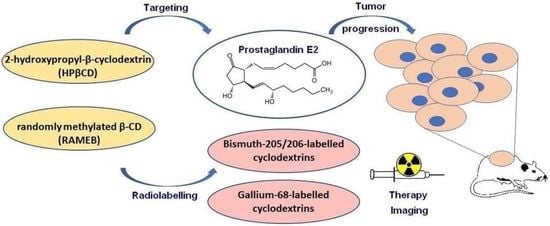
Overview of Prostaglandin E2 (PGE2)-Targeting Radiolabelled Imaging Probes from Preclinical Perspective: Lessons Learned and Road Ahead
Abstract
1. Introduction
1.1. Cyclooxigenase-2 (COX2)/Prostaglandin E2 (PGE2)/PGE2-Receptor (EP) Axis
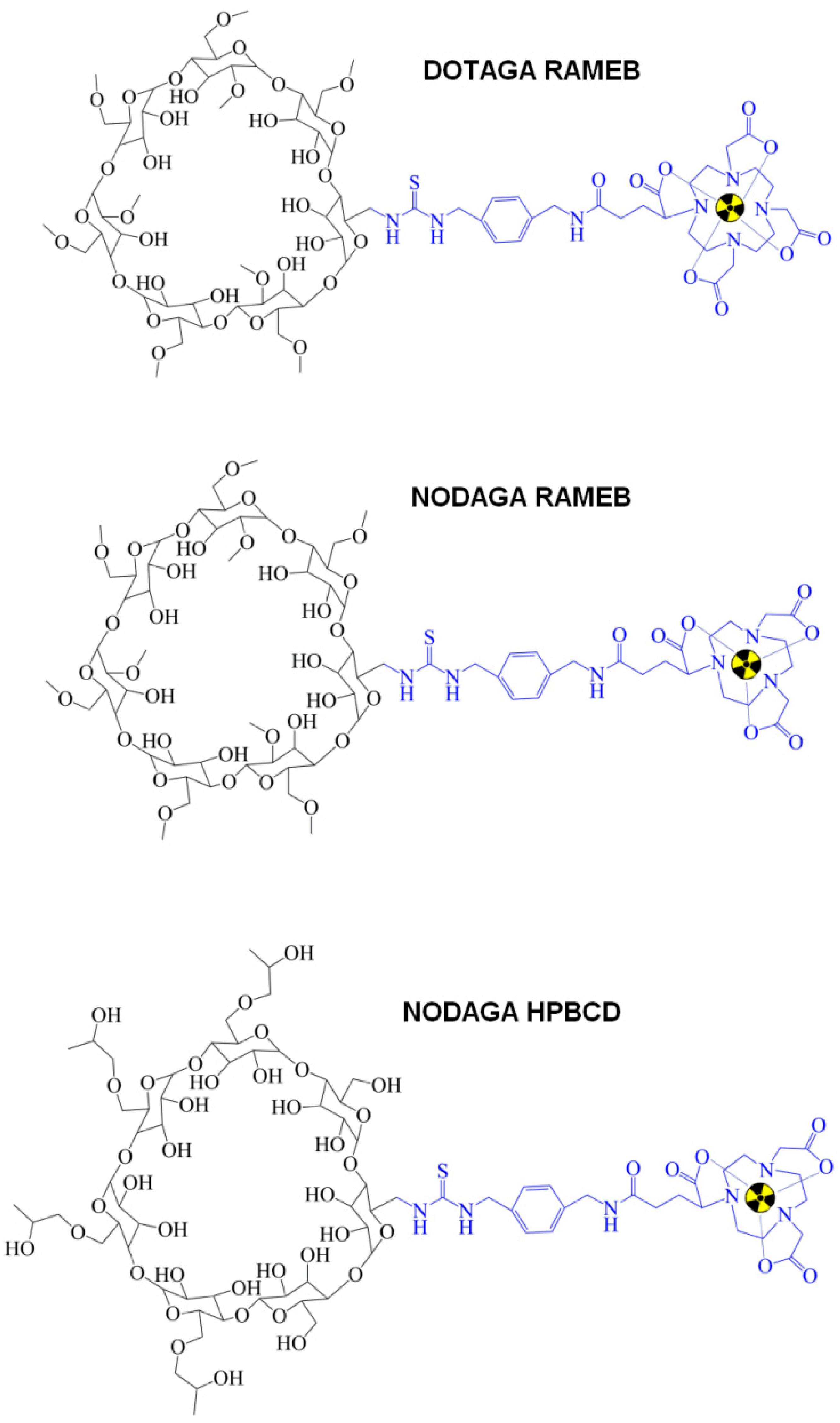
1.2. PGE2 and Cyclodextrins (CDs)
1.3. PGE2pos. Preclinical Tumor Models
2. Insight into Preclinical In Vivo Nuclear Medical Studies
3. Closing Remarks
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Tong, D.; Liu, Q.; Wang, L.A.; Xie, Q.; Pang, J.; Huang, Y.; Wang, L.; Liu, G.; Zhang, D.; Lan, W.; et al. The roles of the COX2/PGE2/EP axis in therapeutic resistance. Cancer Metastasis Rev. 2018, 37, 355–368. [Google Scholar] [CrossRef] [PubMed]
- Wu, W.K.; Sung, J.J.; Lee, C.W.; Yu, J.; Cho, C.H. Cyclooxygenase-2 in tumorigenesis of gastrointestinal cancers: An update on the molecular mechanisms. Cancer. Lett. 2010, 295, 7–16. [Google Scholar] [CrossRef]
- Banu, N.; Buda, A.; Chell, S.; Elder, D.; Moorghen, M.; Paraskeva, C.; Qualtrough, D.; Pignatelli, M. Inhibition of COX-2 with NS-398 decreases colon cancer cell motility through blocking epidermal growth factor receptor transactivation: Possibilities for combination therapy. Cell Prolif. 2007, 40, 768–779. [Google Scholar] [CrossRef] [PubMed]
- Cook, P.J.; Thomas, R.; Kingsley, P.J.; Shimizu, F.; Montrose, D.C.; Marnett, L.J.; Tabar, V.S.; Dannenberg, A.J.; Benezra, R. Cox-2-derived PGE2 induces Id1-dependent radiation resistance and self-renewal in experimental glioblastoma. Neuro. Oncol. 2016, 18, 1379–1389. [Google Scholar] [CrossRef]
- Kim, H.S.; Moon, H.G.; Han, W.; Yom, C.K.; Kim, W.H.; Kim, J.H.; Noh, D.Y. COX2 overexpression is a prognostic marker for stage III breast cancer. Breast. Cancer Res. Treat. 2012, 132, 51–59. [Google Scholar] [CrossRef]
- Liu, Q.; Yuan, W.; Tong, D.; Liu, G.; Lan, W.; Zhang, D.; Xiao, H.; Zhang, Y.; Huang, Z.; Yang, J.; et al. Metformin represses bladder cancer progression by inhibiting stem cell repopulation via COX2/PGE2/STAT3 axis. Oncotarget 2016, 7, 28235–28246. [Google Scholar] [CrossRef]
- Tong, D.; Liu, Q.; Liu, G.; Xu, J.; Lan, W.; Jiang, Y.; Xiao, H.; Zhang, D.; Jiang, J. Metformin inhibits castration-induced EMT in prostate cancer by repressing COX2/PGE2/STAT3 axis. Cancer Lett. 2017, 389, 23–32. [Google Scholar] [CrossRef]
- Chan, A.T.; Ogino, S.; Fuchs, C.S. Aspirin use and survival after diagnosis of colorectal cancer. JAMA 2009, 302, 649–658. [Google Scholar] [CrossRef] [PubMed]
- Harris, R.E.; Beebe-Donk, J.; Alshafie, G.A. Similar reductions in the risk of human colon cancer by selective and nonselective cyclooxygenase-2 (COX-2) inhibitors. BMC. Cancer 2008, 8, 237. [Google Scholar] [CrossRef]
- Yao, M.; Zhou, W.; Sangha, S.; Albert, A.; Chang, A.J.; Liu, T.C.; Wolfe, M.M. Effects of nonselective cyclooxygenase inhibition with low-dose ibuprofen on tumor growth, angiogenesis, metastasis, and survival in a mouse model of colorectal cancer. Clin. Cancer Res. 2005, 11, 1618–1628. [Google Scholar] [CrossRef]
- Tai, H.H.; Ensor, C.M.; Tong, M.; Zhou, H.; Yan, F. Prostaglandin catabolizing enzymes. Prostaglandins Other Lipid Mediat. 2002, 68–69, 483–493. [Google Scholar] [CrossRef]
- Wang, D.; Dubois, R.N. Prostaglandins and cancer. Gut 2006, 55, 115–122. [Google Scholar] [CrossRef] [PubMed]
- Reid, G.; Wielinga, P.; Zelcer, N.; van der Heijden, I.; Kuil, A.; de Haas, M.; Wijnholds, J.; Borst, P. The human multidrug resistance protein MRP4 functions as a prostaglandin efflux transporter and is inhibited by nonsteroidal antiinflammatory drugs. Proc. Natl. Acad. Sci. USA 2003, 100, 9244–9249. [Google Scholar] [CrossRef]
- Kochel, T.J.; Fulton, A.M. Multiple drug resistance-associated protein 4 (MRP4), prostaglandin transporter (PGT), and 15-hydroxyprostaglandin dehydrogenase (15-PGDH) as determinants of PGE2 levels in cancer. Prostaglandins Other Lipid Mediat. 2015, 116–117, 99–103. [Google Scholar] [CrossRef]
- Brown, J.R.; DuBois, R.N. COX-2: A molecular target for colorectal cancer prevention. J. Clin. Oncol. 2005, 23, 2840–2855. [Google Scholar] [CrossRef] [PubMed]
- Madrigal-Martínez, A.; Fernández-Martínez, A.B.; Lucio Cazaña, F.J. Intracrine prostaglandin E2 pro-tumoral actions in prostate epithelial cells originate from non-canonical pathways. J. Cell. Physiol. 2018, 233, 3590–3602. [Google Scholar] [CrossRef] [PubMed]
- Pan, J.; Yang, Q.; Shao, J.; Zhang, L.; Ma, J.; Wang, Y.; Jiang, B.H.; Leng, J.; Bai, X. Cyclooxygenase-2 induced β1-integrin expression in NSCLC and promoted cell invasion via the EP1/MAPK/E2F-1/FoxC2 signal pathway. Sci. Rep. 2016, 6, 33823. [Google Scholar] [CrossRef]
- Ma, X.; Aoki, T.; Tsuruyama, T.; Narumiya, S. Definition of prostaglandin E2-EP2 signals in the colon tumor microenvironment that amplify inflammation and tumor growth. Cancer Res. 2015, 75, 2822–2832. [Google Scholar] [CrossRef]
- Ding, Y.; Tong, M.; Liu, S.; Moscow, J.A.; Tai, H.H. NAD+-linked 15-hydroxyprostaglandin dehydrogenase (15-PGDH) behaves as a tumor suppressor in lung cancer. Carcinogenesis 2005, 26, 65–72. [Google Scholar] [CrossRef]
- Gee, J.R.; Montoya, R.G.; Khaled, H.M.; Sabichi, A.L.; Grossman, H.B. Cytokeratin 20, AN43, PGDH, and COX-2 expression in transitional and squamous cell carcinoma of the bladder. Urol. Oncol. 2003, 21, 266–270. [Google Scholar] [CrossRef]
- Kochel, T.J.; Reader, J.C.; Ma, X.; Kundu, N.; Fulton, A.M. Multiple drug resistance-associated protein (MRP4) exports prostaglandin E2 (PGE2) and contributes to metastasis in basal/triple negative breast cancer. Oncotarget 2017, 8, 6540–6554. [Google Scholar] [CrossRef]
- Del Valle, E.M.M. Cyclodextrins and their uses: A review. Process Biochem. 2004, 39, 1033–1046. [Google Scholar] [CrossRef]
- Loftsson, T.; Jarho, P.; Másson, M.; Järvinen, T. Cyclodextrins in drug delivery. Expert. Opin. Drug. Deliv. 2005, 2, 335–351. [Google Scholar] [CrossRef]
- Szejtli, J. Cyclodextrins and Their INCLUSION Complexes; Academic Publisher: Budapest, Hungary, 1982; p. 395. [Google Scholar]
- Szejtli, J. Introduction and General Overview of Cyclodextrin Chemistry. Chem. Rev. 1998, 98, 1743–1754. [Google Scholar] [CrossRef]
- Hirayama, F.; Kurihara, M.; Uekama, K. Improving the aqueous stability of prostaglandin E2 and prostaglandin A2 by inclusion complexation with methylated-beta-cyclodextrins. Chem. Pharm. Bull. 1984, 32, 4237–4240. [Google Scholar] [CrossRef]
- Sauer, R.S.; Rittner, H.L.; Roewer, N.; Sohajda, T.; Shityakov, S.; Brack, A.; Broscheit, J.A. A Novel Approach for the Control of Inflammatory Pain: Prostaglandin E2 Complexation by Randomly Methylated β-Cyclodextrins. Anesth. Analg. 2017, 124, 675–685. [Google Scholar] [CrossRef]
- Inoue, Y.; Sekiya, N.; Katayama, K.; Narutaki, S.; Yamamoto, M.; Iohara, D.; Hirayama, F.; Uekama, K. Stabilizing effect of β-cyclodextrin on Limaprost, a PGE1 derivative, in Limaprost alfadex tablets (Opalmon) in highly humid conditions. Chem. Pharm. Bull. 2014, 62, 786–792. [Google Scholar] [CrossRef]
- Inoue, Y.; Sekiya, N.; Yamamoto, M.; Iohara, D.; Hirayama, F.; Uekama, K. Formation of the ternary inclusion complex of limaprost with α- and β-cyclodextrins in aqueous solution. Chem. Pharm. Bull. 2015, 63, 318–325. [Google Scholar] [CrossRef]
- Phelps, M.E. Positron emission tomography provides molecular imaging of biological processes. Proc. Natl. Acad. Sci. USA 2000, 97, 9226–9233. [Google Scholar] [CrossRef]
- Hajdu, I.; Angyal, J.; Szikra, D.; Kertész, I.; Malanga, M.; Fenyvesi, É.; Szente, L.; Vecsernyés, M.; Bácskay, I.; Váradi, J.; et al. Radiochemical synthesis and preclinical evaluation of 68Ga-labeled NODAGA-hydroxypropyl-beta-cyclodextrin (68Ga-NODAGA-HPBCD). Eur. J. Pharm. Sci. 2019, 128, 202–208. [Google Scholar] [CrossRef]
- Phelps, M.E. PET: The merging of biology and imaging into molecular imaging. J. Nucl. Med. 2000, 41, 661–681. [Google Scholar]
- Trencsényi, G.; Kis, A.; Szabó, J.P.; Ráti, Á.; Csige, K.; Fenyvesi, É.; Szente, L.; Malanga, M.; Méhes, G.; Emri, M.; et al. In vivo preclinical evaluation of the new 68Ga-labeled beta-cyclodextrin in prostaglandin E2 (PGE2) positive tumor model using positron emission tomography. Int. J. Pharm. 2020, 576, 118954. [Google Scholar] [CrossRef] [PubMed]
- Csige, K.; Szabó, J.P.; Kálmán-Szabó, I.; Dénes, N.S.; Szikra, D.; Képes, Z.; Opposits, G.; Méhes, G.; Kertész, I.; Fenyvesi, F.; et al. In vivo investigation of Gallium-68 and Bismuth-205/206 labeled beta cyclodextrin for targeted alpha therapy of prostaglandin E2 receptor-expressing tumors in mice. Int. J. Pharm. 2022, 625, 122132. [Google Scholar] [CrossRef] [PubMed]
- Szabó, J.P.; Csige, K.; Kálmán-Szabó, I.; Arató, V.; Opposits, G.; Jószai, I.; Kertész, I.; Képes, Z.; Méhes, G.; Fenyvesi, F.; et al. In vivo assessment of tumor targeting potential of 68Ga-labelled randomly methylated beta-cyclodextrin (RAMEB) and 2-hydroxypropyl-β-cyclodextrin (HPβCD) using positron emission tomography. Int. J. Pharm. 2023, 630, 122462. [Google Scholar] [CrossRef]
- Takahashi, T.; Uehara, H.; Ogawa, H.; Umemoto, H.; Bando, Y.; Izumi, K. Inhibition of EP2/EP4 signaling abrogates IGF-1R-mediated cancer cell growth: Involvement of protein kinase C-θ activation. Oncotarget 2015, 6, 4829–4844. [Google Scholar] [CrossRef]
- Gonnermann, D.; Oberg, H.H.; Kellner, C.; Peipp, M.; Sebens, S.; Kabelitz, D.; Wesch, D. Resistance of cyclooxygenase-2 expressing pancreatic ductal adenocarcinoma cells against γδ T cell cytotoxicity. Oncoimmunology 2015, 4, e988460. [Google Scholar] [CrossRef]
- Kim, S.; Han, J.; Lee, S.K.; Hur, S.M.; Koo, M.; Cho, D.H.; Bae, S.Y.; Choi, M.Y.; Shin, I.; Yang, J.H.; et al. Pifithrin-α, an inhibitor of p53 transactivation, up-regulates COX-2 expression through an MAPK-dependent pathway. Pharmacology 2010, 86, 313–319. [Google Scholar] [CrossRef]
- Pi, C.; Jing, P.; Li, B.; Feng, Y.; Xu, L.; Xie, K.; Huang, T.; Xu, X.; Gu, H.; Fang, J. Reversing PD-1 Resistance in B16F10 Cells and Recovering Tumour Immunity Using a COX2 Inhibitor. Cancers 2022, 14, 4134. [Google Scholar] [CrossRef]
- Fedyk, E.R.; Brown, D.M.; Phipps, R.P. PGE2 regulation of B lymphocytes and T helper 1 and T helper 2 cells: Induction of inflammatory versus allergic responses. Adv. Exp. Med. Biol. 1997, 407, 237–242. [Google Scholar]
- Brown, D.M.; Phipps, R.P. Characterization and regulation of prostaglandin E2 receptors on normal and malignant murine B lymphocytes. Cell. Immunol. 1995, 161, 79–87. [Google Scholar] [CrossRef]
- Burchiel, S.W.; Hanson, K.; Warner, N.L. Clonal heterogeneity of cyclic AMP responsiveness: A comparison of malignant murine lymphoid cell lines. Int. J. Immunopharmacol. 1984, 6, 35–42. [Google Scholar] [CrossRef]
- Roper, R.L.; Phipps, R.P. Prostaglandin E2 and cAMP inhibit B lymphocyte activation and simultaneously promote IgE and IgG1 synthesis. J. Immunol. 1992, 149, 2984–2991. [Google Scholar] [CrossRef]
- Fedyk, E.R.; Ripper, J.M.; Brown, D.M.; Phipps, R.P. A molecular analysis of PGE receptor (EP) expression on normal and transformed B lymphocytes: Coexpression of EP1, EP2, EP3beta and EP4. Mol. Immunol. 1996, 5, 33–45. [Google Scholar] [CrossRef] [PubMed]
- Yip-Schneider, M.T.; Barnard, D.S.; Billings, S.D.; Cheng, L.; Heilman, D.K.; Lin, A.; Marshall, S.J.; Crowell, P.L.; Marshall, M.S.; Sweeney, C.J. Cyclooxygenase-2 expression in human pancreatic adenocarcinomas. Carcinogenesis 2000, 21, 139–146. [Google Scholar] [CrossRef]
- Frijlink, H.W.; Visser, J.; Hefting, N.R.; Oosting, R.; Meijer, D.K.; Lerk, C.F. The pharmacokinetics of beta-cyclodextrin and hydroxypropyl-beta-cyclodextrin in the rat. Pharm. Res. 1990, 7, 1248–1252. [Google Scholar] [CrossRef]
- Gould, S.; Scott, R.C. 2-Hydroxypropyl-beta-cyclodextrin (HP-beta-CD): A toxicology review. Food Chem. Toxicol. 2005, 43, 1451–1459. [Google Scholar] [CrossRef]
- Lattuada, L.; Barge, A.; Cravotto, G.; Giovenzana, G.B.; Tei, L. The synthesis and application of polyamino polycarboxylic bifunctional chelating agents. Chem. Soc. Rev. 2011, 40, 3019–3049. [Google Scholar] [CrossRef]
- Okoye, N.C.; Baumeister, J.E.; Najafi, K.F.; Hennkens, H.M.; Jurisson, S.S. Chelators and metal complex stability for radiopharmaceutical applications. Radiochim. Acta 2019, 107, 1087–1120. [Google Scholar] [CrossRef]
- Borgstrom, P.; Oh, P.; Czarny, M.; Racine, B.; Schnitzer, J.E. Co-implanting orthotopic tissue creates stroma microenvironment enhancing growth and angiogenesis of multiple tumors. F1000Research 2013, 2, 129. [Google Scholar] [CrossRef]
- Máté, G.; Kertész, I.; Enyedi, K.N.; Mező, G.; Angyal, J.; Vasas, N.; Kis, A.; Szabó, É.; Emri, M.; Bíró, T.; et al. In vivo imaging of Aminopeptidase N (CD13) receptors in experimental renal tumors using the novel radiotracer (68)Ga-NOTA-c(NGR). Eur. J. Pharm. Sci. 2015, 69, 61–71. [Google Scholar] [CrossRef]
- Logsdon, C.D.; Lu, W. The Significance of Ras Activity in Pancreatic Cancer Initiation. Int. J. Biol. Sci. 2016, 12, 338–346. [Google Scholar] [CrossRef]
- Backlund, M.G.; Mann, J.R.; Wang, D.; Dubois, R.N. Ras up-regulation of cyclooxygenase-2. Methods Enzymol. 2006, 407, 401–410. [Google Scholar]
- Downward, J. Targeting RAS signalling pathways in cancer therapy. Nat. Rev. Cancer 2003, 3, 11–22. [Google Scholar] [CrossRef]
- Yoshitake, R.; Saeki, K.; Eto, S.; Shinada, M.; Nakano, R.; Sugiya, H.; Endo, Y.; Fujita, N.; Nishimura, R.; Nakagawa, T. Aberrant expression of the COX2/PGE2 axis is induced by activation of the RAF/MEK/ERK pathway in BRAFV595E canine urothelial carcinoma. Sci. Rep. 2020, 10, 7826. [Google Scholar] [CrossRef]
- Bos, J.L. Ras oncogenes in human cancer: A review. Cancer Res. 1989, 49, 4682–4689. [Google Scholar]
- Sheng, H.; Shao, J.; Dixon, D.A.; Williams, C.S.; Prescott, S.M.; DuBois, R.N.; Beauchamp, R.D. Transforming growth factor-beta1 enhances Ha-ras-induced expression of cyclooxygenase-2 in intestinal epithelial cells via stabilization of mRNA. J. Biol. Chem. 2000, 275, 6628–6635. [Google Scholar] [CrossRef]
- Wang, D.; Buchanan, F.G.; Wang, H.; Dey, S.K.; DuBois, R.N. Prostaglandin E2 enhances intestinal adenoma growth via activation of the Ras-mitogen-activated protein kinase cascade. Cancer Res. 2005, 65, 1822–1829. [Google Scholar] [CrossRef]
- Zhang, Z.; Sheng, H.; Shao, J.; Beauchamp, R.D.; DuBois, R.N. Posttranscriptional regulation of cyclooxygenase-2 in rat intestinal epithelial cells. Neoplasia 2000, 2, 523–530. [Google Scholar] [CrossRef]

| Investigated Object | Investigated Phenomenon | Radiopharmaceutical | Imaging Technique | Reference |
|---|---|---|---|---|
| healthy BALB/c male mice | diagnostic feasibility, radiochemical synthesis process, proof-of-concept study | [68Ga]Ga-NODAGA-HPBCD | in vivo PET/CT imaging, ex vivo radioactivity determination by gamma counter | Hajdu et al., 2019 [31] |
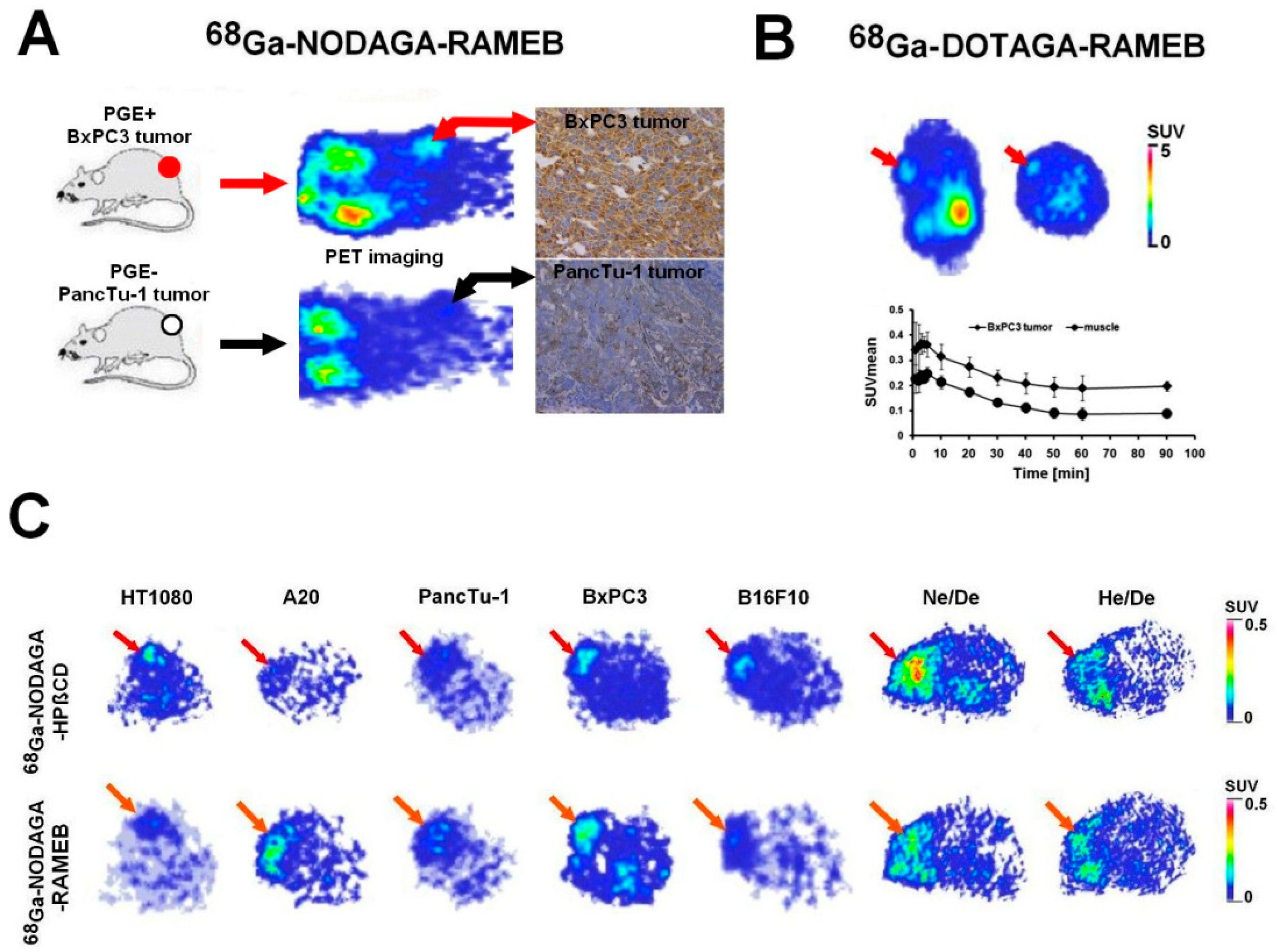
| BxPC-3 and PancTu-1 tumor-bearing CB17 SCID mice | in vivo and ex vivo organ distribution, PGE2 tumor-homing ability | [68Ga]Ga-NODAGA-RAMEB | in vivo static and dynamic PET imaging, ex vivo gamma counter measurements | Trencsényi et al., 2020 [33] |
| CB17 SCID male mice bearing BxPC-3 tumor | ex vivo biodistribution, PGE2-targeting capability, pharmacokinetic profile, biosynthesis | [68Ga]Ga-DOTAGA-RAMEB [205/206Bi]Bi-DOTAGA-RAMEB | in vivo static and dynamic PET acquisition, ex vivo gamma counting | Csige et al., 2022 [34] |
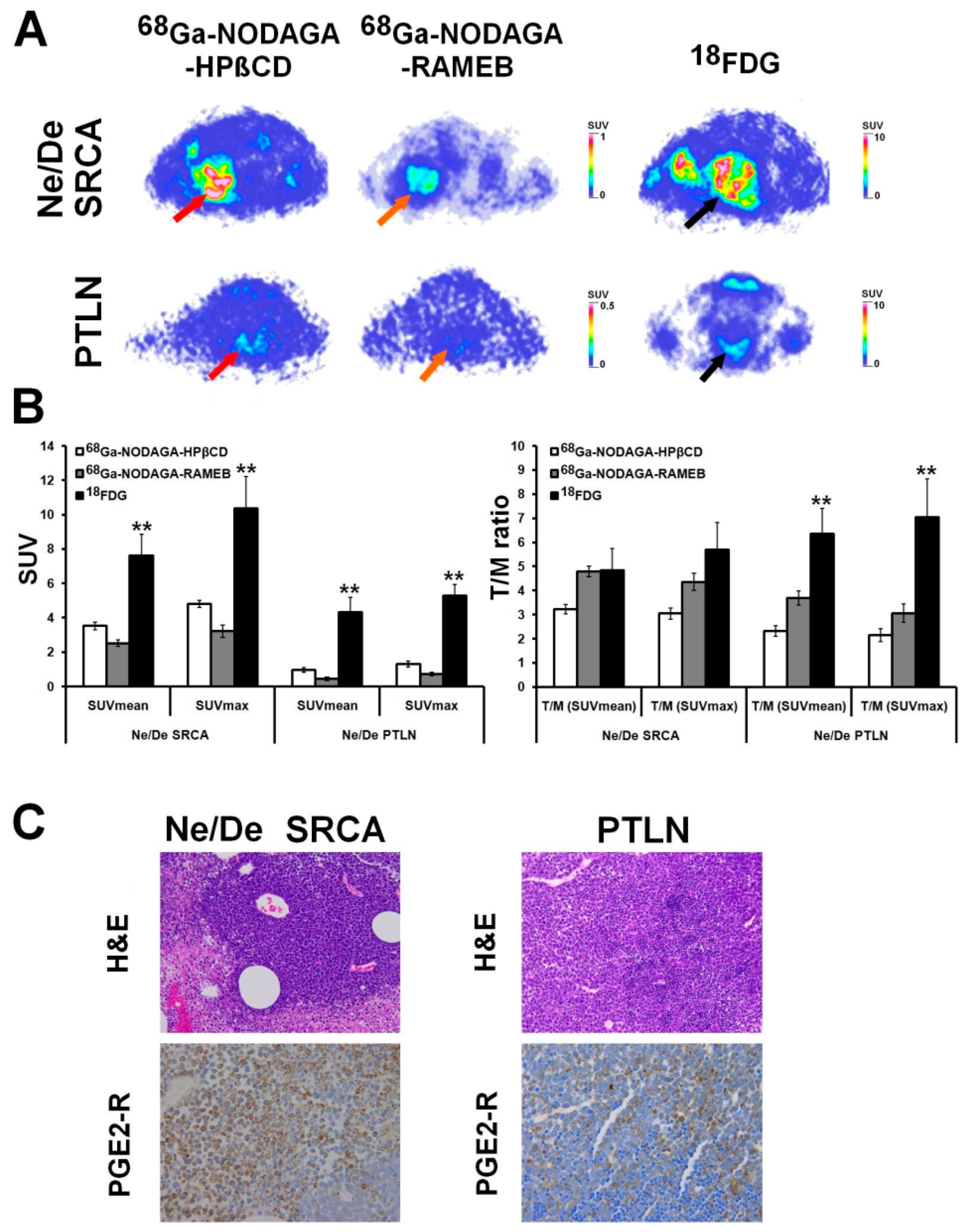
| HT1080, -A20, -PancTu-1, -BxPC-3, -B16-F10 tumor-bearing CB17 SCID male mice and Ne/De, He/De tumor-bearing Fischer-344 female rats | in vivo and ex vivo biodistribution pattern, tumor-targeting competence | [68Ga]Ga-NODAGA-RAMEB, [68Ga]Ga-NODAGA-HPBCD | in vivo static PET imaging, ex vivo gamma counting | Szabó et al., 2023 [35] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Képes, Z.; Dénes, N.; Kertész, I.; Hajdu, I.; Trencsényi, G. Overview of Prostaglandin E2 (PGE2)-Targeting Radiolabelled Imaging Probes from Preclinical Perspective: Lessons Learned and Road Ahead. Int. J. Mol. Sci. 2023, 24, 6942. https://doi.org/10.3390/ijms24086942
Képes Z, Dénes N, Kertész I, Hajdu I, Trencsényi G. Overview of Prostaglandin E2 (PGE2)-Targeting Radiolabelled Imaging Probes from Preclinical Perspective: Lessons Learned and Road Ahead. International Journal of Molecular Sciences. 2023; 24(8):6942. https://doi.org/10.3390/ijms24086942
Chicago/Turabian StyleKépes, Zita, Noémi Dénes, István Kertész, István Hajdu, and György Trencsényi. 2023. "Overview of Prostaglandin E2 (PGE2)-Targeting Radiolabelled Imaging Probes from Preclinical Perspective: Lessons Learned and Road Ahead" International Journal of Molecular Sciences 24, no. 8: 6942. https://doi.org/10.3390/ijms24086942
APA StyleKépes, Z., Dénes, N., Kertész, I., Hajdu, I., & Trencsényi, G. (2023). Overview of Prostaglandin E2 (PGE2)-Targeting Radiolabelled Imaging Probes from Preclinical Perspective: Lessons Learned and Road Ahead. International Journal of Molecular Sciences, 24(8), 6942. https://doi.org/10.3390/ijms24086942