Abstract
Strong evidence suggests a correlation between degeneration and mitochondrial deficiency. Typical cases of degeneration can be observed in physiological phenomena (i.e., ageing) as well as in neurological neurodegenerative diseases and cancer. All these pathologies have the dyshomeostasis of mitochondrial bioenergy as a common denominator. Neurodegenerative diseases show bioenergetic imbalances in their pathogenesis or progression. Huntington’s chorea and Parkinson’s disease are both neurodegenerative diseases, but while Huntington’s disease is genetic and progressive with early manifestation and severe penetrance, Parkinson’s disease is a pathology with multifactorial aspects. Indeed, there are different types of Parkinson/Parkinsonism. Many forms are early-onset diseases linked to gene mutations, while others could be idiopathic, appear in young adults, or be post-injury senescence conditions. Although Huntington’s is defined as a hyperkinetic disorder, Parkinson’s is a hypokinetic disorder. However, they both share a lot of similarities, such as neuronal excitability, the loss of striatal function, psychiatric comorbidity, etc. In this review, we will describe the start and development of both diseases in relation to mitochondrial dysfunction. These dysfunctions act on energy metabolism and reduce the vitality of neurons in many different brain areas.
1. Introduction
The mitochondrion is an evolutionary organelle that originated from the established symbiotic relationship between alpha-proteobacteria and eukaryotic cells [1]. This organelle was stabilized in the host through mitotic cell divisions [1,2]. In light of this hypothesis, it is possible that the mitochondria enhanced the development of life as we know it and represent the common factor through which many organisms function or dysfunction [2,3,4]. Mitochondria are well-known energetic units of eukaryotic cells [2]. They supply energy in the form of ATP to support all cellular functions. In aerobic respiration, through a complex biochemical pathway that involves the addition of different co-factors, the mitochondria aid in the synthesis of ATP molecules from pyruvate [2,3]. In addition, mitochondrial proteomes, which are aggregates of more than 1000 proteins, can support a wide variety of critical biochemical processes, including amino acid metabolism, nucleotide metabolism, protein synthesis, and fatty acid catabolism [3,4].
There is abundant evidence in the literature associating mitochondrial disruption with neurological and neurodegenerative diseases [4,5,6]. The brain requires high-energy consumption in the form of ATP. In fact, it was estimated that a cortical neuron in the human brain could utilize 4.7 billion molecules of ATP/sec. Furthermore, the ATP used at rest corresponds to about 5.7 kg per day [7]. The brain’s high energy demand supports many important functions, such as neurotransmission, cellular activities required for learning and memory, neural plasticity, and synapse development [8]. Since the mitochondrion, through oxidative phosphorylation and the production of ATP, meets all the energy demands of the nervous system, even with a rapid turnover [9], mitochondrial dysfunction with a consequent loss in the energetic capacity may represent a neurodegenerative trigger. Severe bioenergetic dysfunction makes neurons very vulnerable to oxidative stress and predisposed to neuronal cell death [10]. Additionally, neurodegeneration can be seen as an energy disorder, which manifests in different aspects depending on the severity of the process and the area involved [11]. This energetic disorder can lead to different pathologies, but sometimes with opposite symptoms, such as hypokinetic and hyperkinetic disorders. The purpose of this review is to emphasize how mitochondrial dysfunctions, which may be associated with phenomena such as inflammation and degeneration, are actually a common element in two major neurodegenerative diseases. One is purely caused by genetics, while the other results from a combination of genetic and environmental factors: Huntington’s disease (HD) and Parkinson’s disease (PD), respectively.
2. Role of Mitochondria in Brain Energy Metabolism, Calcium Homeostasis, and Signal Transduction
Mitochondria are organelles responsible for several critical processes in neuronal function and dysfunction, including energy metabolism, calcium homeostasis, and signal transduction [1]. The brain consumes about 20% of the total oxygen respired to meet the high metabolic demand of neurons that must maintain ionic gradients across membranes, transport molecules from the soma along axons and dendrites, as well as for neurotransmission [12]. The metabolic activities that generate ATP rely mainly on mitochondrial oxidative phosphorylation (OXPHOS) [1]. During the process of OXPHOS, enzymes of the Krebs cycle utilize acetyl-coenzyme A to reduce the cofactors, nicotinamide adenine dinucleotides (NADH) and flavin adenine dinucleotides (FADH2), that aid energy transfer to the electron transport chain (ETC), embedded within the extensive inner mitochondrial membrane [13,14]. The ETC consists of four complexes that transfer electrons from NADH and FADH2 to O2. Ultimately, the energy released by the transfer of electrons is used for the thermodynamically unfavorable pumping of protons against their concentration gradient from the matrix to the intermembrane space to generate an electrochemical gradient known as mitochondrial membrane potential (ΔΨm) [1]. This membrane potential is essential for the process of energy storage (Figure 1). The protons accumulated in the intermembrane space are then allowed to move according to a concentration gradient back into the matrix by passing through one of the domains of the enzyme ATP synthase (or Complex V), which finally harnesses the released energy to phosphorylate ADP molecules into ATP [13]. Proper neuronal function is strongly linked to the retention of mitochondrial membrane potential and ATP levels [15] (Figure 1).
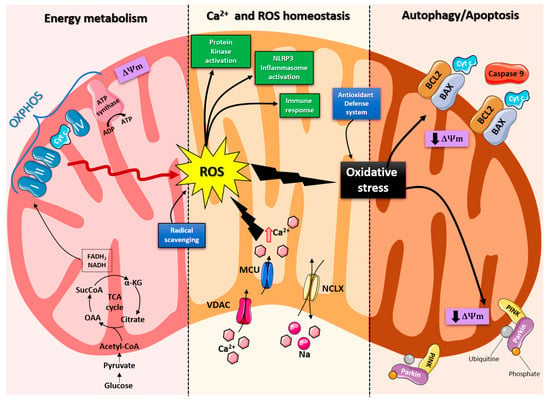
Figure 1.
Schematic representation of the mitochondrial functions. From left: Under physiological conditions, mitochondria supply ATP through OXPHOS. Krebs cycle enzymes use acetyl-coenzyme A to reduce NADH and FADH2, which are used for energy transfer to the electron transport chain (ETC) embedded in the inner mitochondrial membrane. OXPHOS is also an important source of ROS, whose basal levels are maintained by the radical scavenging network. Mitochondria also play a crucial role in calcium homeostasis. The voltage-dependent anion channel (VDAC) and the mitochondrial Ca2+ uniporter complex (MCU) finely control Ca2+ passage across the mitochondrial membranes, while the mitochondrial Na+/Ca2+ exchanger (NCLX) is one of the central units involved in Ca2+ extrusion. Under normal conditions, through ROS generation and redox signaling, mitochondria can control cellular metabolism, physiology, the inflammatory response, and immune function and act as important signaling molecules in the cell by activating various protein kinases. In contrast, the overproduction of ROS and dysregulation of the redox signaling system result in oxidative stress that can lead to mitochondrial damage. Malfunctioning mitochondria can be selectively removed through mitophagy, or, as all other defense mechanisms fail, the neuron can orchestrate its own destruction by activating the intrinsic suicide program of apoptosis.
Indeed, mitochondrial functions go beyond the primary role of ATP production because these organelles are intimately involved in numerous processes operating within the cell, including calcium homeostasis, the generation of free radical species (ROS), steroid synthesis, apoptosis, and cell signaling pathways [16]. Particularly, the direction of the mitochondrial membrane potential (negative internal) has important implications, as it elicits the thermodynamic force supporting the accumulation of metal cations, especially calcium (Ca2+) in the mitochondria [17]. Mitochondrial Ca2+ homeostasis plays a key role in cellular bioenergetics and signaling [18]. Ca2+ passage across the outer mitochondrial membrane (OMM) is mediated by the VDAC channel, whose different selectivity for cations or anions is voltage-dependent [19]. For instance, at low potentials (10 mV), the VDAC channel is highly permeable to anions and able to maintain a low Ca2+ flux. Conversely, increases in the membrane potential (20–30 mV) result in a conformational change that allows a 4- to 10-fold increase in Ca2+ influx [20]. In comparison, the inner mitochondrial membrane (IMM) has considerable Ca2+ permeability: the mitochondrial calcium uniporter (MCU) contributes to the potential-dependent Ca2+ influx into the mitochondrial matrix, while the mitochondrial Na+/Ca2+ exchanger (NCLX) is one of the main units involved in Ca2+ extrusion [21]. In general, cytosolic calcium uptake by mitochondria will occur only if they are exposed to elevated Ca2+ concentrations [22,23] (Figure 1). However, within the cell, Ca2+ is compartmentalized, and the average resting cytosolic concentration is remarkably low [24,25,26]. One of the main stores of cellular calcium is the endoplasmic reticulum (ER), which contributes to orchestrating cellular Ca2+ homeostasis through its interaction with the mitochondria [27]. Notably, Ca2+ uptake in the mitochondria probably occurs only at sites in close proximity between the ER and mitochondria [18]. On the other hand, neuronal mitochondria, unlike those in non-neuronal cells, have a considerably lower threshold for Ca2+ uptake. In addition, ER-mitochondria contacts are critical for Ca2+ uptake in dendritic mitochondria but not in axonal mitochondria [28]. This property of axonal mitochondria is due to the presence of the brain-specific uniporter MICU3, which causes axons to be less dependent on intracellular Ca2+ storage. In the absence of MICU3, synaptic function is impaired [29] (Figure 1).
Mitochondrial calcium-signaling in neurons regulates metabolism and energy production, which are crucial for neurotransmission and sustaining synaptic plasticity [30]. In addition, mitochondrial Ca2+ also drives the production of reactive oxygen species (ROS) [31]. Mitochondria are the primary sources of ROS in cells and actively participate in cellular redox regulation and ROS signaling [32] (Figure 1). ROS, in the form of superoxide, are natural byproducts of normal mitochondrial activity and are naturally converted to H2O2, which is in turn scavenged by the enzyme catalase to produce water [32]. Under normal conditions, through the generation of ROS and redox signaling, mitochondria can control cell metabolism and physiology, as well as inflammatory responses, immune function, autophagy, and stress responses [33,34,35]. Indeed, ROS, and particularly hydrogen peroxide at low concentrations, act as important signaling molecules in the cell, activating several protein kinases, such as PKA, PKC, PI3K, and p38 [36]. Moreover, in immune cells, mitochondrial metabolites and ROS finely regulate signaling pathways and cell fate, thereby orchestrating the immune response [37].
The immune system comprises a diverse family of cells with multiple roles during homeostasis and inflammation, capable of using distinct metabolic programs to undertake their functions. For instance, during the immune response, effector T cells promote aerobic glycolysis, while memory T cells and regulatory T cells promote fatty acid oxidation [38]. Particularly, the mitochondria can modulate the metabolic and physiological states of different types of immune cells [39]. It can also stimulate the innate immune signaling cascade, which can intensify inflammation following cytotoxic stimuli or microbial infection [40]. For example, the innate immune receptor NLRX1, a member of the Nod-like Receptor (NLR) family, is located in the mitochondria and undertakes an important role in maintaining cellular homeostasis following acute mitochondrial injury [41,42]. In addition, it has recently been discovered that mitochondria play a central role in initiating and regulating the NLRP3 (nucleotide-binding domain, leucine-rich repeat family, pyrin domain-containing 3) inflammasome [43]. This multiprotein complex, activated upon infection or cellular stress, leads to the secretion of proinflammatory cytokines, such as interleukin-1β (IL-1β) and IL-18, that trigger an inflammatory form of cell death called pyroptosis [44,45]. Although the mechanisms of NLRP3 inflammasome activation are still debated, it is widely believed that changes in the mitochondrial membrane potential, permeabilization of the outer mitochondrial membrane, and increased formation of mitochondrial ROS are crucial factors in inducing the cytosolic translocation of mitochondrial molecules such as cardiolipin and mitochondrial DNA, which are capable of activating the inflammasome [43]. Indeed, the overproduction of ROS and dysregulation of the redox signaling system result in oxidative stress that can lead to mitochondrial damage, induce mitochondrial DNA mutations, damage the respiratory chain, alter membrane permeability, and affect the Ca2+ homeostasis and mitochondrial defense systems [46]. Accumulating evidence suggests that oxidative stress and mitochondrial injury can result in cellular DNA damage, degradation of proteins and lipids, and the pathogenesis of neurodegenerative diseases [47,48]. Nevertheless, cells have an accurate endogenous antioxidant defense system that can maintain cellular redox homeostasis between ROS production and elimination to ensure normal cellular signaling and redox regulation (Figure 1) [49].
The nuclear factor erythroid 2–related factor 2 (Nrf2) is an emerging therapeutic target, since it is involved in cellular resistance to oxidants. In detail, Nrf2 controls the basal expression of genes coding for enzymes and proteins involved in antioxidant and detoxifying action, repair and removal of damaged proteins and organelles, the inflammatory response, and mitochondrial bioenergetics [50,51]. Additionally, malfunctioning mitochondria can be selectively removed through a conserved cellular recycling process known as mitochondrial autophagy or mitophagy. In fact, the efficient elimination of damaged mitochondria prevents activation of cell death pathways, protects against ROS overproduction, and maintains efficient ATP production [52]. Damaged mitochondria are swallowed into autophagic vesicles that subsequently transport them to lysosomes for destruction. Mitophagy is a strictly regulated process, modulated by mitochondrial fission and fusion proteins, BCL-2 (B-cell lymphoma 2) family proteins [53], and the PINK1/Parkin pathway [54]. As all other defense mechanisms fail, the neuron can orchestrate its own-destruction by activating an intrinsic suicide program, otherwise known as apoptosis [55] (Figure 1). The underlying mechanisms of apoptosis are very sophisticated and engage an energy-dependent cascade of molecular events. In addition, it can follow different molecular pathways, one of which is the intrinsic pathway that involves the mitochondria [56]. Indeed, these organelles represent the site where anti-apoptotic and pro-apoptotic proteins interact and the origin of signals that initiate the activation of caspases, the cysteine proteases capable of cleaving many cellular substrates to disrupt cellular contents [57].
It is therefore understood that mitochondrial integrity and homeostasis are prerequisites for proper cellular functioning, especially for neurons, which are polarized, complex cells with high energy demands [58]. Not surprisingly, neurons have the highest content of mitochondria compared to other cell populations. Mitochondrial functionality is essential for ensuring membrane excitability and performing the complex neurotransmission and plasticity processes.
Firstly, the mitochondria provide the energy needed to undertake a wide range of neuronal functions, such as the maintenance of resting membrane potential, the restoration of ionic balance after depolarization, the cycling of synaptic vesicles, and the transport of proteins and organelles from the soma to distal sites. Interestingly, the mitochondria in axons and dendrites have different morphologies, some are small and sparsely distributed in the former, whereas others are elongated and densely distributed in the latter. In addition, axonal and dendritic mitochondria differ in movement, metabolism, and responses to neuronal activity [59].
Secondly, several lines of evidence support the role of mitochondria in the mobilization and recycling of synaptic vesicles [60]. Neurotransmission is underpinned by endocytosis and the local filling of synaptic vesicles in the presynaptic terminal [61]. Furthermore, the mitochondria support synaptic activity through cytosolic calcium reabsorption, a critical buffering mechanism for establishing and maintaining synaptic activity and preventing neuronal toxicity and excitotoxicity. Moreover, mitochondrial calcium buffering appears to be necessary at the synapse, even when the neuron is at rest: synaptic terminals lacking mitochondria show a higher frequency of spontaneous release of neurotransmitter-containing vesicles [62].
Overall, the importance of the mitochondria in neurons is unequivocal. Therefore, mitochondrial dysfunction leads to a plethora of severe conditions, from impaired neuronal development to various neurodegenerative diseases.
3. Role of Mitochondria in Neurodegenerative Disease
A great deal of evidence shows the effects of mitochondrial dysfunction on degenerative diseases [63]. Diseases caused by mitochondrial alterations often show a neurodegenerative component involving the nervous system. Similarly, mitochondrial defects are frequently observed in tissue samples taken from patients with a neurodegenerative disorder. This evidence reflected the high-energy requirements for all biological processes [64]. The continuous increase in energy demand, mainly due to excess food consumed daily, the constant increase in energy required for thermoregulation, or the increase in energy needed to counteract the environmental toxicity caused by humans, leads to an overwork of the mitochondria, resulting in impaired bioenergy efficiency [64,65]. It was demonstrated that cells exposed to an opulent nutrient environment are inclined to have their mitochondria in a fragmented state. Moreover, the mitochondria observed in cells in malnourished conditions remain for a longer period of time in the associated state [66,67]. This portrays the fact that the mitochondria can change their architecture and, therefore, their bioenergy capacity according to external events.
A degenerative disease is regarded as a type of non-physiological condition whose origins in a tissue or organ worsen over time. Energy failure has been linked to degenerative processes such as cancer, aging, neuroendocrine disease, neurodegenerative disease, and inflammatory diseases. Likewise, results from many studies support the involvement of the mitochondria in neurodegenerative diseases, particularly PD [68,69,70,71,72]. In addition, studies relating to the MPTP toxin first highlighted the role of mitochondrial complex I dysfunction and neurodegeneration in PD [73]. In association with other commonly used toxins, such as rotenone and paraquat, MPTP offered a new insight into defining the effective role of mitochondrial bioenergy in neurodegeneration [74]. Indeed, more than five thousand manuscripts dealing with the association between Parkinson’s disease and mitochondrial alteration can be found on PubMed (Figure 2). In addition, due to studies on PD, particularly genetic Parkinsonism, all mitochondrial dysfunctions leading to cell death have been well defined [75]. The PINK1 gene mutation, responsible for an early onset of Parkinsonism, serves as a good example [76]. This gene codes for the mitochondrial protein, phosphatase, and tensin homolog serine/threonine-protein kinase 1 (PTEN-induced kinase 1) [77]. PTEN-proteins can protect cells against oxidative stress, proton chain dysfunction, and bioenergy failure [76,78]. The PINK1 gene has also been identified as an oncogene with tumor suppressor properties [77,79,80]. Additionally, the PINK1 protective role has been observed in many disorders characterized by progressive inflammation and neurodegeneration, such as Alzheimer’s disease, multiple sclerosis, amyotrophic lateral sclerosis, and HD [77]. In physiological conditions, PINK1 is translocated inside the mitochondria in its mature isoform with the aim to withstand the activity of the mitochondrial chain and to produce (at the level of complex I) the molecules of ATP showing the maximal bioenergetic efficiency (Figure 1). Moreover, PINK1 is also located in the inner mitochondrial membrane and can interact with the chaperone TRAP1 (also recognized as an interactor of the type 1 tumor necrosis factor receptor) to maintain important bioenergetics and proteostatic functions [77,81]. Cleaved PINK1 can interact with other chaperon proteins, and when it is located in the cytosol, it can activate the m-Tork/Atk pathway. In addition, PINK1 can mediate the phosphorylation of another important gene for PD: Parkin. The coupled action of PINK1 and Parkin may induce mitochondrial fusion in order to cause the elimination of dysfunctional mitochondria. Furthermore, PINK1 is also involved in the formation of an autophagosome complex by the activation of beclin protein and may regulate the apoptotic process [77]. The role of the PINK1 gene in PD was well investigated by the use of animal models and cellular cultures of human fibroblasts [82,83,84,85,86,87] (Table 1). These studies have confirmed the role of bioenergetic efficiency in the maintenance of a healthy state or the progression of neurodegeneration. However, the absence of PINK1 results in low bioenergetic efficiency and programmed cell death after each minor stress [86,88]. It is very interesting to note that PINK1 not only has a key role in degeneration, oncological degenerative processes, and neurodegenerative PD but also in the neurodegeneration found in HD. Parkinson’s disease (PD) and HD are two different neurological diseases involving the central nervous system. The symptomatology of both is quite similar (cognitive impairment, limb inflexibility, and problems walking or talking), but while PD results from a combination of genetic and environmental factors, HD is only an inherited genetic disease. Both pathologies have a common factor, which is the loss of bioenergetic efficiency [77,89].
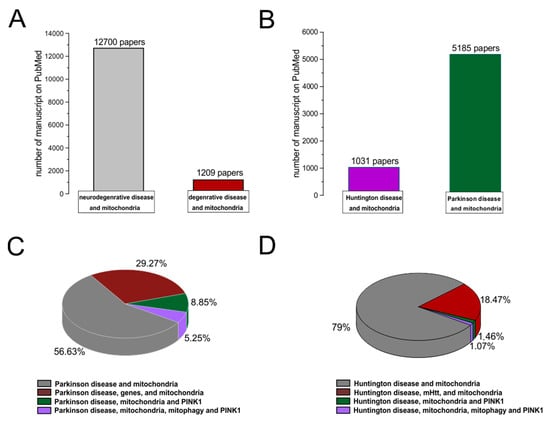
Figure 2.
PubMed evidences of mitochondrial involvement in neuro-degenerative/degenerative diseases. Records were obtained from research articles published until January 2023. Article publications were obtained from the following PubMed searches: “Neurodegenerative disease and mitochondria; degenerative disease and mitochondria (A); HD and mitochondria; PD and mitochondria (B)”. (C,D) To realize the pies on sub-items, we have selected “PD, genes, and mitochondria; PD, PINK1, and mitochondria; PD, PINK1, mitophagy, and mitochondria; HD, mHHT, and mitochondria; HD, mHHT, mitophagy, and mitochondria.

Table 1.
Alteration of mitochondrial bioenergy function identified in genetic mouse models of PD and HD. The table summarizes the major mitochondrial changes found in murine models of PD and HD. The legend of the abbreviations is listed below.
Huntington disease is an incurable degenerative disorder caused by a mutation in the huntingtin gene, where the CAG sequence is excessively repeated. This mutation alters numerous cellular processes and leads to cell apoptosis. One important alteration is caused by the impairment of the mitochondrial metabolism. In a study conducted on the fly model, the formation of an abnormal ring-shaped mitochondria was observed; this particular shape was previously identified in mitophagy-blocked cells in which PINK1 overexpression was able to rescue the regular shape and function of the mitochondria. PINK1 over-expression was able to improve bioenergetic efficiency (increasing ATP levels) and rescue neuronal integrity in the adult drosophila model of HD [89]. HD is a degenerative pathology caused by the pathological expansion of CAG repeats in the Huntington gene, which codes for the Huntingtin protein. The gene is located on chromosome number 4 and is characterized by high levels of polymorphism. Unlike PD, HD has an age range of about 35–44 years and an estimated post-onset life span of 15/18 years. It is also characterized by an advanced motor disability, including chorea [111]. Enough evidence suggests that the mutation may cause an alteration in mitochondrial trafficking, an increase in oxidative stress, dyshomeostasis in intracellular calcium content, an alteration in bioenergetics, and an alteration in a respiratory mitochondrial chain [112,113,114,115]. Huntington’s disease, PD, and Alzheimer’s disease are three neurodegenerative diseases that have 37 common genes and about 40% of whose products act at the mitochondrial level [116]. These neurodegenerative diseases are coupled to a physiological degenerative process called aging or senescence that starts at the mitochondrial level and results in reduced bioenergetic efficiency [117]. Cellular senescence is characterized by heavy changes in cellular metabolism and an increase in pyruvate utilization that may produce differences in phosphorylation states, thereby increasing the activity of the mitochondrial pyruvate dehydrogenase complex and ROS production [118,119]. These features may lead to an impairment of cellular function that induces degeneration, as in the case of cancer or other neurodegenerative processes [120]. Moreover, recent data have demonstrated that key oncogenes and tumor suppressors modulate mitochondrial metabolism and dynamics. Indeed, different types of cancer result in more or less sensitivity to the modulation of mitochondrial function in the lesion caused by the tumor [121]. Therefore, the mitochondria play a cardinal role in many degenerative processes, which are mostly demonstrated in animal models.
4. Mitochondria Bioenergy in Parkinson’s Disease and Huntington Disease in Rodents Animal Models
Parkinson’s and Huntington’s diseases are both neurodegenerative diseases with multiple mitochondrial bioenergy alterations related to metabolism, oxidative stress, dynamics of biogenesis transport, and mitophagy (Table 1). As a result of the close relationship between neurodegeneration and mitochondrial bioenergy, one might presume that changes in mitochondrial homeostasis with the release of calcium and NO, as well as reactive oxygen species (ROS), will affect the unfolded protein response, modulating specific signaling molecules, and reprogramming mitochondrial bioenergy [122] (Table 1). The mouse models used in the study of PD and HD serve as valuable tools in defining the role of mitochondrial bioenergy in the pathogenic mechanisms of these diseases (Table 1).
4.1. Parkinson Disease
4.1.1. Neurotoxin-Induced and Autosomal-Dominant PD Models
Parkinson’s disease animal models are associated with multiple mitochondrial defects for review [122,123,124,125] (Table 1). Indeed, human idiopathic PD patients showed abnormalities in mitochondrial activity with reductions of complex I, NADH: ubiquinone oxidoreductase, and strikingly reduced succinate: cytochrome c oxidoreductase, suggesting an etiological role in the pathogenesis of PD [126].
Chemically, the neurotoxin agent is widely used to recapitulate Parkinsonian features in various animal models [127,128]. In fact, the neurotoxins 1-metil-4-fenil-1,2,3,6-tetraidropiridina (MPTP), rotenone, and paraquat block mitochondrial bioenergetics in dopaminergic neurons and induce Parkinsonian syndrome for review [129,130,131,132,133,134,135]. However, it is important to consider that overexpression of α-synuclein or agents that generate stress or cytosolic acidification show the translocation of α-synuclein into the mitochondria or in the mitochondrial membrane [136,137,138]. α-synuclein is a presynaptic neuronal protein that is linked to familial and idiopathic PD [139]. Both the over-expression and loss-of-function of the SNCA gene contribute to the manifestation of the disease [140], according to the “α-synuclein cascade hypothesis” [141].
Experimental studies in A53T mutant mice, a transgenic animal model that expresses human α-synuclein, show that they develop mitochondrial DNA damage and degeneration with an apoptotic-like death of neocortical, brainstem, and motor neurons [91]. In the same animal model, mitochondrial complex IV activity was reduced significantly in the spinal cord [142]. Additionally, in vivo human α-synuclein expression is associated with a decrease of Drp1 (Dynamin-related protein 1), a major player in the regulation of mitochondrial dynamics and the maintenance of their proper function [91]. On the other hand, the dysfunction of Drp1 in the mitochondria is also associated with enlarged neuronal mitochondria [91]. Other proteins, such as Mitofusin1 (Mfn1) [91], that facilitate mitochondrial fusion are decreased in these models, which correlate with the mitochondrial bioenergy dynamic changes. Previous studies in A53T mice suggest that α-synuclein distresses the mitochondrial morphology and reduces both Mfn1 and Mfn2 in an age-dependent manner [90]. In addition, an in vivo study in midbrain dopaminergic neurons suggested that α-synuclein predominantly accumulates in the central mitochondrial membrane and interacts with complex I, resulting in impaired activity of the mitochondrial electron transport chain [92]. Consistent with these findings, other experimental analyses show that after post-inoculation of human α-synuclein in rats, its accumulation in striatal dopaminergic terminals from the SNpc and early synapse loss were observed [143]. To explain this early synapse loss, a proteomic analysis using a real-time cell metabolic method and isolated synaptosomes was used to investigate the functional and molecular mechanisms. Upon injection in isolated striatal synapses, multiple dysfunctions of mitochondrial bioenergetics and morphology were detected with the upregulation of PRKAG2 and TTR, respectively, which are sensor proteins of the cell energy status and markers of oxidative stress [143]. Moreover, ultrastructural examination of subsequent human α-synuclein accumulation showed an altered expression of Rab5 endocytic and LC3 autophagic proteins that potentially reflect an accumulation of autophagic vesicles [143]. This study allowed the evaluation of an interesting aspect, namely the effect of oxidative stress and the mitochondrial bioenergy alteration on both dopaminergic neuron death and striatal neurons. These could be both responsible for the induction of Parkinsonian syndrome.
The neurons are high-energy consumers and employ most of the energy at the level of the synapse to preserve and restore ionic gradients and for the uptake and recycling of neurotransmitters [93]. Various mouse lines expressing a different type of α-synuclein present impaired dopamine neurotransmission and striatal synaptic plasticity [144]. In rodents, the injection of α-synuclein with an adeno-associated viral vector blocks the induction of long-term potentiation (LTP) and long-term depression (LTD) that are normally expressed in medium spine neurons (SPNs) [135,145,146,147], which are associated with early memory and motor alterations. There is sufficient evidence showing that α-synuclein misfolding and aggregation lead to mitochondrial stress, which appears to be related to the dysfunction of synaptic plasticity. Another interesting aspect is that α-synuclein overexpression via the endoplasmic reticulum (ER), promotes Ca2+ transfer in the mitochondria, while its silencing impairs mitochondrial function by loosening the ER-mitochondria interface [148]. However, the precise mechanism by which α-synuclein accomplished pathological characteristics and specific cell death remains obscure. Nevertheless, it is important to note that α-synuclein plays a pro-apoptotic role in different neuronal cells [149]. The wild-type α-synuclein can defend neurons from apoptosis by inhibiting caspase-3, after which the mutant α-synuclein loses this activity [149]. Caspases-3 actions then cause general damage and degeneration, aggregate in synapses, and persist in neurons without causing acute cell death [150,151,152] (Table 1).
4.1.2. Autosomal-Recessive PD Models
The discovery of many genes related directly or indirectly to the mitochondria in PD underlines the implication of mitochondrial bioenergy in its pathogenesis. For instance, genes linked to early-onset recessive PD include: Parkin [83], PINK1 [76], and DJ-1 [153]. These genes have been strongly implicated in the mitochondrial morphology, damage, and degradation via mitophagy [154], which is involved in the mitochondrial activity and quality control mechanisms [77,155,156,157]. Parkin encodes a protein localized in the cytoplasm that contains an N-terminal ubiquitin-like domain with a function as E3 ubiquitin-protein ligase [158,159,160]. Parkin knockout (KO) mice show alteration in the levels of different proteins involved in detoxification, stress-related chaperones, without neuronal degeneration [161]. Parkin also has neuroprotective effects through the regulation of different cellular processes or pathways, such as mitochondrial swelling and cytochrome c release [100,162,163]. Normally, Parkin resides in the cytoplasm but can translocate to depolarized or damaged mitochondria to mediate their removal by mitophagy in cooperation with PINK1 and potentially other factors [164]. In a mouse model, Parkin KO was reported to cause a decrease in subunits of complexes I and IV with a reduction in peroxide reductases. In addition, the serum also aids in the reduction in antioxidant capacity and increases protein levels of lipid peroxidation [165]. Other studies on Parkin-deficient mice showed an increase in the content of dopamine in the striatum and the consequent reduction in the excitability of striatal SPNs [101]. Deficits in glutamate neurotransmission and amphetamine-induced dopamine release were also observed in other Parkin mutant mice with an increase in the metabolism of dopamine (MAO) [166]. Functional analysis of striatal SPNs showed an impairment of bi-directional corticostriatal synaptic plasticity with the loss of LTD and LTP in Parkin KO mice, while synaptic plasticity was not altered in the hippocampus of these animals [167]. The dopaminergic defect [101,166] may explain the selectively enhanced sensitivity to the striatal group II metabotropic glutamate receptor in cortically evoked excitatory post-synaptic potentials recorded from SPNs. This reinforcing effect is an adaptive change [78]. Furthermore, recent evidence in Parkin-KO rats suggests that the lack of Parkin is also necessary for the maintenance of post-synaptic endocytosis of AMPARs and for the decreased expression of the post-synaptic protein Homer1, which is essential for coupling the AMPA receptor endocytic zones with the post-synaptic density [168]. On the other hand, changes to the numbers or function of ER-mitochondrial contact sites may also affect mitochondrial calcium homeostasis, which is regulated by Parkin [169]. Calcium uptake into the mitochondrial matrix results in enhanced respiratory function, thereby tuning synaptic activity [60,170]. Recent studies also showed that the conditional Parkin KO in adult animals expresses the progressive loss of dopamine neurons. Moreover, the overexpression of PGC-1α leads to the selective loss of DA neurons in the substantia nigra [171].
Another gene linked to early-onset recessive PD is PINK1, which encodes a protein with a serine/threonine kinase catalytic domain, whether cytosolic or mitochondrial-associated [77]. PINK KO mice models exhibited significant functional impairment of synaptic plasticity and mitochondrial morphology without motor deficits or dopaminergic neuronal loss [83]. Consistent with these findings, PINK KO mice models generated with the silencing of the PINK1 gene did not develop dopaminergic neurodegeneration [172]. The PINK1 heterozygous KO mouse also showed selective impairment of LTP with a normal expression of LTD [173]. Nevertheless, low doses of rotenone were sufficient to induce severe alterations of the corticostriatal LTP and LTD [84]. This led to the proposal that the PINK1 homozygous KO model represents a good model to study the effect of gene-environment interaction. Although PINK1 KO rats show mitochondrial alteration, locomotor deficits, and α-synuclein aggregate in several brain regions, such as the cerebral cortex and dorsal striatum, it is also responsible for the degeneration of the substantia nigra [174,175,176,177]. However, this model presents clear symptomatic deficits that occur between 4 months and 9 months [176]. In the same model, using magnetic resonance spectroscopy, the mitochondrial metabolomic alterations increased in the cortex and striatum during the asymptomatic period, which coincides with the normal metabolic alterations [176]. Functional and bioenergetics experiments revealed that mitochondrial alterations in PINK1 KO rats are due to impaired complex I respiration, a dysfunction in ATP synthase, and reduced substrate oxidation [175]. New scientific research performed in animal models with the deletion of PINK1 mice provided an indication that a lack of PINK1 results in impaired synaptic plasticity, which is caspase-mediated [86]. Caspase-3 presents an additional role in neuronal processes, including the regulation of synaptic function and pruning [95,151,152]. Previous studies of PINK1 KO show a loss of LTP and LTD with alterations in dopamine release [83]. Indeed, low activation of caspase-3 is critical in restoring cortical-striatal LTD [86]. However, high activation is involved in degenerative processes [152]. In PINK1 KO mice, analysis with electron microscopy and other functional studies of the striatum revealed significantly enlarged mitochondria and impaired activity [178]. Likewise, the functional examination showed impaired mitochondrial respiration and aconitase activity in the striatum but not in the cerebral cortex [178]. Additional studies of PINK1 KO mice showed a decreased dopamine release in the dorsal striatum in an age-dependent manner with an impairment of the basal mitochondrial respiration [88].
DJ-1 gene mutations cause autosomal recessive early-onset PD. DJ1 encodes a protein of the superfamily that belongs to the ThiJ/PfpI-like. Under normal conditions, DJ-1 is confined throughout the cytoplasm of neurons, with a small amount localized to the mitochondrial matrix and intermembrane space [179], indicating a role in the balance of the mitochondrial physiology [17]. In a study, it was reported that DJ-1 KO mice failed to express PD features such as substantia nigra degeneration or the formation of protein inclusions similar to PINK1 and Parkin KO mice [180]. Recent experiments in DJ-1 KO mice showed increased calcium influx into the neuron during its activity, creating basal mitochondrial oxidant stress [94,181]. Functional analysis of isolated mitochondria showed increased ROS and decreased aconitase activity in DJ-1 KO mice [182]. Additionally, DJ-1 gene deletion revealed a deficit in scavenging because the loss of the DJ-1 pathway resulted in the loss of atypical peroxiredoxin-like activity [183]. In contrast, an independent study of DJ-1 KO mice reported an increase in mitochondrial respiration-dependent H2O2 consumption, mitochondrial Trx activity, total glutathione (GSH and GSSG, respectively) levels, mitochondrial glutaredoxin (GRX) activity, and a decrease in mitochondrial glutathione reductase (GR) activity [96]. Furthermore, new research on the semi-quantitative measurement of cerebral metabolites in DJ-1 KO mice showed significantly increased glutathione (GSH) levels and GSH/glutamate (Glu) ratio in the prefrontal cortex [97] (Table 1). It is important to note that the GSH system is more important than the antioxidant system in ROS detoxification in the brain [98,99,184]. Accumulated scientific information hypothesized that the increased sensitivity to oxidative stress in DJ-1 KO mice and dopaminergic neuronal cells is related to a decrease in ROS scavenging arising from deteriorated peroxidase-like scavenging with a deficiency of Nrf2 transcriptional factors and increased mitochondrial dysfunction due to a complex I deficiency [182,185,186,187,188]. Although DJ-1 KO mice had normal corticostriatal LTP, LTD was absent [101]. Overall, the data from animal models provide a framework that suggests that genes linked to early-onset recessive PD are important for maintaining normal mitochondrial bioenergy function (Figure 3). Gradually, the mutation protein described above, which is related to mitochondrial bioenergy, activates the response to mitigate mitochondrial alterations. Indeed, the genetic inactivation of PINK1/Parkin/DJ-1 with a triple KO mouse exhibits abnormalities in the mitochondrial pathway, activity, or morphology without PD-related phenotypes [189].
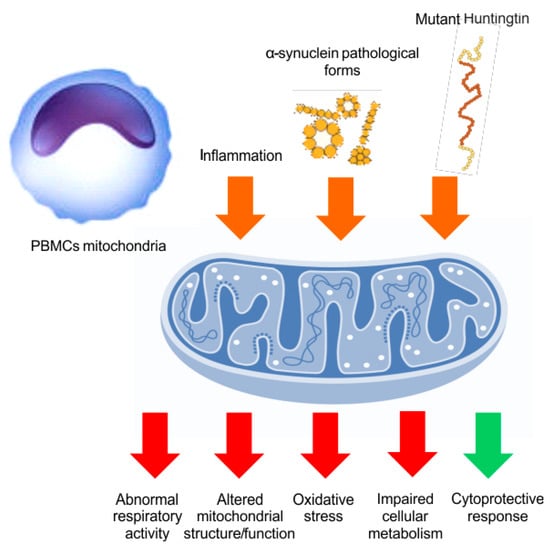
Figure 3.
PBMCs mitochondrial function. In PD and HD, the systemic inflammation and the circulating neurodegeneration-related peptides (orange arrows) might affect the PBMCs mitochondrial function, inducing altered function or morphology, abnormal respiratory activity, increased oxidative stress, and impaired cellular metabolism (red arrows). However, some protective mechanisms could also be triggered (green arrow), especially at early disease stages, although they would be later overwhelmed by clinical-pathological progression.
4.2. Huntington Disease
With regards to HD, several transgenic mice have been engineered to address specific pathological characteristics [190]. Multiple scientific information sources implicate that the gain and/or loss of function of mutant HTT leads to impaired mitochondrial bioenergy with alteration of oxidative stress, dysfunction of mitochondrial trafficking, and mitochondrial calcium dyshomeostasis [115]. In fact, malonate and 3-nitropropionic acid (3-NPA) are considered potent neurotoxins and are used to induce experimental HD in rodents using pharmacological models [191]. These oldest models provided the first piece of evidence that mitochondrial bioenergy, targeting the electron transport chain, is involved in the pathophysiology of HD. However, degenerating mitochondria have been identified in different areas, including the brain, liver, and muscle, in genetic mouse models of HD [192,193,194] (Table 1).
Both R6/1 and R6/2 strains express a single copy of a human genomic fragment that contains 116 and 144 CAG repeats of HTT under the control of the human HTT promoter [195]. These mice express a relatively rapid onset and progression of symptoms that include motor defects and neurodegeneration. Another major contribution to the research was the generation of knock-in mice models where CAG was repeated extensively (CAG94 and CAG140), compared to the human trans gene, for developing HD-associated phenotypes [190]. The R6/2 mice models have been the most widely used because the disease starts earlier and progresses more rapidly compared to the YAC, BAC, and other knock-in lines [196]. Transgenic animal models expressing the amino (N)-terminal fragment of the mutant form of HTT (R6/2) show an increase in 8-hydroxy-2-deoxyguanosine OH(8)dG during the late stages of the illness [197]. Therefore, oxidative stress occurs in the striatum before the onset of motor symptoms. In addition, biochemical analysis performed in the striatum of R6/2 mice at 12 weeks showed a significant reduction in the mitochondrial complex IV activities and a decrease in aconitase in the cerebral cortex [198]. In accordance with these data, new scientific research using synaptosomes isolated from R6/2 mice showed a decrease in the mitochondrial mass with increased ROS production and antioxidant levels in the striatum compared to the cortex [199], indicating oxidative stress. Additionally, in the same model in depolarized conditions, the oxygen consumption rates in synaptosomal cells were significantly amplified, which was accompanied by a clear increase in the mitochondrial proton leak of the striatal synaptosomes [199], indicating synaptic mitochondrial stress. Indeed, synaptic dysfunction occurs in various mouse models with HD, which appears to be associated with the dysfunction of LTD but displays a physiological LTP with lost synaptic depotentiation. These have been directly connected with the onset of cognitive deficits in HD models [103,107,108,200,201]. Using the brain slices from R6/1 mice at different ages, we observed a significant increase in Ca2+ content after glutamate stimulation. Such alterations have an impact on mitochondrial dysfunction with a decrease in NAD(P)H fluorescence and a loss in mitochondrial membrane potential (ΔΨm) [202]. Several groups characterized the reduction in N-acetyl aspartate in R6/2 mice, similar to symptomatic HD patients [102,203,204,205,206], and noticed fluctuations in some metabolites, which suggest the impairment in cellular metabolism and mitochondrial bioenergy [207].
Another interesting contribution to assessing the deficits of mitochondrial function and redox deregulation is the experimental data performed with PET in YAC128 transgenic mice [104]. PET analysis displays a specific accumulation of [64Cu]-ATSM in the striatum with concomitant alterations in mitochondrial respiration and ATP production associated with increased complex II and III activities. In turn, an increase in mitochondrial H2O2 levels in YAC128 mice and defects in Ca2+ handling [104] support an early increase in the striatal susceptibility of mitochondria. Furthermore, functional assays revealed an impairment of the mitochondrial respiratory capacity in the striatum and cortex of R6/1 mice. It is important to note that N-acetylcysteine administration delayed the onset and progression of motor deficits in R6/1 mice [208], which may have reduced both excitotoxicity and oxidative stress in the striatum. A recent study established that voluntary wheel running reduces hindlimb clasping in the R6/1 mouse model. Furthermore, chronic exercise in the R6/1 showed the clasping phenotype with normal mitochondrial respiration in the cortex and striatum, suggesting that mitochondrial dysfunction may not be necessary for the progression of symptoms [105]. At the molecular level, different studies explored the role of the mitochondria in apoptotic cell death processes with elevated caspase activity in HD mice models [106,192]. Interestingly, HTT has been shown to be involved in vitro with caspases-1, 2, and 3 [109,209,210]. In fact, immunostaining analysis of YAC72 mice displays an increase of caspase-2 in the SPNs within the striatum with concomitantly decreased levels of a brain-derived neurotrophic factor in the cortex and striatum at 3 months [209]. This indicates that caspase-2 participates selectively in the neurodegeneration of SNPs in the striatum. In contrast, in YAC128 mice models, the lack of caspase-2 results in protection from the well-validated motor and cognitive features of HD [211]. Moreover, previous studies in transgenic mice expressing exon 1 of the human HTT demonstrate that intracerebroventricular administration of a caspase inhibitor 1 delays disease progression [211] (Table 1).
In conclusion, while HD animal models have not yet provided definitive evidence whether or not mitochondrial bioenergy is critically involved in HTT-induced disease symptoms compared to PD animal models’ genes, mitochondrial bioenergy defects are nonetheless a major component of both diseases progression. On the other hand, the creation of HD mouse models using different strain backgrounds and expressing only a portion of the HTT protein leads to increased difficulty in understanding the compelling findings that were obtained with the genetic models of PD.
5. Mitochondrial Bioenergy in Parkinson’s Disease and Huntington Disease, Based on Human Evidences
Assessment of mitochondrial activity (or dysfunction) in the CNS of living patients is not easy. However, analyzing peripheral tissues and fluids can offer a reliable measure. Although the core pathology in PD and HD is central, a number of substantial abnormalities occur at the systemic or peripheral level. Peripheral blood mononucleate cells (PBMCs) are one of the most reliable “in vivo PD models”. Peripheral blood mononucleate cells present several alterations in critical metabolic pathways and accumulate α- synuclein in pathological forms, well recapitulating PD-related neuropathology [212,213]. In a recent study, the mitochondrial bioenergetics of PBMCs (Figure 2) from PD patients was assessed using the Seahorse Bioscience technology. The results showed a peculiar pattern of mitochondrial respiration, including normal basal respiration, significant augmentation of the maximal and spare respiratory capacities, and a tendency to increase ATP production. The increased spare respiratory capacity was found to follow the disease duration and severity of motor disturbances, revealing some mitochondrial adaptations to the higher bioenergetic requirements occurring at later disease stages [110]. Similarly, consistent findings of greater mitochondrial respiration were also observed in different cell lines obtained from PD patients, such as lymphoblasts (immortalized blood lymphocyte-derived cells) [214] and fibroblasts [215,216]. Therefore, it is reasonable to assume that in PD patients, unlike animal models, mitochondrial activity may increase in a compensatory manner. Indeed, the “nuclear factor erythroid 2-related factor 2” (Nrf2) pathway, a master regulator of cellular defense and mitochondrial activity, can be overexpressed in PD patients’ PBMCs in proportion to the disease duration, suggesting a systemic defensive response to the PD clinical-pathological progression [213]. Otherwise, changes in respiratory activity could reflect defects in mitochondrial structures, metabolism, or respiratory protein functions [217]. In another study, PBMCs showed increased glycolysis and deficits in superoxide dismutase, together with a peculiar mitochondrial vulnerability in the monocyte subpopulation in PD patients’ [218]. Substantial evidence can also be obtained from the assay of mitochondrial dysfunction biomarkers in PD patients’ fluids. Indeed, metabolomic studies performed in blood and cerebrospinal fluid (CSF) disclosed various abnormalities in metabolic pathways related to the mitochondria (in alanine, branched-chain amino acids, fatty acids, and acylcarnitines levels; in steroidogenesis; in the glutathione cycle) [219,220,221]. Likewise, the redox balance-related biomarker levels, such as uric acid [222,223,224,225], catalase, total glutathione [226], nonmercaptalbumin (an oxidized form of albumin) [227], oxidized glutathione [213], oxidized DJ-1 [228], α-klotho [229], lactoperoxidase [230], and heme-oxygenase-1 [231], were altered in the same fluids. Genomic studies performed on peripheral blood showed the down-regulation of genes critical for mitochondrial functions (COX4I1, ATP5A1, and VDAC3) [232], while the analysis of blood-circulating extracellular vesicles demonstrated the reduction in mitochondrial components (i.e., ATP5A, NDUFS3, and SDHB) in PD patients [233]. Furthermore, analysis of mitochondrial bioenergetics has also been performed in peripheral tissues of HD patients, with some controversial findings depending on the matrix or the experimental technique used. Indeed, mutant huntingtin is expressed outside the CNS as well, accounting for the substantial impairment in multiple cellular pathways related to the mitochondria and redox balance [234] (Figure 2). It has also been observed that skin fibroblasts may show some energetic, respiratory, redox, and morphological abnormalities [235,236]. Likewise, several functional or structural mitochondrial defects have been noticed in HD patients lymphoblasts [237]. Finally, increased oxidative damage, reduced antioxidant capacity, and mitochondrial abnormalities have been tracked even in HD patients’ blood through different fluid markers [238].
6. Discussion
Degenerative diseases commonly entail mitochondrial impairment. The mitochondria represent the major source of bioenergy for the cell, and as a consequence, their putative failure leads to a lack of energy. Daily energy metabolism for both humans and organisms is the sum of daily energy expenditure and daily energy intake. Daily energy expenditure may be divided into different categories: (1) energy spent on basal metabolism; (2) energy spent by thermic increment following food intake; (3) energy spent on thermoregulation; and (4) energy spent during daily activities [239]. In particular, energy metabolism is the process of generating energy (ATP) from nutrients, and mitochondria represent the main powerhouse of the cell. To produce energy in the form of ATP and GTP, all the cells, especially the neurons, consume glucose, amino acids, and fatty acids [239]. These nutrients are processed and transferred into the tricarboxylic acid (TCA) cycle, and electrons are stored in their reducing equivalents, NADH and FADH2, through iterative oxidations. NADH and FADH2 serve as carrier molecules that transport electrons into the electron transport chain (ETC), while protons flow down to generate ATP [240]. The capacity of the mitochondria to supply energy in the form of ATP when required could be referred to as bioenergetic efficiency, which may change due to the effects of environmental stress, aging, or pathologies. For example, during aging, the mitochondria undergo alterations in their capacity to produce ATP [240,241]. This is due to the release of a large number of reactive oxygen species (ROS). ROS are able to increase spontaneous DNA mutations and can start the processes that lead to cancer [241,242].
Mitochondrial biogenesis is a process that continuously balances the degradation of dysfunctional mitochondria inside the cells. The disruption of this homeostasis, which may be guaranteed in all functioning cells, represents an additional point that focalizes the loss of energetic function, which may precede cellular loss. This critical balance is called mitochondrial dynamics. During these processes, mitochondrial fusion and fission regulate the number and size of the mitochondria, while mitochondrial biogenesis increases the mitochondrial mass inside the cells by producing ATP as a response to greater energy demand. Mitochondrial biogenesis requires a very multifaceted process that starts with the coordination of the nuclear and mitochondrial expression programs. The regulator for the cited process is the protein PGC-1α, which is able to interact with many transcription factors, such as myocyte enhancer factor 2C (MEF2C), nuclear respiratory factors 1 and 2 (NRF1 and NRF2), peroxisome proliferator-activated receptors (PPAR), estrogen-related receptor alpha (ERRα) and many others involved in coordinating mitochondrial biogenesis and oxidative metabolism [243,244,245]. Moreover, NRF1 and NRF2 cooperate to upregulate the transcription of several nuclear-encoded genes that act in the negative feedback effect on mitochondrial production [244,246]. Selective autophagic degradation, called mitophagy, recruits the damaged mitochondria into a pre-autophagosome structure via a PINK1/Parkin-dependent process. Indeed, the mitochondrial transport system is essential for the distribution of the mitochondria into the cells and, in particular, into the different neuronal structures, which require the synthesis of the mitochondria and their proteins [247,248,249,250].
A consistent number of mitochondria are localized at the presynaptic terminals, to provide ATP for intense synaptic activity [251]. In fact, it was hypothesized that the loss of synaptic plasticity found in PINK1 KO mice was a result of the lack of ATP at the presynaptic terminal, thereby confirming the essential role of mitochondria in this area [252].
Previously, we have referred to the role of the PINK1 gene in early inherited parkinsonism, and these findings are in support of the theory that links mitochondrial dysfunction to PD.
In addition, the mitochondria in aging people show different features [253]; they swell while their numbers dwindle and are unable to replace themselves as quickly in their dysfunctional state [254]. Parkinson’s disease is also an aging disease, in its idiopathic form, and this suggests a link with the common non-genetic form of PD. Therefore, failure in the mitochondria is the starting phase of the entire neurodegenerative process [255].
It was demonstrated in both PD and HD that the presence of environmental stress can increase the production of oxidative species and can also modify the structure of proteins and DNA inside the mitochondria and its nuclei [256]. Usually, HD is considered a hyperkinetic disorder despite the presence of hypokinetic features in the motor symptoms [250]. Conversely, PD is considered a hypokinetic disorder, in which the resultant speech motion disease may be classified as hyperkinetic or hypokinetic dysarthria [257,258]. Both are defined as neurodegenerative disorders causing the gradual loss of neurons, and we can explain why. Concerning HD, the mutant HTT protein was shown to be able to directly impair the ability of PGC-1α to activate target genes related to mitochondrial biogenesis and normal mitochondrial function. The amplification of mHTT proteins enhances mitochondrial depolarization and swelling, which are calcium dependent. In addition, it has been demonstrated that mutant HTT’s ability to combine specifically with the beta-tubulin subunit can adversely affect mitochondrial transport. Moreover, both disorders result in a deficit at the level of the basal ganglia, with peculiar references to the striatum [259] and hippocampal areas [260].
In rodent models of HD, only the progression of the disease leads to neuronal loss. Cell dysfunctions may generate symptoms, and in particular, abnormal synaptic statements due to a lack of ATP [261]. In contrast, in human patients, clinical symptoms occur as a combination of functional and structural changes that may result in a reduction in lactate levels in the brain area as a consequence of an altered mitochondrial metabolism [250].
In PD models, dysfunctions in striatal plasticity are described in detail [135] and are strictly connected to energy failure [132,135].
These areas are linked to movement and cognitive ability. For this reason, it is not a surprise that both diseases can affect cognitive or thinking abilities and motor functions. Studies on rodent models of PD show impairment in the bioenergetic pathway and a block in function in mitochondrial complexes I and III. Additionally in genetics, phenotypic models were induced by toxin exposure in the early stages of the disease as well as in the developmental stage [86,94,122,124,125,238]. On the other hand, studies on animal models of HD show an evident alteration in mitochondrial complexes II and III and describe a reduction in bioenergetic efficiency that was described essentially in the early pathogenic mechanism [262].
Interestingly, this evidence has been confirmed by translational studies in men, in which the association between mitochondrial deficit and biomarkers of PD and HD was shown [252]. Actually, there is still no cure for pathology, and drugs that hinder the development and severity of the diseases do not exist. Consequently, the knowledge of pathogenetic mechanisms and the similarities between these diseases can help us while reviewing the traditional approach in order to develop novel approaches. In fact, new pharmacological approaches focused on mitochondrial target agents could be used in the future to cure degenerative and, in particular, neurodegenerative pathologies.
7. Future Perspectives
Numerous evidences show the role of mitochondria in neurodegenerative diseases. Approximately 1209 papers described the mitochondria’s involvement in non-neurological degenerative processes (cancer, aging, and other pathologies) (Figure 2A,B). Among the published works on PD, 53% (of 5185 papers) talked about mitochondrial involvement in the pathogenesis of the disease, while only 5% focused on the bioenergetic role of the mitochondria and the regulatory and protective role of the PINK1 gene in the mitochondrial machinery (Figure 2C). In HD, on the other hand, 79% (of 1031 works) indicated the involvement of the mitochondria in the progression of symptoms and only 1% highlighted the protective role of the PINK1 gene in the mitochondrial machinery (Figure 2D).
Conversely, many questions remain unresolved, especially about how mitochondrial failures progress and the loss of energy efficiency triggered by environmental events. However, it is clear that in these two illnesses, the role of mitochondria in providing the energetic functions of the neuron is still lacking [4].
Therefore, in-depth studies in this field could provide more insight and knowledge about movement disorders and could offer new therapeutic solutions for pathologies, such as PD and HD, for which only symptomatic cures currently exist.
Author Contributions
Conceptualization: A.T., M.M., T.S. and G.M., bibliography search and collecting: A.T., M.M., T.S. and G.M., resources: P.B.; supervision: P.B.; visualization: G.P.; writing—original draft: A.T., M.M., T.S. and G.M.; writing—review and editing: A.T., M.M., T.S., G.P. and P.B.; table and figure: A.T., M.M., T.S. and G.M. All authors have read and agreed to the published version of the manuscript.
Funding
This work was partially supported by Italian Ministry of Health “Ricerca Finalizzata” grant RF-2019-12370182 to P.B. The funding source had no involvement in the design or writing of the report and in the decision to submit the article for publication.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
The data presented in this study are available in PubMed library.
Acknowledgments
All the authors wish to thank Massimo Tolu for his excellent technical assistance. We thank Diepriye Charls-Davies for skilled editorial assistance.
Conflicts of Interest
The authors declare no conflict of interest. The funders had no role in the design of the revision, in the collection, and interpretation of data, or in the writing of the manuscript.
Abbreviations
| A53T | α-synuclein with a PD-associated mutation |
| ADP | Adenosine diphosphate |
| AMPAR | α-amino-3-hydroxy-5-methyl-4-isoxazolepropionic acid receptor |
| ATP | Adenosine triphosphate |
| ATP5A | ATP synthase alpha-subunit gene |
| ATP5A1 | encodes for a subunit of ATP synthase (complex V) |
| BAC | bacterial artificial chromosome |
| BCL-2 | B-cell lymphoma 2 |
| Ca | calcium |
| COX4I1 | Cytochrome C Oxidase Subunit 4I1 |
| CSF | cerebrospinal fluid |
| DJ-1 | protein of the peptidase C56 family |
| DNA | deoxyribonucleic acid |
| Drp1 | dynamin-related protein 1 |
| ER | endoplasmic reticulum |
| ETC | electron transport chain |
| ETC | electron transport chain |
| FADH2 | flavin adenine dinucleotides |
| Glu | glutamate |
| GR | glutathione reductase |
| GRX | glutaredoxin |
| GSH | glutathione reduced |
| GSSG | Glutathione oxidized |
| GTP | Guanosine-5′-triphosphate |
| HD | Huntington’s disease |
| Homer1 | Homer Scaffold Protein 1 |
| HTT | huntingtin |
| IL-18 | interleukin-18 |
| IL-1β | interleukin-1β |
| IMM | inner mitochondrial membrane |
| KO | knockout |
| LC3 | Microtubule-associated protein 1A/1B-light chain 3 |
| LTD | long-term depression |
| LTP | long-term potentiation |
| MAO | Monoamine oxidases |
| MCU | mitochondrial calcium uniporter |
| Mfn1 | Mitofusin1 |
| Mfn2 | Mitofusin2 |
| MICU3 | mitochondrial calcium uptake family member3 |
| MPTP | 1-metil-4-fenil-1,2,3,6-tetraidropiridina |
| NADH | nicotinamide adenine dinucleotides |
| NCLX | mitochondrial Na+/Ca2+ exchanger |
| NDUFS3 | NADH: Ubiquinone Oxidoreductase Core Subunit S3 |
| NLR | Nod-like Receptor |
| NLRP3 | nucleotide-binding domain, leucine-rich-repeat containing family, pyrin domain-containing 3 |
| NLRX1 | NLR Family Member X1 |
| NO | Nitric oxide |
| 3-NPA | 3-nitropropionic acid |
| Nrf2 | nuclear factor erythroid 2–related factor 2 |
| OMM | outer mitochondrial membrane |
| OXPHOS | mitochondrial oxidative phosphorylation |
| PBMCs | Peripheral blood mononucleate cells |
| PD | Parkinson’s disease |
| PGC-1α | Peroxisome proliferator-activated receptor gamma coactivator 1-alpha |
| PINK1 | PTEN-induced kinase 1 |
| PRKAG2 | protein kinase AMP-activated non-catalytic subunit gamma 2 |
| Rab5 | Ras-related protein5 |
| ROS | generation of free radical species |
| SDHB | Succinate Dehydrogenase Complex, Subunit B |
| SNCA | alpha-synuclein gene |
| SNpc | Substantia nigra pars compacta |
| SPN | medium spine neuron |
| TCA | tricarboxylic acid |
| Trx | Thioredoxin |
| TTR | Transthyretin |
| VDAC | Voltage-dependent anion channel |
| VDAC3 | Voltage-dependent anion-selective channel protein 3 |
| YAC | yeast artificial chromosome |
| ΔΨm | Mitochondrial membrane potential |
References
- Osellame, L.D.; Blacker, T.S.; Duchen, M.R. Cellular and Molecular Mechanisms of Mitochondrial Function. Best Pract. Res. Clin. Endocrinol. Metab. 2012, 26, 711–723. [Google Scholar] [CrossRef] [PubMed]
- Pizzorno, J. Mitochondria-Fundamental to Life and Health. Integr. Med. 2014, 13, 8–15. [Google Scholar]
- Bruce, A. Molecular Biology of the Cell; W. W. Norton & Company: New York, NY, USA, 2022. [Google Scholar]
- Wang, Y.; Xu, E.; Musich, P.R.; Lin, F. Mitochondrial Dysfunction in Neurodegenerative Diseases and the Potential Countermeasure. CNS Neurosci. Ther. 2019, 25, 816–824. [Google Scholar] [CrossRef] [PubMed]
- Golpich, M.; Amini, E.; Mohamed, Z.; Azman Ali, R.; Mohamed Ibrahim, N.; Ahmadiani, A. Mitochondrial Dysfunction and Biogenesis in Neurodegenerative Diseases: Pathogenesis and Treatment. CNS Neurosci. Ther. 2017, 23, 5–22. [Google Scholar] [CrossRef]
- Jadiya, P.; Garbincius, J.F.; Elrod, J.W. Reappraisal of Metabolic Dysfunction in Neurodegeneration: Focus on Mitochondrial Function and Calcium Signaling. Acta Neuropathol. Commun. 2021, 9, 124. [Google Scholar] [CrossRef]
- Zhu, X.-H.; Qiao, H.; Du, F.; Xiong, Q.; Liu, X.; Zhang, X.; Ugurbil, K.; Chen, W. Quantitative Imaging of Energy Expenditure in Human Brain. NeuroImage 2012, 60, 2107–2117. [Google Scholar] [CrossRef]
- Vergara, R.C.; Jaramillo-Riveri, S.; Luarte, A.; Moënne-Loccoz, C.; Fuentes, R.; Couve, A.; Maldonado, P.E. The Energy Homeostasis Principle: Neuronal Energy Regulation Drives Local Network Dynamics Generating Behavior. Front. Comput. Neurosci. 2019, 13, 49. [Google Scholar] [CrossRef]
- Brand, M.D.; Nicholls, D.G. Assessing Mitochondrial Dysfunction in Cells. Biochem. J. 2011, 435, 297–312. [Google Scholar] [CrossRef]
- Akbar, M.; Essa, M.M.; Daradkeh, G.; Abdelmegeed, M.A.; Choi, Y.; Mahmood, L.; Song, B.-J. Mitochondrial Dysfunction and Cell Death in Neurodegenerative Diseases through Nitroxidative Stress. Brain Res. 2016, 1637, 34–55. [Google Scholar] [CrossRef]
- Jellinger, K.A. Basic Mechanisms of Neurodegeneration: A Critical Update. J. Cell. Mol. Med. 2010, 14, 457–487. [Google Scholar] [CrossRef]
- Murali Mahadevan, H.; Hashemiaghdam, A.; Ashrafi, G.; Harbauer, A.B. Mitochondria in Neuronal Health: From Energy Metabolism to Parkinson’s Disease. Adv. Biol. 2021, 5, 2100663. [Google Scholar] [CrossRef] [PubMed]
- Zhao, R.; Jiang, S.; Zhang, L.; Yu, Z. Mitochondrial Electron Transport Chain, ROS Generation and Uncoupling (Review). Int. J. Mol. Med. 2019, 44, 3–15. [Google Scholar] [CrossRef] [PubMed]
- Nolfi-Donegan, D.; Braganza, A.; Shiva, S. Mitochondrial Electron Transport Chain: Oxidative Phosphorylation, Oxidant Production, and Methods of Measurement. Redox Biol. 2020, 37, 101674. [Google Scholar] [CrossRef] [PubMed]
- Trigo, D.; Avelar, C.; Fernandes, M.; Sá, J.; Cruz e Silva, O. Mitochondria, Energy, and Metabolism in Neuronal Health and Disease. FEBS Lett. 2022, 596, 1095–1110. [Google Scholar] [CrossRef]
- McBride, H.M.; Neuspiel, M.; Wasiak, S. Mitochondria: More Than Just a Powerhouse. Curr. Biol. 2006, 16, R551–R560. [Google Scholar] [CrossRef]
- Giorgi, C.; Agnoletto, C.; Bononi, A.; Bonora, M.; De Marchi, E.; Marchi, S.; Missiroli, S.; Patergnani, S.; Poletti, F.; Rimessi, A.; et al. Mitochondrial Calcium Homeostasis as Potential Target for Mitochondrial Medicine. Mitochondrion 2012, 12, 77–85. [Google Scholar] [CrossRef]
- Pathak, T.; Trebak, M. Mitochondrial Ca2+ Signaling. Pharmacol. Ther. 2018, 192, 112–123. [Google Scholar] [CrossRef]
- Shoshan-Barmatz, V.; Ben-Hail, D. VDAC, a Multi-Functional Mitochondrial Protein as a Pharmacological Target. Mitochondrion 2012, 12, 24–34. [Google Scholar] [CrossRef]
- Rostovtseva, T.K.; Bezrukov, S.M.; Hoogerheide, D.P. Regulation of Mitochondrial Respiration by VDAC Is Enhanced by Membrane-Bound Inhibitors with Disordered Polyanionic C-Terminal Domains. Int. J. Mol. Sci. 2021, 22, 7358. [Google Scholar] [CrossRef]
- Jung, H.; Kim, S.Y.; Canbakis Cecen, F.S.; Cho, Y.; Kwon, S.-K. Dysfunction of Mitochondrial Ca2+ Regulatory Machineries in Brain Aging and Neurodegenerative Diseases. Front. Cell Dev. Biol. 2020, 8, 599792. [Google Scholar] [CrossRef]
- Sparagna, G.C.; Gunter, K.K.; Gunter, T.E. A System for Producing and Monitoring in Vitro Calcium Pulses Similar to Those Observed in Vivo. Anal. Biochem. 1994, 219, 96–103. [Google Scholar] [CrossRef] [PubMed]
- Sparagna, G.C.; Gunter, K.K.; Sheu, S.-S.; Gunter, T.E. Mitochondrial Calcium Uptake from Physiological-Type Pulses of Calcium: A description of the rapid uptake mode. J. Biol. Chem. 1995, 270, 27510–27515. [Google Scholar] [CrossRef]
- Grienberger, C.; Konnerth, A. Imaging Calcium in Neurons. Neuron 2012, 73, 862–885. [Google Scholar] [CrossRef] [PubMed]
- Petersen, O.H. Calcium Signal Compartmentalization. Biol. Res. 2002, 35, 177–182. [Google Scholar] [CrossRef] [PubMed]
- Laude, A.J.; Simpson, A.W.M. Compartmentalized Signalling: Ca2+ Compartments, Microdomains and the Many Facets of Ca2+ Signalling. FEBS J. 2009, 276, 1800–1816. [Google Scholar] [CrossRef]
- Panda, S.; Behera, S.; Alam, M.F.; Syed, G.H. Endoplasmic Reticulum & Mitochondrial Calcium Homeostasis: The Interplay with Viruses. Mitochondrion 2021, 58, 227–242. [Google Scholar] [CrossRef]
- Hirabayashi, Y.; Kwon, S.-K.; Paek, H.; Pernice, W.M.; Paul, M.A.; Lee, J.; Erfani, P.; Raczkowski, A.; Petrey, D.S.; Pon, L.A.; et al. ER-Mitochondria Tethering by PDZD8 Regulates Ca2+ Dynamics in Mammalian Neurons. Science 2017, 358, 623–630. [Google Scholar] [CrossRef]
- Ashrafi, G.; de Juan-Sanz, J.; Farrell, R.J.; Ryan, T.A. Molecular Tuning of the Axonal Mitochondrial Ca2+ Uniporter Ensures Metabolic Flexibility of Neurotransmission. Neuron 2020, 105, 678–687.e5. [Google Scholar] [CrossRef]
- Ryan, K.C.; Ashkavand, Z.; Norman, K.R. The Role of Mitochondrial Calcium Homeostasis in Alzheimer’s and Related Diseases. Int. J. Mol. Sci. 2020, 21, 9153. [Google Scholar] [CrossRef]
- Görlach, A.; Bertram, K.; Hudecova, S.; Krizanova, O. Calcium and ROS: A Mutual Interplay. Redox Biol. 2015, 6, 260–271. [Google Scholar] [CrossRef]
- Kuznetsov, A.V.; Margreiter, R.; Ausserlechner, M.J.; Hagenbuchner, J. The Complex Interplay between Mitochondria, ROS and Entire Cellular Metabolism. Antioxidants 2022, 11, 1995. [Google Scholar] [CrossRef]
- Angelova, P.R.; Abramov, A.Y. Functional Role of Mitochondrial Reactive Oxygen Species in Physiology. Free Radic. Biol. Med. 2016, 100, 81–85. [Google Scholar] [CrossRef] [PubMed]
- Angelova, P.R. Sources and Triggers of Oxidative Damage in Neurodegeneration. Free Radic. Biol. Med. 2021, 173, 52–63. [Google Scholar] [CrossRef] [PubMed]
- Tait, S.W.G.; Green, D.R. Mitochondria and Cell Signalling. J. Cell Sci. 2012, 125 Pt 4, 807–815. [Google Scholar] [CrossRef] [PubMed]
- Hordijk, P.L. Regulation of NADPH Oxidases: The Role of Rac Proteins. Circ. Res. 2006, 98, 453–462. [Google Scholar] [CrossRef]
- Angajala, A.; Lim, S.; Phillips, J.B.; Kim, J.-H.; Yates, C.; You, Z.; Tan, M. Diverse Roles of Mitochondria in Immune Responses: Novel Insights Into Immuno-Metabolism. Front. Immunol. 2018, 9, 1605. [Google Scholar] [CrossRef] [PubMed]
- Pearce, E.L.; Pearce, E.J. Metabolic Pathways in Immune Cell Activation and Quiescence. Immunity 2013, 38, 633–643. [Google Scholar] [CrossRef]
- Breda, C.N.d.S.; Davanzo, G.G.; Basso, P.J.; Saraiva Câmara, N.O.; Moraes-Vieira, P.M.M. Mitochondria as Central Hub of the Immune System. Redox Biol. 2019, 26, 101255. [Google Scholar] [CrossRef]
- Chen, Y.; Zhou, Z.; Min, W. Mitochondria, Oxidative Stress and Innate Immunity. Front. Physiol. 2018, 9, 1487. [Google Scholar] [CrossRef]
- Chu, X.; Wu, S.; Raju, R. NLRX1 Regulation Following Acute Mitochondrial Injury. Front. Immunol. 2019, 10, 2431. [Google Scholar] [CrossRef]
- Stokman, G.; Kors, L.; Bakker, P.J.; Rampanelli, E.; Claessen, N.; Teske, G.J.D.; Butter, L.; van Andel, H.; van den Bergh Weerman, M.A.; Larsen, P.W.B.; et al. NLRX1 Dampens Oxidative Stress and Apoptosis in Tissue Injury via Control of Mitochondrial Activity. J. Exp. Med. 2017, 214, 2405–2420. [Google Scholar] [CrossRef] [PubMed]
- Liu, Q.; Zhang, D.; Hu, D.; Zhou, X.; Zhou, Y. The Role of Mitochondria in NLRP3 Inflammasome Activation. Mol. Immunol. 2018, 103, 115–124. [Google Scholar] [CrossRef] [PubMed]
- Lamkanfi, M.; Kanneganti, T.-D. Nlrp3: An Immune Sensor of Cellular Stress and Infection. Int. J. Biochem. Cell Biol. 2010, 42, 792–795. [Google Scholar] [CrossRef]
- He, Y.; Hara, H.; Núñez, G. Mechanism and Regulation of NLRP3 Inflammasome Activation. Trends Biochem. Sci. 2016, 41, 1012–1021. [Google Scholar] [CrossRef] [PubMed]
- Guo, C.; Sun, L.; Chen, X.; Zhang, D. Oxidative Stress, Mitochondrial Damage and Neurodegenerative Diseases. Neural Regen. Res. 2013, 8, 2003–2014. [Google Scholar] [CrossRef]
- Singh, A.; Kukreti, R.; Saso, L.; Kukreti, S. Oxidative Stress: A Key Modulator in Neurodegenerative Diseases. Molecules 2019, 24, 1583. [Google Scholar] [CrossRef]
- Behrouzi, A.; Kelley, M.R.; Fehrenbacher, J.C. Oxidative DNA Damage: A Role in Altering Neuronal Function. J. Cell. Signal. 2022, 3, 160–166. [Google Scholar] [CrossRef]
- Poljsak, B.; Šuput, D.; Milisav, I. Achieving the Balance between ROS and Antioxidants: When to Use the Synthetic Antioxidants. Oxid. Med. Cell. Longev. 2013, 2013, 956792. [Google Scholar] [CrossRef]
- Ma, Q. Role of Nrf2 in Oxidative Stress and Toxicity. Annu. Rev. Pharmacol. Toxicol. 2013, 53, 401–426. [Google Scholar] [CrossRef]
- Dinkova-Kostova, A.T.; Kostov, R.V.; Kazantsev, A.G. The Role of Nrf2 Signaling in Counteracting Neurodegenerative Diseases. FEBS J. 2018, 285, 3576–3590. [Google Scholar] [CrossRef]
- Thomas, R.L.; Gustafsson, A.B. Mitochondrial Autophagy—An Essential Quality Control Mechanism for Myocardial Homeostasis. Circ. J. Off. J. Jpn. Circ. Soc. 2013, 77, 2449–2454. [Google Scholar] [CrossRef]
- Pattingre, S.; Tassa, A.; Qu, X.; Garuti, R.; Liang, X.H.; Mizushima, N.; Packer, M.; Schneider, M.D.; Levine, B. Bcl-2 Antiapoptotic Proteins Inhibit Beclin 1-Dependent Autophagy. Cell 2005, 122, 927–939. [Google Scholar] [CrossRef] [PubMed]
- Narendra, D.P.; Jin, S.M.; Tanaka, A.; Suen, D.-F.; Gautier, C.A.; Shen, J.; Cookson, M.R.; Youle, R.J. PINK1 Is Selectively Stabilized on Impaired Mitochondria to Activate Parkin. PLoS Biol. 2010, 8, e1000298. [Google Scholar] [CrossRef] [PubMed]
- Elmore, S. Apoptosis: A Review of Programmed Cell Death. Toxicol. Pathol. 2007, 35, 495–516. [Google Scholar] [CrossRef] [PubMed]
- Suen, D.-F.; Norris, K.L.; Youle, R.J. Mitochondrial Dynamics and Apoptosis. Genes Dev. 2008, 22, 1577–1590. [Google Scholar] [CrossRef]
- Wang, C.; Youle, R.J. The Role of Mitochondria in Apoptosis. Annu. Rev. Genet. 2009, 43, 95–118. [Google Scholar] [CrossRef]
- Sheng, Z.-H. The Interplay of Axonal Energy Homeostasis and Mitochondrial Trafficking and Anchoring. Trends Cell Biol. 2017, 27, 403–416. [Google Scholar] [CrossRef]
- Seager, R.; Lee, L.; Henley, J.M.; Wilkinson, K.A. Mechanisms and Roles of Mitochondrial Localisation and Dynamics in Neuronal Function. Neuronal Signal. 2020, 4, NS20200008. [Google Scholar] [CrossRef]
- Vos, M.; Lauwers, E.; Verstreken, P. Synaptic Mitochondria in Synaptic Transmission and Organization of Vesicle Pools in Health and Disease. Front. Synaptic Neurosci. 2010, 2, 139. [Google Scholar] [CrossRef]
- Chanaday, N.L.; Cousin, M.A.; Milosevic, I.; Watanabe, S.; Morgan, J.R. The Synaptic Vesicle Cycle Revisited: New Insights into the Modes and Mechanisms. J. Neurosci. 2019, 39, 8209–8216. [Google Scholar] [CrossRef]
- Datta, S.; Jaiswal, M. Mitochondrial Calcium at the Synapse. Mitochondrion 2021, 59, 135–153. [Google Scholar] [CrossRef] [PubMed]
- Orth, M.; Schapira, A.H. Mitochondria and Degenerative Disorders. Am. J. Med. Genet. 2001, 106, 27–36. [Google Scholar] [CrossRef] [PubMed]
- Liesa, M.; Shirihai, O.S. Mitochondrial Dynamics in the Regulation of Nutrient Utilization and Energy Expenditure. Cell Metab. 2013, 17, 491–506. [Google Scholar] [CrossRef] [PubMed]
- Zhang, P.N.; Zhou, M.Q.; Guo, J.; Zheng, H.J.; Tang, J.; Zhang, C.; Liu, Y.N.; Liu, W.J.; Wang, Y.X. Mitochondrial Dysfunction and Diabetic Nephropathy: Nontraditional Therapeutic Opportunities. J. Diabetes Res. 2021, 2021, 1010268. [Google Scholar] [CrossRef]
- Molina, A.J.A.; Wikstrom, J.D.; Stiles, L.; Las, G.; Mohamed, H.; Elorza, A.; Walzer, G.; Twig, G.; Katz, S.; Corkey, B.E.; et al. Mitochondrial Networking Protects Beta-Cells from Nutrient-Induced Apoptosis. Diabetes 2009, 58, 2303–2315. [Google Scholar] [CrossRef] [PubMed]
- Gomes, L.C.; Di Benedetto, G.; Scorrano, L. During Autophagy Mitochondria Elongate, Are Spared from Degradation and Sustain Cell Viability. Nat. Cell Biol. 2011, 13, 589–598. [Google Scholar] [CrossRef]
- Lewin, R. Trail of Ironies to Parkinson’s Disease. Science 1984, 224, 1083–1085. [Google Scholar] [CrossRef]
- Hauser, D.N.; Hastings, T.G. Mitochondrial Dysfunction and Oxidative Stress in Parkinson’s Disease and Monogenic Parkinsonism. Neurobiol. Dis. 2013, 51, 35–42. [Google Scholar] [CrossRef]
- Surmeier, D.J.; Obeso, J.A.; Halliday, G.M. Selective Neuronal Vulnerability in Parkinson Disease. Nat. Rev. Neurosci. 2017, 18, 101–113. [Google Scholar] [CrossRef]
- Park, J.-S.; Davis, R.L.; Sue, C.M. Mitochondrial Dysfunction in Parkinson’s Disease: New Mechanistic Insights and Therapeutic Perspectives. Curr. Neurol. Neurosci. Rep. 2018, 18, 21. [Google Scholar] [CrossRef]
- Magalhães, J.D.; Cardoso, S.M. Mitochondrial Signaling on Innate Immunity Activation in Parkinson Disease. Curr. Opin. Neurobiol. 2023, 78, 102664. [Google Scholar] [CrossRef] [PubMed]
- Sagan, L. On the Origin of Mitosing Cells. J. Theor. Biol. 1967, 14, 225–274. [Google Scholar] [CrossRef] [PubMed]
- Rossi, A.; Pizzo, P. Mitochondrial Bioenergetics and Neurodegeneration: A Paso Doble. Neural Regen. Res. 2021, 16, 686–687. [Google Scholar] [CrossRef] [PubMed]
- Zhang, C.; Chen, S.; Li, X.; Xu, Q.; Lin, Y.; Lin, F.; Yuan, M.; Zi, Y.; Cai, J. Progress in Parkinson’s Disease Animal Models of Genetic Defects: Characteristics and Application. Biomed. Pharmacother. 2022, 155, 113768. [Google Scholar] [CrossRef]
- Valente, E.M.; Abou-Sleiman, P.M.; Caputo, V.; Muqit, M.M.K.; Harvey, K.; Gispert, S.; Ali, Z.; Del Turco, D.; Bentivoglio, A.R.; Healy, D.G.; et al. Hereditary Early-Onset Parkinson’s Disease Caused by Mutations in PINK1. Science 2004, 304, 1158–1160. [Google Scholar] [CrossRef]
- Arena, G.; Valente, E.M. PINK1 in the Limelight: Multiple Functions of an Eclectic Protein in Human Health and Disease. J. Pathol. 2017, 241, 251–263. [Google Scholar] [CrossRef]
- Silvestri, L.; Caputo, V.; Bellacchio, E.; Atorino, L.; Dallapiccola, B.; Valente, E.M.; Casari, G. Mitochondrial Import and Enzymatic Activity of PINK1 Mutants Associated to Recessive Parkinsonism. Hum. Mol. Genet. 2005, 14, 3477–3492. [Google Scholar] [CrossRef]
- Unoki, M.; Nakamura, Y. Growth-Suppressive Effects of BPOZ and EGR2, Two Genes Involved in the PTEN Signaling Pathway. Oncogene 2001, 20, 4457–4465. [Google Scholar] [CrossRef]
- Garber, K. Parkinson’s Disease and Cancer: The Unexplored Connection. J. Natl. Cancer Inst. 2010, 102, 371–374. [Google Scholar] [CrossRef]
- Masgras, I.; Laquatra, C.; Cannino, G.; Serapian, S.A.; Colombo, G.; Rasola, A. The Molecular Chaperone TRAP1 in Cancer: From the Basics of Biology to Pharmacological Targeting. Semin. Cancer Biol. 2021, 76, 45–53. [Google Scholar] [CrossRef]
- Exner, N.; Treske, B.; Paquet, D.; Holmström, K.; Schiesling, C.; Gispert, S.; Carballo-Carbajal, I.; Berg, D.; Hoepken, H.-H.; Gasser, T.; et al. Loss-of-Function of Human PINK1 Results in Mitochondrial Pathology and Can Be Rescued by Parkin. J. Neurosci. 2007, 27, 12413–12418. [Google Scholar] [CrossRef] [PubMed]
- Kitada, T.; Pisani, A.; Porter, D.R.; Yamaguchi, H.; Tscherter, A.; Martella, G.; Bonsi, P.; Zhang, C.; Pothos, E.N.; Shen, J. Impaired Dopamine Release and Synaptic Plasticity in the Striatum of PINK1-Deficient Mice. Proc. Natl. Acad. Sci. USA 2007, 104, 11441–11446. [Google Scholar] [CrossRef] [PubMed]
- Martella, G.; Madeo, G.; Maltese, M.; Vanni, V.; Puglisi, F.; Ferraro, E.; Schirinzi, T.; Valente, E.M.; Bonanni, L.; Shen, J.; et al. Exposure to Low-Dose Rotenone Precipitates Synaptic Plasticity Alterations in PINK1 Heterozygous Knockout Mice. Neurobiol. Dis. 2016, 91, 21–36. [Google Scholar] [CrossRef] [PubMed]
- Imbriani, P.; D’Angelo, V.; Platania, P.; Di Lazzaro, G.; Scalise, S.; Salimei, C.; El Atiallah, I.; Colona, V.L.; Mercuri, N.B.; Bonsi, P.; et al. Ischemic Injury Precipitates Neuronal Vulnerability in Parkinson’s Disease: Insights from PINK1 Mouse Model Study and Clinical Retrospective Data. Park. Relat. Disord. 2020, 74, 57–63. [Google Scholar] [CrossRef] [PubMed]
- Imbriani, P.; Tassone, A.; Meringolo, M.; Ponterio, G.; Madeo, G.; Pisani, A.; Bonsi, P.; Martella, G. Loss of Non-Apoptotic Role of Caspase-3 in the PINK1 Mouse Model of Parkinson’s Disease. Int. J. Mol. Sci. 2019, 20, 3407. [Google Scholar] [CrossRef]
- Brunelli, F.; Valente, E.M.; Arena, G. Mechanisms of Neurodegeneration in Parkinson’s Disease: Keep Neurons in the PINK1. Mech. Ageing Dev. 2020, 189, 111277. [Google Scholar] [CrossRef]
- Zhi, L.; Qin, Q.; Muqeem, T.; Seifert, E.L.; Liu, W.; Zheng, S.; Li, C.; Zhang, H. Loss of PINK1 Causes Age-Dependent Decrease of Dopamine Release and Mitochondrial Dysfunction. Neurobiol. Aging 2019, 75, 1–10. [Google Scholar] [CrossRef]
- Onyango, I.G.; Bennett, J.P.; Stokin, G.B. Regulation of Neuronal Bioenergetics as a Therapeutic Strategy in Neurodegenerative Diseases. Neural Regen. Res. 2021, 16, 1467–1482. [Google Scholar] [CrossRef]
- Xie, W.; Chung, K.K.K. Alpha-Synuclein Impairs Normal Dynamics of Mitochondria in Cell and Animal Models of Parkinson’s Disease. J. Neurochem. 2012, 122, 404–414. [Google Scholar] [CrossRef]
- Portz, P.; Lee, M.K. Changes in Drp1 Function and Mitochondrial Morphology Are Associated with the α-Synuclein Pathology in a Transgenic Mouse Model of Parkinson’s Disease. Cells 2021, 10, 885. [Google Scholar] [CrossRef]
- Chinta, S.J.; Mallajosyula, J.K.; Rane, A.; Andersen, J.K. Mitochondrial α-Synuclein Accumulation Impairs Complex I Function in Dopaminergic Neurons and Results in Increased Mitophagy in Vivo. Neurosci. Lett. 2010, 486, 235–239. [Google Scholar] [CrossRef] [PubMed]
- Metabolic Dysfunction in Parkinson’s Disease: Bioenergetics, Redox Homeostasis and Central Carbon Metabolism. Brain Res. Bull. 2017, 133, 12–30. Available online: https://pubmed.ncbi.nlm.nih.gov/28341600/ (accessed on 6 March 2023). [CrossRef] [PubMed]
- Goldberg, J.A.; Guzman, J.N.; Estep, C.M.; Ilijic, E.; Kondapalli, J.; Sanchez-Padilla, J.; Surmeier, D.J. Calcium Entry Induces Mitochondrial Oxidant Stress in Vagal Neurons at Risk in Parkinson’s Disease. Nat. Neurosci. 2012, 15, 1414–1421. [Google Scholar] [CrossRef]
- Li, Z.; Jo, J.; Jia, J.-M.; Lo, S.-C.; Whitcomb, D.J.; Jiao, S.; Cho, K.; Sheng, M. Caspase-3 Activation via Mitochondria Is Required for Long-Term Depression and AMPA Receptor Internalization. Cell 2010, 141, 859–871. [Google Scholar] [CrossRef] [PubMed]
- Lopert, P.; Patel, M. Brain Mitochondria from DJ-1 Knockout Mice Show Increased Respiration-Dependent Hydrogen Peroxide Consumption. Redox Biol. 2014, 2, 667–672. [Google Scholar] [CrossRef]
- Chen, W.; Liu, H.; Liu, S.; Kang, Y.; Nie, Z.; Lei, H. Altered Prefrontal Neurochemistry in the DJ-1 Knockout Mouse Model of Parkinson’s Disease: Complementary Semi-Quantitative Analyses with in Vivo Magnetic Resonance Spectroscopy and MALDI-MSI. Anal. Bioanal. Chem. 2022, 414, 7977–7987. [Google Scholar] [CrossRef]
- Kirkinezos, I.G.; Moraes, C.T. Reactive Oxygen Species and Mitochondrial Diseases. Semin. Cell Dev. Biol. 2001, 12, 449–457. [Google Scholar] [CrossRef]
- Dringen, R.; Gutterer, J.M.; Hirrlinger, J. Glutathione Metabolism in Brain Metabolic Interaction between Astrocytes and Neurons in the Defense against Reactive Oxygen Species. Eur. J. Biochem. 2000, 267, 4912–4916. [Google Scholar] [CrossRef]
- Darios, F. Parkin Prevents Mitochondrial Swelling and Cytochrome c Release in Mitochondria-Dependent Cell Death. Hum. Mol. Genet. 2003, 12, 517–526. [Google Scholar] [CrossRef]
- Goldberg, M.S.; Pisani, A.; Haburcak, M.; Vortherms, T.A.; Kitada, T.; Costa, C.; Tong, Y.; Martella, G.; Tscherter, A.; Martins, A.; et al. Nigrostriatal Dopaminergic Deficits and Hypokinesia Caused by Inactivation of the Familial Parkinsonism-Linked Gene DJ-1. Neuron 2005, 45, 489–496. [Google Scholar] [CrossRef]
- Tkac, I.; Dubinsky, J.M.; Keene, C.D.; Gruetter, R.; Low, W.C. Neurochemical Changes in Huntington R6/2 Mouse Striatum Detected by in Vivo 1H NMR Spectroscopy. J. Neurochem. 2007, 100, 1397–1406. [Google Scholar] [CrossRef]
- Ghiglieri, V.; Bagetta, V.; Calabresi, P.; Picconi, B. Functional Interactions within Striatal Microcircuit in Animal Models of Huntington’s Disease. Neuroscience 2012, 211, 165–184. [Google Scholar] [CrossRef] [PubMed]
- Lopes, C.; Ferreira, I.L.; Maranga, C.; Beatriz, M.; Mota, S.I.; Sereno, J.; Castelhano, J.; Abrunhosa, A.; Oliveira, F.; De Rosa, M.; et al. Mitochondrial and Redox Modifications in Early Stages of Huntington’s Disease. Redox Biol. 2022, 56, 102424. [Google Scholar] [CrossRef] [PubMed]
- Herbst, E.a.F.; Holloway, G.P. Exercise Training Normalizes Mitochondrial Respiratory Capacity within the Striatum of the R6/1 Model of Huntington’s Disease. Neuroscience 2015, 303, 515–523. [Google Scholar] [CrossRef] [PubMed]
- Gardian, G.; Browne, S.E.; Choi, D.-K.; Klivenyi, P.; Gregorio, J.; Kubilus, J.K.; Ryu, H.; Langley, B.; Ratan, R.R.; Ferrante, R.J.; et al. Neuroprotective Effects of Phenylbutyrate in the N171-82Q Transgenic Mouse Model of Huntington’s Disease. J. Biol. Chem. 2005, 280, 556–563. [Google Scholar] [CrossRef]
- Nithianantharajah, J.; Hannan, A.J. Dysregulation of Synaptic Proteins, Dendritic Spine Abnormalities and Pathological Plasticity of Synapses as Experience-Dependent Mediators of Cognitive and Psychiatric Symptoms in Huntington’s Disease. Neuroscience 2013, 251, 66–74. [Google Scholar] [CrossRef]
- Ghiglieri, V.; Campanelli, F.; Marino, G.; Natale, G.; Picconi, B.; Calabresi, P. Corticostriatal Synaptic Plasticity Alterations in the R6/1 Transgenic Mouse Model of Huntington’s Disease. J. Neurosci. Res. 2019, 97, 1655–1664. [Google Scholar] [CrossRef]
- Wellington, C.L.; Ellerby, L.M.; Hackam, A.S.; Margolis, R.L.; Trifiro, M.A.; Singaraja, R.; McCutcheon, K.; Salvesen, G.S.; Propp, S.S.; Bromm, M.; et al. Caspase Cleavage of Gene Products Associated with Triplet Expansion Disorders Generates Truncated Fragments Containing the Polyglutamine Tract. J. Biol. Chem. 1998, 273, 9158–9167. [Google Scholar] [CrossRef]
- Schirinzi, T.; Salvatori, I.; Zenuni, H.; Grillo, P.; Valle, C.; Martella, G.; Mercuri, N.B.; Ferri, A. Pattern of Mitochondrial Respiration in Peripheral Blood Cells of Patients with Parkinson’s Disease. Int. J. Mol. Sci. 2022, 23, 10863. [Google Scholar] [CrossRef]
- Anderson, K.E.; Marshall, F.J. Behavioral Symptoms Associated with Huntington’s Disease. Adv. Neurol. 2005, 96, 197–208. [Google Scholar]
- Caron, N.S.; Wright, G.E.; Hayden, M.R. Huntington Disease. In GeneReviews®; Adam, M.P., Everman, D.B., Mirzaa, G.M., Pagon, R.A., Wallace, S.E., Bean, L.J., Gripp, K.W., Amemiya, A., Eds.; University of Washington: Seattle, WA, USA, 1993. [Google Scholar]
- Damiano, M.; Galvan, L.; Déglon, N.; Brouillet, E. Mitochondria in Huntington’s Disease. Biochim. Biophys. Acta 2010, 1802, 52–61. [Google Scholar] [CrossRef] [PubMed]
- Rehman, M.U.; Sehar, N.; Dar, N.J.; Khan, A.; Arafah, A.; Rashid, S.; Rashid, S.M.; Ganaie, M.A. Mitochondrial Dysfunctions, Oxidative Stress and Neuroinflammation as Therapeutic Targets for Neurodegenerative Diseases: An Update on Current Advances and Impediments. Neurosci. Biobehav. Rev. 2023, 144, 104961. [Google Scholar] [CrossRef] [PubMed]
- Jurcau, A.; Jurcau, C.M. Mitochondria in Huntington’s Disease: Implications in Pathogenesis and Mitochondrial-Targeted Therapeutic Strategies. Neural Regen. Res. 2023, 18, 1472–1477. [Google Scholar] [CrossRef] [PubMed]
- Kawsar, M.; Taz, T.A.; Paul, B.K.; Ahmed, K.; Habib, M.A.; Bhuyian, T. Identification of Vital Regulatory Genes with Network Pathways among Huntington’s, Parkinson’s, and Alzheimer’s Diseases. Netw. Model. Anal. Health Inform. Bioinform. 2020, 9, 50. [Google Scholar] [CrossRef]
- Li, Z.; Zhang, Z.; Ren, Y.; Wang, Y.; Fang, J.; Yue, H.; Ma, S.; Guan, F. Aging and Age-Related Diseases: From Mechanisms to Therapeutic Strategies. Biogerontology 2021, 22, 165–187. [Google Scholar] [CrossRef]
- Quijano, C.; Cao, L.; Fergusson, M.M.; Romero, H.; Liu, J.; Gutkind, S.; Rovira, I.I.; Mohney, R.P.; Karoly, E.D.; Finkel, T. Oncogene-Induced Senescence Results in Marked Metabolic and Bioenergetic Alterations. Cell Cycle 2012, 11, 1383–1392. [Google Scholar] [CrossRef]
- Sun, N.; Youle, R.J.; Finkel, T. The Mitochondrial Basis of Aging. Mol. Cell 2016, 61, 654–666. [Google Scholar] [CrossRef]
- Moro, L. Mitochondrial Dysfunction in Aging and Cancer. J. Clin. Med. 2019, 8, 1983. [Google Scholar] [CrossRef]
- Boland, M.L.; Chourasia, A.H.; Macleod, K.F. Mitochondrial Dysfunction in Cancer. Front. Oncol. 2013, 3, 292. [Google Scholar] [CrossRef]
- Wang, X.-L.; Feng, S.-T.; Wang, Y.-T.; Yuan, Y.-H.; Li, Z.-P.; Chen, N.-H.; Wang, Z.-Z.; Zhang, Y. Mitophagy, a Form of Selective Autophagy, Plays an Essential Role in Mitochondrial Dynamics of Parkinson’s Disease. Cell. Mol. Neurobiol. 2022, 42, 1321–1339. [Google Scholar] [CrossRef]
- Ryan, B.J.; Hoek, S.; Fon, E.A.; Wade-Martins, R. Mitochondrial Dysfunction and Mitophagy in Parkinson’s: From Familial to Sporadic Disease. Trends Biochem. Sci. 2015, 40, 200–210. [Google Scholar] [CrossRef] [PubMed]
- Büeler, H. Impaired Mitochondrial Dynamics and Function in the Pathogenesis of Parkinson’s Disease. Exp. Neurol. 2009, 218, 235–246. [Google Scholar] [CrossRef] [PubMed]
- Chen, C.; Turnbull, D.M.; Reeve, A.K. Mitochondrial Dysfunction in Parkinson’s Disease—Cause or Consequence? Biology 2019, 8, 38. [Google Scholar] [CrossRef] [PubMed]
- Parker, W.D.; Boyson, S.J.; Parks, J.K. Abnormalities of the Electron Transport Chain in Idiopathic Parkinson’s Disease. Ann. Neurol. 1989, 26, 719–723. [Google Scholar] [CrossRef]
- Thirugnanam, T.; Santhakumar, K. Chemically Induced Models of Parkinson’s Disease. Comp. Biochem. Physiol. Toxicol. Pharmacol. CBP 2022, 252, 109213. [Google Scholar] [CrossRef]
- Bové, J.; Perier, C. Neurotoxin-Based Models of Parkinson’s Disease. Neuroscience 2012, 211, 51–76. [Google Scholar] [CrossRef]
- Exner, N.; Lutz, A.K.; Haass, C.; Winklhofer, K.F. Mitochondrial Dysfunction in Parkinson’s Disease: Molecular Mechanisms and Pathophysiological Consequences. EMBO J. 2012, 31, 3038–3062. [Google Scholar] [CrossRef]
- Meredith, G.E.; Rademacher, D.J. MPTP Mouse Models of Parkinson’s Disease: An Update. J. Park. Dis. 2011, 1, 19–33. [Google Scholar] [CrossRef]
- Zeng, X.-S.; Geng, W.-S.; Jia, J.-J. Neurotoxin-Induced Animal Models of Parkinson Disease: Pathogenic Mechanism and Assessment. ASN Neuro 2018, 10, 1759091418777438. [Google Scholar] [CrossRef]
- Imbriani, P.; Schirinzi, T.; Meringolo, M.; Mercuri, N.B.; Pisani, A. Centrality of Early Synaptopathy in Parkinson’s Disease. Front. Neurol. 2018, 9, 103. [Google Scholar] [CrossRef]
- Imbriani, P.; Sciamanna, G.; Santoro, M.; Schirinzi, T.; Pisani, A. Promising Rodent Models in Parkinson’s Disease. Park. Relat. Disord. 2018, 46 (Suppl. S1), S10–S14. [Google Scholar] [CrossRef] [PubMed]
- Innos, J.; Hickey, M.A. Using Rotenone to Model Parkinson’s Disease in Mice: A Review of the Role of Pharmacokinetics. Chem. Res. Toxicol. 2021, 34, 1223–1239. [Google Scholar] [CrossRef] [PubMed]
- Imbriani, P.; Martella, G.; Bonsi, P.; Pisani, A. Oxidative Stress and Synaptic Dysfunction in Rodent Models of Parkinson’s Disease. Neurobiol. Dis. 2022, 173, 105851. [Google Scholar] [CrossRef] [PubMed]
- McLean, P.J.; Ribich, S.; Hyman, B.T. Subcellular Localization of Alpha-Synuclein in Primary Neuronal Cultures: Effect of Missense Mutations. J. Neural Transm. Suppl. 2000, 7, 53–63. [Google Scholar] [CrossRef]
- Zhang, L.; Zhang, C.; Zhu, Y.; Cai, Q.; Chan, P.; Uéda, K.; Yu, S.; Yang, H. Semi-Quantitative Analysis of Alpha-Synuclein in Subcellular Pools of Rat Brain Neurons: An Immunogold Electron Microscopic Study Using a C-Terminal Specific Monoclonal Antibody. Brain Res. 2008, 1244, 40–52. [Google Scholar] [CrossRef]
- Cole, N.B.; Dieuliis, D.; Leo, P.; Mitchell, D.C.; Nussbaum, R.L. Mitochondrial Translocation of Alpha-Synuclein Is Promoted by Intracellular Acidification. Exp. Cell Res. 2008, 314, 2076–2089. [Google Scholar] [CrossRef]
- Polymeropoulos, M.H.; Higgins, J.J.; Golbe, L.I.; Johnson, W.G.; Ide, S.E.; Di Iorio, G.; Sanges, G.; Stenroos, E.S.; Pho, L.T.; Schaffer, A.A.; et al. Mapping of a Gene for Parkinson’s Disease to Chromosome 4q21-Q23. Science 1996, 274, 1197–1199. [Google Scholar] [CrossRef]
- Koprich, J.B.; Kalia, L.V.; Brotchie, J.M. Animal Models of α-Synucleinopathy for Parkinson Disease Drug Development. Nat. Rev. Neurosci. 2017, 18, 515–529. [Google Scholar] [CrossRef]
- Ingelsson, M. Alpha-Synuclein Oligomers—Neurotoxic Molecules in Parkinson’s Disease and Other Lewy Body Disorders. Front. Neurosci. 2016, 10, 408. [Google Scholar] [CrossRef]
- Martin, L.J.; Pan, Y.; Price, A.C.; Sterling, W.; Copeland, N.G.; Jenkins, N.A.; Price, D.L.; Lee, M.K. Parkinson’s Disease Alpha-Synuclein Transgenic Mice Develop Neuronal Mitochondrial Degeneration and Cell Death. J. Neurosci. 2006, 26, 41–50. [Google Scholar] [CrossRef]
- Merino-Galán, L.; Jimenez-Urbieta, H.; Zamarbide, M.; Rodríguez-Chinchilla, T.; Belloso-Iguerategui, A.; Santamaria, E.; Fernández-Irigoyen, J.; Aiastui, A.; Doudnikoff, E.; Bézard, E.; et al. Striatal Synaptic Bioenergetic and Autophagic Decline in Premotor Experimental Parkinsonism. Brain J. Neurol. 2022, 145, 2092–2107. [Google Scholar] [CrossRef] [PubMed]
- Kurz, A.; Double, K.L.; Lastres-Becker, I.; Tozzi, A.; Tantucci, M.; Bockhart, V.; Bonin, M.; García-Arencibia, M.; Nuber, S.; Schlaudraff, F.; et al. A53T-Alpha-Synuclein Overexpression Impairs Dopamine Signaling and Striatal Synaptic Plasticity in Old Mice. PLoS ONE 2010, 5, e11464. [Google Scholar] [CrossRef] [PubMed]
- Durante, V.; de Iure, A.; Loffredo, V.; Vaikath, N.; De Risi, M.; Paciotti, S.; Quiroga-Varela, A.; Chiasserini, D.; Mellone, M.; Mazzocchetti, P.; et al. Alpha-Synuclein Targets GluN2A NMDA Receptor Subunit Causing Striatal Synaptic Dysfunction and Visuospatial Memory Alteration. Brain J. Neurol. 2019, 142, 1365–1385. [Google Scholar] [CrossRef] [PubMed]
- Tozzi, A.; de Iure, A.; Bagetta, V.; Tantucci, M.; Durante, V.; Quiroga-Varela, A.; Costa, C.; Di Filippo, M.; Ghiglieri, V.; Latagliata, E.C.; et al. Alpha-Synuclein Produces Early Behavioral Alterations via Striatal Cholinergic Synaptic Dysfunction by Interacting With GluN2D N-Methyl-D-Aspartate Receptor Subunit. Biol. Psychiatry 2016, 79, 402–414. [Google Scholar] [CrossRef] [PubMed]
- Tozzi, A.; Sciaccaluga, M.; Loffredo, V.; Megaro, A.; Ledonne, A.; Cardinale, A.; Federici, M.; Bellingacci, L.; Paciotti, S.; Ferrari, E.; et al. Dopamine-Dependent Early Synaptic and Motor Dysfunctions Induced by α-Synuclein in the Nigrostriatal Circuit. Brain J. Neurol. 2021, 144, 3477–3491. [Google Scholar] [CrossRef]
- Calì, T.; Ottolini, D.; Negro, A.; Brini, M. α-Synuclein Controls Mitochondrial Calcium Homeostasis by Enhancing Endoplasmic Reticulum-Mitochondria Interactions. J. Biol. Chem. 2012, 287, 17914–17929. [Google Scholar] [CrossRef]
- Alves Da Costa, C.; Paitel, E.; Vincent, B.; Checler, F. Alpha-Synuclein Lowers P53-Dependent Apoptotic Response of Neuronal Cells: Abolishment by 6-Hydroxydopamine and Implication for Parkinson’s Disease. J. Biol. Chem. 2002, 277, 50980–50984. [Google Scholar] [CrossRef]
- Chan, S.L.; Mattson, M.P. Caspase and Calpain Substrates: Roles in Synaptic Plasticity and Cell Death. J. Neurosci. Res. 1999, 58, 167–190. [Google Scholar] [CrossRef]
- D’Amelio, M.; Cavallucci, V.; Cecconi, F. Neuronal Caspase-3 Signaling: Not Only Cell Death. Cell Death Differ. 2010, 17, 1104–1114. [Google Scholar] [CrossRef]
- Snigdha, S.; Smith, E.D.; Prieto, G.A.; Cotman, C.W. Caspase-3 Activation as a Bifurcation Point between Plasticity and Cell Death. Neurosci. Bull. 2012, 28, 14–24. [Google Scholar] [CrossRef]
- Bonifati, V.; Rizzu, P.; van Baren, M.J.; Schaap, O.; Breedveld, G.J.; Krieger, E.; Dekker, M.C.J.; Squitieri, F.; Ibanez, P.; Joosse, M.; et al. Mutations in the DJ-1 Gene Associated with Autosomal Recessive Early-Onset Parkinsonism. Science 2003, 299, 256–259. [Google Scholar] [CrossRef] [PubMed]
- Thomas, K.J.; McCoy, M.K.; Blackinton, J.; Beilina, A.; van der Brug, M.; Sandebring, A.; Miller, D.; Maric, D.; Cedazo-Minguez, A.; Cookson, M.R. DJ-1 Acts in Parallel to the PINK1/Parkin Pathway to Control Mitochondrial Function and Autophagy. Hum. Mol. Genet. 2011, 20, 40–50. [Google Scholar] [CrossRef] [PubMed]
- Trancikova, A.; Tsika, E.; Moore, D.J. Mitochondrial Dysfunction in Genetic Animal Models of Parkinson’s Disease. Antioxid. Redox Signal. 2012, 16, 896–919. [Google Scholar] [CrossRef] [PubMed]
- Du, F.; Yu, Q.; Yan, S.; Hu, G.; Lue, L.-F.; Walker, D.G.; Wu, L.; Yan, S.F.; Tieu, K.; Yan, S.S. PINK1 Signalling Rescues Amyloid Pathology and Mitochondrial Dysfunction in Alzheimer’s Disease. Brain J. Neurol. 2017, 140, 3233–3251. [Google Scholar] [CrossRef] [PubMed]
- Lazarou, M.; Sliter, D.A.; Kane, L.A.; Sarraf, S.A.; Wang, C.; Burman, J.L.; Sideris, D.P.; Fogel, A.I.; Youle, R.J. The Ubiquitin Kinase PINK1 Recruits Autophagy Receptors to Induce Mitophagy. Nature 2015, 524, 309–314. [Google Scholar] [CrossRef]
- Lücking, C.B.; Dürr, A.; Bonifati, V.; Vaughan, J.; De Michele, G.; Gasser, T.; Harhangi, B.S.; Meco, G.; Denèfle, P.; Wood, N.W.; et al. Association between Early-Onset Parkinson’s Disease and Mutations in the Parkin Gene. N. Engl. J. Med. 2000, 342, 1560–1567. [Google Scholar] [CrossRef]
- Moore, D.J. Parkin: A Multifaceted Ubiquitin Ligase. Biochem. Soc. Trans. 2006, 34 Pt 5, 749–753. [Google Scholar] [CrossRef]
- Xiong, H.; Wang, D.; Chen, L.; Choo, Y.S.; Ma, H.; Tang, C.; Xia, K.; Jiang, W.; Ronai, Z.; Zhuang, X.; et al. Parkin, PINK1, and DJ-1 Form a Ubiquitin E3 Ligase Complex Promoting Unfolded Protein Degradation. J. Clin. Investig. 2009, 119, 650–660. [Google Scholar] [CrossRef]
- Periquet, M.; Corti, O.; Jacquier, S.; Brice, A. Proteomic Analysis of Parkin Knockout Mice: Alterations in Energy Metabolism, Protein Handling and Synaptic Function. J. Neurochem. 2005, 95, 1259–1276. [Google Scholar] [CrossRef]
- Shin, J.-H.; Ko, H.S.; Kang, H.; Lee, Y.; Lee, Y.-I.; Pletinkova, O.; Troconso, J.C.; Dawson, V.L.; Dawson, T.M. PARIS (ZNF746) Repression of PGC-1α Contributes to Neurodegeneration in Parkinson’s Disease. Cell 2011, 144, 689–702. [Google Scholar] [CrossRef]
- Jo, A.; Lee, Y.; Kam, T.-I.; Kang, S.-U.; Neifert, S.; Karuppagounder, S.S.; Khang, R.; Kang, H.; Park, H.; Chou, S.-C.; et al. PARIS Farnesylation Prevents Neurodegeneration in Models of Parkinson’s Disease. Sci. Transl. Med. 2021, 13, eaax8891. [Google Scholar] [CrossRef] [PubMed]
- Narendra, D.; Tanaka, A.; Suen, D.-F.; Youle, R.J. Parkin-Induced Mitophagy in the Pathogenesis of Parkinson Disease. Autophagy 2009, 5, 706–708. [Google Scholar] [CrossRef] [PubMed]
- Palacino, J.J.; Sagi, D.; Goldberg, M.S.; Krauss, S.; Motz, C.; Wacker, M.; Klose, J.; Shen, J. Mitochondrial Dysfunction and Oxidative Damage in Parkin-Deficient Mice. J. Biol. Chem. 2004, 279, 18614–18622. [Google Scholar] [CrossRef]
- Itier, J.-M.; Ibanez, P.; Mena, M.A.; Abbas, N.; Cohen-Salmon, C.; Bohme, G.A.; Laville, M.; Pratt, J.; Corti, O.; Pradier, L.; et al. Parkin Gene Inactivation Alters Behaviour and Dopamine Neurotransmission in the Mouse. Hum. Mol. Genet. 2003, 12, 2277–2291. [Google Scholar] [CrossRef] [PubMed]
- Kitada, T.; Pisani, A.; Karouani, M.; Haburcak, M.; Martella, G.; Tscherter, A.; Platania, P.; Wu, B.; Pothos, E.N.; Shen, J. Impaired Dopamine Release and Synaptic Plasticity in the Striatum of Parkin −/− Mice. J. Neurochem. 2009, 110, 613–621. [Google Scholar] [CrossRef] [PubMed]
- Cortese, G.P.; Zhu, M.; Williams, D.; Heath, S.; Waites, C.L. Parkin Deficiency Reduces Hippocampal Glutamatergic Neurotransmission by Impairing AMPA Receptor Endocytosis. J. Neurosci. 2016, 36, 12243–12258. [Google Scholar] [CrossRef]
- Calì, T.; Ottolini, D.; Negro, A.; Brini, M. Enhanced Parkin Levels Favor ER-Mitochondria Crosstalk and Guarantee Ca(2+) Transfer to Sustain Cell Bioenergetics. Biochim. Biophys. Acta 2013, 1832, 495–508. [Google Scholar] [CrossRef]
- Bianchi, K.; Rimessi, A.; Prandini, A.; Szabadkai, G.; Rizzuto, R. Calcium and Mitochondria: Mechanisms and Functions of a Troubled Relationship. Biochim. Biophys. Acta 2004, 1742, 119–131. [Google Scholar] [CrossRef]
- Jo, D.; Song, J. Irisin Acts via the PGC-1α and BDNF Pathway to Improve Depression-like Behavior. Clin. Nutr. Res. 2021, 10, 292–302. [Google Scholar] [CrossRef]
- Zhou, H.; Falkenburger, B.H.; Schulz, J.B.; Tieu, K.; Xu, Z.; Xia, X.G. Silencing of the Pink1 Gene Expression by Conditional RNAi Does Not Induce Dopaminergic Neuron Death in Mice. Int. J. Biol. Sci. 2007, 3, 242–250. [Google Scholar] [CrossRef]
- Madeo, G.; Schirinzi, T.; Martella, G.; Latagliata, E.C.; Puglisi, F.; Shen, J.; Valente, E.M.; Federici, M.; Mercuri, N.B.; Puglisi-Allegra, S.; et al. PINK1 Heterozygous Mutations Induce Subtle Alterations in Dopamine-Dependent Synaptic Plasticity. Mov. Disord. 2014, 29, 41–53. [Google Scholar] [CrossRef] [PubMed]
- Dave, K.D.; De Silva, S.; Sheth, N.P.; Ramboz, S.; Beck, M.J.; Quang, C.; Switzer, R.C.; Ahmad, S.O.; Sunkin, S.M.; Walker, D.; et al. Phenotypic Characterization of Recessive Gene Knockout Rat Models of Parkinson’s Disease. Neurobiol. Dis. 2014, 70, 190–203. [Google Scholar] [CrossRef] [PubMed]
- Stauch, K.L.; Villeneuve, L.M.; Purnell, P.R.; Ottemann, B.M.; Emanuel, K.; Fox, H.S. Loss of Pink1 Modulates Synaptic Mitochondrial Bioenergetics in the Rat Striatum Prior to Motor Symptoms: Concomitant Complex I Respiratory Defects and Increased Complex II-Mediated Respiration. Proteom. Clin. Appl. 2016, 10, 1205–1217. [Google Scholar] [CrossRef]
- Villeneuve, L.M.; Purnell, P.R.; Boska, M.D.; Fox, H.S. Early Expression of Parkinson’s Disease-Related Mitochondrial Abnormalities in PINK1 Knockout Rats. Mol. Neurobiol. 2016, 53, 171–186. [Google Scholar] [CrossRef] [PubMed]
- Creed, R.B.; Goldberg, M.S. Analysis of α-Synuclein Pathology in PINK1 Knockout Rat Brains. Front. Neurosci. 2018, 12, 1034. [Google Scholar] [CrossRef]
- Gautier, C.A.; Kitada, T.; Shen, J. Loss of PINK1 Causes Mitochondrial Functional Defects and Increased Sensitivity to Oxidative Stress. Proc. Natl. Acad. Sci. USA 2008, 105, 11364–11369. [Google Scholar] [CrossRef]
- Zhang, L.; Shimoji, M.; Thomas, B.; Moore, D.J.; Yu, S.-W.; Marupudi, N.I.; Torp, R.; Torgner, I.A.; Ottersen, O.P.; Dawson, T.M.; et al. Mitochondrial Localization of the Parkinson’s Disease Related Protein DJ-1: Implications for Pathogenesis. Hum. Mol. Genet. 2005, 14, 2063–2073. [Google Scholar] [CrossRef] [PubMed]
- Edzamko, N.; Zhou, J.; Huang, Y.; Halliday, G.M. Parkinson’s Disease-Implicated Kinases in the Brain; Insights into Disease Pathogenesis. Front. Mol. Neurosci. 2014, 7, 57. [Google Scholar] [CrossRef]
- Guzman, J.N.; Sanchez-Padilla, J.; Wokosin, D.; Kondapalli, J.; Ilijic, E.; Schumacker, P.T.; Surmeier, D.J. Oxidant Stress Evoked by Pacemaking in Dopaminergic Neurons Is Attenuated by DJ-1. Nature 2010, 468, 696–700. [Google Scholar] [CrossRef]
- Heo, J.Y.; Park, J.H.; Kim, S.J.; Seo, K.S.; Han, J.S.; Lee, S.H.; Kim, J.M.; Park, J.I.; Park, S.K.; Lim, K.; et al. DJ-1 Null Dopaminergic Neuronal Cells Exhibit Defects in Mitochondrial Function and Structure: Involvement of Mitochondrial Complex I Assembly. PLoS ONE 2012, 7, e32629. [Google Scholar] [CrossRef]
- Andres-Mateos, E.; Perier, C.; Zhang, L.; Blanchard-Fillion, B.; Greco, T.M.; Thomas, B.; Ko, H.S.; Sasaki, M.; Ischiropoulos, H.; Przedborski, S.; et al. DJ-1 Gene Deletion Reveals That DJ-1 Is an Atypical Peroxiredoxin-like Peroxidase. Proc. Natl. Acad. Sci. USA 2007, 104, 14807–14812. [Google Scholar] [CrossRef] [PubMed]
- Dringen, R.; Hirrlinger, J. Glutathione Pathways in the Brain. Biol. Chem. 2003, 384, 505–516. [Google Scholar] [CrossRef] [PubMed]
- Junn, E.; Jang, W.H.; Zhao, X.; Jeong, B.S.; Mouradian, M.M. Mitochondrial Localization of DJ-1 Leads to Enhanced Neuroprotection. J. Neurosci. Res. 2009, 87, 123–129. [Google Scholar] [CrossRef] [PubMed]
- Irrcher, I.; Aleyasin, H.; Seifert, E.L.; Hewitt, S.J.; Chhabra, S.; Phillips, M.; Lutz, A.K.; Rousseaux, M.W.C.; Bevilacqua, L.; Jahani-Asl, A.; et al. Loss of the Parkinson’s Disease-Linked Gene DJ-1 Perturbs Mitochondrial Dynamics. Hum. Mol. Genet. 2010, 19, 3734–3746. [Google Scholar] [CrossRef] [PubMed]
- Im, J.-Y.; Lee, K.-W.; Woo, J.-M.; Junn, E.; Mouradian, M.M. DJ-1 Induces Thioredoxin 1 Expression through the Nrf2 Pathway. Hum. Mol. Genet. 2012, 21, 3013–3024. [Google Scholar] [CrossRef]
- Dolgacheva, L.P.; Berezhnov, A.V.; Fedotova, E.I.; Zinchenko, V.P.; Abramov, A.Y. Role of DJ-1 in the Mechanism of Pathogenesis of Parkinson’s Disease. J. Bioenerg. Biomembr. 2019, 51, 175–188. [Google Scholar] [CrossRef]
- Kitada, T.; Tong, Y.; Gautier, C.A.; Shen, J. Absence of Nigral Degeneration in Aged Parkin/DJ-1/PINK1 Triple Knockout Mice. J. Neurochem. 2009, 111, 696–702. [Google Scholar] [CrossRef]
- Farshim, P.P.; Bates, G.P. Mouse Models of Huntington’s Disease. Methods Mol. Biol. 2018, 1780, 97–120. [Google Scholar] [CrossRef]
- Pouladi, M.A.; Morton, A.J.; Hayden, M.R. Choosing an Animal Model for the Study of Huntington’s Disease. Nat. Rev. Neurosci. 2013, 14, 708–721. [Google Scholar] [CrossRef]
- Yu, Z.-X.; Li, S.-H.; Evans, J.; Pillarisetti, A.; Li, H.; Li, X.-J. Mutant Huntingtin Causes Context-Dependent Neurodegeneration in Mice with Huntington’s Disease. J. Neurosci. 2003, 23, 2193–2202. [Google Scholar] [CrossRef]
- Choo, Y.S.; Johnson, G.V.W.; MacDonald, M.; Detloff, P.J.; Lesort, M. Mutant Huntingtin Directly Increases Susceptibility of Mitochondria to the Calcium-Induced Permeability Transition and Cytochrome c Release. Hum. Mol. Genet. 2004, 13, 1407–1420. [Google Scholar] [CrossRef]
- Panov, A.V.; Gutekunst, C.-A.; Leavitt, B.R.; Hayden, M.R.; Burke, J.R.; Strittmatter, W.J.; Greenamyre, J.T. Early Mitochondrial Calcium Defects in Huntington’s Disease Are a Direct Effect of Polyglutamines. Nat. Neurosci. 2002, 5, 731–736. [Google Scholar] [CrossRef] [PubMed]
- Mangiarini, L.; Sathasivam, K.; Seller, M.; Cozens, B.; Harper, A.; Hetherington, C.; Lawton, M.; Trottier, Y.; Lehrach, H.; Davies, S.W.; et al. Exon 1 of the HD Gene with an Expanded CAG Repeat Is Sufficient to Cause a Progressive Neurological Phenotype in Transgenic Mice. Cell 1996, 87, 493–506. [Google Scholar] [CrossRef]
- Menalled, L.; El-Khodor, B.F.; Patry, M.; Suárez-Fariñas, M.; Orenstein, S.J.; Zahasky, B.; Leahy, C.; Wheeler, V.; Yang, X.W.; MacDonald, M.; et al. Systematic Behavioral Evaluation of Huntington’s Disease Transgenic and Knock-in Mouse Models. Neurobiol. Dis. 2009, 35, 319–336. [Google Scholar] [CrossRef] [PubMed]
- Bogdanov, M.B.; Andreassen, O.A.; Dedeoglu, A.; Ferrante, R.J.; Beal, M.F. Increased Oxidative Damage to DNA in a Transgenic Mouse Model of Huntington’s Disease. J. Neurochem. 2001, 79, 1246–1249. [Google Scholar] [CrossRef] [PubMed]
- Tabrizi, S.J.; Workman, J.; Hart, P.E.; Mangiarini, L.; Mahal, A.; Bates, G.; Cooper, J.M.; Schapira, A.H. Mitochondrial Dysfunction and Free Radical Damage in the Huntington R6/2 Transgenic Mouse. Ann. Neurol. 2000, 47, 80–86. [Google Scholar] [CrossRef] [PubMed]
- Petersen, M.H.; Willert, C.W.; Andersen, J.V.; Madsen, M.; Waagepetersen, H.S.; Skotte, N.H.; Nørremølle, A. Progressive Mitochondrial Dysfunction of Striatal Synapses in R6/2 Mouse Model of Huntington’s Disease. J. Huntingt. Dis. 2022, 11, 121–140. [Google Scholar] [CrossRef] [PubMed]
- Milnerwood, A.J.; Raymond, L.A. Corticostriatal Synaptic Function in Mouse Models of Huntington’s Disease: Early Effects of Huntingtin Repeat Length and Protein Load. J. Physiol. 2007, 585 Pt 3, 817–831. [Google Scholar] [CrossRef]
- Giralt, A.; Saavedra, A.; Alberch, J.; Pérez-Navarro, E. Cognitive Dysfunction in Huntington’s Disease: Humans, Mouse Models and Molecular Mechanisms. J. Huntingt. Dis. 2012, 1, 155–173. [Google Scholar] [CrossRef]
- Rosenstock, T.R.; Bertoncini, C.R.A.; Teles, A.V.; Hirata, H.; Fernandes, M.J.S.; Smaili, S.S. Glutamate-Induced Alterations in Ca2+ Signaling Are Modulated by Mitochondrial Ca2+ Handling Capacity in Brain Slices of R6/1 Transgenic Mice. Eur. J. Neurosci. 2010, 32, 60–70. [Google Scholar] [CrossRef]
- Clark, J.B. N-Acetyl Aspartate: A Marker for Neuronal Loss or Mitochondrial Dysfunction. Dev. Neurosci. 1998, 20, 271–276. [Google Scholar] [CrossRef] [PubMed]
- Jenkins, B.G.; Klivenyi, P.; Kustermann, E.; Andreassen, O.A.; Ferrante, R.J.; Rosen, B.R.; Beal, M.F. Nonlinear Decrease over Time in N-Acetyl Aspartate Levels in the Absence of Neuronal Loss and Increases in Glutamine and Glucose in Transgenic Huntington’s Disease Mice. J. Neurochem. 2000, 74, 2108–2119. [Google Scholar] [CrossRef] [PubMed]
- Andreassen, O.A.; Dedeoglu, A.; Ferrante, R.J.; Jenkins, B.G.; Ferrante, K.L.; Thomas, M.; Friedlich, A.; Browne, S.E.; Schilling, G.; Borchelt, D.R.; et al. Creatine Increase Survival and Delays Motor Symptoms in a Transgenic Animal Model of Huntington’s Disease. Neurobiol. Dis. 2001, 8, 479–491. [Google Scholar] [CrossRef]
- Jenkins, B.G.; Andreassen, O.A.; Dedeoglu, A.; Leavitt, B.; Hayden, M.; Borchelt, D.; Ross, C.A.; Ferrante, R.J.; Beal, M.F. Effects of CAG Repeat Length, HTT Protein Length and Protein Context on Cerebral Metabolism Measured Using Magnetic Resonance Spectroscopy in Transgenic Mouse Models of Huntington’s Disease. J. Neurochem. 2005, 95, 553–562. [Google Scholar] [CrossRef]
- Browne, S.E. Mitochondria and Huntington’s Disease Pathogenesis: Insight from Genetic and Chemical Models. Ann. N. Y. Acad. Sci. 2008, 1147, 358–382. [Google Scholar] [CrossRef] [PubMed]
- Wright, D.J.; Renoir, T.; Smith, Z.M.; Frazier, A.E.; Francis, P.S.; Thorburn, D.R.; McGee, S.L.; Hannan, A.J.; Gray, L.J. N-Acetylcysteine Improves Mitochondrial Function and Ameliorates Behavioral Deficits in the R6/1 Mouse Model of Huntington’s Disease. Transl. Psychiatry 2015, 5, e492. [Google Scholar] [CrossRef] [PubMed]
- Hermel, E.; Gafni, J.; Propp, S.S.; Leavitt, B.R.; Wellington, C.L.; Young, J.E.; Hackam, A.S.; Logvinova, A.V.; Peel, A.L.; Chen, S.F.; et al. Specific Caspase Interactions and Amplification Are Involved in Selective Neuronal Vulnerability in Huntington’s Disease. Cell Death Differ. 2004, 11, 424–438. [Google Scholar] [CrossRef]
- Ona, V.O.; Li, M.; Vonsattel, J.P.; Andrews, L.J.; Khan, S.Q.; Chung, W.M.; Frey, A.S.; Menon, A.S.; Li, X.J.; Stieg, P.E.; et al. Inhibition of Caspase-1 Slows Disease Progression in a Mouse Model of Huntington’s Disease. Nature 1999, 399, 263–267. [Google Scholar] [CrossRef]
- Carroll, J.B.; Southwell, A.L.; Graham, R.K.; Lerch, J.P.; Ehrnhoefer, D.E.; Cao, L.-P.; Zhang, W.-N.; Deng, Y.; Bissada, N.; Henkelman, R.M.; et al. Mice Lacking Caspase-2 Are Protected from Behavioral Changes, but Not Pathology, in the YAC128 Model of Huntington Disease. Mol. Neurodegener. 2011, 6, 59. [Google Scholar] [CrossRef]
- Avenali, M.; Cerri, S.; Ongari, G.; Ghezzi, C.; Pacchetti, C.; Tassorelli, C.; Valente, E.M.; Blandini, F. Profiling the Biochemical Signature of GBA-Related Parkinson’s Disease in Peripheral Blood Mononuclear Cells. Mov. Disord. 2021, 36, 1267–1272. [Google Scholar] [CrossRef]
- Petrillo, S.; Schirinzi, T.; Di Lazzaro, G.; D’Amico, J.; Colona, V.L.; Bertini, E.; Pierantozzi, M.; Mari, L.; Mercuri, N.B.; Piemonte, F.; et al. Systemic Activation of Nrf2 Pathway in Parkinson’s Disease. Mov. Disord. 2020, 35, 180–184. [Google Scholar] [CrossRef] [PubMed]
- Annesley, S.J.; Lay, S.T.; De Piazza, S.W.; Sanislav, O.; Hammersley, E.; Allan, C.Y.; Francione, L.M.; Bui, M.Q.; Chen, Z.-P.; Ngoei, K.R.W.; et al. Immortalized Parkinson’s Disease Lymphocytes Have Enhanced Mitochondrial Respiratory Activity. Dis. Models Mech. 2016, 9, 1295–1305. [Google Scholar] [CrossRef] [PubMed]
- Haylett, W.; Swart, C.; van der Westhuizen, F.; van Dyk, H.; van der Merwe, L.; van der Merwe, C.; Loos, B.; Carr, J.; Kinnear, C.; Bardien, S. Altered Mitochondrial Respiration and Other Features of Mitochondrial Function in Parkin-Mutant Fibroblasts from Parkinson’s Disease Patients. Park. Dis. 2016, 2016, 1819209. [Google Scholar] [CrossRef]
- Antony, P.M.A.; Kondratyeva, O.; Mommaerts, K.; Ostaszewski, M.; Sokolowska, K.; Baumuratov, A.S.; Longhino, L.; Poulain, J.F.; Grossmann, D.; Balling, R.; et al. Fibroblast Mitochondria in Idiopathic Parkinson’s Disease Display Morphological Changes and Enhanced Resistance to Depolarization. Sci. Rep. 2020, 10, 1569. [Google Scholar] [CrossRef]
- Fais, M.; Dore, A.; Galioto, M.; Galleri, G.; Crosio, C.; Iaccarino, C. Parkinson’s Disease-Related Genes and Lipid Alteration. Int. J. Mol. Sci. 2021, 22, 7630. [Google Scholar] [CrossRef]
- Smith, A.M.; Depp, C.; Ryan, B.J.; Johnston, G.I.; Alegre-Abarrategui, J.; Evetts, S.; Rolinski, M.; Baig, F.; Ruffmann, C.; Simon, A.K.; et al. Mitochondrial Dysfunction and Increased Glycolysis in Prodromal and Early Parkinson’s Blood Cells. Mov. Disord. Off. J. Mov. Disord. Soc. 2018, 33, 1580–1590. [Google Scholar] [CrossRef]
- Havelund, J.F.; Heegaard, N.H.H.; Færgeman, N.J.K.; Gramsbergen, J.B. Biomarker Research in Parkinson’s Disease Using Metabolite Profiling. Metabolites 2017, 7, 42. [Google Scholar] [CrossRef]
- Willkommen, D.; Lucio, M.; Moritz, F.; Forcisi, S.; Kanawati, B.; Smirnov, K.S.; Schroeter, M.; Sigaroudi, A.; Schmitt-Kopplin, P.; Michalke, B. Metabolomic Investigations in Cerebrospinal Fluid of Parkinson’s Disease. PLoS ONE 2018, 13, e0208752. [Google Scholar] [CrossRef]
- Saiki, S.; Hatano, T.; Fujimaki, M.; Ishikawa, K.-I.; Mori, A.; Oji, Y.; Okuzumi, A.; Fukuhara, T.; Koinuma, T.; Imamichi, Y.; et al. Decreased Long-Chain Acylcarnitines from Insufficient β-Oxidation as Potential Early Diagnostic Markers for Parkinson’s Disease. Sci. Rep. 2017, 7, 7328. [Google Scholar] [CrossRef]
- Ascherio, A.; Schwarzschild, M.A. The Epidemiology of Parkinson’s Disease: Risk Factors and Prevention. Lancet Neurol. 2016, 15, 1257–1272. [Google Scholar] [CrossRef]
- Schirinzi, T.; Di Lazzaro, G.; Sancesario, G.M.; Summa, S.; Petrucci, S.; Colona, V.L.; Bernardini, S.; Pierantozzi, M.; Stefani, A.; Mercuri, N.B.; et al. Young-Onset and Late-Onset Parkinson’s Disease Exhibit a Different Profile of Fluid Biomarkers and Clinical Features. Neurobiol. Aging 2020, 90, 119–124. [Google Scholar] [CrossRef] [PubMed]
- Schirinzi, T.; Vasco, G.; Zanni, G.; Petrillo, S.; Piemonte, F.; Castelli, E.; Bertini, E.S. Serum Uric Acid in Friedreich Ataxia. Clin. Biochem. 2018, 54, 139–141. [Google Scholar] [CrossRef] [PubMed]
- Schirinzi, T.; Di Lazzaro, G.; Colona, V.L.; Imbriani, P.; Alwardat, M.; Sancesario, G.M.; Martorana, A.; Pisani, A. Assessment of Serum Uric Acid as Risk Factor for Tauopathies. J. Neural Transm. 2017, 124, 1105–1108. [Google Scholar] [CrossRef]
- Wei, Z.; Li, X.; Li, X.; Liu, Q.; Cheng, Y. Oxidative Stress in Parkinson’s Disease: A Systematic Review and Meta-Analysis. Front. Mol. Neurosci. 2018, 11, 236. [Google Scholar] [CrossRef]
- Ueno, S.-I.; Hatano, T.; Okuzumi, A.; Saiki, S.; Oji, Y.; Mori, A.; Koinuma, T.; Fujimaki, M.; Takeshige-Amano, H.; Kondo, A.; et al. Nonmercaptalbumin as an Oxidative Stress Marker in Parkinson’s and PARK2 Disease. Ann. Clin. Transl. Neurol. 2020, 7, 307–317. [Google Scholar] [CrossRef]
- Yamagishi, Y.; Saigoh, K.; Saito, Y.; Ogawa, I.; Mitsui, Y.; Hamada, Y.; Samukawa, M.; Suzuki, H.; Kuwahara, M.; Hirano, M.; et al. Diagnosis of Parkinson’s Disease and the Level of Oxidized DJ-1 Protein. Neurosci. Res. 2018, 128, 58–62. [Google Scholar] [CrossRef] [PubMed]
- Sancesario, G.M.; Di Lazzaro, G.; Grillo, P.; Biticchi, B.; Giannella, E.; Alwardat, M.; Pieri, M.; Bernardini, S.; Mercuri, N.B.; Pisani, A.; et al. Biofluids Profile of α-Klotho in Patients with Parkinson’s Disease. Park. Relat. Disord. 2021, 90, 62–64. [Google Scholar] [CrossRef] [PubMed]
- Fernández-Espejo, E.; Rodriguez de Fonseca, F.; Suárez, J.; Martín de Pablos, Á. Cerebrospinal Fluid Lactoperoxidase Level Is Enhanced in Idiopathic Parkinson’s Disease, and Correlates with Levodopa Equivalent Daily Dose. Brain Res. 2021, 1761, 147411. [Google Scholar] [CrossRef] [PubMed]
- Sun, W.; Zheng, J.; Ma, J.; Wang, Z.; Shi, X.; Li, M.; Huang, S.; Hu, S.; Zhao, Z.; Li, D. Increased Plasma Heme Oxygenase-1 Levels in Patients With Early-Stage Parkinson’s Disease. Front. Aging Neurosci. 2021, 13, 621508. [Google Scholar] [CrossRef]
- Shamir, R.; Klein, C.; Amar, D.; Vollstedt, E.-J.; Bonin, M.; Usenovic, M.; Wong, Y.C.; Maver, A.; Poths, S.; Safer, H.; et al. Analysis of Blood-Based Gene Expression in Idiopathic Parkinson Disease. Neurology 2017, 89, 1676–1683. [Google Scholar] [CrossRef]
- Picca, A.; Guerra, F.; Calvani, R.; Marini, F.; Biancolillo, A.; Landi, G.; Beli, R.; Landi, F.; Bernabei, R.; Bentivoglio, A.R.; et al. Mitochondrial Signatures in Circulating Extracellular Vesicles of Older Adults with Parkinson’s Disease: Results from the EXosomes in PArkiNson’s Disease (EXPAND) Study. J. Clin. Med. 2020, 9, 504. [Google Scholar] [CrossRef] [PubMed]
- Paul, B.D.; Snyder, S.H. Impaired Redox Signaling in Huntington’s Disease: Therapeutic Implications. Front. Mol. Neurosci. 2019, 12, 68. [Google Scholar] [CrossRef] [PubMed]
- Jędrak, P.; Mozolewski, P.; Węgrzyn, G.; Więckowski, M.R. Mitochondrial Alterations Accompanied by Oxidative Stress Conditions in Skin Fibroblasts of Huntington’s Disease Patients. Metab. Brain Dis. 2018, 33, 2005–2017. [Google Scholar] [CrossRef] [PubMed]
- Vanisova, M.; Stufkova, H.; Kohoutova, M.; Rakosnikova, T.; Krizova, J.; Klempir, J.; Rysankova, I.; Roth, J.; Zeman, J.; Hansikova, H. Mitochondrial Organization and Structure Are Compromised in Fibroblasts from Patients with Huntington’s Disease. Ultrastruct. Pathol. 2022, 46, 462–475. [Google Scholar] [CrossRef] [PubMed]
- Neueder, A.; Orth, M. Mitochondrial Biology and the Identification of Biomarkers of Huntington’s Disease. Neurodegener. Dis. Manag. 2020, 10, 243–255. [Google Scholar] [CrossRef] [PubMed]
- Chen, C.-M.; Wu, Y.-R.; Cheng, M.-L.; Liu, J.-L.; Lee, Y.-M.; Lee, P.-W.; Soong, B.-W.; Chiu, D.T.-Y. Increased Oxidative Damage and Mitochondrial Abnormalities in the Peripheral Blood of Huntington’s Disease Patients. Biochem. Biophys. Res. Commun. 2007, 359, 335–340. [Google Scholar] [CrossRef]
- Spinelli, J.B.; Zaganjor, E. Mitochondrial Efficiency Directs Cell Fate. Nat. Cell Biol. 2022, 24, 125–126. [Google Scholar] [CrossRef]
- Ahmad, M.; Wolberg, A.; Kahwaji, C.I. Biochemistry, Electron Transport Chain; StatPearls Publishing: Treasure Island, FL, USA, 2022. [Google Scholar]
- McAdam, E.; Brem, R.; Karran, P. Oxidative Stress-Induced Protein Damage Inhibits DNA Repair and Determines Mutation Risk and Therapeutic Efficacy. Mol. Cancer Res. MCR 2016, 14, 612–622. [Google Scholar] [CrossRef]
- Korovila, I.; Hugo, M.; Castro, J.P.; Weber, D.; Höhn, A.; Grune, T.; Jung, T. Proteostasis, Oxidative Stress and Aging. Redox Biol. 2017, 13, 550–567. [Google Scholar] [CrossRef]
- Wu, Z.; Puigserver, P.; Andersson, U.; Zhang, C.; Adelmant, G.; Mootha, V.; Troy, A.; Cinti, S.; Lowell, B.; Scarpulla, R.C.; et al. Mechanisms Controlling Mitochondrial Biogenesis and Respiration through the Thermogenic Coactivator PGC-1. Cell 1999, 98, 115–124. [Google Scholar] [CrossRef]
- Scarpulla, R.C. Transcriptional Paradigms in Mammalian Mitochondrial Biogenesis and Function. Physiol. Rev. 2008, 88, 611–638. [Google Scholar] [CrossRef] [PubMed]
- Scarpulla, R.C. Metabolic Control of Mitochondrial Biogenesis through the PGC-1 Family Regulatory Network. Biochim. Biophys. Acta 2011, 1813, 1269–1278. [Google Scholar] [CrossRef] [PubMed]
- Virbasius, J.V.; Scarpulla, R.C. Activation of the Human Mitochondrial Transcription Factor A Gene by Nuclear Respiratory Factors: A Potential Regulatory Link between Nuclear and Mitochondrial Gene Expression in Organelle Biogenesis. Proc. Natl. Acad. Sci. USA 1994, 91, 1309–1313. [Google Scholar] [CrossRef] [PubMed]
- Saxton, W.M.; Hollenbeck, P.J. The Axonal Transport of Mitochondria. J. Cell Sci. 2012, 125 Pt 9, 2095–2104. [Google Scholar] [CrossRef]
- Schwarz, T.L. Mitochondrial Trafficking in Neurons. Cold Spring Harb. Perspect. Biol. 2013, 5, a011304. [Google Scholar] [CrossRef]
- Misgeld, T.; Schwarz, T.L. Mitostasis in Neurons: Maintaining Mitochondria in an Extended Cellular Architecture. Neuron 2017, 96, 651–666. [Google Scholar] [CrossRef]
- Franco-Iborra, S.; Vila, M.; Perier, C. Mitochondrial Quality Control in Neurodegenerative Diseases: Focus on Parkinson’s Disease and Huntington’s Disease. Front. Neurosci. 2018, 12, 342. [Google Scholar] [CrossRef]
- Keating, D.J. Mitochondrial Dysfunction, Oxidative Stress, Regulation of Exocytosis and Their Relevance to Neurodegenerative Diseases. J. Neurochem. 2008, 104, 298–305. [Google Scholar] [CrossRef]
- Schirinzi, T.; Madeo, G.; Martella, G.; Maltese, M.; Picconi, B.; Calabresi, P.; Pisani, A. Early Synaptic Dysfunction in Parkinson’s Disease: Insights from Animal Models: Early Synaptic Dysfunction in PD. Mov. Disord. 2016, 31, 802–813. [Google Scholar] [CrossRef]
- Gerencser, A.A.; Doczi, J.; Töröcsik, B.; Bossy-Wetzel, E.; Adam-Vizi, V. Mitochondrial Swelling Measurement in Situ by Optimized Spatial Filtering: Astrocyte-Neuron Differences. Biophys. J. 2008, 95, 2583–2598. [Google Scholar] [CrossRef]
- O’Sullivan, J.D.B.; Bullen, A.; Mann, Z.F. Mitochondrial Form and Function in Hair Cells. Heart Res. 2023, 428, 108660. [Google Scholar] [CrossRef] [PubMed]
- Woo, J.; Cho, H.; Seol, Y.; Kim, S.H.; Park, C.; Yousefian-Jazi, A.; Hyeon, S.J.; Lee, J.; Ryu, H. Power Failure of Mitochondria and Oxidative Stress in Neurodegeneration and Its Computational Models. Antioxidants 2021, 10, 229. [Google Scholar] [CrossRef] [PubMed]
- Polyzos, A.A.; McMurray, C.T. The Chicken or the Egg: Mitochondrial Dysfunction as a Cause or Consequence of Toxicity in Huntington’s Disease. Mech. Ageing Dev. 2017, 161 Pt A, 181–197. [Google Scholar] [CrossRef]
- Lang, A.E.; Espay, A.J. Disease Modification in Parkinson’s Disease: Current Approaches, Challenges, and Future Considerations. Mov. Disord. 2018, 33, 660–677. [Google Scholar] [CrossRef] [PubMed]
- Lee, L.-H.N.; Huang, C.-S.; Chuang, H.-H.; Lai, H.-J.; Yang, C.-K.; Yang, Y.-C.; Kuo, C.-C. An Electrophysiological Perspective on Parkinson’s Disease: Symptomatic Pathogenesis and Therapeutic Approaches. J. Biomed. Sci. 2021, 28, 85. [Google Scholar] [CrossRef]
- Jamwal, S.; Kumar, P. Insight Into the Emerging Role of Striatal Neurotransmitters in the Pathophysiology of Parkinson’s Disease and Huntington’s Disease: A Review. Curr. Neuropharmacol. 2019, 17, 165–175. [Google Scholar] [CrossRef]
- Terreros-Roncal, J.; Moreno-Jiménez, E.P.; Flor-García, M.; Rodríguez-Moreno, C.B.; Trinchero, M.F.; Cafini, F.; Rábano, A.; Llorens-Martín, M. Impact of Neurodegenerative Diseases on Human Adult Hippocampal Neurogenesis. Science 2021, 374, 1106–1113. [Google Scholar] [CrossRef]
- Levine, M.S.; Cepeda, C.; Hickey, M.A.; Fleming, S.M.; Chesselet, M.-F. Genetic Mouse Models of Huntington’s and Parkinson’s Diseases: Illuminating but Imperfect. Trends Neurosci. 2004, 27, 691–697. [Google Scholar] [CrossRef] [PubMed]
- Carmo, C.; Naia, L.; Lopes, C.; Rego, A.C. Mitochondrial Dysfunction in Huntington’s Disease. Adv. Exp. Med. Biol. 2018, 1049, 59–83. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).