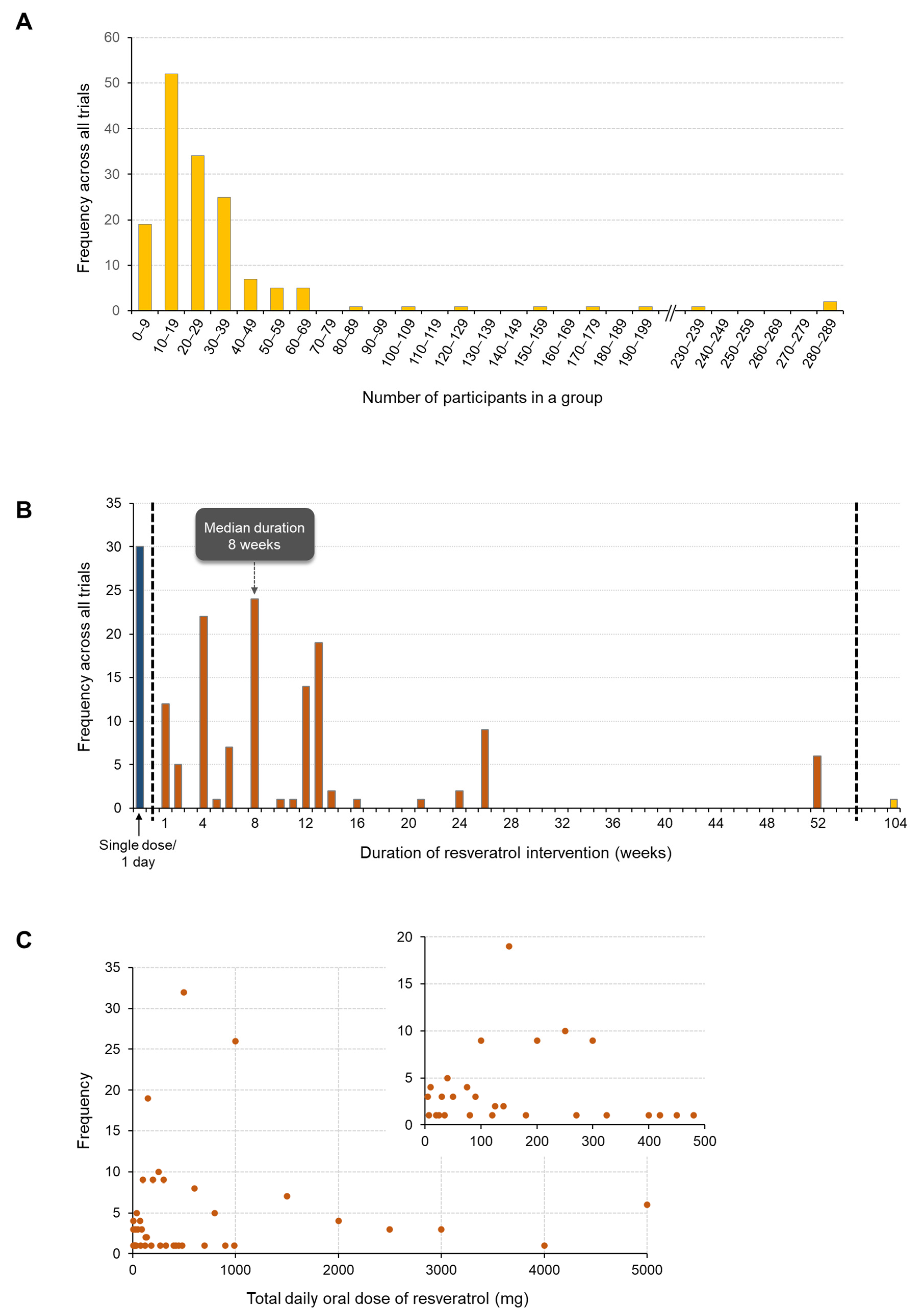
3.4. Duration and Size of Trials
3.5. RSV Dose
3.6. Safety Reporting
3.7. Types of Trial Outcomes
4. Discussion
4.1. Challenges and Knowledge Gaps
4.2. Encouraging Findings and Opportunities
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Jang, M.; Cai, L.; Udeani, G.O.; Slowing, K.V.; Thomas, C.F.; Beecher, C.W.; Fong, H.H.; Farnsworth, N.R.; Kinghorn, A.D.; Mehta, R.G.; et al. Cancer chemopreventive activity of resveratrol, a natural product derived from grapes. Science 1997, 275, 218–220. [Google Scholar] [CrossRef]
- Walle, T.; Hsieh, F.; DeLegge, M.H.; Oatis, J.E., Jr.; Walle, U.K. High absorption but very low bioavailability of oral resveratrol in humans. Drug Metab. Dispos. 2004, 32, 1377–1382. [Google Scholar] [CrossRef]
- Apostolidou, C.; Adamopoulos, K.; Iliadis, S.; Kourtidou-Papadeli, C. Alterations of antioxidant status in asymptomatic hypercholesterolemic individuals after resveratrol intake. Int. J. Food Sci. Nutr. 2015, 67, 541–552. [Google Scholar] [CrossRef]
- Chekalina, N.I. Resveratrol has a positive effect on parameters of central hemodynamics and myocardial ischemia in patients with stable coronary heart disease. Wiad. Lek. 2017, 70, 286–291. [Google Scholar]
- Chen, J.; Bai, Q.; Zhao, Z.; Sui, H.; Xie, X. Resveratrol improves delayed r-tPA treatment outcome by reducing MMPs. Acta Neurol. Scand. 2016, 134, 54–60. [Google Scholar] [CrossRef]
- Ding, J.; Kang, Y.; Fan, Y.; Chen, Q. Efficacy of resveratrol to supplement oral nifedipine treatment in pregnancy-induced preeclampsia. Endocr. Connect. 2017, 6, 595–600. [Google Scholar] [CrossRef]
- Djurica, D.; Ren, J.; Holt, R.R.; Feng, X.; Carlson, C.R.; Shindel, A.W.; Keen, C.L.; Hackman, R.M. A single intake of a resveratrol-arginine conjugate improves microvascular function compared to trans-resveratrol in postmenopausal women. PharmaNutrition 2016, 4, 132–138. [Google Scholar] [CrossRef]
- Fodor, K.; Tit, D.M.; Pasca, B.; Bustea, C.; Uivarosan, D.; Endres, L.; Iovan, C.; Abdel-Daim, M.M.; Bungau, S. Long-Term Resveratrol Supplementation as a Secondary Prophylaxis for Stroke. Oxidative Med. Cell. Longev. 2018, 2018, 4147320. [Google Scholar] [CrossRef]
- Gal, R.; Deres, L.; Horvath, O.; Eros, K.; Sandor, B.; Urban, P.; Soos, S.; Marton, Z.; Sumegi, B.; Toth, K.; et al. Resveratrol Improves Heart Function by Moderating Inflammatory Processes in Patients with Systolic Heart Failure. Antioxidants 2020, 9, 1108. [Google Scholar] [CrossRef]
- Gal, R.; Praksch, D.; Kenyeres, P.; Rabai, M.; Toth, K.; Halmosi, R.; Habon, T. Hemorheological Alterations in Patients with Heart Failure with Reduced Ejection Fraction Treated by Resveratrol. Cardiovasc. Ther. 2020, 2020, 7262474. [Google Scholar] [CrossRef]
- Lixia, G.; Haiyun, Z.; Xia, Z. The clinical effects of resveratrol on atherosclerosis treatment and its effect on the expression of NADPH oxidase complex genes in vascular smooth muscle cell line. Cell. Mol. Biol. 2021, 67, 148–152. [Google Scholar] [CrossRef]
- Magyar, K.; Halmosi, R.; Palfi, A.; Feher, G.; Czopf, L.; Fulop, A.; Battyany, I.; Sumegi, B.; Toth, K.; Szabados, E. Cardioprotection by resveratrol: A human clinical trial in patients with stable coronary artery disease. Clin. Hemorheol. Microcirc. 2012, 50, 179–187. [Google Scholar] [CrossRef]
- Marques, B.C.A.A.; Trindade, M.; Aquino, J.C.F.; Cunha, A.R.; Gismondi, R.O.; Neves, M.F.; Oigman, W. Beneficial effects of acute trans-resveratrol supplementation in treated hypertensive patients with endothelial dysfunction. Clin. Exp. Hypertens. 2018, 40, 218–223. [Google Scholar] [CrossRef]
- McDermott, M.M.; Leeuwenburgh, C.; Guralnik, J.M.; Tian, L.; Sufit, R.; Zhao, L.; Criqui, M.H.; Kibbe, M.R.; Stein, J.H.; Lloyd-Jones, D.; et al. Effect of Resveratrol on Walking Performance in Older People With Peripheral Artery Disease: The RESTORE Randomized Clinical Trial. JAMA Cardiol. 2017, 2, 902–907. [Google Scholar] [CrossRef]
- Militaru, C.; Donoiu, I.; Craciun, A.; Scorei, I.D.; Bulearca, A.M.; Scorei, R.I. Oral resveratrol and calcium fructoborate supplementation in subjects with stable angina pectoris: Effects on lipid profiles, inflammation markers, and quality of life. Nutrition 2013, 29, 178–183. [Google Scholar] [CrossRef]
- van der Made, S.M.; Plat, J.; Mensink, R.P. Resveratrol does not influence metabolic risk markers related to cardiovascular health in overweight and slightly obese subjects: A randomized, placebo-controlled crossover trial. PLoS ONE 2015, 10, e0118393. [Google Scholar] [CrossRef]
- van der Made, S.M.; Plat, J.; Mensink, R.P. Trans-Resveratrol Supplementation and Endothelial Function during the Fasting and Postprandial Phase: A Randomized Placebo-Controlled Trial in Overweight and Slightly Obese Participants. Nutrients 2017, 9, 596. [Google Scholar] [CrossRef]
- Wong, R.H.X.; Howe, P.R.C.; Buckley, J.D.; Coates, A.M.; Kunz, I.; Berry, N.M. Acute resveratrol supplementation improves flow-mediated dilatation in overweight/obese individuals with mildly elevated blood pressure. Nutr. Metab. Cardiovasc. Dis. 2011, 21, 851–856. [Google Scholar] [CrossRef]
- Wong, R.H.X.; Berry, N.M.; Coates, A.M.; Buckley, J.D.; Bryan, J.; Kunz, I.; Howe, P.R.C. Chronic resveratrol consumption improves brachial flow-mediated dilatation in healthy obese adults. J. Hypertens. 2013, 31, 1819–1827. [Google Scholar] [CrossRef]
- Bashmakov, Y.K.; Assaad-Khalil, S.H.; Abou Seif, M.; Udumyan, R.; Megallaa, M.; Rohoma, K.H.; Zeitoun, M.; Petyaev, I.M. Resveratrol promotes foot ulcer size reduction in type 2 diabetes patients. ISRN Endocrinol. 2014, 2014, 816307. [Google Scholar] [CrossRef]
- Bhatt, J.K.; Thomas, S.; Nanjan, M.J. Resveratrol supplementation improves glycemic control in type 2 diabetes mellitus. Nutr. Res. 2012, 32, 537–541. [Google Scholar] [CrossRef]
- Bhatt, J.K.; Nanjan, M.J. Resveratrol supplementation in patients with type 2 diabetes mellitus: A prospective, open label, randomized controlled trial. Int. Res. J. Pharm. 2013, 4, 245–249. [Google Scholar] [CrossRef]
- Bo, S.; Ponzo, V.; Ciccone, G.; Evangelista, A.; Saba, F.; Goitre, I.; Procopio, M.; Pagano, G.F.; Cassader, M.; Gambino, R. Six months of resveratrol supplementation has no measurable effect in type 2 diabetic patients. A randomized, double blind, placebo-controlled trial. Pharmacol. Res. 2016, 111, 896–905. [Google Scholar] [CrossRef]
- Bo, S.; Ponzo, V.; Evangelista, A.; Ciccone, G.; Goitre, I.; Saba, F.; Procopio, M.; Cassader, M.; Gambino, R. Effects of 6 months of resveratrol versus placebo on pentraxin 3 in patients with type 2 diabetes mellitus: A double-blind randomized controlled trial. Acta Diabetol. 2017, 54, 499–507. [Google Scholar] [CrossRef]
- Bo, S.; Togliatto, G.; Gambino, R.; Ponzo, V.; Lombardo, G.; Rosato, R.; Cassader, M.; Brizzi, M.F. Impact of sirtuin-1 expression on H3K56 acetylation and oxidative stress: A double-blind randomized controlled trial with resveratrol supplementation. Acta Diabetol. 2018, 55, 331–340. [Google Scholar] [CrossRef]
- Gambino, R.; Fanni, G.; Togliatto, G.; Ponzo, V.; Goitre, I.; Cassader, M.; Brizzi, M.F.; Bo, S. Rs12778366 single nucleotide polymorphism of Sirtuin 1 (SIRT1) and response to resveratrol supplementation in patients with type 2 diabetes mellitus. Acta Diabetol. 2019, 56, 963–966. [Google Scholar] [CrossRef]
- Bo, S.; Gambino, R.; Ponzo, V.; Cioffi, I.; Goitre, I.; Evangelista, A.; Ciccone, G.; Cassader, M.; Procopio, M. Effects of resveratrol on bone health in type 2 diabetic patients. A double-blind randomized-controlled trial. Nutr. Diabetes 2018, 8, 51. [Google Scholar] [CrossRef]
- Brasnyo, P.; Molnar, G.A.; Mohas, M.; Marko, L.; Laczy, B.; Cseh, J.; Mikolas, E.; Szijarto, I.A.; Merei, A.; Halmai, R.; et al. Resveratrol improves insulin sensitivity, reduces oxidative stress and activates the Akt pathway in type 2 diabetic patients. Br. J. Nutr. 2011, 106, 383–389. [Google Scholar] [CrossRef]
- de Ligt, M.; Bruls, Y.M.H.; Hansen, J.; Habets, M.-F.; Havekes, B.; Nascimento, E.B.M.; Moonen-Kornips, E.; Schaart, G.; Schrauwen-Hinderling, V.B.; van Marken Lichtenbelt, W.; et al. Resveratrol improves ex vivo mitochondrial function but does not affect insulin sensitivity or brown adipose tissue in first degree relatives of patients with type 2 diabetes. Mol. Metab. 2018, 12, 39–47. [Google Scholar] [CrossRef]
- Boswijk, E.; de Ligt, M.; Habets, M.-F.J.; Mingels, A.M.A.; van Marken Lichtenbelt, W.D.; Mottaghy, F.M.; Schrauwen, P.; Wildberger, J.E.; Bucerius, J. Resveratrol treatment does not reduce arterial inflammation in males at risk of type 2 diabetes: A randomized crossover trial. Nucl. Med. 2022, 61, 33–41. [Google Scholar] [CrossRef]
- Goh, K.P.; Lee, H.Y.; Lau, D.P.; Supaat, W.; Chan, Y.H.; Koh, A.F. Effects of resveratrol in patients with type 2 diabetes mellitus on skeletal muscle SIRT1 expression and energy expenditure. Int. J. Sport Nutr. Exerc. Metab. 2014, 24, 2–13. [Google Scholar] [CrossRef]
- Hoseini, A.; Namazi, G.; Farrokhian, A.; Reiner, Z.; Aghadavod, E.; Bahmani, F.; Asemi, Z. The effects of resveratrol on metabolic status in patients with type 2 diabetes mellitus and coronary heart disease. Food Funct. 2019, 10, 6042–6051. [Google Scholar] [CrossRef]
- Khodabandehloo, H.; Seyyedebrahimi, S.; Esfahani, E.N.; Razi, F.; Meshkani, R. Resveratrol supplementation decreases blood glucose without changing the circulating CD14+CD16+ monocytes and inflammatory cytokines in patients with type 2 diabetes: A randomized, double-blind, placebo-controlled study. Nutr. Res. 2018, 54, 40–51. [Google Scholar] [CrossRef]
- Ma, N.; Zhang, Y. Effects of resveratrol therapy on glucose metabolism, insulin resistance, inflammation, and renal function in the elderly patients with type 2 diabetes mellitus: A randomized controlled clinical trial protocol. Medicine 2022, 101, e30049. [Google Scholar] [CrossRef]
- Mahjabeen, W.; Khan, D.A.; Mirza, S.A. Role of resveratrol supplementation in regulation of glucose hemostasis, inflammation and oxidative stress in patients with diabetes mellitus type 2: A randomized, placebo-controlled trial. Complement. Ther. Med. 2022, 66, 102819. [Google Scholar] [CrossRef]
- Movahed, A.; Nabipour, I.; Lieben Louis, X.; Thandapilly, S.J.; Yu, L.; Kalantarhormozi, M.; Rekabpour, S.J.; Netticadan, T. Antihyperglycemic effects of short term resveratrol supplementation in type 2 diabetic patients. Evid. Based Complement. Altern. Med. 2013, 2013, 851267. [Google Scholar] [CrossRef]
- Sattarinezhad, A.; Roozbeh, J.; Shirazi Yeganeh, B.; Omrani, G.R.; Shams, M. Resveratrol reduces albuminuria in diabetic nephropathy: A randomized double-blind placebo-controlled clinical trial. Diabetes Metab. 2019, 45, 53–59. [Google Scholar] [CrossRef]
- Seyyedebrahimi, S.; Khodabandehloo, H.; Nasli Esfahani, E.; Meshkani, R. The effects of resveratrol on markers of oxidative stress in patients with type 2 diabetes: A randomized, double-blind, placebo-controlled clinical trial. Acta Diabetol. 2018, 55, 341–353. [Google Scholar] [CrossRef]
- Thazhath, S.S.; Wu, T.; Bound, M.J.; Checklin, H.L.; Standfield, S.; Jones, K.L.; Horowitz, M.; Rayner, C.K. Administration of resveratrol for 5 wk has no effect on glucagon-like peptide 1 secretion, gastric emptying, or glycemic control in type 2 diabetes: A randomized controlled trial. Am. J. Clin. Nutr. 2016, 103, 66–70. [Google Scholar] [CrossRef]
- Timmers, S.; de Ligt, M.; Phielix, E.; van de Weijer, T.; Hansen, J.; Moonen-Kornips, E.; Schaart, G.; Kunz, I.; Hesselink, M.K.C.; Schrauwen-Hinderling, V.B.; et al. Resveratrol as Add-on Therapy in Subjects With Well-Controlled Type 2 Diabetes: A Randomized Controlled Trial. Diabetes Care 2016, 39, 2211–2217. [Google Scholar] [CrossRef]
- Abdollahi, S.; Salehi-Abargouei, A.; Toupchian, O.; Sheikhha, M.H.; Fallahzadeh, H.; Rahmanian, M.; Tabatabaie, M.; Mozaffari-Khosravi, H. The Effect of Resveratrol Supplementation on Cardio-Metabolic Risk Factors in Patients with Type 2 Diabetes: A Randomized, Double-Blind Controlled Trial. Phytother. Res. 2019, 33, 3153–3162. [Google Scholar] [CrossRef]
- Tabatabaie, M.; Abdollahi, S.; Salehi-Abargouei, A.; Clark, C.C.T.; Karimi-Nazari, E.; Fallahzadeh, H.; Rahmanian, M.; Mozaffari-Khosravi, H. The effect of resveratrol supplementation on serum levels of asymmetric de-methyl-arginine and paraoxonase 1 activity in patients with type 2 diabetes: A randomized, double-blind controlled trial. Phytother. Res. 2020, 34, 2023–2031. [Google Scholar] [CrossRef]
- Toupchian, O.; Abdollahi, S.; Salehi-Abargouei, A.; Heshmati, J.; Clark, C.C.T.; Sheikhha, M.H.; Fallahzadeh, H.; Mozaffari-Khosravi, H. The effects of resveratrol supplementation on PPARalpha, p16, p53, p21 gene expressions, and sCD163/sTWEAK ratio in patients with type 2 diabetes mellitus: A double-blind controlled randomized trial. Phytother. Res. 2021, 35, 3205–3213. [Google Scholar] [CrossRef]
- Ali Sangouni, A.; Abdollahi, S.; Mozaffari-Khosravi, H. Effect of resveratrol supplementation on hepatic steatosis and cardiovascular indices in overweight subjects with type 2 diabetes: A double-blind, randomized controlled trial. BMC Cardiovasc. Disord. 2022, 22, 212. [Google Scholar] [CrossRef]
- de Ligt, M.; Bergman, M.; Fuentes, R.M.; Essers, H.; Moonen-Kornips, E.; Havekes, B.; Schrauwen-Hinderling, V.B.; Schrauwen, P. No effect of resveratrol supplementation after 6 months on insulin sensitivity in overweight adults: A randomized trial. Am. J. Clin. Nutr. 2020, 112, 1029–1038. [Google Scholar] [CrossRef]
- Goncalinho, G.H.F.; Roggerio, A.; Goes, M.F.d.S.; Avakian, S.D.; Leal, D.P.; Strunz, C.M.C.; de Padua Mansur, A. Comparison of Resveratrol Supplementation and Energy Restriction Effects on Sympathetic Nervous System Activity and Vascular Reactivity: A Randomized Clinical Trial. Molecules 2021, 26, 3168. [Google Scholar] [CrossRef]
- Pollack, R.M.; Barzilai, N.; Anghel, V.; Kulkarni, A.S.; Golden, A.; O’Broin, P.; Sinclair, D.A.; Bonkowski, M.S.; Coleville, A.J.; Powell, D.; et al. Resveratrol Improves Vascular Function and Mitochondrial Number but Not Glucose Metabolism in Older Adults. J. Gerontol. Ser. A Biol. Sci. Med. Sci. 2017, 72, 1703–1709. [Google Scholar] [CrossRef]
- Poulsen, M.M.; Vestergaard, P.F.; Clasen, B.F.; Radko, Y.; Christensen, L.P.; Stodkilde-Jorgensen, H.; Moller, N.; Jessen, N.; Pedersen, S.B.; Jorgensen, J.O.L. High-dose resveratrol supplementation in obese men: An investigator-initiated, randomized, placebo-controlled clinical trial of substrate metabolism, insulin sensitivity, and body composition. Diabetes 2013, 62, 1186–1195. [Google Scholar] [CrossRef]
- Clasen, B.F.; Poulsen, M.M.; Escande, C.; Pedersen, S.B.; Moller, N.; Chini, E.N.; Jessen, N.; Jorgensen, J.O.L. Growth hormone signaling in muscle and adipose tissue of obese human subjects: Associations with measures of body composition and interaction with resveratrol treatment. J. Clin. Endocrinol. Metab. 2014, 99, E2565–E2573. [Google Scholar] [CrossRef][Green Version]
- Simental-Mendia, L.E.; Guerrero-Romero, F. Effect of resveratrol supplementation on lipid profile in subjects with dyslipidemia: A randomized double-blind, placebo-controlled trial. Nutrition 2019, 58, 7–10. [Google Scholar] [CrossRef]
- Williams, C.B.; Hughes, M.C.; Edgett, B.A.; Scribbans, T.D.; Simpson, C.A.; Perry, C.G.R.; Gurd, B.J. An examination of resveratrol’s mechanisms of action in human tissue: Impact of a single dose in vivo and dose responses in skeletal muscle ex vivo. PLoS ONE 2014, 9, e102406. [Google Scholar] [CrossRef]
- Yoshino, J.; Conte, C.; Fontana, L.; Mittendorfer, B.; Imai, S.-i.; Schechtman, K.B.; Gu, C.; Kunz, I.; Rossi Fanelli, F.; Patterson, B.W.; et al. Resveratrol supplementation does not improve metabolic function in nonobese women with normal glucose tolerance. Cell Metab. 2012, 16, 658–664. [Google Scholar] [CrossRef]
- Zhou, Y.; Zeng, Y.; Pan, Z.; Jin, Y.; Li, Q.; Pang, J.; Wang, X.; Chen, Y.; Yang, Y.; Ling, W. A Randomized Trial on Resveratrol Supplement Affecting Lipid Profile and Other Metabolic Markers in Subjects with Dyslipidemia. Nutrients 2023, 15, 492. [Google Scholar] [CrossRef]
- Zortea, K.; Franco, V.C.; Francesconi, L.P.; Cereser, K.M.M.; Lobato, M.I.R.; Belmonte-de-Abreu, P.S. Resveratrol Supplementation in Schizophrenia Patients: A Randomized Clinical Trial Evaluating Serum Glucose and Cardiovascular Risk Factors. Nutrients 2016, 8, 73. [Google Scholar] [CrossRef]
- Zortea, K.; Franco, V.C.; Guimaraes, P.; Belmonte-de-Abreu, P.S. Resveratrol Supplementation Did Not Improve Cognition in Patients with Schizophrenia: Results from a Randomized Clinical Trial. Front. Psychiatry 2016, 7, 159. [Google Scholar] [CrossRef]
- Asghari, S.; Rafraf, M.; Farzin, L.; Asghari-Jafarabadi, M.; Ghavami, S.-M.; Somi, M.-H. Effects of Pharmacologic Dose of Resveratrol Supplementation on Oxidative/Antioxidative Status Biomarkers in Nonalcoholic Fatty Liver Disease Patients: A Randomized, Double-Blind, Placebo-Controlled Trial. Adv. Pharm. Bull. 2018, 8, 307–317. [Google Scholar] [CrossRef]
- Asghari, S.; Asghari-Jafarabadi, M.; Somi, M.-H.; Ghavami, S.-M.; Rafraf, M. Comparison of Calorie-Restricted Diet and Resveratrol Supplementation on Anthropometric Indices, Metabolic Parameters, and Serum Sirtuin-1 Levels in Patients With Nonalcoholic Fatty Liver Disease: A Randomized Controlled Clinical Trial. J. Am. Coll. Nutr. 2018, 37, 223–233. [Google Scholar] [CrossRef]
- Chachay, V.S.; Macdonald, G.A.; Martin, J.H.; Whitehead, J.P.; O’Moore-Sullivan, T.M.; Lee, P.; Franklin, M.; Klein, K.; Taylor, P.J.; Ferguson, M.; et al. Resveratrol does not benefit patients with nonalcoholic fatty liver disease. Clin. Gastroenterol. Hepatol. 2014, 12, 2092–2096. [Google Scholar] [CrossRef]
- Chen, S.; Zhao, X.; Ran, L.; Wan, J.; Wang, X.; Qin, Y.; Shu, F.; Gao, Y.; Yuan, L.; Zhang, Q.; et al. Resveratrol improves insulin resistance, glucose and lipid metabolism in patients with non-alcoholic fatty liver disease: A randomized controlled trial. Dig. Liver Dis. 2015, 47, 226–232. [Google Scholar] [CrossRef]
- Faghihzadeh, F.; Adibi, P.; Rafiei, R.; Hekmatdoost, A. Resveratrol supplementation improves inflammatory biomarkers in patients with nonalcoholic fatty liver disease. Nutr. Res. 2014, 34, 837–843. [Google Scholar] [CrossRef]
- Faghihzadeh, F.; Adibi, P.; Hekmatdoost, A. The effects of resveratrol supplementation on cardiovascular risk factors in patients with non-alcoholic fatty liver disease: A randomised, double-blind, placebo-controlled study. Br. J. Nutr. 2015, 114, 796–803. [Google Scholar] [CrossRef]
- Heeboll, S.; Kreuzfeldt, M.; Hamilton-Dutoit, S.; Kjaer Poulsen, M.; Stodkilde-Jorgensen, H.; Moller, H.J.; Jessen, N.; Thorsen, K.; Kristina Hellberg, Y.; Bonlokke Pedersen, S.; et al. Placebo-controlled, randomised clinical trial: High-dose resveratrol treatment for non-alcoholic fatty liver disease. Scand. J. Gastroenterol. 2016, 51, 456–464. [Google Scholar] [CrossRef]
- Kantartzis, K.; Fritsche, L.; Bombrich, M.; Machann, J.; Schick, F.; Staiger, H.; Kunz, I.; Schoop, R.; Lehn-Stefan, A.; Heni, M.; et al. Effects of resveratrol supplementation on liver fat content in overweight and insulin-resistant subjects: A randomized, double-blind, placebo-controlled clinical trial. Diabetes Obes. Metab. 2018, 20, 1793–1797. [Google Scholar] [CrossRef]
- Poulsen, M.K.; Nellemann, B.; Bibby, B.M.; Stodkilde-Jorgensen, H.; Pedersen, S.B.; Gronbaek, H.; Nielsen, S. No effect of resveratrol on VLDL-TG kinetics and insulin sensitivity in obese men with nonalcoholic fatty liver disease. Diabetes Obes. Metab. 2018, 20, 2504–2509. [Google Scholar] [CrossRef]
- Batista-Jorge, G.C.; Barcala-Jorge, A.S.; Silveira, M.F.; Lelis, D.F.; Andrade, J.M.O.; de Paula, A.M.B.; Guimaraes, A.L.S.; Santos, S.H.S. Oral resveratrol supplementation improves Metabolic Syndrome features in obese patients submitted to a lifestyle-changing program. Life Sci. 2020, 256, 117962. [Google Scholar] [CrossRef]
- Mendez-del Villar, M.; Gonzalez-Ortiz, M.; Martinez-Abundis, E.; Perez-Rubio, K.G.; Lizarraga-Valdez, R. Effect of resveratrol administration on metabolic syndrome, insulin sensitivity, and insulin secretion. Metab. Syndr. Relat. Disord. 2014, 12, 497–501. [Google Scholar] [CrossRef]
- Arzola-Paniagua, M.A.; Garcia-Salgado Lopez, E.R.; Calvo-Vargas, C.G.; Guevara-Cruz, M. Efficacy of an orlistat-resveratrol combination for weight loss in subjects with obesity: A randomized controlled trial. Obesity 2016, 24, 1454–1463. [Google Scholar] [CrossRef]
- Mahmood, W.A.; Mshimesh, B.A.R.; Khazaal, F.A.K.; Jasim, S.Y.; Mahmood, A.A. Potential effects of resveratrol on obesity-related nephropathy in Iraqi obese women. J. Pharm. Sci. Res. 2018, 10, 999–1005. [Google Scholar]
- Timmers, S.; Konings, E.; Bilet, L.; Houtkooper, R.H.; van de Weijer, T.; Goossens, G.H.; Hoeks, J.; van der Krieken, S.; Ryu, D.; Kersten, S.; et al. Calorie restriction-like effects of 30 days of resveratrol supplementation on energy metabolism and metabolic profile in obese humans. Cell Metab. 2011, 14, 612–622. [Google Scholar] [CrossRef]
- Konings, E.; Timmers, S.; Boekschoten, M.V.; Goossens, G.H.; Jocken, J.W.; Afman, L.A.; Muller, M.; Schrauwen, P.; Mariman, E.C.; Blaak, E.E. The effects of 30 days resveratrol supplementation on adipose tissue morphology and gene expression patterns in obese men. Int. J. Obes. 2014, 38, 470–473. [Google Scholar] [CrossRef]
- Knop, F.K.; Konings, E.; Timmers, S.; Schrauwen, P.; Holst, J.J.; Blaak, E.E. Thirty days of resveratrol supplementation does not affect postprandial incretin hormone responses, but suppresses postprandial glucagon in obese subjects. Diabet. Med. 2013, 30, 1214–1218. [Google Scholar] [CrossRef]
- van Polanen, N.; Zacharewicz, E.; de Ligt, M.; Timmers, S.; Moonen-Kornips, E.; Schaart, G.; Hoeks, J.; Schrauwen, P.; Hesselink, M.K.C. Resveratrol-induced remodelling of myocellular lipid stores: A study in metabolically compromised humans. Physiol. Rep. 2021, 9, e14692. [Google Scholar] [CrossRef]
- de Ligt, M.; Hesselink, M.K.C.; Jorgensen, J.; Hoebers, N.; Blaak, E.E.; Goossens, G.H. Resveratrol supplementation reduces ACE2 expression in human adipose tissue. Adipocyte 2021, 10, 408–411. [Google Scholar] [CrossRef]
- Alway, S.E.; McCrory, J.L.; Kearcher, K.; Vickers, A.; Frear, B.; Gilleland, D.L.; Bonner, D.E.; Thomas, J.M.; Donley, D.A.; Lively, M.W.; et al. Resveratrol Enhances Exercise-Induced Cellular and Functional Adaptations of Skeletal Muscle in Older Men and Women. J. Gerontol. Ser. A Biol. Sci. Med. Sci. 2017, 72, 1595–1606. [Google Scholar] [CrossRef]
- Gliemann, L.; Schmidt, J.F.; Olesen, J.; Bienso, R.S.; Peronard, S.L.; Grandjean, S.U.; Mortensen, S.P.; Nyberg, M.; Bangsbo, J.; Pilegaard, H.; et al. Resveratrol blunts the positive effects of exercise training on cardiovascular health in aged men. J. Physiol. 2013, 591, 5047–5059. [Google Scholar] [CrossRef]
- Gliemann, L.; Olesen, J.; Bienso, R.S.; Schmidt, J.F.; Akerstrom, T.; Nyberg, M.; Lindqvist, A.; Bangsbo, J.; Hellsten, Y. Resveratrol modulates the angiogenic response to exercise training in skeletal muscles of aged men. Am. J. Physiol. Heart Circ. Physiol. 2014, 307, H1111–H1119. [Google Scholar] [CrossRef]
- Olesen, J.; Gliemann, L.; Bienso, R.; Schmidt, J.; Hellsten, Y.; Pilegaard, H. Exercise training, but not resveratrol, improves metabolic and inflammatory status in skeletal muscle of aged men. J. Physiol. 2014, 592, 1873–1886. [Google Scholar] [CrossRef]
- Harper, S.A.; Bassler, J.R.; Peramsetty, S.; Yang, Y.; Roberts, L.M.; Drummer, D.; Mankowski, R.T.; Leeuwenburgh, C.; Ricart, K.; Patel, R.P.; et al. Resveratrol and exercise combined to treat functional limitations in late life: A pilot randomized controlled trial. Exp. Gerontol. 2021, 143, 111111. [Google Scholar] [CrossRef]
- Laupheimer, M.W.; Perry, M.; Benton, S.; Malliaras, P.; Maffulli, N. Resveratrol exerts no effect on inflammatory response and delayed onset muscle soreness after a marathon in male athletes.: A randomised, double-blind, placebo-controlled pilot feasibility study. Transl. Med. 2014, 10, 38–42. [Google Scholar]
- Lokken, N.; Khawajazada, T.; Storgaard, J.H.; Raaschou-Pedersen, D.; Christensen, M.E.; Hornsyld, T.M.; Krag, T.; Orngreen, M.C.; Vissing, J. No effect of resveratrol in patients with mitochondrial myopathy: A cross-over randomized controlled trial. J. Inherit. Metab. Dis. 2021, 44, 1186–1198. [Google Scholar] [CrossRef]
- Macedo, R.C.S.; Vieira, A.; Marin, D.P.; Otton, R. Effects of chronic resveratrol supplementation in military firefighters undergo a physical fitness test—A placebo-controlled, double blind study. Chem. Biol. Interact. 2014, 227, 89–95. [Google Scholar] [CrossRef]
- Nicolau, A.L.A.; Peres, G.B.; de Souza Silva, J.; Nunes, S.H.; Fortes, T.M.L.; Suffredini, I.B. Pilot project. Resveratrol intake by physical active and sedentary older adult women and blood pressure. Exp. Gerontol. 2022, 166, 111883. [Google Scholar] [CrossRef]
- Scribbans, T.D.; Ma, J.K.; Edgett, B.A.; Vorobej, K.A.; Mitchell, A.S.; Zelt, J.G.E.; Simpson, C.A.; Quadrilatero, J.; Gurd, B.J. Resveratrol supplementation does not augment performance adaptations or fibre-type-specific responses to high-intensity interval training in humans. Appl. Physiol. Nutr. Metab. 2014, 39, 1305–1313. [Google Scholar] [CrossRef]
- Storgaard, J.H.; Lokken, N.; Madsen, K.L.; Voermans, N.C.; Laforet, P.; Nadaj-Pakleza, A.; Tard, C.; van Hall, G.; Vissing, J.; Orngreen, M.C. No effect of resveratrol on fatty acid oxidation or exercise capacity in patients with fatty acid oxidation disorders: A randomized clinical cross-over trial. J. Inherit. Metab. Dis. 2022, 45, 517–528. [Google Scholar] [CrossRef]
- Bagen, H.; Liu, X.; Han, J. The anti-inflammation effects of resveratrol for patients after oral implantology. Biomed. Res. 2018, 29, 1841–1844. [Google Scholar] [CrossRef]
- Bo, S.; Ciccone, G.; Castiglione, A.; Gambino, R.; De Michieli, F.; Villois, P.; Durazzo, M.; Cavallo-Perin, P.; Cassader, M. Anti-inflammatory and antioxidant effects of resveratrol in healthy smokers a randomized, double-blind, placebo-controlled, cross-over trial. Curr. Med. Chem. 2013, 20, 1323–1331. [Google Scholar] [CrossRef]
- Di Pierro, D.; Ciaccio, C.; Sbardella, D.; Tundo, G.R.; Bernardini, R.; Curatolo, P.; Galasso, C.; Pironi, V.; Coletta, M.; Marini, S. Effects of oral administration of common antioxidant supplements on the energy metabolism of red blood cells. Attenuation of oxidative stress-induced changes in Rett syndrome erythrocytes by CoQ10. Mol. Cell. Biochem. 2020, 463, 101–113. [Google Scholar] [CrossRef]
- Vicari, E.; Arancio, A.; Catania, V.E.; Vicari, B.O.; Sidoti, G.; Castiglione, R.; Malaguarnera, M. Resveratrol reduces inflammation-related prostate fibrosis. Int. J. Med. Sci. 2020, 17, 1864–1870. [Google Scholar] [CrossRef]
- Zhang, D.; Zhang, Y.; Zhu, B.; Zhang, H.; Sun, Y.; Sun, C. Resveratrol may reverse the effects of long-term occupational exposure to electromagnetic fields on workers of a power plant. Oncotarget 2017, 8, 47497–47506. [Google Scholar] [CrossRef]
- Hussain, S.A.; Marouf, B.H.; Ali, Z.S.; Ahmmad, R.S. Efficacy and safety of co-administration of resveratrol with meloxicam in patients with knee osteoarthritis: A pilot interventional study. Clin. Interv. Aging 2018, 13, 1621–1630. [Google Scholar] [CrossRef]
- Khojah, H.M.; Ahmed, S.; Abdel-Rahman, M.S.; Elhakeim, E.H. Resveratrol as an effective adjuvant therapy in the management of rheumatoid arthritis: A clinical study. Clin. Rheumatol. 2018, 37, 2035–2042. [Google Scholar] [CrossRef]
- Marouf, B.H.; Hussain, S.A.; Ali, Z.S.; Ahmmad, R.S. Clinical efficacy of resveratrol as an adjuvant with meloxican in the treatment of knee osteoarthritis patients: A double-blind, randomised, placebo-controlled trial. Braz. J. Pharm. Sci. 2018, 54, e17773. [Google Scholar] [CrossRef]
- Marouf, B.H.; Hussain, S.A.; Ali, Z.S.; Ahmmad, R.S. Resveratrol Supplementation Reduces Pain and Inflammation in Knee Osteoarthritis Patients Treated with Meloxicam: A Randomized Placebo-Controlled Study. J. Med. Food 2018, 21, 1253–1259. [Google Scholar] [CrossRef]
- Marouf, B.H.; Hussain, S.A.; Ali, Z.S. Correlation between serum pro inflammatory cytokines and clinical scores of knee osteoarthritic patients using resveratrol as a supplementary therapy with meloxicam. Indian J. Pharmacol. 2021, 53, 270–277. [Google Scholar] [CrossRef]
- Evans, H.M.; Howe, P.R.C.; Wong, R.H.X. Effects of Resveratrol on Cognitive Performance, Mood and Cerebrovascular Function in Post-Menopausal Women; A 14-Week Randomised Placebo-Controlled Intervention Trial. Nutrients 2017, 9, 27. [Google Scholar] [CrossRef]
- Wong, R.H.X.; Evans, H.M.; Howe, P.R.C. Resveratrol supplementation reduces pain experience by postmenopausal women. Menopause 2017, 24, 916–922. [Google Scholar] [CrossRef]
- Kennedy, D.O.; Wightman, E.L.; Reay, J.L.; Lietz, G.; Okello, E.J.; Wilde, A.; Haskell, C.F. Effects of resveratrol on cerebral blood flow variables and cognitive performance in humans: A double-blind, placebo-controlled, crossover investigation. Am. J. Clin. Nutr. 2010, 91, 1590–1597. [Google Scholar] [CrossRef]
- Thaung Zaw, J.J.; Howe, P.R.; Wong, R.H. Long-term effects of resveratrol on cognition, cerebrovascular function and cardio-metabolic markers in postmenopausal women: A 24-month randomised, double-blind, placebo-controlled, crossover study. Clin. Nutr. 2021, 40, 820–829. [Google Scholar] [CrossRef]
- Thaung Zaw, J.J.; Howe, P.R.C.; Wong, R.H.X. Long-term resveratrol supplementation improves pain perception, menopausal symptoms, and overall well-being in postmenopausal women: Findings from a 24-month randomized, controlled, crossover trial. Menopause 2020, 28, 40–49. [Google Scholar] [CrossRef]
- Thaung Zaw, J.J.; Howe, P.R.C.; Wong, R.H.X. Sustained Cerebrovascular and Cognitive Benefits of Resveratrol in Postmenopausal Women. Nutrients 2020, 12, 828. [Google Scholar] [CrossRef]
- Wong, R.H.; Thaung Zaw, J.J.; Xian, C.J.; Howe, P.R. Regular Supplementation With Resveratrol Improves Bone Mineral Density in Postmenopausal Women: A Randomized, Placebo-Controlled Trial. J. Bone Miner. Res. 2020, 35, 2121–2131. [Google Scholar] [CrossRef]
- Wightman, E.L.; Reay, J.L.; Haskell, C.F.; Williamson, G.; Dew, T.P.; Kennedy, D.O. Effects of resveratrol alone or in combination with piperine on cerebral blood flow parameters and cognitive performance in human subjects: A randomised, double-blind, placebo-controlled, cross-over investigation. Br. J. Nutr. 2014, 112, 203–213. [Google Scholar] [CrossRef]
- Wong, R.H.X.; Nealon, R.S.; Scholey, A.; Howe, P.R.C. Low dose resveratrol improves cerebrovascular function in type 2 diabetes mellitus. Nutr. Metab. Cardiovasc. Dis. 2016, 26, 393–399. [Google Scholar] [CrossRef]
- Wong, R.H.X.; Raederstorff, D.; Howe, P.R.C. Acute Resveratrol Consumption Improves Neurovascular Coupling Capacity in Adults with Type 2 Diabetes Mellitus. Nutrients 2016, 8, 425. [Google Scholar] [CrossRef]
- Hendouei, F.; Sanjari Moghaddam, H.; Mohammadi, M.R.; Taslimi, N.; Rezaei, F.; Akhondzadeh, S. Resveratrol as adjunctive therapy in treatment of irritability in children with autism: A double-blind and placebo-controlled randomized trial. J. Clin. Pharm. Ther. 2020, 45, 324–334. [Google Scholar] [CrossRef]
- Rafeiy-Torghabeh, M.; Ashraf-Ganjouei, A.; Moradi, K.; Bagheri, S.; Mohammadi, M.-R.; Akhondzadeh, S. Resveratrol adjunct to methylphenidate improves symptoms of attention-deficit/hyperactivity disorder: A randomized, double-blinded, placebo-controlled clinical trial. Eur. Child Adolesc. Psychiatry 2020, 30, 799–807. [Google Scholar] [CrossRef]
- Samaei, A.; Moradi, K.; Bagheri, S.; Ashraf-Ganjouei, A.; Alikhani, R.; Mousavi, S.B.; Rezaei, F.; Akhondzadeh, S. Resveratrol Adjunct Therapy for Negative Symptoms in Patients With Stable Schizophrenia: A Double-Blind, Randomized Placebo-Controlled Trial. Int. J. Neuropsychopharmacol. 2020, 23, 775–782. [Google Scholar] [CrossRef]
- Gu, J.; Li, Z.; Chen, H.; Xu, X.; Li, Y.; Gui, Y. Neuroprotective Effect of Trans-Resveratrol in Mild to Moderate Alzheimer Disease: A Randomized, Double-Blind Trial. Neurol. Ther. 2021, 10, 905–917. [Google Scholar] [CrossRef]
- Turner, R.S.; Thomas, R.G.; Craft, S.; van Dyck, C.H.; Mintzer, J.; Reynolds, B.A.; Brewer, J.B.; Rissman, R.A.; Raman, R.; Aisen, P.S.; et al. A randomized, double-blind, placebo-controlled trial of resveratrol for Alzheimer disease. Neurology 2015, 85, 1383–1391. [Google Scholar] [CrossRef]
- Moussa, C.; Hebron, M.; Huang, X.; Ahn, J.; Rissman, R.A.; Aisen, P.S.; Turner, R.S. Resveratrol regulates neuro-inflammation and induces adaptive immunity in Alzheimer’s disease. J. Neuroinflamm. 2017, 14, 1. [Google Scholar] [CrossRef]
- Almeida, L.; Vaz-da-Silva, M.; Falcao, A.; Soares, E.; Costa, R.; Loureiro, A.I.; Fernandes-Lopes, C.; Rocha, J.F.; Nunes, T.; Wright, L.; et al. Pharmacokinetic and safety profile of trans-resveratrol in a rising multiple-dose study in healthy volunteers. Mol. Nutr. Food Res. 2009, 53 (Suppl. 1), S7–S15. [Google Scholar] [CrossRef]
- Anton, S.D.; Embry, C.; Marsiske, M.; Lu, X.; Doss, H.; Leeuwenburgh, C.; Manini, T.M. Safety and metabolic outcomes of resveratrol supplementation in older adults: Results of a twelve-week, placebo-controlled pilot study. Exp. Gerontol. 2014, 57, 181–187. [Google Scholar] [CrossRef]
- Anton, S.D.; Ebner, N.; Dzierzewski, J.M.; Zlatar, Z.Z.; Gurka, M.J.; Dotson, V.M.; Kirton, J.; Mankowski, R.T.; Marsiske, M.; Manini, T.M. Effects of 90 Days of Resveratrol Supplementation on Cognitive Function in Elders: A Pilot Study. J. Altern. Complement. Med. 2018, 24, 725–732. [Google Scholar] [CrossRef]
- Howells, L.M.; Berry, D.P.; Elliott, P.J.; Jacobson, E.W.; Hoffmann, E.; Hegarty, B.; Brown, K.; Steward, W.P.; Gescher, A.J. Phase I randomized, double-blind pilot study of micronized resveratrol (SRT501) in patients with hepatic metastases—Safety, pharmacokinetics, and pharmacodynamics. Cancer Prev. Res. 2011, 4, 1419–1425. [Google Scholar] [CrossRef]
- Kodarahmian, M.; Amidi, F.; Moini, A.; Kashani, L.; Shabani Nashtaei, M.; Pazhohan, A.; Bahramrezai, M.; Berenjian, S.; Sobhani, A. The modulating effects of Resveratrol on the expression of MMP-2 and MMP-9 in endometriosis women: A randomized exploratory trial. Gynecol. Endocrinol. 2019, 35, 719–726. [Google Scholar] [CrossRef]
- Khodarahmian, M.; Amidi, F.; Moini, A.; Kashani, L.; Salahi, E.; Danaii-mehrabad, S.; Nashtaei, M.S.; Mojtahedi, M.F.; Esfandyari, S.; Sobhani, A. A randomized exploratory trial to assess the effects of resveratrol on VEGF and TNF-α 2 expression in endometriosis women. J. Reprod. Immunol. 2021, 143, 103248. [Google Scholar] [CrossRef]
- Mendes da Silva, D.; Gross, L.A.; Neto, E.d.P.G.; Lessey, B.A.; Savaris, R.F. The Use of Resveratrol as an Adjuvant Treatment of Pain in Endometriosis: A Randomized Clinical Trial. J. Endocr. Soc. 2017, 1, 359–369. [Google Scholar] [CrossRef]
- Bahramrezaie, M.; Amidi, F.; Aleyasin, A.; Saremi, A.; Aghahoseini, M.; Brenjian, S.; Khodarahmian, M.; Pooladi, A. Effects of resveratrol on VEGF & HIF1 genes expression in granulosa cells in the angiogenesis pathway and laboratory parameters of polycystic ovary syndrome: A triple-blind randomized clinical trial. J. Assist. Reprod. Genet. 2019, 36, 1701–1712. [Google Scholar] [CrossRef]
- Banaszewska, B.; Wrotynska-Barczynska, J.; Spaczynski, R.Z.; Pawelczyk, L.; Duleba, A.J. Effects of Resveratrol on Polycystic Ovary Syndrome: A Double-blind, Randomized, Placebo-controlled Trial. J. Clin. Endocrinol. Metab. 2016, 101, 4322–4328. [Google Scholar] [CrossRef]
- Brenjian, S.; Moini, A.; Yamini, N.; Kashani, L.; Faridmojtahedi, M.; Bahramrezaie, M.; Khodarahmian, M.; Amidi, F. Resveratrol treatment in patients with polycystic ovary syndrome decreased pro-inflammatory and endoplasmic reticulum stress markers. Am. J. Reprod. Immunol. 2020, 83, e13186. [Google Scholar] [CrossRef]
- Mansour, A.; Samadi, M.; Sanginabadi, M.; Gerami, H.; Karimi, S.; Hosseini, S.; Shirzad, N.; Hekmatdoost, A.; Mahdavi-Gorabi, A.; Mohajeri-Tehrani, M.R.; et al. Effect of resveratrol on menstrual cyclicity, hyperandrogenism and metabolic profile in women with PCOS. Clin. Nutr. 2021, 40, 4106–4112. [Google Scholar] [CrossRef] [PubMed]
- Lin, C.-T.; Sun, X.-Y.; Lin, A.-X. Supplementation with high-dose trans-resveratrol improves ultrafiltration in peritoneal dialysis patients: A prospective, randomized, double-blind study. Ren. Fail. 2016, 38, 214–221. [Google Scholar] [CrossRef] [PubMed]
- Saldanha, J.F.; Leal, V.O.; Rizzetto, F.; Grimmer, G.H.; Ribeiro-Alves, M.; Daleprane, J.B.; Carraro-Eduardo, J.C.; Mafra, D. Effects of Resveratrol Supplementation in Nrf2 and NF-kappaB Expressions in Nondialyzed Chronic Kidney Disease Patients: A Randomized, Double-Blind, Placebo-Controlled, Crossover Clinical Trial. J. Ren. Nutr. 2016, 26, 401–406. [Google Scholar] [CrossRef] [PubMed]
- Alvarenga, L.; Cardozo, L.F.M.F.; Leal, V.d.O.; Kemp, J.A.; Saldanha, J.F.; Ribeiro-Alves, M.; Meireles, T.; Nakao, L.S.; Mafra, D. Can Resveratrol Supplementation Reduce Uremic Toxin Plasma Levels From the Gut Microbiota in Nondialyzed Patients With Chronic Kidney Disease? J. Ren. Nutr. 2022, 32, 685–691. [Google Scholar] [CrossRef] [PubMed]
- Dzator, J.S.A.; Howe, P.R.C.; Coupland, K.G.; Wong, R.H.X. A Randomised, Double-Blind, Placebo-Controlled Crossover Trial of Resveratrol Supplementation for Prophylaxis of Hormonal Migraine. Nutrients 2022, 14, 1763. [Google Scholar] [CrossRef] [PubMed]
- Ma, H.; Qiao, Z. Analysis of the efficacy of resveratrol treatment in patients with scarred uterus. Exp. Ther. Med. 2018, 15, 5410–5414. [Google Scholar] [CrossRef] [PubMed]
- Ornstrup, M.J.; Harslof, T.; Kjaer, T.N.; Langdahl, B.L.; Pedersen, S.B. Resveratrol increases bone mineral density and bone alkaline phosphatase in obese men: A randomized placebo-controlled trial. J. Clin. Endocrinol. Metab. 2014, 99, 4720–4729. [Google Scholar] [CrossRef]
- Kjaer, T.N.; Ornstrup, M.J.; Poulsen, M.M.; Jorgensen, J.O.L.; Hougaard, D.M.; Cohen, A.S.; Neghabat, S.; Richelsen, B.; Pedersen, S.B. Resveratrol reduces the levels of circulating androgen precursors but has no effect on, testosterone, dihydrotestosterone, PSA levels or prostate volume. A 4-month randomised trial in middle-aged men. Prostate 2015, 75, 1255–1263. [Google Scholar] [CrossRef]
- Kjaer, T.N.; Ornstrup, M.J.; Poulsen, M.M.; Stodkilde-Jorgensen, H.; Jessen, N.; Jorgensen, J.O.L.; Richelsen, B.; Pedersen, S.B. No Beneficial Effects of Resveratrol on the Metabolic Syndrome: A Randomized Placebo-Controlled Clinical Trial. J. Clin. Endocrinol. Metab. 2017, 102, 1642–1651. [Google Scholar] [CrossRef]
- Korsholm, A.S.; Kjaer, T.N.; Ornstrup, M.J.; Pedersen, S.B. Comprehensive metabolomic analysis in blood, urine, fat, and muscle in men with metabolic syndrome: A randomized, placebo-controlled clinical trial on the effects of resveratrol after four months’ treatment. Int. J. Mol. Sci. 2017, 18, 554. [Google Scholar] [CrossRef] [PubMed]
- Golshah, A.; Mirzaeei, S.; Nikkerdar, N.; Ghorbani, F. Gingivitis effectiveness of emulgel containing 2% resveratrol in orthodontic patients: An 8-week randomized clinical trial. Int. J. Dent. 2021, 2021, 6615900. [Google Scholar] [CrossRef]
- Zhang, Q.; Xu, S.; Xu, W.; Zhou, Y.; Luan, H.; Wang, D. Resveratrol decreases local inflammatory markers and systemic endotoxin in patients with aggressive periodontitis. Medicine 2022, 101, e29393. [Google Scholar] [CrossRef]
- Samsami-Kor, M.; Daryani, N.E.; Asl, P.R.; Hekmatdoost, A. Anti-Inflammatory Effects of Resveratrol in Patients with Ulcerative Colitis: A Randomized, Double-Blind, Placebo-controlled Pilot Study. Arch. Med. Res. 2015, 46, 280–285. [Google Scholar] [CrossRef]
- Samsamikor, M.; Daryani, N.E.; Asl, P.R.; Hekmatdoost, A. Resveratrol Supplementation and Oxidative/Anti-Oxidative Status in Patients with Ulcerative Colitis: A Randomized, Double-Blind, Placebo-controlled Pilot Study. Arch. Med. Res. 2016, 47, 304–309. [Google Scholar] [CrossRef]
- Beijers, R.J.; Gosker, H.R.; Sanders, K.J.; de Theije, C.; Kelders, M.; Clarke, G.; Cryan, J.F.; van den Borst, B.; Schols, A.M. Resveratrol and metabolic health in COPD: A proof-of-concept randomized controlled trial. Clin. Nutr. 2020, 39, 2989–2997. [Google Scholar] [CrossRef]
- Malaguarnera, G.; Pennisi, M.; Bertino, G.; Motta, M.; Borzì, A.M.; Vicari, E.; Bella, R.; Drago, F.; Malaguarnera, M. Resveratrol in Patients with Minimal Hepatic Encephalopathy. Nutrients 2018, 10, 329. [Google Scholar] [CrossRef]
- Martinez, A.M.; Sordia-Hernandez, L.H.; Morales, J.A.; Merino, M.; Vidal, O.; Garcia Garza, M.R.; Valdes, O. A randomized clinical study assessing the effects of the antioxidants, resveratrol or SC1002, a hydrogen sulfide prodrug, on idiopathic oligoasthenozoospermia. Asian Pac. J. Reprod. 2015, 4, 106–111. [Google Scholar] [CrossRef]
- Qiang, L.; Di, Y.; Jiang, Z.; Xu, J. Resveratrol improves efficacy of oral amoxicillin against childhood fast breathing pneumonia in a randomized placebo-controlled double blind clinical trial. Microb. Pathog. 2018, 114, 209–212. [Google Scholar] [CrossRef]
- Shi, G.; Hua, M.; Xu, Q.; Ren, T. Resveratrol improves treatment outcome and laboratory parameters in patients with Takayasu arteritis: A randomized double-blind and placebo-controlled trial. Immunobiology 2017, 222, 164–168. [Google Scholar] [CrossRef]
- Renaud, S.; de Lorgeril, M. Wine, alcohol, platelets, and the French paradox for coronary heart disease. Lancet 1992, 339, 1523–1526. [Google Scholar] [CrossRef]
- Catalgol, B.; Batirel, S.; Taga, Y.; Ozer, N.K. Resveratrol: French paradox revisited. Front. Pharmacol. 2012, 3, 141. [Google Scholar] [CrossRef]
- Fragopoulou, E.; Antonopoulou, S. The French paradox three decades later: Role of inflammation and thrombosis. Clin. Chim. Acta 2020, 510, 160–169. [Google Scholar] [CrossRef]
- Brown, K.; Aburido, G.; Britton, R.G. Resveratrol for Cancer Prevention: Current Gaps and Opportunities. In Natural Products for Cancer Chemoprevention; Pezzuto, J., Vang, O., Eds.; Springer: Cham, Switzerland, 2020; pp. 19–47. [Google Scholar]
- Nunes, T.; Almeida, L.; Rocha, J.-F.; Falcao, A.; Fernandes-Lopes, C.; Loureiro, A.I.; Wright, L.; Vaz-da-Silva, M.; Soares-da-Silva, P. Pharmacokinetics of trans-resveratrol following repeated administration in healthy elderly and young subjects. J. Clin. Pharmacol. 2009, 49, 1477–1482. [Google Scholar] [CrossRef]
- la Porte, C.; Voduc, N.; Zhang, G.; Seguin, I.; Tardiff, D.; Singhal, N.; Cameron, D.W. Steady-State pharmacokinetics and tolerability of trans-resveratrol 2000 mg twice daily with food, quercetin and alcohol (ethanol) in healthy human subjects. Clin. Pharmacokinet. 2010, 49, 449–454. [Google Scholar] [CrossRef]
- Brown, V.A.; Patel, K.R.; Viskaduraki, M.; Crowell, J.A.; Perloff, M.; Booth, T.D.; Vasilinin, G.; Sen, A.; Schinas, A.M.; Piccirilli, G.; et al. Repeat dose study of the cancer chemopreventive agent resveratrol in healthy volunteers: Safety, pharmacokinetics, and effect on the insulin-like growth factor axis. Cancer Res. 2010, 70, 9003–9011. [Google Scholar] [CrossRef]
- Vaz-da-Silva, M.; Loureiro, A.I.; Falcao, A.; Nunes, T.; Rocha, J.F.; Fernandes-Lopes, C.; Soares, E.; Wright, L.; Almeida, L.; Soares-da-Silva, P. Effect of food on the pharmacokinetic profile of trans-resveratrol. Int. J. Clin. Pharmacol. Ther. 2008, 46, 564–570. [Google Scholar] [CrossRef]
- Kemper, C.; Benham, D.; Brothers, S.; Wahlestedt, C.; Volmar, C.-H.; Bennett, D.; Hayward, M. Safety and pharmacokinetics of a highly bioavailable resveratrol preparation (JOTROL TM). AAPS Open 2022, 8, 11. [Google Scholar] [CrossRef]
- Marchezan, J.; Deckmann, I.; da Fonseca, G.C.; Margis, R.; Riesgo, R.; Gottfried, C. Resveratrol Treatment of Autism Spectrum Disorder-A Pilot Study. Clin. Neuropharmacol. 2022, 45, 122–127. [Google Scholar] [CrossRef]
- Kawamura, K.; Fukumura, S.; Nikaido, K.; Tachi, N.; Kozuka, N.; Seino, T.; Hatakeyama, K.; Mori, M.; Ito, Y.M.; Takami, A.; et al. Resveratrol improves motor function in patients with muscular dystrophies: An open-label, single-arm, phase IIa study. Sci. Rep. 2020, 10, 20585. [Google Scholar] [CrossRef]
- Chow, H.H.S.; Garland, L.L.; Hsu, C.-H.; Vining, D.R.; Chew, W.M.; Miller, J.A.; Perloff, M.; Crowell, J.A.; Alberts, D.S. Resveratrol modulates drug- and carcinogen-metabolizing enzymes in a healthy volunteer study. Cancer Prev. Res. 2010, 3, 1168–1175. [Google Scholar] [CrossRef]
- Popat, R.; Plesner, T.; Davies, F.; Cook, G.; Cook, M.; Elliott, P.; Jacobson, E.; Gumbleton, T.; Oakervee, H.; Cavenagh, J. A phase 2 study of SRT501 (resveratrol) with bortezomib for patients with relapsed and or refractory multiple myeloma. Br. J. Haematol. 2013, 160, 714–717. [Google Scholar] [CrossRef]
- Bedada, S.K.; Neerati, P. Resveratrol Pretreatment Affects CYP2E1 Activity of Chlorzoxazone in Healthy Human Volunteers. Phytother. Res. 2016, 30, 463–468. [Google Scholar] [CrossRef]
- Bedada, S.K.; Nearati, P. Effect of resveratrol on the pharmacokinetics of carbamazepine in healthy human volunteers. Phytother. Res. 2015, 29, 701–706. [Google Scholar] [CrossRef]
- Iglesias-Aguirre, C.E.; Vallejo, F.; Beltran, D.; Aguilar-Aguilar, E.; Puigcerver, J.; Alajarin, M.; Berna, J.; Selma, M.V.; Espín, J.C. Lunularin Producers versus Non-producers: Novel Human Metabotypes Associated with the Metabolism of Resveratrol by the Gut Microbiota. J. Agric. Food Chem. 2022, 70, 10521–10531. [Google Scholar] [CrossRef]
- Boocock, D.J.; Faust, G.E.S.; Patel, K.R.; Schinas, A.M.; Brown, V.A.; Ducharme, M.P.; Booth, T.D.; Crowell, J.A.; Perloff, M.; Gescher, A.J.; et al. Phase I dose escalation pharmacokinetic study in healthy volunteers of resveratrol, a potential cancer chemopreventive agent. Cancer Epidemiol. Biomark. Prev. 2007, 16, 1246–1252. [Google Scholar] [CrossRef]
- Cai, H.; Scott, E.; Kholghi, A.; Andreadi, C.; Rufini, A.; Karmokar, A.; Britton, R.G.; Horner-Glister, E.; Greaves, P.; Jawad, D.; et al. Cancer chemoprevention: Evidence of a nonlinear dose response for the protective effects of resveratrol in humans and mice. Sci. Transl. Med. 2015, 7, 298ra117. [Google Scholar] [CrossRef]
- Briskey, D.; Rao, A. Trans-Resveratrol Oral Bioavailability in Humans Using LipiSperse TM Dispersion Technology. Pharmaceutics 2020, 12, 1190. [Google Scholar] [CrossRef]
- Brotons-Canto, A.; Gonzalez-Navarro, C.J.; Gurrea, J.; Gonzalez-Ferrero, C.; Irache, J.M. Zein nanoparticles improve the oral bioavailability of resveratrol in humans. J. Drug Deliv. Sci. Technol. 2020, 57, 101704. [Google Scholar] [CrossRef]
- Iannitti, R.G.; Floridi, A.; Lazzarini, A.; Tantucci, A.; Russo, R.; Ragonese, F.; Monarca, L.; Caglioti, C.; Spogli, R.; Leonardi, L.; et al. Resveratrol Supported on Magnesium DiHydroxide (Resv@MDH) Represents an Oral Formulation of Resveratrol With Better Gastric Absorption and Bioavailability Respect to Pure Resveratrol. Front. Nutr. 2020, 7, 570047. [Google Scholar] [CrossRef]
- Jang, J.Y.; Im, E.; Kim, N.D. Mechanism of Resveratrol-Induced Programmed Cell Death and New Drug Discovery against Cancer: A Review. Int. J. Mol. Sci. 2022, 23, 13689. [Google Scholar] [CrossRef]
- Tabrizi, R.; Tamtaji, O.R.; Lankarani, K.B.; Mirhosseini, N.; Akbari, M.; Dadgostar, E.; Peymani, P.; Asemi, Z. The effects of resveratrol supplementation on biomarkers of inflammation and oxidative stress among patients with metabolic syndrome and related disorders: A systematic review and meta-analysis of randomized controlled trials. Food Funct. 2018, 9, 6116–6128. [Google Scholar] [CrossRef]
- Rafiee, S.; Mohammadi, H.; Ghavami, A.; Sadeghi, E.; Safari, Z.; Askari, G. Efficacy of resveratrol supplementation in patients with nonalcoholic fatty liver disease: A systematic review and meta-analysis of clinical trials. Complement. Ther. Clin. Pract. 2021, 42, 101281. [Google Scholar] [CrossRef]
- Hosseini, H.; Koushki, M.; Khodabandehloo, H.; Fathi, M.; Panahi, G.; Teimouri, M.; Majidi, Z.; Meshkani, R. The effect of resveratrol supplementation on C-reactive protein (CRP) in type 2 diabetic patients: Results from a systematic review and meta-analysis of randomized controlled trials. Complement. Ther. Med. 2020, 49, 102251. [Google Scholar] [CrossRef]
- Zhou, Q.; Wang, Y.; Han, X.; Fu, S.; Zhu, C.; Chen, Q. Efficacy of Resveratrol Supplementation on Glucose and Lipid Metabolism: A Meta-Analysis and Systematic Review. Front. Physiol. 2022, 13, 795980. [Google Scholar] [CrossRef]
- Cao, X.; Liao, W.; Xia, H.; Wang, S.; Sun, G. The Effect of Resveratrol on Blood Lipid Profile: A Dose-Response Meta-Analysis of Randomized Controlled Trials. Nutrients 2022, 14, 3755. [Google Scholar] [CrossRef]
- Delpino, F.M.; Figueiredo, L.M. Resveratrol supplementation and type 2 diabetes: A systematic review and meta-analysis. Crit Rev. Food Sci. Nutr. 2022, 62, 4465–4480. [Google Scholar] [CrossRef]
- Delpino, F.M.; Figueiredo, L.M.; Caputo, E.L.; Mintem, G.C.; Gigante, D.P. What is the effect of resveratrol on obesity? A systematic review and meta-analysis. Clin. Nutr. ESPEN 2021, 41, 59–67. [Google Scholar] [CrossRef]
- COLO-PREVENT—Do Simple Drugs (Aspirin or Aspirin Plus Metformin) or Food Supplements (Resveratrol) Reduce the Occurrence of Bowel Polyps (Small Growths on the Bowel Lining), Which in Turn Reduce Bowel Cancer Risk? Available online: https://www.isrctn.com/ISRCTN13526628 (accessed on 31 December 2023).
- Alonso, C.; Marti, M.; Barba, C.; Carrer, V.; Rubio, L.; Coderch, L. Skin permeation and antioxidant efficacy of topically applied resveratrol. Arch. Dermatol. Res. 2017, 309, 423–431. [Google Scholar] [CrossRef]
- Amiot, M.J.; Romier, B.; Dao, T.-M.A.; Fanciullino, R.; Ciccolini, J.; Burcelin, R.; Pechere, L.; Emond, C.; Savouret, J.-F.; Seree, E. Optimization of trans-Resveratrol bioavailability for human therapy. Biochimie 2013, 95, 1233–1238. [Google Scholar] [CrossRef]
- Andrade, J.M.O.; Barcala-Jorge, A.S.; Batista-Jorge, G.C.; Paraiso, A.F.; de Freitas, K.M.; Lelis, D.d.F.; Guimaraes, A.L.S.; de Paula, A.M.B.; Santos, S.H.S. Effect of resveratrol on expression of genes involved thermogenesis in mice and humans. Biomed. Pharmacother. 2019, 112, 108634. [Google Scholar] [CrossRef]
- Bailey, H.H.; Johnson, J.J.; Lozar, T.; Scarlett, C.O.; Wollmer, B.W.; Kim, K.; Havinghurst, T.; Ahmad, N. A randomized, double-blind, dose-ranging, pilot trial of piperine with resveratrol on the effects on serum levels of resveratrol. Eur. J. Cancer Prev. 2021, 30, 285–290. [Google Scholar] [CrossRef]
- Bedada, S.K.; Yellu, N.R.; Neerati, P. Effect of Resveratrol Treatment on the Pharmacokinetics of Diclofenac in Healthy Human Volunteers. Phytother. Res. 2016, 30, 397–401. [Google Scholar] [CrossRef]
- Blanchard, O.L.; Friesenhahn, G.; Javors, M.A.; Smoliga, J.M. Development of a lozenge for oral transmucosal delivery of trans-resveratrol in humans: Proof of concept. PLoS ONE 2014, 9, e90131. [Google Scholar] [CrossRef]
- Bode, L.M.; Bunzel, D.; Huch, M.; Cho, G.-S.; Ruhland, D.; Bunzel, M.; Bub, A.; Franz, C.M.A.P.; Kulling, S.E. In vivo and in vitro metabolism of trans-resveratrol by human gut microbiota. Am. J. Clin. Nutr. 2013, 97, 295–309. [Google Scholar] [CrossRef]
- Boocock, D.J.; Patel, K.R.; Faust, G.E.S.; Normolle, D.P.; Marczylo, T.H.; Crowell, J.A.; Brenner, D.E.; Booth, T.D.; Gescher, A.; Steward, W.P. Quantitation of trans-resveratrol and detection of its metabolites in human plasma and urine by high performance liquid chromatography. J. Chromatogr. B Analyt. Technol. Biomed. Life Sci. 2007, 848, 182–187. [Google Scholar] [CrossRef]
- Cai, H.; Scott, E.N.; Britton, R.G.; Parrott, E.; Ognibene, T.J.; Malfatti, M.; Khan, M.; Steward, W.P.; Brown, K. Distribution and metabolism of [14C]-resveratrol in human prostate tissue after oral administration of a “dietary-achievable” or “pharmacological” dose: What are the implications for anticancer activity? Am. J. Clin. Nutr. 2021, 113, 1115–1125. [Google Scholar] [CrossRef]
- Chekalina, N.I.; Kazakov, Y.M.; Mamontova, T.V.; Vesnina, L.E.; Kaidashev, I.P. Resveratrol more effectively than quercetin reduces endothelium degeneration and level of necrosis factor alpha in patients with coronary artery disease. Wiad. Lek. 2016, 69, 475–479. [Google Scholar]
- Chow, H.H.S.; Garland, L.L.; Heckman-Stoddard, B.M.; Hsu, C.-H.; Butler, V.D.; Cordova, C.A.; Chew, W.M.; Cornelison, T.L. A pilot clinical study of resveratrol in postmenopausal women with high body mass index: Effects on systemic sex steroid hormones. J. Transl. Med. 2014, 12, 223. [Google Scholar] [CrossRef]
- Crandall, J.P.; Oram, V.; Trandafirescu, G.; Reid, M.; Kishore, P.; Hawkins, M.; Cohen, H.W.; Barzilai, N. Pilot study of resveratrol in older adults with impaired glucose tolerance. J. Gerontol. Ser. A Biol. Sci. Med. Sci. 2012, 67, 1307–1312. [Google Scholar] [CrossRef]
- De Groote, D.; Van Belleghem, K.; Deviere, J.; Van Brussel, W.; Mukaneza, A.; Amininejad, L. Effect of the intake of resveratrol, resveratrol phosphate, and catechin-rich grape seed extract on markers of oxidative stress and gene expression in adult obese subjects. Ann. Nutr. Metab. 2012, 61, 15–24. [Google Scholar] [CrossRef]
- Diaz, M.; Avila, A.; Degens, H.; Coeckelberghs, E.; Vanhees, L.; Cornelissen, V.; Azzawi, M. Acute resveratrol supplementation in coronary artery disease: Towards patient stratification. Scand. Cardiovasc. J. 2019, 54, 14–19. [Google Scholar] [CrossRef]
- Fabbrocini, G.; Staibano, S.; De Rosa, G.; Battimiello, V.; Fardella, N.; Ilardi, G.; La Rotonda, M.I.; Longobardi, A.; Mazzella, M.; Siano, M.; et al. Resveratrol-containing gel for the treatment of acne vulgaris: A single-blind, vehicle-controlled, pilot study. Am. J. Clin. Dermatol. 2011, 12, 133–141. [Google Scholar] [CrossRef]
- Gualdoni, G.A.; Kovarik, J.J.; Hofer, J.; Dose, F.; Pignitter, M.; Doberer, D.; Steinberger, P.; Somoza, V.; Wolzt, M.; Zlabinger, G.J. Resveratrol enhances TNF-α production in human monocytes upon bacterial stimulation. Biochim. Et Biophys. Acta (BBA) Gen. Subj. 2014, 1840, 95–105. [Google Scholar] [CrossRef]
- Gualdoni, G.A.; Fuchs, D.; Zlabinger, G.J.; Gostner, J.M. Resveratrol intake enhances indoleamine-2,3-dioxygenase activity in humans. Pharmacol. Rep. 2016, 68, 1065–1068. [Google Scholar] [CrossRef]
- Pignitter, M.; Schueller, K.; Burkon, A.; Knorr, V.; Esefelder, L.; Doberer, D.; Wolzt, M.; Somoza, V. Concentration-dependent effects of resveratrol and metabolites on the redox status of human erythrocytes in single-dose studies. J. Nutr. Biochem. 2016, 27, 164–170. [Google Scholar] [CrossRef]
- Gupta, N.; Zhang, B.; Zhou, Y.; McCormack, F.X.; Ingledue, R.; Robbins, N.; Kopras, E.J.; McMahan, S.; Singla, A.; Swigris, J.; et al. Safety and Efficacy of Combined Resveratrol and Sirolimus in Lymphangioleiomyomatosis. Chest 2023, 163, 1144–1155. [Google Scholar] [CrossRef]
- Huang, C.C.; Liu, C.C.; Tsao, J.P.; Hsu, C.L.; Cheng, I.S. Effects of oral resveratrol supplementation on glycogen replenishment and mitochondria biogenesis in exercised human skeletal muscle. Nutrients 2020, 12, 3721. [Google Scholar] [CrossRef]
- Tsao, J.-P.; Liu, C.-C.; Wang, H.-F.; Bernard, J.R.; Huang, C.-C.; Cheng, I.S. Oral Resveratrol supplementation attenuates exercise-induced Interleukin-6 but not Oxidative Stress after a high intensity cycling challenge in adults. Int. J. Med. Sci. 2021, 18, 2137–2145. [Google Scholar] [CrossRef]
- Iglesias-Aguirre, C.E.; Avila-Galvez, M.A.; Lopez de Las Hazas, M.-C.; Davalos, A.; Espin, J.C. Exosome-Containing Extracellular Vesicles Contribute to the Transport of Resveratrol Metabolites in the Bloodstream: A Human Pharmacokinetic Study. Nutrients 2022, 14, 3632. [Google Scholar] [CrossRef]
- Joseph, A.; Balakrishnan, A.; Shanmughan, P.; Maliakel, B.; Illathu Madhavamenon, K. Micelle/Hydrogel Composite as a “Natural Self-Emulsifying Reversible Hybrid Hydrogel (N’SERH)” Enhances the Oral Bioavailability of Free (Unconjugated) Resveratrol. ACS Omega 2022, 7, 12835–12845. [Google Scholar] [CrossRef]
- Maia, H., Jr.; Haddad, C.; Pinheiro, N.; Casoy, J. Advantages of the association of resveratrol with oral contraceptives for management of endometriosis-related pain. Int. J. Women’s Health 2012, 4, 543–549. [Google Scholar] [CrossRef]
- Mansur, A.P.; Roggerio, A.; Goes, M.F.S.; Avakian, S.D.; Leal, D.P.; Maranhao, R.C.; Strunz, C.M.C. Serum concentrations and gene expression of sirtuin 1 in healthy and slightly overweight subjects after caloric restriction or resveratrol supplementation: A randomized trial. Int. J. Cardiol. 2017, 227, 788–794. [Google Scholar] [CrossRef]
- Roggerio, A.; Strunz, C.M.C.; Pacanaro, A.P.; Leal, D.P.; Takada, J.Y.; Avakian, S.D.; Mansur, A.d.P. Gene Expression of Sirtuin-1 and Endogenous Secretory Receptor for Advanced Glycation End Products in Healthy and Slightly Overweight Subjects after Caloric Restriction and Resveratrol Administration. Nutrients 2018, 10, 937. [Google Scholar] [CrossRef]
- Marouf, B.H. Effect of Resveratrol on Serum Levels of Type II Collagen and Aggrecan in Patients with Knee Osteoarthritis: A Pilot Clinical Study. BioMed Res. Int. 2021, 2021, 3668568. [Google Scholar] [CrossRef]
- Movahed, A.; Raj, P.; Nabipour, I.; Mahmoodi, M.; Ostovar, A.; Kalantarhormozi, M.; Netticadan, T. Efficacy and Safety of Resveratrol in Type 1 Diabetes Patients: A Two-Month Preliminary Exploratory Trial. Nutrients 2020, 12, 161. [Google Scholar] [CrossRef]
- Ochiai, A.; Kuroda, K.; Ikemoto, Y.; Ozaki, R.; Nakagawa, K.; Nojiri, S.; Takeda, S.; Sugiyama, R. Influence of resveratrol supplementation on IVF-embryo transfer cycle outcomes. Reprod. Biomed. Online 2019, 39, 205–210. [Google Scholar] [CrossRef]
- Patel, K.R.; Brown, V.A.; Jones, D.J.L.; Britton, R.G.; Hemingway, D.; Miller, A.S.; West, K.P.; Booth, T.D.; Perloff, M.; Crowell, J.A.; et al. Clinical pharmacology of resveratrol and its metabolites in colorectal cancer patients. Cancer Res. 2010, 70, 7392–7399. [Google Scholar] [CrossRef]
- Patel, K.R.; Andreadi, C.; Britton, R.G.; Horner-Glister, E.; Karmokar, A.; Sale, S.; Brown, V.A.; Brenner, D.E.; Singh, R.; Steward, W.P.; et al. Sulfate metabolites provide an intracellular pool for resveratrol generation and induce autophagy with senescence. Sci. Transl. Med. 2013, 5, 205ra133. [Google Scholar] [CrossRef]
- Radko, Y.; Christensen, K.B.; Christensen, L.P. Semi-preparative isolation of dihydroresveratrol-3-O-beta-d-glucuronide and four resveratrol conjugates from human urine after oral intake of a resveratrol-containing dietary supplement. J. Chromatogr. B Anal. Technol. Biomed. Life Sci. 2013, 930, 54–61. [Google Scholar] [CrossRef]
- Sergides, C.; Chirila, M.; Silvestro, L.; Pitta, D.; Pittas, A. Bioavailability and safety study of resveratrol 500 mg tablets in healthy male and female volunteers. Exp. Ther. Med. 2016, 11, 164–170. [Google Scholar] [CrossRef]
- Tani, H.; Hikami, S.; Iizuna, S.; Yoshimatsu, M.; Asama, T.; Ota, H.; Kimura, Y.; Tatefuji, T.; Hashimoto, K.; Higaki, K. Pharmacokinetics and safety of resveratrol derivatives in humans after oral administration of melinjo (Gnetum gnemon L.) seed extract powder. J. Agric. Food Chem. 2014, 62, 1999–2007. [Google Scholar] [CrossRef]
- Theodotou, M.; Fokianos, K.; Mouzouridou, A.; Konstantinou, C.; Aristotelous, A.; Prodromou, D.; Chrysikou, A. The effect of resveratrol on hypertension: A clinical trial. Exp. Ther. Med. 2017, 13, 295–301. [Google Scholar] [CrossRef]
- Theodotou, M.; Fokianos, K.; Moniatis, D.; Kadlenic, R.; Chrysikou, A.; Aristotelous, A.; Mouzouridou, A.; Diakides, J.; Stavrou, E. Effect of resveratrol on non-alcoholic fatty liver disease. Exp. Ther. Med. 2019, 18, 559–565. [Google Scholar] [CrossRef]
- Wagemaker, T.A.L.; Maia Campos, P.M.B.G.; Shimizu, K.; Kyotani, D.; Yoshida, D. Antioxidant-based topical formulations influence on the inflammatory response of Japanese skin: A clinical study using non-invasive techniques. Eur. J. Pharm. Biopharm. 2017, 117, 195–202. [Google Scholar] [CrossRef]
- Yiu, E.M.; Tai, G.; Peverill, R.E.; Lee, K.J.; Croft, K.D.; Mori, T.A.; Scheiber-Mojdehkar, B.; Sturm, B.; Praschberger, M.; Vogel, A.P.; et al. An open-label trial in Friedreich ataxia suggests clinical benefit with high-dose resveratrol, without effect on frataxin levels. J. Neurol. 2015, 262, 1344–1353. [Google Scholar] [CrossRef]
- Zhang, B.; Zhang, Y.; Liu, X.; Zhao, C.; Yin, J.; Li, X.; Zhang, X.; Wang, J.; Wang, S. Distinctive anti-inflammatory effects of resveratrol, dihydroresveratrol, and 3-(4-hydroxyphenyl)-propionic acid on DSS-induced colitis in pseudo-germ-free mice. Food Chem. 2023, 400, 133904. [Google Scholar] [CrossRef]

| Study (Trial Registration Identification, Where Reported) | Primary Aim or Outcome | Participants | Dose, Schedule, and Formulation | Number of Participants | Adverse Events | Main Findings | |
|---|---|---|---|---|---|---|---|
| Cardiovascular disease | |||||||
| 1 | Apostolidou 2015 [3] | Changes in blood lipid levels, vitamin E, and total anti-oxidant capacity (TAC) | Healthy volunteers with asymptomatic hypercholesterolemia and normal cholesterol levels | RSV 150 mg or placebo capsule once daily. Crossover design with 30 days of RSV/placebo; 30 days washout then 30 days placebo/RSV | 40 in total with 20 in each group | None stated | RSV caused a direct antioxidant effect in normal cholesterol individuals. In asymptomatic hypercholesterolaemic individuals, RSV facilitated an increase in vitamin E |
| 2 | Chekalina 2017 [4] | Effects on parameters of central hemodynamics and myocardial ischemia in patients with stable coronary heart disease | Patients with coronary heart disease: stable angina pectoris, FC II, and healthy individuals made up the control group | 100 mg RSV daily for 2 months. The pharmaceutical form of RSV is not stated | 30 on RSV plus standard therapy; 55 on standard therapy alone | None stated | RSV significantly improved left ventricular systolic function (ejection fraction) and significantly reduced the number of premature atrial and ventricular contractions |
| 3 | Chen 2016 [5] | Effect of RSV on improving treatment outcomes of delayed recombinant tissue plasminogen activator (r-tPA) administration. The primary outcome was at least a 4-point improvement from baseline in the NIH stroke scale (NIHSS) score or a complete resolution of symptoms | Brain ischaemic stroke patients | 2.5 mg/kg RSV or placebo was given by intravenous bolus injection and then infusion over 60 min | 154 on RSV; 158 on placebo | Zero reported | RSV co-administration with r-tPA treatment significantly improved NIHSS scores |
| 4 | Ding 2017 [6] | Does RSV provide an effective adjuvant to nifedipine in decreasing blood pressure in severe pre-eclampsia? | Women with pregnancy-induced pre-eclampsia | All patients received nifedipine plus either a 50 mg RSV or placebo capsule every 15 min until blood pressure was ≤150/100 mmHg (maximum of 250 mg RSV) | 174 on RSV; 175 on placebo | 23 women reported AEs in the RSV group vs. 28 in the control group. No significant differences for maternal or neonatal AEs. RSV AEs were nausea; vomiting; maternal tachycardia; mild headache; dizziness; and chest pain | RSV + nifedipine group needed significantly less time to control blood pressure, delayed the time before a new hypertensive crisis, and resulted in a reduction in the number of doses to control blood pressure compared with the placebo + nifedipine group |
| 5 | Djurica 2016 [7] | Primary: reactive hyperaemia index (RHI) with platelet reactivity and plasma nitrate/nitrite | Healthy post-menopausal women | Crossover design; a single dose of 90 mg RSV or an RSV-arginine conjugate in capsule form with a minimum of 1 week washout | Thirty-seven and twenty-five women in two different studies that compared the two formulations both with a crossover design | Not stated | RSV-Arg significantly increased RHI and reduced platelet reactivity compared with RSV |
| 6 | Fodor 2018 [8] | Effects on BP, weight status, glucose, and lipid profile | Patients who had a first stroke in the last 12 months | RSV 100 mg or 200 mg once daily for 12 months. A control group (and RSV groups) received standard care only. The pharmaceutical form of RSV is not stated | 81 on 100 mg; 55 on 200 mg; and 92 in the control group | Not stated | RSV significantly decreased blood pressure, body mass index, all parameters of lipid profile, and glucose (in non-diabetic patients) compared with the control group |
| 7 | Gal 2020 [9] | Effects on left ventricular function and exercise capacity | Patients with systolic heart failure | 100 mg RSV or placebo taken in capsule form daily for 3 months | 30 on RSV; 30 on placebo | Not stated | RSV significantly improved systolic and diastolic left ventricular function, global longitudinal strain, exercise capacity, ventilation parameters, and quality of life vs. placebo |
| Gal 2020 [10] | Effects on hemorheological parameters in patients with heart failure with reduced ejection fraction | RSV significantly improved red blood cell aggregation compared to baseline | |||||
| 8 | Lixia 2021 [11] | Clinical effects of RSV on the treatment of atherosclerosis | Patients prone to atherosclerosis | 100 mg RSV or placebo, plus 20 mg atorvastatin (standard therapy) daily for 12 months. The type of RSV formulation used is not stated | 60 in each of the two groups | Not stated | In participants who took RSV plus atorvastatin daily, there was a significant decrease in both systolic and diastolic blood pressure, cholesterol, triglyceride, and low-density lipoprotein (LDL), and a significant increase in aspartate transaminase. There were no significant changes observed in these parameters for participants in the placebo + atorvastatin group after 12 months |
| 9 | Magyar 2012 [12] | Endothelium-dependent vasodilatation on systolic and diastolic left ventricular function | Patients with myocardial infarction and angiographically verified coronary artery disease | 10 mg RSV or placebo capsule once daily for 3 months | 20 on RSV; 20 on placebo | Not stated | RSV significantly improved left ventricular diastolic function and endothelial function as measured by flow-mediated dilatation |
| 10 | Marques 2018 (NCT02616822) [13] | Flow-mediated dilation | Hypertensive patients with endothelial dysfunction | 300 mg RSV or placebo in capsule form as a single dose in a crossover study | 24 | Zero reported | RSV promoted improved endothelial function, especially in women and those with higher LDL-cholesterol |
| 11 | McDermott 2017 (NCT02246660) [14] | Improve six-minute walk performance at 6-month follow-up | Older people with peripheral arterial disease | 125 mg, 500 mg RSV, or placebo capsule once daily for 6 months | 21 on 125 mg, 23 on 500 mg, and 22 on placebo | 14 in the 125 mg group; 27 in the 500 mg group; and 13 in the placebo group. No SAEs were attributable to RSV. AEs were diarrhoea; abdominal pain; and pruritic exanthem | RSV did not improve walking performance |
| 12 | Militaru 2013 (ISRCTN0233780) [15] | Effects on inflammation biomarkers (high-sensitivity C-reactive protein), left ventricular function markers (N-terminal prohormone of brain natriuretic peptide), and lipid markers (total cholesterol, low-density lipoprotein cholesterol, high-density lipoprotein-cholesterol, and triacylglycerols) | Patients with stable angina pectoris | 10 mg RSV capsule daily for 60 days. Other groups had 10 mg RSV plus calcium fructoborate, calcium fructoborate alone, or placebo | Twenty-nine patients per group with four study groups | Zero reported | RSV significantly decreased high-sensitivity C-reactive protein in all groups, with brain natriuretic peptide significantly decreased by RSV and by calcium fructoborate alone. This effect was enhanced by their combination |
| 13 | van der Made 2015 (NCT01364961) [16] | Effect on apolipoprotein A-I plasma concentrations | Overweight and slightly obese subjects with low HDL cholesterol concentrations | 75 mg RSV or placebo capsule twice daily for 4 weeks, with at least a 4-week washout | 45 participants; crossover design | Zero reported | RSV had no effect on apolipoprotein A-1 plasma concentrations |
| van der Made 2017 (Reanalysis of samples) [17] | Effects on endothelial function in the fasting state and postprandial phase | RSV had no effect on flow-mediated dilation in the fasting or postprandial state | |||||
| 14 | Wong 2011 [18] | Whether RSV could acutely improve FMD in a dose–dependent manner | Overweight individuals with mildly elevated blood pressure | Each participant consumed three doses of RSV (30, 90, and 270 mg) and placebo in capsule form at weekly intervals | 19 participants; crossover design | Not stated | RSV (30, 90, 270 mg doses) significantly increased FMD compared to placebo. There was a significant linear relationship between log10 of RSV dose and acute FMD response |
| 15 | Wong 2013 (ACTRN12611000060943) [19] | Whether there is a sustained effect on FMD after 6 weeks of daily RSV | Obese but otherwise healthy men and post-menopausal women | Initially randomised to either 75 mg RSV or a placebo capsule daily for 6 weeks in a crossover trial | 28 participants in total | Zero reported | RSV significantly increased FMD |
| Type 2 Diabetes Mellitus, including glucose control | |||||||
| 16 | Bashmakov 2014 (ACTRN12610000629033) [20] | The healing rate of foot ulcers in type 2 diabetics | T2DM patients with diabetic foot syndrome | 50 mg RSV or placebo capsule morning and evening for 60 days | 14 on RSV; 10 on placebo | None stated | RSV decreased diabetic ulcer size compared with placebo and marginally improved performance in the foot pressure test |
| 17 | Bhatt 2012 (CTRI/2011/05/ 00173) [21] | HbA1c levels | Patients with T2DM | 250 mg RSV capsule once daily for 3 months plus standard of care (anti-hypoglycaemic drugs as required). The control group received the standard of care only | 30 on RSV; 32 in the control group | None stated | RSV significantly improved mean haemoglobin A1c |
| Bhatt 2013 [22] | Effects of RSV in patients with T2DM on a range of anthropo-metric and biochemical markers | RSV significantly decreased body weight, systolic blood pressure, cholesterol, triglyceride, urea nitrogen, and total protein | |||||
| 18 | Bo 2016 (NCT02244879) [23] | Effects on CRP | Individuals with T2DM | RSV at a dose of 40 mg or 500 mg once daily, or placebo, given in capsule form for 6 months | 65 in each RSV group; 62 on placebo | Zero reported | RSV caused a non-significant dose–dependent reduction in CRP compared with placebo |
| Bo 2017 [24] | Circulating levels of PTX3 | RSV caused a dose–dependent increase in PTX3 and total anti-oxidant status (TAS). | |||||
| Bo 2018 [25] | Association between changes in SIRT-1 level and variation in H3K56ac value | RSV (500 mg) significantly increased SIRT1. RSV significantly decreased H3K56ac and body fat percentage in the highest SIRT-1 tertial | |||||
| Gambino 2019 [26] | Impact of rs12778366 (an SNP located in the promoter region of SIRT1) on the response to RSV supplementation in T2DM patients—the primary outcome was SIRT1 protein levels in PBMN cells | SIRT1 decreased in variant C-allele carriers but increased in T-allele homozygotes. Differences between C-allele carriers and T-allele homozygotes were significant in the RSV arm (40 mg) | |||||
| Bo 2018 [27] | Bone mineral density; circulating concentrations of calcium metabolism biomarkers | RSV (500 mg) caused a significant change in whole-body BMD, whole-body BMC, whole-body T-score, and serum phosphorus compared with placebo between baseline and study end | |||||
| 19 | Brasnyo 2011 [28] | Is RSV beneficial for controlling and/or improving insulin resistance? | Male patients with T2DM | 5 mg RSV or placebo capsule twice daily for 4 weeks | 10 on RSV; 9 on placebo | Zero reported | RSV significantly decreased insulin resistance |
| 20 | deLigt 2018 (NCT02129595) [29] | Improved insulin sensitivity and muscle mitochondrial function | Overweight males at increased risk of developing T2DM | Crossover RCT; participants took 150 mg RSV or placebo in capsule form once daily for 30 or 34 days with a washout of at least 30 days between interventions | 13; crossover study | None stated | RSV significantly improved ex vivo muscle mitochondrial function on a fatty acid-derived substrate but did not improve insulin sensitivity |
| Boswijk 2022 [30] | Reduction in arterial inflammation, assessed by 18F-FDG uptake measured by PET | Eight participants out of the original fifteen underwent an additional 18F-FDG PET scan and are included in this study | Arterial 18F-FDG uptake was non-significantly higher after RSV in comparison to placebo | ||||
| 21 | Goh 2014 (NCT01677611) [31] | SIRT expression in muscle and energy expenditure | Male patients with T2DM | RSV or placebo 500 mg starting dose; the dose was increased by 500 mg/day every 3 days to a maximum dose of 3 g per day in three divided doses if there was no hypoglycaemia. The duration was 12 weeks. Formulation of RSV was not stated but compliance was monitored by ‘pill counting’ | 5 on RSV; 5 on placebo | Four AEs reported. The proportion of patients with AEs did not significantly differ between groups. AEs were mild elevation of ALT in one patient on RSV; diarrhoea and mild hypoglycaemia, which resolved spontaneously in one patient on RSV; mild cellulitis at biopsy site in one patient per group | RSV significantly increased SIRT1 expression and p-AMPK to AMPK ratio compared with placebo |
| 22 | Hoseini 2019 (IRCT20181029041490) [32] | Insulin resistance | Patients with T2DM and proven 2- and 3-vessel coronary heart disease | 500 mg RSV or placebo in capsule form once daily for 4 weeks | 30 on RSV; 30 on placebo | Zero reported | RSV significantly reduced fasting glucose, insulin, and insulin resistance. RSV significantly increased insulin sensitivity |
| 23 | Khodabandehloo 2018 (RCT2015080223336N2) [33] | Changes in the percentage of CD14+ CD16+ monocytes; changes to blood glucose | Patients with T2DM | 400 mg RSV or placebo capsule twice daily for 8 weeks | 25 on RSV; 24 on placebo | Zero reported | RSV caused a significant reduction in fasting blood glucose and blood pressure. There was no change in the percentage of CD14+ CD16+ monocytes |
| 24 | Ma 2022 (MDJMU.201905191 × 1) [34] | Effects of RSV on blood glucose parameters, insulin resistance, nutrient sensing systems, and renal function | Elderly patients with T2DM | 500 mg RSV or placebo once daily for 6 months. No information was given on the formulation of RSV used | 238 on RSV; 234 on placebo | Although the following RSV-related side effects were reported: diarrhoea (28), constipation (6), muscle cramps/pain (12), fatigue (30), memory loss (4), allergies/upper respiratory infection (4), difficulty swallowing (3), rash (5), headache (4), there were no statistically significant differences in AEs reported from participants on RSV compared to placebo | RSV greatly improved glucose metabolism, insulin tolerance, and insulin metabolism compared to placebo. It significantly decreased levels of glycated haemoglobin/HbA1c, C-reactive protein, IL-6, TNF-α, and IL-1β compared to placebo and improved blood glucose parameters, lipid profile, and renal function |
| 25 | Mahjabeen 2022 (SLCTR/2018/019) [35] | Markers of glucose homeostasis, inflammation, and oxidative stress | Adults (18–70 years) with T2DM of duration ≥5 years, HbA1c 7–12%, and treated with oral hypoglycaemic agents for at least 1 year | One 200 mg capsule RSV or placebo per day for 24 weeks | 55 in each group | Zero reported | Significant reductions comparing RSV and placebo groups in HbA1c, fasting insulin, HOMA-IR, hs-CRP, TNF-α, IL-6, and malondialdehyde |
| 26 | Movahed 2013 (IRCT201111198129N1) [36] | Blood glucose lowering effect | Patients with T2DM | 500 mg RSV or placebo capsule twice daily for 45 days | 34 on RSV; 32 on placebo | Not stated | RSV significantly decreased fasting blood glucose, haemoglobin A1c, insulin, and insulin resistance |
| 27 | Sattarinezhad 2019 (NCT02704494) [37] | Changes in urine albumin/creatinine ratio, estimated glomerular filtration rate (eGFR), and serum creatinine levels | Patients with T2DM and newly confirmed albuminuria | 250 mg RSV or placebo capsule twice daily for 90 days | 32 on RSV; 32 on placebo | Two AEs consisting of mild dyspepsia | RSV significantly decreased the mean urine albumin/creatinine ratio. eGFR and serum creatinine were unchanged |
| 28 | Seyyedebrahimi 2018 (IRCT2015072523336N1) [38] | Oxidative stress indices in plasma and peripheral blood mononuclear cells | Patients with T2DM | 800 mg RSV or placebo in capsule form daily for 2 months | 23 on RSV; 25 on placebo | Zero reported | RSV significantly reduced plasma protein carbonyl content and PBMCs O2 level and significantly increased plasma total anti-oxidant capacity and total thiol content. The expression of Nrf2 and SOD were significantly increased after RSV consumption |
| 29 | Thazhath 2016 (ACTRN12613000717752) [39] | Effects on GLP-1 secretion, gastric emptying, and glycaemic control in T2DM | Patients with T2DM | 500 mg RSV or placebo capsule twice daily for 5 weeks | 14; crossover study | Zero reported | RSV did not affect GLP-1 secretion, glycaemic control, gastric emptying, body weight, or energy intake |
| 30 | Timmers 2016 (NCT01638780) [40] | Effect on insulin sensitivity compared with placebo | Men with well-controlled T2DM | 75 mg RSV or placebo capsule twice daily for 30 days | 17 participants; crossover design | Zero reported | RSV did not improve hepatic or peripheral insulin sensitivity |
| Cardiometabolic disease | |||||||
| 31 | Abdollahi 2019 (IRCT20171118037528N1) [41] | Glycaemic status, lipid profile, and body composition | Individuals with T2DM | 500 mg RSV capsule twice daily for 8 weeks and matched placebo | 38 on RSV; 38 on placebo | Zero reported | RSV significantly decreased fasting blood sugar and significantly increased high-density lipoprotein compared with placebo. No significant differences in anthropometric measures were observed |
| Tabatabaie 2020 [42] | Effects on serum levels of ADMA and PON1 activity | RSV significantly decreased serum ADMA and significantly improved PON1 enzyme activity compared with placebo | |||||
| Toupchian 2021; [43] describes the primary objectives for the trial in Abdollahi 2019 [41], which reports different outcomes | Effects on the gene expression of PPARα, p16, p53, p21 and serum levels of sCD163/sTWEAK ratio | RSV significantly increased p53 and p21 mRNA expression and significantly decreased serum sCD163/sTWEAK ratio compared with placebo | |||||
| 32 | AliSangouni 2022 (IRCT20171118037528N1) [44] | Effect on hepatic steatosis indices, levels of lipid accumulation product (LAP), and visceral adiposity index (VAI), as well as cardiovascular indices, Casterlli risk index I (CRI-I) and CRI-II, and atherogenic coefficient (AC) | Patients with T2DM | 2 × 500 mg capsules RSV or placebo per day for 8 weeks | 38 in each group | Zero reported | No significant change in any of the indices measured, LAP, VAI, CRI-I, CRI-II, and AC following RSV, compared to placebo and baseline values |
| 33 | deLigt 2020 (NCT02565979) [45] | Effect on insulin sensitivity | Overweight adults | 75 mg RSV or placebo capsule twice daily for 6 months | 21 on RSV; 21 on placebo | Zero reported | RSV had no effect on insulin sensitivity |
| 34 | Goncalinho 2021 (NCT01668836) [46] | Effects of RSV compared to energy restriction (ER) diet on vascular reactivity and sympathetic nervous system activity (measured by plasma noradrenaline) | Adults aged between 50 and 65 years with a normal clinical history, physical examination, and resting electrocardiogram | Participants were randomised to 250 mg RSV capsule twice daily or an energy restriction diet (1000 kcal/day) for 30 days | 24 in each group | Not stated | RSV does not improve cardiometabolic risk factors, sympathetic activity, or endothelial function. The only effects reported were an increase in apoB and total cholesterol in the RSV group compared to baseline, with no change in plasma noradrenaline |
| 35 | Pollack 2017 [47] | Characterise the effects on metabolism, vascular function, and mitochondrial biogenesis | Adults without T2DM | Crossover study design that also included placebo. Initially, 1.5 g RSV in capsule form was taken twice daily by the first nine participants, but the dose was reduced due to gastrointestinal side effects to 1 g twice daily, for 6 weeks | 30 participants; crossover study | Three AEs. One SAE of GI symptoms at 3 g/day; one patient requiring hospitalisation. Subsequent dose de-escalation to 2 g/day with no further side effects | RSV improved the fasting reactive hyperaemia index. RSV increased mitochondrial number, and RNA-Seq analysis showed that it significantly perturbs mitochondrial dysfunction and oxidative phosphorylation pathways |
| 36 | Poulsen 2013 (NCT01150955) [48] | Impact of high-dose RSV on energy and substrate metabolism, insulin sensitivity, ectopic fat disposition, 24 h ambulatory blood pressure, and inflammatory and metabolic biomarkers in obese human subjects | Obese healthy male volunteers | 500 mg RSV or placebo tablet three times each day for 4 weeks | 13 on RSV; 12 on placebo | Four in RSV and four in placebo groups. RSV AEs were flatulence, reflux, and rash. | RSV had no significant effect on insulin sensitivity, endogenous glucose production, turnover and oxidation rates of glucose, blood pressure, resting energy expenditure, oxidation rates of lipid, ectopic or visceral fat content, or inflammatory and metabolic parameters |
| Clasen 2014 [49] | Effects of RSV on growth hormone signalling in skeletal muscle and adipose tissue | RSV had no significant effect on growth hormone-induced STAT5b phosphorylation or the transcription level of CISH, SOCS2, or IGF-1 in muscle or fat | |||||
| 37 | Simental-Mendia 2019 [50] | Effects on lipid profile | Healthy adults with a new diagnosis of dyslipaemia | 100 mg RSV or placebo capsule once daily for 2 months | 35 on RSV; 36 on placebo | Zero reported | RSV significantly decreased total cholesterol and triacylglycerol |
| 38 | Williams 2014 [51] | RSV effects of AMPK/SIRT1 axis in human skeletal muscle and adipose tissue | Overweight participants with less than one hour per week of structured exercise at enrolment | Participants consumed a meal plus a capsule containing 300 mg RSV or placebo, with an interval of at least 7 days between | Eight participants; crossover design | Not stated | RSV had no effect on nuclear SIRT1 activity, AMPK phosphorylation, ACC, or PKA in either skeletal muscle or adipose tissue |
| 39 | Yoshino 2012 (NCT00823381) [52] | Effect on insulin sensitivity and global gene expression and molecular changes in adipose tissue and skeletal muscle | Caucasian post- menopausal non-obese women | 75 mg RSV or placebo capsule once daily for 12 weeks | 15 on RSV; 15 on placebo | Zero reported | RSV had no effect on liver, skeletal muscle, or adipose tissue insulin sensitivity. RSV did not affect AMPK, Sirt1, NAMPT, and PGC-1α in either skeletal muscle or adipose tissue |
| 40 | Zhou 2023 (NCT04886297) [53] | Changes in the lipid profile | Individuals with dyslipidaemia | RSV (100, 300, or 600 mg) or placebo in capsule form daily for 8 weeks | 41 on 100 mg RSV, 43 on 300 mg, 41 on 600 mg, and 43 on placebo | The placebo and RSV were generally well-tolerated, and no serious adverse reactions were reported. Most participants stopped taking the supplements for personal reasons, while the remaining few stopped due to mild adverse effects, with one person each in the placebo and 100 mg RSV groups reporting stomach ache and one person on 600 mg reporting headache | No significant change to the serum lipid profile with RSV intervention compared to placebo. A significant reduction in serum uric acid at 300 mg and 600 mg RSV compared to placebo. No significant differences between RSV and placebo groups for other metabolic markers, glucose, insulin, or oxidative stress biomarkers |
| 41 | Zortea 2016 (NCT 02062190) [54] | Efficacy of RSV on serum glucose and CVD risk factors | Patients with schizophrenia | 200 mg RSV or placebo once daily for 4 weeks. No details were given on the formulation used, but compliance was monitored by ‘pill counting’ | Ten on RSV; nine on placebo | Zero reported | RSV did not affect glucose or CVD risk factors |
| Zortea 2016 [55] | Effects on cognition | RSV did not significantly improve neuropsychology performance measures and psychopathology severity after 1 month | |||||
| Non-Alcoholic Fatty Liver Disease (NAFLD) | |||||||
| 42 | Asghari 2018 [56] | Whether RSV can improve oxidative/anti-oxidative status in patients with NAFLD | NAFLD patients | 600 mg RSV or placebo given in capsule form daily for 12 weeks | 30 on RSV; 30 on placebo | None stated | RSV did not cause significant changes to any of the outcomes |
| 43 | Asghari 2018 (RCT201511233664N16) [57] | Effect on anthropo-metric indices and metabolic factors and comparison with calorie restriction | Patients with NAFLD | 600 mg RSV or placebo daily given in capsule form; also compared with a calorie-restricted diet for 12 weeks | 30 on RSV; 30 on placebo; 30 on calorie restriction | Zero reported | RSV significantly reduced weight and BMI compared with placebo |
| 44 | Chachay 2014 (ACTRN12612001135808) [58] | Insulin resistance | Overweight or obese diagnosed with NAFLD | 1.5 g RSV or placebo in capsule form each morning and evening (total daily dose 3 g) for 8 weeks | 10 on RSV 10 on placebo | None stated | RSV did not improve any features of NAFLD but increased hepatic stress |
| 45 | Chen 2015 (ChiCTR-TRC-12002378) [59] | Effect of RSV on insulin resistance, glucose, and lipid metabolism | Patients with NAFLD | 300 mg RSV or placebo in capsule form twice daily for 3 months | 30 on RSV; 30 on placebo | Zero reported | RSV significantly decreased aspartate aminotransferase, glucose, low-density lipoprotein cholesterol, alanine aminotransferase, total cholesterol, and the homeostasis model assessment insulin resistance index |
| 46 | Faghihzadeh 2014 (NCT02030977) [60] | The primary outcome was ALT, but the focus was on inflammatory biomarkers | Patients with NAFLD | 500 mg RSV capsule once daily for 12 weeks. All patients were advised to follow an energy-balanced diet and physical activity recommendations during the trial | 25 on RSV; 25 on placebo | Zero reported | RSV significantly decreased alanine aminotransferase, inflammatory cytokines, nuclear factor κB activity, serum cytokeratin-18, and hepatic steatosis grade vs. placebo |
| Faghihzadeh 2015 [61] | The same primary outcome was reported in the 2014 paper above. Emphasis is on CV risk factors, and BMI, WC, cholesterol, and glucose were also reported | RSV significantly reduced alanine aminotransferase (ALT) and hepatic steatosis compared with placebo. There were no significant changes in blood pressure, insulin resistance markers, and TAG in either group | |||||
| 47 | Heeboll 2016 (NCT01464801) [62] | Assess whether RSV would lower plasma transaminases, liver fat content, and the histological NAFLD activity score (NAS) with superiority to placebo | Patients with transaminasemia and suspected NAFLD | 500 mg RSV or placebo tablet three times a day for 6 months | 15 on RSV; 13 on placebo | 33 AEs with RSV; 14 with placebo. RSV is generally well-tolerated, with the total number of patients reporting AEs similar between the RSV (9/15) and placebo group (6/13). AEs were constipation; diarrhoea; abdominal pain; nausea; heartburn; flatulence; less appetite; more appetite; less fatigue; more fatigue; miscellaneous; Bicytopen fever; (see SAE below) dizziness; and hot flushes. There was one SAE in the RSV arm: febrile leukopenia and thrombocytopenia after 10 days of RSV treatment, which recurred upon repeated exposure to RSV | RSV caused a significant decrease in liver lipid content, but there was no difference from placebo |
| 48 | Kantartzis 2018 (NCT01635114) [63] | Change in liver fat content | Overweight and insulin-resistant subjects | 150 mg RSV or placebo in capsule form once daily for 12 weeks | 54 on RSV; 54 on placebo | 73 (RSV); 43 (placebo). There were no safety issues with RSV. The proportion of patients reporting AEs was similar between the groups. The numbers of subjects having at least one AE were 19/54 for placebo and 24/54 for RSV. AEs for RSV were infections and infestations; immune system disorders; metabolism and nutrition disorders; nervous system disorders; vascular disorders; gastrointestinal disorders; respiratory, thoracic and mediastinal disorders; skin and subcutaneous tissue disorders; musculoskeletal and connective tissue disorders; renal and urinary disorders; reproductive system and breast disorders; and injury, poisoning, and procedural complications | Placebo reduced liver fat content, but RSV did not |
| 49 | Poulsen 2018 (NCT01446276) [64] | Long-term effects of high-dose RSV on basal and insulin-mediated very-low-density lipoprotein triglyceride, palmitate and glucose kinetics, and liver fat content in men with NAFLD | Non-diabetic, upper-body obese men with confirmed non-alcoholic fatty liver disease | 500 mg RSV or placebo three times each day for 6 months. No details on the formulation used, but tablets are mentioned | Eight on RSV; eight on placebo | Not stated | RSV had no significant effect on basal or insulin-mediated VLDL-TG secretion or its oxidation or clearance rates, palmitate turnover, glucose turnover, body composition, and liver fat content compared with placebo treatment |
| Metabolic syndrome | |||||||
| 50 | Batista-Jorge 2020 [65] | Effects on biochemical and physical parameters in combination with changes to diet and exercise regimen | Obese patients with metabolic syndrome | 250 mg RSV or placebo capsule daily in combination with a physical activity programme + diet for 3 months | 13 on RSV; 12 on placebo | None stated | RSV improved total cholesterol, high-density lipoprotein cholesterol, very-low-density lipoprotein cholesterol, urea, creatinine, and albumin vs. placebo. Anthropometric parameters were significantly different after 3 months of physical activity for both placebo and RSV |
| 51 | Mendez-del Villar 2014 [66] | Effects on metabolic syndrome, insulin secretion, and insulin sensitivity | Patients with metabolic syndrome | 500 mg RSV or placebo capsules three times each day for 90 days | 12 on RSV; 12 on placebo | Zero reported | RSV significantly decreases weight, BMI, fat mass, WC, AUC of insulin, and total insulin secretion |
| Obesity | |||||||
| 52 | Arzola-Paniagua 2016 (National Clinical Trial Registry of Mexico 33300410A0152) [67] | Body weight loss alone and in combination with orlistat | Obese adults | 100 mg RSV capsule three times per day with meals, or orlistat alone, the combination of RSV and orlistat, or placebo. All participants were also on an energy-reduced diet | 40 on RSV alone; 40 on orlistat alone; 41 on the combination; 40 on placebo | Related and non-related AEs were observed in the intervention groups, with no differences among the groups. No SAEs were reported. The number of patients withdrawing due to AEs was three in the orlistat + RSV group, six in the RSV group, two in the orlistat group, and four in the placebo group. AEs consisted of abdominal pain; constipation; diarrhoea; nausea; and steatorrhoea | RSV + orlistat caused significant weight loss compared to placebo. RSV + orlistat significantly decreased BMI, waist circumference, fat mass, triglycerides, leptin, and the leptin/adiponectin ratio |
| 53 | Mahmood 2018 [68] | Obesity-related nephropathy | Obese female patients | 1 g RSV in capsule form twice daily for 8 weeks | 24 on RSV plus standard treatment; 22 on standard treatment of orlistat, metformin, and fluoxetine | Not stated | RSV significantly reduced serum and urinary creatinine, microalbuminuria, collagen IV, and alpha glutathione-S-transferase (alpha GST), with the estimated glomerular filtration rate (eGFR) significantly elevated. RSV significantly reduced glutamic oxaloacetic transaminase (SGOT), glutamic pyruvic transaminase (SGPT) total cholesterol (TC), triglycerides (TGs), very-low-density lipoprotein (VLDL-c), and resistin and interleukin-6 (IL-6). RSV significantly increased high-density lipoprotein (HDL-c), serum adiponectin, and superoxide dismutase 1 (SOD1) |
| 54 | Timmers 2011 (NCT 00998504) [69] | Whole-body energy expenditure, substrate utilization, ectopic lipid storage, and mitochondrial function, and lipolysis in adipose tissue and skeletal muscle | Obese but otherwise healthy male volunteers | RSV 150 mg or placebo capsule once daily for 30 days. Crossover design with a 4-week washout | 11 participants; crossover design | Zero reported | RSV activated AMPK, increased SIRT1 and PGC-1α protein levels, increased citrate synthase activity, and improved muscle mitochondrial respiration ex vivo. RSV increased intramyocellular lipid levels and decreased intrahepatic lipid content, circulating glucose, triglycerides, alanine-aminotransferase, and inflammation markers |
| Konings 2014 (Reanalysis of samples from Timmers 2011) [70] | Adipose tissue morphology and transcriptional profile | RSV significantly decreased adipocyte size and downregulated Wnt and Notch. RSV upregulated cell cycle regulatory genes lysosomal/phagosomal pathway and transcription factor EB | |||||
| Knop 2013 (Reanalysis of samples from Timmers 2011) [71] | Postprandial incretin hormone and glucagon responses | RSV significantly suppressed postprandial glucagon responses without affecting fasting glucagon levels | |||||
| vanPolanen 2021 (Reanalysis of samples from 3 trials: Timmers 2011, 2016, de Ligt 2018, NCT00998504, NCT01638780 and NCT02129595) [72] | Intramyocellular lipid (IMCL) storage | RSV significantly increased IMCL in predominantly type I muscle fibres in the subsarcolemmal and intermyofibrillar region and in PLIN5-coated lipid droplets | |||||
| deLigt 2021 (Reanalysis) [73] | Impact of RSV on the expression of renin-angiotensin-system components in adipose tissue and skeletal muscle compared to placebo in people with obesity | RSV significantly decreased the gene expression of angiotensin-converting enzyme 2 (~40%) and leptin (~30%) in abdominal subcutaneous adipose tissue compared to placebo but did not alter angiotensinogen, ACE, or angiotensin II type 1 receptor (AT1R) expression in AT or skeletal muscles. It was suggested that the effect on ACE2 might dampen SARS-CoV-2 spread in COVID-19 | |||||
| Response to exercise training | |||||||
| 55 | Alway 2017 [74] | Mitochondrial density; muscle fatigue resistance; cardiovascular function | Healthy older adults | 500 mg RSV or placebo capsule once daily (+exercise) for 12 weeks | 19 on RSV; 16 on placebo | None stated | RSV increased mitochondrial density and muscle fatigue resistance compared with placebo. RSV combined with exercise might provide a better approach for reversing sarcopenia than exercise alone |
| 56 | Gliemann 2013 [75] | Effect on training-induced improvements in cardiovascular health parameters | Healthy, aged, physically inactive men | RSV 250 mg or placebo tablets each morning for 8 weeks. Both groups undertook a programme of high-intensity exercise training | 14 on RSV; 13 on placebo | Not stated, but see Olesen below | Placebo + exercise was more effective in increasing maximal oxygen uptake than RSV + exercise |
| Gliemann 2014 [76] | To assess whether RSV would potentiate an angiogenic response to exercise training | RSV or placebo 250 mg each morning for 8 weeks. | Same study as 2013 but also incorporates another study where individuals followed a normal sedentary lifestyle—nine on RSV, seven on placebo | RSV supplementation did not increase the capillary-to-fibre ratio or muscle VEGF protein. Muscle TIMP-1 protein levels were lower in the RSV group than the placebo group | |||
| Olesen 2014 [77] | Metabolic adaptations and anti-inflammatory effects in skeletal muscle of healthy aged subjects similar to exercise training | No participants reported any significant side effects throughout the intervention (covers both studies) | RSV did not elicit metabolic improvements and impaired exercise training-induced improvements in markers of oxidative stress and inflammation in skeletal muscle | ||||
| 57 | Harper 2021 (NCT02523274) [78] | Safety, feasibility, and potential efficacy of RSV combined with exercise training on indices of physical function and skeletal muscle mitochondrial function | One capsule twice daily for 12 weeks plus exercise. Equivalent to 500 mg RSV once or twice daily, or placebo (participants took a combination of RSV and placebo capsules, as required) | Twenty per group (three groups) | AE frequency and type were similar between groups. There were nineteen AEs related or possibly related to RSV across the two dose groups. AEs were gastrointestinal issues, musculoskeletal, and dizziness | RSV + exercise was safe and feasible for older adults with functional limitations and may improve skeletal muscle mitochondrial function and mobility-related indices of physical function | |
| 58 | Laupheimer 2014 [79] | Effects on immune response and delayed onset muscle soreness | Male athletes | 200 mg RSV or placebo in tablet form three times each day for 7 days prior to running a marathon | Four on RSV; four on placebo | Zero reported | RSV did not cause any significant changes compared with placebo |
| 59 | Lokken 2021 (NCT03728777) [80] | Exercise capacity, judged by the change in heart rate (HR) during constant load cycling and changes in maximum oxidative capacity | 500 mg RSV or placebo capsule twice daily for 8 weeks; crossover design | 10 participants; crossover trial | Both RSV and placebo treatments were well-tolerated, with all AEs remitting spontaneously. No SAEs were reported. AEs observed for RSV and placebo were mild gastrointestinal symptoms, including diarrhoea | RSV did not cause any significant differences in HR | |
| 60 | Macedo 2014 [81] | Plasma metabolic response and certain indicators of oxidative stress—comparing response to a fitness test pre/post-intervention (anti-oxidant system and oxidative stress biomarkers) | Military firefighters | 100 mg RSV or placebo capsule once daily for 3 months | 30 on RSV; 30 on placebo | Not stated | RSV decreased IL-6 and TNF-a post-fitness test. RSV decreased glutathione peroxidase activity in both pre- and post-fitness tests. |
| 61 | Nicolau 2022 [82] | Alterations in anthropometric parameters, heart measures, blood analytes, leukogram, and plaquetogram indices | Female participants aged between 60 and 80 | 300 mg RSV or placebo capsule once daily for 60 days | Participants were randomised into four groups based on their level of activity at baseline: no exercise with placebo (n = 15), no exercise with RSV (n = 16), exercise (n = 17), and exercise with RSV (n = 14) | Not stated | RSV was not associated with any significant changes in many of the parameters assessed. Blood pressure was increased after 60 days in sedentary women compared to those who exercised, although values remained within the normal range. Mean corpuscular volume and red blood cell distribution width were significantly improved in the groups receiving RSV and exercise compared to the sedentary group on RSV |
| 62 | Scribbans 2014 [83] | (i) Effects of RSV on exercise-mediated increases in aerobic capacity in young healthy men; (ii) to examine if RSV impacts exercise-induced adaption in anaerobic capacity and submaximal substrate utilization; and (iii) to determine if RSV impacts skeletal muscle gene expression or adaptations in oxidative/glycolytic capacity in a fibre-specific manner | Healthy, recreationally active men | 150 mg RSV or placebo capsule once daily for 4 weeks | Eight on RSV; eight on placebo | Not stated | RSV had no significant effects on any outcomes compared to placebo |
| 63 | Storgaard 2022 [84] | Heart rate and fatty acid oxidation, measured using a stable isotope technique during constant workload exercise | Patients with fatty acid oxidation disorders—genetically verified deficiency in very long-chain acyl-CoA dehydrogenase (VLCAD) or carnitine palmitoyl transferase (CPT) II | 500 mg RSV or placebo capsule twice daily for 8 weeks each in a crossover study, with a 4-week washout in between | Eight participants in a crossover design | 13 reported in total as follows: RSV treatment: headache (2); stomach ache/diarrhoea (4); pins and needles (1); eczema (1); respiratory infection (1) Placebo: headache (1); loss of appetite/diarrhoea (1); fatigue (1); respiratory infection (1) | Neither heart rate nor fatty acid oxidation differed at the end of exercise after treatment with RSV versus placebo |
| General anti-inflammatory/anti-oxidant effects | |||||||
| 64 | BaGen 2018 [85] | Effects on inflammatory and anti-inflammation factors after oral implantology | Patients with oral implants | 2 mg/kg/d of RSV or placebo for 4 weeks. No information was given on the formulation | 82 on RSV; 80 on placebo | None stated | RSV decreased mRNA and serum levels of IL-1β, IL-17A, tumour necrosis factor-alpha (TNF-α), intercellular adhesion molecule-1 (ICAM-1), and immunoglobulin A1 (IgA1). RSV increased mRNA and serum levels of IL-2, IL-6, and IL-10 |
| 65 | Bo 2013 [86] | Levels of inflammatory and oxidative mediators | Healthy smoking volunteers | 500 mg RSV tablet or placebo each morning after fasting overnight for 30 days. A crossover study with a 30-day washout between periods of RSV/placebo | 50; with 25 in each group randomised to RSV or placebo first | Zero reported | RSV significantly reduced C-reactive protein (CRP) and triglycerides and increased total anti-oxidant status. |
| 66 | DiPierro 2020 [87] | Energy metabolism and oxidative status in RBC | Healthy volunteers | 325 mg RSV once daily for 30 days. RSV was taken orally but the pharmaceutical form is not stated | Three | Zero reported | RSV did not significantly change to ATP/ADP ratio or RBC NAD levels |
| 67 | Vicari 2020 [88] | Changes in the NIH-CPSI (prostatic symptom index) total score and IPSS (prostate symptom score) total score | 19.8 mg RSV or placebo tablet twice daily for 2 months | 32 on RSV; 32 on placebo | Not stated | RSV showed significant symptomatic improvement of all NIH-CPSI and IPSS subscale scores | |
| 68 | Zhang 2017 [89] | Whether RSV can mitigate the effects of occupational ELF-EMF exposure on several inflammatory biomarkers and biomarkers of oxidative stress | Workers exposed to electromagnetic fields and a control population | 500 mg RSV or placebo capsule twice daily for 12 months | Within the two groups, participants were randomised into RSV or placebo. In the exposed population, 314 were allocated RSV and 289 placebo. For the control population, the numbers were 263 and 214, respectively | Not stated | RSV significantly reversed the adverse impacts of ELF-EMF and significantly decreased urinary 8-OHdG and F2-isoprostane levels compared with the reference group |
| Arthritis related disease | |||||||
| 69 | Hussain 2018 [90] | Assessment of lipid-haematological profile, liver and kidney functions, and a comprehensive short-term follow-up of the clinical and physical examinations, vital signs, body weight alteration, and occurrence of drug-related adverse events. Efficacy was a secondary objective | Patients with knee osteoarthritis | 500 mg RSV or placebo in capsule form once daily for 3 months. All participants also had meloxicam as the standard of care | 55 on RSV; 55 on placebo | Zero reported | RSV + meloxicam significantly reduced total cholesterol and triglyceride levels compared with placebo. RSV + meloxicam significantly decreased serum levels of GOT, GPT, and ALP, but this was also observed for placebo + meloxicam |
| 70 | Khojah 2018 [91] | Biochemical and clinical markers of RA | Patients with rheumatoid arthritis | 1 g RSV in capsule form once daily for 3 months | 50 on resveratrol plus standard treatment; 50 on standard treatment alone | Zero reported | The 28-joint count for swelling and tenderness, and the disease activity score assessment for 28 joints were significantly lowered by resveratrol. Serum levels of C-reactive protein, the erythrocyte sedimentation rate, undercarboxylated osteocalcin, matrix metalloproteinase-3, tumour necrosis factor alpha, and interleukin-6 were significantly decreased by resveratrol |
| 71 | Marouf 2018 [92] | Clinical scores of knee osteoarthritis | Patients with knee osteoarthritis | 500 mg RSV or placebo in capsule form once daily for 90 days | 60 on RSV; 40 on placebo. Both groups had meloxicam as the standard treatment | Not stated | RSV + meloxicam improves pain and symptom scores in patients with mild-to-moderate knee osteoarthritis compared with placebo |
| Marouf 2018, same study as above but with a few extra patients [93] | Pain severity and biochemical markers of inflammation | 60 on RSV as above; 50 on placebo (an extra 10) | RSV caused a significant time-dependent decrease in pain severity | ||||
| 72 | Marouf 2021 [94] | Correlation between pro-inflammatory markers and clinical outcomes when RSV is used as an add-on therapy to meloxicam | Patients with painful knee osteoarthritis | 500 mg RSV or placebo daily taken in capsule form, in addition to the standard therapy of 15 mg meloxicam for 90 days | 50 patients in the RSV group; 32 in the placebo group | Not stated | RSV significantly improves both the Knee Injury and Osteoarthritis Outcome Score (KOOS) and the Western Ontario and McMaster Universities Osteoarthritis Index (WOMAC) at the end of treatment compared with baseline values and those reported in the control group post-intervention. The addition of RSV to meloxicam also significantly reduces TNF-α, IL-1β, and IL-6 serum levels compared with day 0 and the placebo group. However, there was only a weak non-significant correlation between serum biomarkers and clinical outcomes |
| Cognitive and cerebrovascular function | |||||||
| 73 | Evans 2017 (ANZCTR12615000291583) [95] | Cerebrovascular responsive-ness and cognitive performance | Post-menopausal women | RSV or placebo 75 mg capsule twice daily for 14 weeks | 39 on RSV; 41 on placebo | Not stated (see below) | RSV increased CVR in response to hypercapnic and cognitive stimuli. Cognitive tasks and overall cognitive performance significantly improved |
| Wong 2017 [96] | Whether RSV could improve aspects of overall well-being such as pain perception, menopausal symptoms, sleep quality, perceived QoL, and depressive symptoms | Zero reported | RSV caused a significant reduction in pain and improved total well-being vs. placebo. Improvements correlated with improved cerebrovascular function | ||||
| 74 | Kennedy 2010 [97] | Modulation of mental function and increased cerebral blood flow | Healthy men | Reports two studies: PK and assessing cognitive effects. In the PK study, participants took a single dose of 250 mg or 500 mg RSV (with placebo for blinding) with ~7 days in between. In the second study, participants took single doses of 250 mg, 500 mg RSV, or placebo on three separate days in a crossover design. The order was randomly allocated, and the study was blind. RSV was taken in capsule form | Nine in the PK study and twenty-four in the second study | Not stated | RSV caused dose–dependent increases in cerebral blood flow. There was no change in cognitive function |
| 75 | ThaungZaw 2021 (ACTRN12616000679482p) [98] | Cognitive performance | Post-menopausal women | 75 mg RSV or placebo capsule twice daily for 12 months; crossover design | Numbers described in this final report of the trial are 63 who started on RSV and 62 on placebo, which gives a total of 125 participants that received RSV | 19 AEs. Eight AEs for placebo and four for RSV in the first supplementation phase (previously published). In the second phase of the study, two AEs for RSV and five AEs for placebo were reported. RSV AEs were itching, menses, prolapsed bladder, a pre-scheduled left eye operation (first phase), the exacerbation of gastric reflux, and pre-scheduled surgery for a posterior heart valve stent insertion (second phase). AEs were not necessarily related to RSV | RSV significantly improved overall cognitive performance compared to placebo |
| ThaungZaw 2020; Reports secondary endpoints from completed RESHAW trial [99] | Effects on aspects of overall well-being, such as pain perception, menopausal symptoms, mood and depressive symptoms, sleep quality, and perceived quality of life | RSV significantly reduced composite pain score and was associated with significant improvements in cerebrovascular responsiveness to hypercapnia, somatic menopausal symptoms, and general well-being | |||||
| ThaungZaw 2020; 12-month interim analysis of RESHAW trial [100] | Cerebrovascular function, cognitive performance, and cardiometabolic parameters | 73 initially randomised to RSV; 73 randomised to placebo. Crossover study—this is the interim analysis at 12 months of a 2-year study | 12 AEs in total. Four AEs in RSV were itching, menses, prolapsed bladder, and pre-scheduled left eye operation. Four AEs in the placebo group were itching, exacerbation of gastric reflux, constipation, and pre-scheduled breast reduction procedure. Four SAEs in the placebo group were urinary tract infection, bowel blockage, oesophageal tear repair, and pre-scheduled operation on the lumbar spine | RSV significantly improved overall cognitive performance and attenuated decline in CVR to cognitive stimuli, which was associated with a significant reduction in fasting blood glucose | |||
| Wong 2020; Reanalysis of ThuangZaw 2020 at the 12-month timepoint [101] | Treatment-induced changes to bone parameters | RSV improved bone density in the lumbar spine and neck of the femur and decreased C-terminal telopeptide type-1 collagen levels (bone resorption marker) compared with placebo. The increase in bone mineral density resulted in an improvement in T-score and a reduction in the 10-year probability of hip fracture risk | |||||
| 76 | Wightman 2014 (NCT01331382) [102] | To ascertain whether piperine is capable of enhancing the bioefficacy of RSV with regard to cerebral blood flow and cognitive performance in healthy adults | Healthy adults | Each participant received a single dose of placebo, RSV (250 mg) alone, and RSV plus 20 mg piperine in capsule form on separate days at least a week apart | 23 participants; crossover design | Not stated | RSV+ piperine significantly enhanced cerebral blood flow |
| 77 | Wong 2016 (ACTRN126140008916) [103] | To determine the most efficacious dose of RSV to improve cerebral vasodilator responsiveness (CVR) in T2DM | Dementia-free older adults with non-insulin-dependent T2DM | Each participant took three different doses of RSV (75, 150, and 300 mg) and a placebo, in capsule form, on a single occasion at weekly intervals | 38 participants; crossover design | Zero reported | RSV significantly increased CVR in the middle cerebral artery at all doses. A RSV 75 mg dose was efficacious in the peripheral cerebral arteries |
| Wong 2016 [104] | Effects of RSV on neurovascular coupling capacity (CVR to cognitive stimuli), cognitive performance, and correlations with plasma concentrations | RSV (75 mg) significantly improved neurovascular coupling capacity, which correlated with total plasma RSV levels. RSV (75 and 300 mg) enhanced performance on the multi-tasking test battery | |||||
| Mental health and neurodevelopmental conditions | |||||||
| 78 | Hendouei 2019 (IRCT20090117001556N104) [105] | Mean change in the score for irritability subscale from baseline/screening to week 10 | Children with autism spectrum disorder | RSV 250 mg or placebo tablet twice daily for 10 weeks. Both groups also had risperidone twice daily | 35 on RSV; 35 on placebo | There was no significant difference in the frequency of AEs between the two groups. There were 48 AEs with RSV + resperidone, with the most frequent side effects being restlessness, constipation, and diarrhoea. The most frequent side effects in the placebo group were restlessness, increased appetite, and constipation | RSV did not cause any difference in primary or secondary outcomes compared with placebo |
| 79 | Rafeiy-Torghabeh 2020 (IRCT20090117001556N115) [106] | Main ADHD symptoms, evaluated using the Teacher and Parent versions of ADHD-RS-IV | Children with attention-deficit/hyperactivity disorder | 250 mg RSV or placebo tablet twice daily for 8 weeks | 33 on RSV; 30 on placebo. Both groups received methylphenidate as a standard treatment | 38 (RSV + methyl-phenidate); 47 (placebo + methyl-phenidate). Headache; insomnia; drowsiness; fatigue; dry mouth; decreased appetite; nausea; vomiting; diarrhoea; abdominal pain. The most common complications in both groups were decreased appetite (RSV: 20%; placebo: 27%) and headache (RSV: 17%; placebo: 23%). There was no statistical significance between groups | RSV + methylphenidate resulted in a significant improvement in the time–treatment interaction on all three subscales of the Parent ADHD-RS |
| 80 | Samaei 2020 (IRCT20090117001556N103) [107] | Differences in positive and negative syndrome scale scores from baseline to week 8 between the treatment groups | Patients with schizophrenia | 200 mg RSV or placebo capsule for 8 weeks | 26 on RSV; 26 on placebo | 43 AEs. All AEs were tolerable with mild to moderate severity. The frequency of AEs did not significantly differ between RSV and placebo groups. AEs were headache (4), constipation (7), diarrhoea (5), fatigue (3), nausea (3), increased appetite (9), abdominal pain (6), and nervousness (6) | RSV improves negative, general psychopathology, and total scores to a greater extent than placebo |
| Dementia and Alzheimer’s disease | |||||||
| 81 | Gu 2021 (SRRSH2017-0075A) [108] | The antagonistic effects of RSV on moderate to mild Alzheimer’s disease | Patients with Alzheimer’s disease | 500 mg RSV or placebo once daily for 52 weeks. RSV was taken orally but the type of formulation is not stated | 15 on RSV; 15 on placebo | 88 AEs reported in total; however, for each type, they were equally distributed between the RSV and placebo groups. For example, six infections and infestations were reported in each of the RSV and placebo groups and six nervous system disorders were also described in each group | In the RSV group, there was no significant change in plasma or CSF levels of Aβ40 over the 52 weeks compared to baseline, whilst significant decreases were observed in patients on placebo. There was a significant reduction in brain volume and levels of matrix metallopeptidase 9 in the CSF of patients on RSV compared to placebo. These findings indicate potential neuroprotective effects of RSV |
| 82 | Turner 2015 (NCT01504854) [109] | (1) Safety and tolerability; (2) effect on plasma and CSF Ab42 and Ab40, CSF tau and phospho-tau 181, and volumetric MRI; and (3) examine pharmacokinetics | Patients with mild to moderate dementia due to Alzheimer’s disease | 500 mg RSV or placebo capsule once daily with a dose escalation by 500 mg increments every 13 weeks, ending with 1000 mg twice daily for 52 weeks | 64 on RSV; 55 on placebo | A total of 657 AEs (490 mild, 139 moderate, 28 severe) were reported (355 on drug, 302 on placebo). A total of 113 out of 119 (95%) participants reported at least one AE. Thirty-six SAEs were reported (nineteen on drug, seventeen on placebo), including three deaths (one on drug, two on placebo), which were not related to the study drug. There were no differences between groups for SAEs. The most common AEs were nausea and diarrhoea (in 42% of individuals on RSV vs. 33% on placebo) | RSV significantly decreased CSF Ab40 and plasma Ab40 levels at week 52. RSV increased brain volume loss |
| Moussa 2017 (Reanalysis of samples) [110] | Effects on pro- and anti-inflammatory cytokines, chemokines, and metalloproteinases in CSF and plasma | The study analysed a subset of patients (19 per group) from the trial | RSV reduced CSF MMP9 and increased macrophage-derived chemokine (MDC), interleukin (IL)-4, and fibroblast growth factor (FGF)-2 compared to placebo at 52 weeks | ||||
| Pharmacokinetics, ADME, and safety | |||||||
| 83 | Almeida 2009 [111] | Safety and PK | Healthy volunteers | 25, 50, 100, and 150 mg RSV or placebo given in capsule form 6 times a day for 48 h | Eight on each dose (total on RSV was thirty-two, with eight on placebo) | Mild AEs—fifteen for RSV (nine possibly related) and three for placebo; AEs were headache, myalgia, somnolence, epididymitis, dizziness, and occipital headache | Cmax was 3.89, 7.39, 23.1, and 63.8 ng/mL for each dose, respectively. T1/2 was 1–3 h following single-dose, and 2–5 h for repeated dosing. Repeated administration was well-tolerated. Bioavailability was higher after morning administration |
| 84 | Anton 2014 (NCT01126229) [112] | Safety and metabolic effects | Healthy, overweight, older adults | 150 mg, 500 mg RSV or placebo given in capsule form twice daily for 90 days | 14 on 300 mg RSV, 13 on 1 g RSV daily, and 12 on placebo | 17 AEs at 300 mg/day; 12 at 1000 mg/day; 10 in the placebo group. AEs were diarrhoea (5); constipation (3); muscle cramps/pain (4); fatigue (4); memory loss (4); allergies/URI (3); difficulty swallowing (2); rash (2); headache (3); others (7) | RSV significantly decreased blood glucose levels post-treatment compared with placebo |
| Anton 2018 [113] | Cognitive outcomes | RSV (1000 mg/day) improved psychomotor speed compared to participants taking 300 mg/day or placebo | |||||
| 85 | Howells 2011 (NCT00920803) [114] | Safety and pharmacokinetics | Patients with colorectal liver metastases | 5 g SRT501 microparticle RSV powder or placebo sachet mixed with liquid to make a suspension, which is drunk once daily for ~14 days | Six on RSV; three on placebo | Seventeen on RSV; three on placebo. Primarily GI and mild in grade. AEs were anal pruritus; diarrhoea; nausea; chills; lethargy; peripheral neuropathy; rash; skin irritation; flushing | SRT501 was well-tolerated. Mean plasma RSV levels following a single dose of SRT501 were 1942 ± 1422 ng/mL, exceeding those published for equivalent doses of nonmicronised RSV by 3.6-fold |
| Endometriosis | |||||||
| 86 | Kodarahmian 2019 [115] | Endometrial and serum levels of MMP2 and MMP9 | Women with endometriosis | 400 mg RSV or placebo twice daily for 12–14 weeks. The type of formulation was not stated | 17 on RSV; 17 on a placebo | Not stated, but see below | RSV decreased the mRNA and protein of both MMP-2 and -9 |
| Khodarahmian 2021 [116] | VEGF and TNF-alpha 2 expression in the eutopic endometrium | Zero reported | RSV caused a significant decrease in VEGF and TNF-α gene and protein levels compared with placebo | ||||
| 87 | Mendes da Silva 2017 (NCT02475564) [117] | Pain score | Endometriosis patients | 40 mg RSV or placebo capsule once daily for 42 days | 22 on RSV; 22 on placebo | Not stated | RSV is not superior to placebo for the treatment of pain in endometriosis |
| Polycystic ovarian syndrome | |||||||
| 88 | Bahramrezaie 2019 (IRCT2016030126860N) [118] | Effect of RSV on the expression of the VEGF and HIF1 genes in granulosa cells in the angiogenesis pathway of PCOS patients | Infertile patients with PCOS | 800 mg RSV or placebo daily given in capsule form for 40 days | 31 on RSV; 31 on a placebo | Zero reported | RSV significantly decreased the expression of VEGF and HIF1 genes in granulosa cells vs. the placebo |
| 89 | Banaszewska 2016 (NCT01720459) [119] | Effects on the endocrine and metabolic function of women with PCOS | Individuals with PCOS | 1500 mg RSV daily for 3 months. The formulation is described as micronised RSV or placebo in pill form | 17 on RSV; 17 on a placebo | Two AEs for RSV. AEs were transient numbness of hands in two patients | RSV significantly decreased total testosterone, dehydroepiandrosterone sulfate, and fasting insulin. RSV significantly increased the insulin sensitivity index |
| 90 | Brenjian 2020 (IRCT2016041827453 N1) [120] | Effects of RSV treatment on pro-inflammatory and ER stress markers | Patients with PCOS | 800 mg RSV or placebo in capsule form once daily for 40 days | 22 on RSV; 22 on placebo | None stated | RSV decreased serum levels of IL-6, IL-1β, TNF-α, IL-18, NF-κB, and CRP. RSV significantly increased the gene expression of ATF4 and ATF6 and significantly decreased levels of CHOP, GRP78, and XBP1 |
| 91 | Mansour 2021 (IRCT2017061917139N2) [121] | Reduction in testosterone from baseline to 3 months. As additional indicators of androgen excess in PCOS, changes in FAI, SHBG, DHEA, LH, and FS from baseline to 3 months | Women in the age range 18–40 years diagnosed with PCOS | 1 g RSV or placebo in capsule form once daily for 3 months | 39 on RSV; 39 on placebo | Zero reported | RSV did not cause any significant differences compared to placebo in terms of testosterone, ovarian, and adrenal androgens, sex hormone binding globulin (SHBG) levels, the free androgen index (FAI), glycoinsulinemic metabolism, and lipid profile |
| Renal disease | |||||||
| 92 | Lin 2016 [122] | Whether RSV has beneficial effects on ultrafiltration (UF) and angiogenesis | Patients undergoing peritoneal dialysis | 150 mg or 450 mg RSV, or placebo in capsule form, once daily for 12 weeks | 24 on each dose of RSV; 24 on placebo | Four patients discontinued RSV. AEs were diarrhoea; constipation; muscle cramps/pain; fatigue; headache; memory loss | RSV (both dose groups) caused a significant improvement in mean net UF volume and UF rate compared to placebo. High-dose RSV significantly reduced appearance rates of VEGF, Flk-1, and Ang-2 compared to placebo |
| 93 | Saldanha 2016 (NCT02433925) [123] | Nrf2 and NF-kB expression | Non-dialysed patients with chronic kidney disease | 500 mg RSV or placebo capsule once daily for 4 weeks. Crossover design with an 8-week washout | Twenty in a crossover trial (eleven on placebo first, nine on RSV first) | Zero reported | RSV does not significantly change NRF2 or NFkB |
| Alvarenga 2022 [124] | Plasma levels of uremic toxins, indoxyl sulfate (IS), p-cresyl sulfate (pCS), and indole-3-acetic acid (IAA) | RSV did not reduce the plasma levels of IS, pCS, and IAA in non-dialyzed patients with chronic kidney disease | |||||
| Gynaecological and menstrual disorders | |||||||
| 94 | Dzator 2022 (ACTRN12620000180910) [125] | The hormonal migraine burden index—HMBI (number of days with menstrual migraine per month) | Healthy volunteers aged between 18 to 50 with a regular hormonal cycle length between 21 and 35 days and suffer from migraine | 75 mg RSV capsule twice daily or placebo, each for a period of 3 months | 31 women, crossover study | Not stated | No significant difference in the HMBI or other outcomes assessing migraine-related disability and quality of life when comparing RSV and placebo treatments |
| 95 | Ma 2018 [126] | Remodelling of the scarred uterus and improved pregnancy outcomes | Women with a scarred uterus | 10 mg RSV or placebo once daily for 3 months. No information was given on the formulation of RSV used | 46 on RSV; 32 on placebo | Not stated | RSV reduced uterus scarring in 87.36% of patients and downregulated the plasma levels of β-human chorionic gonadotropin. RSV promoted remodelling of the scarred uterus, regeneration of the endometrium, and improved pregnancy outcomes |
| Bone maintenance | |||||||
| 96 | Ornstrup 2014 (NCT01412645) [127] | Change in bone alkaline phosphatase (BAP) | Men with metabolic syndrome | 75 mg or 500 mg RSV, or placebo in tablet form twice daily for 16 weeks | 23 on 75 mg RSV; 25 on 500 mg RSV; 26 on placebo | RSV high group—seven; RSV low group—three; placebo group—four. Gastrointestinal complaints were mild and primarily related to increased frequency and/or softer stool | High-dose RSV significantly increased BAP vs. placebo at all time points |
| Kjaer 2015 [128] | Circulating androgens and prostate size | Reports lower numbers of patients than original study: 21 per group for both 75 mg and 500 mg RSV and 24 on placebo | High-dose RSV decreased serum androstenedione and dehydroepiandrosterone-sulphate (DHEAS) and significantly decreased dehydroepiandrosterone (DHEA) | ||||
| Kjaer 2017 [129] | Inflammatory markers in plasma and adipose tissue, glycaemic status, circulating lipid parameters, blood pressure, and body composition | Same participant numbers as Kjaer 2015 | High-dose RSV significantly increased total cholesterol, low-density lipoprotein (LDL) cholesterol, and fructosamine | ||||
| Korsholm 2017 [130] | Metabolomic analysis on blood, urine, adipose tissue, and skeletal muscle tissue in middle-aged men with metabolic syndrome | Just involves the 500 mg RSV and placebo groups | RSV reduced sulfated androgen precursors in blood, adipose tissue, and muscle tissue, and increased these metabolites in urine. Markers of muscle turnover were increased with increased intracellular glycerol and the accumulation of long-chain saturated, monounsaturated, and polyunsaturated (n3 and n6) free fatty acids. RSV affected urinary derivatives of aromatic amino acids, reflecting the composition of the gut microbiota | ||||
| Periodontitis | |||||||
| 97 | Golshah 2021 (IRCT20130812014333N91) [131] | The efficiency of Emulgel containing resveratrol in improving clinical gingival inflammatory status; criteria included bleeding on probing (BOP), the gingival index (GI), the hyperplastic index (HI), and probing pocket depth (PPD) | Individuals aged between 12 and 25 years old who received fixed orthodontic treatment, before which they had no clinical signs of gingivitis or periodontitis | Participants were randomised to topical Emugel containing 2% RSV, a control group, and a placebo group receiving a similar Emugel product without RSV. The gel (5 mL) was massaged on the gums for 30 s every night after brushing teeth for 8 weeks | 26 on RSV; 24 in the control group; 23 in the Emugel placebo group | Zero reported | Emulgel-containing resveratrol is effective in improving gingival health in orthodontic patients for 8 weeks and can reduce gingival inflammation. The HI and GI scores were significantly decreased at 4 and 8 weeks after the start of the study in the RSV group |
| 98 | Zhang 2022 [132] | Improvements in symptoms of periodontitis, measured by clinical attachment level (CAL), the bleeding index (BI), the oral hygiene index-simplified (OHI-S), and probing pocket depth (PPD) | Individuals with periodontitis | Patients randomised to 125 mg, 250 mg, or 500 mg RSV daily, or placebo, in capsule form, for 8 weeks | Forty participants in each of the four groups | There were no significant differences in the AEs observed between the RSV and placebo-treated patients. The most common AEs were nausea and diarrhoea. In total, there were 64 AEs in the RSV groups, but the frequency was not dose-related. Eighteen AEs were reported in the 500 mg high dose group (nausea six; diarrhoea nine; hypotension one; proteinuria two), twenty-two were reported in patients on 250 mg (nausea eight; diarrhoea ten; hypotension one; proteinuria three) and there were eight AEs in the 125 mg dose group (nausea eight; diarrhoea eleven; hypotension two; proteinuria three). Values are not reported for the placebo group | Symptoms of periodontitis determined by CAL, BI, OHI-S, and PPD were improved by all doses of RSV compared to placebo. RSV also significantly decreased specific systemic and local inflammatory markers compared to placebo |
| Ulcerative colitis | |||||||
| 99 | Samsami-Kor 2015 (RCT201209154010N10) [133] | Inflammatory biomarkers and quality of life | Patients with mild to moderate active ulcerative colitis | 500 mg RSV or placebo capsule for 6 weeks | 25 on RSV; 24 on placebo | Zero reported | RSV significantly reduced plasma levels of TNF-α, hs-CRP, and the activity of NF-kB in PBMCs. RSV significantly decreased the clinical colitis activity index score and increased the inflammatory bowel disease questionnaire—9 (IBDQ-9) score |
| Samsamikor 2016 (same study as above, but with more patients included) [134] | Whether RSV can improve oxidative/anti-oxidative status in subjects with UC | 28 in each group | RSV increased serum SOD and TAC and significantly decreased serum MDA. RSV significantly decreased disease activity and increased the quality of life | ||||
| Other | |||||||
| 100 | Beijers 2020 (NCT02245932) [135] | Effects on skeletal muscle mitochondrial function | Patients with chronic obstructive pulmonary disease | 75 mg RSV or placebo capsule twice daily for 4 weeks | 11 on RSV; 10 on placebo | Zero reported | RSV did not have any significant effects on mitochondrial function |
| 101 | Malaguarnera 2018 [136] | Liver health, health-related quality of life (HRQL) and a reduction in depression and anxiety in patients with MHE | Patients with Minimal Hepatic Encephalopathy (MHE) | 18.8 mg RSV or a placebo tablet once daily for 90 days | 35 on RSV; 35 on placebo, which also contained N-acetylcysteine | Zero reported | RSV caused a significant decrease in the Back Depression Inventory (BDI) and state-trait anxiety inventory (STAI). RSV significantly improved physical function, body pain, general health, vitality, and social function |
| 102 | Martinez 2015 [137] | Sperm number, motility, or reduced abnormalities | Males with infertility | 50 mg RSV daily or placebo in capsule form for 75 days | 18 on RSV, 18 on placebo, and 18 on another investigational product—a hydrogen sulfide prodrug | Zero reported in the RSV group | RSV did not cause any significant change to any parameters |
| 103 | Qiang 2018 [138] | Clinical efficacy in improving treatment outcome of oral amoxicillin against childhood fast breathing pneumonia. The primary outcome was defined as treatment failure up to day 3 | Children diagnosed with fast-breathing pneumonia | 20–60 mg RSV depending on body weight, twice daily for 3 days. The control group had placebo and all patients had amoxicillin. No information was given on the RSV formulation used | 282 on RSV; 282 on placebo, both with amoxicillin | Zero reported | RSV + amoxycillin revealed decreased treatment failure rates vs. placebo + amoxycillin |
| 104 | Shi 2017 [139] | Disease activity was determined using the Birmingham Vascular Activity Score (BVAS) | Patients with acute Takayasu arteritis | 250 mg RSV or placebo capsule once daily for 3 months | 121 on RSV; 121 on placebo | Not stated | RSV significantly decreased BVAS scores after week 6 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Brown, K.; Theofanous, D.; Britton, R.G.; Aburido, G.; Pepper, C.; Sri Undru, S.; Howells, L. Resveratrol for the Management of Human Health: How Far Have We Come? A Systematic Review of Resveratrol Clinical Trials to Highlight Gaps and Opportunities. Int. J. Mol. Sci. 2024, 25, 747. https://doi.org/10.3390/ijms25020747
Brown K, Theofanous D, Britton RG, Aburido G, Pepper C, Sri Undru S, Howells L. Resveratrol for the Management of Human Health: How Far Have We Come? A Systematic Review of Resveratrol Clinical Trials to Highlight Gaps and Opportunities. International Journal of Molecular Sciences. 2024; 25(2):747. https://doi.org/10.3390/ijms25020747
Chicago/Turabian StyleBrown, Karen, Despoina Theofanous, Robert G. Britton, Grandezza Aburido, Coral Pepper, Shanthi Sri Undru, and Lynne Howells. 2024. "Resveratrol for the Management of Human Health: How Far Have We Come? A Systematic Review of Resveratrol Clinical Trials to Highlight Gaps and Opportunities" International Journal of Molecular Sciences 25, no. 2: 747. https://doi.org/10.3390/ijms25020747
APA StyleBrown, K., Theofanous, D., Britton, R. G., Aburido, G., Pepper, C., Sri Undru, S., & Howells, L. (2024). Resveratrol for the Management of Human Health: How Far Have We Come? A Systematic Review of Resveratrol Clinical Trials to Highlight Gaps and Opportunities. International Journal of Molecular Sciences, 25(2), 747. https://doi.org/10.3390/ijms25020747





