Screening of the Anti-Neurodegenerative Activity of Caffeic Acid after Introduction into Inorganic Metal Delivery Systems to Increase Its Solubility as the Result of a Mechanosynthetic Approach
Abstract
1. Introduction
2. Results and Discussion
3. Materials and Methods
3.1. Materials
3.2. The Preparation of the CA Solid Dispersions
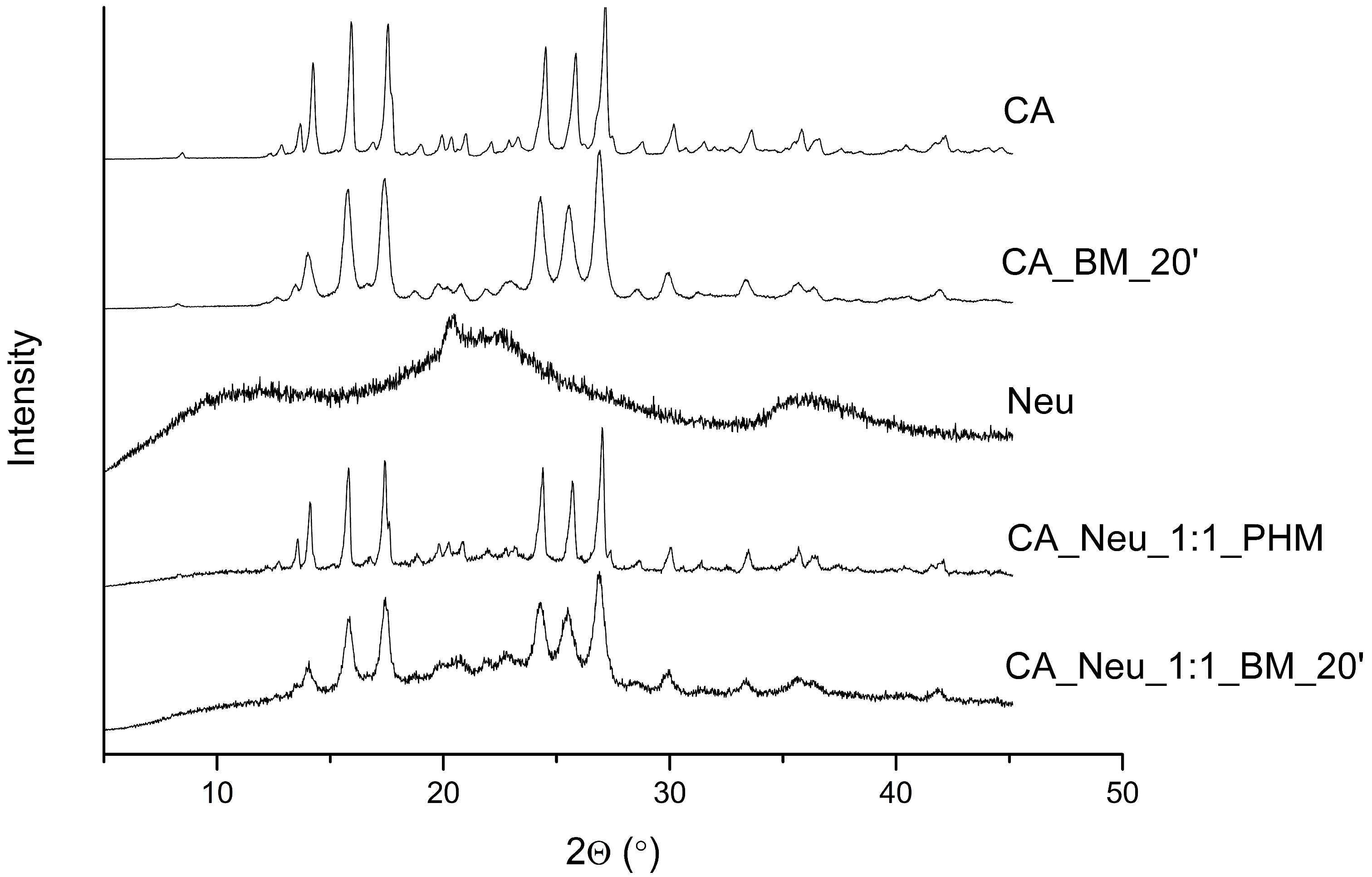
3.3. X-ray Powder Diffraction of the CA and CA Systems
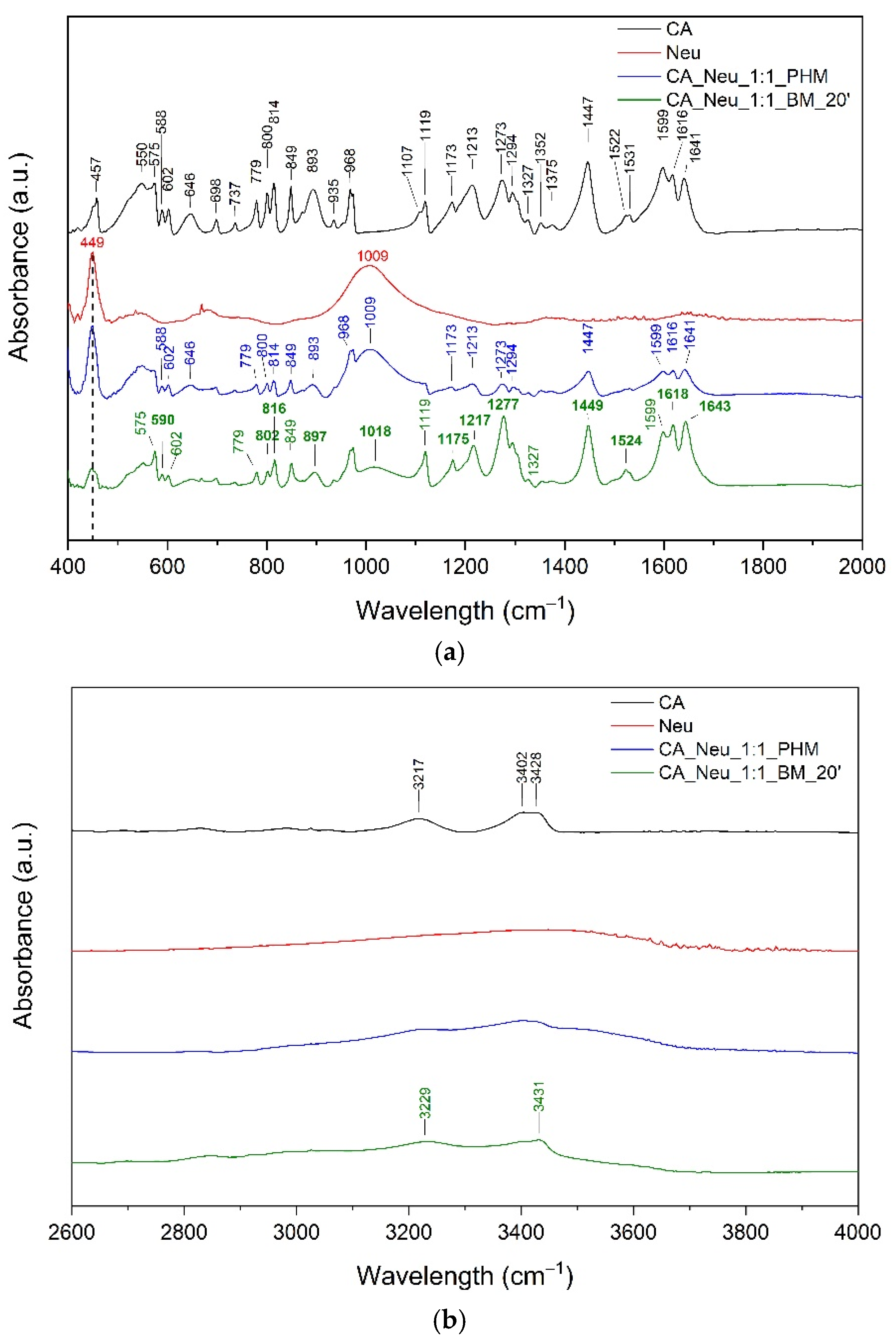
3.4. Fourier-Transform Infrared Spectroscopy of the CA and CA Systems
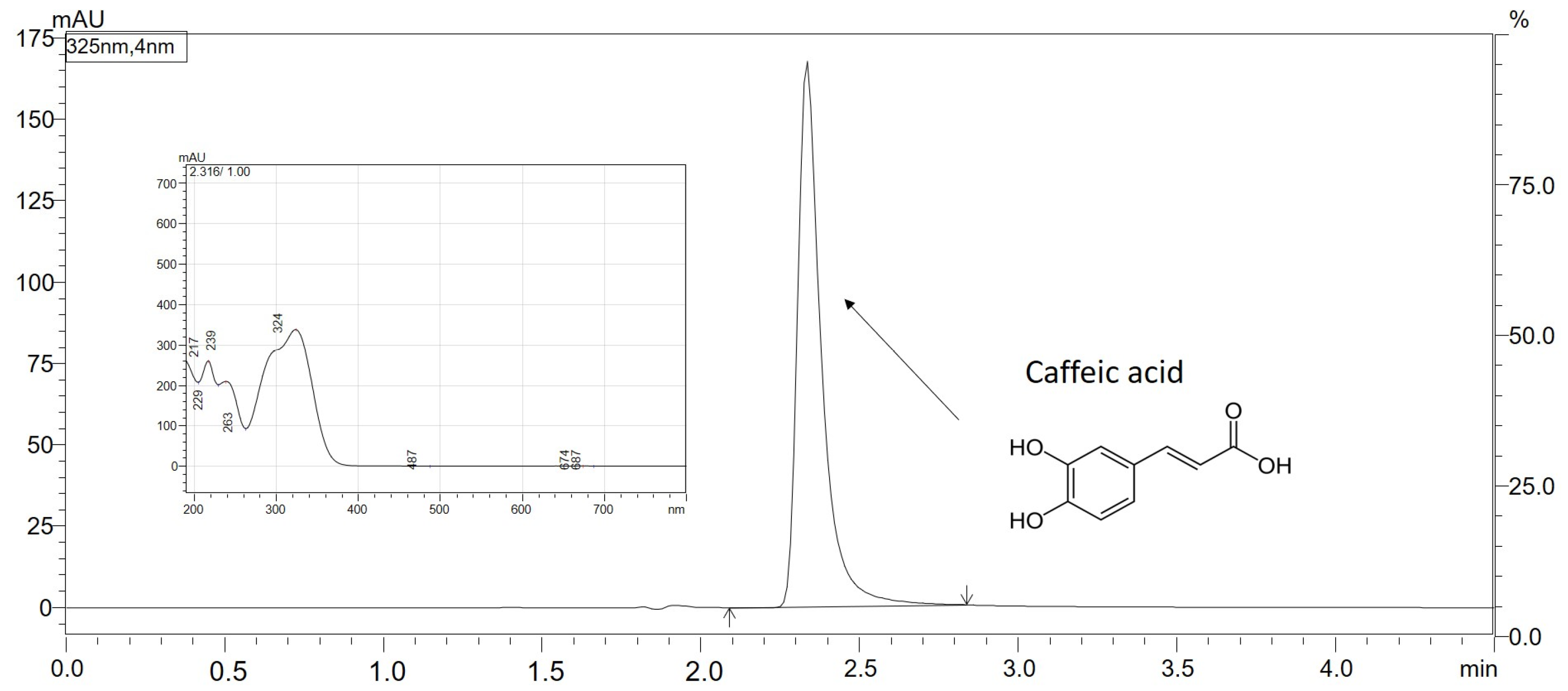
3.5. Chromatographic Conditions of the CA Separation
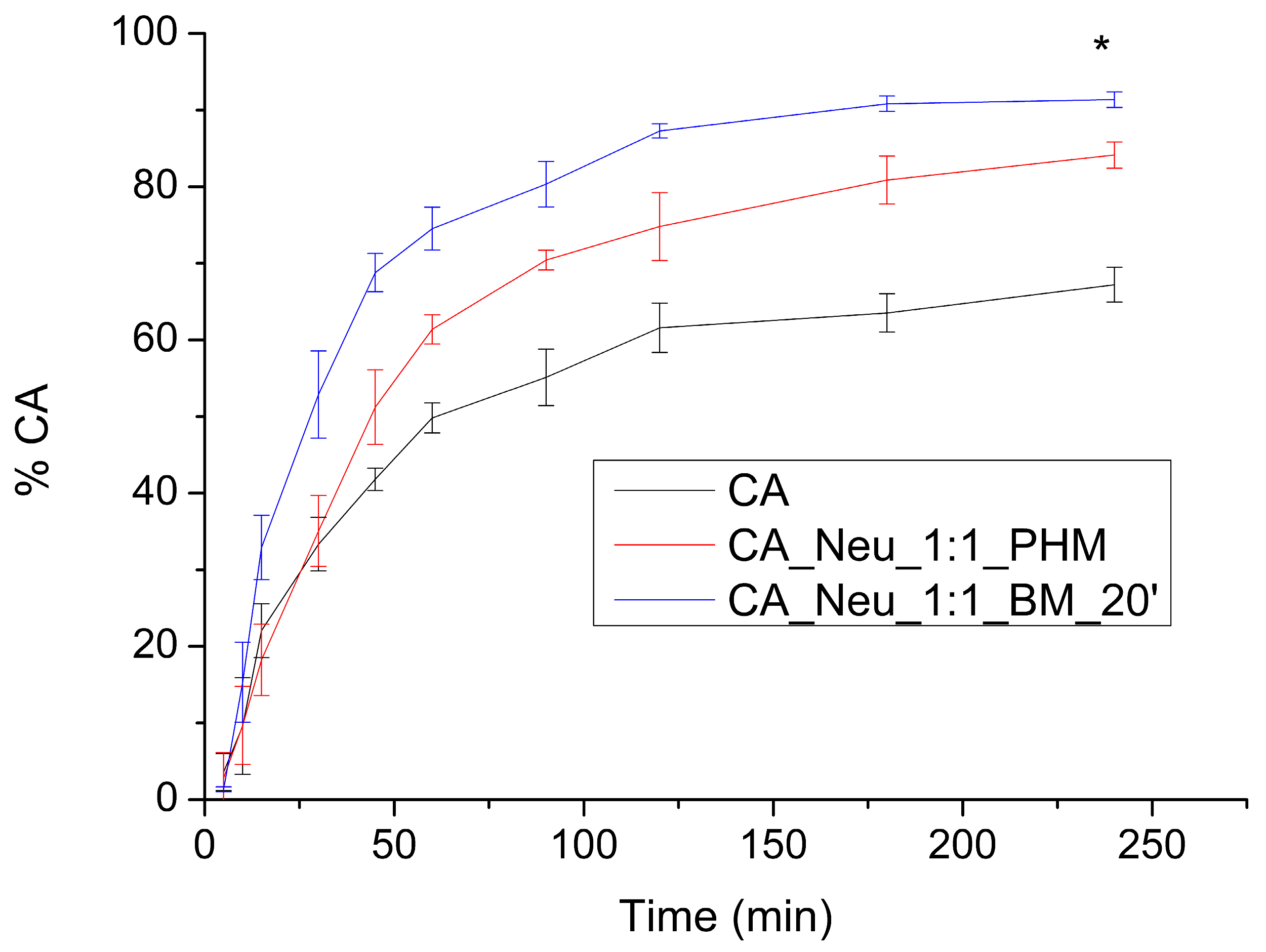
3.6. Solubility of CA in Pure Form and after Introduction into the Inorganic Metal Delivery System
3.7. The Apparent Solubility of CA from the Inorganic Metal Delivery System
3.8. Gastrointestinal and Blood-Brain Barrier Membranes Permeability of the CA from the Inorganic Metal Delivery System
3.9. Biological Activity of the CA from the Inorganic Metal Delivery System
3.9.1. Antioxidant Activity
3.9.2. Determination of Enzymes Influencing the Development of Neurodegenerative Diseases Inhibition
3.9.3. Molecular Docking Study
3.10. Statistical Analysis
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| ABTS | 2,2′-azino-bis(3-ethylbenzothiazoline-6-sulfonic acid) |
| AChE | Acetylcholinesterase |
| ANOVA | one-way analysis of variance |
| ATCI | acetylthiocholine iodide |
| ATR-FTIR | Attenuated Total Reflectance Fourier Transform Infrared spectroscopy |
| BBB | blood-brain barrier |
| BChE | Butyrylcholinesterase |
| BM | ball milling |
| BTCI | butyrylthiocholine iodide |
| CA | caffeic acid |
| CD | Cyclodextrin |
| CUPRAC | cupric reducing antioxidant capacity |
| DMSO | dimethyl sulfoxide |
| DPPH | 2,2-diphenyl-1-picrylhydrazyl |
| DTNB | 5,5′-dithio-bis-(2-nitrobenzoic) acid |
| EUD.L100 | eudragit L100 |
| FD | freeze-drying |
| FRAP | ferric reducing antioxidant power |
| GIT | gastrointestinal tract |
| GOH | gohsenol EG-05PW |
| HPLC | high-performance liquid chromatography |
| HP-β-CD | 2-hydroxypropyl-β-cyclodextrin |
| KOLL.VA64 | kollidon VA 64 |
| NEU | neusilin US2 |
| PAMPA | parallel artificial membrane permeability assay |
| PHM | physical mixture |
| RPM | rotations per minute |
| TNB | 3-carboxy-4-nitrothiolate |
| TPTZ | 2,4,6-tris(2-pyridyl)-1,3,5-triazine |
| XRPD | X-ray Powder Diffraction |
References
- Alam, M.; Ahmed, S.; Elasbali, A.M.; Adnan, M.; Alam, S.; Hassan, M.I.; Pasupuleti, V.R. Therapeutic Implications of Caffeic Acid in Cancer and Neurological Diseases. Front. Oncol. 2022, 12, 860508. [Google Scholar] [CrossRef] [PubMed]
- Muhammad Abdul Kadar, N.N.; Ahmad, F.; Teoh, S.L.; Yahaya, M.F. Caffeic Acid on Metabolic Syndrome: A Review. Molecules 2021, 26, 5490. [Google Scholar] [CrossRef] [PubMed]
- Khan, F.A.; Maalik, A.; Murtaza, G. Inhibitory Mechanism against Oxidative Stress of Caffeic Acid. J. Food Drug Anal. 2016, 24, 695–702. [Google Scholar] [CrossRef]
- Kilani-Jaziri, S.; Mokdad-Bzeouich, I.; Krifa, M.; Nasr, N.; Ghedira, K.; Chekir-Ghedira, L. Immunomodulatory and Cellular Anti-Oxidant Activities of Caffeic, Ferulic, and p-Coumaric Phenolic Acids: A Structure-Activity Relationship Study. Drug Chem. Toxicol. 2017, 40, 416–424. [Google Scholar] [CrossRef] [PubMed]
- Khan, F.; Bamunuarachchi, N.I.; Tabassum, N.; Kim, Y.-M. Caffeic Acid and Its Derivatives: Antimicrobial Drugs toward Microbial Pathogens. J. Agric. Food Chem. 2021, 69, 2979–3004. [Google Scholar] [CrossRef]
- Colonnello, A.; Aguilera-Portillo, G.; Rubio-López, L.C.; Robles-Bañuelos, B.; Rangel-López, E.; Cortez-Núñez, S.; Evaristo-Priego, Y.; Silva-Palacios, A.; Galván-Arzate, S.; García-Contreras, R.; et al. Comparing the Neuroprotective Effects of Caffeic Acid in Rat Cortical Slices and Caenorhabditis Elegans: Involvement of Nrf2 and SKN-1 Signaling Pathways. Neurotox. Res. 2020, 37, 326–337. [Google Scholar] [CrossRef]
- Takeda, H.; Tsuji, M.; Miyamoto, J.; Masuya, J.; Iimori, M.; Matsumiya, T. Caffeic Acid Produces Antidepressive-and/or Anxiolytic-like Effects through Indirect Modulation of the [Alpha] 1A-Adrenoceptor System in Mice. Neuroreport 2003, 14, 1067–1070. [Google Scholar] [CrossRef]
- Pelinson, L.P.; Assmann, C.E.; Palma, T.V.; da Cruz, I.B.M.; Pillat, M.M.; Mânica, A.; Stefanello, N.; Weis, G.C.C.; de Oliveira Alves, A.; de Andrade, C.M.; et al. Antiproliferative and Apoptotic Effects of Caffeic Acid on SK-Mel-28 Human Melanoma Cancer Cells. Mol. Biol. Rep. 2019, 46, 2085–2092. [Google Scholar] [CrossRef]
- Xu, W.; Luo, Q.; Wen, X.; Xiao, M.; Mei, Q. Antioxidant and Anti-Diabetic Effects of Caffeic Acid in a Rat Model of Diabetes. Trop. J. Pharm. Res. 2020, 19, 1227–1232. [Google Scholar] [CrossRef]
- Semis, H.S.; Gur, C.; Ileriturk, M.; Kaynar, O.; Kandemir, F.M. Investigation of the Anti-Inflammatory Effects of Caffeic Acid Phenethyl Ester in a Model of λ-Carrageenan–Induced Paw Edema in Rats. Hum. Exp. Toxicol. 2021, 40, S721–S738. [Google Scholar] [CrossRef]
- Agunloye, O.M.; Oboh, G. Modulatory Effect of Caffeic Acid on Cholinesterases Inhibitory Properties of Donepezil. J. Complement. Integr. Med. 2017, 15, 20170016. [Google Scholar] [CrossRef] [PubMed]
- Iwasaki, Y.; Manabe, R.; Kimoto, M.; Fukuda, M.; Mase, N.; Miyazawa, M.; Hosokawa, K.; Kamei, J. Copper-Induced Interactions of Caffeic Acid and Sinapic Acid to Generate New Compounds in Artificial Biological Fluid Conditions. Antioxidants 2022, 11, 1307. [Google Scholar] [CrossRef]
- Anwar, J.; Spanevello, R.M.; Thomé, G.; Stefanello, N.; Schmatz, R.; Gutierres, J.; Vieira, J.; Baldissarelli, J.; Carvalho, F.B.; da Rosa, M.M.; et al. Effects of Caffeic Acid on Behavioral Parameters and on the Activity of Acetylcholinesterase in Different Tissues from Adult Rats. Pharmacol. Biochem. Behav. 2012, 103, 386–394. [Google Scholar] [CrossRef] [PubMed]
- Dornlakorn, O.; Saenno, A.; Anosri, T.; Kaewngam, S.; Suwannakot, K. The Neuroprotective Effect of Caffeic Acid against L-Methionine Induced Memory Deficits in Adult Rats. Srinagarind Med. J. 2021, 36, 591–596. [Google Scholar]
- Raviteja, S. Protective Role of Caffeic Acid in Cognitive Dysfunction and Oxidative Stress Induced by Colchicine in Rats. Indian J. Pharm. Educ. Res. 2021, 55, s457–s467. [Google Scholar] [CrossRef]
- Shiozawa, R.; Inoue, Y.; Murata, I.; Kanamoto, I. Effect of Antioxidant Activity of Caffeic Acid with Cyclodextrins Using Ground Mixture Method. Asian J. Pharm. Sci. 2018, 13, 24–33. [Google Scholar] [CrossRef]
- Górnas, P.; Neunert, G.; Baczyński, K.; Polewski, K. Beta-Cyclodextrin Complexes with Chlorogenic and Caffeic Acids from Coffee Brew: Spectroscopic, Thermodynamic and Molecular Modelling Study. Food Chem. 2009, 114, 190–196. [Google Scholar] [CrossRef]
- Zhang, M.; Li, J.; Zhang, L.; Chao, J. Preparation and Spectral Investigation of Inclusion Complex of Caffeic Acid with Hydroxypropyl-Beta-Cyclodextrin. Spectrochim. Acta A Mol. Biomol. Spectrosc. 2009, 71, 1891–1895. [Google Scholar] [CrossRef]
- García-Padial, M.; Martínez-Ohárriz, M.C.; Navarro-Blasco, I.; Zornoza, A. The Role of Cyclodextrins in ORAC-Fluorescence Assays. Antioxidant Capacity of Tyrosol and Caffeic Acid with Hydroxypropyl-β-Cyclodextrin. J. Agric. Food Chem. 2013, 61, 12260–12264. [Google Scholar] [CrossRef]
- Nunes, C.; Maricato, É.; Cunha, Â.; Nunes, A.; da Silva, J.A.L.; Coimbra, M.A. Chitosan-Caffeic Acid-Genipin Films Presenting Enhanced Antioxidant Activity and Stability in Acidic Media. Carbohydr. Polym. 2013, 91, 236–243. [Google Scholar] [CrossRef]
- Kaczmarek-Szczepańska, B.; Sosik, A.; Małkowska, A.; Zasada, L.; Michalska-Sionkowska, M. Chitosan-Based Films Enriched by Caffeic Acid with Poly(Ethylene Glycol)—A Physicochemical and Antibacterial Properties Evaluation. Int. J. Biol. Macromol. 2021, 192, 728–735. [Google Scholar] [CrossRef] [PubMed]
- Hallan, S.S.; Sguizzato, M.; Drechsler, M.; Mariani, P.; Montesi, L.; Cortesi, R.; Björklund, S.; Ruzgas, T.; Esposito, E. The Potential of Caffeic Acid Lipid Nanoparticulate Systems for Skin Application: In Vitro Assays to Assess Delivery and Antioxidant Effect. Nanomaterials 2021, 11, 171. [Google Scholar] [CrossRef] [PubMed]
- Fathi, M.; Mirlohi, M.; Varshosaz, J.; Madani, G. Novel Caffeic Acid Nanocarrier: Production, Characterization, and Release Modeling. J. Nanomater. 2013, 2013, e434632. [Google Scholar] [CrossRef]
- Zeren, S.; Sahin, S.; Sumnu, G. Encapsulation of Caffeic Acid in Carob Bean Flour and Whey Protein-Based Nanofibers via Electrospinning. Foods 2022, 11, 1860. [Google Scholar] [CrossRef]
- Chuysinuan, P.; Pavasant, P.; Supaphol, P. Preparation and Characterization of Caffeic Acid-Grafted Electrospun Poly(L-Lactic Acid) Fiber Mats for Biomedical Applications. ACS Appl. Mater. Interfaces 2012, 4, 3031–3040. [Google Scholar] [CrossRef]
- Mangrulkar, S.; Shah, P.; Navnage, S.; Mazumdar, P.; Chaple, D. Phytophospholipid Complex of Caffeic Acid: Development, In Vitro Characterization, and In Vivo Investigation of Antihyperlipidemic and Hepatoprotective Action in Rats. AAPS PharmSciTech 2021, 22, 28. [Google Scholar] [CrossRef]
- Sguizzato, M.; Mariani, P.; Ferrara, F.; Drechsler, M.; Hallan, S.S.; Huang, N.; Simelière, F.; Khunti, N.; Cortesi, R.; Marchetti, N.; et al. Nanoparticulate Gels for Cutaneous Administration of Caffeic Acid. Nanomaterials 2020, 10, 961. [Google Scholar] [CrossRef]
- Kfoury, M.; Geagea, C.; Ruellan, S.; Greige-Gerges, H.; Fourmentin, S. Effect of Cyclodextrin and Cosolvent on the Solubility and Antioxidant Activity of Caffeic Acid. Food Chem. 2019, 278, 163–169. [Google Scholar] [CrossRef]
- Jha, D.K.; Shah, D.S.; Amin, P.D. Thermodynamic Aspects of the Preparation of Amorphous Solid Dispersions of Naringenin with Enhanced Dissolution Rate. Int. J. Pharm. 2020, 583, 119363. [Google Scholar] [CrossRef]
- Altaani, B.; Khanfar, M.; Abualsuod, O. Enhancement of the Release of Curcumin by the Freeze Drying Technique Using Inulin and Neusilin as Carriers. Int. J. Appl. Pharm. 2018, 10, 42–48. [Google Scholar] [CrossRef]
- Liu, J.; Wang, X.; Bai, R.; Zhang, N.; Kan, J.; Jin, C. Synthesis, Characterization, and Antioxidant Activity of Caffeic-Acid-Grafted Corn Starch. Starch-Stärke 2018, 70, 1700141. [Google Scholar] [CrossRef]
- Acharya, M.; Mishra, S.; Sahoo, R.N.; Mallick, S. Infrared Spectroscopy for Analysis of Co-Processed Ibuprofen and Magnesium Trisilicate at Milling and Freeze Drying. Acta Chim. Slov. 2017, 64, 45–54. [Google Scholar] [CrossRef]
- Kanjanabat, S.; Pongjanyakul, T. Preparation and Characterization of Nicotine-Magnesium Aluminum Silicate Complex-Loaded Sodium Alginate Matrix Tablets for Buccal Delivery. AAPS PharmSciTech 2011, 12, 683–692. [Google Scholar] [CrossRef] [PubMed]
- Dangre, P.V.; Tattu, A.D.; Borikar, S.P.; Surana, S.J.; Chalikwar, S.S. Development and Statistical Optimization of Alginate-Neusilin US2 Micro-Composite Beads to Elicit Gastric Stability and Sustained Action of Hesperidin. Int. J. Biol. Macromol. 2021, 171, 514–526. [Google Scholar] [CrossRef] [PubMed]
- Mallick, S.; Pattnaik, S.; Swain, K.; De, P.K.; Saha, A.; Ghoshal, G.; Mondal, A. Formation of Physically Stable Amorphous Phase of Ibuprofen by Solid State Milling with Kaolin. Eur. J. Pharm. Biopharm. 2008, 68, 346–351. [Google Scholar] [CrossRef] [PubMed]
- Takahashi, K.; Uegaki, Y.; Hirai, N.; Nakase, T.T. Development of Controlled- Release Solid Dispersion Granules Containing a Poorly Water-Soluble Drug, Porous Calcium Silicate, and the Water-Soluble Polymer Polyvinylpyrrolidone. J. Pharm. Pharmacol. 2018, 6, 1–7. [Google Scholar]
- Doan, V.; Köppe, R.; Kasai, P.H. Dimerization of Carboxylic Acids and Salts: An IR Study in Perfluoropolyether Media. J. Am. Chem. Soc. 1997, 119, 9810–9815. [Google Scholar] [CrossRef]
- Krupa, A.; Majda, D.; Jachowicz, R.; Mozgawa, W. Solid-State Interaction of Ibuprofen and Neusilin US2. Thermochim. Acta 2010, 509, 12–17. [Google Scholar] [CrossRef]
- Kararli, T.T.; Needham, T.E.; Seul, C.J.; Finnegan, P.M. Solid-State Interaction of Magnesium Oxide and Ibuprofen to Form a Salt. Pharm Res 1989, 6, 804–808. [Google Scholar] [CrossRef]
- Gupta, M.K.; Vanwert, A.; Bogner, R.H. Formation of Physically Stable Amorphous Drugs by Milling with Neusilin. J. Pharm. Sci. 2003, 92, 536–551. [Google Scholar] [CrossRef]
- Vadher, A.H.; Parikh, J.R.; Parikh, R.H.; Solanki, A.B. Preparation and Characterization of Co-Grinded Mixtures of Aceclofenac and Neusilin US2 for Dissolution Enhancement of Aceclofenac. AAPS PharmSciTech 2009, 10, 606–614. [Google Scholar] [CrossRef] [PubMed]
- Vertuccio, L.; Guadagno, L.; D’Angelo, A.; Viola, V.; Raimondo, M.; Catauro, M. Sol-Gel Synthesis of Caffeic Acid Entrapped in Silica/Polyethylene Glycol Based Organic-Inorganic Hybrids: Drug Delivery and Biological Properties. Appl. Sci. 2023, 13, 2164. [Google Scholar] [CrossRef]
- Alam, M.A.; Subhan, N.; Hossain, H.; Hossain, M.; Reza, H.M.; Rahman, M.M.; Ullah, M.O. Hydroxycinnamic Acid Derivatives: A Potential Class of Natural Compounds for the Management of Lipid Metabolism and Obesity. Nutr. Metab. 2016, 13, 27. [Google Scholar] [CrossRef] [PubMed]
- Lafay, S.; Gil-Izquierdo, A. Bioavailability of Phenolic Acids. Phytochem. Rev. 2008, 7, 301–311. [Google Scholar] [CrossRef]
- Birková, A.; Hubková, B.; Bolerázska, B.; Mareková, M.; Čižmárová, B. Caffeic Acid: A Brief Overview of Its Presence, Metabolism, and Bioactivity. Bioact. Compd. Health Dis. 2020, 3, 74–81. [Google Scholar] [CrossRef]
- Wang, S.-J.; Zeng, J.; Yang, B.-K.; Zhong, Y.-M. Bioavailability of Caffeic Acid in Rats and Its Absorption Properties in the Caco-2 Cell Model. Pharm. Biol. 2014, 52, 1150–1157. [Google Scholar] [CrossRef]
- Jana, S.; Rastogi, H. Effects of Caffeic Acid and Quercetin on In Vitro Permeability, Metabolism and In Vivo Pharmacokinetics of Melatonin in Rats: Potential for Herb-Drug Interaction. Eur. J. Drug Metab. Pharm. 2017, 42, 781–791. [Google Scholar] [CrossRef]
- Prasadani, W.C.; Senanayake, C.M.; Jayathilaka, N.; Ekanayake, S.; Seneviratne, K.N. Effect of Three Edible Oils on the Intestinal Absorption of Caffeic Acid: An in Vivo and in Vitro Study. PLoS ONE 2017, 12, e0179292. [Google Scholar] [CrossRef]
- Grabska-Kobylecka, I.; Kaczmarek-Bak, J.; Figlus, M.; Prymont-Przyminska, A.; Zwolinska, A.; Sarniak, A.; Wlodarczyk, A.; Glabinski, A.; Nowak, D. The Presence of Caffeic Acid in Cerebrospinal Fluid: Evidence That Dietary Polyphenols Can Cross the Blood-Brain Barrier in Humans. Nutrients 2020, 12, 1531. [Google Scholar] [CrossRef]
- Castro Dias, M.; Mapunda, J.A.; Vladymyrov, M.; Engelhardt, B. Structure and Junctional Complexes of Endothelial, Epithelial and Glial Brain Barriers. Int. J. Mol. Sci. 2019, 20, 5372. [Google Scholar] [CrossRef]
- Hoosain, F.G.; Choonara, Y.E.; Tomar, L.K.; Kumar, P.; Tyagi, C.; du Toit, L.C.; Pillay, V. Bypassing P-Glycoprotein Drug Efflux Mechanisms: Possible Applications in Pharmacoresistant Schizophrenia Therapy. Biomed. Res. Int. 2015, 2015, 484963. [Google Scholar] [CrossRef]
- Bellettato, C.M.; Scarpa, M. Possible Strategies to Cross the Blood–Brain Barrier. Ital. J. Pediatr. 2018, 44, 131. [Google Scholar] [CrossRef] [PubMed]
- Hersh, D.S.; Wadajkar, A.S.; Roberts, N.; Perez, J.G.; Connolly, N.P.; Frenkel, V.; Winkles, J.A.; Woodworth, G.F.; Kim, A.J. Evolving Drug Delivery Strategies to Overcome the Blood Brain Barrier. Curr. Pharm. Des. 2016, 22, 1177–1193. [Google Scholar] [CrossRef]
- Teleanu, R.I.; Preda, M.D.; Niculescu, A.-G.; Vladâcenco, O.; Radu, C.I.; Grumezescu, A.M.; Teleanu, D.M. Current Strategies to Enhance Delivery of Drugs across the Blood–Brain Barrier. Pharmaceutics 2022, 14, 987. [Google Scholar] [CrossRef] [PubMed]
- Zeiadeh, I.; Najjar, A.; Karaman, R. Strategies for Enhancing the Permeation of CNS-Active Drugs through the Blood-Brain Barrier: A Review. Molecules 2018, 23, 1289. [Google Scholar] [CrossRef] [PubMed]
- Andrade, S.; Pereira, M.C.; Loureiro, J.A. Caffeic Acid Loaded into Engineered Lipid Nanoparticles for Alzheimer’s Disease Therapy. Colloids Surf. B Biointerfaces 2023, 225, 113270. [Google Scholar] [CrossRef]
- Akomolafe, S.F.; Akinyemi, A.J.; Ogunsuyi, O.B.; Oyeleye, S.I.; Oboh, G.; Adeoyo, O.O.; Allismith, Y.R. Effect of Caffeine, Caffeic Acid and Their Various Combinations on Enzymes of Cholinergic, Monoaminergic and Purinergic Systems Critical to Neurodegeneration in Rat Brain—In Vitro. NeuroToxicology 2017, 62, 6–13. [Google Scholar] [CrossRef]
- Kumar, A.; Ekavali, A.S. A Review on Alzheimer’s Disease Pathophysiology and Its Management: An Update. Pharmacol. Rep. 2015, 67, 195–203. [Google Scholar] [CrossRef]
- Lee, K.H.; Cha, M.; Lee, B.H. Neuroprotective Effect of Antioxidants in the Brain. Int. J. Mol. Sci. 2020, 21, 7152. [Google Scholar] [CrossRef]
- Mohseni, M.; Sahebkar, A.; Askari, G.; Johnston, T.P.; Alikiaii, B.; Bagherniya, M. The Clinical Use of Curcumin on Neurological Disorders: An Updated Systematic Review of Clinical Trials. Phytother. Res. 2021, 35, 6862–6882. [Google Scholar] [CrossRef]
- Thongchai, K.; Chuysinuan, P.; Thanyacharoen, T.; Techasakul, S.; Ummartyotin, S. Characterization, Release, and Antioxidant Activity of Caffeic Acid-Loaded Collagen and Chitosan Hydrogel Composites. J. Mater. Res. Technol. 2020, 9, 6512–6520. [Google Scholar] [CrossRef]
- Katuwavila, N.P.; Perera, A.D.L.C.; Karunaratne, V.; Amaratunga, G.A.J.; Karunaratne, D.N. Improved Delivery of Caffeic Acid through Liposomal Encapsulation. J. Nanomater. 2016, 2016, e9701870. [Google Scholar] [CrossRef]
- Tosheva, A.; Petrov, P.; Grancharov, G.; Yoncheva, K.; Tzankova, D.; Tzankova, V.; Aluani, D. In Vitro Evaluation of Antioxidant Activity and Biocompatibility of Caffeic Acid Phenethyl Ester Loaded in Polymeric Micelles. Mol. Cell. Toxicol. 2023, 19, 89–98. [Google Scholar] [CrossRef]
- Nakagawa, R.; Ohnishi, T.; Kobayashi, H.; Yamaoka, T.; Yajima, T.; Tanimura, A.; Kato, T.; Yoshizawa, K. Long-Term Effect of Galantamine on Cognitive Function in Patients with Alzheimer’s Disease versus a Simulated Disease Trajectory: An Observational Study in the Clinical Setting. Neuropsychiatr. Dis. Treat. 2017, 13, 1115–1124. [Google Scholar] [CrossRef]
- Liu, Y.; Zhang, Y.; Zheng, X.; Fang, T.; Yang, X.; Luo, X.; Guo, A.; Newell, K.A.; Huang, X.-F.; Yu, Y. Galantamine Improves Cognition, Hippocampal Inflammation, and Synaptic Plasticity Impairments Induced by Lipopolysaccharide in Mice. J. Neuroinflammation 2018, 15, 112. [Google Scholar] [CrossRef]
- Gotur, A.; Prasad, K.; Srivastava, A.; Vibha, D.; Pandit, A.; Rajan, R. Rivastigmine for Cognitive Impairment in Multiple Sclerosis: A Parallel Group Randomised Open Label Study with Blinded End Point Assessment (5201). Neurology 2021, 96. [Google Scholar]
- Sul, D.; Kim, H.-S.; Lee, D.; Joo, S.S.; Hwang, K.W.; Park, S.-Y. Protective Effect of Caffeic Acid against Beta-Amyloid-Induced Neurotoxicity by the Inhibition of Calcium Influx and Tau Phosphorylation. Life Sci. 2009, 84, 257–262. [Google Scholar] [CrossRef]
- Chang, W.; Huang, D.; Lo, Y.M.; Tee, Q.; Kuo, P.; Wu, J.S.; Huang, W.; Shen, S. Protective Effect of Caffeic Acid against Alzheimer’s Disease Pathogenesis via Modulating Cerebral Insulin Signaling, β-Amyloid Accumulation, and Synaptic Plasticity in Hyperinsulinemic Rats. J. Agric. Food Chem. 2019, 67, 7684–7693. [Google Scholar] [CrossRef]
- Saenno, R.; Dornlakorn, O.; Anosri, T.; Kaewngam, S.; Sirichoat, A.; Aranarochana, A.; Pannangrong, W.; Wigmore, P.; Welbat, J.U. Caffeic Acid Alleviates Memory and Hippocampal Neurogenesis Deficits in Aging Rats Induced by D-Galactose. Nutrients 2022, 14, 2169. [Google Scholar] [CrossRef]
- Wang, S.; Kong, X.; Chen, Z.; Wang, G.; Zhang, J.; Wang, J. Role of Natural Compounds and Target Enzymes in the Treatment of Alzheimer’s Disease. Molecules 2022, 27, 4175. [Google Scholar] [CrossRef]
- Oboh, G.; Agunloye, O.M.; Akinyemi, A.J.; Ademiluyi, A.O.; Adefegha, S.A. Comparative Study on the Inhibitory Effect of Caffeic and Chlorogenic Acids on Key Enzymes Linked to Alzheimer’s Disease and Some pro-Oxidant Induced Oxidative Stress in Rats’ Brain-In Vitro. Neurochem. Res. 2013, 38, 413–419. [Google Scholar] [CrossRef] [PubMed]
- Işık, M.; Beydemir, Ş. The Impact of Some Phenolic Compounds on Serum Acetylcholinesterase: Kinetic Analysis of an Enzyme/Inhibitor Interaction and Molecular Docking Study. J. Biomol. Struct. Dyn. 2021, 39, 6515–6523. [Google Scholar] [CrossRef]
- Stasiłowicz, A.; Tykarska, E.; Lewandowska, K.; Kozak, M.; Miklaszewski, A.; Kobus-Cisowska, J.; Szymanowska, D.; Plech, T.; Jenczyk, J.; Cielecka-Piontek, J. Hydroxypropyl-β-Cyclodextrin as an Effective Carrier of Curcumin–Piperine Nutraceutical System with Improved Enzyme Inhibition Properties. J. Enzym. Inhib. Med. Chem. 2020, 35, 1811–1821. [Google Scholar] [CrossRef] [PubMed]
- PL-Grid Project. Available online: https://www.plgrid.pl/ (accessed on 30 January 2022).
- Frisch, M.J.; Trucks, G.W.; Schlegel, H.B.; Scuseria, G.; Robb, M.; Cheeseman, J.; Scalmani, G.; Barone, V.; Mennucci, B.; Petersson, G. Gaussian 09, Revision C. 01; Gaussian, Inc.: Wallingford, CT, USA, 2009. [Google Scholar]
- Dennington, R.; Keith, T.; Millam, J. GaussView, Version 5; Semichem Inc.: Shawnee Mission, KS, USA, 2009. [Google Scholar]
- Hens, B.; Tsume, Y.; Bermejo Sanz, M.; Paixao, P.; Koenigsknecht, M.; Baker, J.; Hasler, W.; Lionberger, R.; Fan, J.; Dickens, J.; et al. Low Buffer Capacity and Alternating Motility Along The Human Gastrointestinal Tract: Implications for In Vivo Dissolution and Absorption of Ionizable Drugs. Mol. Pharm. 2017, 14, 4281–4294. [Google Scholar] [CrossRef]
- Prior, A.; Frutos, P.; Correa, C. Comparison of Dissolution Profiles: Current Guidelines. In Proceedings of the VI Congreso SEFIG, Granada, Spain, 9–11 February 2003; Volume 3, pp. 507–509. [Google Scholar]
- Fischer, H.; Kansy, M.; Avdeef, A.; Senner, F. Permeation of Permanently Positive Charged Molecules through Artificial Membranes--Influence of Physico-Chemical Properties. Eur. J. Pharm. Sci. 2007, 31, 32–42. [Google Scholar] [CrossRef]
- Di, L.; Kerns, E.H.; Fan, K.; McConnell, O.J.; Carter, G.T. High Throughput Artificial Membrane Permeability Assay for Blood-Brain Barrier. Eur. J. Med. Chem. 2003, 38, 223–232. [Google Scholar] [CrossRef] [PubMed]
- Re, R.; Pellegrini, N.; Proteggente, A.; Pannala, A.; Yang, M.; Rice-Evans, C. Antioxidant Activity Applying an Improved ABTS Radical Cation Decolorization Assay. Free. Radic. Biol. Med. 1999, 26, 1231–1237. [Google Scholar] [CrossRef]
- Kikowska, M.A.; Chmielewska, M.; Włodarczyk, A.; Studzińska-Sroka, E.; Żuchowski, J.; Stochmal, A.; Kotwicka, M.; Thiem, B. Effect of Pentacyclic Triterpenoids-Rich Callus Extract of Chaenomeles Japonica (Thunb.) Lindl. Ex Spach on Viability, Morphology, and Proliferation of Normal Human Skin Fibroblasts. Molecules 2018, 23, 3009. [Google Scholar] [CrossRef]
- Ellman, G.L.; Courtney, K.D.; Andres, V.; Featherstone, R.M. A New and Rapid Colorimetric Determination of Acetylcholinesterase Activity. Biochem. Pharmacol. 1961, 7, 88–95. [Google Scholar] [CrossRef]
- Lim, T.Y.; Lim, Y.Y.; Yule, C.M. Evaluation of Antioxidant, Antibacterial and Anti-Tyrosinase Activities of Four Macaranga Species. Food Chem. 2009, 114, 594–599. [Google Scholar] [CrossRef]
- Morris, G.M.; Huey, R.; Lindstrom, W.; Sanner, M.F.; Belew, R.K.; Goodsell, D.S.; Olson, A.J. AutoDock4 and AutoDockTools4: Automated Docking with Selective Receptor Flexibility. J. Comput. Chem. 2009, 30, 2785–2791. [Google Scholar] [CrossRef]
- Najafi, Z.; Kamari-aliabadi, A.; Sabourian, R.; Hajimahmoodi, M.; Chehardoli, G. Synthesis and Molecular Modeling of New 2-Benzylidenethiobarbituric Acid Derivatives as Potent Tyrosinase Inhibitors Agents. J. Chin. Chem. Soc. 2022, 69, 692–702. [Google Scholar] [CrossRef]
- O’Boyle, N.M.; Banck, M.; James, C.A.; Morley, C.; Vandermeersch, T.; Hutchison, G.R. Open Babel: An Open Chemical Toolbox. J. Cheminformatics 2011, 3, 33. [Google Scholar] [CrossRef] [PubMed]
- Adasme, M.; Linnemann, K.; Bolz, S.; Kaiser, F.; Salentin, S.; Haupt, V.; Schroeder, M. PLIP 2021: Expanding the Scope of the Protein-Ligand Interaction Profiler to DNA and RNA. Nucleic Acids Res. 2021, 49, W530–W534. [Google Scholar] [CrossRef] [PubMed]
- Schrödinger LLC. The PyMOL Molecular Graphics System, Version 1.8; Schrödinger LLC: New York, NY, USA, 2015. [Google Scholar]



| The Substance/System | Permeability Coefficient (cm s−1) | ||
|---|---|---|---|
| pH 1.2 | pH 6.8 | BBB | |
| CA | 7.405 × 10−6 ± 2.872 × 10−7 | 1.445 × 10−7 ± 3.486 × 10−8 | 1.142 × 10−6 ± 2.006 × 10−7 |
| CA_Neu_1:1_PHM | 7.546 × 10−6 ± 7.741 × 10−8 | 1.322 × 10−7 ± 2.431 × 10−8 | - |
| CA_Neu_1:1_BM_20′ | 8.850 × 10−6 ± 1.765 × 10−7 (*) | 1.812 × 10−7 ± 8.183 × 10−9 | - |
| The Substance/System | Enzyme Inhibitory Potential | ||
|---|---|---|---|
| AChE | BChE | Tyrosinase | |
| CA | N/D | N/D | 2.657 ± 0.039% |
| CA_Neu_1:1_PHM | 11.854 ± 0.396% (*) | 8.644 ± 0.121% (*) | 29.443 ± 0.150% (*) |
| CA_Neu_1:1_BM_20′ | 18.444 ± 0.429% (*) | 13.011 ± 0.209% (*) | 58.658 ± 0.173% (*) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Stasiłowicz-Krzemień, A.; Rosiak, N.; Miklaszewski, A.; Cielecka-Piontek, J. Screening of the Anti-Neurodegenerative Activity of Caffeic Acid after Introduction into Inorganic Metal Delivery Systems to Increase Its Solubility as the Result of a Mechanosynthetic Approach. Int. J. Mol. Sci. 2023, 24, 9218. https://doi.org/10.3390/ijms24119218
Stasiłowicz-Krzemień A, Rosiak N, Miklaszewski A, Cielecka-Piontek J. Screening of the Anti-Neurodegenerative Activity of Caffeic Acid after Introduction into Inorganic Metal Delivery Systems to Increase Its Solubility as the Result of a Mechanosynthetic Approach. International Journal of Molecular Sciences. 2023; 24(11):9218. https://doi.org/10.3390/ijms24119218
Chicago/Turabian StyleStasiłowicz-Krzemień, Anna, Natalia Rosiak, Andrzej Miklaszewski, and Judyta Cielecka-Piontek. 2023. "Screening of the Anti-Neurodegenerative Activity of Caffeic Acid after Introduction into Inorganic Metal Delivery Systems to Increase Its Solubility as the Result of a Mechanosynthetic Approach" International Journal of Molecular Sciences 24, no. 11: 9218. https://doi.org/10.3390/ijms24119218
APA StyleStasiłowicz-Krzemień, A., Rosiak, N., Miklaszewski, A., & Cielecka-Piontek, J. (2023). Screening of the Anti-Neurodegenerative Activity of Caffeic Acid after Introduction into Inorganic Metal Delivery Systems to Increase Its Solubility as the Result of a Mechanosynthetic Approach. International Journal of Molecular Sciences, 24(11), 9218. https://doi.org/10.3390/ijms24119218









