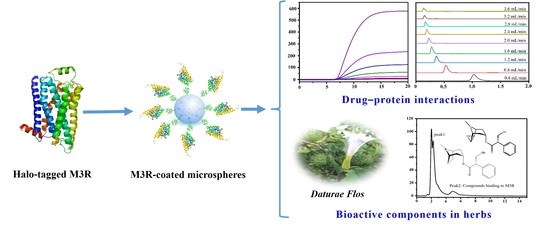
Immobilization of M3 Muscarinic Receptor to Rapidly Analyze Drug—Protein Interactions and Bioactive Components in a Natural Plant
Abstract
1. Introduction
2. Results and Discussion
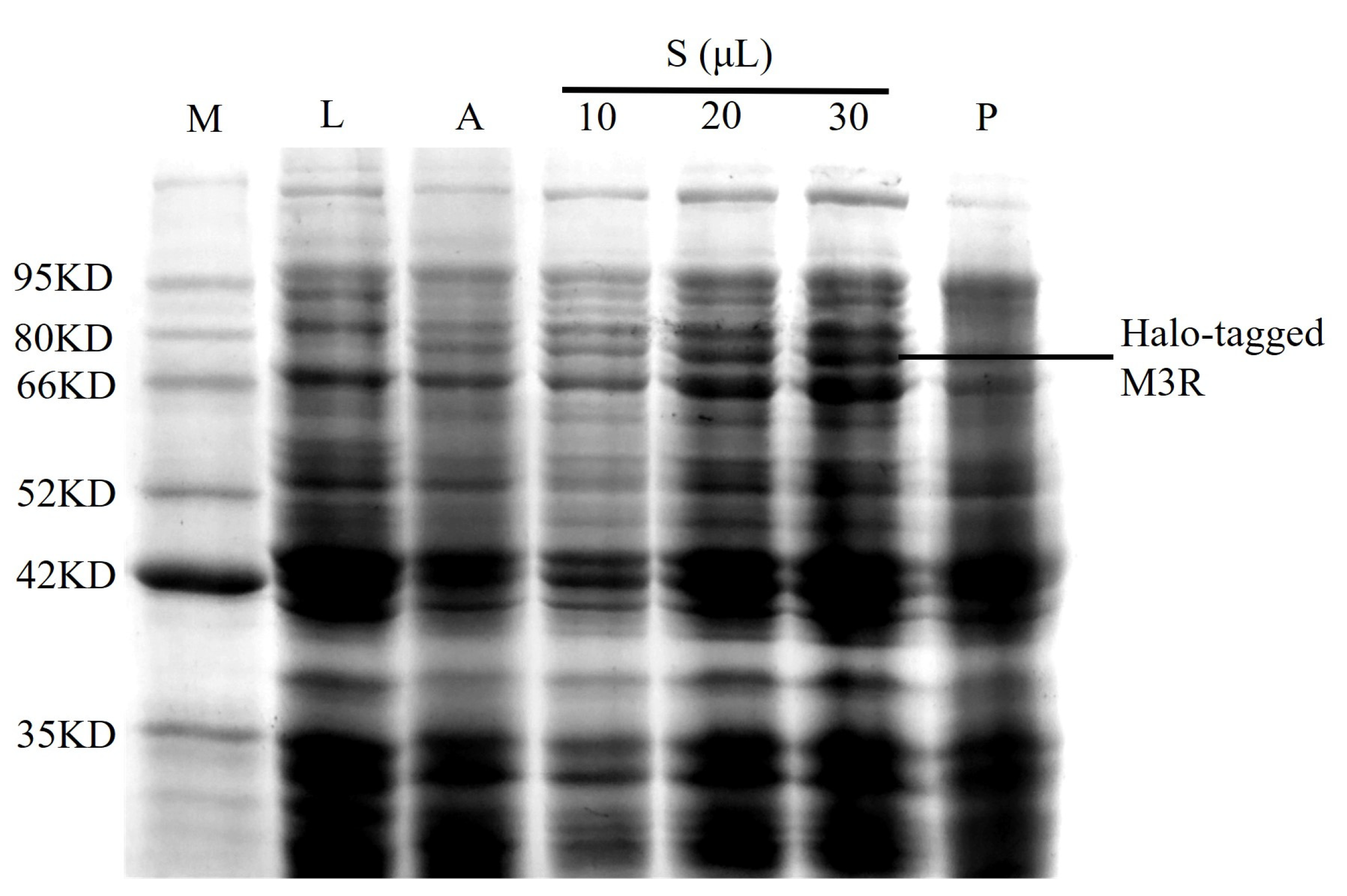
2.1. Expression of Halo-Tagged M3R
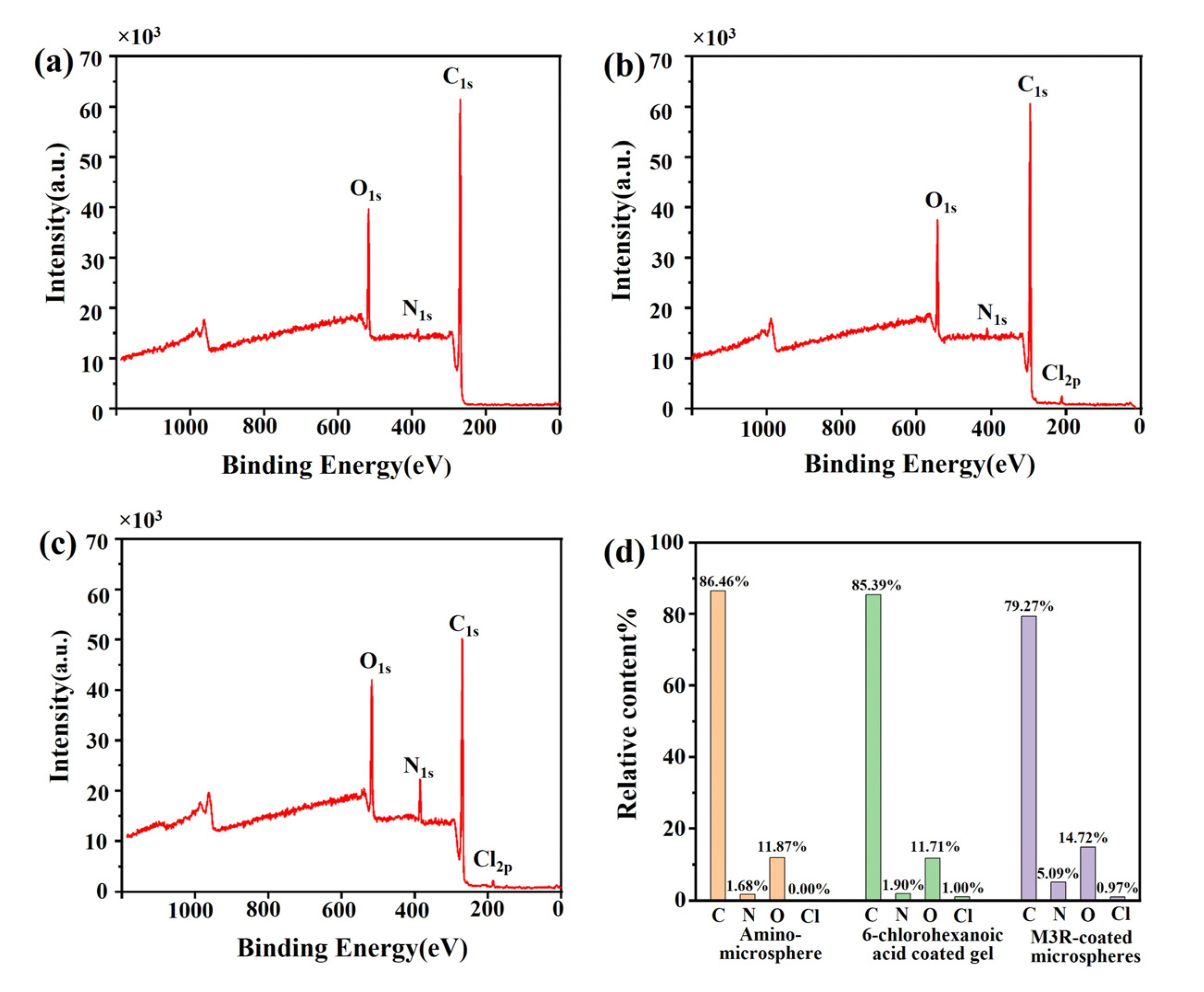
2.2. Specificity and Stability Assessments of the M3R-Immobilized Column
2.3. M3R-Immobilized Column for Determination of the Binding Coefficient of Drug–Receptor Interactions
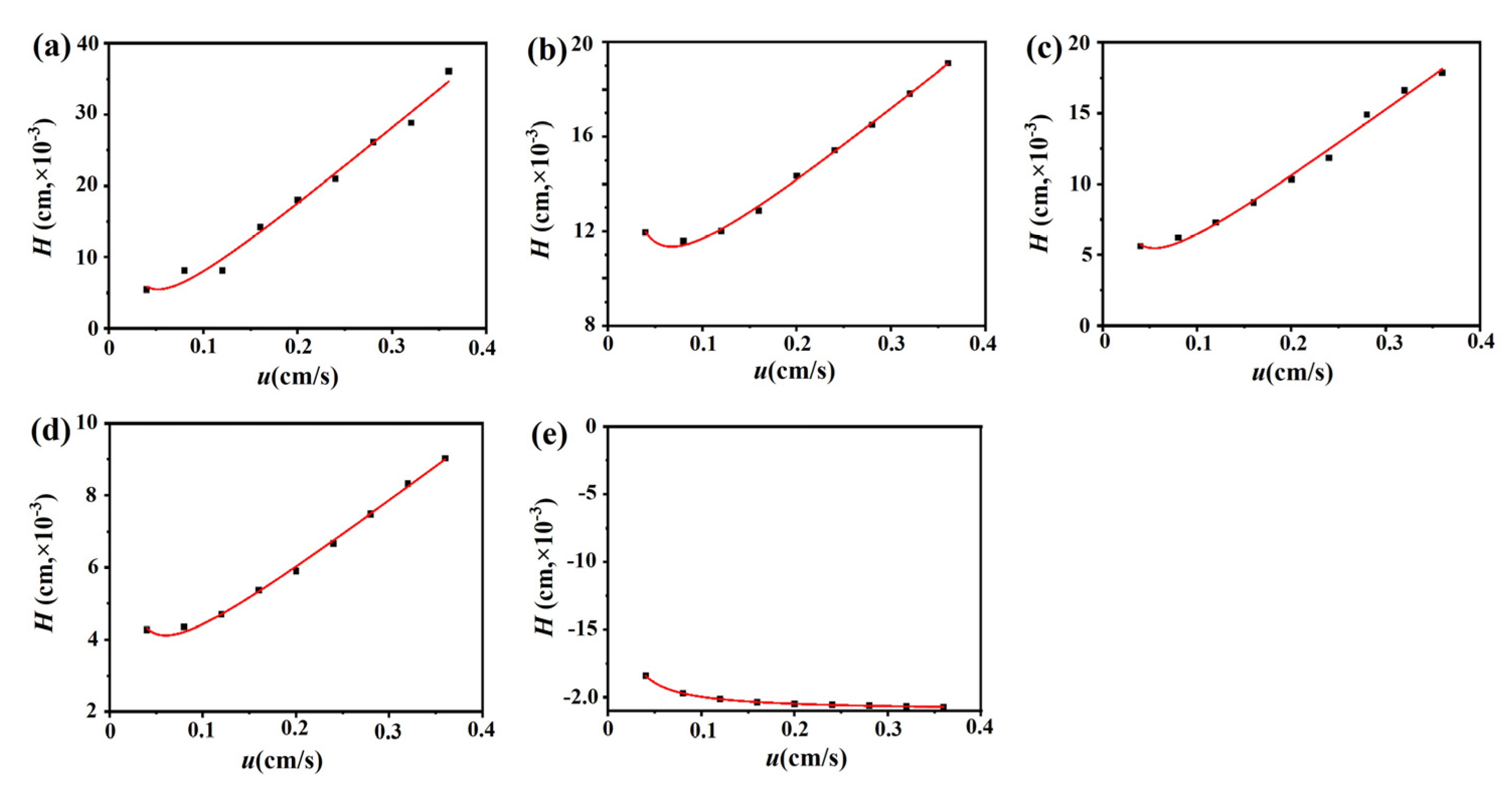
2.3.1. Association Constants and Binding Site Numbers
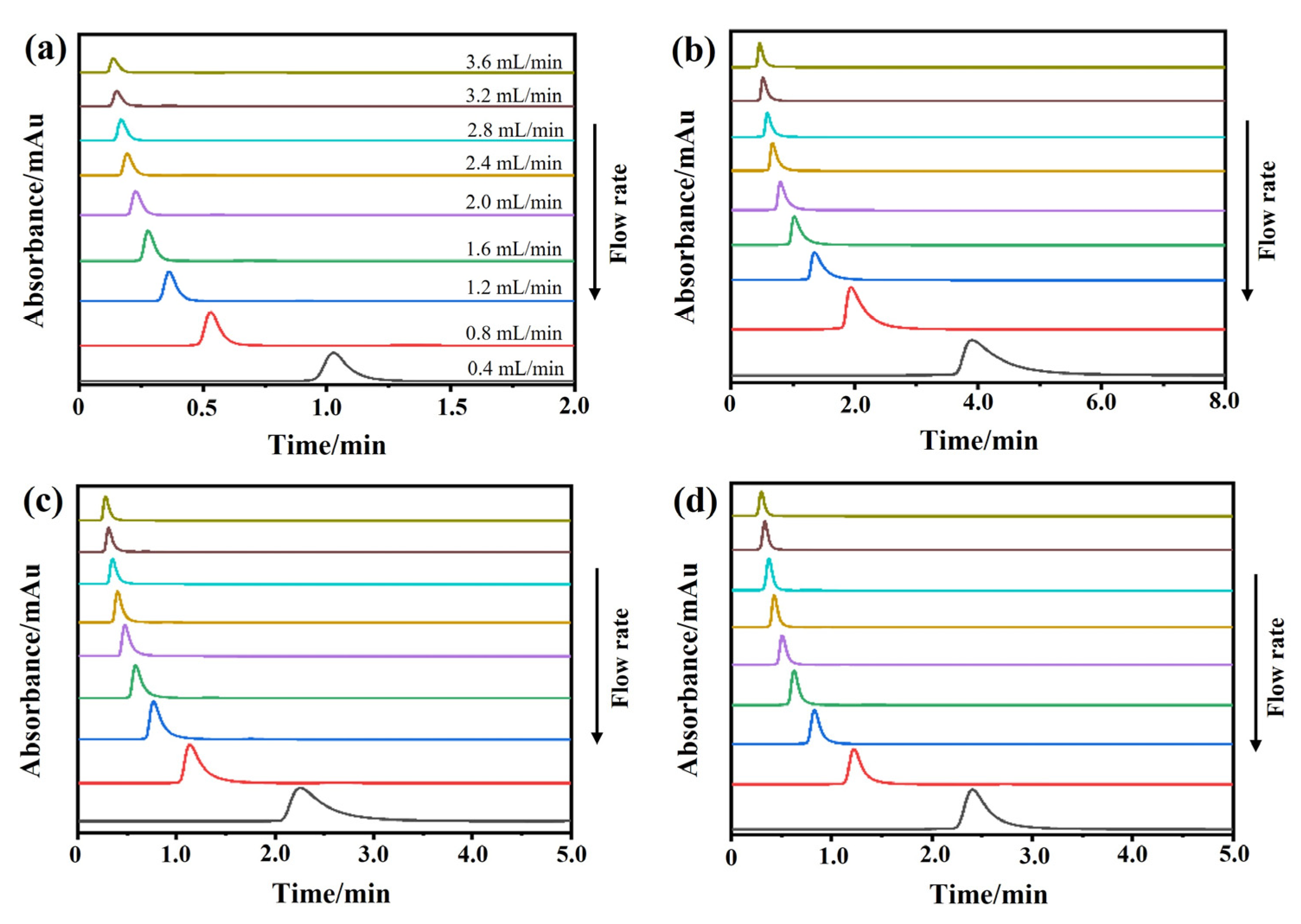
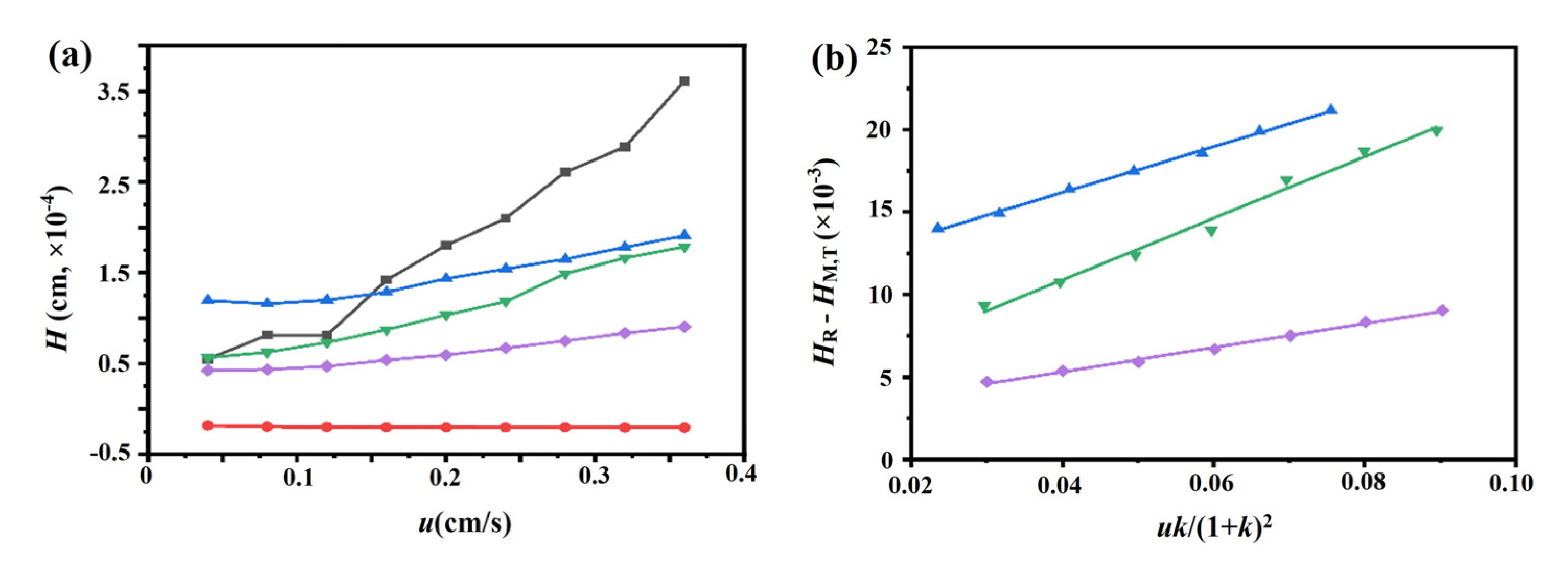
2.3.2. Dissociation Rate Constants
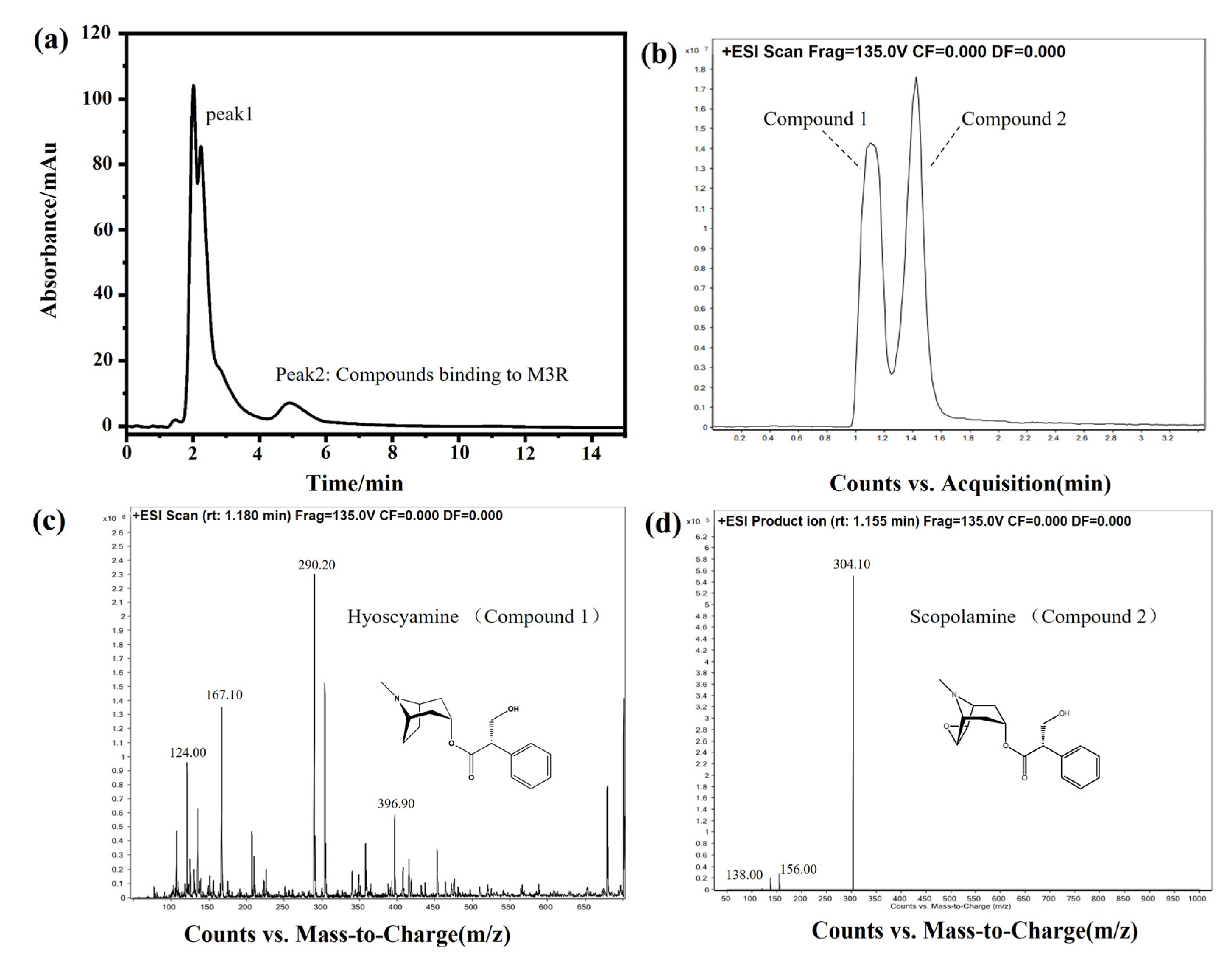
2.4. Identification of Bioactive Compounds in the DF Extract
3. Materials and Methods
3.1. Chemicals and Instruments
3.2. Preparation of the M3R-Immobilized Column
3.3. Characterization of the M3R-Immobilized Column
3.4. Stability and Specificity Analyses of the M3R-Immobilized Column
3.5. Receptor–Drug Interaction Analysis
3.5.1. Frontal Analysis
3.5.2. Peak Profiling Method
3.6. Analysis of the M3R-Binding Bioactive Components in the DF Extract
3.6.1. Preparation of the DF Extract
3.6.2. Identification of Active Compounds in the DF Extract
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Zhang, X.F.; He, C.L.; Wang, M.; Zhou, Q.T.; Yang, D.H.; Zhu, Y.; Feng, W.B.; Zhang, H.; Dai, A.T.; Chu, X.J.; et al. Structures of the human cholecystokinin receptors bound to agonists and antagonists. Nat. Prod. Rep. 2021, 17, 1230–1237. [Google Scholar] [CrossRef]
- Soriano-Ursua, M.A.; Trujillo-Ferrara, J.; Correa-Basurto, J.; Vilar, S. Recent Structural Advances of beta 1 and beta 2 Adrenoceptors Yield Keys for Ligand Recognition and Drug Design. J. Med. Chem. 2013, 56, 8207–8223. [Google Scholar] [CrossRef] [PubMed]
- Rapposelli, S.; Gaudio, E.; Bertozzi, F.; Gul, S. Editorial: Protein-Protein Interactions: Drug Discovery for the Future. Front. Chem. 2021, 9, 811190. [Google Scholar] [CrossRef]
- Sun, C.R.; Fu, J.D.; He, S.; Pan, Y.J. An integrative approach for the isolation, screening and analysis of antitumor agents by liquid chromatography combined with mass spectrometry. Anal. Chim. Acta 2009, 655, 86–91. [Google Scholar] [CrossRef] [PubMed]
- Guo, J.L.; Lin, H.; Wang, J.C.; Lin, Y.J.; Zhang, T.T.; Jiang, Z.J. Recent advances in bio-affinity chromatography for screening bioactive compounds from natural products. J. Pharm. Biomed. Anal. 2019, 165, 182–197. [Google Scholar] [CrossRef]
- Aristotelous, T.; Hopkins, A.L.; Navratilova, I. Surface plasmon resonance analysis of seven-transmembrane receptors. Methods Enzymol. 2015, 556, 499–525. [Google Scholar]
- Zhang, Z.H.; Chen, L.F.; Zhong, F.S.; Wang, D.Y.; Jiang, J.X.; Zhang, S.L.; Jiang, H.L.; Zheng, M.Y.; Li, X.T. Graph neural network approaches for drug-target interactions. Curr. Opin. Struct. Biol. 2022, 73, 102327. [Google Scholar] [CrossRef] [PubMed]
- Wani, T.A.; Alanazi, M.M.; Alsaif, N.A.; Bakheit, A.H.; Zargar, S.; Alsalami, O.M.; Khan, A.A. Interaction characterization of a tyrosine kinase inhibitor erlotinib with a model transport protein in the presence of quercetin: A drug–protein and drug–drug interaction investigation using multi-spectroscopic and computational approaches. Molecules 2022, 27, 1265. [Google Scholar] [CrossRef]
- Najafi, V.; Yoosefian, M.; Hassani, Z. Development of venetoclax performance using its new derivatives on BCL-2 protein inhibition. Cell Biochem. Funct. 2023, 41, 58–66. [Google Scholar] [CrossRef]
- Yoosefian, M.; Moghani, M.Z.; Juan, A. In silico evaluation of atazanavir as a potential HIV main protease inhibitor and its comparison with new designed analogs. Comput. Biol. Med. 2022, 145, 105523. [Google Scholar] [CrossRef]
- Li, Q.; Yin, G.W.; Wang, J.; Li, L.K.; Liang, Q.; Zhao, X.; Chen, Y.Y.; Zheng, X.H.; Zhao, X.F. An emerging paradigm to develop analytical methods based on immobilized transmembrane proteins and its applications in drug discovery. Trac-Trends Anal. Chem. 2022, 157, 116728. [Google Scholar] [CrossRef]
- Gao, J.; Yuan, X.Y.; Zheng, X.X.; Zhao, X.; Wang, T.T.; Liang, Q.; Xiao, C.N.; Wang, J.; Li, Q.; Zhao, X.F. Two-point immobilization of a conformation-specific beta(2)-adrenoceptor for recognizing the receptor agonists or antagonists inspired by binding-induced DNA assembly. Biomater. Sci. 2021, 9, 7934–7943. [Google Scholar] [CrossRef] [PubMed]
- Li, Q.; Ning, X.H.; An, Y.X.; Stanley, B.J.; Liang, Y.; Wang, J.; Zeng, K.Z.; Fei, F.H.; Liu, T.; Sun, H.M.; et al. Reliable analysis of the interaction between specific ligands and immobilized beta-2-adrenoceptor by adsorption energy distribution. Anal. Chem. 2018, 90, 7903–7911. [Google Scholar] [CrossRef] [PubMed]
- Brauner-Osborne, H.; Brann, M.R. Pharmacology of muscarinic acetylcholine receptor subtypes (m1–m5): High throughput assays in mammalian cells. Eur. J. Pharmacol. 1996, 295, 93–102. [Google Scholar] [CrossRef]
- Staus, D.P.; Hu, H.L.; Robertson, M.J.; Kleinhenz, A.L.W.; Wingler, L.M.; Capel, W.D.; Latorraca, N.R.; Lefkowitz, R.J.; Skiniotis, G. Structure of the M2 muscarinic receptor-beta-arrestin complex in a lipid nanodisc. Nature 2020, 579, 297–302. [Google Scholar] [CrossRef]
- Belmonte, K.E. Cholinergic pathways in the lungs and anticholinergic therapy for chronic obstructive pulmonary disease. Proc. Am. Math. Soc. 2005, 2, 297–304. [Google Scholar] [CrossRef]
- Tanaka, H.; Negoro, K.; Koike, T.; Tsukamoto, I.; Yokoyama, K.; Maeda, J.; Inagaki, Y.; Shimoshige, Y.; Ino, K.; Ishizu, K.; et al. Discovery and structure-activity relationships study of positive allosteric modulators of the M3 muscarinic acetylcholine receptor. Bioorg. Med. Chem. 2020, 28, 115531. [Google Scholar] [CrossRef]
- Fisher, T.J.; Vincent, S.G.; Gomeza, J.; Yamada, M.; Wess, J. Loss of vagally mediated bradycardia and bronchoconstriction in mice lacking M2 or M3 muscarinic acetylcholine receptors. FASEB J. 2004, 18, 711–713. [Google Scholar] [CrossRef]
- Liu, H.T.; Hofmann, J.; Fish, J.; Schaake, B.; Eitel, E.; Bartuschat, A.; Kaindl, J.; Rampp, H.; Banerjee, A.; Hubner, H.; et al. Structure-guided development of selective M3 muscarinic acetylcholine receptor antagonists. Proc. Natl. Acad. Sci. USA 2008, 115, 12046–12050. [Google Scholar] [CrossRef]
- Wess, J. Molecular biology of muscarinic acetylcholine receptors. Crit. Rev. Neurobiol. 1996, 10, 69–99. [Google Scholar] [CrossRef]
- Talbert, A.M.; Tranter, G.E.; Holmes, E.; Francis, P.L. Determination of drug–plasma protein binding kinetics and equilibria by chromatographic profiling: Exemplification of the method using L-Tryptophan and Albumin. Anal. Chem. 2002, 74, 446–452. [Google Scholar] [CrossRef] [PubMed]
- Wang, C.F.; Ge, J.W.; Zhang, J.W.; Guo, T.; Chi, L.D.; He, Z.G.; Xu, X.; York, P.; Sun, L.X.; Li, H.Y. Multianalyte determination of the kinetic rate constants of drug-cyclodextrin supermolecules by high performance affinity chromatography. J. Chromatogr. A 2014, 1359, 287–295. [Google Scholar] [CrossRef]
- Zhang, J.W.; Li, H.Y.; Sun, L.X.; Wang, C.F. Determination of the kinetic rate constant of cyclodextrin supramolecular systems by high-performance affinity chromatography. Methods Mol. Biol. 2015, 1286, 309–319. [Google Scholar]
- Sykes, D.A.; Dowling, M.R.; Charlton, S.J. Exploring the mechanism of agonist efficacy: A relationship between efficacy and agonist dissociation rate at the muscarinic M3 receptor. Mol. Pharmacol. 2009, 76, 543–551. [Google Scholar] [CrossRef] [PubMed]
- Hage, D.S.; Anguizola, J.A.; Jackson, A.J.; Matsuda, R.; Papastavros, E.; Pfaunmiller, E.; Tong, Z.H.; Vargas-Badilla, J.; Yoo, M.J.; Zheng, X.W. Chromatographic analysis of drug interactions in the serum proteoteome. Anal. Methods 2011, 3, 1449–1460. [Google Scholar] [CrossRef] [PubMed]
- He, Y.T.; Luo, J.G.; Kong, L.Y. Preparative separation of atropine and scopolamine from Daturae metelis Flos using pH-zone-refining counter-current chromatography with counter-rotation and dual-mode elution procedure. J. Sep. Sci. 2011, 34, 806–811. [Google Scholar] [CrossRef]
- Kuang, H.X.; Yang, B.Y.; Xia, Y.G.; Feng, W.S. Chemical constituents from the flower of Datura metel L. Arch. Pharmacal Res. 2008, 31, 1094–1097. [Google Scholar] [CrossRef]
- Zhu, J.J.; Deng, Y.J.; He, Y.S.; Wang, R.; Kuang, H.X.; Wang, Q.H. Research progress on chemical constituents, pharmacological effect and clinical applications of Daturae Flos. Chin. J. Exp. Tradit. Med. 2021, 27, 201–209. [Google Scholar]
- England, C.G.; Luo, H.M.; Cai, W.B. HaloTag technology: A versatile platform for biomedical applications. Bioconjugate Chem. 2015, 26, 975–986. [Google Scholar] [CrossRef]
- Zeng, K.Z.; Li, Q.; Wang, J.; Yin, G.W.; Zhang, Y.J.; Xiao, C.N.; Fan, T.P.; Zhao, X.F.; Zheng, X.H. One-step methodology for the direct covalent capture of GPCRs from complex matrices onto solid surfaces based on the bioorthogonal reaction between haloalkane dehalogenase and chloroalkanes. Chem. Sci. 2018, 9, 446–456. [Google Scholar] [CrossRef]
- Zhao, X.F.; Lu, H.Y.; Huang, J.J.; Zheng, J.B.; Zheng, X.H.; Zhang, Y.Y. Binding interaction between prazosin and immobilized receptor by frontal analysis. Chromatographia 2012, 75, 411–415. [Google Scholar] [CrossRef]
- Calleri, E.; Temporini, C.; Massolini, G. Frontal affinity chromatography in characterizing immobilized receptors. J. Pharm. Biomed. Anal. 2011, 54, 911–925. [Google Scholar] [CrossRef] [PubMed]
- Schiel, J.E.; Ohnmacht, C.M.; Hage, D.S. Measurement of drug-protein dissociation rates by high-performance affinity chromatography and peak profiling. Anal. Chem. 2009, 81, 4320–4333. [Google Scholar] [CrossRef]
- Chen, J.Z.; Schiel, J.E.; Hage, D.S. Non-competitive peak analysis of drug-protein dissociation by high-performance affinity chromatography. J. Sep. Sci. 2009, 32, 1632–1641. [Google Scholar] [CrossRef] [PubMed]
- Li, H.Y.; Ge, J.W.; Guo, T.; Yang, S.; He, Z.G.; York, P.; Sun, L.X.; Xu, X.; Zhang, J.W. Determination of the kinetic rate constant of cyclodextrin supramolecular systems by high performance affinity chromatography. J. Chromatogr. A 2013, 1305, 139–148. [Google Scholar] [CrossRef] [PubMed]

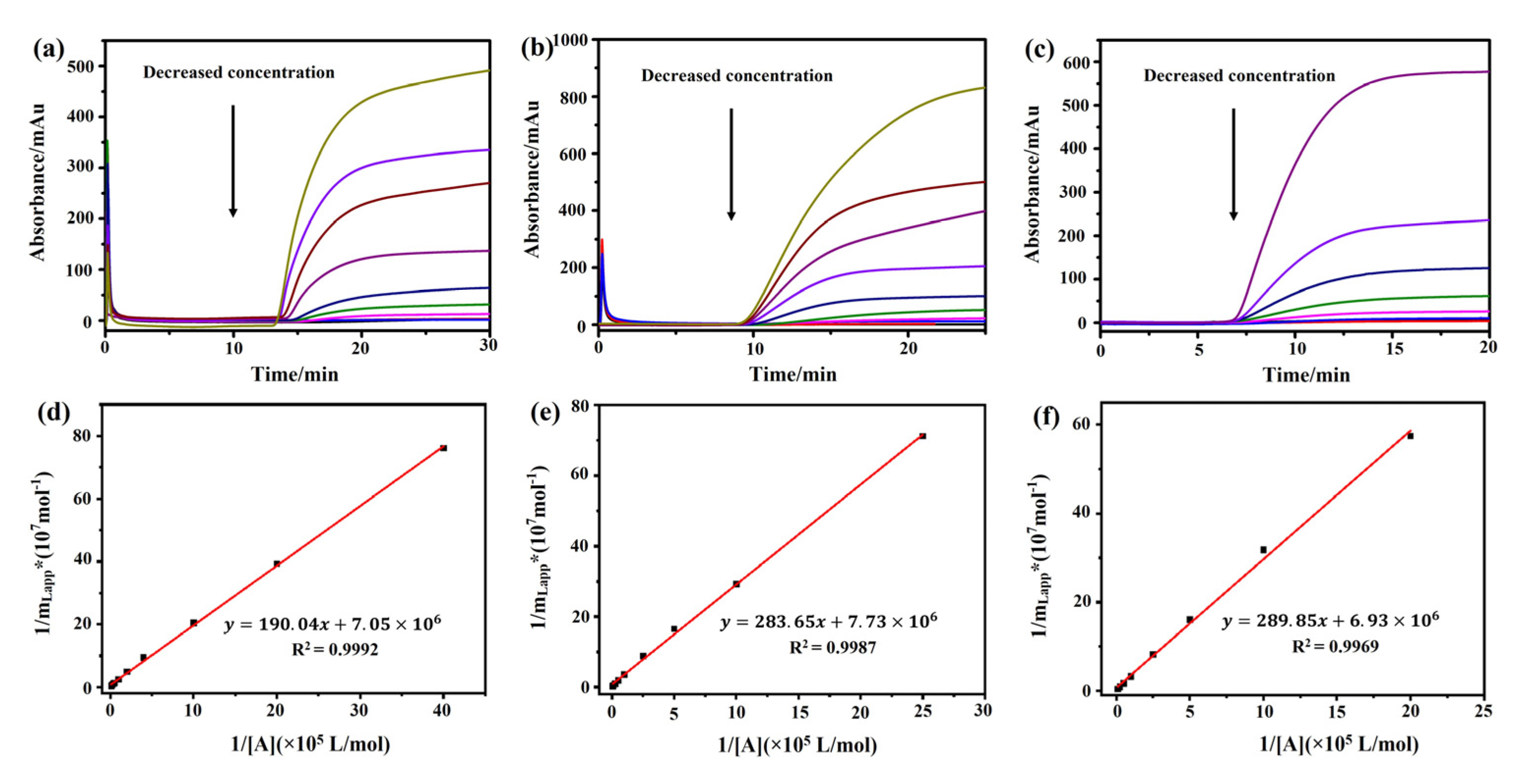

| Ligands | KA by Frontal Analysis (M−1) | mL by Frontal Analysis (M) | kd by the Peak Profiling Method (min−1) | kd by the Radioligand Binding Assay (min−1) |
|---|---|---|---|---|
| Atropine sulfate | (3.71 ± 0.03) × 104 | (1.42 ± 0.45) × 10−7 | 14.28 ± 0.17 | 1.12 ± 0.13 |
| Pilocarpine | (2.73 ± 0.04) × 104 | (1.29 ± 0.30) × 10−7 | 27.47 ± 0.65 | 15.3 ± 2.30 |
| (−)-Scopolamine hydrochloride | (2.39 ± 0.03) × 104 | (1.44 ± 0.19) × 10−7 | 10.70 ± 0.35 | 0.31 ± 0.03 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Fan, H.; Huang, X.; Zhang, Z.; Wang, T.; Wang, L.; Zhang, Y. Immobilization of M3 Muscarinic Receptor to Rapidly Analyze Drug—Protein Interactions and Bioactive Components in a Natural Plant. Int. J. Mol. Sci. 2023, 24, 7171. https://doi.org/10.3390/ijms24087171
Fan H, Huang X, Zhang Z, Wang T, Wang L, Zhang Y. Immobilization of M3 Muscarinic Receptor to Rapidly Analyze Drug—Protein Interactions and Bioactive Components in a Natural Plant. International Journal of Molecular Sciences. 2023; 24(8):7171. https://doi.org/10.3390/ijms24087171
Chicago/Turabian StyleFan, Hushuai, Xiaomin Huang, Ziru Zhang, Ting Wang, Ludan Wang, and Yajun Zhang. 2023. "Immobilization of M3 Muscarinic Receptor to Rapidly Analyze Drug—Protein Interactions and Bioactive Components in a Natural Plant" International Journal of Molecular Sciences 24, no. 8: 7171. https://doi.org/10.3390/ijms24087171
APA StyleFan, H., Huang, X., Zhang, Z., Wang, T., Wang, L., & Zhang, Y. (2023). Immobilization of M3 Muscarinic Receptor to Rapidly Analyze Drug—Protein Interactions and Bioactive Components in a Natural Plant. International Journal of Molecular Sciences, 24(8), 7171. https://doi.org/10.3390/ijms24087171