D-Chiro-Inositol and Myo-Inositol Induce WAT/BAT Trans-Differentiation in Two Different Human Adipocyte Models (SGBS and LiSa-2)
Abstract
1. Introduction
2. Results
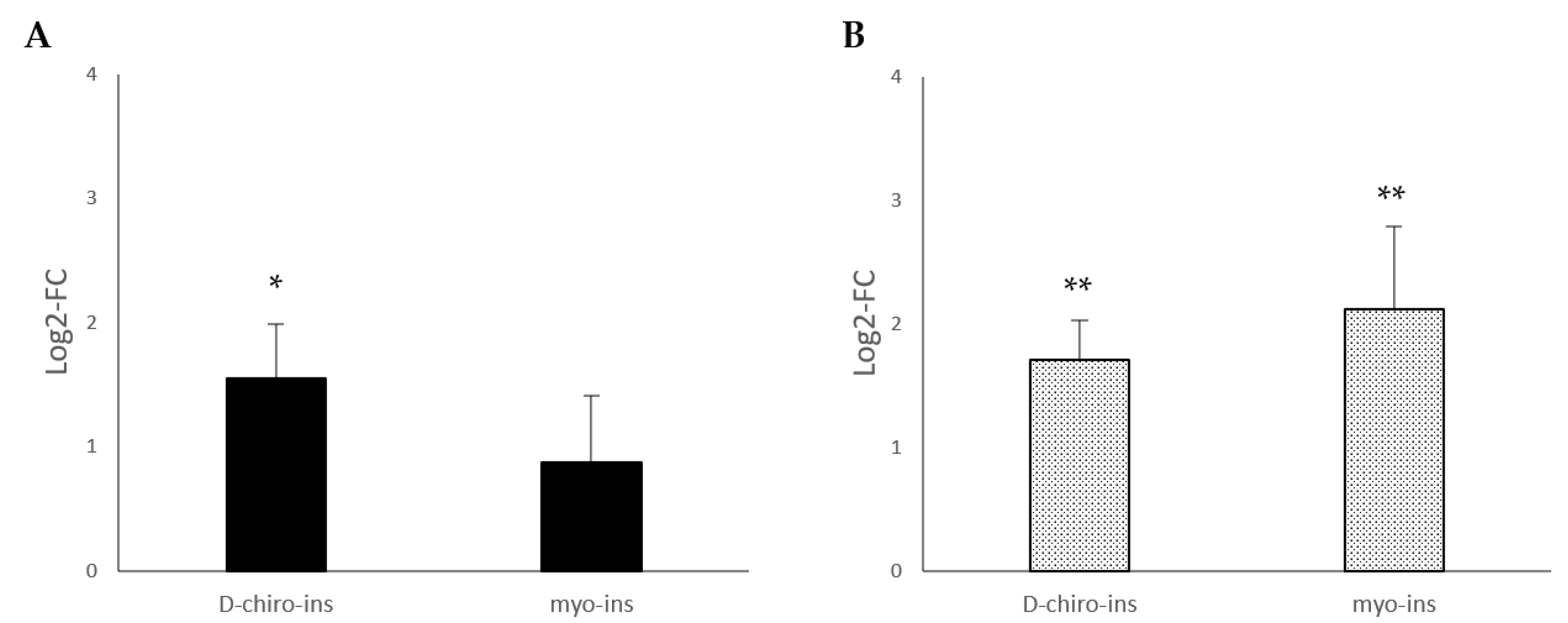
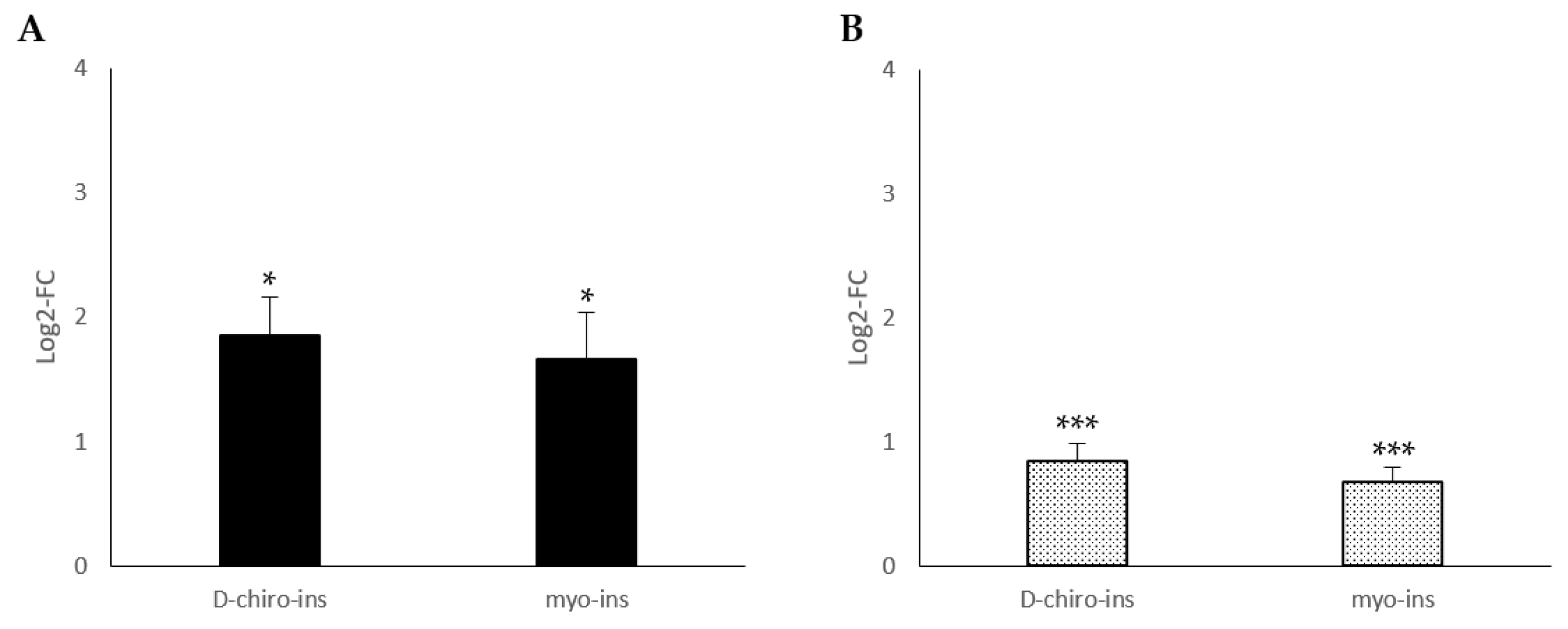
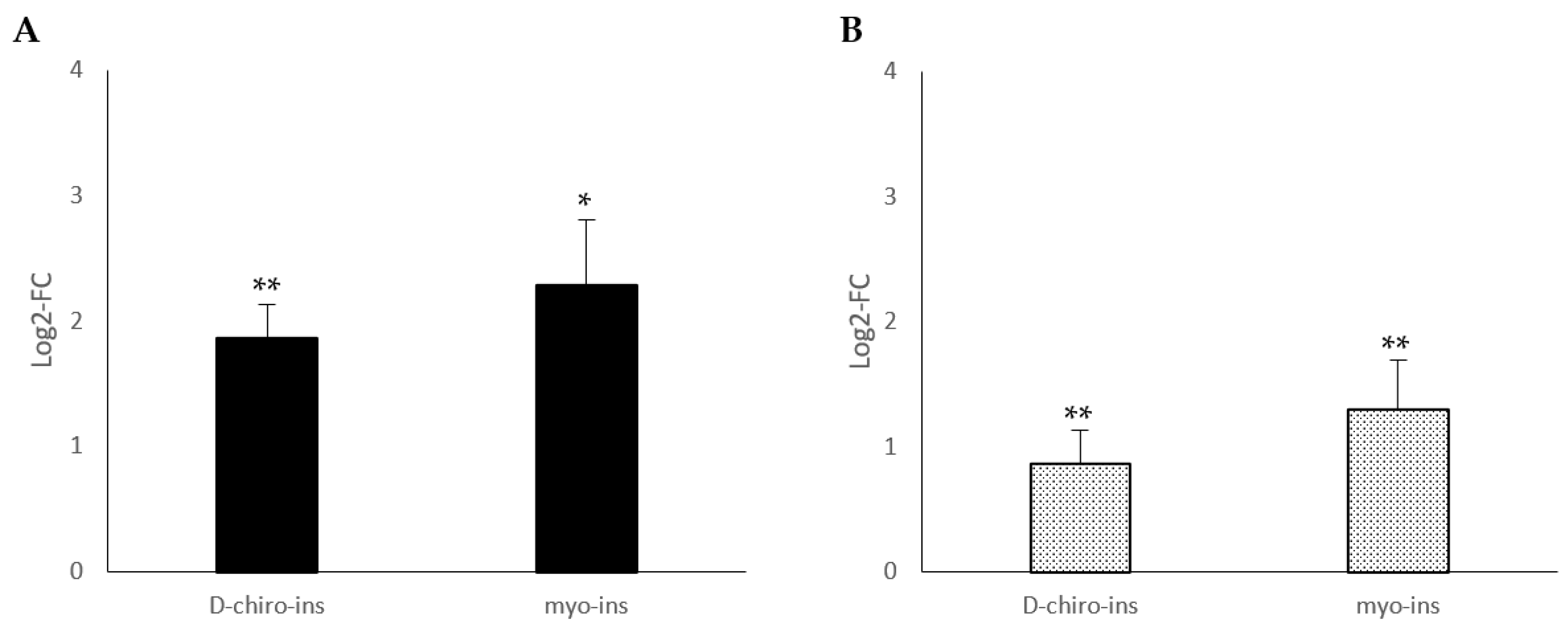
2.1. Modulation of UCP-1 mRNA
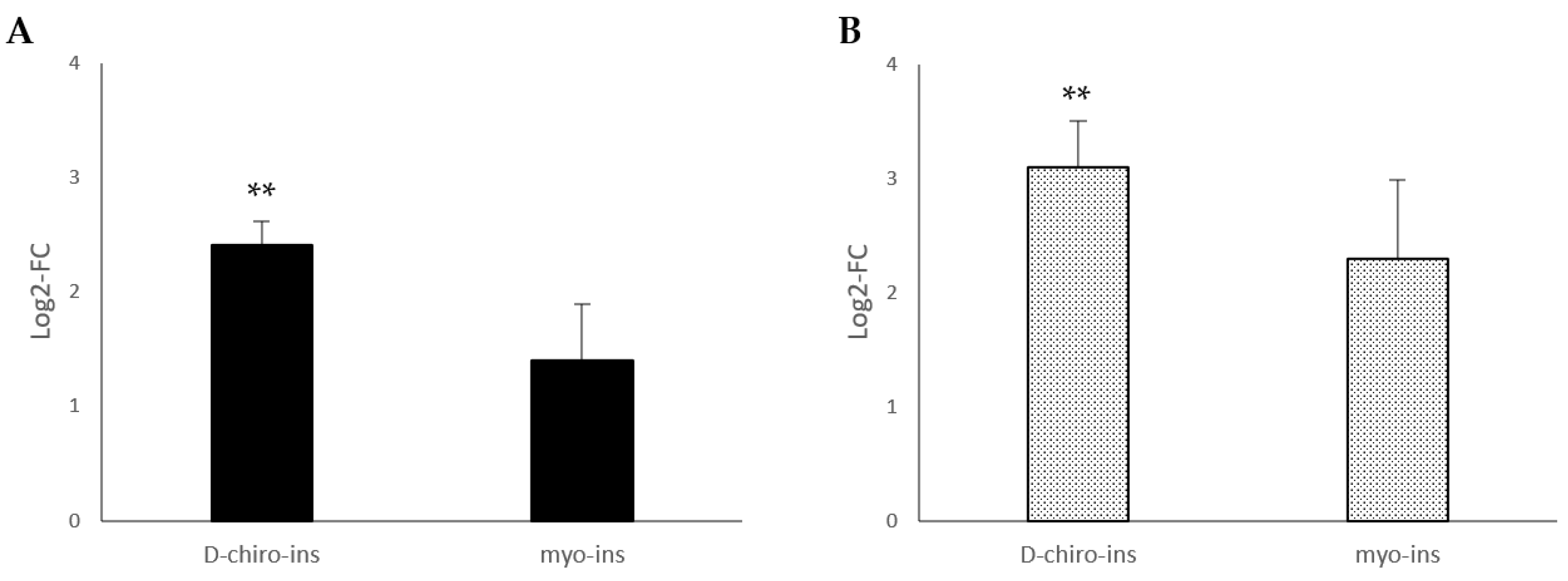
2.2. Increase in Mitochondrial DNA Copy Number
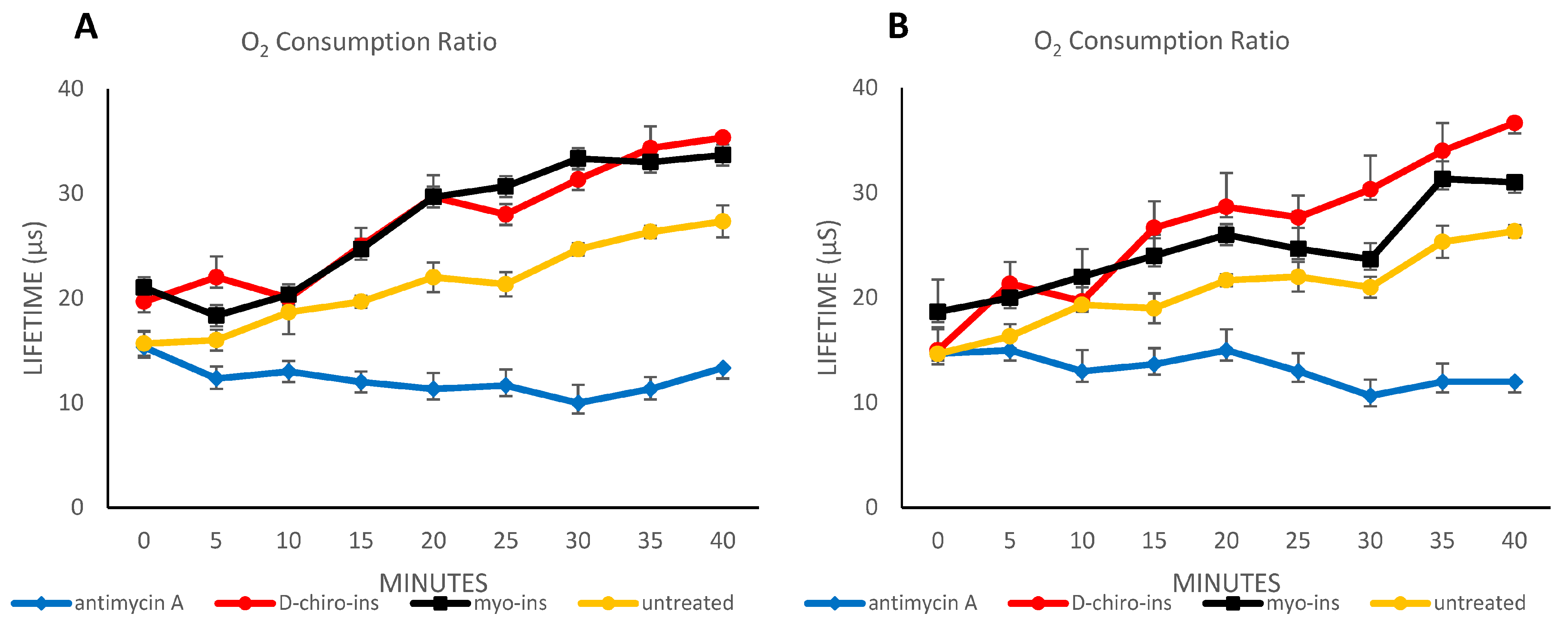
2.3. Oximetry Assessment
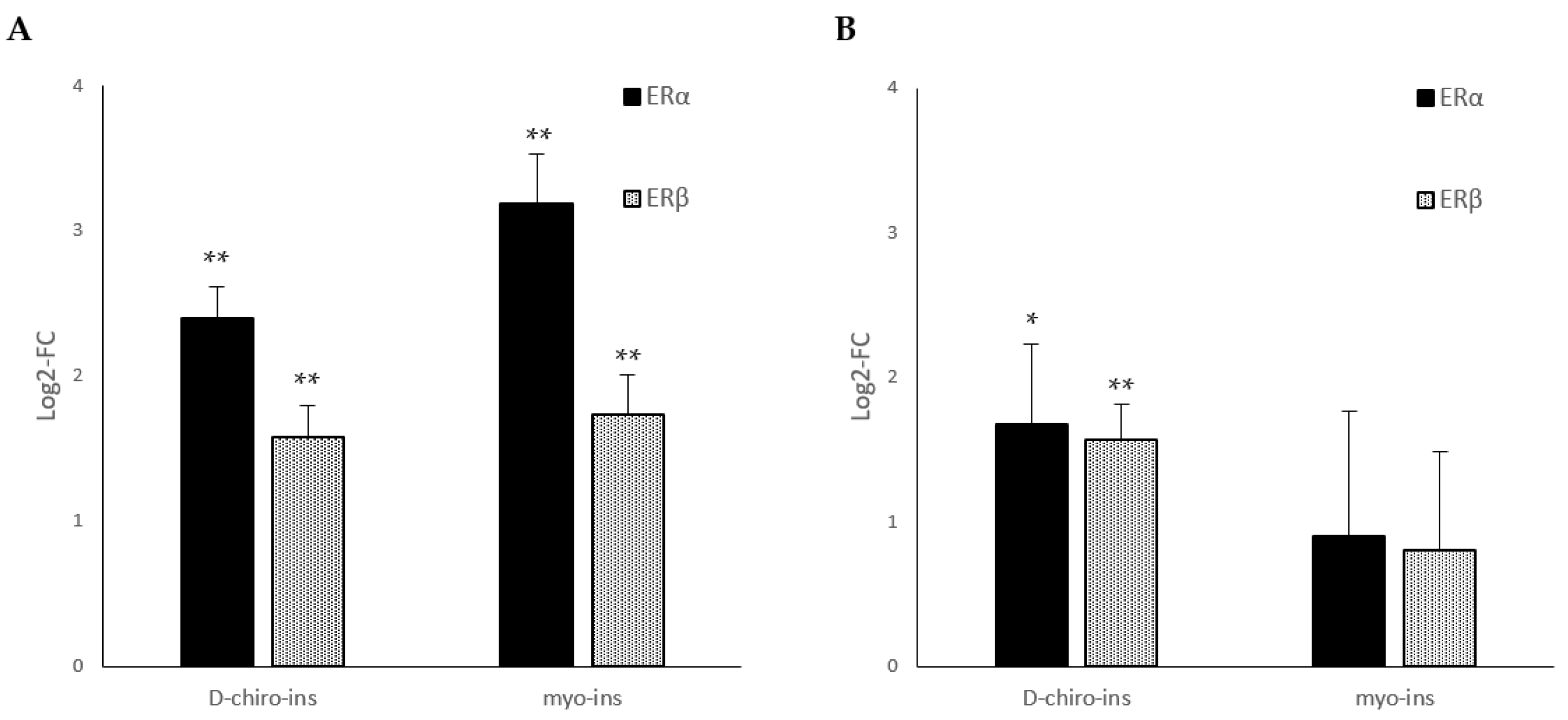
2.4. Expression of Estrogen Receptors (ERα and ERβ) mRNAs
2.5. Increase in PPAR-γ Gene Expression
3. Discussion
4. Materials and Methods
4.1. Cell Culture SGBS
Adipocyte Differentiation Process
4.2. Cell Culture LiSa-2
4.3. Treatments
4.4. RNA Isolation, RT-PCR, qPCR
4.5. Mitochondrial and Nuclear DNA Isolation and mitDNA Quantification by qPCR
4.6. Ex Vivo Oxygen Consumption Rate Measurement
4.7. Statistical Analysis and Data Presentation
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Afshin, A.; Forouzanfar, M.H.; Reitsma, M.B.; Sur, P.; Estep, K.; Lee, A.; Marczak, L.; Mokdad, A.H.; Moradi-Lakeh, M.; Naghavi, M.; et al. Health Effects of Overweight and Obesity in 195 Countries over 25 Years. N. Engl. J. Med. 2017, 377, 13–27. [Google Scholar] [CrossRef] [PubMed]
- World Health Organization. Available online: https://www.who.int/ (accessed on 18 November 2022).
- Nnodim, J.O. Development of adipose tissues. Anat. Rec. 1987, 219, 331–337. [Google Scholar] [CrossRef] [PubMed]
- Klaus, S. Functional differentiation of white and brown adipocytes. Bioessays 1997, 19, 215–223. [Google Scholar] [CrossRef]
- Cannon, B.; Nedergaard, J. Brown adipose tissue: Function and physiological significance. Physiol. Rev. 2004, 84, 277–359. [Google Scholar] [CrossRef]
- Giralt, M.; Villarroya, F. White, brown, beige/brite: Different adipose cells for different functions? Endocrinology 2013, 154, 2992–3000. [Google Scholar] [CrossRef] [PubMed]
- Rosenwald, M.; Wolfrum, C. The origin and definition of brite versus white and classical brown adipocytes. Adipocyte 2014, 3, 4–9. [Google Scholar] [CrossRef] [PubMed]
- Dragano, N.R.V.; Fernø, J.; Diéguez, C.; López, M.; Milbank, E. Recent Updates on Obesity Treatments: Available Drugs and Future Directions. Neuroscience 2020, 437, 215–239. [Google Scholar] [CrossRef] [PubMed]
- Pak, Y.; Huang, L.C.; Lilley, K.J.; Larner, J. In vivo conversion of [3H]myoinositol to [3H]chiroinositol in rat tissues. J. Biol. Chem. 1992, 267, 16904–16910. [Google Scholar] [CrossRef]
- Facchinetti, F.; Appetecchia, M.; Aragona, C.; Bevilacqua, A.; Bezerra Espinola, M.S.; Bizzarri, M.; D’Anna, R.; Dewailly, D.; Diamanti-Kandarakis, E.; Hernández Marín, I.; et al. Experts’ opinion on inositols in treating polycystic ovary syndrome and non-insulin dependent diabetes mellitus: A further help for human reproduction and beyond. Expert Opin. Drug Metab. Toxicol. 2020, 16, 255–274. [Google Scholar] [CrossRef] [PubMed]
- Dinicola, S.; Unfer, V.; Facchinetti, F.; Soulage, C.O.; Greene, N.D.; Bizzarri, M.; Laganà, A.S.; Chan, S.Y.; Bevilacqua, A.; Pkhaladze, L.; et al. Inositols: From Established Knowledge to Novel Approaches. Int. J. Mol. Sci. 2021, 22, 10575. [Google Scholar] [CrossRef]
- Milewska, E.M.; Czyzyk, A.; Meczekalski, B.; Genazzani, A.D. Inositol and human reproduction. From cellular metabolism to clinical use. Gynecol. Endocrinol. 2016, 32, 690–695. [Google Scholar] [CrossRef] [PubMed]
- Facchinetti, F.; Espinola, M.S.B.; Dewailly, D.; Ozay, A.C.; Prapas, N.; Vazquez-Levin, M.; Wdowiak, A.; Unfer, V. Breakthroughs in the Use of Inositols for Assisted Reproductive Treatment (ART). Trends Endocrinol. Metab. 2020, 31, 570–579. [Google Scholar] [CrossRef]
- Hayashi, E.; Maeda, T.; Tomita, T. The effect of myo-inositol deficiency on lipid metabolism in rats. I. The alteration of lipid metabolism in myo-inositol deficient rats. Biochim. Biophys. Acta 1974, 360, 134–145. [Google Scholar] [CrossRef] [PubMed]
- Lee, B.-J.; Lee, K.-J.; Lim, S.-J.; Lee, S.-M. Dietary myo-inositol requirement for Olive flounder, Paralichthys olivaceus (Temminch et Schlegel). Aquac. Res. 2008, 40, 83–90. [Google Scholar] [CrossRef]
- Khosravi, S.; Lim, S.-J.; Rahimnejad, S.; Kim, S.-S.; Lee, B.-J.; Kim, K.-W.; Han, H.-S.; Lee, K.-J. Dietary myo-inositol requirement of parrot fish, Oplegnathus fasciatus. Aquaculture 2015, 436, 1–7. [Google Scholar] [CrossRef]
- Fan, C.; Zhang, D.; Zhang, J.; Li, J.; Wang, Y.; Gao, L.; Han, S. The effect of D-chiro-inositol on renal protection in diabetic mice. Aging 2022, 14, 3416–3424. [Google Scholar] [CrossRef]
- Montt-Guevara, M.M.; Finiguerra, M.; Marzi, I.; Fidecicchi, T.; Ferrari, A.; Genazzani, A.D.; Simoncini, T. D-Chiro-Inositol Regulates Insulin Signaling in Human Adipocytes. Front. Endocrinol. 2021, 12, 660815. [Google Scholar] [CrossRef]
- Wabitsch, M.; Brüderlein, S.; Melzner, I.; Braun, M.; Mechtersheimer, G.; Möller, P. LiSa-2, a novel human liposarcoma cell line with a high capacity for terminal adipose differentiation. Int. J. Cancer 2000, 88, 889–894. [Google Scholar] [CrossRef]
- Nicholls, D.G.; Locke, R.M. Thermogenic mechanisms in brown fat. Physiol. Rev. 1984, 64, 1–64. [Google Scholar] [CrossRef]
- Nedergaard, J.; Golozoubova, V.; Matthias, A.; Asadi, A.; Jacobsson, A.; Cannon, B. UCP1: The only protein able to mediate adaptive non-shivering thermogenesis and metabolic inefficiency. Biochim. Biophys. Acta 2001, 1504, 82–106. [Google Scholar] [CrossRef]
- Liu, X.; Zheng, Z.; Zhu, X.; Meng, M.; Li, L.; Shen, Y.; Chi, Q.; Wang, D.; Zhang, Z.; Li, C.; et al. Brown adipose tissue transplantation improves whole-body energy metabolism. Cell Res. 2013, 23, 851–854. [Google Scholar] [CrossRef]
- Wei, G.; Sun, H.; Liu, J.L.; Dong, K.; Liu, J.; Zhang, M. Indirubin, a small molecular deriving from connectivity map (CMAP) screening, ameliorates obesity-induced metabolic dysfunction by enhancing brown adipose thermogenesis and white adipose browning. Nutr. Metab. 2020, 17, 21. [Google Scholar] [CrossRef] [PubMed]
- Vijgen, G.H.; Bouvy, N.D.; Teule, G.J.; Brans, B.; Schrauwen, P.; van Marken Lichtenbelt, W.D. Brown adipose tissue in morbidly obese subjects. PLoS ONE 2011, 6, e17247. [Google Scholar] [CrossRef]
- Al-Qahtani, S.M.; Bryzgalova, G.; Valladolid-Acebes, I.; Korach-André, M.; Dahlman-Wright, K.; Efendić, S.; Berggren, P.O.; Portwood, N. 17β-Estradiol suppresses visceral adipogenesis and activates brown adipose tissue-specific gene expression. Horm. Mol. Biol. Clin. Investig. 2017, 29, 13–26. [Google Scholar] [CrossRef] [PubMed]
- Santos, R.S.; Frank, A.P.; Fátima, L.A.; Palmer, B.F.; Öz, O.K.; Clegg, D.J. Activation of estrogen receptor alpha induces beiging of adipocytes. Mol. Metab. 2018, 18, 51–59. [Google Scholar] [CrossRef] [PubMed]
- Nilsson, M.; Dahlman, I.; Rydén, M.; Nordström, E.A.; Gustafsson, J.A.; Arner, P.; Dahlman-Wright, K. Oestrogen receptor alpha gene expression levels are reduced in obese compared to normal weight females. Int. J. Obes. 2007, 31, 900–907. [Google Scholar] [CrossRef]
- Zhou, Z.; Moore, T.M.; Drew, B.G.; Ribas, V.; Wanagat, J.; Civelek, M.; Segawa, M.; Wolf, D.M.; Norheim, F.; Seldin, M.M.; et al. Estrogen receptor α controls metabolism in white and brown adipocytes by regulating Polg1 and mitochondrial remodeling. Sci. Transl. Med. 2020, 12, eaax8096. [Google Scholar] [CrossRef]
- Rodríguez-Cuenca, S.; Monjo, M.; Gianotti, M.; Proenza, A.M.; Roca, P. Expression of mitochondrial biogenesis-signaling factors in brown adipocytes is influenced specifically by 17beta-estradiol, testosterone, and progesterone. Am. J. Physiol. Endocrinol. Metab. 2007, 292, E340–E346. [Google Scholar] [CrossRef] [PubMed]
- Quarta, C.; Mazza, R.; Pasquali, R.; Pagotto, U. Role of sex hormones in modulation of brown adipose tissue activity. J. Mol. Endocrinol. 2012, 49, R1–R7. [Google Scholar] [CrossRef]
- Yoshioka, K.; Yoshida, T.; Wakabayashi, Y.; Nishioka, H.; Kondo, M. Reduced brown adipose tissue thermogenesis of obese rats after ovariectomy. Endocrinol. Jpn. 1988, 35, 537–543. [Google Scholar] [CrossRef]
- Pedersen, S.B.; Bruun, J.M.; Kristensen, K.; Richelsen, B. Regulation of UCP1, UCP2, and UCP3 mRNA expression in brown adipose tissue, white adipose tissue, and skeletal muscle in rats by estrogen. Biochem. Biophys. Res. Commun. 2001, 288, 191–197. [Google Scholar] [CrossRef] [PubMed]
- Martínez de Morentin, P.B.; González-García, I.; Martins, L.; Lage, R.; Fernández-Mallo, D.; Martínez-Sánchez, N.; Ruíz-Pino, F.; Liu, J.; Morgan, D.A.; Pinilla, L.; et al. Estradiol regulates brown adipose tissue thermogenesis via hypothalamic AMPK. Cell Metab. 2014, 20, 41–53. [Google Scholar] [CrossRef]
- Zhang, W.; Schmull, S.; Du, M.; Liu, J.; Lu, Z.; Zhu, H.; Xue, S.; Lian, F. Estrogen Receptor α and β in Mouse: Adipose-Derived Stem Cell Proliferation, Migration, and Brown Adipogenesis In Vitro. Cell. Physiol. Biochem. 2016, 38, 2285–2299. [Google Scholar] [CrossRef] [PubMed]
- Monastra, G.; Vazquez-Levin, M.; Bezerra Espinola, M.S.; Bilotta, G.; Laganà, A.S.; Unfer, V. D-chiro-inositol, an aromatase down-modulator, increases androgens and reduces estrogens in male volunteers: A pilot study. Basic Clin. Androl. 2021, 31, 13. [Google Scholar] [CrossRef] [PubMed]
- Nordio, M.; Kumanov, P.; Chiefari, A.; Puliani, G. D-Chiro-Inositol improves testosterone levels in older hypogonadal men with low-normal testosterone: A pilot study. Basic Clin. Androl. 2021, 31, 28. [Google Scholar] [CrossRef] [PubMed]
- Gambioli, R.; Forte, G.; Aragona, C.; Bevilacqua, A.; Bizzarri, M.; Unfer, V. The use of D-chiro-Inositol in clinical practice. Eur. Rev. Med. Pharmacol. Sci. 2021, 25, 438–446. [Google Scholar] [CrossRef]
- Gambioli, R.; Montanino Oliva, M.; Nordio, M.; Chiefari, A.; Puliani, G.; Unfer, V. New Insights into the Activities of D-Chiro-Inositol: A Narrative Review. Biomedicines 2021, 9, 1378. [Google Scholar] [CrossRef]
- Montanino Oliva, M.; Gambioli, R.; Forte, G.; Porcaro, G.; Aragona, C.; Unfer, V. Unopposed estrogens: Current and future perspectives. Eur. Rev. Med. Pharmacol. Sci. 2022, 26, 2975–2989. [Google Scholar] [CrossRef]
- Semple, R.K.; Chatterjee, V.K.; O’Rahilly, S. PPAR gamma and human metabolic disease. J. Clin. Investig. 2006, 116, 581–589. [Google Scholar] [CrossRef]
- Gervois, P.; Torra, I.P.; Fruchart, J.C.; Staels, B. Regulation of lipid and lipoprotein metabolism by PPAR activators. Clin. Chem. Lab. Med. 2000, 38, 3–11. [Google Scholar] [CrossRef]
- Zhang, Y.; Li, C.; Zhang, W.; Zheng, X.; Chen, X. Decreased Insulin Resistance by Myo-Inositol Is Associated with Suppressed Interleukin 6/Phospho-STAT3 Signaling in a Rat Polycystic Ovary Syndrome Model. J. Med. Food 2020, 23, 375–387. [Google Scholar] [CrossRef] [PubMed]
- Klusóczki, Á.; Veréb, Z.; Vámos, A.; Fischer-Posovszky, P.; Wabitsch, M.; Bacso, Z.; Fésüs, L.; Kristóf, E. Differentiating SGBS adipocytes respond to PPARγ stimulation, irisin and BMP7 by functional browning and beige characteristics. Sci. Rep. 2019, 9, 5823. [Google Scholar] [CrossRef] [PubMed]
- Mukherjee, R.; Jow, L.; Croston, G.E.; Paterniti, J.R., Jr. Identification, characterization, and tissue distribution of human peroxisome proliferator-activated receptor (PPAR) isoforms PPARgamma2 versus PPARgamma1 and activation with retinoid X receptor agonists and antagonists. J. Biol. Chem. 1997, 272, 8071–8076. [Google Scholar] [CrossRef] [PubMed]
- Mueller, E.; Drori, S.; Aiyer, A.; Yie, J.; Sarraf, P.; Chen, H.; Hauser, S.; Rosen, E.D.; Ge, K.; Roeder, R.G.; et al. Genetic analysis of adipogenesis through peroxisome proliferator-activated receptor gamma isoforms. J. Biol. Chem. 2002, 277, 41925–41930. [Google Scholar] [CrossRef] [PubMed]
- Sabichi, A.L.; Subbarayan, V.; Llansa, N.; Lippman, S.M.; Menter, D.G. Peroxisome proliferator-activated receptor-gamma suppresses cyclooxygenase-2 expression in human prostate cells. Cancer Epidemiol. Biomark. Prev. 2004, 13, 1704–1709. [Google Scholar] [CrossRef]
- Tontonoz, P.; Hu, E.; Graves, R.A.; Budavari, A.I.; Spiegelman, B.M. mPPAR gamma 2: Tissue-specific regulator of an adipocyte enhancer. Genes Dev. 1994, 8, 1224–1234. [Google Scholar] [CrossRef]
- Fischer-Posovszky, P.; Newell, F.S.; Wabitsch, M.; Tornqvist, H.E. Human SGBS cells—A unique tool for studies of human fat cell biology. Obes. Facts 2008, 1, 184–189. [Google Scholar] [CrossRef]
- Wabitsch, M.; Brenner, R.E.; Melzner, I.; Braun, M.; Möller, P.; Heinze, E.; Debatin, K.M.; Hauner, H. Characterization of a human preadipocyte cell strain with high capacity for adipose differentiation. Int. J. Obes. Relat. Metab. Disord. 2001, 25, 8–15. [Google Scholar] [CrossRef]
- Garzon, S.; Laganà, A.S.; Monastra, G. Risk of reduced intestinal absorption of myo-inositol caused by D-chiro-inositol or by glucose transporter inhibitors. Expert. Opin. Drug Metab. Toxicol. 2019, 15, 697–703. [Google Scholar] [CrossRef]
- Ranaldi, G.; Ferruzza, S.; Natella, F.; Unfer, V.; Sambuy, Y.; Monastra, G. Enhancement of D-chiro-inositol transport across intestinal cells by alpha-Lactalbumin peptides. Eur. Rev. Med. Pharmacol. Sci. 2020, 24, 10143–10154. [Google Scholar] [CrossRef]
- Monastra, G.; Sambuy, Y.; Ferruzza, S.; Ferrari, D.; Ranaldi, G. Alpha-lactalbumin Effect on Myo-inositol Intestinal Absorption: In Vivo and In Vitro. Curr. Drug Deliv. 2018, 15, 1305–1311. [Google Scholar] [CrossRef] [PubMed]
- Rousset, S.; Mozo, J.; Dujardin, G.; Emre, Y.; Masscheleyn, S.; Ricquier, D.; Cassard-Doulcier, A.M. UCP2 is a mitochondrial transporter with an unusual very short half-life. FEBS Lett. 2007, 581, 479–482. [Google Scholar] [CrossRef] [PubMed]
- Moazed, B.; Desautels, M. Differentiation-dependent expression of cathepsin D and importance of lysosomal proteolysis in the degradation of UCP1 in brown adipocytes. Can. J. Physiol. Pharmacol. 2002, 80, 515–525. [Google Scholar] [CrossRef]
- Hawthorne, J.N. Inositol lipid metabolism and cell membrane. Biochem. J. 1972, 128, 19P–20P. [Google Scholar] [CrossRef] [PubMed]
- Kulterer, O.C.; Herz, C.T.; Prager, M.; Schmöltzer, C.; Langer, F.B.; Prager, G.; Marculescu, R.; Kautzky-Willer, A.; Hacker, M.; Haug, A.R.; et al. Brown Adipose Tissue Prevalence Is Lower in Obesity but Its Metabolic Activity Is Intact. Front. Endocrinol. 2022, 13, 858417. [Google Scholar] [CrossRef]
| Gene | Protein Name | Sequence 5′→3′ | Amplicon Length | NCBI Ref | |
|---|---|---|---|---|---|
| ACTB | β-Actin | F: AGAAGGATTCCTATGTGGGGG | R: CATGTCGTCCCAGTTGGTGAC | 101 | NM_001101.5 |
| ESR1 | ER-α | F: GATGCTGAGCCCCCATACT | R: CACACGGCACAGTAGCGAG | 128 | NM_001122742.2 |
| ESR2 | ER-β | F: AGCTCAGCCTGTTCGA | R: TCTACGCATTTCCCCTCATCC | 151 | NM_001437.3 |
| UCP-1 | UCP-1 | F: GGAAAGAAACAGCACCTAGTTT | R: CGTCAAGCCTTCGGTTGTTGCTA | 197 | NM_021833.5 |
| PPARg-1 | PPAR-γ1 | F: CGTGGCCGCAGATTTGAA | R: CTTCCATTACGGAGAGATCCAC | 166 | NM_138712.5 |
| PPARg-2 | PPAR-γ2 | F: GGTGAAACTCTGGGAGATTCT | R: CTCTGTGTCAACCATGGTCA | 102 | NM_015869.5 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Monastra, G.; Gambioli, R.; Unfer, V.; Forte, G.; Maymo-Masip, E.; Comitato, R. D-Chiro-Inositol and Myo-Inositol Induce WAT/BAT Trans-Differentiation in Two Different Human Adipocyte Models (SGBS and LiSa-2). Int. J. Mol. Sci. 2023, 24, 7421. https://doi.org/10.3390/ijms24087421
Monastra G, Gambioli R, Unfer V, Forte G, Maymo-Masip E, Comitato R. D-Chiro-Inositol and Myo-Inositol Induce WAT/BAT Trans-Differentiation in Two Different Human Adipocyte Models (SGBS and LiSa-2). International Journal of Molecular Sciences. 2023; 24(8):7421. https://doi.org/10.3390/ijms24087421
Chicago/Turabian StyleMonastra, Giovanni, Riccardo Gambioli, Vittorio Unfer, Gianpiero Forte, Elsa Maymo-Masip, and Raffaella Comitato. 2023. "D-Chiro-Inositol and Myo-Inositol Induce WAT/BAT Trans-Differentiation in Two Different Human Adipocyte Models (SGBS and LiSa-2)" International Journal of Molecular Sciences 24, no. 8: 7421. https://doi.org/10.3390/ijms24087421
APA StyleMonastra, G., Gambioli, R., Unfer, V., Forte, G., Maymo-Masip, E., & Comitato, R. (2023). D-Chiro-Inositol and Myo-Inositol Induce WAT/BAT Trans-Differentiation in Two Different Human Adipocyte Models (SGBS and LiSa-2). International Journal of Molecular Sciences, 24(8), 7421. https://doi.org/10.3390/ijms24087421








