Effects of Donor Ages and Propagation Methods on Seedling Growth of Platycladus orientalis (L.) Franco in Winter
Abstract
1. Introduction
2. Results
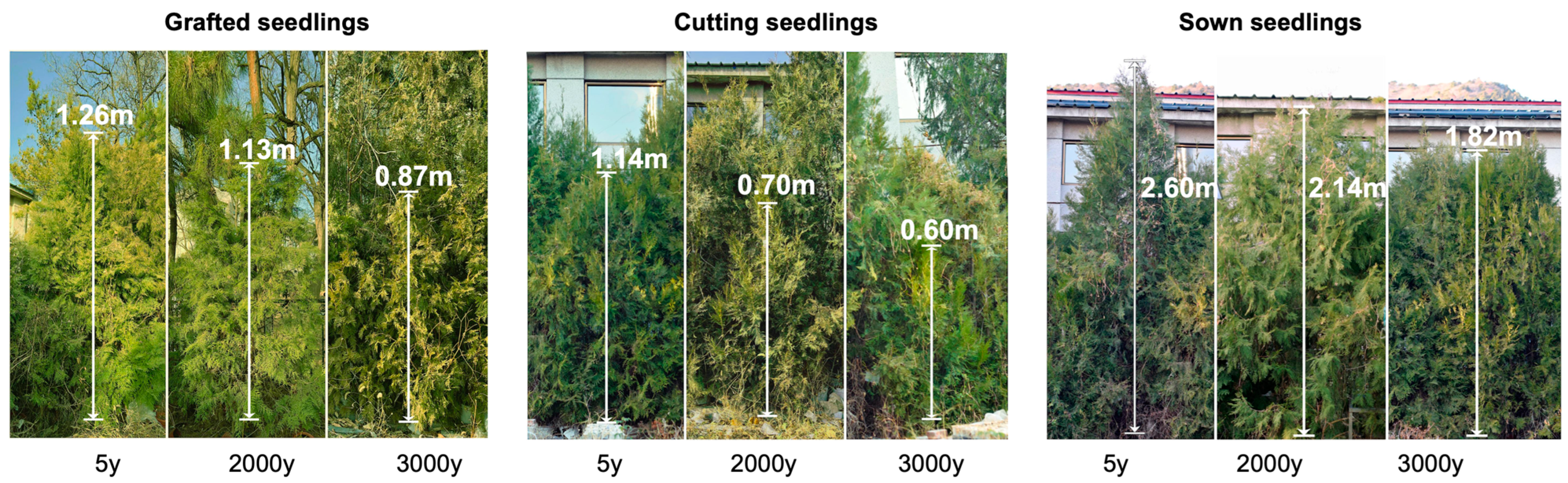
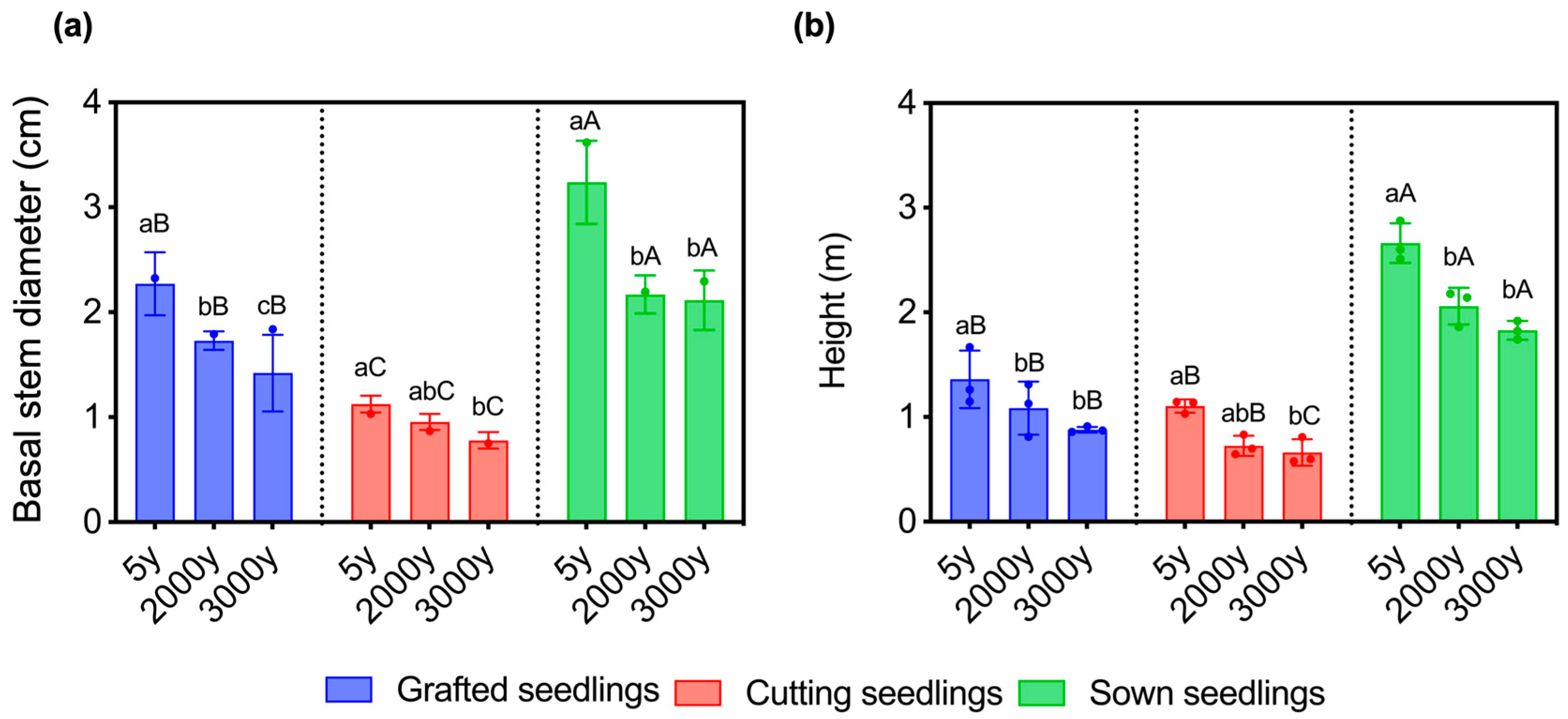
2.1. Age of Donors and Propagation Methods Affect the Growth of Seedlings in Winter
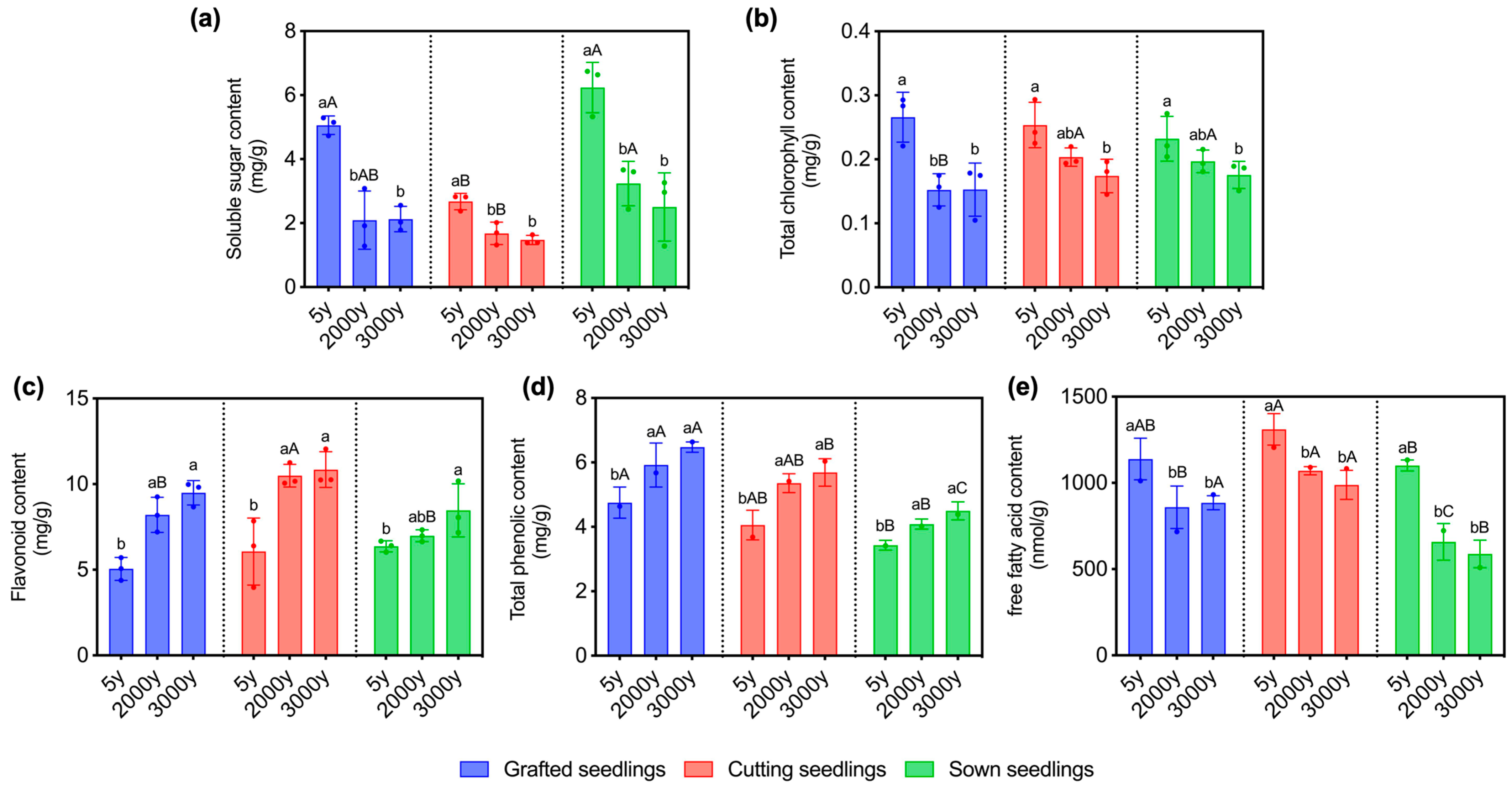
2.2. Age of Donors and Propagation Methods Affect the Soluble Sugar and Fatty Acid Contents of Seedlings in Winter
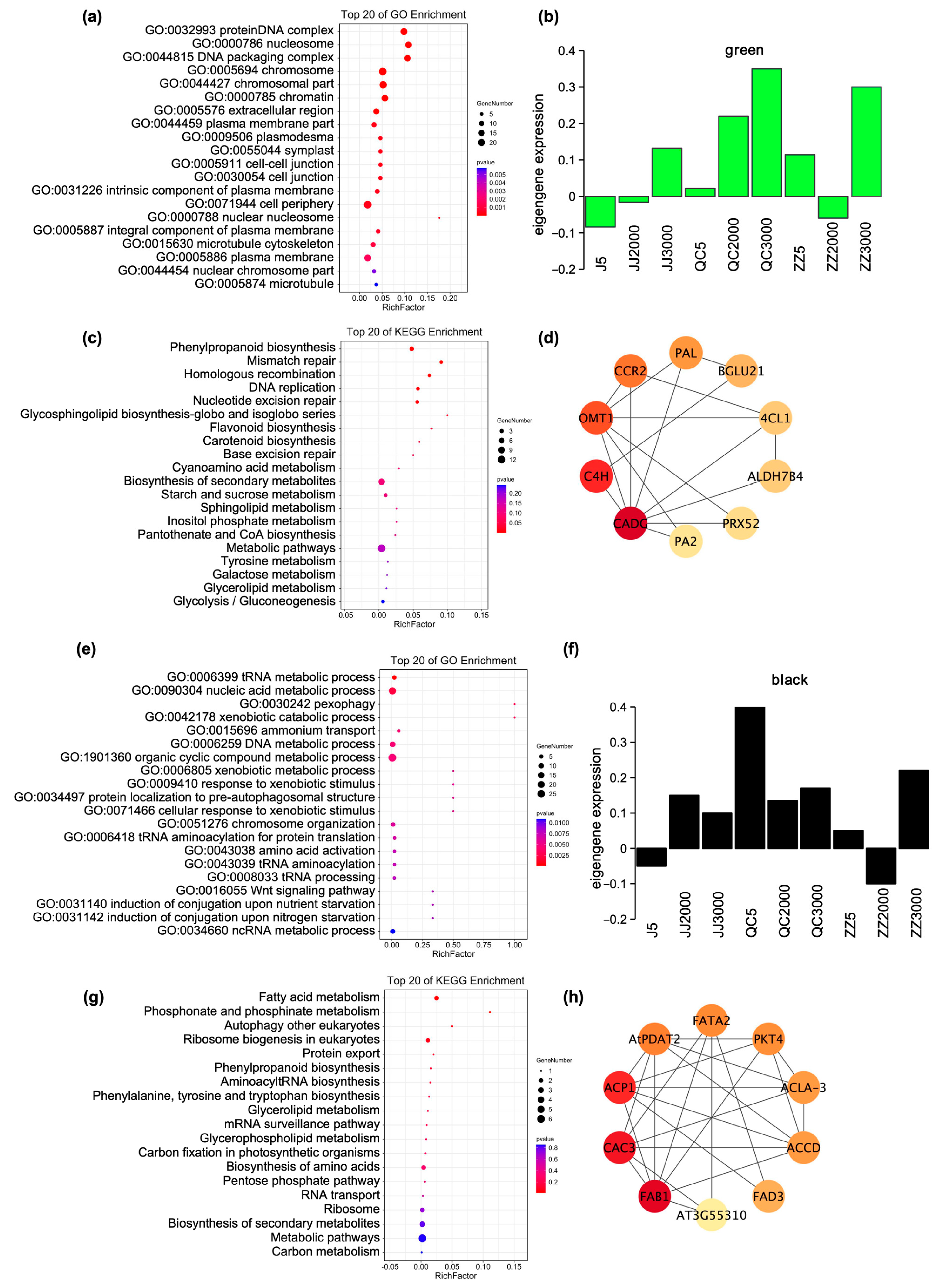
2.3. Age of Donors and Propagation Methods Affect the Expression of Genes Related to Phenylpropanoid Biosynthesis and Fatty Acid Metabolism of Seedlings in Winter
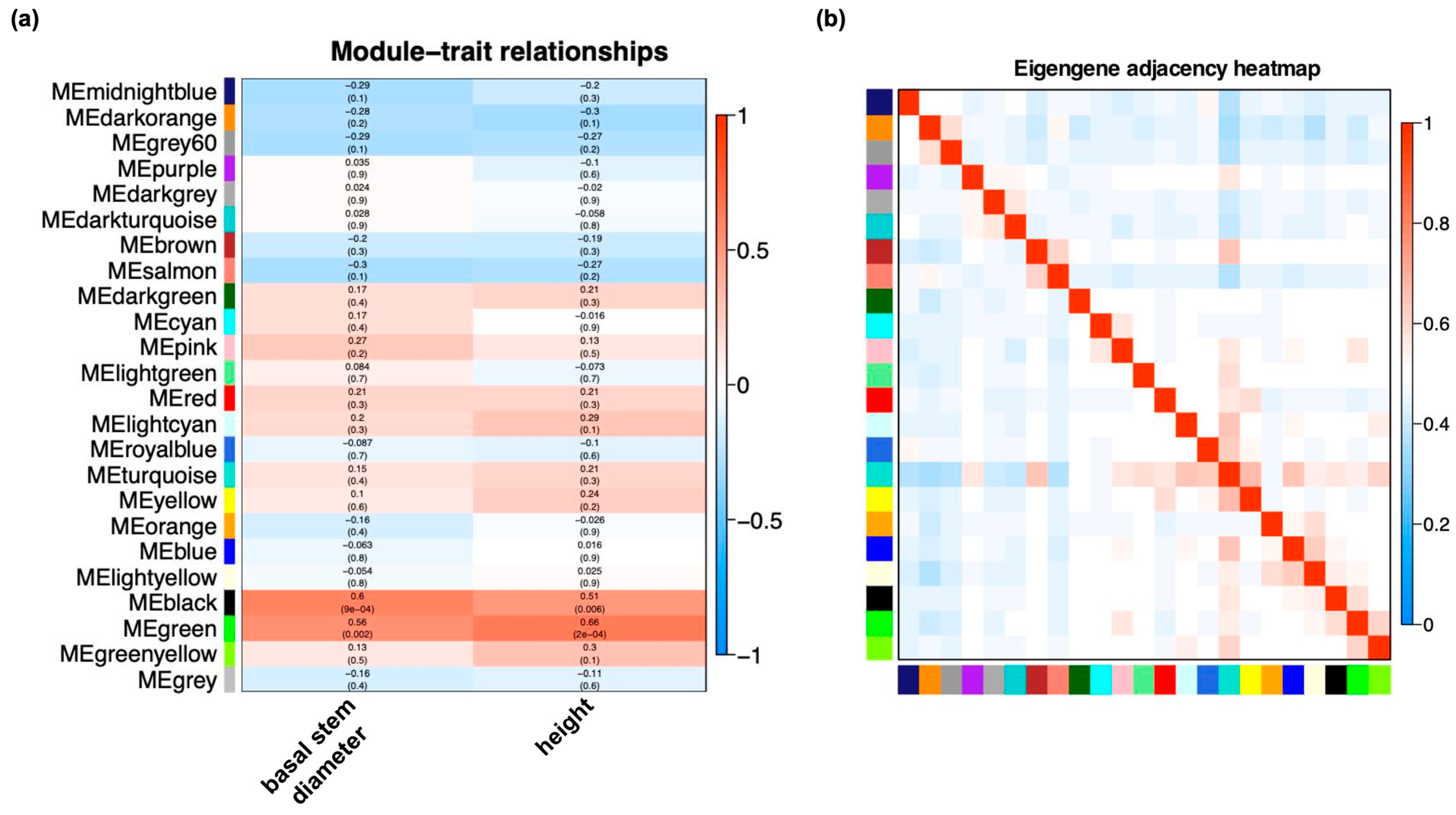
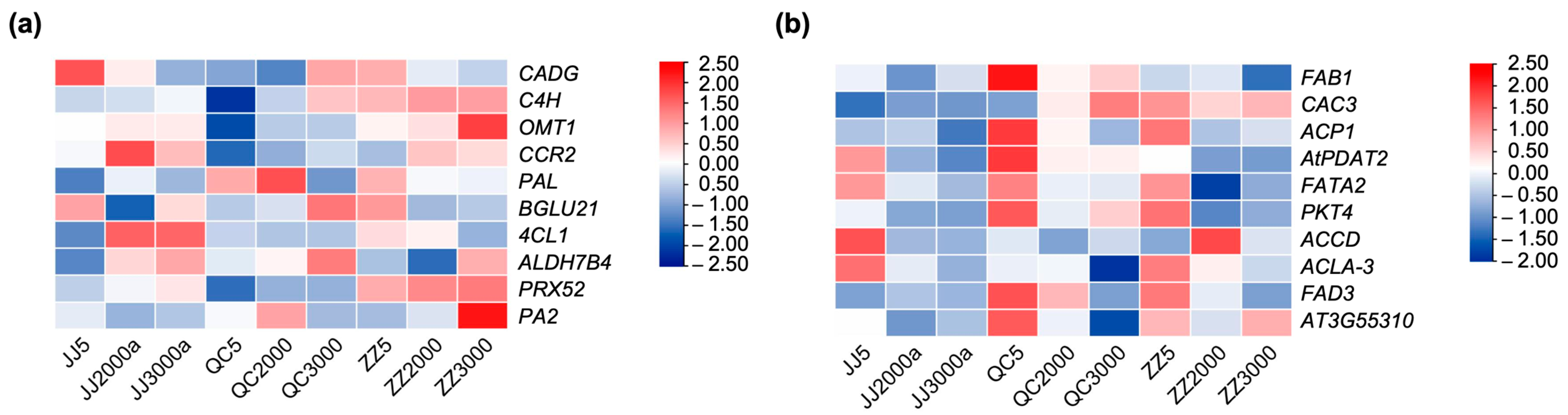
2.4. Identification of Hub Genes of Co-Expressed Modules
2.5. qRT-PCR Validation of Gene Expression Profiles of DEGs
3. Discussion
4. Materials and Methods
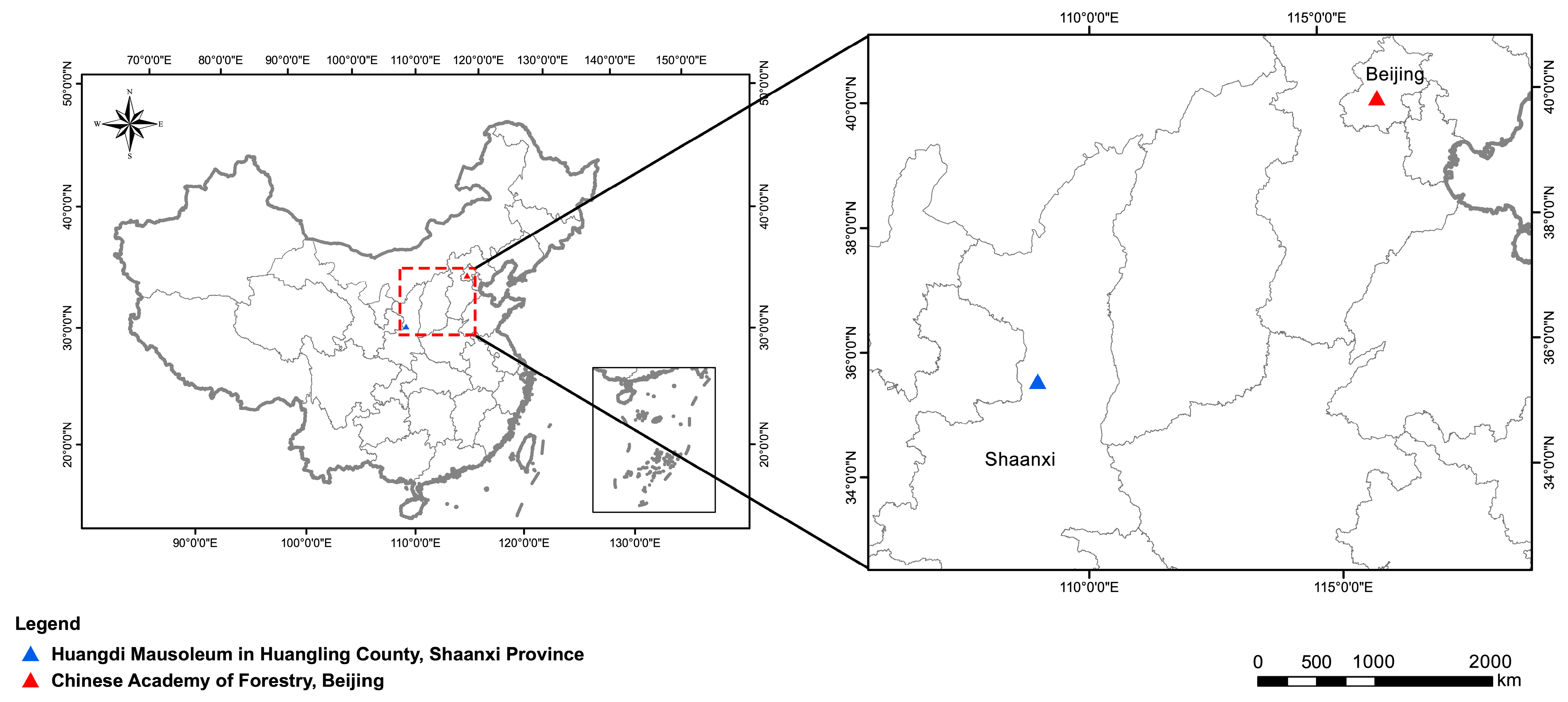
4.1. Plant Materials and Growth Conditions
4.2. Phenotypic Measurements
4.3. Determination of Soluble Sugar and Chlorophyll Contents
4.4. Determination of Flavonoids, Total Phenolics and Fatty Acids
4.5. RNA Sequencing Analysis
4.6. Quantitative Real-Time PCR Analysis
4.7. Statistical Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Chang, E.; Yao, X.; Zhang, J.; Deng, N.; Jiang, Z.; Shi, S. De novo characterization of Platycladus orientalis transcriptome 2 and analysis of its gene expression during aging. PeerJ Prepr. 2017, 5, e2866v1. [Google Scholar] [CrossRef]
- Chang, E.; Zhang, J.; Deng, N.; Yao, X.; Liu, J.; Zhao, X.; Jiang, Z.; Shi, S. Transcriptome differences between 20- and 3,000-year-old Platycladus orientalis reveal that ROS are involved in senescence regulation. Electron. J. Biotechnol. 2017, 29, 68–77. [Google Scholar] [CrossRef]
- Shi, X.; Wang, X.; Cheng, F.; Cao, H.; Liang, H.; Lu, J.; Kong, Q.; Bie, Z. iTRAQ-based quantitative proteomics analysis of cold stress-induced mechanisms in grafted watermelon seedlings. J. Proteom. 2019, 192, 311–320. [Google Scholar] [CrossRef] [PubMed]
- Viherä-Aarnio, A.; Ryynänen, L. Growth, crown structure and seed production of birch seedlings, grafts and micropropagated plants. Silva Fenn. 1995, 29, 3–12. [Google Scholar] [CrossRef]
- Tsobeng, A.; Asaah, E.; Tchoundjeu, Z.; Van Damme, P.; Ofori, D.; Jamnadass, R. Growth, flowering and fruiting of stecklings, grafts and seedlings of Allanblackia floribunda Oliver (Clusiaceae). Agrofor. Syst. 2017, 91, 259–270. [Google Scholar] [CrossRef]
- Andrade Júnior, S.D.; Alexandre, R.S.; Schmildt, E.R.; Partelli, F.L.; Ferrão, M.A.G.; Mauri, A.L. Comparison between grafting and cutting as vegetative propagation methods for conilon coffee plants. Acta Sci.-Agron. 2013, 35, 461–469. [Google Scholar] [CrossRef]
- Xu, J.; Zhang, M.; Liu, G.; Yang, X.; Hou, X. Comparative transcriptome profiling of chilling stress responsiveness in grafted watermelon seedlings. Plant Physiol. Biochem. 2016, 109, 561–570. [Google Scholar] [CrossRef]
- Welander, N.T.; Gemmel, P.; Hellgren, O.; Ottosson, B. The consequences of freezing temperatures followed by high irradiance on in vivo chlorophyll fluorescence and growth in Picea abies. Physiol. Plant. 1994, 91, 121–127. [Google Scholar] [CrossRef]
- Zhang, S.; Zhang, L.; Chai, Y.; Wang, F.; Li, Y.; Su, L.; Zhao, Z. Physiology and proteomics research on the leaves of ancient Platycladus orientalis (L.) during winter. J. Proteomics 2015, 126, 263–278. [Google Scholar] [CrossRef]
- Castañeda-Saucedo, M.C.; Tapia-Campos, E.; Ramírez-Anaya, J. del P.; Beltrán, J. Growth and development of stevia cuttings during propagation with hormones in different months of the year. Plants 2020, 9, 294. [Google Scholar] [CrossRef]
- Bauters, L.; Stojilković, B.; Gheysen, G. Pathogens pulling the strings: Effectors manipulating salicylic acid and phenylpropanoid biosynthesis in plants. Mol. Plant Pathol. 2021, 22, 1436–1448. [Google Scholar] [CrossRef]
- Song, Y.; Feng, J.; Liu, D.; Long, C. Different phenylalanine pathway responses to cold stress based on metabolomics and transcriptomics in Tartary buckwheat landraces. J. Agric. Food Chem. 2022, 70, 687–698. [Google Scholar] [CrossRef] [PubMed]
- Gong, X.-X.; Yan, B.-Y.; Hu, J.; Yang, C.-P.; Li, Y.-J.; Liu, J.-P.; Liao, W.-B. Transcriptome profiling of rubber tree (Hevea brasiliensis) discovers candidate regulators of the cold stress response. Genes Genom. 2018, 40, 1181–1197. [Google Scholar] [CrossRef] [PubMed]
- Zou, K.; Liu, X.; Zhang, D.; Yang, Q.; Fu, S.; Meng, D.; Chang, W.; Li, R.; Yin, H.; Liang, Y. Flavonoid biosynthesis is likely more susceptible to elevation and tree age than other branch pathways involved in phenylpropanoid biosynthesis in Ginkgo leaves. Front. Plant Sci. 2019, 10, 983. [Google Scholar] [CrossRef] [PubMed]
- Tian, J.; Tian, L.; Chen, M.; Chen, Y.; Wei, A. Low temperature affects fatty acids profiling and key synthesis genes expression patterns in Zanthoxylum bungeanum maxim. Int. J. Mol. Sci. 2022, 23, 2319. [Google Scholar] [CrossRef] [PubMed]
- Sharma, A.; Shahzad, B.; Rehman, A.; Bhardwaj, R.; Landi, M.; Zheng, B. Response of phenylpropanoid pathway and the role of polyphenols in plants under abiotic stress. Molecules 2019, 24, 2452. [Google Scholar] [CrossRef]
- Martz, F.; Kiviniemi, S.; Palva, T.E.; Sutinen, M.-L. Contribution of omega-3 fatty acid desaturase and 3-ketoacyl-ACP synthase II (KASII) genes in the modulation of glycerolipid fatty acid composition during cold acclimation in birch leaves. J. Exp. Bot. 2006, 57, 897–909. [Google Scholar] [CrossRef]
- Wang, X.; Yu, C.; Liu, Y.; Yang, L.; Li, Y.; Yao, W.; Cai, Y.; Yan, X.; Li, S.; Cai, Y.; et al. GmFAD3A, a ω-3 fatty acid desaturase gene, enhances cold tolerance and seed germination rate under low temperature in rice. Int. J. Mol. Sci. 2019, 20, 3796. [Google Scholar] [CrossRef]
- Li, H.; Guo, Y.; Lan, Z.; Xu, K.; Chang, J.; Ahammed, G.J.; Ma, J.; Wei, C.; Zhang, X. Methyl jasmonate mediates melatonin-induced cold tolerance of grafted watermelon plants. Hortic. Res. 2021, 8, 57. [Google Scholar] [CrossRef]
- Londo, J.P.; Kovaleski, A.P.; Lillis, J.A. Divergence in the transcriptional landscape between low temperature and freeze shock in cultivated grapevine (Vitis vinifera). Hortic. Res. 2018, 5, 10. [Google Scholar] [CrossRef]
- Branen, J.K.; Chiou, T.-J.; Engeseth, N.J. Overexpression of Acyl carrier protein-1 alters fatty acid composition of leaf tissue in Arabidopsis. Plant Physiol. 2001, 127, 222–229. [Google Scholar] [CrossRef] [PubMed]
- Brouwer, D.J.; Duke, S.H.; Osborn, T.C. Comparison of seedling and cuttings for evaluating winter hardiness in Alfalfa. Crop Sci. 1998, 38, 1704–1707. [Google Scholar] [CrossRef]
- Fu, S.; Chen, J.; Wu, X.; Gao, H.; Lü, G. Comprehensive evaluation of low temperature and salt tolerance in grafted and rootstock seedlings combined with yield and quality of grafted tomato. Horticulturae 2022, 8, 595. [Google Scholar] [CrossRef]
- Jaikumar, N.S.; Snapp, S.S.; Sharkey, T.D. Older Thinopyrum intermedium (Poaceae) plants exhibit superior photosynthetic tolerance to cold stress and greater increases in two photosynthetic enzymes under freezing stress compared with young plants. J. Exp. Bot. 2016, 67, 4743–4753. [Google Scholar] [CrossRef] [PubMed]
- Singh, I.; Kumar, U.; Singh, S.K.; Gupta, C.; Singh, M.; Kushwaha, S.R. Physiological and biochemical effect of 24-epibrassinoslide on cold tolerance in maize seedlings. Physiol. Mol. Biol. Plants 2012, 18, 229–236. [Google Scholar] [CrossRef]
- Swamy, S.L.; Puri, S.; Singh, A.K. Effect of auxins (IBA and NAA) and season on rooting of juvenile and mature hardwood cuttings of Robinia pseudoacacia and Grewia optiva. New For. 2002, 23, 143–157. [Google Scholar] [CrossRef]
- Donaldson, J.R.; Stevens, M.T.; Barnhill, H.R.; Lindroth, R.L. Age-related shifts in leaf chemistry of clonal aspen (Populus tremuloides). J. Chem. Ecol. 2006, 32, 1415–1429. [Google Scholar] [CrossRef]
- Turfan, N.; Alay, M.; Sariyildiz, T. Effect of tree age on chemical compounds of ancient Anatolian black pine (Pinus nigra subsp. pallasiana) needles in Northwest Turkey. IForest-Biogeosci. For. 2018, 11, 406. [Google Scholar] [CrossRef]
- Dong, N.-Q.; Lin, H.-X. Contribution of phenylpropanoid metabolism to plant development and plant–environment interactions. J. Integr. Plant Biol. 2021, 63, 180–209. [Google Scholar] [CrossRef]
- Wang, X.; Liu, Y.; Han, Z.; Chen, Y.; Huai, D.; Kang, Y.; Wang, Z.; Yan, L.; Jiang, H.; Lei, Y.; et al. Integrated transcriptomics and metabolomics analysis reveal key metabolism pathways contributing to cold tolerance in peanut. Front. Plant Sci. 2021, 12, 752474. [Google Scholar] [CrossRef]
- Zhu, Y.; Huang, J.; Wang, K.; Wang, B.; Sun, S.; Lin, X.; Song, L.; Wu, A.; Li, H. Characterization of lignin structures in Phyllostachys edulis (moso bamboo) at different ages. Polymers 2020, 12, 187. [Google Scholar] [CrossRef]
- Ďurkovič, J.; Kaňuchová, A.; Kačík, F.; Solár, R.; Lengyelová, A. Genotype- and age-dependent patterns of lignin and cellulose in regenerants derived from 80-year-old trees of black mulberry (Morus nigra L.). Plant Cell Tissue Organ Cult. PCTOC 2012, 108, 359–370. [Google Scholar] [CrossRef]
- Xu, D.; Yuan, H.; Tong, Y.; Zhao, L.; Qiu, L.; Guo, W.; Shen, C.; Liu, H.; Yan, D.; Zheng, B. Comparative proteomic analysis of the graft unions in hickory (Carya cathayensis) provides insights into response mechanisms to grafting process. Front. Plant Sci. 2017, 8, 676. [Google Scholar] [CrossRef] [PubMed]
- Upchurch, R.G. Fatty acid unsaturation, mobilization, and regulation in the response of plants to stress. Biotechnol. Lett. 2008, 30, 967–977. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Z.; Fan, J.; Yang, P.; Wang, Z.; Opiyo, S.O.; Mackey, D.; Xia, Y. Involvement of Arabidopsis acyl carrier protein 1 in PAMP-triggered immunity. Mol. Plant-Microbe Interact. 2022, 35, 681–693. [Google Scholar] [CrossRef]
- Rodríguez, M.F.R.; Sánchez-García, A.; Salas, J.J.; Garcés, R.; Martínez-Force, E. Characterization of soluble acyl-ACP desaturases from Camelina sativa, Macadamia tetraphylla and Dolichandra unguis-cati. J. Plant Physiol. 2015, 178, 35–42. [Google Scholar] [CrossRef]
- Yuan, L.; Mao, X.; Zhao, K.; Ji, X.; Ji, C.; Xue, J.; Li, R. Characterisation of phospholipid: Diacylglycerol acyltransferases (PDATs) from Camelina sativa and their roles in stress responses. Biol. Open 2017, 6, 1024–1034. [Google Scholar] [CrossRef]
- Hernández, M.L.; Moretti, S.; Sicardo, M.D.; García, Ú.; Pérez, A.; Sebastiani, L.; Martínez-Rivas, J.M. Distinct physiological roles of three phospholipid: Diacylglycerol acyltransferase genes in olive fruit with respect to oil accumulation and the response to abiotic stress. Front. Plant Sci. 2021, 12, 751959. [Google Scholar] [CrossRef]
- Wang, J.; Liu, Z.; Liu, H.; Peng, D.; Zhang, J.; Chen, M. Linum usitatissimum FAD2A and FAD3A enhance seed polyunsaturated fatty acid accumulation and seedling cold tolerance in Arabidopsis thaliana. Plant Sci. 2021, 311, 111014. [Google Scholar] [CrossRef]
- Yang, Z.; Ohlrogge, J.B. Turnover of fatty acids during natural senescence of Arabidopsis, Brachypodium, and switchgrass and in Arabidopsis β-oxidation mutants. Plant Physiol. 2009, 150, 1981–1989. [Google Scholar] [CrossRef]
- Zhou, Z.; Wang, M.-J.; Zhao, S.-T.; Hu, J.-J.; Lu, M.-Z. Changes in freezing tolerance in hybrid poplar caused by up- and down-regulation of PtFAD2 gene expression. Transgenic Res. 2010, 19, 647–654. [Google Scholar] [CrossRef] [PubMed]
- Wu, L.; Deng, Z.; Cao, L.; Meng, L. Effect of plant density on yield and quality of perilla sprouts. Sci. Rep. 2020, 10, 9937. [Google Scholar] [CrossRef] [PubMed]
- Jia, Z.; Tang, M.; Wu, J. The determination of flavonoid contents in mulberry and their scavenging effects on superoxide radicals. Food Chem. 1999, 64, 555–559. [Google Scholar] [CrossRef]
- Cicco, N.; Lanorte, M.T.; Paraggio, M.; Viggiano, M.; Lattanzio, V. A reproducible, rapid and inexpensive Folin–Ciocalteu micro-method in determining phenolics of plant methanol extracts. Microchem. J. 2009, 91, 107–110. [Google Scholar] [CrossRef]
- Veerapagu, M.; Narayanan, D.A.S.; Ponmurugan, K.; Jeya, K.R. Screening selection identification production and optimization of bacterial lipase from oil spilled soil. Asian J. Pharm. Clin. Res. 2013, 6, 62–67. [Google Scholar]
- Kim, D.; Pertea, G.; Trapnell, C.; Pimentel, H.; Kelley, R.; Salzberg, S.L. TopHat2: Accurate alignment of transcriptomes in the presence of insertions, deletions and gene fusions. Genome Biol. 2013, 14, R36. [Google Scholar] [CrossRef]
- Trapnell, C.; Roberts, A.; Goff, L.; Pertea, G.; Kim, D.; Kelley, D.R.; Pimentel, H.; Salzberg, S.L.; Rinn, J.L.; Pachter, L. Differential gene and transcript expression analysis of RNA-seq experiments with TopHat and Cufflinks. Nat. Protoc. 2012, 7, 562–578. [Google Scholar] [CrossRef]
- Wang, L.; Feng, Z.; Wang, X.; Wang, X.; Zhang, X. DEGseq: An R package for identifying differentially expressed genes from RNA-seq data. Bioinformatics 2010, 26, 136–138. [Google Scholar] [CrossRef]
- Conway, J.R.; Lex, A.; Gehlenborg, N. UpSetR: An R package for the visualization of intersecting sets and their properties. Bioinformatics 2017, 33, 2938–2940. [Google Scholar] [CrossRef]
- Langfelder, P.; Horvath, S. WGCNA: An R package for weighted correlation network analysis. BMC Bioinform. 2008, 9, 559. [Google Scholar] [CrossRef]
- Chen, C.; Chen, H.; Zhang, Y.; Thomas, H.R.; Frank, M.H.; He, Y.; Xia, R. TBtools: An integrative toolkit developed for interactive analyses of big biological data. Mol. Plant 2020, 13, 1194–1202. [Google Scholar] [CrossRef] [PubMed]
- Goh, H.-H.; Abu Bakar, S.; Kamal Azlan, N.D.; Zainal, Z.; Mohd Noor, N. Transcriptional reprogramming during Garcinia-type recalcitrant seed germination of Garcinia mangostana. Sci. Hortic. 2019, 257, 108727. [Google Scholar] [CrossRef]
- Marum, L.; Miguel, A.; Ricardo, C.P.; Miguel, C. Reference gene selection for quantitative real-time PCR normalization in Quercus suber. PLoS ONE 2012, 7, e35113. [Google Scholar] [CrossRef]
- Livak, K.J.; Schmittgen, T.D. Analysis of relative gene expression data using real-time quantitative PCR and the 2−ΔΔCT method. Methods 2001, 25, 402–408. [Google Scholar] [CrossRef] [PubMed]



| Items | Number |
|---|---|
| Total unigenes | 204,798 |
| Total base | 156,177,968 |
| Average length (bp) | 762.6 |
| N50 | 1428 |
| GC percent | 38.68% |
| BUSCO score | C: 85.6% |
| Number of transcripts <300 bp | 74,617 (36%) |
| Number of transcripts between 300 and 500 bp | 56,732 (28%) |
| Number of transcripts between 500 and 1000 bp | 36,205 (18%) |
| Number of transcripts between 1000 and 2000 bp | 19,647 (10%) |
| Number of transcripts >2000 bp | 17,597 (9%) |
| Database | Number of Unigenes | Percentage (%) |
|---|---|---|
| Annotated in NR | 62,859 | 30.69% |
| Annotated in NT | 28,559 | 13.94% |
| Annotated in KO | 10,136 | 4.95% |
| Annotated in SwissProt | 41,489 | 20.26% |
| Annotated in Pfam | 59,635 | 29.12% |
| Annotated in GO | 34,306 | 16.75% |
| Annotated in COG/KOG | 34,431 | 16.81% |
| Annotated in all Databases | 3262 | 1.59% |
| Annotated in at least one Database | 79,514 | 38.83% |
| Total Unigenes | 204,798 | 100% |
| Annotated in NR | 62,859 | 30.69% |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Dong, Y.; Xiao, W.; Guo, W.; Liu, Y.; Nie, W.; Huang, R.; Tan, C.; Jia, Z.; Liu, J.; Jiang, Z.; et al. Effects of Donor Ages and Propagation Methods on Seedling Growth of Platycladus orientalis (L.) Franco in Winter. Int. J. Mol. Sci. 2023, 24, 7170. https://doi.org/10.3390/ijms24087170
Dong Y, Xiao W, Guo W, Liu Y, Nie W, Huang R, Tan C, Jia Z, Liu J, Jiang Z, et al. Effects of Donor Ages and Propagation Methods on Seedling Growth of Platycladus orientalis (L.) Franco in Winter. International Journal of Molecular Sciences. 2023; 24(8):7170. https://doi.org/10.3390/ijms24087170
Chicago/Turabian StyleDong, Yao, Wenfa Xiao, Wei Guo, Yifu Liu, Wen Nie, Ruizhi Huang, Cancan Tan, Zirui Jia, Jianfeng Liu, Zeping Jiang, and et al. 2023. "Effects of Donor Ages and Propagation Methods on Seedling Growth of Platycladus orientalis (L.) Franco in Winter" International Journal of Molecular Sciences 24, no. 8: 7170. https://doi.org/10.3390/ijms24087170
APA StyleDong, Y., Xiao, W., Guo, W., Liu, Y., Nie, W., Huang, R., Tan, C., Jia, Z., Liu, J., Jiang, Z., & Chang, E. (2023). Effects of Donor Ages and Propagation Methods on Seedling Growth of Platycladus orientalis (L.) Franco in Winter. International Journal of Molecular Sciences, 24(8), 7170. https://doi.org/10.3390/ijms24087170





