Synaptic Targets of Glycinergic Neurons in Laminae I–III of the Spinal Dorsal Horn
Abstract
1. Introduction
2. Results
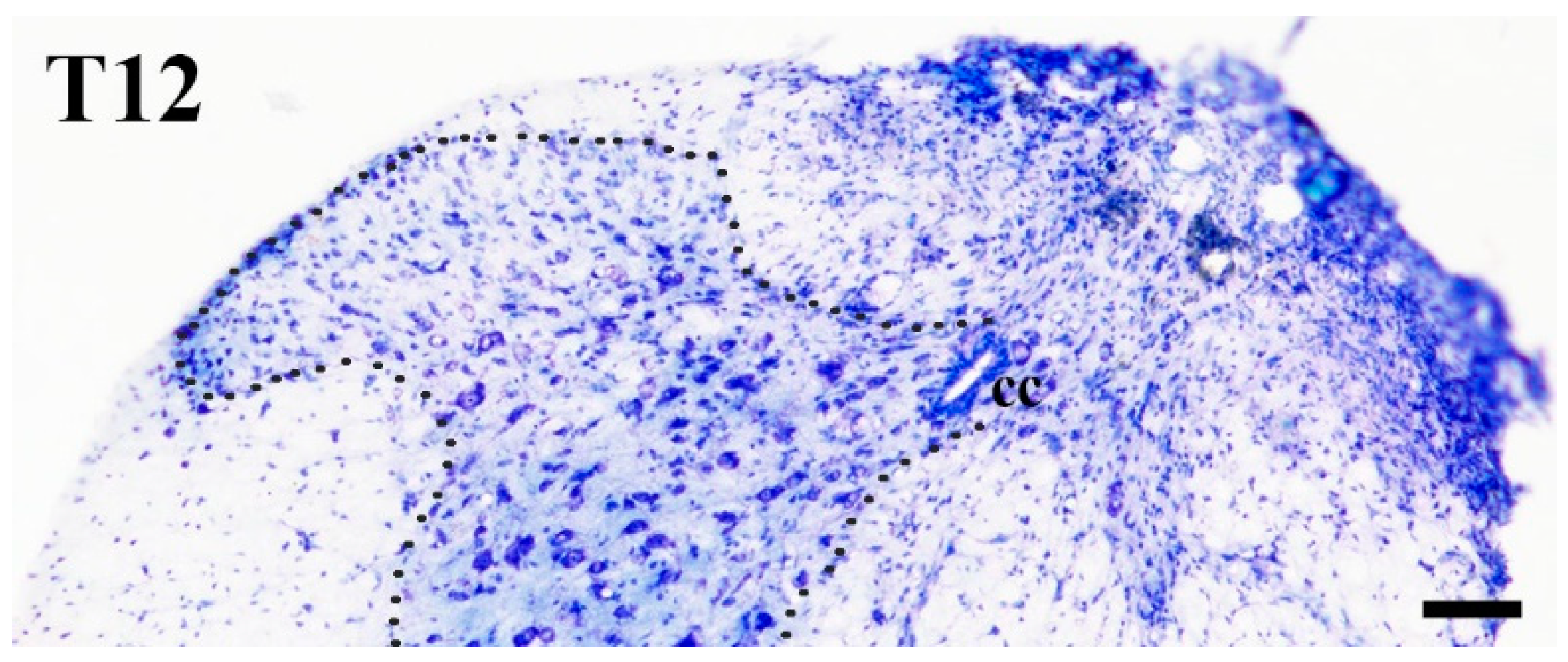
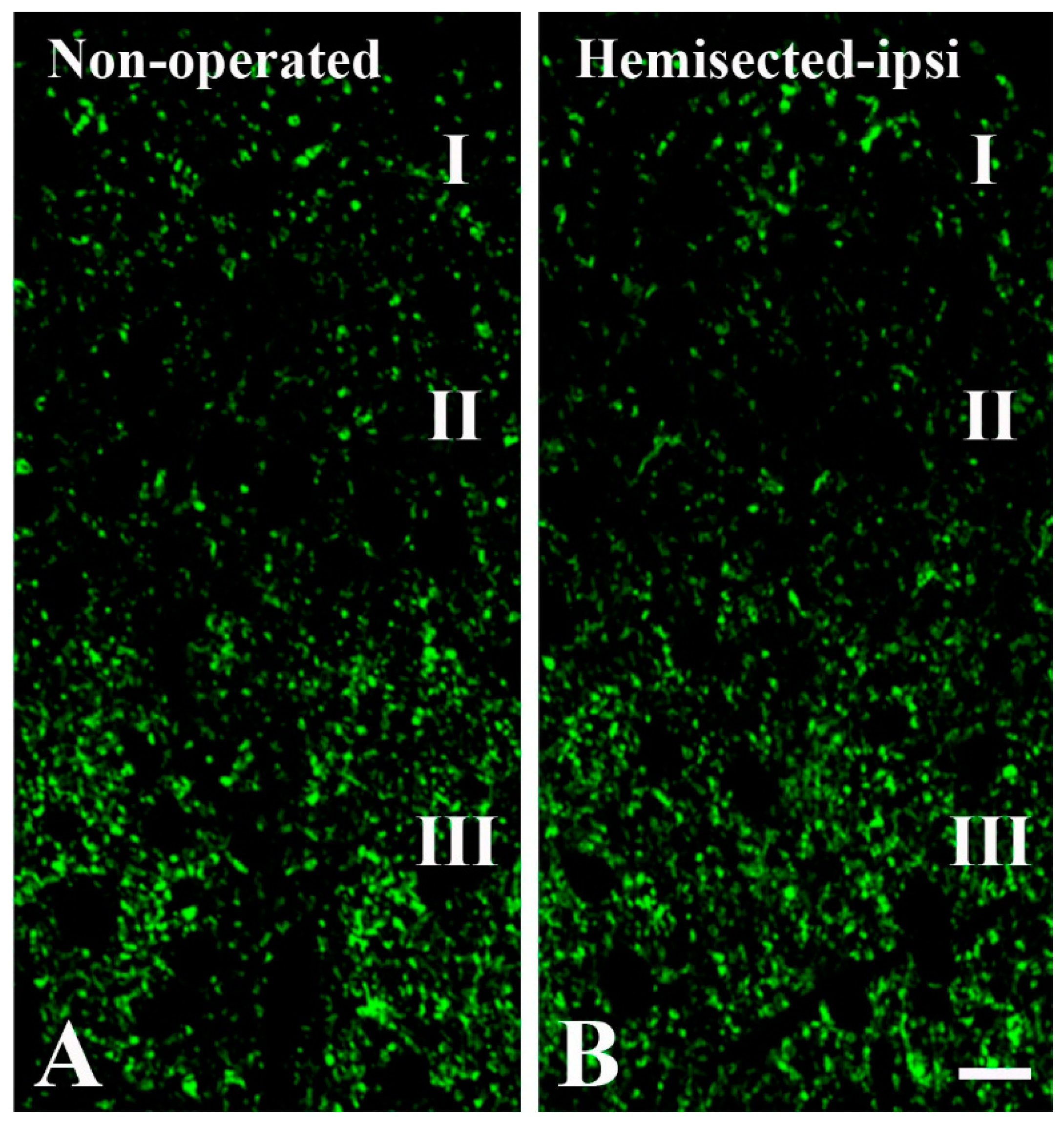
2.1. Elimination of GlyT2 Expressing Axon Terminals Descending from Higher Brain Centers to the Lumbar Spinal Cord
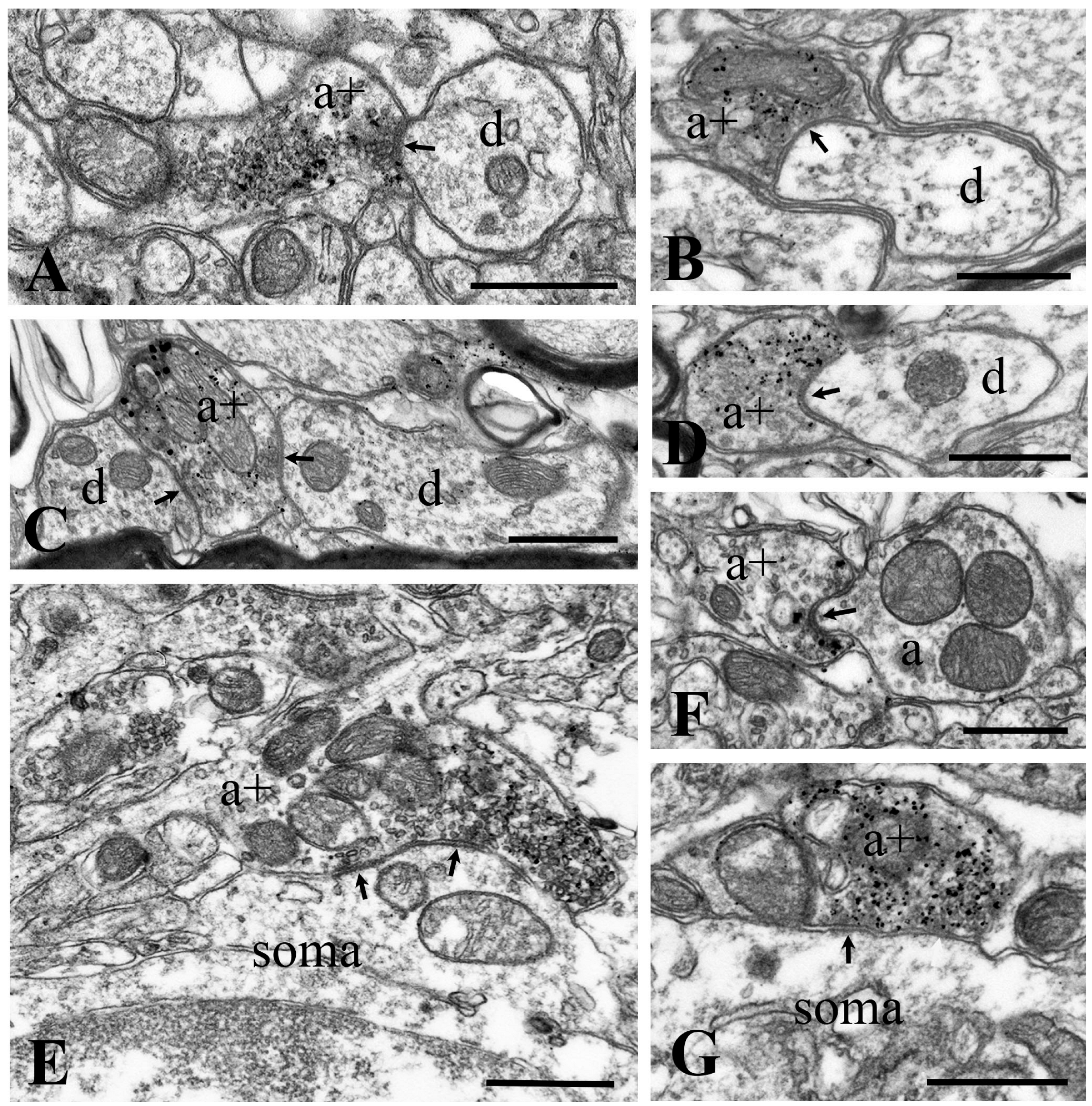
2.2. Synapses Formed by GlyT2 Immunoreactive Axon Terminals
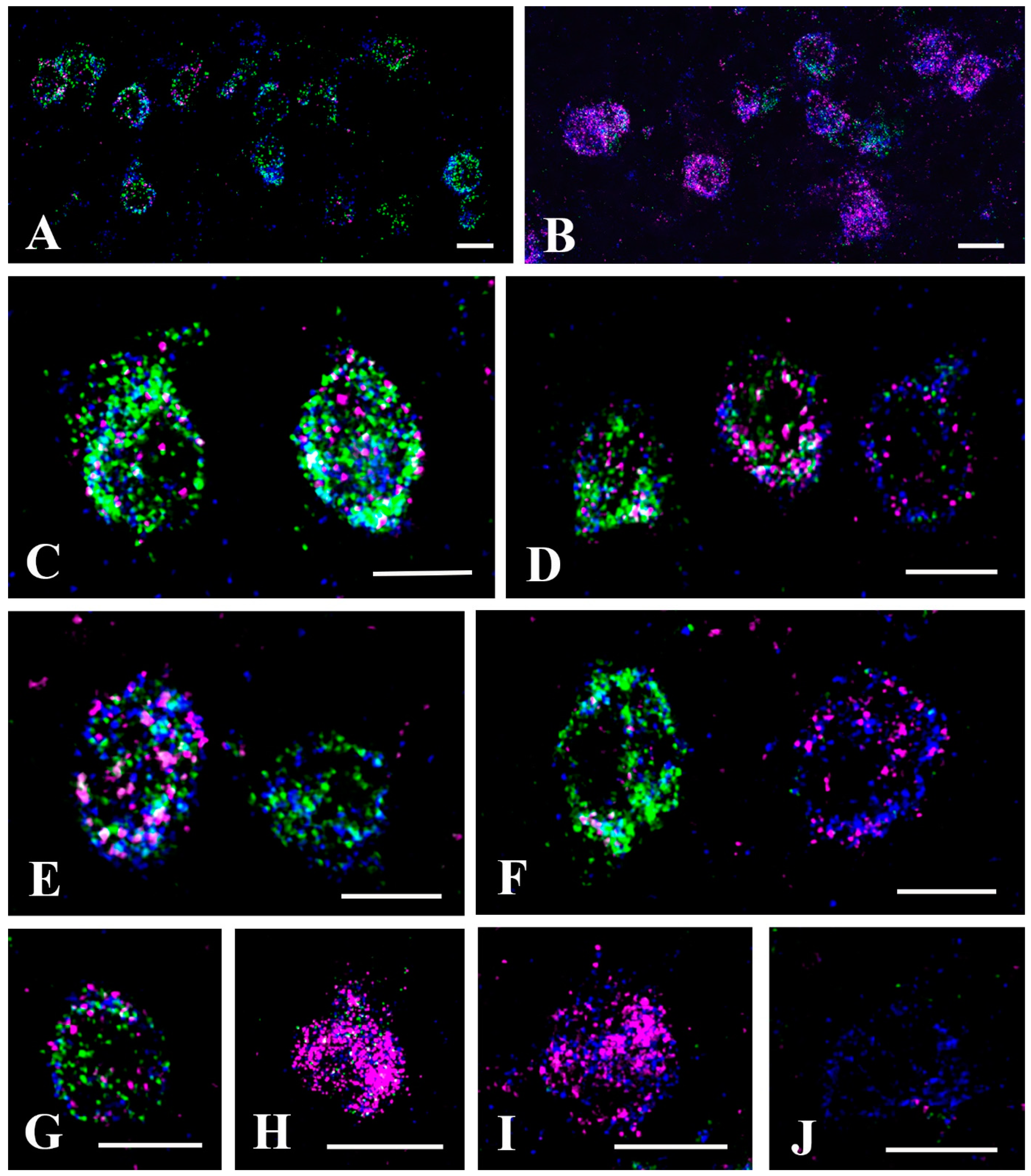
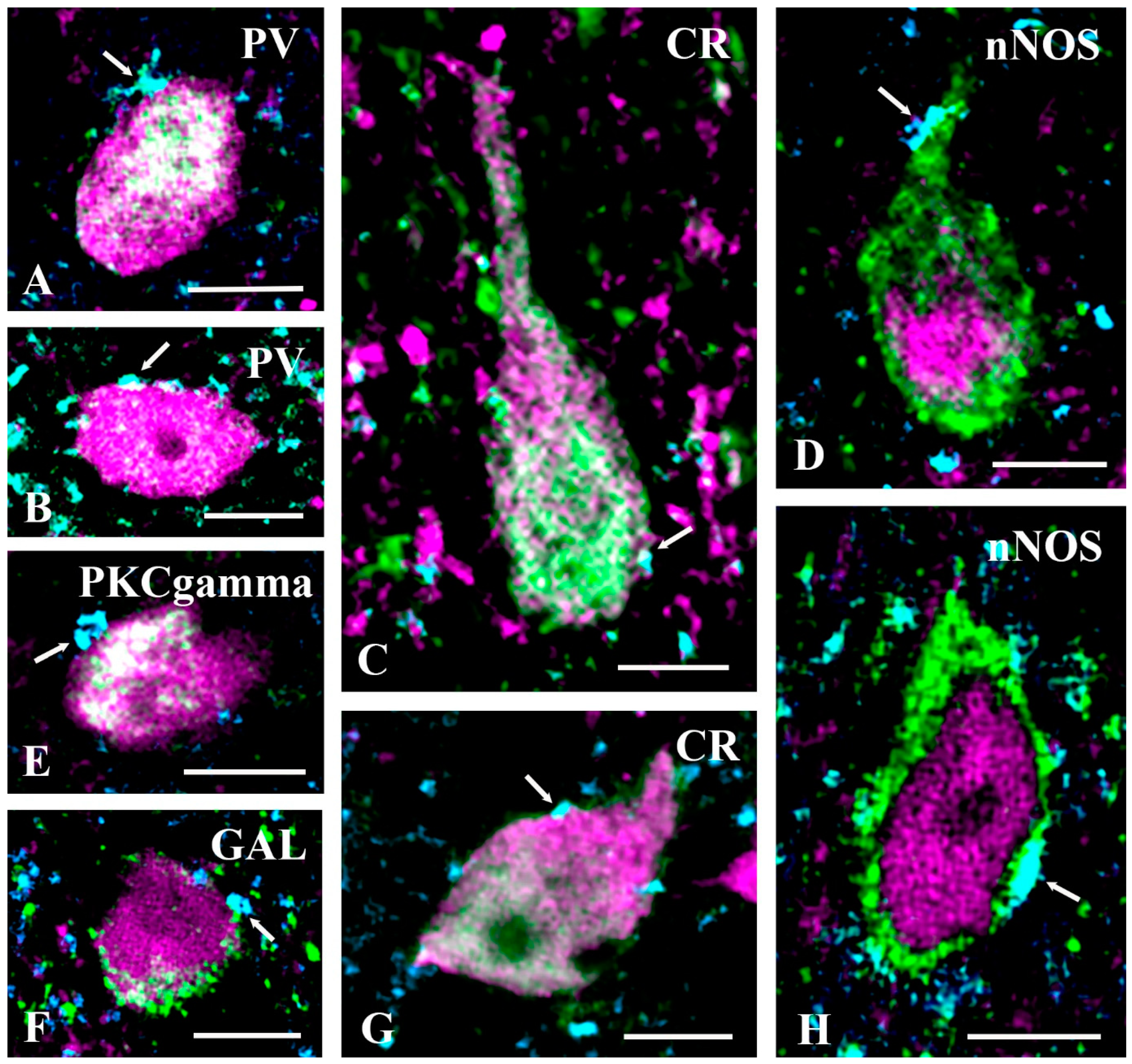
2.3. Neurochemical Identification of Neurons Receiving Axosomatic Glycinergic Inputs
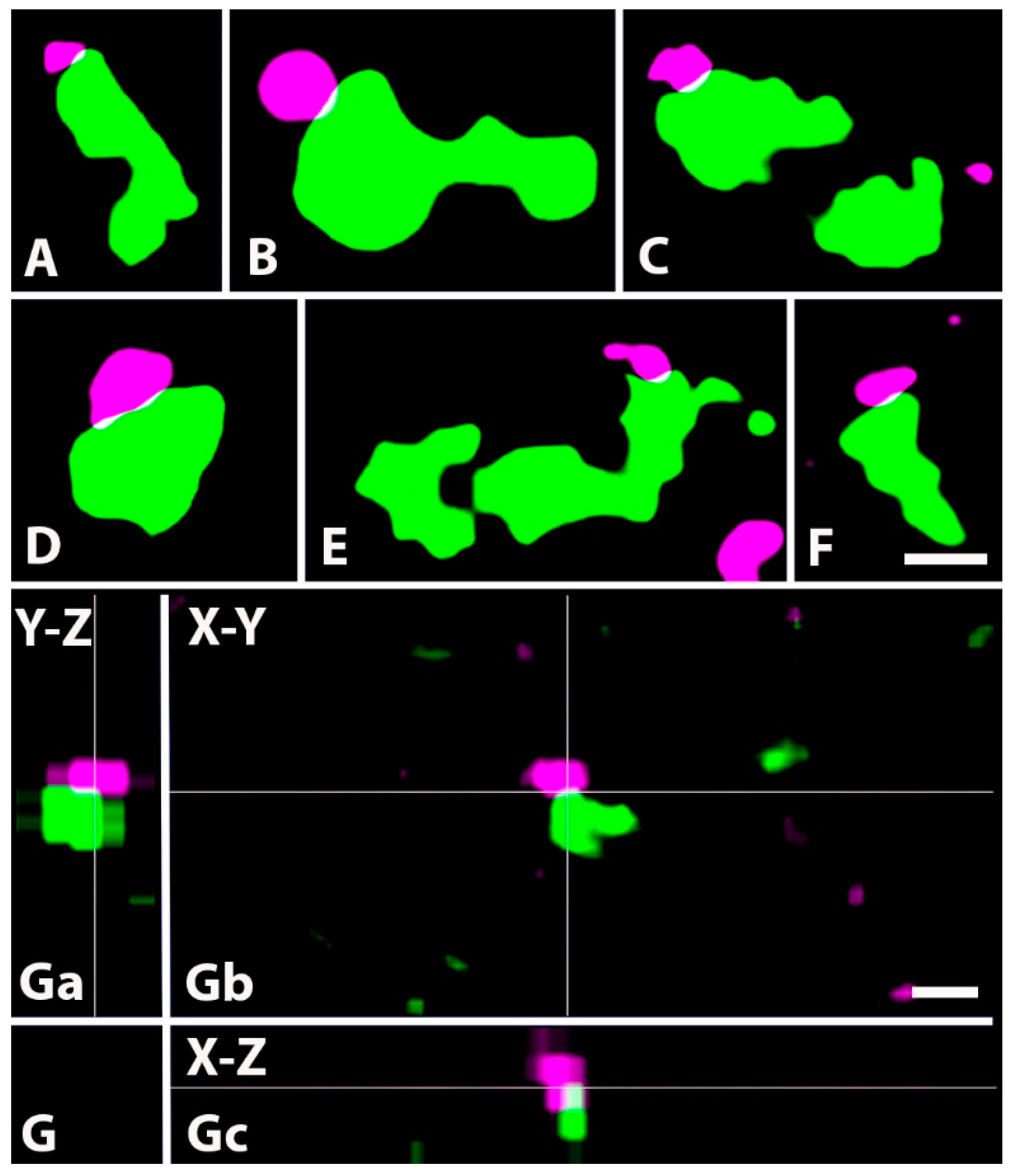
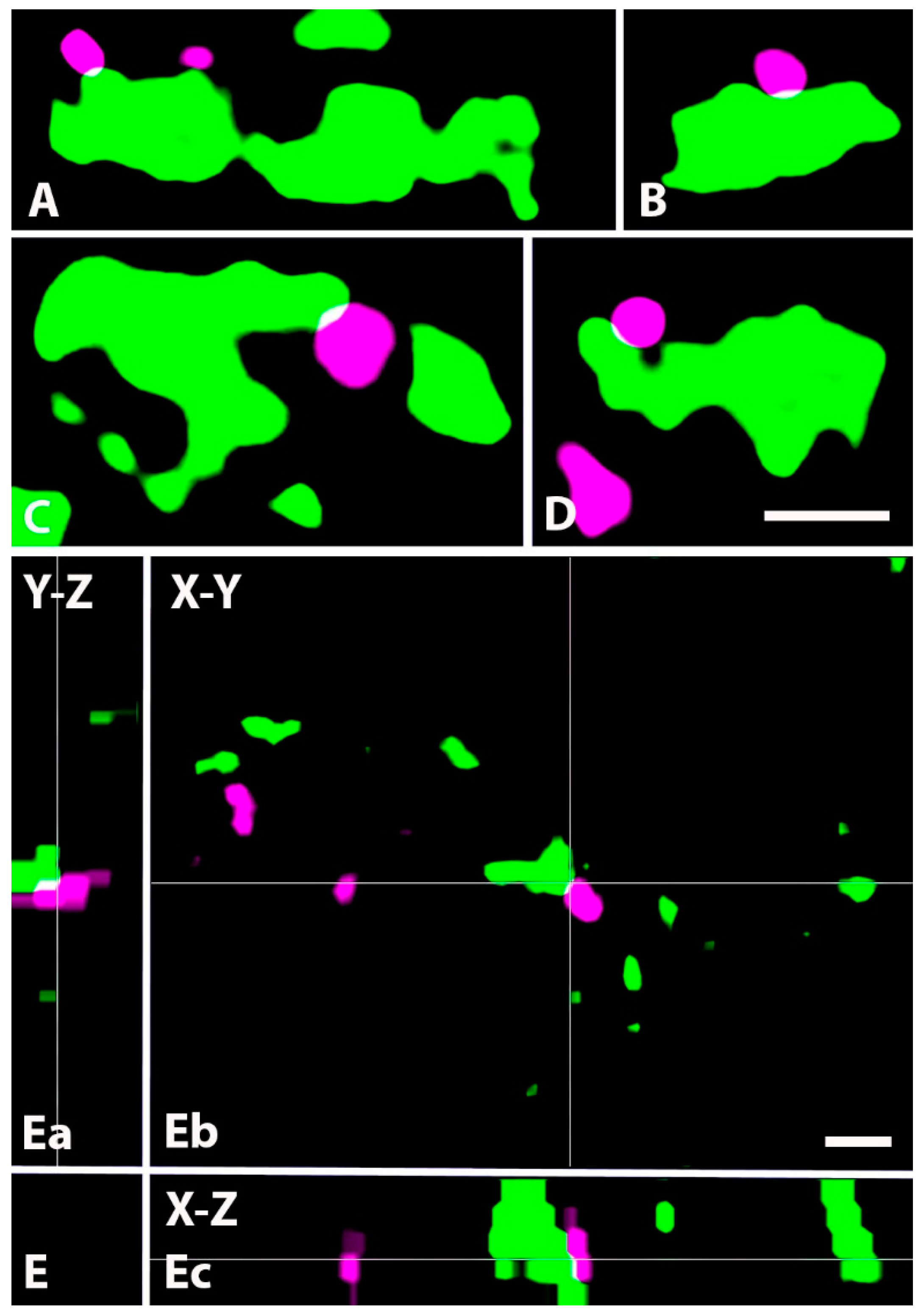
2.4. Neurochemical Identification of Axon Terminals Receiving Glycinergic Axo-Axonic Inputs
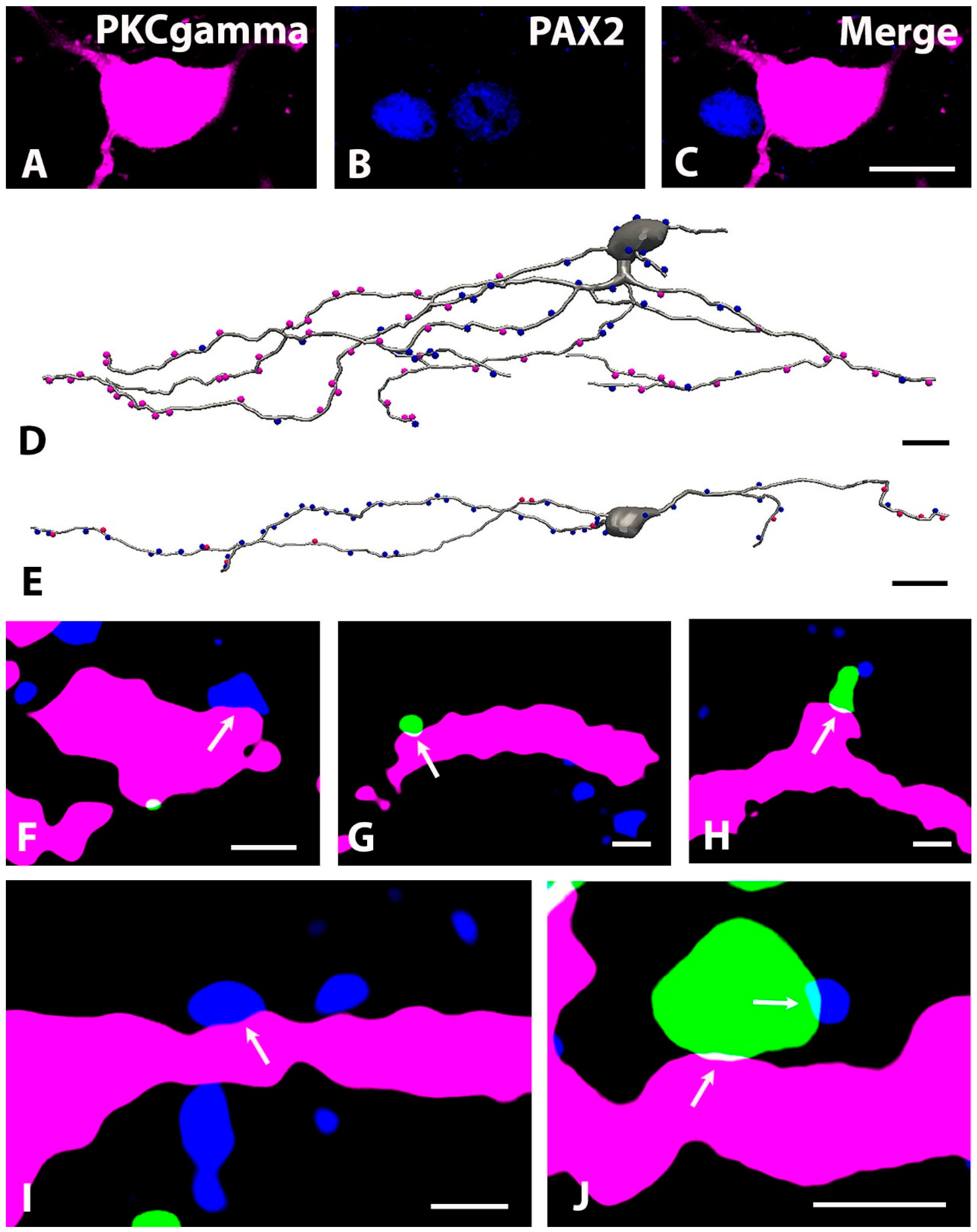
2.5. Distribution of GlyT2 Immunostained Axon Terminals on the Somato-Dendritic Compartment of PKCγ-Expressing Neurons
3. Discussion
3.1. GABAergic versus Glycinergic Inhibition in Laminae I–III of the Spinal Dorsal Horn
3.2. Synaptic Targets of Glycinergic Axon Terminals of Spinal Origin in Laminae I–III of the Spinal Dorsal Horn
3.2.1. Postsynaptic versus Presynaptic Inhibition
3.2.2. Targets of Postsynaptic Glycinergic Inhibition
3.2.3. Targets of Presynaptic Glycinergic Inhibition
4. Material and Methods
4.1. Animals
4.2. Hemisection of the Spinal Cord
4.3. Preparation of Tissue Sections
4.3.1. For Immunohistochemistry
4.3.2. For In Situ Hybridization
4.4. Immunohistochemistry for Light Microscopy
4.4.1. Single Immunostaining of Sections Obtained from Wild-Type Animals
4.4.2. Double Immunostaining of Sections Obtained from Wild-Type Animals
4.4.3. Double Immunostaining on Sections Obtained from PAX2:Cre-tdTomato Transgenic Animals
4.4.4. Triple Immunostaining on Sections Obtained from the PKCγ:Cre/ERT2-tdTomato Transgenic Animals
4.5. Immunohistochemistry for Electron Microscopy
4.6. Multiplex Fluorescent In Situ Hybridization
4.7. Confocal Microscopy
4.8. Neurolucida Reconstruction
4.9. Statistical Analysis
4.9.1. Changes in the Numbers of GlyT2 Immunostained Boutons Following Hemisection of the Spinal Cord
4.9.2. Colocalization of tdTomato, GAD1/2, and GlyT2 mRNAs
4.9.3. Expression of PAX2 in the Cell Bodies of Neurons Immunostained for Various Neuronal Markers
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Foster, E.; Wildner, H.; Tudeau, L.; Haueter, S.; Ralvenius, W.T.; Jegen, M.; Johannssen, H.; Hösli, L.; Haenraets, K.; Ghanem, A.; et al. Targeted ablation, silencing, and activation establish glycinergic dorsal horn neurons as key components of a spinal gate for pain and itch. Neuron 2015, 85, 1289–1304. [Google Scholar] [CrossRef]
- Zeilhofer, H.U.; Acuña, M.A.; Gingras, J.; Yévenes, G.E. Glycine receptors and glycine transporters: Targets for novel analgesics? Cell Mol. Life Sci. 2018, 75, 447–465. [Google Scholar] [CrossRef]
- Zeilhofer, H.U.; Werynska, K.; Gingras, J.; Yévenes, G.E. Glycine Receptors in Spinal Nociceptive Control-An Update. Biomolecules 2021, 11, 846. [Google Scholar] [CrossRef] [PubMed]
- Todd, A.J.; Sullivan, A.C. Light microscope study of the coexistence of GABA-like and glycine-like immunoreactivities in the spinal cord of the rat. J. Comp. Neurol. 1990, 296, 496–505. [Google Scholar] [CrossRef]
- Mitchell, K.; Spike, R.C.; Todd, A.J. An immunocytochemical study of glycine receptor and GABA in laminae I–III of rat spinal dorsal horn. J. Neurosci. 1993, 13, 2371–2381. [Google Scholar] [CrossRef] [PubMed]
- Todd, A.J. GABA and glycine in synaptic glomeruli of the rat spinal dorsal horn. Eur. J. Neurosci. 1996, 8, 2492–2498. [Google Scholar] [CrossRef] [PubMed]
- Todd, A.J.; Spike, R.C.; Chong, D.; Neilson, M. The relationship between glycine and gephyrin in synapses of the rat spinal cord. Eur. J. Neurosci. 1995, 7, 1–11. [Google Scholar] [CrossRef]
- Todd, A.J.; Watt, C.; Spike, R.C.; Sieghart, W. Colocalization of GABA, glycine, and their receptors at synapses in the rat spinal cord. J. Neurosci. 1996, 16, 974–982. [Google Scholar] [CrossRef]
- Chéry, N.; de Koninck, Y. Junctional versus extrajunctional glycine and GABA(A) receptor-mediated IPSCs in identified lamina I neurons of the adult rat spinal cord. J. Neurosci. 1999, 19, 7342–7355. [Google Scholar] [CrossRef]
- Keller, A.F.; Coull, J.A.; Chery, N.; Poisbeau, P.; De Koninck, Y. Region-specific developmental specialization of GABA-glycine cosynapses in laminas I–II of the rat spinal dorsal horn. J. Neurosci. 2001, 21, 7871–7880. [Google Scholar] [CrossRef]
- Takazawa, T.; MacDermott, A.B. Glycinergic and GABAergic tonic inhibition fine tune inhibitory control in regionally distinct subpopulations of dorsal horn neurons. J. Physiol. 2010, 588 Pt 14, 2571–2587. [Google Scholar] [CrossRef] [PubMed]
- Lu, Y.; Dong, H.; Gao, Y.; Gong, Y.; Ren, Y.; Gu, N.; Zhou, S.; Xia, N.; Sun, Y.Y.; Ji, R.R.; et al. A feed-forward spinal cord glycinergic neural circuit gates mechanical allodynia. J. Clin. Investig. 2013, 123, 4050–4062. [Google Scholar] [CrossRef] [PubMed]
- Punnakkal, P.; von Schoultz, C.; Haenraets, K.; Wildner, H.; Zeilhofer, H.U. Morphological, biophysical and synaptic properties of glutamatergic neurons of the mouse spinal dorsal horn. J. Physiol. 2014, 592, 759–776. [Google Scholar] [CrossRef]
- Takazawa, T.; Choudhury, P.; Tong, C.K.; Conway, C.M.; Scherrer, G.; Flood, P.D.; Mukai, J.; MacDermott, A.B. Inhibition Mediated by Glycinergic and GABAergic Receptors on Excitatory Neurons in Mouse Superficial Dorsal Horn Is Location-Specific but Modified by Inflammation. J. Neurosci. 2017, 37, 2336–2348. [Google Scholar] [CrossRef]
- Aubrey, K.R.; Supplisson, S. Heterogeneous Signaling at GABA and Glycine Co- releasing Terminals. Front. Synaptic Neurosci. 2018, 10, 40. [Google Scholar] [CrossRef]
- El Khoueiry, C.; Alba-Delgado, C.; Antri, M.; Gutierrez-Mecinas, M.; Todd, A.J.; Artola, A.; Dallel, R. GABAA and Glycine Receptor-Mediated Inhibitory Synaptic Transmission onto Adult Rat Lamina IIi PKCγ-Interneurons: Pharmacological but Not Anatomical Specialization. Cells 2022, 11, 1356. [Google Scholar] [CrossRef] [PubMed]
- Zeilhofer, H.U. The glycinergic control of spinal pain processing. Cell Mol. Life Sci. 2005, 62, 2027–2035. [Google Scholar] [CrossRef]
- Torsney, C.; MacDermott, A.B. Disinhibition opens the gate to pathological pain signaling in superficial neurokinin 1 receptor-expressing neurons in rat spinal cord. J. Neurosci. 2006, 26, 1833–1843. [Google Scholar] [CrossRef]
- Miraucourt, L.S.; Dallel, R.; Voisin, D.L. Glycine inhibitory dysfunction turns touch into pain through PKCgamma interneurons. PLoS ONE 2007, 2, e1116. [Google Scholar] [CrossRef]
- Miraucourt, L.S.; Moisset, X.; Dallel, R.; Voisin, D.L. Glycine inhibitory dysfunction induces a selectively dynamic, morphine-resistant, and neurokinin 1 receptor- independent mechanical allodynia. J. Neurosci. 2009, 29, 2519–2527. [Google Scholar] [CrossRef]
- Zeilhofer, H.U.; Benke, D.; Yevenes, G.E. Chronic pain states: Pharmacological strategies to restore diminished inhibitory spinal pain control. Annu. Rev. Pharmacol. Toxicol. 2012, 52, 111–133. [Google Scholar] [CrossRef] [PubMed]
- Peirs, C.; Seal, R.P. Neural circuits for pain: Recent advances and current views. Science 2016, 354, 578–584. [Google Scholar] [CrossRef] [PubMed]
- Inquimbert, P.; Rodeau, J.L.; Schlichter, R. Differential contribution of GABAergic and glycinergic components to inhibitory synaptic transmission in lamina II and laminae III–IV of the young rat spinal cord. Eur. J. Neurosci. 2007, 26, 2940–2949. [Google Scholar] [CrossRef]
- Mitchell, E.A.; Gentet, L.J.; Dempster, J.; Belelli, D. GABAA and glycine receptor- mediated transmission in rat lamina II neurones: Relevance to the analgesic actions of neuroactive steroids. J. Physiol. 2007, 583 Pt 3, 1021–1040. [Google Scholar] [CrossRef]
- Anderson, W.B.; Graham, B.A.; Beveridge, N.J.; Tooney, P.A.; Brichta, A.M.; Callister, R.J. Different forms of glycine- and GABA(A)-receptor mediated inhibitory synaptic transmission in mouse superficial and deep dorsal horn neurons. Mol. Pain 2009, 5, 65. [Google Scholar] [CrossRef] [PubMed]
- Miranda, C.O.; Hegedüs, K.; Wildner, H.; Zeilhofer, H.U.; Antal, M. Morphological and neurochemical characterization of glycinergic neurons in laminae I–IV of the mouse spinal dorsal horn. J. Comp. Neurol. 2022, 530, 607–626. [Google Scholar] [CrossRef] [PubMed]
- Antal, M.; Petkó, M.; Polgár, E.; Heizmann, C.W.; Storm-Mathisen, J. Direct evidence of an extensive GABAergic innervation of the spinal dorsal horn by fibres descending from the rostral ventromedial medulla. Neuroscience 1996, 73, 509–518. [Google Scholar] [CrossRef] [PubMed]
- Hossaini, M.; Goos, J.A.; Kohli, S.K.; Holstege, J.C. Distribution of glycine/GABA neurons in the ventromedial medulla with descending spinal projections and evidence for an ascending glycine/GABA projection. PLoS ONE 2012, 7, e35293. [Google Scholar] [CrossRef]
- Spike, R.C.; Watt, C.; Zafra, F.; Todd, A.J. An ultrastructural study of the glycine transporter GLYT2 and its association with glycine in the superficial laminae of the rat spinal dorsal horn. Neuroscience 1997, 77, 543–551. [Google Scholar] [CrossRef]
- Núñez, E.; Pérez-Siles, G.; Rodenstein, L.; Alonso-Torres, P.; Zafra, F.; Jiménez, E.; Aragón, C.; López-Corcuera, B. Subcellular localization of the neuronal glycine transporter GLYT2 in brainstem. Traffic 2009, 10, 829–843. [Google Scholar] [CrossRef]
- Ribeiro-da-Silva, A.; Coimbra, A. Two types of synaptic glomeruli and their distribution in laminae I–III of the rat spinal cord. J. Comp. Neurol. 1982, 209, 176–186. [Google Scholar] [CrossRef] [PubMed]
- Ribeiro-da-Silva, A.; Tagari, P.; Cuello, A.C. Morphological characterization of substance P-like immunoreactive glomeruli in the superficial dorsal horn of the rat spinal cord and trigeminal subnucleus caudalis: A quantitative study. J. Comp. Neurol. 1989, 281, 415–497. [Google Scholar] [CrossRef]
- Antal, M.; Freund, T.F.; Polgár, E. Calcium-binding proteins, parvalbumin- and calbindin-D 28k-immunoreactive neurons in the rat spinal cord and dorsal root ganglia: A light and electron microscopic study. J. Comp. Neurol. 1990, 295, 467–484. [Google Scholar] [CrossRef]
- Todd, A.J. Neuronal circuitry for pain processing in the dorsal horn. Nat. Rev. Neurosci. 2010, 11, 823–836. [Google Scholar] [CrossRef] [PubMed]
- Todd, A.J. Identifying functional populations among the interneurons in laminae I–III of the spinal dorsal horn. Mol. Pain 2017, 13, 1744806917693003. [Google Scholar] [CrossRef]
- Peirs, C.; Williams, S.P.; Zhao, X.; Wlash, C.E.; Gedeon, J.Y.; Cagle, N.E.; Goldring, A.C.; Hioki, H.; Liu, Z.; Marell, P.S.; et al. Dorsal Horn Circuits for Persistent Mechanical Pain. Neuron 2015, 87, 797–812. [Google Scholar] [CrossRef] [PubMed]
- Abraira, V.E.; Kuehn, E.D.; Chirila, A.M.; Springel, M.W.; Toliver, A.A.; Zimmerman, A.L.; Orefice, L.L.; Boyle, K.A.; Bai, L.; Song, B.J.; et al. The Cellular and Synaptic Architecture of the Mechanosensory Dorsal Horn. Cell 2017, 168, 295–310.e219. [Google Scholar] [CrossRef]
- Boyle, K.A.; Gutierrez-Mecinas, M.; Polgár, E.; Mooney, N.; O’Connor, E.; Furuta, T.; Watanabe, M.; Todd, A.J. A quantitative study of neurochemically defined populations of inhibitory interneurons in the superficial dorsal horn of the mouse spinal cord. Neuroscience 2017, 363, 120–133. [Google Scholar] [CrossRef]
- Smith, K.M.; Browne, T.J.; Davis, O.C.; Coyle, A.; Boyle, K.A.; Watanabe, M.; Dickinson, S.A.; Iredale, J.A.; Gradwell, M.A.; Jobling, P.; et al. Calretinin positive neurons form an excitatory amplifier network in the spinal cord dorsal horn. eLife 2019, 8, e49190. [Google Scholar] [CrossRef]
- Hughes, D.I.; Todd, A.J. Central Nervous System Targets: Inhibitory Interneurons in the Spinal Cord. Neurotherapeutics 2020, 17, 874–885. [Google Scholar] [CrossRef]
- Smith, K.M.; Boyle, K.A.; Madden, J.F.; Dickinson, S.A.; Jobling, P.; Callister, R.J.; Hughes, D.I.; Graham, B.A. Functional heterogeneity of calretinin-expressing neurons in the mouse superficial dorsal horn: Implications for spinal pain processing. J. Physiol. 2015, 593, 4319–4339. [Google Scholar] [CrossRef]
- Gutierrez-Mecinas, M.; Davis, O.; Polgár, E.; Shahzad, M.; Navarro-Batista, K.; Furuta, T.; Watanabe, M.; Hughes, D.I.; Todd, A.J. Expression of Calretinin Among Different Neurochemical Classes of Interneuron in the Superficial Dorsal Horn of the Mouse Spinal Cord. Neuroscience 2019, 398, 171–181. [Google Scholar] [CrossRef] [PubMed]
- Gradwell, M.A.; Boyle, K.A.; Browne, T.J.; Bell, A.M.; Leonardo, J.; Peralta Reyes, F.S.; Dickie, A.C.; Smith, K.M.; Callister, R.J.; Dayas, C.V.; et al. Diversity of inhibitory and excitatory parvalbumin interneuron circuits in the dorsal horn. Pain 2022, 163, e432–e452. [Google Scholar] [CrossRef] [PubMed]
- Alaynick, W.A.; Jessell, T.M.; Pfaff, S.L. SnapShot: Spinal cord development. Cell 2011, 146, 178. [Google Scholar] [CrossRef]
- Balázs, A.; Mészár, Z.; Hegedűs, K.; Kenyeres, A.; Hegyi, Z.; Dócs, K.; Antal, M. Development of putative inhibitory neurons in the embryonic and postnatal mouse superficial spinal dorsal horn. Brain Struct. Funct. 2017, 222, 2157–2171. [Google Scholar] [CrossRef] [PubMed]
- Larsson, M. Pax2 is persistently expressed by GABAergic neurons throughout the adult rat dorsal horn. Neurosci. Lett. 2017, 638, 96–101. [Google Scholar] [CrossRef]
- Zeilhofer, H.U.; Studler, B.; Arabadzisz, D.; Schweizer, C.; Ahmadi, S.; Layh, B.; Bösl, M.R.; Fritschy, J.M. Glycinergic neurons expressing enhanced green fluorescent protein in bacterial artificial chromosome transgenic mice. J. Comp. Neurol. 2005, 482, 123–141. [Google Scholar] [CrossRef] [PubMed]
- Simmons, D.R.; Spike, R.C.; Todd, A.J. Galanin is contained in GABAergic neurons in the rat spinal dorsal horn. Neurosci. Lett. 1995, 187, 119–122. [Google Scholar] [CrossRef]
- Polgár, E.; Fowler, J.H.; McGill, M.M.; Todd, A.J. The types of neuron which contain protein kinase C gamma in rat spinal cord. Brain Res. 1999, 833, 71–80. [Google Scholar] [CrossRef]
- Hantman, A.W.; Perl, E.R. Molecular and genetic features of a labeled class of spinal substantia gelatinosa neurons in a transgenic mouse. J. Comp. Neurol. 2005, 492, 90–100. [Google Scholar] [CrossRef] [PubMed]
- Sardella, T.C.; Polgár, E.; Watanabe, M.; Todd, A.J. A quantitative study of neuronal nitric oxide synthase expression in laminae I–III of the rat spinal dorsal horn. Neuroscience 2011, 192, 708–720. [Google Scholar] [CrossRef] [PubMed]
- Tiong, S.Y.; Polgár, E.; van Kralingen, J.C.; Watanabe, M.; Todd, A.J. Galanin- immunoreactivity identifies a distinct population of inhibitory interneurons in laminae I- III of the rat spinal cord. Mol. Pain 2011, 7, 36. [Google Scholar] [CrossRef]
- Smith, K.M.; Boyle, K.A.; Mustapa, M.; Jobling, P.; Callister, R.J.; Hughes, D.I.; Graham, B.A. Distinct forms of synaptic inhibition and neuromodulation regulate calretinin-positive neuron excitability in the spinal cord dorsal horn. Neuroscience 2016, 326, 10–21. [Google Scholar] [CrossRef]
- Duan, B.; Cheng, L.; Bourane, S.; Britz, O.; Padilla, C.; Garcia-Campmany, L.; Krashes, M.; Knowlton, W.; Velasquez, T.; Ren, X.; et al. Identification of spinal circuits transmitting and gating mechanical pain. Cell 2014, 159, 1417–1432. [Google Scholar] [CrossRef]
- Li, J.L.; Fujiyama, F.; Kaneko, T.; Mizuno, N. Expression of vesicular glutamate transporters, VGluT1 and VGluT2, in axon terminals of nociceptive primary afferent fibers in the superficial layers of the medullary and spinal dorsal horns of the rat. J. Comp. Neurol. 2003, 457, 236–249. [Google Scholar] [CrossRef] [PubMed]
- Oliveira, A.L.; Hydling, F.; Olsson, E.; Shi, T.; Edwards, R.H.; Fujiyama, F.; Kaneko, T.; Hökfelt, T.; Cullheim, S.; Meister, B. Cellular localization of three vesicular glutamate transporter mRNAs and proteins in rat spinal cord and dorsal root ganglia. Synapse 2003, 50, 117–129. [Google Scholar] [CrossRef]
- Todd, A.J.; Hughes, D.I.; Polgár, E.; Nagy, G.G.; Mackie, M.; Ottersen, O.P.; Maxwell, D.J. The expression of vesicular glutamate transporters VGLUT1 and VGLUT2 in neurochemically defined axonal populations in the rat spinal cord with emohasis ont he dorsal horn. Eur. J. Neurosci. 2003, 17, 13–27. [Google Scholar] [CrossRef] [PubMed]
- Willis, W.D.; Coggeshall, R.E. Primaty afferent neurons and the spinal dorsal horn. In Sensory Mechanisms of the Spinal Cord; Kluwer Academic/Plenum: New York, NY, USA, 2004; Volume 1. [Google Scholar]
- Ribeiro-de-Silva, A.; De Korninck, Y. Morphological and neurochemical organization of the spinal dorsal horn. In Science of Pain; Basbaum, A.I., Bushnell, M.C., Eds.; Elsevier: Amsterdam, The Netherlands, 2009; pp. 279–310. [Google Scholar]
- Todd, A.J.; Koerber, H.R. Neuroanatomical substrates of spinal nociception. In Wall and Melzack’s Textbook of Pain; McMahon, S.B., Koltzenburg, M., Tracy, I., Turk, D.C., Eds.; Elsevier: Amsterdam, The Netherlands, 2013; pp. 77–93. [Google Scholar]
- Neumann, S.; Braz, J.M.; Skinner, K.; Llewellyn-Smith, I.J.; Basbaum, A.I. Innocuous, not noxious, input activates PKCgamma interneurons of the spinal dorsal horn via myelinated afferent fibers. J. Neurosci. 2008, 28, 7936–7944. [Google Scholar] [CrossRef]
- Peirs, C.; Patil, S.; Bouali-Benazzouz, R.; Artola, A.; Landry, M.; Dallel, R. Protein kinase C gamma interneurons in the rat medullary dorsal horn: Distribution and synaptic inputs to these neurons, and subcellular localization of the enzyme. J. Comp. Neurol. 2014, 522, 393–413. [Google Scholar] [CrossRef] [PubMed]
- Peirs, C.; Dallel, R.; Todd, A.J. Recent advances in our understanding of the organization of dorsal horn neuron populations and their contribution to cutaneous mechanical allodynia. J. Neural Transm. 2020, 127, 505–525. [Google Scholar] [CrossRef]
- Peirs, C.; Williams, S.G.; Zhao, X.; Arokiaraj, C.M.; Ferreira, D.W.; Noh, M.C.; Smith, K.M.; Halder, P.; Corrigan, K.A.; Gedeon, J.Y.; et al. Mechanical Allodynia Circuitry in the Dorsal Horn Is Defined by the Nature of the Injury. Neuron 2021, 109, 73–90.e77. [Google Scholar] [CrossRef] [PubMed]
- Artola, A.; Voisin, D.; Dallel, R. PKCγ interneurons, a gateway to pathological pain in the dorsal horn. J. Neural Transm. 2020, 127, 527–540. [Google Scholar] [CrossRef] [PubMed]
- Mori, M.; Kose, A.; Tsujino, T.; Tanaka, C. Immunocytochemical localization of protein kinase C subspecies in the rat spinal cord: Light and electron microscopic study. J. Comp. Neurol. 1990, 299, 167–177. [Google Scholar] [CrossRef] [PubMed]
- Malmberg, A.B.; Chen, C.; Tonegawa, S.; Basbaum, A.I. Preserved acute pain and reduced neuropathic pain in mice lacking PKCgamma. Science 1997, 278, 279–283. [Google Scholar] [CrossRef] [PubMed]
- Gobel, S. Golgi studies of the neurons in layer II of the dorsal horn of the medulla (trigeminal nucleus caudalis). J. Comp. Neurol. 1978, 180, 395–413. [Google Scholar] [CrossRef] [PubMed]
- Grudt, T.J.; Perl, E.R. Correlations between neuronal morphology and electrophysiological features in the rodent superficial dorsal horn. J. Physiol. 2002, 540 Pt 1, 189–207. [Google Scholar] [CrossRef]
- Liu, P.; Zhang, X.; He, X.; Jiang, Z.; Wang, Q.; Lu, Y. Spinal GABAergic neurons are under feed-forward inhibitory control driven by A. Mol. Pain 2021, 17, 1744806921992620. [Google Scholar] [CrossRef]
- Gomeza, J.; Ohno, K.; Hülsmann, S.; Armsen, W.; Eulenburg, V.; Richter, D.W.; Laube, B.; Betz, H. Deletion of the mouse glycine transporter 2 results in a hyperekplexia phenotype and postnatal lethality. Neuron 2003, 40, 797–806. [Google Scholar] [CrossRef]
- Rousseau, F.; Aubrey, K.R.; Supplisson, S. The glycine transporter GlyT2 controls the dynamics of synaptic vesicle refilling in inhibitory spinal cord neurons. J. Neurosci. 2008, 28, 9755–9768. [Google Scholar] [CrossRef]
- Lynch, J.W. Molecular structure and function of the glycine receptor chloride channel. Physiol. Rev. 2004, 84, 1051–1095. [Google Scholar] [CrossRef]
- Callister, R.J.; Graham, B.A. Early history of glycine receptor biology in Mammalian spinal cord circuits. Front. Mol. Neurosci. 2010, 3, 13. [Google Scholar] [CrossRef]
- Fink, A.J.; Croce, K.R.; Huang, Z.J.; Abbott, L.F.; Jessell, T.M.; Azim, E. Presynaptic inhibition of spinal sensory feedback ensures smooth movement. Nature 2014, 509, 43–48. [Google Scholar] [CrossRef] [PubMed]
- Todd, A.J. An electron microscope study of glycine-like immunoreactivity in laminae I–III of the spinal dorsal horn of the rat. Neuroscience 1990, 39, 387–394. [Google Scholar] [CrossRef] [PubMed]
- Hughes, D.I.; Sikander, S.; Kinnon, C.M.; Boyle, K.A.; Watanabe, M.; Callister, R.J.; Graham, B.A. Morphological, neurochemical and electrophysiological features of parvalbumin-expressing cells: A likely source of axo-axonic inputs in the mouse spinal dorsal horn. J. Physiol. 2012, 590, 3927–3951. [Google Scholar] [CrossRef] [PubMed]
- Petitjean, H.; Pawlowski, S.A.; Fraine, S.L.; Sharif, B.; Hamad, D.; Fatima, T.; Berg, J.; Brown, C.M.; Jan, L.Y.; Ribeiro-da-Silva, A.; et al. Dorsal Horn Parvalbumin Neurons Are Gate-Keepers of Touch-Evoked Pain after Nerve Injury. Cell Rep. 2015, 13, 1246–1257. [Google Scholar] [CrossRef]
- Imlach, W.L.; Bhola, R.F.; Mohammadi, S.A.; Christie, M.J. Glycinergic dysfunction in a subpopulation of dorsal horn interneurons in a rat model of neuropathic pain. Sci. Rep. 2016, 6, 37104. [Google Scholar] [CrossRef]
- Gradwell, M.A.; Boyle, K.A.; Callister, R.J.; Hughes, D.I.; Graham, B.A. Heteromeric α/β glycine receptors regulate excitability in parvalbumin-expressing dorsal horn neurons through phasic and tonic glycinergic inhibition. J. Physiol. 2017, 595, 7185–7202. [Google Scholar] [CrossRef]
- Zheng, Y.; Liu, P.; Bai, L.; Trimmer, J.S.; Bean, B.P.; Ginty, D.D. Deep Sequencing of Somatosensory Neurons Reveals Molecular Determinants of Intrinsic Physiological Properties. Neuron 2019, 103, 598–616.e597. [Google Scholar] [CrossRef]
- Kalló, I.; Butler, J.A.; Barkovics-Kalló, M.; Goubillon, M.L.; Coen, C.W. Oestrogen receptor beta-immunoreactivity in gonadotropin releasing hormone-expressing neurones: Regulation by oestrogen. J. Neuroendocrinol. 2001, 13, 741–748. [Google Scholar] [CrossRef]
- Bardóczi, Z.; Pál, B.; Kőszeghy, Á.; Wilheim, T.; Watanabe, M.; Záborszky, L.; Liposits, Z.; Kalló, I. Glycinergic Input to the Mouse Basal Forebrain Cholinergic Neurons. J. Neurosci. 2017, 37, 9534–9549. [Google Scholar] [CrossRef] [PubMed]
- McClung, J.R. Rexed’s laminae as it applies to the rat cervical spinal cord. Exp. Neurol. 1978, 58, 145–148. [Google Scholar] [CrossRef] [PubMed]
- Molander, C.; Xu, Q.; Grant, G. The cytoarchitectonic organization of the spinal cord in the rat. I. The lower throcacic and lumbosacral cord. J. Comp. Neurol. 1984, 230, 133–141. [Google Scholar] [CrossRef] [PubMed]
- McNeill, D.L.; Chung, K.; Hulsebosch, C.E.; Bolender, R.P.; Coggeshall, R.E. Numbers of synapses in laminae I–IV of the rat dorsal horn. J. Comp. Neurol. 1988, 278, 453–460. [Google Scholar] [CrossRef] [PubMed]
- Sengul, G.; Watson, C.; Tanaka, I.; Paxinos, G. Atlas of the Spinal Cord of the Rat, Mouse, Marmoset, Rhesus, and Human; Elsevier: Amsterdam, The Netherlands, 2013. [Google Scholar]


| 1 | 2 | 3 | 4 | 5 | 6 | 7 | 8 | |
|---|---|---|---|---|---|---|---|---|
| tdTomato+ GlyT2+ GAD+ | tdTomato+ GlyT2+ GAD− | tdTomato+ GlyT2− GAD+ | Ʃ1–3 | tdTomato+ GlyT2− GAD− | Ʃ4–5 | tdTomato− GlyT2+ and/or GAD+ | Ʃ6–7 | |
| Laminae I–III | 261 | 0 | 128 | 389 | 19 | 408 | 9 | 417 |
| Lamina IV | 589 | 24 | 43 | 656 | 9 | 665 | 2 | 667 |
| Marker | CaB | PKCγ | CR | GAL | nNOS | PV | ||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| No. | % | No. | % | No. | % | No. | % | No. | % | No. | % | |
| marker+/tdTomato+ | 11 | 3.1 | 32 | 13.2 | 62 | 17.9 | 19 | 90.5 | 36 | 58.1 | 87 | 45.3 |
| marker+/tdTomato− | 345 | 96.9 | 210 | 86.8 | 284 | 82.1 | 2 | 9.5 | 26 | 41.9 | 105 | 54.7 |
| total | 356 | 100 | 242 | 100 | 346 | 100 | 21 | 100 | 62 | 100 | 192 | 11 |
| Target | Host Species | Dilution | Catalog # | Company | RRID |
|---|---|---|---|---|---|
| GlyT2 | Guinea pig | 1:40,000 | 272-004 | Synaptic Systems | AB_2619998 |
| Calbindin | Rabbit | 1:10,000 | CB38 | SWANT | AB_10000340 |
| Calretinin | Rabbit | 1:5000 | 7697 | SWANT | AB_2721226 |
| CGRP | Rabbit | 1:3000 | T-4239 | Peninsula | AB_518150 |
| Galanin | Rabbit | 1:10,000 | T-4334 | Peninsula | AB_518348 |
| nNOS | Rabbit | 1:4000 | AB76067 | Abcam | AB_2152469 |
| Parvalbumin | Rabbit | 1:60,000 | PV27 | SWANT | AB_2631173 |
| PAX2 | Rabbit | 1:200 | 71-6000 | ThermoFisher Scientific | AB_2533990 |
| PKC-γ | Rabbit | 1:2000 | AB71558 | Abcam | AB_1281066 |
| VGLUT1 | Rabbit | 1:2000 | AB227805 | Abcam | AB_2868428 |
| VGLUT1 | Goat | 1:5000 | 135 307 | Synaptic Systems | AB_2619821 |
| VGLUT2 | Rabbit | 1:1000 | AB216463 | Abcam | AB_2893024 |
| b-IB4 | - | 1:2000 | I21414 | ThermoFisher Scientific |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Miranda, C.O.; Hegedüs, K.; Kis, G.; Antal, M. Synaptic Targets of Glycinergic Neurons in Laminae I–III of the Spinal Dorsal Horn. Int. J. Mol. Sci. 2023, 24, 6943. https://doi.org/10.3390/ijms24086943
Miranda CO, Hegedüs K, Kis G, Antal M. Synaptic Targets of Glycinergic Neurons in Laminae I–III of the Spinal Dorsal Horn. International Journal of Molecular Sciences. 2023; 24(8):6943. https://doi.org/10.3390/ijms24086943
Chicago/Turabian StyleMiranda, Camila Oliveira, Krisztina Hegedüs, Gréta Kis, and Miklós Antal. 2023. "Synaptic Targets of Glycinergic Neurons in Laminae I–III of the Spinal Dorsal Horn" International Journal of Molecular Sciences 24, no. 8: 6943. https://doi.org/10.3390/ijms24086943
APA StyleMiranda, C. O., Hegedüs, K., Kis, G., & Antal, M. (2023). Synaptic Targets of Glycinergic Neurons in Laminae I–III of the Spinal Dorsal Horn. International Journal of Molecular Sciences, 24(8), 6943. https://doi.org/10.3390/ijms24086943






