The Role of Low-Level Laser Therapy in Bone Healing: Systematic Review
Abstract
1. Introduction
2. Materials and Methods
- Identifying the research question
- Identifying relevant studies
- Electronic database search
- ((“low level light therapy” [MeSH Terms] OR (“low level” [All Fields] AND “light” [All Fields] AND “therapy” [All Fields]) OR “low level light therapy” [All Fields] OR (“low” [All Fields] AND “level” [All Fields] AND “laser” [All Fields] AND “therapy” [All Fields]) OR “low level laser therapy” [All Fields]) AND (“bone and bones” [MeSH Terms] OR (“bone” [All Fields] AND “bones” [All Fields]) OR “bone and bones” [All Fields] OR “bone” [All Fields] OR (“mesenchymal stem cells” [MeSH Terms] OR (“mesenchymal” [All Fields] AND “stem” [All Fields] AND “cells” [All Fields]) OR “mesenchymal stem cells” [All Fields]))) AND ((fft [Filter]) AND (English [Filter]) AND (2002: 2022 [pdat])) [953];
- (“low level laser therapy”/exp OR “lllt (low level laser therapy)” OR “low-intensity (therapeutic) laser therapy (lilt)” OR “laser biostimulation” OR “laser therapy, low-level” OR “low energy laser therapy” OR “low energy laser treatment” OR “low intensity laser therapy” OR “low intensity laser treatment” OR “low level laser therapy” OR “low level laser treatment” OR “low level light therapy” OR “low power laser therapy” OR “low power laser treatment” OR “low-level laser therapy” OR “low-level laser therapy (lllt)” OR “low-level light therapy” OR “photo biomodulation therapy” OR “photo-bio-modulation therapy” OR “photo-biomodulation (pbm) therapy” OR “photo-biomodulation therapy (pbmt)” OR “photobiomodulation (pbm) therapy” OR “photobiomodulation therapy” OR “photobiomodulation therapy (pbm)” OR “photobiomodulation therapy (pbmt)” OR “soft laser therapy” OR “therapeutic laser therapy”) AND (“bone”/exp OR “mesenchymal stem cells”) AND [2002–2022]/py AND [english]/lim [1069].
- Other sources
- Study inclusion
- Data extraction
- Study selection
3. Results
3.1. Low-Level Laser Therapy (LLLT)
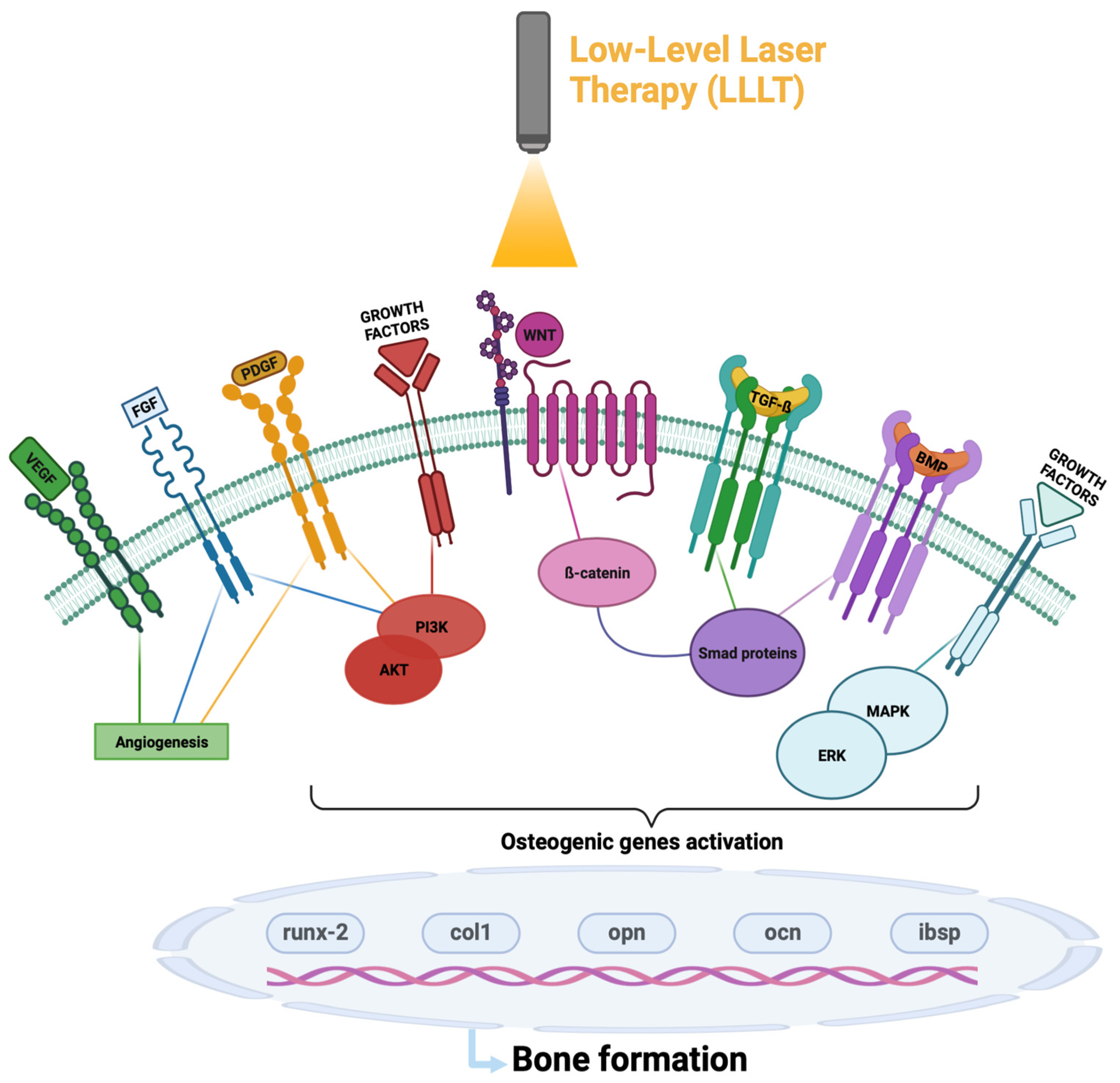
3.2. Molecular Pathways LLT-Activated on Bone Healing
3.3. In Vitro Studies
3.3.1. Inflammatory Phase
- WNT/β-catenin is a signaling pathway that plays a crucial role in the progression of bone regeneration; in particular, its activation stabilizes the cytoplasmic β-catenin, allowing its nuclear translocation and degrades the protein complex formed by axin, adenomatosis polyposis coli (APC), protein phosphatase 2A (PP2A), glycogen synthase kinase 3 (GSK3) and casein kinase 1 α (CK1α) responsible for the destruction of ß-catenin [20]. Once activated and translocated to the nucleus, β-catenin interacts with several DNA-bound proteins, including LEF and TFC, to induce the expression of osteogenic markers and the differentiation of pre-osteoblasts [35]. The inflammatory process negatively affects this signaling pathway, since the presence of pro-inflammatory cytokines, including TNF, stimulates the expression of Dickkopf-related protein 1 (DKK-1), an antagonist of the WNT signaling pathway that prevents its activation [36]. Laser treatment, particularity with a wavelength of 830 nm, increases the translocation of β-catenin from the cytoplasm to the nucleus, increasing the activation of the pathway and accelerating the bone regeneration process [25].
- NF-kB is a transcription factor present in the cytoplasm, which, following the phosphorylation of its inhibitor complex IkBα, can translocate into the nucleus and through gene transcription, promoting the inflammation process. Treatment, with a red laser with a wavelength of 660 nm of adipose-derived stem cells (ADSCs), showed a reduction in phosphorylated IkBα and NF-kB concentration in the nucleus, highlighting the ability of LLLT to negatively regulate the process of inflammation via NF-kB inhibition [27].
3.3.2. Angio-Mesenchymal Phase
- VEGF is one of the main regulators of angiogenesis, able to promote the migration and proliferation of endothelial cells. A subclass of endothelial vessels, termed H-type vessels due to high expression of CD31 and endomucin, positively influence osteogenesis by resisting surrounding osteoprogenitors that express high levels of osterix, a promoter of bone formation. It has been reported through the literature that irradiation of human bone marrow mesenteric stem cells with an 808 nm laser increased both VEGF expression and H-type vessel formation, leading to an overall enhancement of the angiogenic process [16,29].
- FGF contributes to the regulation of several important cellular processes; by activating the PI3K-AKT signaling pathway, it inhibits cell apoptosis by promoting its proliferation, while by stimulating the MAPK/ERK pathway it induces osteoblast differentiation. It is also involved in the process of angiogenesis and wound healing, and studies in the literature have shown a correlation between irradiation with a 660 nm laser and an increase in FGF expression [28].
- PDGF is a factor that plays a role in the regulation of the mitogenic, angiogenic and proliferative activity of MSC cells. Thanks to its dimerization and the activation of several signaling proteins including phospholipase C, the kinases Src, PI3-kinase and the phosphatase SHP2, it also recalls different growth factors. A significant increase in this factor was observed when treating MSC cells with a laser with a wavelength of 636 nm [26].
- ROS/HIF-1α pathway activation promotes cell differentiation and endothelial angiogenesis, two key mechanisms of bone repair. Recent studies have shown that the GaAlAs laser treatment with 4.5 J/cm2 of BMSC cells, in simple culture and more significantly if in co-culture with HUVECs, led to activation of the ROS/HIF-1α pathway [16].
3.3.3. Bone Formation Phase
- Runt-related transcription factor (runx-2) has a relevant role in the recruitment of MSCs and in their differentiation into osteoblasts; it also induces the expression of the bone matrix genes collagen type 1 (col1a1), osteopontin (opn), osteocalcin (ibsp) and osteocalcin (ocn) [16,35]. Studies in the literature have shown that laser therapy directs MSCs towards osteogenic differentiation versus adipogenic differentiation [25]. Moreover, an increase in BMSC proliferation and differentiation in osteoblasts was observed after irradiation with a GaAlAs diode laser with a wavelength of 810 nm and an energy density between 2 and 4 J/cm2 [30].
- BMP/TGF-β has a key role in the osteogenic process; in particular, TGF- β plays a crucial role in osteoblast proliferation, while BMP has a remarkable ability in bone formation as well as being fundamental in the maturation process of osteogenesis [23]. In the literature, different studies reported a correlation between LLLT and the increase in TGF- β and BMP expression. In particular, it was observed that the irradiation of osteoblast-like cells (human osteosarcoma cell line MG-63) with LLLT led to an increase in BMP [31] and promoted osteogenic differentiation through the activation of ROS, which in turn activated TGF-β 1 [16]. This correlation has also been found in hypoxic conditions by treating osteoblasts with a GaAlAs laser [32].
- The PI3K/Akt/mTOR signaling pathway, thanks to the activation cascade of its protein effectors, is one of the main pathways involved in cell survival, regulating cell cycle, motility, apoptosis, metabolism and cell differentiation, as well as the mechanisms of transcription and translation. It would also seem to have a correlation with bone regeneration, and in fact there are some studies in the literature showing a possible involvement in bone formation and remodeling [23]. Through these studies, it was observed that exposure to a laser treatment with a wavelength of 650 nm and an energy density between 1 and 4 J/cm2 of the HUVECs cell line increased the phosphorylation of PI3K, AKT and mTOR, allowing greater activation and functionality of the signaling pathway [33]. An interesting study confirmed the role of LLLT on increasing the Akt expression levels and its activated phosphorylated form on osteoblasts irradiated with 635 nm as compared to untreated cells [34].
- The MAPK kinase pathway plays an important role in signal transduction by controlling several cellular processes, including cell proliferation and differentiation as well as responses to cellular stress. Furthermore, it also regulates bone formation, inducing osteogenic differentiation and controlling the vitality and functions of osteoblasts. In particular, the use of a laser with a wavelength of 910 nm on the MC3T3-E1 cell line allowed an increase in ERK phosphorylation, increasing the activity of the pathway [11].
3.4. In Vivo Studies
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| ADSCs | Adipose-derived stem cells |
| ATP | Adenosine triphosphate |
| BMP | Bone morphogenetic proteins |
| BMSC | Bone marrow Stem Cells |
| CCO | Cytochrome c oxidase |
| col1a1 | Collagen type I gene |
| DKK-1 | Dickkopf-Related Protein 1 |
| FGF | Fibroblast Growth Factors |
| HUVEC | Human Umbilical Vein Endothelial Cells |
| ibsp | Bone sialoprotein 2 gene |
| IkBα | Inhibitors of NF-kB |
| LLLT | Low-level laser therapy |
| MSC | Mesenchymal stem cells |
| NF-kB | Nuclear Factor kappa B |
| ocn | Osteocalcin gene |
| opn | Osteopontin gene |
| PDGF | Platelet-Derived Growth Factor |
| PMNs | Polymorphonuclear neutrophils |
| Runx-2 | Runt-related transcription factor gene |
| TGF-ß | Tumor growth factor- ß |
| TNFα | Tumor necrosis factor-α |
| VEGF | Vascular Endothelial Growth Factor |
| AKT | AKT Serine/Threonine Kinase 1 |
| PI3K | Phosphatidylinositol-4,5-Bisphosphate 3-Kinase Catalytic Subunit Delta |
References
- Sant’Anna, E.F.; de Souza Araújo, M.T.; Nojima, L.I.; da Cunha, A.C.; da Silveira, B.L.; Marquezan, M. High-Intensity Laser Application in Orthodontics. Dental Press J. Orthod. 2017, 22, 99–109. [Google Scholar] [CrossRef]
- Hanna, R.; Pawelczyk-Madalińska, M.; Sălăgean, T.; Nap, M.E.; Bordea, I.R.; Benedicenti, S. A Novel Concept of Combined High-Level-Laser Treatment and Transcutaneous Photobiomodulation Therapy Utilisation in Orthodontic Periodontal Interface Management. Sensors 2022, 22, 2263. [Google Scholar] [CrossRef] [PubMed]
- Prados-Frutos, J.C.; Rodríguez-Molinero, J.; Prados-Privado, M.; Torres, J.H.; Rojo, R. Lack of Clinical Evidence on Low-Level Laser Therapy (LLLT) on Dental Titanium Implant: A Systematic Review. Lasers Med. Sci. 2016, 31, 383–392. [Google Scholar] [CrossRef]
- Moshkovska, T. It Is Time to Test Low Level Laser Therapy in Great Britain. Postgrad. Med. J. 2005, 81, 436–441. [Google Scholar] [CrossRef]
- Avci, P.; Gupta, A.; Sadasivam, M.; Pam, Z.; Pam, N.; Hamblin, M.R. Low-Level Laser (Light) Therapy (LLLT) in Skin: Stimulating, Healing, Restoring. Semin. Cutan. Med. Surg. 2013, 32, 41–52. [Google Scholar] [PubMed]
- Nampo, F.K.; Cavalheri, V.; Ramos, S.D.P.; Camargo, E.A. Effect of Low-Level Phototherapy on Delayed Onset Muscle Soreness: A Systematic Review and Meta-Analysis. Lasers Med. Sci. 2016, 31, 165–177. [Google Scholar] [CrossRef] [PubMed]
- Cardoso, M.V.; do Vale Placa, R.; Sant’Ana, A.C.P.; Greghi, S.L.A.; Zangrando, M.S.R.; de Rezende, M.L.R.; Oliveira, R.C.; Damante, C.A. Laser and LED Photobiomodulation Effects in Osteogenic or Regular Medium on Rat Calvaria Osteoblasts Obtained by Newly Forming Bone Technique. Lasers Med. Sci. 2021, 36, 541–553. [Google Scholar] [CrossRef] [PubMed]
- Hou, J.; Zhang, H.; Yuan, X.; Li, J.; Wei, Y.; Hu, S. In Vitro Effects of Low-Level Laser Irradiation for Bone Marrow Mesenchymal Stem Cells: Proliferation, Growth Factors Secretion and Myogenic Differentiation. Lasers Surg. Med. 2008, 40, 726–733. [Google Scholar] [CrossRef] [PubMed]
- Gavish, L.; Perez, L.; Gertz, S.D. Low-Level Laser Irradiation Modulates Matrix Metalloproteinase Activity and Gene Expression in Porcine Aortic Smooth Muscle Cells. Lasers Surg. Med. 2006, 38, 779–786. [Google Scholar] [CrossRef]
- Dimitriou, R.; Jones, E.; McGonagle, D.; Giannoudis, P.V. Bone Regeneration: Current Concepts and Future Directions. BMC Med 2011, 9, 66. [Google Scholar] [CrossRef]
- Kunimatsu, R.; Gunji, H.; Tsuka, Y.; Yoshimi, Y.; Awada, T.; Sumi, K.; Nakajima, K.; Kimura, A.; Hiraki, T.; Abe, T.; et al. Effects of High-Frequency near-Infrared Diode Laser Irradiation on the Proliferation and Migration of Mouse Calvarial Osteoblasts. Lasers Med. Sci. 2018, 33, 959–966. [Google Scholar] [CrossRef]
- Page, M.J.; McKenzie, J.E.; Bossuyt, P.M.; Boutron, I.; Hoffmann, T.C.; Mulrow, C.D.; Shamseer, L.; Tetzlaff, J.M.; Akl, E.A.; Brennan, S.E.; et al. The PRISMA 2020 Statement: An Updated Guideline for Reporting Systematic Reviews. BMJ 2021, 372, n71. [Google Scholar] [CrossRef]
- de Oliveira, G.J.P.L.; Aroni, M.A.T.; Pinotti, F.E.; Marcantonio, E.; Marcantonio, R.A.C. Low-Level Laser Therapy (LLLT) in Sites Grafted with Osteoconductive Bone Substitutes Improves Osseointegration. Lasers Med. Sci. 2020, 35, 1519–1529. [Google Scholar] [CrossRef]
- Karu, T.; Pyatibrat, L.; Kalendo, G. Irradiation with He–Ne Laser Increases ATP Level in Cells Cultivated in Vitro. J. Photochem. Photobiol. B Biol. 1995, 27, 219–223. [Google Scholar] [CrossRef]
- Harris, D.M. Editorial Comment Biomolecular Mechanisms of Laser Biostimulation. J. Clin. Laser Med. Surg. 1991, 9, 277–280. [Google Scholar] [CrossRef]
- Bai, J.; Li, L.; Kou, N.; Bai, Y.; Zhang, Y.; Lu, Y.; Gao, L.; Wang, F. Low Level Laser Therapy Promotes Bone Regeneration by Coupling Angiogenesis and Osteogenesis. Stem Cell Res. Ther. 2021, 12, 432. [Google Scholar] [CrossRef]
- Karu, T.I.; Kolyakov, S.F. Exact Action Spectra for Cellular Responses Relevant to Phototherapy. Photomed. Laser Surg. 2005, 23, 355–361. [Google Scholar] [CrossRef]
- Wang, X.; Wang, Y.; Gou, W.; Lu, Q.; Peng, J.; Lu, S. Role of Mesenchymal Stem Cells in Bone Regeneration and Fracture Repair: A Review. Int. Orthop. (SICOT) 2013, 37, 2491–2498. [Google Scholar] [CrossRef]
- Schett, G. Effects of Inflammatory and Anti-Inflammatory Cytokines on the Bone: Cytokine Effects on Bone. Eur. J. Clin. Investig. 2011, 41, 1361–1366. [Google Scholar] [CrossRef]
- Caliogna, L.; Medetti, M.; Bina, V.; Brancato, A.M.; Castelli, A.; Jannelli, E.; Ivone, A.; Gastaldi, G.; Annunziata, S.; Mosconi, M.; et al. Pulsed Electromagnetic Fields in Bone Healing: Molecular Pathways and Clinical Applications. Int. J. Mol. Sci. 2021, 22, 7403. [Google Scholar] [CrossRef]
- Yun, Y.-R.; Won, J.E.; Jeon, E.; Lee, S.; Kang, W.; Jo, H.; Jang, J.-H.; Shin, U.S.; Kim, H.-W. Fibroblast Growth Factors: Biology, Function, and Application for Tissue Regeneration. J. Tissue Eng. 2010, 1, 218142. [Google Scholar] [CrossRef] [PubMed]
- Fayaz, H.C.; Giannoudis, P.V.; Vrahas, M.S.; Smith, R.M.; Moran, C.; Pape, H.C.; Krettek, C.; Jupiter, J.B. The Role of Stem Cells in Fracture Healing and Nonunion. Int. Orthop. (SICOT) 2011, 35, 1587–1597. [Google Scholar] [CrossRef]
- Majidinia, M.; Sadeghpour, A.; Yousefi, B. The Roles of Signaling Pathways in Bone Repair and Regeneration. J. Cell. Physiol. 2018, 233, 2937–2948. [Google Scholar] [CrossRef]
- Mountziaris, P.M.; Mikos, A.G. Modulation of the Inflammatory Response for Enhanced Bone Tissue Regeneration. Tissue Eng. Part B Rev. 2008, 14, 179–186. [Google Scholar] [CrossRef] [PubMed]
- Zhang, R.-F.; Wang, Q.; Zhang, A.-A.; Xu, J.-G.; Zhai, L.-D.; Yang, X.-M.; Liu, X.-T. Low-Level Laser Irradiation Promotes the Differentiation of Bone Marrow Stromal Cells into Osteoblasts through the APN/Wnt/β-Catenin Pathway. Eur. Rev. Med. Pharmacol. Sci. 2018, 22, 2860–2868. [Google Scholar] [CrossRef]
- Yin, K.; Zhu, R.; Wang, S.; Zhao, R.C. Low-Level Laser Effect on Proliferation, Migration, and Antiapoptosis of Mesenchymal Stem Cells. Stem Cells Dev. 2017, 26, 762–775. [Google Scholar] [CrossRef] [PubMed]
- Wu, J.-Y.; Chen, C.-H.; Wang, C.-Z.; Ho, M.-L.; Yeh, M.-L.; Wang, Y.-H. Low-Power Laser Irradiation Suppresses Inflammatory Response of Human Adipose-Derived Stem Cells by Modulating Intracellular Cyclic AMP Level and NF-ΚB Activity. PLoS ONE 2013, 8, e54067. [Google Scholar] [CrossRef]
- Park, I.-S.; Chung, P.-S.; Ahn, J.C. Enhancement of Ischemic Wound Healing by Spheroid Grafting of Human Adipose-Derived Stem Cells Treated with Low-Level Light Irradiation. PLoS ONE 2015, 10, e0122776. [Google Scholar] [CrossRef]
- Rodríguez-Merchán, E.C. A Review of Recent Developments in the Molecular Mechanisms of Bone Healing. Int. J. Mol. Sci. 2021, 22, 767. [Google Scholar] [CrossRef]
- Soleimani, M.; Abbasnia, E.; Fathi, M.; Sahraei, H.; Fathi, Y.; Kaka, G. The Effects of Low-Level Laser Irradiation on Differentiation and Proliferation of Human Bone Marrow Mesenchymal Stem Cells into Neurons and Osteoblasts—An in Vitro Study. Lasers Med. Sci. 2012, 27, 423–430. [Google Scholar] [CrossRef]
- Manzano-Moreno, F.J.; Medina-Huertas, R.; Ramos-Torrecillas, J.; García-Martínez, O.; Ruiz, C. The Effect of Low-Level Diode Laser Therapy on Early Differentiation of Osteoblast via BMP-2/TGF-Β1 and Its Receptors. J. Cranio-Maxillofac. Surg. 2015, 43, 1926–1932. [Google Scholar] [CrossRef] [PubMed]
- Pyo, S.-J.; Song, W.-W.; Kim, I.-R.; Park, B.-S.; Kim, C.-H.; Shin, S.-H.; Chung, I.-K.; Kim, Y.-D. Low-Level Laser Therapy Induces the Expressions of BMP-2, Osteocalcin, and TGF-Β1 in Hypoxic-Cultured Human Osteoblasts. Lasers Med. Sci. 2013, 28, 543–550. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Xu, Q.; Shi, M.; Gan, P.; Huang, Q.; Wang, A.; Tan, G.; Fang, Y.; Liao, H. Low-Level Laser Therapy Induces Human Umbilical Vascular Endothelial Cell Proliferation, Migration and Tube Formation through Activating the PI3K/Akt Signaling Pathway. Microvasc. Res. 2020, 129, 103959. [Google Scholar] [CrossRef] [PubMed]
- Tani, A.; Chellini, F.; Giannelli, M.; Nosi, D.; Zecchi-Orlandini, S.; Sassoli, C. Red (635 Nm), Near-Infrared (808 Nm) and Violet-Blue (405 Nm) Photobiomodulation Potentiality on Human Osteoblasts and Mesenchymal Stromal Cells: A Morphological and Molecular In Vitro Study. Int. J. Mol. Sci. 2018, 19, 1946. [Google Scholar] [CrossRef] [PubMed]
- Hanna, R.; Agas, D.; Benedicenti, S.; Ferrando, S.; Laus, F.; Cuteri, V.; Lacava, G.; Sabbieti, M.G.; Amaroli, A. A Comparative Study Between the Effectiveness of 980 Nm Photobiomodulation Delivered by Hand-Piece With Gaussian vs. Flat-Top Profiles on Osteoblasts Maturation. Front. Endocrinol. 2019, 10, 92. [Google Scholar] [CrossRef] [PubMed]
- Diarra, D.; Stolina, M.; Polzer, K.; Zwerina, J.; Ominsky, M.S.; Dwyer, D.; Korb, A.; Smolen, J.; Hoffmann, M.; Scheinecker, C.; et al. Dickkopf-1 Is a Master Regulator of Joint Remodeling. Nat. Med. 2007, 13, 156–163. [Google Scholar] [CrossRef]
- Tim, C.R.; Bossini, P.S.; Kido, H.W.; Malavazi, I.; von Zeska Kress, M.R.; Carazzolle, M.F.; Parizotto, N.A.; Rennó, A.C. Effects of Low Level Laser Therapy on Inflammatory and Angiogenic Gene Expression during the Process of Bone Healing: A Microarray Analysis. J. Photochem. Photobiol. B Biol. 2016, 154, 8–15. [Google Scholar] [CrossRef]
- Usumez, A.; Cengiz, B.; Oztuzcu, S.; Demir, T.; Aras, M.H.; Gutknecht, N. Effects of Laser Irradiation at Different Wavelengths (660, 810, 980, and 1,064 Nm) on Mucositis in an Animal Model of Wound Healing. Lasers Med. Sci. 2014, 29, 1807–1813. [Google Scholar] [CrossRef]
- AboElsaad, N.S.; Soory, M.; Gadalla, L.M.A.; Ragab, L.I.; Dunne, S.; Zalata, K.R.; Louca, C. Effect of Soft Laser and Bioactive Glass on Bone Regeneration in the Treatment of Infra-Bony Defects (a Clinical Study). Lasers Med. Sci. 2009, 24, 387–395. [Google Scholar] [CrossRef]
- da Fonseca, E.V.; Bussadori, S.K.; da Silva Martinho, L.F.C.; de Sousa Melo, M.C.; de Andrade, F.L.; Gonçalves, M.L.L.; Mesquita-Ferrari, R.A.; Horliana, A.C.R.T.; Fernandes, K.P.S. Evaluation of Photobiomodulation Effects on Pain, Edema, Paresthesia, and Bone Regeneration after Surgically Assisted Rapid Maxillary Expansion: Study Protocol for a Randomized, Controlled, and Double Blind Clinical Trial. Medicine 2019, 98, e17756. [Google Scholar] [CrossRef]
- Marques, L.; Holgado, L.A.; Francischone, L.A.; Ximenez, J.P.B.; Okamoto, R.; Kinoshita, A. New LLLT Protocol to Speed up the Bone Healing Process—Histometric and Immunohistochemical Analysis in Rat Calvarial Bone Defect. Lasers Med. Sci. 2015, 30, 1225–1230. [Google Scholar] [CrossRef]
- Fekrazad, R.; Sadeghi Ghuchani, M.; Eslaminejad, M.B.; Taghiyar, L.; Kalhori, K.A.M.; Pedram, M.S.; Shayan, A.M.; Aghdami, N.; Abrahamse, H. The Effects of Combined Low Level Laser Therapy and Mesenchymal Stem Cells on Bone Regeneration in Rabbit Calvarial Defects. J. Photochem. Photobiol. B Biol. 2015, 151, 180–185. [Google Scholar] [CrossRef]
- Sakata, S.; Kunimatsu, R.; Tsuka, Y.; Nakatani, A.; Gunji, H.; Yanoshita, M.; Kado, I.; Ito, S.; Putranti, N.A.R.; Prasetya, R.C.; et al. High-Frequency near-Infrared Diode Laser Irradiation Suppresses IL-1β-Induced Inflammatory Cytokine Expression and NF-ΚB Signaling Pathways in Human Primary Chondrocytes. Lasers Med. Sci. 2022, 37, 1193–1201. [Google Scholar] [CrossRef]
- Oliveira, F.A.; Matos, A.A.; Santesso, M.R.; Tokuhara, C.K.; Leite, A.L.; Bagnato, V.S.; Machado, M.A.A.M.; Peres-Buzalaf, C.; Oliveira, R.C. Low Intensity Lasers Differently Induce Primary Human Osteoblast Proliferation and Differentiation. J. Photochem. Photobiol. B Biol. 2016, 163, 14–21. [Google Scholar] [CrossRef]

| Parameter | LED Range |
|---|---|
| Wavelength (nm) | 500–1000 |
| Power density (J/cm2) | 0.5–30 |
| Energy (mW) | 5–100 |
| Irradiation time (s) | 3–1440 |
| Repetition rate (d) | 1–60 |
| Distance from sample (cm) | 0–14 |
| Laser Wavelength | Laser Power Density | Target | Effects | Bone Phase Target | Ref. |
|---|---|---|---|---|---|
| 830 nm | 7.64 J/cm2 | BMSCs | WNT-ß-catenin activation | Inflammatory phase | [25] |
| 636 nm | 5 J/cm2 | MSCs | PDGF increase | Angio-mesenchymal phase | [26] |
| 660 nm | 4–8 J/cm2 | ADSCs | NF-kB inhibition | Inflammatory phase | [27] |
| 660 nm | 6 J/cm2 | ADSCs | FGF increase | Angio-mesenchymal phase | [28] |
| 808 nm | 4.5 J/cm2 | BMSC | VEGF increase | Angio-mesenchymal phase | [16,29] |
| 808 nm | 4.5 J/cm2 | Co-culture of BMSC and HUVECs | ROS/HIF-1α activation | Angio-mesenchymal phase | [16] |
| 810 nm | 2–4 J/cm2 | BMSC | ALP activity increase | Bone formation phase | [30] |
| 940 nm | NA | MG-63 cell line | BMP increase | Bone formation phase | [31] |
| 808 nm | 1.2, 2.4, and 3.6 J/cm2 | Human fetal osteoblasts | BMP crease | Bone formation phase | [32] |
| 650 nm | 1–4 J/cm2 | HUVEC | PI3K/Akt/mTOR activation | Bone formation phase | [33] |
| 635 nm | 0,4 J/cm2 | Human osteoblast | PI3K/Akt/mTOR activation | Bone formation phase | [34] |
| 910 nm | 2.85 J/cm2 | MC3T3-E1 | MAPK kinase pathway activation | Bone formation phase | [11] |
| Laser Wavelength | Laser Power Density | Target | Effects | Bone Phase Target | Ref. |
|---|---|---|---|---|---|
| 830 nm | NA | rats | Decrease of pro-inflammatory interleukins (IL-1, IL6, IL8, IL18) | Inflammation phase | [37] |
| 830 nm | NA | rats | VEGF increase | Angio-mesenchymal phase | [37] |
| 1064 nm | 8 J/cm2 | rats | PDGF and FGF increase | Angio-mesenchymal phase | [38] |
| 808 nm | 354 J/cm2 | rats | BMP2, OCN and ALP increase | Bone formation | [13] |
| 830 nm | 16 J/cm2 | Human periodontal infra-bony defects | Radiological evidence | Bone formation | [39] |
| 660 nm | 2.7 J/cm2 | Human surgical disjunction of the maxilla | Accelerating of the repair process. | Bone formation | [40] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Berni, M.; Brancato, A.M.; Torriani, C.; Bina, V.; Annunziata, S.; Cornella, E.; Trucchi, M.; Jannelli, E.; Mosconi, M.; Gastaldi, G.; et al. The Role of Low-Level Laser Therapy in Bone Healing: Systematic Review. Int. J. Mol. Sci. 2023, 24, 7094. https://doi.org/10.3390/ijms24087094
Berni M, Brancato AM, Torriani C, Bina V, Annunziata S, Cornella E, Trucchi M, Jannelli E, Mosconi M, Gastaldi G, et al. The Role of Low-Level Laser Therapy in Bone Healing: Systematic Review. International Journal of Molecular Sciences. 2023; 24(8):7094. https://doi.org/10.3390/ijms24087094
Chicago/Turabian StyleBerni, Micaela, Alice Maria Brancato, Camilla Torriani, Valentina Bina, Salvatore Annunziata, Elena Cornella, Michelangelo Trucchi, Eugenio Jannelli, Mario Mosconi, Giulia Gastaldi, and et al. 2023. "The Role of Low-Level Laser Therapy in Bone Healing: Systematic Review" International Journal of Molecular Sciences 24, no. 8: 7094. https://doi.org/10.3390/ijms24087094
APA StyleBerni, M., Brancato, A. M., Torriani, C., Bina, V., Annunziata, S., Cornella, E., Trucchi, M., Jannelli, E., Mosconi, M., Gastaldi, G., Caliogna, L., Grassi, F. A., & Pasta, G. (2023). The Role of Low-Level Laser Therapy in Bone Healing: Systematic Review. International Journal of Molecular Sciences, 24(8), 7094. https://doi.org/10.3390/ijms24087094









