Recent Insight into the Role of Sphingosine-1-Phosphate Lyase in Neurodegeneration
Abstract
1. Introduction
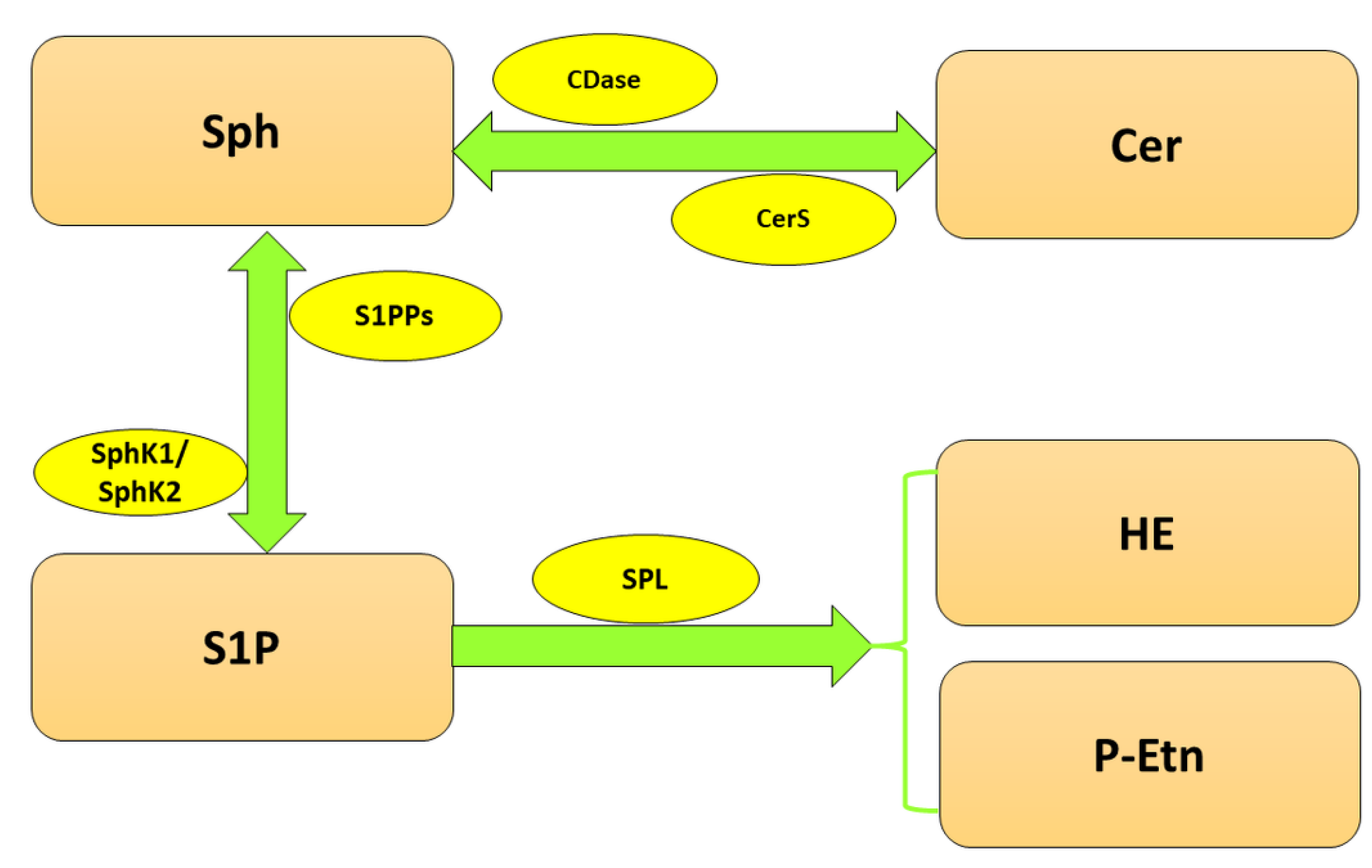
2. SPL in the Nervous System
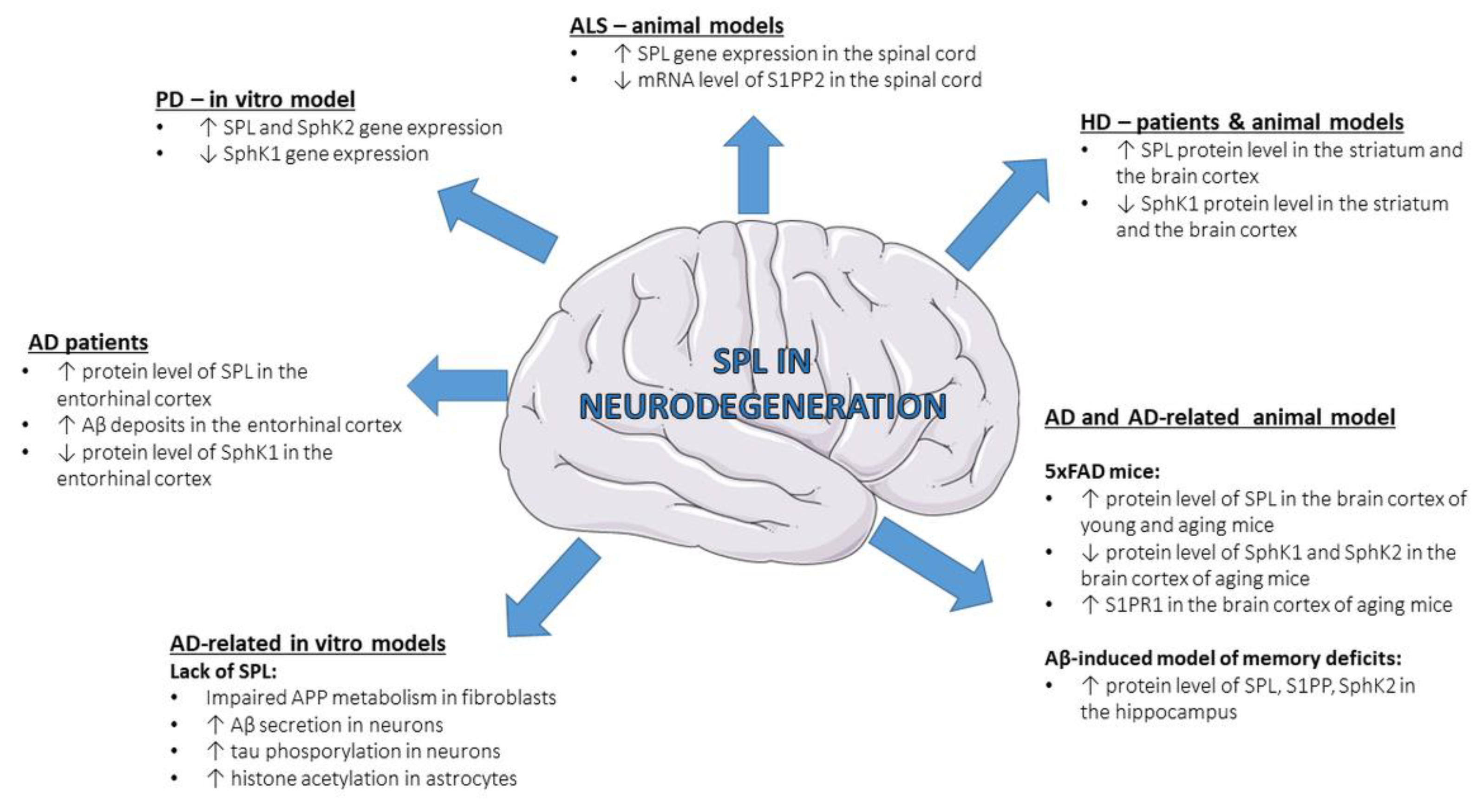
3. SPL in Neurodegenerative Diseases
3.1. Alzheimer’s Disease
3.2. Parkinson’s Disease
3.3. Huntington’s Disease
3.4. Amyotrophic Lateral Sclerosis
4. SPL in Neuroinflammation
5. SPL Inhibitors
5.1. Sphingosine Analogues
5.1.1. Fingolimod
5.1.2. Other Sphingosine Analogues
5.2. Non-Lipidic Direct Inhibitors of SPL
5.3. Functional Antagonists
5.3.1. 4-Deoxypyridoxine
5.3.2. 2-Acetyl-4(5)-(1,2,3,4-tetrahydroxybutyl) Imidazole
5.3.3. LX2931 and LX2932
6. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Hannun, Y.A.; Obeid, L.M. Principles of Bioactive Lipid Signalling: Lessons from Sphingolipids. Nat. Rev. Mol. Cell Biol. 2008, 9, 139–150. [Google Scholar] [CrossRef] [PubMed]
- Strub, G.M.; Maceyka, M.; Hait, N.C.; Milstien, S.; Spiegel, S. Extracellular and Intracellular Actions of Sphingosine-1-Phosphate. Adv. Exp. Med. Biol. 2010, 688, 141–155. [Google Scholar] [CrossRef] [PubMed]
- Donati, C.; Meacci, E.; Nuti, F.; Becciolini, L.; Farnararo, M.; Bruni, P. Sphingosine 1-Phosphate Regulates Myogenic Differentiation: A Major Role for S1P2 Receptor. FASEB J. 2005, 19, 449–451. [Google Scholar] [CrossRef]
- Donati, C.; Cencetti, F.; Nincheri, P.; Bernacchioni, C.; Brunelli, S.; Clementi, E.; Cossu, G.; Bruni, P. Sphingosine 1-Phosphate Mediates Proliferation and Survival of Mesoangioblasts. Stem Cells 2007, 25, 1713–1719. [Google Scholar] [CrossRef]
- Calise, S.; Blescia, S.; Cencetti, F.; Bernacchioni, C.; Donati, C.; Bruni, P. Sphingosine 1-Phosphate Stimulates Proliferation and Migration of Satellite Cells: Role of S1P Receptors. Biochim. Biophys. Acta 2012, 1823, 439–450. [Google Scholar] [CrossRef] [PubMed]
- Hait, N.C.; Allegood, J.; Maceyka, M.; Strub, G.M.; Harikumar, K.B.; Singh, S.K.; Luo, C.; Marmorstein, R.; Kordula, T.; Milstien, S.; et al. Regulation of Histone Acetylation in the Nucleus by Sphingosine-1-Phosphate. Science 2009, 325, 1254–1257. [Google Scholar] [CrossRef] [PubMed]
- Lépine, S.; Allegood, J.C.; Park, M.; Dent, P.; Milstien, S.; Spiegel, S. Sphingosine-1-Phosphate Phosphohydrolase-1 Regulates ER Stress-Induced Autophagy. Cell Death Differ. 2011, 18, 350–361. [Google Scholar] [CrossRef]
- Strub, G.M.; Paillard, M.; Liang, J.; Gomez, L.; Allegood, J.C.; Hait, N.C.; Maceyka, M.; Price, M.M.; Chen, Q.; Simpson, D.C.; et al. Sphingosine-1-Phosphate Produced by Sphingosine Kinase 2 in Mitochondria Interacts with Prohibitin 2 to Regulate Complex IV Assembly and Respiration. FASEB J. 2011, 25, 600–612. [Google Scholar] [CrossRef]
- Shen, Z.; Liu, C.; Liu, P.; Zhao, J.; Xu, W. Sphingosine 1-Phosphate (S1P) Promotes Mitochondrial Biogenesis in Hep G2 Cells by Activating Peroxisome Proliferator-Activated Receptor γ Coactivator 1α (PGC-1α). Cell Stress Chaperones 2014, 19, 541–548. [Google Scholar] [CrossRef]
- Diaz Escarcega, R.; McCullough, L.D.; Tsvetkov, A.S. The Functional Role of Sphingosine Kinase 2. Front. Mol. Biosci. 2021, 8, 683767. [Google Scholar] [CrossRef]
- Dawoody Nejad, L.; Stumpe, M.; Rauch, M.; Hemphill, A.; Schneiter, R.; Bütikofer, P.; Serricchio, M. Mitochondrial Sphingosine-1-Phosphate Lyase Is Essential for Phosphatidylethanolamine Synthesis and Survival of Trypanosoma Brucei. Sci. Rep. 2020, 10, 8268. [Google Scholar] [CrossRef] [PubMed]
- Di Pardo, A.; Maglione, V. Sphingolipid Metabolism: A New Therapeutic Opportunity for Brain Degenerative Disorders. Front. Neurosci. 2018, 12, 249. [Google Scholar] [CrossRef] [PubMed]
- Ebenezer, D.L.; Ramchandran, R.; Fu, P.; Mangio, L.A.; Suryadevara, V.; Ha, A.W.; Berdyshev, E.; Van Veldhoven, P.P.; Kron, S.J.; Schumacher, F.; et al. Nuclear Sphingosine-1-Phosphate Lyase Generated ∆2-Hexadecenal Is A Regulator of HDAC Activity and Chromatin Remodeling in Lung Epithelial Cells. Cell Biochem. Biophys. 2021, 79, 575–592. [Google Scholar] [CrossRef] [PubMed]
- Ikeda, M.; Kihara, A.; Igarashi, Y. Sphingosine-1-Phosphate Lyase SPL Is an Endoplasmic Reticulum-Resident, Integral Membrane Protein with the Pyridoxal 5’-Phosphate Binding Domain Exposed to the Cytosol. Biochem. Biophys. Res. Commun. 2004, 325, 338–343. [Google Scholar] [CrossRef] [PubMed]
- Choi, Y.-J.; Saba, J.D. Sphingosine Phosphate Lyase Insufficiency Syndrome (SPLIS): A Novel Inborn Error of Sphingolipid Metabolism. Adv. Biol. Regul. 2019, 71, 128–140. [Google Scholar] [CrossRef]
- Van Veldhoven, P.P.; Gijsbers, S.; Mannaerts, G.P.; Vermeesch, J.R.; Brys, V. Human Sphingosine-1-Phosphate Lyase: CDNA Cloning, Functional Expression Studies and Mapping to Chromosome 10q22(1). Biochim. Biophys. Acta 2000, 1487, 128–134. [Google Scholar] [CrossRef]
- Kumar, A.; Saba, J.D. Lyase to Live by: Sphingosine Phosphate Lyase as a Therapeutic Target. Expert Opin. Ther. Targets 2009, 13, 1013–1025. [Google Scholar] [CrossRef]
- Bourquin, F.; Riezman, H.; Capitani, G.; Grütter, M.G. Structure and Function of Sphingosine-1-Phosphate Lyase, a Key Enzyme of Sphingolipid Metabolism. Structure 2010, 18, 1054–1065. [Google Scholar] [CrossRef]
- Weiler, S.; Braendlin, N.; Beerli, C.; Bergsdorf, C.; Schubart, A.; Srinivas, H.; Oberhauser, B.; Billich, A. Orally Active 7-Substituted (4-Benzylphthalazin-1-Yl)-2-Methylpiperazin-1-Yl]Nicotinonitriles as Active-Site Inhibitors of Sphingosine 1-Phosphate Lyase for the Treatment of Multiple Sclerosis. J. Med. Chem. 2014, 57, 5074–5084. [Google Scholar] [CrossRef]
- Tissue Expression of SGPL1—Summary—The Human Protein Atlas. Available online: https://www.proteinatlas.org/ENSG00000166224-SGPL1/tissue (accessed on 25 February 2023).
- Schmahl, J.; Raymond, C.S.; Soriano, P. PDGF Signaling Specificity Is Mediated through Multiple Immediate Early Genes. Nat. Genet. 2007, 39, 52–60. [Google Scholar] [CrossRef]
- Taylor, V.A.; Stone, H.K.; Schuh, M.P.; Zhao, X.; Setchell, K.D.; Erkan, E. Disarranged Sphingolipid Metabolism from Sphingosine-1-Phosphate Lyase Deficiency Leads to Congenital Nephrotic Syndrome. Kidney Int. Rep. 2019, 4, 1763–1769. [Google Scholar] [CrossRef] [PubMed]
- Janecke, A.R.; Xu, R.; Steichen-Gersdorf, E.; Waldegger, S.; Entenmann, A.; Giner, T.; Krainer, I.; Huber, L.A.; Hess, M.W.; Frishberg, Y.; et al. Deficiency of the Sphingosine-1-Phosphate Lyase SGPL1 Is Associated with Congenital Nephrotic Syndrome and Congenital Adrenal Calcifications. Hum. Mutat. 2017, 38, 365–372. [Google Scholar] [CrossRef]
- Lovric, S.; Goncalves, S.; Gee, H.Y.; Oskouian, B.; Srinivas, H.; Choi, W.-I.; Shril, S.; Ashraf, S.; Tan, W.; Rao, J.; et al. Mutations in Sphingosine-1-Phosphate Lyase Cause Nephrosis with Ichthyosis and Adrenal Insufficiency. J. Clin. Investig. 2017, 127, 912–928. [Google Scholar] [CrossRef] [PubMed]
- Prasad, R.; Hadjidemetriou, I.; Maharaj, A.; Meimaridou, E.; Buonocore, F.; Saleem, M.; Hurcombe, J.; Bierzynska, A.; Barbagelata, E.; Bergadá, I.; et al. Sphingosine-1-Phosphate Lyase Mutations Cause Primary Adrenal Insufficiency and Steroid-Resistant Nephrotic Syndrome. J. Clin. Investig. 2017, 127, 942–953. [Google Scholar] [CrossRef] [PubMed]
- Bamborschke, D.; Pergande, M.; Becker, K.; Koerber, F.; Dötsch, J.; Vierzig, A.; Weber, L.T.; Cirak, S. A Novel Mutation in Sphingosine-1-Phosphate Lyase Causing Congenital Brain Malformation. Brain Dev. 2018, 40, 480–483. [Google Scholar] [CrossRef]
- Linhares, N.D.; Arantes, R.R.; Araujo, S.A.; Pena, S.D.J. Nephrotic Syndrome and Adrenal Insufficiency Caused by a Variant in SGPL1. Clin. Kidney J. 2018, 11, 462–467. [Google Scholar] [CrossRef]
- Maharaj, A.; Theodorou, D.; Banerjee, I.I.; Metherell, L.A.; Prasad, R.; Wallace, D. A Sphingosine-1-Phosphate Lyase Mutation Associated with Congenital Nephrotic Syndrome and Multiple Endocrinopathy. Front. Pediatr. 2020, 8, 151. [Google Scholar] [CrossRef]
- Maharaj, A.; Williams, J.; Bradshaw, T.; Güran, T.; Braslavsky, D.; Casas, J.; Chan, L.F.; Metherell, L.A.; Prasad, R. Sphingosine-1-Phosphate Lyase (SGPL1) Deficiency Is Associated with Mitochondrial Dysfunction. J. Steroid Biochem. Mol. Biol. 2020, 202, 105730. [Google Scholar] [CrossRef]
- Bektas, M.; Allende, M.L.; Lee, B.G.; Chen, W.; Amar, M.J.; Remaley, A.T.; Saba, J.D.; Proia, R.L. Sphingosine 1-Phosphate Lyase Deficiency Disrupts Lipid Homeostasis in Liver. J. Biol. Chem. 2010, 285, 10880–10889. [Google Scholar] [CrossRef]
- Vienken, H.; Mabrouki, N.; Grabau, K.; Claas, R.F.; Rudowski, A.; Schömel, N.; Pfeilschifter, J.; Lütjohann, D.; van Echten-Deckert, G.; Meyer Zu Heringdorf, D. Characterization of Cholesterol Homeostasis in Sphingosine-1-Phosphate Lyase-Deficient Fibroblasts Reveals a Niemann-Pick Disease Type C-like Phenotype with Enhanced Lysosomal Ca2+ Storage. Sci. Rep. 2017, 7, 43575. [Google Scholar] [CrossRef]
- Brizuela, L.; Ader, I.; Mazerolles, C.; Bocquet, M.; Malavaud, B.; Cuvillier, O. First Evidence of Sphingosine 1-Phosphate Lyase Protein Expression and Activity Downregulation in Human Neoplasm: Implication for Resistance to Therapeutics in Prostate Cancer. Mol. Cancer Ther. 2012, 11, 1841–1851. [Google Scholar] [CrossRef]
- Oskouian, B.; Sooriyakumaran, P.; Borowsky, A.D.; Crans, A.; Dillard-Telm, L.; Tam, Y.Y.; Bandhuvula, P.; Saba, J.D. Sphingosine-1-Phosphate Lyase Potentiates Apoptosis via P53- and P38-Dependent Pathways and Is Down-Regulated in Colon Cancer. Proc. Natl. Acad. Sci. USA 2006, 103, 17384–17389. [Google Scholar] [CrossRef]
- Colié, S.; Van Veldhoven, P.P.; Kedjouar, B.; Bedia, C.; Albinet, V.; Sorli, S.-C.; Garcia, V.; Djavaheri-Mergny, M.; Bauvy, C.; Codogno, P.; et al. Disruption of Sphingosine 1-Phosphate Lyase Confers Resistance to Chemotherapy and Promotes Oncogenesis through Bcl-2/Bcl-XL Upregulation. Cancer Res. 2009, 69, 9346–9353. [Google Scholar] [CrossRef] [PubMed]
- Kumar, A.; Byun, H.-S.; Bittman, R.; Saba, J.D. The Sphingolipid Degradation Product Trans-2-Hexadecenal Induces Cytoskeletal Reorganization and Apoptosis in a JNK-Dependent Manner. Cell. Signal. 2011, 23, 1144–1152. [Google Scholar] [CrossRef]
- Cohen, D.T.; Wales, T.E.; McHenry, M.W.; Engen, J.R.; Walensky, L.D. Site-Dependent Cysteine Lipidation Potentiates the Activation of Proapoptotic BAX. Cell Rep. 2020, 30, 3229–3239.e6. [Google Scholar] [CrossRef]
- Upadhyaya, P.; Kumar, A.; Byun, H.-S.; Bittman, R.; Saba, J.D.; Hecht, S.S. The Sphingolipid Degradation Product Trans-2-Hexadecenal Forms Adducts with DNA. Biochem. Biophys. Res. Commun. 2012, 424, 18–21. [Google Scholar] [CrossRef]
- Schumacher, F.; Neuber, C.; Finke, H.; Nieschalke, K.; Baesler, J.; Gulbins, E.; Kleuser, B. The Sphingosine 1-Phosphate Breakdown Product, (2E)-Hexadecenal, Forms Protein Adducts and Glutathione Conjugates In Vitro. J. Lipid Res. 2017, 58, 1648–1660. [Google Scholar] [CrossRef]
- Shadyro, O.; Lisovskaya, A.; Semenkova, G.; Edimecheva, I.; Amaegberi, N. Free-Radical Destruction of Sphingolipids Resulting in 2-Hexadecenal Formation. Lipid Insights 2015, 8, 1–9. [Google Scholar] [CrossRef]
- Semenkova, G.N.; Amaegberi, N.V.; Lisovskaya, A.G.; Pinchuk, S.V.; Poleshko, A.G.; Shadyro, O.I. 2-Hexadecenal Regulates ROS Production and Induces Apoptosis in Polymorphonuclear Leucocytes. Cell Biochem. Biophys. 2022, 81, 77–86. [Google Scholar] [CrossRef] [PubMed]
- Kennedy, E.P.; Weiss, S.B. The Function of Cytidine Coenzymes in the Biosynthesis of Phospholipides. J. Biol. Chem. 1956, 222, 193–214. [Google Scholar] [CrossRef] [PubMed]
- Zhang, K.; Pompey, J.M.; Hsu, F.-F.; Key, P.; Bandhuvula, P.; Saba, J.D.; Turk, J.; Beverley, S.M. Redirection of Sphingolipid Metabolism toward de Novo Synthesis of Ethanolamine in Leishmania. EMBO J. 2007, 26, 1094–1104. [Google Scholar] [CrossRef] [PubMed]
- Meng, H.; Lee, V.M. Differential Expression of Sphingosine-1-Phosphate Receptors 1-5 in the Developing Nervous System. Dev. Dyn. 2009, 238, 487–500. [Google Scholar] [CrossRef] [PubMed]
- Edsall, L.C.; Pirianov, G.G.; Spiegel, S. Involvement of Sphingosine 1-Phosphate in Nerve Growth Factor-Mediated Neuronal Survival and Differentiation. J. Neurosci. 1997, 17, 6952–6960. [Google Scholar] [CrossRef]
- Meng, H.; Yuan, Y.; Lee, V.M. Loss of Sphingosine Kinase 1/S1P Signaling Impairs Cell Growth and Survival of Neurons and Progenitor Cells in the Developing Sensory Ganglia. PLoS ONE 2011, 6, e27150. [Google Scholar] [CrossRef]
- Harada, J.; Foley, M.; Moskowitz, M.A.; Waeber, C. Sphingosine-1-Phosphate Induces Proliferation and Morphological Changes of Neural Progenitor Cells. J. Neurochem. 2004, 88, 1026–1039. [Google Scholar] [CrossRef]
- Ye, Y.; Zhao, Z.; Xu, H.; Zhang, X.; Su, X.; Yang, Y.; Yu, X.; He, X. Activation of Sphingosine 1-Phosphate Receptor 1 Enhances Hippocampus Neurogenesis in a Rat Model of Traumatic Brain Injury: An Involvement of MEK/Erk Signaling Pathway. Neural Plast. 2016, 2016, 8072156. [Google Scholar] [CrossRef]
- Toman, R.E.; Payne, S.G.; Watterson, K.R.; Maceyka, M.; Lee, N.H.; Milstien, S.; Bigbee, J.W.; Spiegel, S. Differential Transactivation of Sphingosine-1-Phosphate Receptors Modulates NGF-Induced Neurite Extension. J. Cell Biol. 2004, 166, 381–392. [Google Scholar] [CrossRef]
- Zhang, Y.H.; Vasko, M.R.; Nicol, G.D. Intracellular Sphingosine 1-Phosphate Mediates the Increased Excitability Produced by Nerve Growth Factor in Rat Sensory Neurons. J. Physiol. 2006, 575, 101–113. [Google Scholar] [CrossRef]
- Mizugishi, K.; Yamashita, T.; Olivera, A.; Miller, G.F.; Spiegel, S.; Proia, R.L. Essential Role for Sphingosine Kinases in Neural and Vascular Development. Mol. Cell. Biol. 2005, 25, 11113–11121. [Google Scholar] [CrossRef] [PubMed]
- Kimura, A.; Ohmori, T.; Ohkawa, R.; Madoiwa, S.; Mimuro, J.; Murakami, T.; Kobayashi, E.; Hoshino, Y.; Yatomi, Y.; Sakata, Y. Essential Roles of Sphingosine 1-Phosphate/S1P1 Receptor Axis in the Migration of Neural Stem Cells toward a Site of Spinal Cord Injury. Stem Cells 2007, 25, 115–124. [Google Scholar] [CrossRef]
- Tran, C.; Heng, B.; Teo, J.D.; Humphrey, S.J.; Qi, Y.; Couttas, T.A.; Stefen, H.; Brettle, M.; Fath, T.; Guillemin, G.J.; et al. Sphingosine 1-Phosphate but Not Fingolimod Protects Neurons against Excitotoxic Cell Death by Inducing Neurotrophic Gene Expression in Astrocytes. J. Neurochem. 2020, 153, 173–188. [Google Scholar] [CrossRef] [PubMed]
- Moore, A.N.; Kampfl, A.W.; Zhao, X.; Hayes, R.L.; Dash, P.K. Sphingosine-1-Phosphate Induces Apoptosis of Cultured Hippocampal Neurons That Requires Protein Phosphatases and Activator Protein-1 Complexes. Neuroscience 1999, 94, 405–415. [Google Scholar] [CrossRef] [PubMed]
- Hagen, N.; Hans, M.; Hartmann, D.; Swandulla, D.; van Echten-Deckert, G. Sphingosine-1-Phosphate Links Glycosphingolipid Metabolism to Neurodegeneration via a Calpain-Mediated Mechanism. Cell Death Differ. 2011, 18, 1356–1365. [Google Scholar] [CrossRef]
- Atkinson, D.; Nikodinovic Glumac, J.; Asselbergh, B.; Ermanoska, B.; Blocquel, D.; Steiner, R.; Estrada-Cuzcano, A.; Peeters, K.; Ooms, T.; De Vriendt, E.; et al. Sphingosine 1-Phosphate Lyase Deficiency Causes Charcot-Marie-Tooth Neuropathy. Neurology 2017, 88, 533–542. [Google Scholar] [CrossRef] [PubMed]
- Mitroi, D.N.; Deutschmann, A.U.; Raucamp, M.; Karunakaran, I.; Glebov, K.; Hans, M.; Walter, J.; Saba, J.; Gräler, M.; Ehninger, D.; et al. Sphingosine 1-Phosphate Lyase Ablation Disrupts Presynaptic Architecture and Function via an Ubiquitin- Proteasome Mediated Mechanism. Sci. Rep. 2016, 6, 37064. [Google Scholar] [CrossRef]
- Hagen, N.; Van Veldhoven, P.P.; Proia, R.L.; Park, H.; Merrill, A.H., Jr.; van Echten-Deckert, G. Subcellular Origin of Sphingosine 1-Phosphate Is Essential for Its Toxic Effect in Lyase-Deficient Neurons. J. Biol. Chem. 2009, 284, 11346–11353. [Google Scholar] [CrossRef]
- Moruno-Manchon, J.F.; Uzor, N.-E.; Blasco-Conesa, M.P.; Mannuru, S.; Putluri, N.; Furr-Stimming, E.E.; Tsvetkov, A.S. Inhibiting Sphingosine Kinase 2 Mitigates Mutant Huntingtin-Induced Neurodegeneration in Neuron Models of Huntington Disease. Hum. Mol. Genet. 2017, 26, 1305–1317. [Google Scholar] [CrossRef]
- Komatsu, M.; Waguri, S.; Chiba, T.; Murata, S.; Iwata, J.; Tanida, I.; Ueno, T.; Koike, M.; Uchiyama, Y.; Kominami, E.; et al. Loss of Autophagy in the Central Nervous System Causes Neurodegeneration in Mice. Nature 2006, 441, 880–884. [Google Scholar] [CrossRef]
- Wu, Y.; Li, X.; Zhu, J.X.; Xie, W.; Le, W.; Fan, Z.; Jankovic, J.; Pan, T. Resveratrol-Activated AMPK/SIRT1/Autophagy in Cellular Models of Parkinson’s Disease. Neurosignals 2011, 19, 163–174. [Google Scholar] [CrossRef]
- Yang, D.-S.; Stavrides, P.; Mohan, P.S.; Kaushik, S.; Kumar, A.; Ohno, M.; Schmidt, S.D.; Wesson, D.; Bandyopadhyay, U.; Jiang, Y.; et al. Reversal of Autophagy Dysfunction in the TgCRND8 Mouse Model of Alzheimer’s Disease Ameliorates Amyloid Pathologies and Memory Deficits. Brain 2011, 134, 258–277. [Google Scholar] [CrossRef]
- Hara, T.; Nakamura, K.; Matsui, M.; Yamamoto, A.; Nakahara, Y.; Suzuki-Migishima, R.; Yokoyama, M.; Mishima, K.; Saito, I.; Okano, H.; et al. Suppression of Basal Autophagy in Neural Cells Causes Neurodegenerative Disease in Mice. Nature 2006, 441, 885–889. [Google Scholar] [CrossRef]
- Moruno Manchon, J.F.; Uzor, N.-E.; Dabaghian, Y.; Furr-Stimming, E.E.; Finkbeiner, S.; Tsvetkov, A.S. Cytoplasmic Sphingosine-1-Phosphate Pathway Modulates Neuronal Autophagy. Sci. Rep. 2015, 5, 15213. [Google Scholar] [CrossRef] [PubMed]
- Mitroi, D.N.; Karunakaran, I.; Gräler, M.; Saba, J.D.; Ehninger, D.; Ledesma, M.D.; van Echten-Deckert, G. SGPL1 (Sphingosine Phosphate Lyase 1) Modulates Neuronal Autophagy via Phosphatidylethanolamine Production. Autophagy 2017, 13, 885–899. [Google Scholar] [CrossRef] [PubMed]
- Tanida, I.; Ueno, T.; Kominami, E. LC3 and Autophagy. Methods Mol. Biol. 2008, 445, 77–88. [Google Scholar] [CrossRef]
- Rubinsztein, D.C.; Shpilka, T.; Elazar, Z. Mechanisms of Autophagosome Biogenesis. Curr. Biol. 2012, 22, R29–R34. [Google Scholar] [CrossRef] [PubMed]
- Friedman, L.G.; Lachenmayer, M.L.; Wang, J.; He, L.; Poulose, S.M.; Komatsu, M.; Holstein, G.R.; Yue, Z. Disrupted Autophagy Leads to Dopaminergic Axon and Dendrite Degeneration and Promotes Presynaptic Accumulation of α-Synuclein and LRRK2 in the Brain. J. Neurosci. 2012, 32, 7585–7593. [Google Scholar] [CrossRef]
- Dominguez, G.; Maddelein, M.-L.; Pucelle, M.; Nicaise, Y.; Maurage, C.-A.; Duyckaerts, C.; Cuvillier, O.; Delisle, M.-B. Neuronal Sphingosine Kinase 2 Subcellular Localization Is Altered in Alzheimer’s Disease Brain. Acta Neuropathol. Commun. 2018, 6, 25. [Google Scholar] [CrossRef]
- Jęśko, H.; Wencel, P.L.; Lukiw, W.J.; Strosznajder, R.P. Modulatory Effects of Fingolimod (FTY720) on the Expression of Sphingolipid Metabolism-Related Genes in an Animal Model of Alzheimer’s Disease. Mol. Neurobiol. 2019, 56, 174–185. [Google Scholar] [CrossRef] [PubMed]
- Lei, M.; Teo, J.D.; Song, H.; McEwen, H.P.; Yup Lee, J.; Couttas, T.A.; Duncan, T.; Chesworth, R.; Bertz, J.; Przybyla, M.; et al. Sphingosine Kinase 2 Potentiates Amyloid Deposition but Protects against Hippocampal Volume Loss and Demyelination in a Mouse Model of Alzheimer’s Disease. J. Neurosci. 2019, 39, 9645–9659. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Yang, G. Bone Marrow Mesenchymal Stem Cells-Derived Exosomes Reduce Aβ Deposition and Improve Cognitive Function Recovery in Mice with Alzheimer’s Disease by Activating Sphingosine Kinase/Sphingosine-1-Phosphate Signaling Pathway. Cell Biol. Int. 2021, 45, 775–784. [Google Scholar] [CrossRef]
- Motyl, J.; Przykaza, Ł.; Boguszewski, P.M.; Kosson, P.; Strosznajder, J.B. Pramipexole and Fingolimod Exert Neuroprotection in a Mouse Model of Parkinson’s Disease by Activation of Sphingosine Kinase 1 and Akt Kinase. Neuropharmacology 2018, 135, 139–150. [Google Scholar] [CrossRef] [PubMed]
- Pépin, É.; Jalinier, T.; Lemieux, G.L.; Massicotte, G.; Cyr, M. Sphingosine-1-Phosphate Receptors Modulators Decrease Signs of Neuroinflammation and Prevent Parkinson’s Disease Symptoms in the 1-Methyl-4-Phenyl-1,2,3,6-Tetrahydropyridine Mouse Model. Front. Pharmacol. 2020, 11, 77. [Google Scholar] [CrossRef] [PubMed]
- Schwedhelm, E.; Englisch, C.; Niemann, L.; Lezius, S.; von Lucadou, M.; Marmann, K.; Böger, R.; Peine, S.; Daum, G.; Gerloff, C.; et al. Sphingosine-1-Phosphate, Motor Severity, and Progression in Parkinson’s Disease (MARK-PD). Mov. Disord. 2021, 36, 2178–2182. [Google Scholar] [CrossRef] [PubMed]
- Pirhaji, L.; Milani, P.; Leidl, M.; Curran, T.; Avila-Pacheco, J.; Clish, C.B.; White, F.M.; Saghatelian, A.; Fraenkel, E. Revealing Disease-Associated Pathways by Network Integration of Untargeted Metabolomics. Nat. Methods 2016, 13, 770–776. [Google Scholar] [CrossRef]
- Di Pardo, A.; Basit, A.; Armirotti, A.; Amico, E.; Castaldo, S.; Pepe, G.; Marracino, F.; Buttari, F.; Digilio, A.F.; Maglione, V. De Novo Synthesis of Sphingolipids Is Defective in Experimental Models of Huntington’s Disease. Front. Neurosci. 2017, 11, 698. [Google Scholar] [CrossRef]
- Di Pardo, A.; Amico, E.; Basit, A.; Armirotti, A.; Joshi, P.; Neely, M.D.; Vuono, R.; Castaldo, S.; Digilio, A.F.; Scalabrì, F.; et al. Defective Sphingosine-1-Phosphate Metabolism Is a Druggable Target in Huntington’s Disease. Sci. Rep. 2017, 7, 5280. [Google Scholar] [CrossRef]
- Pirhaji, L.; Milani, P.; Dalin, S.; Wassie, B.T.; Dunn, D.E.; Fenster, R.J.; Avila-Pacheco, J.; Greengard, P.; Clish, C.B.; Heiman, M.; et al. Identifying Therapeutic Targets by Combining Transcriptional Data with Ordinal Clinical Measurements. Nat. Commun. 2017, 8, 623. [Google Scholar] [CrossRef]
- Di Pardo, A.; Pepe, G.; Castaldo, S.; Marracino, F.; Capocci, L.; Amico, E.; Madonna, M.; Giova, S.; Jeong, S.K.; Park, B.-M.; et al. Stimulation of Sphingosine Kinase 1 (SPHK1) Is Beneficial in a Huntington’s Disease Pre-Clinical Model. Front. Mol. Neurosci. 2019, 12, 100. [Google Scholar] [CrossRef]
- Henriques, A.; Croixmarie, V.; Bouscary, A.; Mosbach, A.; Keime, C.; Boursier-Neyret, C.; Walter, B.; Spedding, M.; Loeffler, J.-P. Sphingolipid Metabolism Is Dysregulated at Transcriptomic and Metabolic Levels in the Spinal Cord of an Animal Model of Amyotrophic Lateral Sclerosis. Front. Mol. Neurosci. 2018, 10, 433. [Google Scholar] [CrossRef] [PubMed]
- Gutner, U.A.; Shupik, M.A.; Maloshitskaya, O.A.; Sokolov, S.A.; Rezvykh, A.P.; Funikov, S.Y.; Lebedev, A.T.; Ustyugov, A.A.; Alessenko, A.V. Changes in the Metabolism of Sphingoid Bases in the Brain and Spinal Cord of Transgenic FUS(1-359) Mice, a Model of Amyotrophic Lateral Sclerosis. Biochemistry 2019, 84, 1166–1176. [Google Scholar] [CrossRef]
- Mohassel, P.; Donkervoort, S.; Lone, M.A.; Nalls, M.; Gable, K.; Gupta, S.D.; Foley, A.R.; Hu, Y.; Saute, J.A.M.; Moreira, A.L.; et al. Childhood Amyotrophic Lateral Sclerosis Caused by Excess Sphingolipid Synthesis. Nat. Med. 2021, 27, 1197–1204. [Google Scholar] [CrossRef] [PubMed]
- Zhou, J.; Tawk, M.; Tiziano, F.D.; Veillet, J.; Bayes, M.; Nolent, F.; Garcia, V.; Servidei, S.; Bertini, E.; Castro-Giner, F.; et al. Spinal Muscular Atrophy Associated with Progressive Myoclonic Epilepsy Is Caused by Mutations in ASAH1. Am. J. Hum. Genet. 2012, 91, 5–14. [Google Scholar] [CrossRef] [PubMed]
- Filosto, M.; Aureli, M.; Castellotti, B.; Rinaldi, F.; Schiumarini, D.; Valsecchi, M.; Lualdi, S.; Mazzotti, R.; Pensato, V.; Rota, S.; et al. ASAH1 Variant Causing a Mild SMA Phenotype with No Myoclonic Epilepsy: A Clinical, Biochemical and Molecular Study. Eur. J. Hum. Genet. 2016, 24, 1578–1583. [Google Scholar] [CrossRef] [PubMed]
- McKhann, G.M.; Knopman, D.S.; Chertkow, H.; Hyman, B.T.; Jack, C.R.; Kawas, C.H.; Klunk, W.E.; Koroshetz, W.J.; Manly, J.J.; Mayeux, R.; et al. The Diagnosis of Dementia Due to Alzheimer’s Disease: Recommendations from the National Institute on Aging-Alzheimer’s Association Workgroups on Diagnostic Guidelines for Alzheimer’s Disease. Alzheimers Dement. 2011, 7, 263–269. [Google Scholar] [CrossRef] [PubMed]
- Levenson, R.W.; Sturm, V.E.; Haase, C.M. Emotional and Behavioral Symptoms in Neurodegenerative Disease: A Model for Studying the Neural Bases of Psychopathology. Annu. Rev. Clin. Psychol. 2014, 10, 581–606. [Google Scholar] [CrossRef]
- Jahn, H. Memory Loss in Alzheimer’s Disease. Dialogues Clin. Neurosci. 2013, 15, 445–454. [Google Scholar] [CrossRef]
- Priller, C.; Bauer, T.; Mitteregger, G.; Krebs, B.; Kretzschmar, H.A.; Herms, J. Synapse Formation and Function Is Modulated by the Amyloid Precursor Protein. J. Neurosci. 2006, 26, 7212–7221. [Google Scholar] [CrossRef]
- Karaca, I.; Tamboli, I.Y.; Glebov, K.; Richter, J.; Fell, L.H.; Grimm, M.O.; Haupenthal, V.J.; Hartmann, T.; Gräler, M.H.; van Echten-Deckert, G.; et al. Deficiency of Sphingosine-1-Phosphate Lyase Impairs Lysosomal Metabolism of the Amyloid Precursor Protein. J. Biol. Chem. 2014, 289, 16761–16772. [Google Scholar] [CrossRef]
- Takasugi, N.; Sasaki, T.; Suzuki, K.; Osawa, S.; Isshiki, H.; Hori, Y.; Shimada, N.; Higo, T.; Yokoshima, S.; Fukuyama, T.; et al. BACE1 Activity Is Modulated by Cell-Associated Sphingosine-1-Phosphate. J. Neurosci. 2011, 31, 6850–6857. [Google Scholar] [CrossRef]
- Ceccom, J.; Loukh, N.; Lauwers-Cances, V.; Touriol, C.; Nicaise, Y.; Gentil, C.; Uro-Coste, E.; Pitson, S.; Maurage, C.A.; Duyckaerts, C.; et al. Reduced Sphingosine Kinase-1 and Enhanced Sphingosine 1-Phosphate Lyase Expression Demonstrate Deregulated Sphingosine 1-Phosphate Signaling in Alzheimer’s Disease. Acta Neuropathol. Commun. 2014, 2, 12. [Google Scholar] [CrossRef]
- Couttas, T.A.; Kain, N.; Daniels, B.; Lim, X.Y.; Shepherd, C.; Kril, J.; Pickford, R.; Li, H.; Garner, B.; Don, A.S. Loss of the Neuroprotective Factor Sphingosine 1-Phosphate Early in Alzheimer’s Disease Pathogenesis. Acta Neuropathol. Commun. 2014, 2, 9. [Google Scholar] [CrossRef] [PubMed]
- Jung, Y.; Lopez-Benitez, J.; Tognoni, C.M.; Carreras, I.; Dedeoglu, A. Dysregulation of Sphingosine-1-Phosphate (S1P) and S1P Receptor 1 Signaling in the 5xFAD Mouse Model of Alzheimer’s Disease. Brain Res. 2023, 1799, 148171. [Google Scholar] [CrossRef] [PubMed]
- Kolahdooz, Z.; Nasoohi, S.; Asle-Rousta, M.; Ahmadiani, A.; Dargahi, L. Sphingosin-1-Phosphate Receptor 1: A Potential Target to Inhibit Neuroinflammation and Restore the Sphingosin-1-Phosphate Metabolism. Can. J. Neurol. Sci. 2015, 42, 195–202. [Google Scholar] [CrossRef]
- Ding, H.; Dolan, P.J.; Johnson, G.V.W. Histone Deacetylase 6 Interacts with the Microtubule-Associated Protein Tau. J. Neurochem. 2008, 106, 2119–2130. [Google Scholar] [CrossRef] [PubMed]
- Gräff, J.; Rei, D.; Guan, J.-S.; Wang, W.-Y.; Seo, J.; Hennig, K.M.; Nieland, T.J.F.; Fass, D.M.; Kao, P.F.; Kahn, M.; et al. An Epigenetic Blockade of Cognitive Functions in the Neurodegenerating Brain. Nature 2012, 483, 222–226. [Google Scholar] [CrossRef] [PubMed]
- Lithner, C.U.; Lacor, P.N.; Zhao, W.-Q.; Mustafiz, T.; Klein, W.L.; Sweatt, J.D.; Hernandez, C.M. Disruption of Neocortical Histone H3 Homeostasis by Soluble Aβ: Implications for Alzheimer’s Disease. Neurobiol. Aging 2013, 34, 2081–2090. [Google Scholar] [CrossRef]
- Alam, S.; Piazzesi, A.; Abd El Fatah, M.; Raucamp, M.; van Echten-Deckert, G. Neurodegeneration Caused by S1P-Lyase Deficiency Involves Calcium-Dependent Tau Pathology and Abnormal Histone Acetylation. Cells 2020, 9, 2189. [Google Scholar] [CrossRef]
- Ihlefeld, K.; Claas, R.F.; Koch, A.; Pfeilschifter, J.M.; Meyer Zu Heringdorf, D. Evidence for a Link between Histone Deacetylation and Ca2+ Homoeostasis in Sphingosine-1-Phosphate Lyase-Deficient Fibroblasts. Biochem. J. 2012, 447, 457–464. [Google Scholar] [CrossRef]
- Pyszko, J.A.; Strosznajder, J.B. The Key Role of Sphingosine Kinases in the Molecular Mechanism of Neuronal Cell Survival and Death in an Experimental Model of Parkinson’s Disease. Folia Neuropathol. 2014, 52, 260–269. [Google Scholar] [CrossRef]
- Bunner, K.D.; Rebec, G.V. Corticostriatal Dysfunction in Huntington’s Disease: The Basics. Front. Hum. Neurosci. 2016, 10, 317. [Google Scholar] [CrossRef]
- Chaves-Filho, A.B.; Pinto, I.F.D.; Dantas, L.S.; Xavier, A.M.; Inague, A.; Faria, R.L.; Medeiros, M.H.G.; Glezer, I.; Yoshinaga, M.Y.; Miyamoto, S. Alterations in Lipid Metabolism of Spinal Cord Linked to Amyotrophic Lateral Sclerosis. Sci. Rep. 2019, 9, 11642. [Google Scholar] [CrossRef] [PubMed]
- Fernández-Beltrán, L.C.; Godoy-Corchuelo, J.M.; Losa-Fontangordo, M.; Williams, D.; Matias-Guiu, J.; Corrochano, S. A Transcriptomic Meta-Analysis Shows Lipid Metabolism Dysregulation as an Early Pathological Mechanism in the Spinal Cord of SOD1 Mice. Int. J. Mol. Sci. 2021, 22, 9553. [Google Scholar] [CrossRef] [PubMed]
- Dodge, J.C.; Treleaven, C.M.; Pacheco, J.; Cooper, S.; Bao, C.; Abraham, M.; Cromwell, M.; Sardi, S.P.; Chuang, W.-L.; Sidman, R.L.; et al. Glycosphingolipids Are Modulators of Disease Pathogenesis in Amyotrophic Lateral Sclerosis. Proc. Natl. Acad. Sci. USA 2015, 112, 8100–8105. [Google Scholar] [CrossRef] [PubMed]
- Henriques, A.; Croixmarie, V.; Priestman, D.A.; Rosenbohm, A.; Dirrig-Grosch, S.; D’Ambra, E.; Huebecker, M.; Hussain, G.; Boursier-Neyret, C.; Echaniz-Laguna, A.; et al. Amyotrophic Lateral Sclerosis and Denervation Alter Sphingolipids and Up-Regulate Glucosylceramide Synthase. Hum. Mol. Genet. 2015, 24, 7390–7405. [Google Scholar] [CrossRef] [PubMed]
- Clark, L.F.; Kodadek, T. The Immune System and Neuroinflammation as Potential Sources of Blood-Based Biomarkers for Alzheimer’s Disease, Parkinson’s Disease, and Huntington’s Disease. ACS Chem. Neurosci. 2016, 7, 520–527. [Google Scholar] [CrossRef] [PubMed]
- Bjelobaba, I.; Savic, D.; Lavrnja, I. Multiple Sclerosis and Neuroinflammation: The Overview of Current and Prospective Therapies. Curr. Pharm. Des. 2017, 23, 693–730. [Google Scholar] [CrossRef] [PubMed]
- Marogianni, C.; Sokratous, M.; Dardiotis, E.; Hadjigeorgiou, G.M.; Bogdanos, D.; Xiromerisiou, G. Neurodegeneration and Inflammation-An Interesting Interplay in Parkinson’s Disease. Int. J. Mol. Sci. 2020, 21, 8421. [Google Scholar] [CrossRef]
- Valadão, P.A.C.; Santos, K.B.S.; e Vieira, T.H.F.; e Cordeiro, T.M.; Teixeira, A.L.; Guatimosim, C.; de Miranda, A.S. Inflammation in Huntington’s Disease: A Few New Twists on an Old Tale. J. Neuroimmunol. 2020, 348, 577380. [Google Scholar] [CrossRef]
- Saba, J.; Couselo, F.L.; Bruno, J.; Carniglia, L.; Durand, D.; Lasaga, M.; Caruso, C. Neuroinflammation in Huntington’s Disease: A Starring Role for Astrocyte and Microglia. Curr. Neuropharmacol. 2022, 20, 1116–1143. [Google Scholar] [CrossRef]
- Billich, A.; Baumruker, T.; Beerli, C.; Bigaud, M.; Bruns, C.; Calzascia, T.; Isken, A.; Kinzel, B.; Loetscher, E.; Metzler, B.; et al. Partial Deficiency of Sphingosine-1-Phosphate Lyase Confers Protection in Experimental Autoimmune Encephalomyelitis. PLoS ONE 2013, 8, e59630. [Google Scholar] [CrossRef]
- Karunakaran, I.; Alam, S.; Jayagopi, S.; Frohberger, S.J.; Hansen, J.N.; Kuehlwein, J.; Hölbling, B.V.; Schumak, B.; Hübner, M.P.; Gräler, M.H.; et al. Neural Sphingosine 1-Phosphate Accumulation Activates Microglia and Links Impaired Autophagy and Inflammation. Glia 2019, 67, 1859–1872. [Google Scholar] [CrossRef]
- Stepanovska, B.; Lange, A.I.; Schwalm, S.; Pfeilschifter, J.; Coldewey, S.M.; Huwiler, A. Downregulation of S1P Lyase Improves Barrier Function in Human Cerebral Microvascular Endothelial Cells Following an Inflammatory Challenge. Int. J. Mol. Sci. 2020, 21, 1240. [Google Scholar] [CrossRef]
- Salimi, H.; Klein, R.S. Disruption of the Blood-Brain Barrier During Neuroinflammatory and Neuroinfectious Diseases. In Neuroimmune Diseases; Springer: Berlin/Heidelberg, Germany, 2019; pp. 195–234. [Google Scholar] [CrossRef]
- Bandhuvula, P.; Tam, Y.Y.; Oskouian, B.; Saba, J.D. The Immune Modulator FTY720 Inhibits Sphingosine-1-Phosphate Lyase Activity. J. Biol. Chem. 2005, 280, 33697–33700. [Google Scholar] [CrossRef] [PubMed]
- Berdyshev, E.V.; Goya, J.; Gorshkova, I.; Prestwich, G.D.; Byun, H.-S.; Bittman, R.; Natarajan, V. Characterization of Sphingosine-1-Phosphate Lyase Activity by Electrospray Ionization-Liquid Chromatography/Tandem Mass Spectrometry Quantitation of (2E)-Hexadecenal. Anal. Biochem. 2011, 408, 12–18. [Google Scholar] [CrossRef] [PubMed]
- Park, S.M.; Angel, C.E.; McIntosh, J.D.; Brooks, A.E.S.; Middleditch, M.; Chen, C.-J.J.; Ruggiero, K.; Cebon, J.; Rod Dunbar, P. Sphingosine-1-Phosphate Lyase Is Expressed by CD68+ Cells on the Parenchymal Side of Marginal Reticular Cells in Human Lymph Nodes. Eur. J. Immunol. 2014, 44, 2425–2436. [Google Scholar] [CrossRef]
- Stoffel, W.; Grol, M. Chemistry and Biochemistry of 1-Desoxysphinganine 1-Phosphonate (Dihydrosphingosine-1-Phosphonate). Chem. Phys. Lipids 1974, 13, 372–388. [Google Scholar] [CrossRef] [PubMed]
- Boumendjel, A.; Miller, S.P.F. Synthesis of an Inhibitor of Sphingosine-1-Phosphate Lyase. Tetrahedron Lett. 1994, 35, 819–822. [Google Scholar] [CrossRef]
- Triola, G.; Fabrias, G.; Dragusin, M.; Niederhausen, L.; Broere, R.; Llebaria, A.; van Echten-Deckert, G. Specificity of the Dihydroceramide Desaturase Inhibitor N-[(1R,2S)-2-Hydroxy-1-Hydroxymethyl-2-(2-Tridecyl-1-Cyclopropenyl)Ethyl]Octanamide (GT11) in Primary Cultured Cerebellar Neurons. Mol. Pharmacol. 2004, 66, 1671–1678. [Google Scholar] [CrossRef]
- Bagdanoff, J.T.; Donoviel, M.S.; Nouraldeen, A.; Tarver, J.; Fu, Q.; Carlsen, M.; Jessop, T.C.; Zhang, H.; Hazelwood, J.; Nguyen, H.; et al. Inhibition of Sphingosine-1-Phosphate Lyase for the Treatment of Autoimmune Disorders. J. Med. Chem. 2009, 52, 3941–3953. [Google Scholar] [CrossRef]
- Loetscher, E.; Schneider, K.; Beerli, C.; Billich, A. Assay to Measure the Secretion of Sphingosine-1-Phosphate from Cells Induced by S1P Lyase Inhibitors. Biochem. Biophys. Res. Commun. 2013, 433, 345–348. [Google Scholar] [CrossRef]
- Schümann, J.; Grevot, A.; Ledieu, D.; Wolf, A.; Schubart, A.; Piaia, A.; Sutter, E.; Côté, S.; Beerli, C.; Pognan, F.; et al. Reduced Activity of Sphingosine-1-Phosphate Lyase Induces Podocyte-Related Glomerular Proteinuria, Skin Irritation, and Platelet Activation. Toxicol. Pathol. 2015, 43, 694–703. [Google Scholar] [CrossRef] [PubMed]
- Harris, C.M.; Mittelstadt, S.; Banfor, P.; Bousquet, P.; Duignan, D.B.; Gintant, G.; Hart, M.; Kim, Y.; Segreti, J. Sphingosine-1-Phosphate (S1P) Lyase Inhibition Causes Increased Cardiac S1P Levels and Bradycardia in Rats. J. Pharmacol. Exp. Ther. 2016, 359, 151–158. [Google Scholar] [CrossRef] [PubMed]
- Schwab, S.R.; Pereira, J.P.; Matloubian, M.; Xu, Y.; Huang, Y.; Cyster, J.G. Lymphocyte Sequestration through S1P Lyase Inhibition and Disruption of S1P Gradients. Science 2005, 309, 1735–1739. [Google Scholar] [CrossRef]
- Karuppuchamy, T.; Tyler, C.J.; Lundborg, L.R.; Pérez-Jeldres, T.; Kimball, A.K.; Clambey, E.T.; Jedlicka, P.; Rivera-Nieves, J. Sphingosine-1-Phosphate Lyase Inhibition Alters the S1P Gradient and Ameliorates Crohn’s-Like Ileitis by Suppressing Thymocyte Maturation. Inflamm. Bowel Dis. 2020, 26, 216–228. [Google Scholar] [CrossRef] [PubMed]
- Lee, H.; Park, H.S.; Hong, S.H.; Choi, O.K.; Cho, S.-D.; Park, J.; Oh, J.E.; Chung, S.S.; Jung, H.S.; Park, K.S. 4-Deoxypyridoxine Improves the Viability of Isolated Pancreatic Islets Ex Vivo. Islets 2013, 5, 116–121. [Google Scholar] [CrossRef]
- Hemdan, N.Y.A.; Weigel, C.; Reimann, C.-M.; Gräler, M.H. Modulating Sphingosine 1-Phosphate Signaling with DOP or FTY720 Alleviates Vascular and Immune Defects in Mouse Sepsis. Eur. J. Immunol. 2016, 46, 2767–2777. [Google Scholar] [CrossRef]
- Bassi, R.; Anelli, V.; Giussani, P.; Tettamanti, G.; Viani, P.; Riboni, L. Sphingosine-1-Phosphate Is Released by Cerebellar Astrocytes in Response to BFGF and Induces Astrocyte Proliferation through Gi-Protein-Coupled Receptors. Glia 2006, 53, 621–630. [Google Scholar] [CrossRef]
- Kleinjan, A.; van Nimwegen, M.; Leman, K.; Hoogsteden, H.C.; Lambrecht, B.N. Topical Treatment Targeting Sphingosine-1-Phosphate and Sphingosine Lyase Abrogates Experimental Allergic Rhinitis in a Murine Model. Allergy 2013, 68, 204–212. [Google Scholar] [CrossRef]
- Gorshkova, I.A.; Wang, H.; Orbelyan, G.A.; Goya, J.; Natarajan, V.; Beiser, D.G.; Vanden Hoek, T.L.; Berdyshev, E.V. Inhibition of Sphingosine-1-Phosphate Lyase Rescues Sphingosine Kinase-1-Knockout Phenotype Following Murine Cardiac Arrest. Life Sci. 2013, 93, 359–366. [Google Scholar] [CrossRef]
- Bandhuvula, P.; Honbo, N.; Wang, G.-Y.; Jin, Z.-Q.; Fyrst, H.; Zhang, M.; Borowsky, A.D.; Dillard, L.; Karliner, J.S.; Saba, J.D. S1P Lyase: A Novel Therapeutic Target for Ischemia-Reperfusion Injury of the Heart. Am. J. Physiol. Heart Circ. Physiol. 2011, 300, H1753–H1761. [Google Scholar] [CrossRef]
- Klyachkin, Y.M.; Nagareddy, P.R.; Ye, S.; Wysoczynski, M.; Asfour, A.; Gao, E.; Sunkara, M.; Brandon, J.A.; Annabathula, R.; Ponnapureddy, R.; et al. Pharmacological Elevation of Circulating Bioactive Phosphosphingolipids Enhances Myocardial Recovery After Acute Infarction. Stem Cells Transl. Med. 2015, 4, 1333–1343. [Google Scholar] [CrossRef] [PubMed]
- Baranowska, U.; Holownia, A.; Chabowski, A.; Baranowski, M. Pharmacological Inhibition of Sphingosine-1-Phosphate Lyase Partially Reverses Spatial Memory Impairment in Streptozotocin-Diabetic Rats. Mol. Cell. Neurosci. 2020, 107, 103526. [Google Scholar] [CrossRef] [PubMed]
- Pepe, G.; Capocci, L.; Marracino, F.; Realini, N.; Lenzi, P.; Martinello, K.; Bovier, T.F.; Bichell, T.J.; Scarselli, P.; Di Cicco, C.; et al. Treatment with THI, an Inhibitor of Sphingosine-1-Phosphate Lyase, Modulates Glycosphingolipid Metabolism and Results Therapeutically Effective in Experimental Models of Huntington’s Disease. Mol. Ther. 2022, 31, 282–299. [Google Scholar] [CrossRef]
- Bagdanoff, J.T.; Donoviel, M.S.; Nouraldeen, A.; Carlsen, M.; Jessop, T.C.; Tarver, J.; Aleem, S.; Dong, L.; Zhang, H.; Boteju, L.; et al. Inhibition of Sphingosine 1-Phosphate Lyase for the Treatment of Rheumatoid Arthritis: Discovery of (E)-1-(4-((1R,2S,3R)-1,2,3,4-Tetrahydroxybutyl)-1H-Imidazol-2-Yl)Ethanone Oxime (LX2931) and (1R,2S,3R)-1-(2-(Isoxazol-3-Yl)-1H-Imidazol-4-Yl)Butane-1,2,3,4-Tetraol (LX2932). J. Med. Chem. 2010, 53, 8650–8662. [Google Scholar] [CrossRef]
- Veltman, M.; Stolarczyk, M.; Radzioch, D.; Wojewodka, G.; De Sanctis, J.B.; Dik, W.A.; Dzyubachyk, O.; Oravecz, T.; de Kleer, I.; Scholte, B.J. Correction of Lung Inflammation in a F508del CFTR Murine Cystic Fibrosis Model by the Sphingosine-1-Phosphate Lyase Inhibitor LX2931. Am. J. Physiol. Lung Cell. Mol. Physiol. 2016, 311, L1000–L1014. [Google Scholar] [CrossRef]
- Cencetti, F.; Bruno, G.; Bernacchioni, C.; Japtok, L.; Puliti, E.; Donati, C.; Bruni, P. Sphingosine 1-Phosphate Lyase Blockade Elicits Myogenic Differentiation of Murine Myoblasts Acting via Spns2/S1P2 Receptor Axis. Biochim. Biophys. Acta Mol. Cell. Biol. Lipids 2020, 1865, 158759. [Google Scholar] [CrossRef]
- Weske, S.; Vaidya, M.; von Wnuck Lipinski, K.; Keul, P.; Manthe, K.; Burkhart, C.; Haberhauer, G.; Heusch, G.; Levkau, B. Agonist-Induced Activation of the S1P Receptor 2 Constitutes a Novel Osteoanabolic Therapy for the Treatment of Osteoporosis in Mice. Bone 2019, 125, 1–7. [Google Scholar] [CrossRef] [PubMed]
- Finney, C.A.; Hawkes, C.A.; Kain, D.C.; Dhabangi, A.; Musoke, C.; Cserti-Gazdewich, C.; Oravecz, T.; Liles, W.C.; Kain, K.C. S1P Is Associated with Protection in Human and Experimental Cerebral Malaria. Mol. Med. 2011, 17, 717–725. [Google Scholar] [CrossRef]
- Fujita, T.; Inoue, K.; Yamamoto, S.; Ikumoto, T.; Sasaki, S.; Toyama, R.; Chiba, K.; Hoshino, Y.; Okumoto, T. Fungal Metabolites. Part 11. A Potent Immunosuppressive Activity Found in Isaria Sinclairii Metabolite. J. Antibiot. 1994, 47, 208–215. [Google Scholar] [CrossRef]
- Adachi, K.; Kohara, T.; Nakao, N.; Arita, M.; Chiba, K.; Mishina, T.; Sasaki, S.; Fujita, T. Design, Synthesis, and Structure-Activity Relationships of 2-Substituted-2-Amino-1,3-Propanediols: Discovery of a Novel Immunosuppressant, FTY720. Bioorganic Med. Chem. Lett. 1995, 5, 853–856. [Google Scholar] [CrossRef]
- Chun, J.; Kihara, Y.; Jonnalagadda, D.; Blaho, V.A. Fingolimod: Lessons Learned and New Opportunities for Treating Multiple Sclerosis and Other Disorders. Annu. Rev. Pharmacol. Toxicol. 2019, 59, 149–170. [Google Scholar] [CrossRef] [PubMed]
- Kiuchi, M.; Adachi, K.; Kohara, T.; Minoguchi, M.; Hanano, T.; Aoki, Y.; Mishina, T.; Arita, M.; Nakao, N.; Ohtsuki, M.; et al. Synthesis and Immunosuppressive Activity of 2-Substituted 2-Aminopropane-1,3-Diols and 2-Aminoethanols. J. Med. Chem. 2000, 43, 2946–2961. [Google Scholar] [CrossRef] [PubMed]
- Troncoso, P.; Stepkowski, S.M.; Wang, M.E.; Qu, X.; Chueh, S.C.; Clark, J.; Kahan, B.D. Prophylaxis of Acute Renal Allograft Rejection Using FTY720 in Combination with Subtherapeutic Doses of Cyclosporine. Transplantation 1999, 67, 145–151. [Google Scholar] [CrossRef]
- Budde, K.; Schmouder, R.L.; Brunkhorst, R.; Nashan, B.; Lücker, P.W.; Mayer, T.; Choudhury, S.; Skerjanec, A.; Kraus, G.; Neumayer, H.H. First Human Trial of FTY720, a Novel Immunomodulator, in Stable Renal Transplant Patients. J. Am. Soc. Nephrol. 2002, 13, 1073–1083. [Google Scholar] [CrossRef]
- Mulgaonkar, S.; Tedesco, H.; Oppenheimer, F.; Walker, R.; Kunzendorf, U.; Russ, G.; Knoflach, A.; Patel, Y.; Ferguson, R. FTYA121 study group FTY720/Cyclosporine Regimens in de Novo Renal Transplantation: A 1-Year Dose-Finding Study. Am. J. Transplant. 2006, 6, 1848–1857. [Google Scholar] [CrossRef]
- Salvadori, M.; Budde, K.; Charpentier, B.; Klempnauer, J.; Nashan, B.; Pallardo, L.M.; Eris, J.; Schena, F.P.; Eisenberger, U.; Rostaing, L.; et al. FTY720 versus MMF with Cyclosporine in de Novo Renal Transplantation: A 1-Year, Randomized Controlled Trial in Europe and Australasia. Am. J. Transplant. 2006, 6, 2912–2921. [Google Scholar] [CrossRef] [PubMed]
- Sharma, S.; Mathur, A.G.; Pradhan, S.; Singh, D.B.; Gupta, S. Fingolimod (FTY720): First Approved Oral Therapy for Multiple Sclerosis. J. Pharmacol. Pharmacother. 2011, 2, 49–51. [Google Scholar] [CrossRef] [PubMed]
- Paugh, S.W.; Payne, S.G.; Barbour, S.E.; Milstien, S.; Spiegel, S. The Immunosuppressant FTY720 Is Phosphorylated by Sphingosine Kinase Type 2. FEBS Lett. 2003, 554, 189–193. [Google Scholar] [CrossRef]
- Zemann, B.; Kinzel, B.; Müller, M.; Reuschel, R.; Mechtcheriakova, D.; Urtz, N.; Bornancin, F.; Baumruker, T.; Billich, A. Sphingosine Kinase Type 2 Is Essential for Lymphopenia Induced by the Immunomodulatory Drug FTY720. Blood 2006, 107, 1454–1458. [Google Scholar] [CrossRef]
- Matloubian, M.; Lo, C.G.; Cinamon, G.; Lesneski, M.J.; Xu, Y.; Brinkmann, V.; Allende, M.L.; Proia, R.L.; Cyster, J.G. Lymphocyte Egress from Thymus and Peripheral Lymphoid Organs Is Dependent on S1P Receptor 1. Nature 2004, 427, 355–360. [Google Scholar] [CrossRef]
- Chiba, K. FTY720, a New Class of Immunomodulator, Inhibits Lymphocyte Egress from Secondary Lymphoid Tissues and Thymus by Agonistic Activity at Sphingosine 1-Phosphate Receptors. Pharmacol. Ther. 2005, 108, 308–319. [Google Scholar] [CrossRef] [PubMed]
- Ingwersen, J.; Aktas, O.; Kuery, P.; Kieseier, B.; Boyko, A.; Hartung, H.-P. Fingolimod in Multiple Sclerosis: Mechanisms of Action and Clinical Efficacy. Clin. Immunol. 2012, 142, 15–24. [Google Scholar] [CrossRef] [PubMed]
- Constantinescu, V.; Akgün, K.; Ziemssen, T. Current Status and New Developments in Sphingosine-1-Phosphate Receptor Antagonism: Fingolimod and More. Expert Opin. Drug Metab. Toxicol. 2022, 18, 675–693. [Google Scholar] [CrossRef] [PubMed]
- Allende, M.L.; Dreier, J.L.; Mandala, S.; Proia, R.L. Expression of the Sphingosine 1-Phosphate Receptor, S1P1, on T-Cells Controls Thymic Emigration. J. Biol. Chem. 2004, 279, 15396–15401. [Google Scholar] [CrossRef] [PubMed]
- Lo, C.G.; Xu, Y.; Proia, R.L.; Cyster, J.G. Cyclical Modulation of Sphingosine-1-Phosphate Receptor 1 Surface Expression during Lymphocyte Recirculation and Relationship to Lymphoid Organ Transit. J. Exp. Med. 2005, 201, 291–301. [Google Scholar] [CrossRef] [PubMed]
- Chiba, K.; Matsuyuki, H.; Maeda, Y.; Sugahara, K. Role of Sphingosine 1-Phosphate Receptor Type 1 in Lymphocyte Egress from Secondary Lymphoid Tissues and Thymus. Cell Mol. Immunol. 2006, 3, 11–19. [Google Scholar] [PubMed]
- Pham, T.H.M.; Okada, T.; Matloubian, M.; Lo, C.G.; Cyster, J.G. S1P1 Receptor Signaling Overrides Retention Mediated by G Alpha I-Coupled Receptors to Promote T Cell Egress. Immunity 2008, 28, 122–133. [Google Scholar] [CrossRef]
- Mullershausen, F.; Zecri, F.; Cetin, C.; Billich, A.; Guerini, D.; Seuwen, K. Persistent Signaling Induced by FTY720-Phosphate Is Mediated by Internalized S1P1 Receptors. Nat. Chem. Biol. 2009, 5, 428–434. [Google Scholar] [CrossRef]
- Ntranos, A.; Hall, O.; Robinson, D.P.; Grishkan, I.V.; Schott, J.T.; Tosi, D.M.; Klein, S.L.; Calabresi, P.A.; Gocke, A.R. FTY720 Impairs CD8 T-Cell Function Independently of the Sphingosine-1-Phosphate Pathway. J. Neuroimmunol. 2014, 270, 13–21. [Google Scholar] [CrossRef]
- Mazzola, M.A.; Raheja, R.; Murugaiyan, G.; Rajabi, H.; Kumar, D.; Pertel, T.; Regev, K.; Griffin, R.; Aly, L.; Kivisakk, P.; et al. Identification of a Novel Mechanism of Action of Fingolimod (FTY720) on Human Effector T Cell Function through TCF-1 Upregulation. J. Neuroinflamm. 2015, 12, 245. [Google Scholar] [CrossRef]
- Baer, A.; Colon-Moran, W.; Bhattarai, N. Characterization of the Effects of Immunomodulatory Drug Fingolimod (FTY720) on Human T Cell Receptor Signaling Pathways. Sci. Rep. 2018, 8, 10910. [Google Scholar] [CrossRef] [PubMed]
- Foster, C.A.; Howard, L.M.; Schweitzer, A.; Persohn, E.; Hiestand, P.C.; Balatoni, B.; Reuschel, R.; Beerli, C.; Schwartz, M.; Billich, A. Brain Penetration of the Oral Immunomodulatory Drug FTY720 and Its Phosphorylation in the Central Nervous System during Experimental Autoimmune Encephalomyelitis: Consequences for Mode of Action in Multiple Sclerosis. J. Pharmacol. Exp. Ther. 2007, 323, 469–475. [Google Scholar] [CrossRef] [PubMed]
- Sunter, G.; Enver, E.O.; Akbarzade, A.; Turan, S.; Vatansever, P.; Gunal, D.I.; Haklar, G.; Bereket, A.; Agan, K.; Guran, T. Acquired Modification of Sphingosine-1-Phosphate Lyase Activity Is Not Related to Adrenal Insufficiency. BMC Neurol. 2018, 18, 48. [Google Scholar] [CrossRef]
- Sinkeldam, E.J.; de Groot, A.P.; van den Berg, H.; Chappel, C.I. The Effect of Pyridoxine on the Number of Lymphocytes in the Blood of Rats Fed Caramel Colour (III). Food Chem. Toxicol. 1988, 26, 195–203. [Google Scholar] [CrossRef]
- Frydas, S.; Karagouni, E.; Dotsika, E.; Reale, M.; Barbacane, R.C.; Vlemmas, I.; Anogianakis, G.; Trakatellis, A.; Conti, P. Generation of TNF Alpha, IFN Gamma, IL-6, IL-4 and IL-10 in Mouse Serum from Trichinellosis: Effect of the Anti-Inflammatory Compound 4-Deoxypyridoxine (4-DPD). Immunol. Lett. 1996, 49, 179–184. [Google Scholar] [CrossRef] [PubMed]
- Kroeplien, U.; Rosdorfer, J.; Van der Greef, J.; Long, R.C.; Goldstein, J.H. 2-Acetyl-4(5)-(1,2,3,4-Tetrahydroxybutyl)Imidazole: Detection in Commercial Caramel Color III and Preparation by a Model Browning Reaction. J. Org. Chem. 1985, 50, 1131–1133. [Google Scholar] [CrossRef]
- Bradbury, M.G.; Qiu, M.R.; Parish, C.R. The Immunomodulatory Compound 2-Acetyl-4-Tetrahydroxybutyl Imidazole Causes Sequestration of Lymphocytes in Non-Lymphoid Tissues. Immunol. Cell. Biol. 1997, 75, 497–502. [Google Scholar] [CrossRef] [PubMed]
| Inhibitor | Experimental Model | Results | Reference |
|---|---|---|---|
| Sphingosine analogues | |||
| Fingolimod (FTY720) | HEK293 cell line, 4-week-old FVB mice | ↓ SPL activity; no effect on SPL gene expression; no effect on SPL protein level | [115] |
| Mouse liver (total tissue and microsomes) | dose-dependent ↓ SPL activity; IC50 = 52.4 μM | [116] | |
| CD68+ antigen-presenting cells from human monocytes | ↓ hexadecenal (HE) production | [117] | |
| 1-desoxysphinganine 1-phosphonate | Rat liver microsomes | inhibitory constant (Ki) = 5 μmol/L ↓ hexadecanal production (end product of dihydrosphingosine-1-phosphate (dhS1P) degradation) | [118] |
| 2-vinyl dihydrosphingosine-1-phosphate (2VS1P) | Rat liver microsomes | IC50 = 2.4 μM | [119] |
| N-[(1R,2S)-2-hydroxy-1-hydroxymethyl-2-(2-tridecyl-1-cyclopentenyl) ethyl] octanamide (GT11) | Murine primary cultured cerebellar neurons | high concentrations of GT11: ↓ SPL activity; accumulation of phosphorylated long-chain bases (S1P, dhS1P); no effect on SPL gene expression | [120] |
| Non-lipidic direct SPL inhibitors | |||
| oxopyridylpyrimidine | HepG2 cells | IC50 = 2.1 μM; ↑ S1P level | [121] |
| (R)-6-(4-(4-Benzyl-7-chlorophthalazin- 1-yl)-2-methylpiperazin-1-yl)nicotinonitrile (Compound 31) | (1) purified human SPL (2) HEK293T cells | (1) IC50 = 0.214 μM; (2) concentration-dependent ↑ secreted S1P | [122] |
| experimental immune encephalomyelitis (EAE) (rat model of MS) | ↑ S1P level in lymph nodes, ↑ peripheral lymphopenia, ↓ T cell migration into central nervous system (CNS) | [19] | |
| EAE (rat model of MS) | platelets activation, skin irritation, kidney toxicity | [123] | |
| Female and male Sprague-Dawley rats | ↑ S1P level in a cardiac tissue, bradycardia | [124] | |
| Functional antagonists | |||
| 4-deoxypyridoxine (DOP) | C57Bl/6 (B6) mice, Ly5.2+ Boy/J mice, RAG2-deficient (129 background) mice | ↓ hexadecanal generation in thymus; ↑ lymphoid S1P concentration | [125] |
| TNFΔARE mice (TNF-driven model of chronic ileitis) | ↓ ileal mRNA level of TNF, IL-6, IL-12, IL-17, IFN-γ; ↓ chronic ileitis, ↓ SPL activity in the ileum | [126] | |
| Pancreatic islets from Sprague Dawley rats, Pancreatic islets from C57BL/6 mice, mouse insulinoma cell line MIN-6, rat insulinoma cell line INS-1 | dose-dependent ↓ level of cleaved caspase 3; ↑ viability | [127] | |
| 10–11-week-old C57BL/6J mice with sepsis induced by intraperitoneal injection of a microbiologically-defined human stool suspension | ↓ plasma level of IL-6, TNF-α, monocyte chemoattractant protein (MCP-1) and IL-10; ↑ vascular barrier stability; ↓ immune cell infiltration; ↑ survival; ↑ lymphopenia | [128] | |
| Primary culture of cerebellar astrocytes from 8-day-old Wistar rats | ↑ cellular S1P level; no effect on cell proliferation; no effect on extracellular S1P level | [129] | |
| mouse embryonic fibroblasts (MEFs) | ↑ acetylation of histone 3 at lysine 9 (H3K9) | [99] | |
| 2-acetyl-4(5)-(1,2,3,4-tetrahydroxybutyl) imidazole (THI) | C57Bl/6 (B6) mice, Ly5.2+ Boy/J mice, RAG2-deficient (129 background) mice | ↓ hexadecanal generation in thymus; ↑ lymphoid S1P concentration | [125] |
| BALB/c mice sensitized intraperitoneally with ovalbumin (murine model of allergic rhinitis) | ↓ IL-4 level in cervical lymph nodes; ↓ eosinophil and mast cell number in lamina propria of the nasal mucosa | [130] | |
| SphK1 knockout (SphK1-KO) C57BL/6 mice treated intravenously with KCl (murine model of cardiac arrest) | ↑ survival rate; ↑ S1P level in plasma and heart tissue; ↓ plasma level of 22:0 ceramide; ↑ S1PR2 expression | [131] | |
| Ex vivo murine model of ischemia-reperfusion | ↑ cardiac and plasma S1P level; ↓ infarct size, | [132] | |
| C57BL/6 mice with ligated left anterior descending artery (murine model of myocardial infarction) | ↑ plasma level of S1P and ceramide-1-phosphate (C1P); ↑ bone marrow derived stem/progenitor cells (BMSPCs); ↑ expression of genes involved in stem cell survival and myocardial homing | [133] | |
| Male Wistar rats treated intraperitoneally with streptozotocin (rat model of streptozotocin-induced diabetes) | ↑ S1P level in hippocampus, prefrontal cortex, cerebellum and striatum; ↑ ceramide level in hippocampus and cerebellum; ↑ spatial memory | [134] | |
| (1) R6/2 mice (murine transgenic model of HD) (2) Q128HD-FL transgenic flies (D. melanogaster HD model) | only chronic THI administration: (1) ↓ motor deficits; ↑ prosurvival signaling; ↑ white matter integrity; ↑ proper synaptic activity; (2) ↑ locomotor function; ↑ lifespan | [135] | |
| mouse primary cortical neurons | ↑ Aβ secretion | [90] | |
| (E)-1-(4-((1 R, 2 S, 3 R)-1,2,3,4-Tetrahydroxybutyl)-1 H -imidazol-2-yl)ethanone oxime (LX2931) | 129/SvEvBrd x C57BL/6 F1 mice treated with chicken collagen II emulsified in complete Freund’s adjuvant (murine model of collagen-induced arthritis) | ↓ circulating lymphocytes; ↓ inflammation, erosion, synovial hyperplasia and exudate formation | [136] |
| Cftrtm1EUR F508del CFTR mice (murine model of cystic fibrosis) | ↑ S1P level in lungs; ↓ level of IFN-γ, IL-12, IL-10, keratinocyte-derived cytokines (KC) in lungs; ↓ production of an inflammation-responsive mucin Muc5AC | [137] | |
| C2C12 cell line (murine myoblasts) | ↑ S1P level; ↑ protein level of myogenin and caveolin 3 | [138] | |
| Ovariectomized 12-week- old C57Bl6J mice (murine model of postmenopausal osteoporosis) | ↓ osteopenia; ↑ osteoblast activity; ↑ cortical thickness; ↑ mechanical bone parameters (ultimate force and stiffness) | [139] | |
| 6- to 8-week-old female C57BL/6 mice infected with Plasmodium berghei ANKA strain (murine model of cerebral malaria) | delay of symptoms onset; ↓ IFN-γ plasma level 5 days after infection | [140] | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wieczorek, I.; Strosznajder, R.P. Recent Insight into the Role of Sphingosine-1-Phosphate Lyase in Neurodegeneration. Int. J. Mol. Sci. 2023, 24, 6180. https://doi.org/10.3390/ijms24076180
Wieczorek I, Strosznajder RP. Recent Insight into the Role of Sphingosine-1-Phosphate Lyase in Neurodegeneration. International Journal of Molecular Sciences. 2023; 24(7):6180. https://doi.org/10.3390/ijms24076180
Chicago/Turabian StyleWieczorek, Iga, and Robert Piotr Strosznajder. 2023. "Recent Insight into the Role of Sphingosine-1-Phosphate Lyase in Neurodegeneration" International Journal of Molecular Sciences 24, no. 7: 6180. https://doi.org/10.3390/ijms24076180
APA StyleWieczorek, I., & Strosznajder, R. P. (2023). Recent Insight into the Role of Sphingosine-1-Phosphate Lyase in Neurodegeneration. International Journal of Molecular Sciences, 24(7), 6180. https://doi.org/10.3390/ijms24076180






