Growth-Restricted Fetuses and Offspring Reveal Adverse Sex-Specific Metabolic Responses in Preeclamptic Mice Expressing Human sFLT1
Abstract
1. Introduction
2. Results
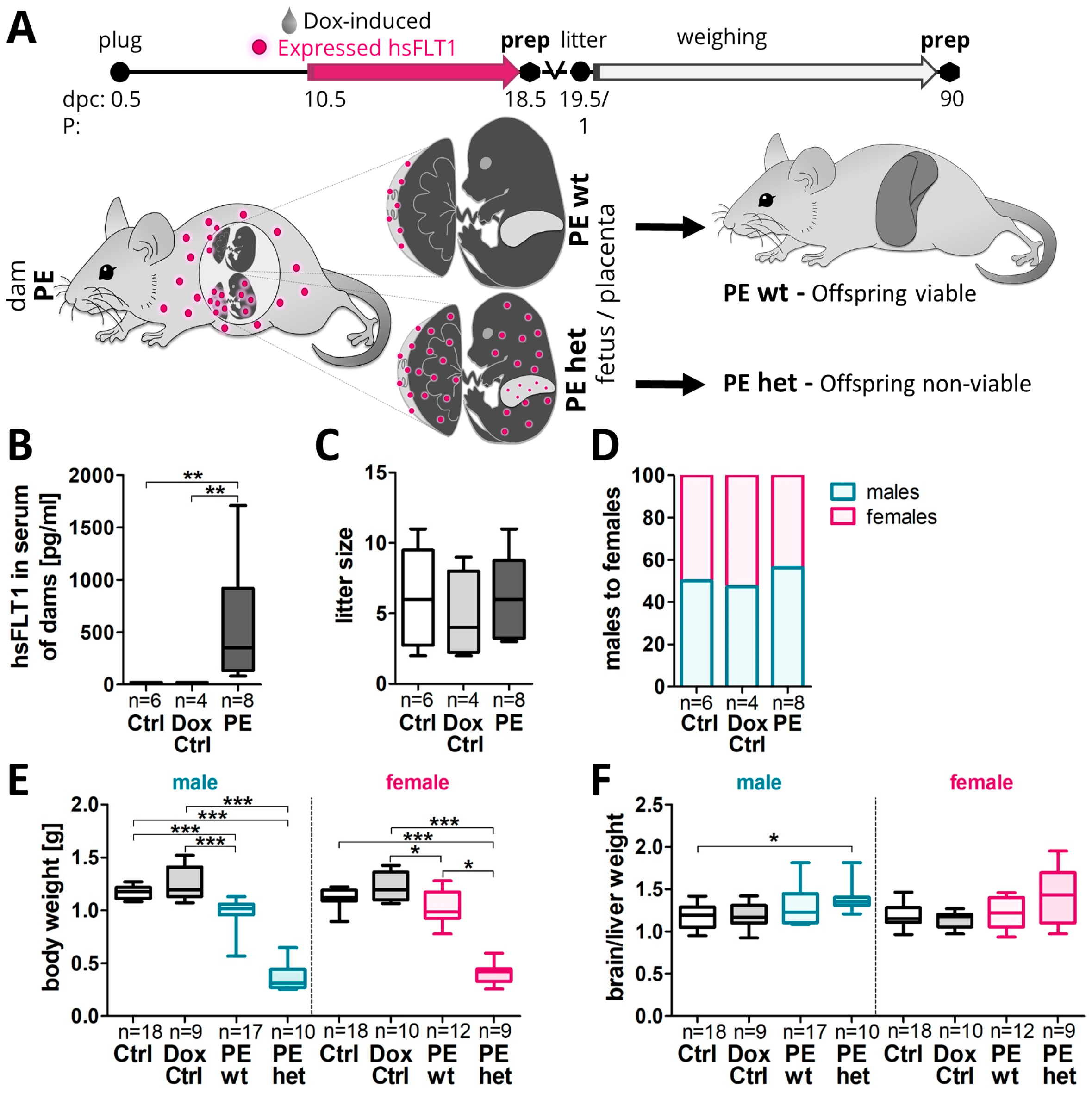
2.1. Asymmetrical Growth Restriction Was More Pronounced in Male Than Female Fetuses upon Systemic Human sFLT1 Overexpression at 18.5 dpc
- Fetal liver development and function upon systemic sFLT1 overexpression in pregnancy
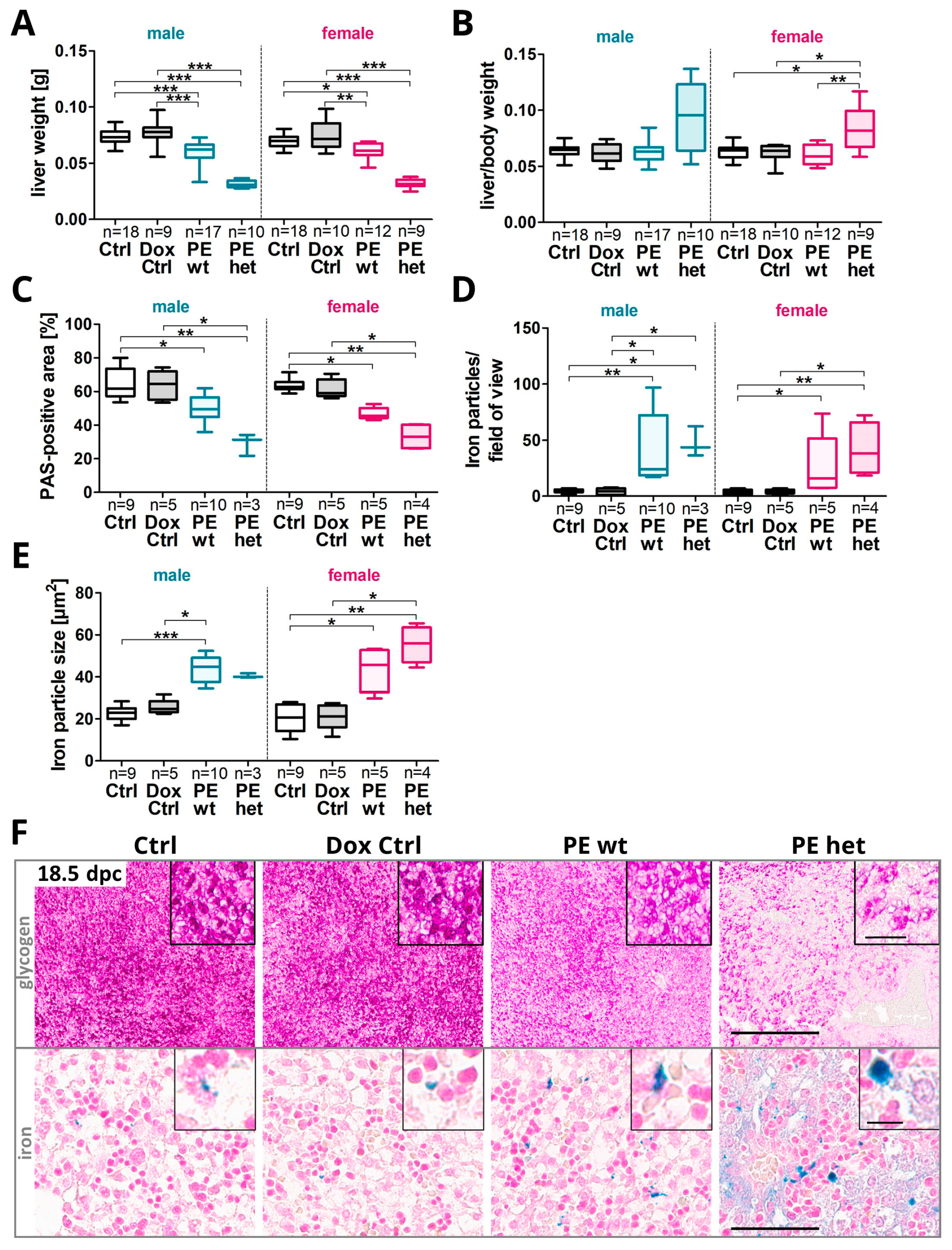
2.2. Fetal Livers Showed a Reduced Glycogen Storage Capacity and Increased Hemorrhages upon Systemic Human sFLT1 at 18.5 dpc
2.3. Systemic Human sFLT1 Leads to Altered Fetal Hepatic Gene Expression Metabolic Profiles at 18.5 dpc
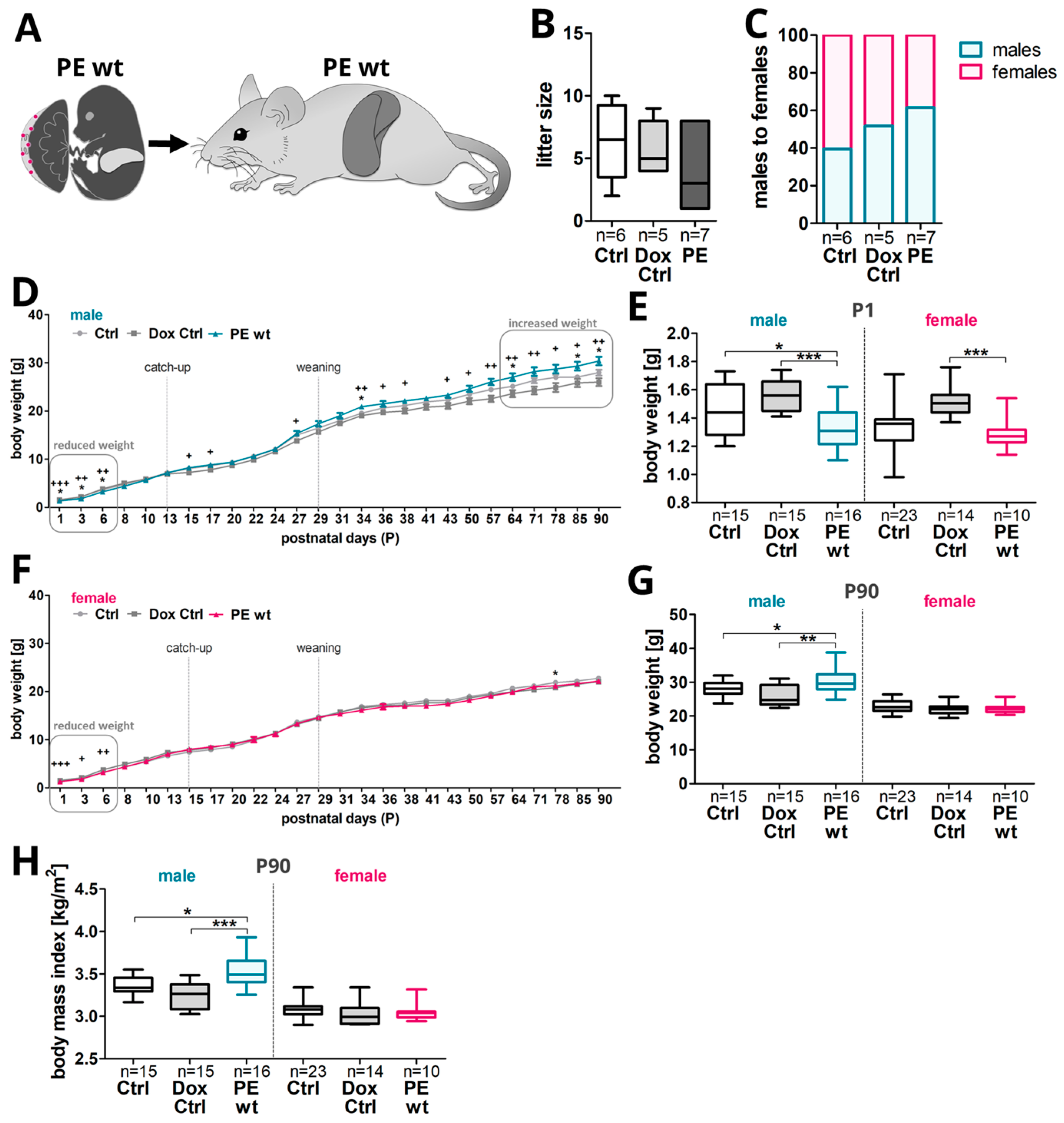
2.4. Postnatal Weight Gain Was Increased in Male Adult Offspring after Preeclampsia-Affected Pregnancy
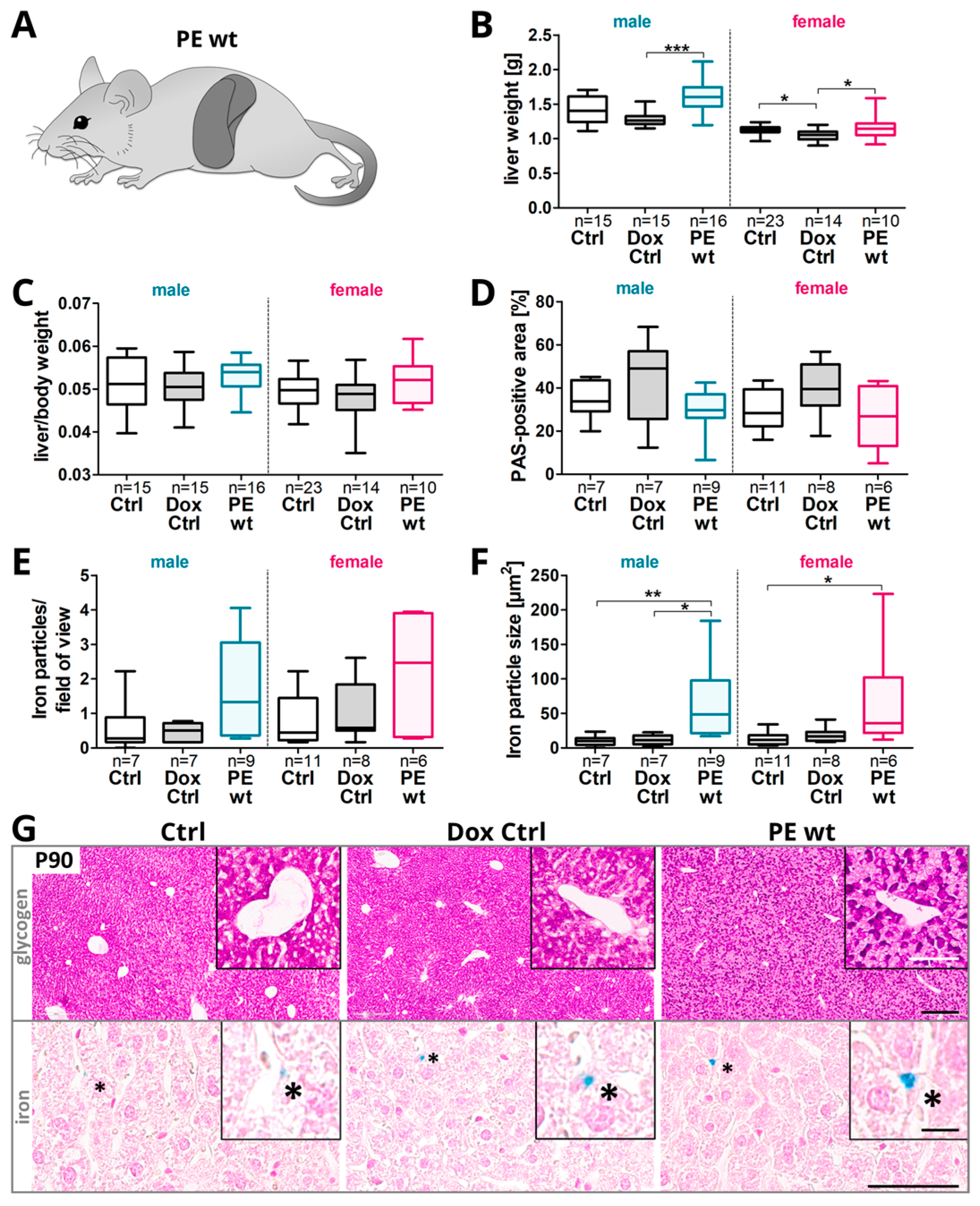
- Postnatal phenotype in adult offspring upon previous systemic sFLT1 overexpression regarding weight gain and liver metabolism
2.5. Changes in the Hepatic Glycogen and Iron Presence in Adult Offspring after Preeclampsia-Affected Pregnancy
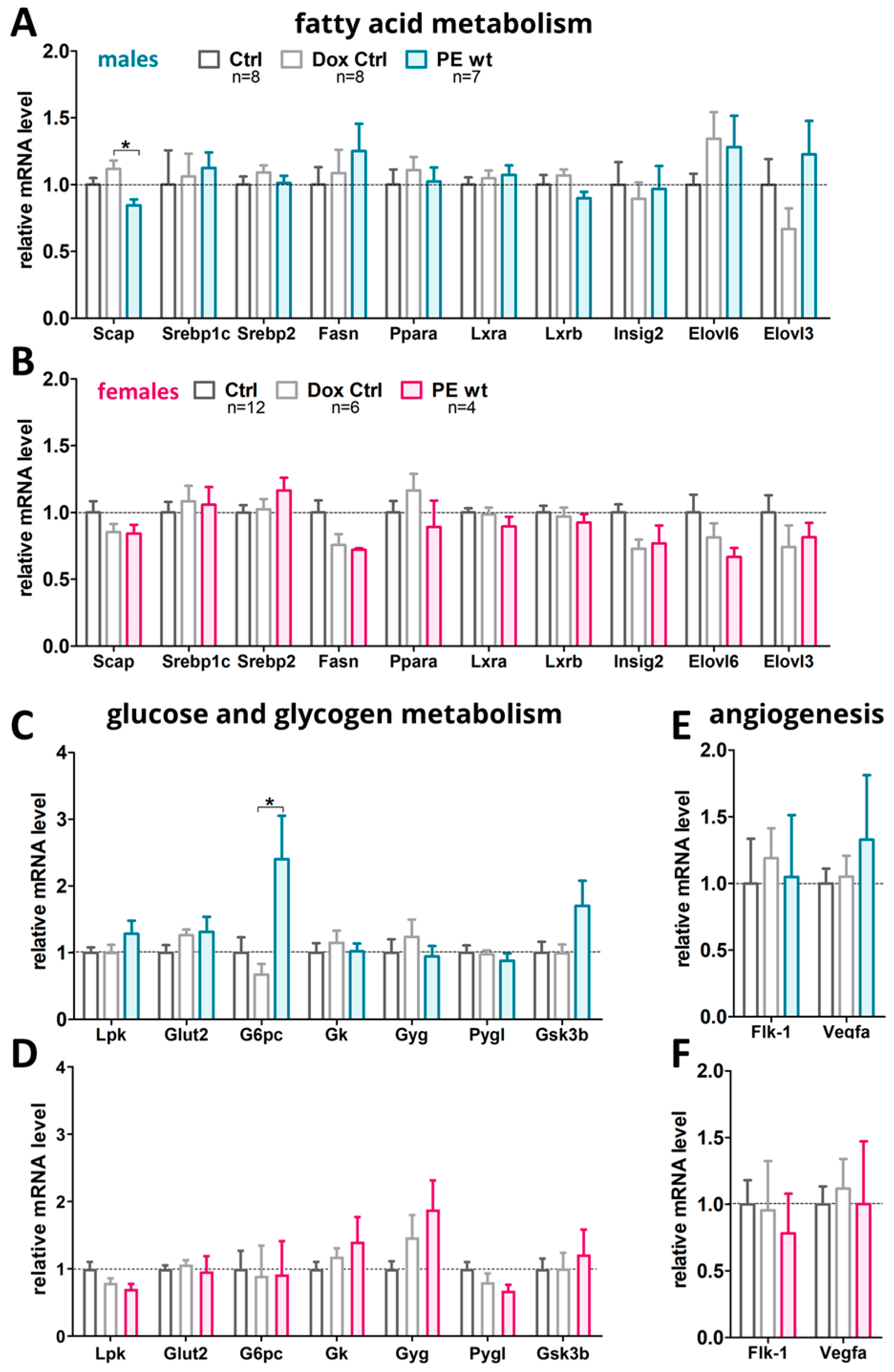
2.6. Hepatic Metabolic Gene Expression Profiles in Adult Offspring after Preeclampsia-Affected Pregnancy
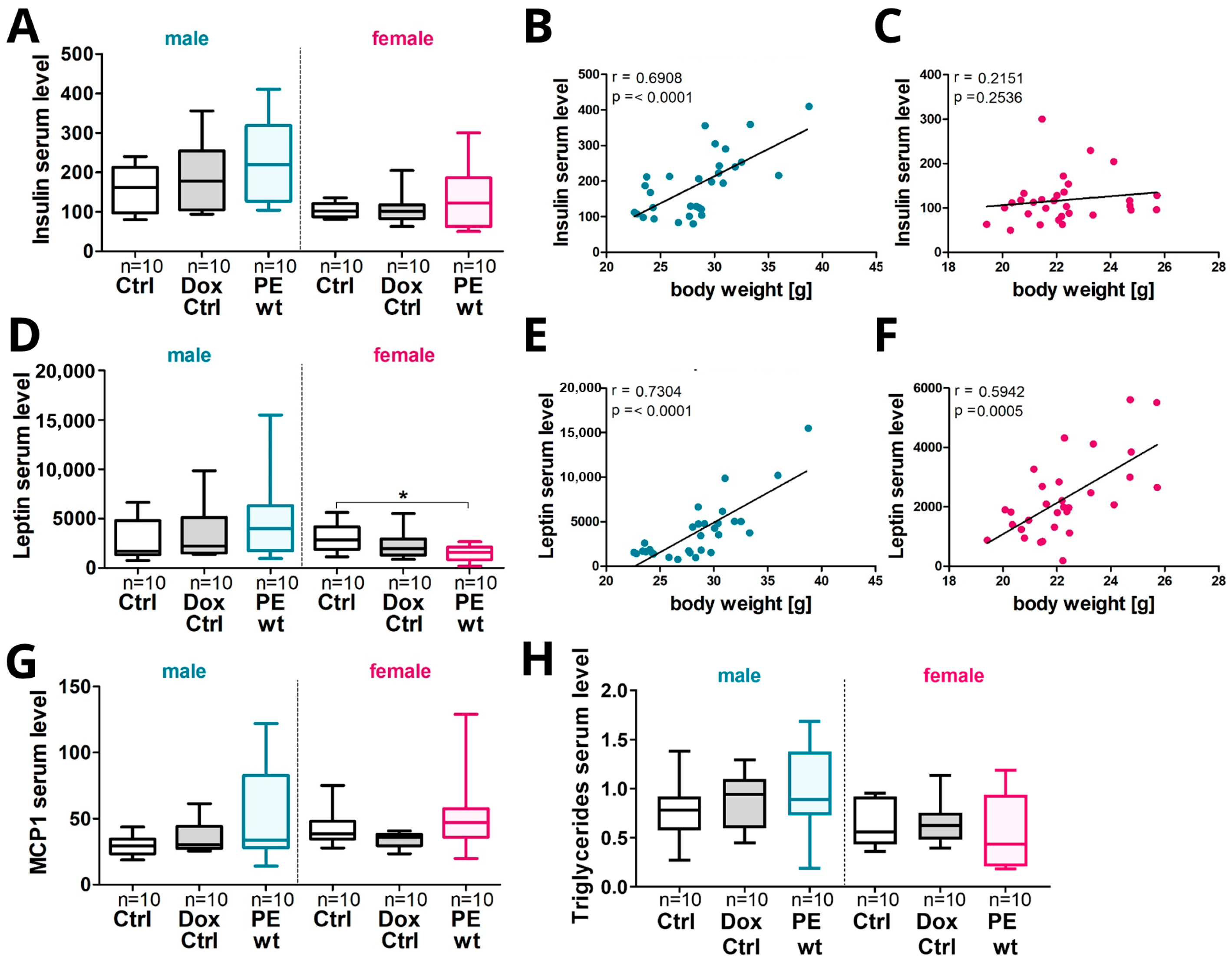
2.7. Increased Offspring Postnatal Weight Gain in Males Was Associated with an Increase in Serum Hormones, Cytokines and Triglyceride Levels
3. Discussion
3.1. Systemic sFLT1 Overexpression during Pregnancy in Mice Leads to a Changed Pathophysiology in the Fetal Liver
3.2. Systemic sFLT1 Overexpression during Pregnancy in Mice Leads to Increased Postnatal Weight Gain in the Adult Male Offspring
4. Materials and Methods
4.1. Animals
4.2. Body Mass Index Calculation
4.3. Tissue Preparation
4.4. Serum hsFLT1 Measurements via Brahms Kryptor
4.5. Serum Hormone, Cytokine, and Triglyceride Measurements
4.6. Hematoxylin and Eosin Staining
4.7. Periodic Acid Schiff’s Reaction
4.8. Perls Prussian Blue Reaction
4.9. Quantitative Image Analysis
4.10. Genomic DNA Isolation, Genotyping and Sex Determination
4.11. RNA Extraction, cDNA Synthesis and Quantitative Polymerase Chain Reaction
4.12. Statistics
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Barker, D.J. The fetal and infant origins of adult disease. BMJ 1990, 301, 1111. [Google Scholar] [CrossRef] [PubMed]
- Barker, D.J. The developmental origins of adult disease. J. Am. Coll. Nutr. 2004, 23 (Suppl. S6), 588S–595S. [Google Scholar] [CrossRef] [PubMed]
- Turbeville, H.R.; Sasser, J.M. Preeclampsia beyond pregnancy: Long-term consequences for mother and child. Am. J. Physiol. Renal Physiol. 2020, 318, F1315–F1326. [Google Scholar] [CrossRef] [PubMed]
- Whitaker, K.W.; Totoki, K.; Reyes, T.M. Metabolic adaptations to early life protein restriction differ by offspring sex and post-weaning diet in the mouse. Nutr. Metab. Cardiovasc. Dis. 2012, 22, 1067–1074. [Google Scholar] [CrossRef]
- Hyatt, M.A.; Gardner, D.S.; Sebert, S.; Wilson, V.; Davidson, N.; Nigmatullina, Y.; Chan, L.L.; Budge, H.; Symonds, M.E. Suboptimal maternal nutrition, during early fetal liver development, promotes lipid accumulation in the liver of obese offspring. Reproduction 2011, 141, 119–126. [Google Scholar] [CrossRef]
- Girardi, G.; Bremer, A.A. The Intersection of Maternal Metabolic Syndrome, Adverse Pregnancy Outcomes, and Future Metabolic Health for the Mother and Offspring. Metab. Syndr. Relat. Disord. 2022, 20, 251–254. [Google Scholar] [CrossRef]
- Bi, S.; Zhang, L.; Huang, L.; Li, Y.; Liang, Y.; Huang, M.; Huang, B.; Liang, J.; Gu, S.; Chen, J.; et al. Long-term effects of preeclampsia on metabolic and biochemical outcomes in offspring: What can be expected from a meta-analysis? Obes. Rev. 2022, 23, e13411. [Google Scholar] [CrossRef]
- Burton, G.J.; Redman, C.W.; Roberts, J.M.; Moffett, A. Pre-eclampsia: Pathophysiology and clinical implications. BMJ 2019, 366, l2381. [Google Scholar] [CrossRef]
- Steegers, E.A.; von Dadelszen, P.; Duvekot, J.J.; Pijnenborg, R. Pre-eclampsia. Lancet 2010, 376, 631–644. [Google Scholar] [CrossRef]
- Staff, A.C. The two-stage placental model of preeclampsia: An update. J. Reprod. Immunol. 2019, 134–135, 1–10. [Google Scholar] [CrossRef]
- Maynard, S.E.; Min, J.Y.; Merchan, J.; Lim, K.H.; Li, J.; Mondal, S.; Libermann, T.A.; Morgan, J.P.; Sellke, F.W.; Stillman, I.E.; et al. Excess placental soluble fms-like tyrosine kinase 1 (sFlt1) may contribute to endothelial dysfunction, hypertension, and proteinuria in preeclampsia. J. Clin. Investig. 2003, 111, 649–658. [Google Scholar] [CrossRef] [PubMed]
- Verlohren, S.; Stepan, H.; Dechend, R. Angiogenic growth factors in the diagnosis and prediction of pre-eclampsia. Clin. Sci. 2012, 122, 43–52. [Google Scholar] [CrossRef]
- Shibuya, M. Vascular endothelial growth factor receptor-1 (VEGFR-1/Flt-1): A dual regulator for angiogenesis. Angiogenesis 2006, 9, 225–230; discussion 231. [Google Scholar] [CrossRef]
- Kuhnel, E.; Kleff, V.; Stojanovska, V.; Kaiser, S.; Waldschutz, R.; Herse, F.; Plosch, T.; Winterhager, E.; Gellhaus, A. Placental-Specific Overexpression of sFlt-1 Alters Trophoblast Differentiation and Nutrient Transporter Expression in an IUGR Mouse Model. J. Cell. Biochem. 2017, 118, 1316–1329. [Google Scholar] [CrossRef] [PubMed]
- Vogtmann, R.; Kuhnel, E.; Dicke, N.; Verkaik-Schakel, R.N.; Plosch, T.; Schorle, H.; Stojanovska, V.; Herse, F.; Koninger, A.; Kimmig, R.; et al. Human sFLT1 Leads to Severe Changes in Placental Differentiation and Vascularization in a Transgenic hsFLT1/rtTA FGR Mouse Model. Front. Endocrinol. 2019, 10, 165. [Google Scholar] [CrossRef] [PubMed]
- Stojanovska, V.; Dijkstra, D.J.; Vogtmann, R.; Gellhaus, A.; Scherjon, S.A.; Plosch, T. A double-hit pre-eclampsia model results in sex-specific growth restriction patterns. Dis. Model. Mech. 2019, 12, dmm035980. [Google Scholar] [CrossRef]
- Stojanovska, V.; Sharma, N.; Dijkstra, D.J.; Scherjon, S.A.; Jager, A.; Schorle, H.; Plosch, T. Placental insufficiency contributes to fatty acid metabolism alterations in aged female mouse offspring. Am. J. Physiol. Regul. Integr. Comp. Physiol. 2018, 315, R1107–R1114. [Google Scholar] [CrossRef]
- Tunster, S.J.; Watson, E.D.; Fowden, A.L.; Burton, G.J. Placental glycogen stores and fetal growth: Insights from genetic mouse models. Reproduction 2020, 159, R213–R235. [Google Scholar] [CrossRef]
- Youssef, L.; Crovetto, F.; Simoes, R.V.; Miranda, J.; Paules, C.; Blasco, M.; Palomo, M.; Garcia-Caldero, H.; Tura-Ceide, O.; Dantas, A.P.; et al. The Interplay between Pathophysiological Pathways in Early-Onset Severe Preeclampsia Unveiled by Metabolomics. Life 2022, 12, 86. [Google Scholar] [CrossRef]
- Vogtmann, R.; Heupel, J.; Herse, F.; Matin, M.; Hagmann, H.; Bendix, I.; Kraker, K.; Dechend, R.; Winterhager, E.; Kimmig, R.; et al. Circulating Maternal sFLT1 (Soluble fms-Like Tyrosine Kinase-1) Is Sufficient to Impair Spiral Arterial Remodeling in a Preeclampsia Mouse Model. Hypertension 2021, 78, 1067–1079. [Google Scholar] [CrossRef]
- Vogtmann, R.; Burk, L.V.; Serdar, M.; Kimmig, R.; Bendix, I.; Gellhaus, A. Systemic Maternal Human sFLT1 Overexpression Leads to an Impaired Foetal Brain Development of Growth-Restricted Foetuses upon Experimental Preeclampsia. Oxidative Med. Cell. Longev. 2022, 2022, 3024032. [Google Scholar] [CrossRef] [PubMed]
- Washburn, L.; Nixon, P.; Russell, G.; Snively, B.M.; O’Shea, T.M. Adiposity in adolescent offspring born prematurely to mothers with preeclampsia. J. Pediatr. 2013, 162, 912–917.e1. [Google Scholar] [CrossRef] [PubMed]
- Kaplan, J.B.; Kalra, A.; Biggins, S.W. Liver Anatomy and Function. In Radiation Therapy for Liver Tumors; Springer: Berlin/Heidelberg, Germany, 2017; pp. 3–11. [Google Scholar]
- Giancotti, A.; Monti, M.; Nevi, L.; Safarikia, S.; D’Ambrosio, V.; Brunelli, R.; Pajno, C.; Corno, S.; Di Donato, V.; Musella, A. Functions and the emerging role of the foetal liver into regenerative medicine. Cells 2019, 8, 914. [Google Scholar] [CrossRef]
- Stallmach, T.; Karolyi, L.; Lichtlen, P.; Maurer, M.; Hebisch, G.; Joller, H.; Marti, H.H.; Gassmann, M. Fetuses from preeclamptic mothers show reduced hepatic erythropoiesis. Pediatr. Res. 1998, 43, 349–354. [Google Scholar] [CrossRef] [PubMed]
- Noguchi, T.; Iritani, N.; Tanaka, T. Molecular mechanism of induction of key enzymes related to lipogenesis. Proc. Soc. Exp. Biol. Med. 1992, 200, 206–209. [Google Scholar] [CrossRef]
- Parchem, J.G.; Kanasaki, K.; Kanasaki, M.; Sugimoto, H.; Xie, L.; Hamano, Y.; Lee, S.B.; Gattone, V.H.; Parry, S.; Strauss, J.F.; et al. Loss of placental growth factor ameliorates maternal hypertension and preeclampsia in mice. J. Clin. Investig. 2018, 128, 5008–5017. [Google Scholar] [CrossRef]
- Sato, E.; Tsunokuni, Y.; Kaneko, M.; Saigusa, D.; Saito, R.; Shimma, S.; Sekimoto, A.; Kawana, Y.; Oe, Y.; Ito, S.; et al. Metabolomics of a mouse model of preeclampsia induced by overexpressing soluble fms-like tyrosine kinase 1. Biochem. Biophys. Res. Commun. 2020, 527, 1064–1071. [Google Scholar] [CrossRef]
- Stojanovska, V.; Holwerda, K.M.; van der Graaf, A.M.; Verkaik-Schakel, R.N.; Boekschoten, M.V.; Faas, M.M.; Scherjon, S.A.; Plosch, T. In utero sFlt-1 exposure differentially affects gene expression patterns in fetal liver. J. Dev. Orig. Health Dis. 2019, 10, 353–361. [Google Scholar] [CrossRef]
- Luo, J.; Yang, H.; Song, B.L. Mechanisms and regulation of cholesterol homeostasis. Nat. Rev. Mol. Cell Biol. 2020, 21, 225–245. [Google Scholar] [CrossRef]
- Oishi, Y.; Spann, N.J.; Link, V.M.; Muse, E.D.; Strid, T.; Edillor, C.; Kolar, M.J.; Matsuzaka, T.; Hayakawa, S.; Tao, J.; et al. SREBP1 Contributes to Resolution of Pro-inflammatory TLR4 Signaling by Reprogramming Fatty Acid Metabolism. Cell Metab. 2017, 25, 412–427. [Google Scholar] [CrossRef]
- Jakobsson, A.; Westerberg, R.; Jacobsson, A. Fatty acid elongases in mammals: Their regulation and roles in metabolism. Prog. Lipid Res. 2006, 45, 237–249. [Google Scholar] [CrossRef]
- Bradbury, M.W. Lipid metabolism and liver inflammation. I. Hepatic fatty acid uptake: Possible role in steatosis. Am. J. Physiol. Gastrointest. Liver Physiol. 2006, 290, G194–G198. [Google Scholar] [CrossRef]
- Magee, T.R.; Han, G.; Cherian, B.; Khorram, O.; Ross, M.G.; Desai, M. Down-regulation of transcription factor peroxisome proliferator-activated receptor in programmed hepatic lipid dysregulation and inflammation in intrauterine growth-restricted offspring. Am. J. Obstet. Gynecol. 2008, 199, 271.e1–271.e5. [Google Scholar] [CrossRef] [PubMed]
- Matsuzaka, T.; Shimano, H. Elovl6: A new player in fatty acid metabolism and insulin sensitivity. J. Mol. Med. 2009, 87, 379–384. [Google Scholar] [CrossRef] [PubMed]
- Neuman, R.I.; Hesselink, E.R.M.; Saleh, L.; van den Meiracker, A.H.; Danser, A.H.J.; Visser, W. Angiogenic markers are elevated in women with acute fatty liver of pregnancy. Ultrasound Obstet. Gynecol. 2020, 56, 465–466. [Google Scholar] [CrossRef] [PubMed]
- Abascal-Saiz, A.; Duque-Alcorta, M.; Fioravantti, V.; Antolin, E.; Fuente-Luelmo, E.; Haro, M.; Ramos-Alvarez, M.P.; Perdomo, G.; Bartha, J.L. The Relationship between Angiogenic Factors and Energy Metabolism in Preeclampsia. Nutrients 2022, 14, 2172. [Google Scholar] [CrossRef]
- Gil-Acevedo, L.A.; Ceballos, G.; Torres-Ramos, Y.D. Foetal lipoprotein oxidation and preeclampsia. Lipids Health Dis. 2022, 21, 51. [Google Scholar] [CrossRef]
- Leon-Reyes, G.; Espino, Y.S.S.; Medina-Navarro, R.; Guzman-Grenfell, A.M.; Medina-Urrutia, A.X.; Fuentes-Garcia, S.; Hicks, G.J.J.; Torres-Ramos, Y.D. Oxidative modifications of foetal LDL-c and HDL-c lipoproteins in preeclampsia. Lipids Health Dis. 2018, 17, 110. [Google Scholar] [CrossRef]
- Carmeliet, P.; Ferreira, V.; Breier, G.; Pollefeyt, S.; Kieckens, L.; Gertsenstein, M.; Fahrig, M.; Vandenhoeck, A.; Harpal, K.; Eberhardt, C.; et al. Abnormal blood vessel development and lethality in embryos lacking a single VEGF allele. Nature 1996, 380, 435–439. [Google Scholar] [CrossRef]
- Lee, D.C.; Romero, R.; Kim, J.S.; Tarca, A.L.; Montenegro, D.; Pineles, B.L.; Kim, E.; Lee, J.; Kim, S.Y.; Draghici, S.; et al. miR-210 targets iron-sulfur cluster scaffold homologue in human trophoblast cell lines: Siderosis of interstitial trophoblasts as a novel pathology of preterm preeclampsia and small-for-gestational-age pregnancies. Am. J. Pathol. 2011, 179, 590–602. [Google Scholar] [CrossRef]
- Pico, C.; Palou, M.; Pomar, C.A.; Rodriguez, A.M.; Palou, A. Leptin as a key regulator of the adipose organ. Rev. Endocr. Metab. Disord. 2022, 23, 13–30. [Google Scholar] [CrossRef] [PubMed]
- Bytautiene, E.; Tamayo, E.; Kechichian, T.; Drever, N.; Gamble, P.; Hankins, G.D.; Saade, G.R. Prepregnancy obesity and sFlt1-induced preeclampsia in mice: Developmental programming model of metabolic syndrome. Am. J. Obstet. Gynecol. 2011, 204, 398.e1–398.e8. [Google Scholar] [CrossRef] [PubMed]
- Byers, B.D.; Betancourt, A.; Lu, F.; Hankins, G.D.; Longo, M.; Saade, G.R.; Bytautiene, E. The effect of prepregnancy obesity and sFlt-1-induced preeclampsia-like syndrome on fetal programming of adult vascular function in a mouse model. Am. J. Obstet. Gynecol. 2009, 200, 432.e1–432.e7. [Google Scholar] [CrossRef] [PubMed]
- Rueda-Clausen, C.F.; Dolinsky, V.W.; Morton, J.S.; Proctor, S.D.; Dyck, J.R.; Davidge, S.T. Hypoxia-induced intrauterine growth restriction increases the susceptibility of rats to high-fat diet-induced metabolic syndrome. Diabetes 2011, 60, 507–516. [Google Scholar] [CrossRef]
- Hassanzadeh-Taheri, M.; Mohammadifard, M.; Erfanian, Z.; Hosseini, M. The maternal reduced uteroplacental perfusion model of preeclampsia induces sexually dimorphic metabolic responses in rat offspring. Biol. Sex Differ. 2022, 13, 48. [Google Scholar] [CrossRef]
- Yang, S.W.; Oh, M.J.; Park, K.V.; Han, S.W.; Kim, H.S.; Sohn, I.S.; Kwon, H.S.; Cho, G.J.; Hwang, H.S. Risk of Early Childhood Obesity in Offspring of Women with Preeclampsia: A Population-Based Study. J. Clin. Med. 2021, 10, 3758. [Google Scholar] [CrossRef]
- Tramunt, B.; Smati, S.; Grandgeorge, N.; Lenfant, F.; Arnal, J.F.; Montagner, A.; Gourdy, P. Sex differences in metabolic regulation and diabetes susceptibility. Diabetologia 2020, 63, 453–461. [Google Scholar] [CrossRef]
- Christians, J.K. The Placenta’s Role in Sexually Dimorphic Fetal Growth Strategies. Reprod. Sci. 2022, 29, 1895–1907. [Google Scholar] [CrossRef]
- Lee, Y.H.; Cherkerzian, S.; Seidman, L.J.; Papandonatos, G.D.; Savitz, D.A.; Tsuang, M.T.; Goldstein, J.M.; Buka, S.L. Maternal Bacterial Infection During Pregnancy and Offspring Risk of Psychotic Disorders: Variation by Severity of Infection and Offspring Sex. Am. J. Psychiatry 2020, 177, 66–75. [Google Scholar] [CrossRef]
- Tobi, E.W.; Lumey, L.H.; Talens, R.P.; Kremer, D.; Putter, H.; Stein, A.D.; Slagboom, P.E.; Heijmans, B.T. DNA methylation differences after exposure to prenatal famine are common and timing- and sex-specific. Hum. Mol. Genet. 2009, 18, 4046–4053. [Google Scholar] [CrossRef]
- Lillycrop, K.A.; Phillips, E.S.; Torrens, C.; Hanson, M.A.; Jackson, A.A.; Burdge, G.C. Feeding pregnant rats a protein-restricted diet persistently alters the methylation of specific cytosines in the hepatic PPAR alpha promoter of the offspring. Br. J. Nutr. 2008, 100, 278–282. [Google Scholar] [CrossRef] [PubMed]
- Schindelin, J.; Arganda-Carreras, I.; Frise, E.; Kaynig, V.; Longair, M.; Pietzsch, T.; Preibisch, S.; Rueden, C.; Saalfeld, S.; Schmid, B.; et al. Fiji: An open-source platform for biological-image analysis. Nat. Methods 2012, 9, 676–682. [Google Scholar] [CrossRef] [PubMed]

| Maternal Groups | Fetal Groups | ||
|---|---|---|---|
| Ctrl Genotype: hsFLT1/rtTA | No hsFLT1 expression, without Dox treatment | Ctrl Genotype: hsFLT1/rtTA | No hsFLT1 expression |
| Dox Ctrl Genotype: hsFLT1 | No hsFLT1 expression, with Dox treatment | Dox Ctrl Genotype: hsFLT1 | No hsFLT1 expression |
| PE Genoype: hsFLT1/rtTA | Systemic hsFLT1 overexpression, with Dox treatment | PE wt Genotype: hsFLT1 PE het Genotype: hsFLT1/rtTA | Exclusive maternal systemic hsFLT1 overexpression Combined maternal and fetal systemic hsFLT1 overexpression |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Vogtmann, R.; Bao, M.; Dewan, M.V.; Riedel, A.; Kimmig, R.; Felderhoff-Müser, U.; Bendix, I.; Plösch, T.; Gellhaus, A. Growth-Restricted Fetuses and Offspring Reveal Adverse Sex-Specific Metabolic Responses in Preeclamptic Mice Expressing Human sFLT1. Int. J. Mol. Sci. 2023, 24, 6885. https://doi.org/10.3390/ijms24086885
Vogtmann R, Bao M, Dewan MV, Riedel A, Kimmig R, Felderhoff-Müser U, Bendix I, Plösch T, Gellhaus A. Growth-Restricted Fetuses and Offspring Reveal Adverse Sex-Specific Metabolic Responses in Preeclamptic Mice Expressing Human sFLT1. International Journal of Molecular Sciences. 2023; 24(8):6885. https://doi.org/10.3390/ijms24086885
Chicago/Turabian StyleVogtmann, Rebekka, Mian Bao, Monia Vanessa Dewan, Alina Riedel, Rainer Kimmig, Ursula Felderhoff-Müser, Ivo Bendix, Torsten Plösch, and Alexandra Gellhaus. 2023. "Growth-Restricted Fetuses and Offspring Reveal Adverse Sex-Specific Metabolic Responses in Preeclamptic Mice Expressing Human sFLT1" International Journal of Molecular Sciences 24, no. 8: 6885. https://doi.org/10.3390/ijms24086885
APA StyleVogtmann, R., Bao, M., Dewan, M. V., Riedel, A., Kimmig, R., Felderhoff-Müser, U., Bendix, I., Plösch, T., & Gellhaus, A. (2023). Growth-Restricted Fetuses and Offspring Reveal Adverse Sex-Specific Metabolic Responses in Preeclamptic Mice Expressing Human sFLT1. International Journal of Molecular Sciences, 24(8), 6885. https://doi.org/10.3390/ijms24086885








