Azido-Ceramides, a Tool to Analyse SARS-CoV-2 Replication and Inhibition—SARS-CoV-2 Is Inhibited by Ceramides
Abstract
1. Introduction
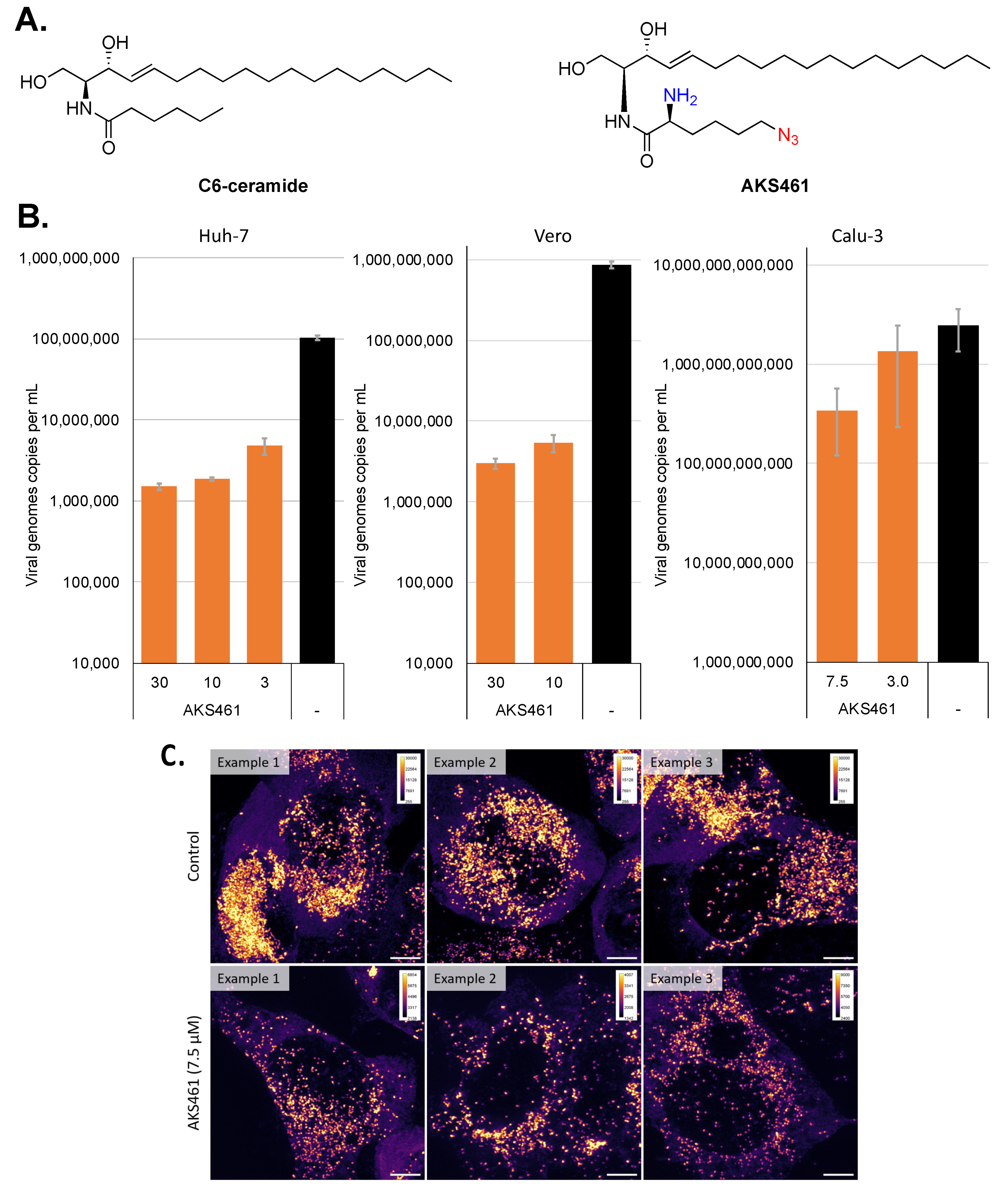
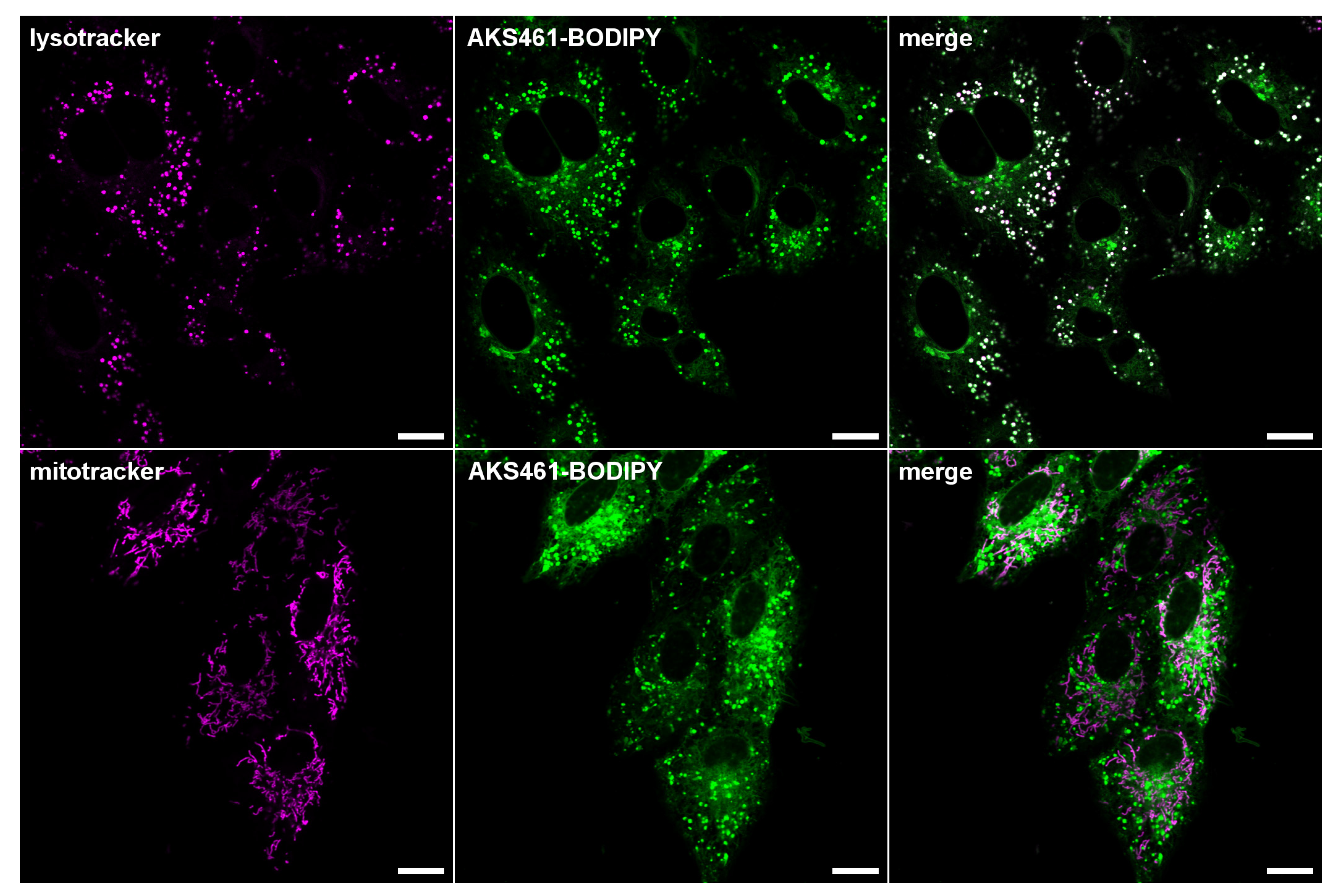
2. Results and Discussion
3. Materials and Methods
3.1. Synthesis
General Experimental Information
- (a)
- tert-Butyl ((S)-6-azido-1-(((2S,3R)-1,3-dihydroxy-(E)-octadec-4-ene-2-yl)amino)-1-oxohexane-2-yl)carbamate 1
- (b)
- (S)-2-Amino-6-azido-N-((2S,3R)-1,3-dihydroxy-(E)-octadec-4-ene-2-yl)hexanamide (α-NH2-ω-N3-C6-ceramide (AKS461))
3.2. Viral Infection and RNA Quantification
3.3. Cytotoxicity and Cellular Proliferation Assays
3.4. FISH Labelling
3.5. FISH Microscopy and Quantification
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Hillen, H.S.; Kokic, G.; Farnung, L.; Dienemann, C.; Tegunov, D.; Cramer, P. Structure of replicating SARS-CoV-2 polymerase. Nature 2020, 584, 154–156. [Google Scholar] [CrossRef] [PubMed]
- Sternberg, A.; Naujokat, C. Structural features of coronavirus SARS-CoV-2 spike protein: Targets for vaccination. Life Sci. 2020, 257, 118056. [Google Scholar] [CrossRef] [PubMed]
- Liu, L.; Wang, P.; Nair, M.S.; Yu, J.; Rapp, M.; Wang, Q.; Luo, Y.; Chan, J.F.; Sahi, V.; Figueroa, A.; et al. Potent neutralizing antibodies against multiple epitopes on SARS-CoV-2 spike. Nature 2020, 584, 450–456. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.; Lin, D.; Kusov, Y.; Nian, Y.; Ma, Q.; Wang, J.; von Brunn, A.; Leyssen, P.; Lanko, K.; Neyts, J.; et al. alpha-Ketoamides as Broad-Spectrum Inhibitors of Coronavirus and Enterovirus Replication: Structure-Based Design, Synthesis, and Activity Assessment. J. Med. Chem. 2020, 63, 4562–4578. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.; Lin, D.; Sun, X.; Curth, U.; Drosten, C.; Sauerhering, L.; Becker, S.; Rox, K.; Hilgenfeld, R. Crystal structure of SARS-CoV-2 main protease provides a basis for design of improved alpha-ketoamide inhibitors. Science 2020, 368, 409–412. [Google Scholar] [CrossRef]
- Breidenbach, J.; Lemke, C.; Pillaiyar, T.; Schakel, L.; Al Hamwi, G.; Diett, M.; Gedschold, R.; Geiger, N.; Lopez, V.; Mirza, S.; et al. Targeting the Main Protease of SARS-CoV-2: From the Establishment of High Throughput Screening to the Design of Tailored Inhibitors. Angew. Chem. Int. Ed Engl. 2021, 60, 10423–10429. [Google Scholar] [CrossRef]
- Geiger, N.; Diesendorf, V.; Roll, V.; Konig, E.M.; Obernolte, H.; Sewald, K.; Breidenbach, J.; Pillaiyar, T.; Gutschow, M.; Muller, C.E.; et al. Cell Type-Specific Anti-Viral Effects of Novel SARS-CoV-2 Main Protease Inhibitors. Int. J. Mol. Sci. 2023, 24, 3972. [Google Scholar] [CrossRef]
- Wang, M.; Cao, R.; Zhang, L.; Yang, X.; Liu, J.; Xu, M.; Shi, Z.; Hu, Z.; Zhong, W.; Xiao, G. Remdesivir and chloroquine effectively inhibit the recently emerged novel coronavirus (2019-nCoV) in vitro. Cell Res. 2020, 30, 269–271. [Google Scholar] [CrossRef]
- Gordon, D.E.; Jang, G.M.; Bouhaddou, M.; Xu, J.; Obernier, K.; White, K.M.; O’Meara, M.J.; Rezelj, V.V.; Guo, J.Z.; Swaney, D.L.; et al. A SARS-CoV-2 protein interaction map reveals targets for drug repurposing. Nature 2020, 583, 459–468. [Google Scholar] [CrossRef]
- Saxena, A. Drug targets for COVID-19 therapeutics: Ongoing global efforts. J. Biosci. 2020, 45, 87. [Google Scholar] [CrossRef]
- Hannun, Y.A.; Obeid, L.M. Sphingolipids and their metabolism in physiology and disease. Nat. Rev. Mol. Cell Biol. 2018, 19, 175–191. [Google Scholar] [CrossRef] [PubMed]
- Ogretmen, B. Sphingolipid metabolism in cancer signalling and therapy. Nat. Rev. Cancer 2018, 18, 33–50. [Google Scholar] [CrossRef] [PubMed]
- Schneider-Schaulies, J.; Schneider-Schaulies, S. Sphingolipids in viral infection. Biol. Chem. 2015, 396, 585–595. [Google Scholar] [CrossRef] [PubMed]
- Lang, J.; Bohn, P.; Bhat, H.; Jastrow, H.; Walkenfort, B.; Cansiz, F.; Fink, J.; Bauer, M.; Olszewski, D.; Ramos-Nascimento, A.; et al. Acid ceramidase of macrophages traps herpes simplex virus in multivesicular bodies and protects from severe disease. Nat. Commun. 2020, 11, 1338. [Google Scholar] [CrossRef] [PubMed]
- Solger, F.; Kunz, T.C.; Fink, J.; Paprotka, K.; Pfister, P.; Hagen, F.; Schumacher, F.; Kleuser, B.; Seibel, J.; Rudel, T. A Role of Sphingosine in the Intracellular Survival of Neisseria gonorrhoeae. Front. Cell Infect. Microbiol. 2020, 10, 215. [Google Scholar] [CrossRef] [PubMed]
- Pata, M.O.; Hannun, Y.A.; Ng, C.K. Plant sphingolipids: Decoding the enigma of the Sphinx. New Phytol. 2010, 185, 611–630. [Google Scholar] [CrossRef]
- Utermohlen, O.; Herz, J.; Schramm, M.; Kronke, M. Fusogenicity of membranes: The impact of acid sphingomyelinase on innate immune responses. Immunobiology 2008, 213, 307–314. [Google Scholar] [CrossRef]
- Piper, R.C.; Katzmann, D.J. Biogenesis and function of multivesicular bodies. Annu. Rev. Cell Dev. Biol. 2007, 23, 519–547. [Google Scholar] [CrossRef]
- Trajkovic, K.; Hsu, C.; Chiantia, S.; Rajendran, L.; Wenzel, D.; Wieland, F.; Schwille, P.; Brugger, B.; Simons, M. Ceramide triggers budding of exosome vesicles into multivesicular endosomes. Science 2008, 319, 1244–1247. [Google Scholar] [CrossRef]
- Ohanian, J.; Ohanian, V. Sphingolipids in mammalian cell signalling. Cell Mol. Life Sci. 2001, 58, 2053–2068. [Google Scholar] [CrossRef]
- Spiegel, S.; Merrill, A.H., Jr. Sphingolipid metabolism and cell growth regulation. FASEB J. 1996, 10, 1388–1397. [Google Scholar] [CrossRef]
- Tirodkar, T.S.; Voelkel-Johnson, C. Sphingolipids in apoptosis. Exp. Oncol. 2012, 34, 231–242. [Google Scholar]
- Hannun, Y.A.; Obeid, L.M. Principles of bioactive lipid signalling: Lessons from sphingolipids. Nat. Rev. Mol. Cell Biol. 2008, 9, 139–150. [Google Scholar] [CrossRef] [PubMed]
- Gault, C.R.; Obeid, L.M.; Hannun, Y.A. An overview of sphingolipid metabolism: From synthesis to breakdown. Adv. Exp. Med. Biol. 2010, 688, 1–23. [Google Scholar] [CrossRef] [PubMed]
- Schloer, S.; Brunotte, L.; Goretzko, J.; Mecate-Zambrano, A.; Korthals, N.; Gerke, V.; Ludwig, S.; Rescher, U. Targeting the endolysosomal host-SARS-CoV-2 interface by clinically licensed functional inhibitors of acid sphingomyelinase (FIASMA) including the antidepressant fluoxetine. Emerg. Microbes Infect. 2020, 9, 2245–2255. [Google Scholar] [CrossRef] [PubMed]
- Carpinteiro, A.; Edwards, M.J.; Hoffmann, M.; Kochs, G.; Gripp, B.; Weigang, S.; Adams, C.; Carpinteiro, E.; Gulbins, A.; Keitsch, S.; et al. Pharmacological Inhibition of Acid Sphingomyelinase Prevents Uptake of SARS-CoV-2 by Epithelial Cells. Cell Rep. Med. 2020, 1, 100142. [Google Scholar] [CrossRef]
- Kornhuber, J.; Hoertel, N.; Gulbins, E. The acid sphingomyelinase/ceramide system in COVID-19. Mol. Psychiatry 2021, 27, 307–314. [Google Scholar] [CrossRef] [PubMed]
- Carpinteiro, A.; Gripp, B.; Hoffmann, M.; Pohlmann, S.; Hoertel, N.; Edwards, M.J.; Kamler, M.; Kornhuber, J.; Becker, K.A.; Gulbins, E. Inhibition of acid sphingomyelinase by ambroxol prevents SARS-CoV-2 entry into epithelial cells. J. Biol. Chem. 2021, 296, 100701. [Google Scholar] [CrossRef]
- Geiger, N.; Kersting, L.; Schlegel, J.; Stelz, L.; Fahr, S.; Diesendorf, V.; Roll, V.; Sostmann, M.; Konig, E.M.; Reinhard, S.; et al. The Acid Ceramidase Is a SARS-CoV-2 Host Factor. Cells 2022, 11, 2532. [Google Scholar] [CrossRef]
- Gotz, R.; Kunz, T.C.; Fink, J.; Solger, F.; Schlegel, J.; Seibel, J.; Kozjak-Pavlovic, V.; Rudel, T.; Sauer, M. Nanoscale imaging of bacterial infections by sphingolipid expansion microscopy. Nat. Commun. 2020, 11, 6173. [Google Scholar] [CrossRef]
- Rensen, E.; Pietropaoli, S.; Mueller, F.; Weber, C.; Souquere, S.; Sommer, S.; Isnard, P.; Rabant, M.; Gibier, J.B.; Terzi, F.; et al. Sensitive visualization of SARS-CoV-2 RNA with CoronaFISH. Life Sci. Alliance 2022, 5, e202101124. [Google Scholar] [CrossRef] [PubMed]
- Ghosh, S.; Dellibovi-Ragheb, T.A.; Kerviel, A.; Pak, E.; Qiu, Q.; Fisher, M.; Takvorian, P.M.; Bleck, C.; Hsu, V.W.; Fehr, A.R.; et al. beta-Coronaviruses Use Lysosomes for Egress Instead of the Biosynthetic Secretory Pathway. Cell 2020, 183, 1520–1535. [Google Scholar] [CrossRef] [PubMed]
- Gorshkov, K.; Chen, C.Z.; Bostwick, R.; Rasmussen, L.; Tran, B.N.; Cheng, Y.S.; Xu, M.; Pradhan, M.; Henderson, M.; Zhu, W.; et al. The SARS-CoV-2 Cytopathic Effect Is Blocked by Lysosome Alkalizing Small Molecules. ACS Infect. Dis. 2021, 7, 1389–1408. [Google Scholar] [CrossRef] [PubMed]
- Bolte, S.; Cordelieres, F.P. A guided tour into subcellular colocalization analysis in light microscopy. J. Microsc. 2006, 224, 213–232. [Google Scholar] [CrossRef] [PubMed]
- Zimniak, M.; Kirschner, L.; Hilpert, H.; Geiger, N.; Danov, O.; Oberwinkler, H.; Steinke, M.; Sewald, K.; Seibel, J.; Bodem, J. The serotonin reuptake inhibitor Fluoxetine inhibits SARS-CoV-2 in human lung tissue. Sci. Rep. 2021, 11, 5890. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Brenner, D.; Geiger, N.; Schlegel, J.; Diesendorf, V.; Kersting, L.; Fink, J.; Stelz, L.; Schneider-Schaulies, S.; Sauer, M.; Bodem, J.; et al. Azido-Ceramides, a Tool to Analyse SARS-CoV-2 Replication and Inhibition—SARS-CoV-2 Is Inhibited by Ceramides. Int. J. Mol. Sci. 2023, 24, 7281. https://doi.org/10.3390/ijms24087281
Brenner D, Geiger N, Schlegel J, Diesendorf V, Kersting L, Fink J, Stelz L, Schneider-Schaulies S, Sauer M, Bodem J, et al. Azido-Ceramides, a Tool to Analyse SARS-CoV-2 Replication and Inhibition—SARS-CoV-2 Is Inhibited by Ceramides. International Journal of Molecular Sciences. 2023; 24(8):7281. https://doi.org/10.3390/ijms24087281
Chicago/Turabian StyleBrenner, Daniela, Nina Geiger, Jan Schlegel, Viktoria Diesendorf, Louise Kersting, Julian Fink, Linda Stelz, Sibylle Schneider-Schaulies, Markus Sauer, Jochen Bodem, and et al. 2023. "Azido-Ceramides, a Tool to Analyse SARS-CoV-2 Replication and Inhibition—SARS-CoV-2 Is Inhibited by Ceramides" International Journal of Molecular Sciences 24, no. 8: 7281. https://doi.org/10.3390/ijms24087281
APA StyleBrenner, D., Geiger, N., Schlegel, J., Diesendorf, V., Kersting, L., Fink, J., Stelz, L., Schneider-Schaulies, S., Sauer, M., Bodem, J., & Seibel, J. (2023). Azido-Ceramides, a Tool to Analyse SARS-CoV-2 Replication and Inhibition—SARS-CoV-2 Is Inhibited by Ceramides. International Journal of Molecular Sciences, 24(8), 7281. https://doi.org/10.3390/ijms24087281





