The Effect of Metformin and Carbohydrate-Controlled Diet on DNA Methylation and Gene Expression in the Endometrium of Women with Polycystic Ovary Syndrome
Abstract
1. Introduction
2. Results
2.1. Demographic and Clinical Characteristics of Participants
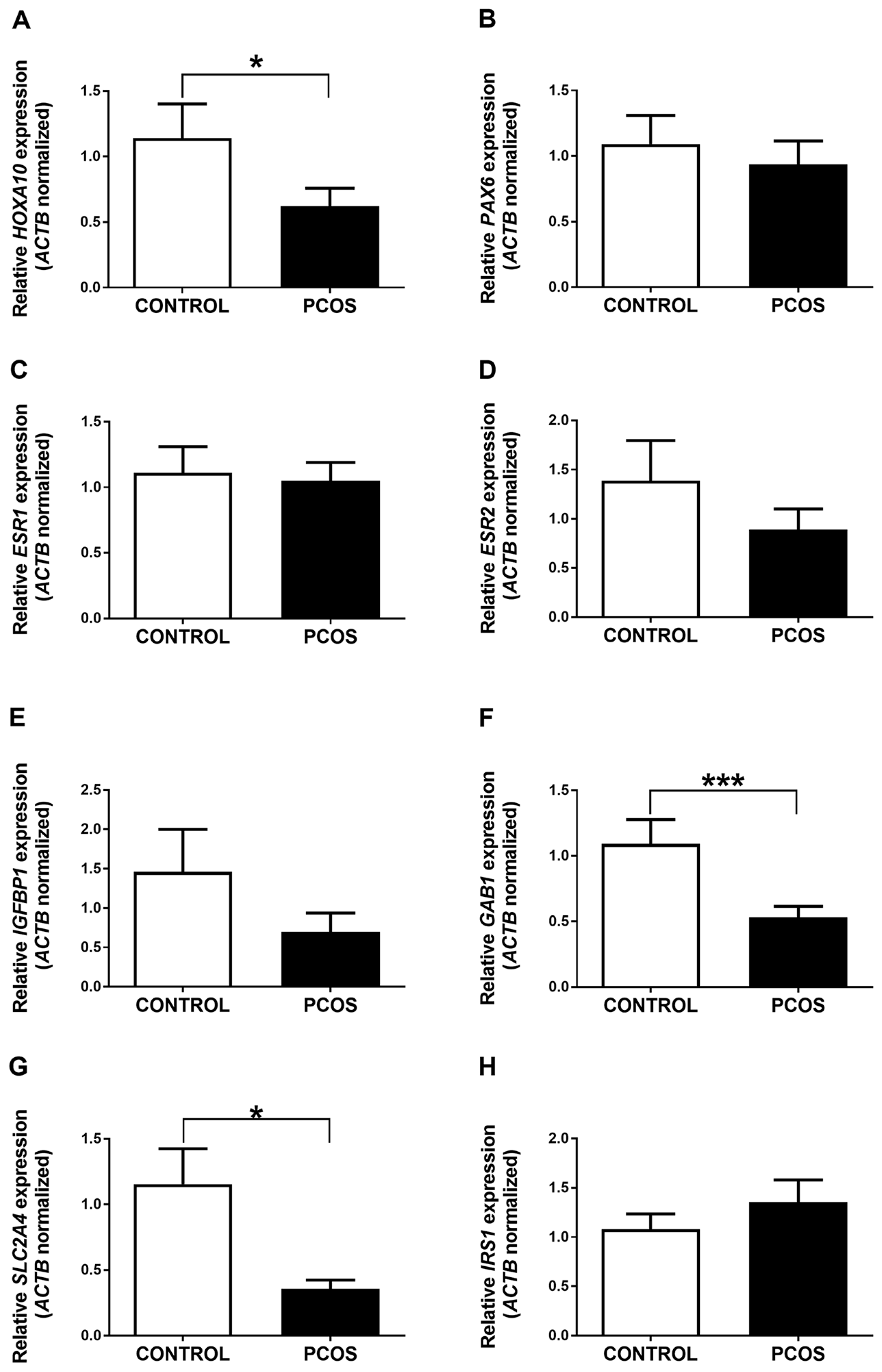
2.2. HOXA10, GAB1, and SLC2A4 Genes Are Differentially Expressed in the Endometrium of PCOS Patients and Women without the Disease
2.3. The Promoter of the HOXA10 Gene Is Differentially Methylated in the Endometrium of PCOS Patients and Women without the Disease
2.4. Intervention with Metformin and a Carbohydrate-Controlled Diet Improves Hormonal and Metabolic Profiles in PCOS Women
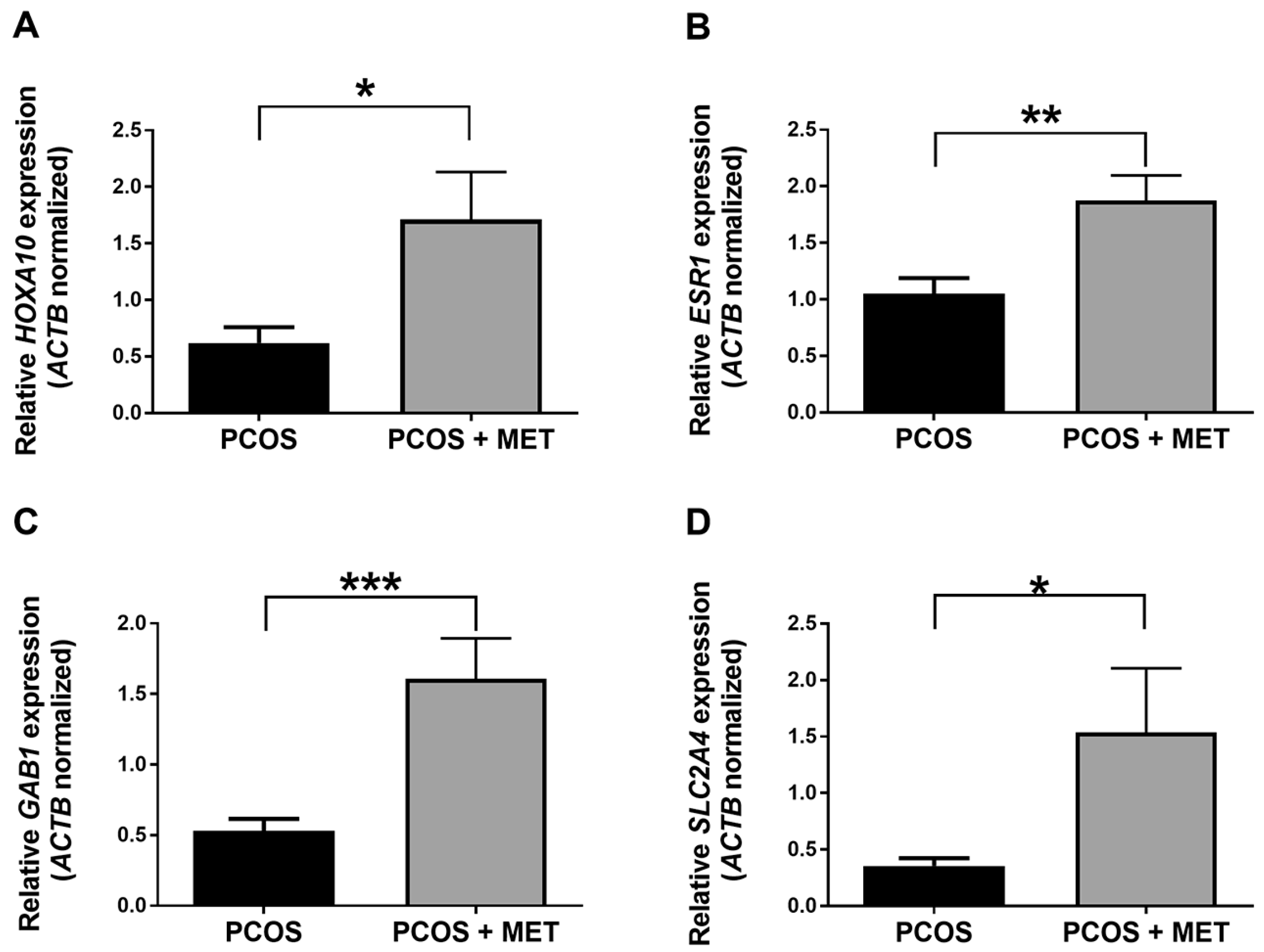
2.5. Intervention with Metformin and a Carbohydrate-Controlled Diet Induces the Expression of HOXA10, ESR1, GAB1, and SLC2A4 Genes in the Endometrium of PCOS Women
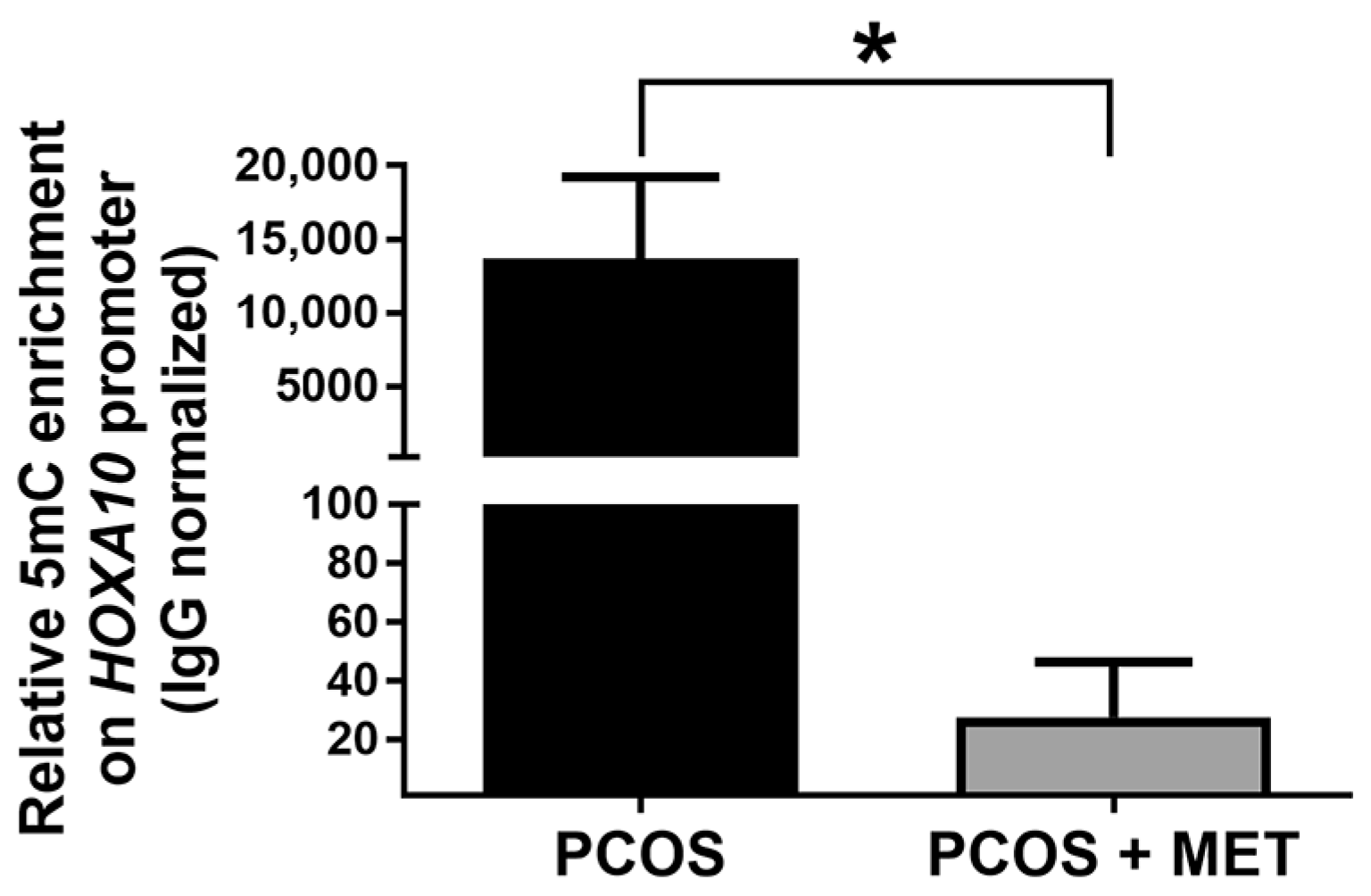
2.6. Effect of Intervention with Metformin and a Carbohydrate-Controlled Diet on DNA Methylation in the Endometrium of PCOS Women
3. Discussion
3.1. Effect of the Intervention with Metformin and a Carbohydrate-Controlled Diet in Metabolic and Clinical Variables in PCOS Women
3.2. Effect of the Intervention with Metformin and a Carbohydrate-Controlled Diet in the Expression of Genes Related to Endometrial Function in the Endometrium of PCOS Women
3.3. Effect of the Intervention with Metformin and a Carbohydrate-Controlled Diet on the DNA Methylation Levels of Genes Related to Endometrial Function in the Endometrium of PCOS Women
4. Materials and Methods
4.1. Inclusion and Exclusion Criteria for Patient Recruitment
4.2. Sample Collection and Metabolic Intervention
4.3. Measurement of Hormone and Glucose Serum Levels
4.4. DNA and RNA Isolation
4.5. RT-qPCR
4.6. Methylated DNA Immunoprecipitation (MeDIP)-qPCR
4.7. Statistical Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- McCartney, C.R.; Marshall, J.C. Polycystic Ovary Syndrome. N. Engl. J. Med. 2016, 375, 1398–1399. [Google Scholar] [CrossRef] [PubMed]
- Sirmans, S.M.; Pate, K.A. Epidemiology, diagnosis, and management of polycystic ovary syndrome. Clin. Epidemiol. 2013, 6, 1–13. [Google Scholar] [CrossRef] [PubMed]
- Teede, H.; Deeks, A.; Moran, L. Polycystic ovary syndrome: A complex condition with psychological, reproductive and metabolic manifestations that impacts on health across the lifespan. BMC Med. 2010, 8, 41. [Google Scholar] [CrossRef] [PubMed]
- Evans, J.; Salamonsen, L.A.; Winship, A.; Menkhorst, E.; Nie, G.; Gargett, C.E.; Dimitriadis, E. Fertile ground: Human endometrial programming and lessons in health and disease. Nat. Rev. Endocrinol. 2016, 12, 654–667. [Google Scholar] [CrossRef]
- Szydlarska, D.; Machaj, M.; Jakimiuk, A. History of discovery of polycystic ovary syndrome. Adv. Clin. Exp. Med. 2017, 26, 555–558. [Google Scholar] [CrossRef]
- Liao, B.; Qiao, J.; Pang, Y. Central Regulation of PCOS: Abnormal Neuronal-Reproductive-Metabolic Circuits in PCOS Pathophysiology. Front. Endocrinol. (Lausanne) 2021, 12, 667422. [Google Scholar] [CrossRef]
- Trikudanathan, S. Polycystic ovarian syndrome. Med. Clin. North. Am. 2015, 99, 221–235. [Google Scholar] [CrossRef]
- Xu, Y.; Qiao, J. Association of Insulin Resistance and Elevated Androgen Levels with Polycystic Ovarian Syndrome (PCOS): A Review of Literature. J. Healthc. Eng. 2022, 2022, 9240569. [Google Scholar] [CrossRef]
- Piltonen, T.T.; Chen, J.C.; Khatun, M.; Kangasniemi, M.; Liakka, A.; Spitzer, T.; Tran, N.; Huddleston, H.; Irwin, J.C.; Giudice, L.C. Endometrial stromal fibroblasts from women with polycystic ovary syndrome have impaired progesterone-mediated decidualization, aberrant cytokine profiles and promote enhanced immune cell migration in vitro. Hum. Reprod. 2015, 30, 1203–1215. [Google Scholar] [CrossRef]
- Bellver, J.; Simon, C. Implantation failure of endometrial origin: What is new? Curr. Opin. Obstet. Gynecol. 2018, 30, 229–236. [Google Scholar] [CrossRef]
- Savaris, R.F.; Groll, J.M.; Young, S.L.; DeMayo, F.J.; Jeong, J.W.; Hamilton, A.E.; Giudice, L.C.; Lessey, B.A. Progesterone resistance in PCOS endometrium: A microarray analysis in clomiphene citrate-treated and artificial menstrual cycles. J. Clin. Endocrinol. Metab. 2011, 96, 1737–1746. [Google Scholar] [CrossRef] [PubMed]
- Qiao, J.; Wang, L.; Li, R.; Zhang, X. Microarray evaluation of endometrial receptivity in Chinese women with polycystic ovary syndrome. Reprod. Biomed. Online 2008, 17, 425–435. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.Y.; Song, H.; Kim, H.; Kang, H.J.; Jun, J.H.; Hong, S.R.; Koong, M.K.; Kim, I.S. Transcriptional profiling with a pathway-oriented analysis identifies dysregulated molecular phenotypes in the endometrium of patients with polycystic ovary syndrome. J. Clin. Endocrinol. Metab. 2009, 94, 1416–1426. [Google Scholar] [CrossRef] [PubMed]
- Senturk, S.; Celik, O.; Dalkilic, S.; Hatirnaz, S.; Celik, N.; Unlu, C.; Otlu, B. Laparoscopic Ovarian Drilling Improves Endometrial Homeobox Gene Expression in PCOS. Reprod. Sci. 2020, 27, 675–680. [Google Scholar] [CrossRef] [PubMed]
- Cermik, D.; Selam, B.; Taylor, H.S. Regulation of HOXA-10 expression by testosterone in vitro and in the endometrium of patients with polycystic ovary syndrome. J. Clin. Endocrinol. Metab. 2003, 88, 238–243. [Google Scholar] [CrossRef] [PubMed]
- Ekanayake, D.L.; Malopolska, M.M.; Schwarz, T.; Tuz, R.; Bartlewski, P.M. The roles and expression of HOXA/Hoxa10 gene: A prospective marker of mammalian female fertility? Reprod. Biol. 2022, 22, 100647. [Google Scholar] [CrossRef]
- Bellver, J.; Martinez-Conejero, J.A.; Labarta, E.; Alama, P.; Melo, M.A.; Remohi, J.; Pellicer, A.; Horcajadas, J.A. Endometrial gene expression in the window of implantation is altered in obese women especially in association with polycystic ovary syndrome. Fertil. Steril. 2011, 95, 2335–2341.e8. [Google Scholar] [CrossRef]
- Roemer, K.L.; Young, S.L.; Savaris, R.F. Characterization of GAB1 expression over the menstrual cycle in women with and without polycystic ovarian syndrome provides a new insight into its pathophysiology. J. Clin. Endocrinol. Metab. 2014, 99, E2162–E2168. [Google Scholar] [CrossRef]
- Ujvari, D.; Hulchiy, M.; Calaby, A.; Nybacka, A.; Bystrom, B.; Hirschberg, A.L. Lifestyle intervention up-regulates gene and protein levels of molecules involved in insulin signaling in the endometrium of overweight/obese women with polycystic ovary syndrome. Hum. Reprod. 2014, 29, 1526–1535. [Google Scholar] [CrossRef]
- Vrhovac Madunić, I.; Karin-Kujundžić, V.; Madunić, J.; Šola, I.M.; Šerman, L. Endometrial Glucose Transporters in Health and Disease. Front. Cell. Dev. Biol. 2021, 9, 703671. [Google Scholar] [CrossRef]
- Villavicencio, A.; Bacallao, K.; Avellaira, C.; Gabler, F.; Fuentes, A.; Vega, M. Androgen and estrogen receptors and co-regulators levels in endometria from patients with polycystic ovarian syndrome with and without endometrial hyperplasia. Gynecol. Oncol. 2006, 103, 307–314. [Google Scholar] [CrossRef] [PubMed]
- Hulchiy, M.; Nybacka, A.; Sahlin, L.; Hirschberg, A.L. Endometrial Expression of Estrogen Receptors and the Androgen Receptor in Women With Polycystic Ovary Syndrome: A Lifestyle Intervention Study. J. Clin. Endocrinol. Metab. 2016, 101, 561–571. [Google Scholar] [CrossRef] [PubMed]
- Zheng, X.R.; Pan, X.; Zhang, J.; Cao, X. Hyperinsulinemia-induced PAX6 expression promotes endometrial epithelial cell proliferation via negatively modulating p27 signaling. Biomed. Pharmacother. 2018, 97, 802–808. [Google Scholar] [CrossRef]
- Liu, Y.N.; Qin, Y.; Wu, B.; Peng, H.; Li, M.; Luo, H.; Liu, L.L. DNA methylation in polycystic ovary syndrome: Emerging evidence and challenges. Reprod. Toxicol. 2022, 111, 11–19. [Google Scholar] [CrossRef] [PubMed]
- McAllister, J.M.; Jones, M.R.; Brower, M.A.; Xu, N.; Cui, J.; Mengesha, E.; Chen, Y.-D.I.; Taylor, K.D.; Azziz, R.; Goodarzi, M.O. Systems Genetics Reveals the Functional Context of PCOS Loci and Identifies Genetic and Molecular Mechanisms of Disease Heterogeneity. PLoS Genet. 2015, 11, e1005455. [Google Scholar]
- Tan, Q. Deciphering the DNA Methylome of Polycystic Ovary Syndrome. Mol. Diagn. Ther. 2020, 24, 245–250. [Google Scholar] [CrossRef]
- Eiras, M.C.; Pinheiro, D.P.; Romcy, K.A.M.; Ferriani, R.A.; Reis, R.M.D.; Furtado, C.L.M. Polycystic Ovary Syndrome: The Epigenetics Behind the Disease. Reprod. Sci. 2022, 29, 680–694. [Google Scholar] [CrossRef]
- Zhong, X.; Jin, F.; Huang, C.; Du, M.; Gao, M.; Wei, X. DNA methylation of AMHRII and INSR gene is associated with the pathogenesis of Polycystic Ovary Syndrome (PCOS). Technol. Health Care 2021, 29, 11–25. [Google Scholar] [CrossRef]
- Pan, J.-X.; Tan, Y.-J.; Wang, F.-F.; Hou, N.-N.; Xiang, Y.-Q.; Zhang, J.-Y.; Liu, Y.; Qu, F.; Meng, Q.; Xu, J.; et al. Aberrant expression and DNA methylation of lipid metabolism genes in PCOS: A new insight into its pathogenesis. Clin. Epigenet. 2018, 10, 6. [Google Scholar] [CrossRef]
- Yu, Y.Y.; Sun, C.X.; Liu, Y.K.; Li, Y.; Wang, L.; Zhang, W. Promoter methylation of CYP19A1 gene in Chinese polycystic ovary syndrome patients. Gynecol. Obstet. Invest. 2013, 76, 209–213. [Google Scholar] [CrossRef]
- Lin, Y.; Xing, F.Q.; Ou, Z.Y.; Liang, J.L.; Wen, A.M.; Chen, Y. Relation between insulin resistance and insulin receptor gene methylation in the endometrium of patients with polycystic ovary syndrome. Nan Fang. Yi Ke Da Xue Xue Bao 2011, 31, 867–870. [Google Scholar] [PubMed]
- Vázquez-Martínez, E.R.; Gómez-Viais, Y.I.; García-Gómez, E.; Reyes-Mayoral, C.; Reyes-Muñoz, E.; Camacho-Arroyo, I.; Cerbón, M. DNA methylation in the pathogenesis of polycystic ovary syndrome. Reproduction 2019, 158, R27–R40. [Google Scholar] [CrossRef]
- Yuan, L.; Wu, H.; Huang, W.; Bi, Y.; Qin, A.; Yang, Y. The function of metformin in endometrial receptivity (ER) of patients with polycyclic ovary syndrome (PCOS): A systematic review and meta-analysis. Reprod. Biol. Endocrinol. 2021, 19, 89. [Google Scholar] [CrossRef] [PubMed]
- Zhai, J.; Liu, C.X.; Tian, Z.R.; Jiang, Q.H.; Sun, Y.P. Effects of metformin on the expression of GLUT4 in endometrium of obese women with polycystic ovary syndrome. Biol. Reprod. 2012, 87, 29. [Google Scholar] [CrossRef]
- Shao, R.; Li, X.; Feng, Y.; Lin, J.F.; Billig, H. Direct effects of metformin in the endometrium: A hypothetical mechanism for the treatment of women with PCOS and endometrial carcinoma. J. Exp. Clin. Cancer Res. 2014, 33, 41. [Google Scholar] [CrossRef]
- Orostica, M.L.; Astorga, I.; Plaza-Parrochia, F.; Poblete, C.; Carvajal, R.; Garcia, V.; Romero, C.; Vega, M. Metformin Treatment Regulates the Expression of Molecules Involved in Adiponectin and Insulin Signaling Pathways in Endometria from Women with Obesity-Associated Insulin Resistance and PCOS. Int. J. Mol. Sci. 2022, 23, 3922. [Google Scholar] [CrossRef] [PubMed]
- Zhong, T.; Men, Y.; Lu, L.; Geng, T.; Zhou, J.; Mitsuhashi, A.; Shozu, M.; Maihle, N.J.; Carmichael, G.G.; Taylor, H.S.; et al. Metformin alters DNA methylation genome-wide via the H19/SAHH axis. Oncogene 2017, 36, 2345–2354. [Google Scholar] [CrossRef] [PubMed]
- Yan, L.; Zhou, J.; Gao, Y.; Ghazal, S.; Lu, L.; Bellone, S.; Yang, Y.; Liu, N.; Zhao, X.; Santin, A.D.; et al. Regulation of tumor cell migration and invasion by the H19/let-7 axis is antagonized by metformin-induced DNA methylation. Oncogene 2015, 34, 3076–3084. [Google Scholar] [CrossRef]
- Xue, Z.; Li, J.; Feng, J.; Han, H.; Zhao, J.; Zhang, J.; Han, Y.; Wu, X.; Zhang, Y. Research Progress on the Mechanism Between Polycystic Ovary Syndrome and Abnormal Endometrium. Front. Physiol. 2021, 12, 788772. [Google Scholar] [CrossRef]
- Palomba, S.; Piltonen, T.T.; Giudice, L.C. Endometrial function in women with polycystic ovary syndrome: A comprehensive review. Hum. Reprod. Update 2021, 27, 584–618. [Google Scholar] [CrossRef]
- Bruni, V.; Capozzi, A.; Lello, S. The Role of Genetics, Epigenetics and Lifestyle in Polycystic Ovary Syndrome Development: The State of the Art. Reprod. Sci. 2022, 29, 668–679. [Google Scholar] [CrossRef] [PubMed]
- Qu, F.; Wang, F.F.; Yin, R.; Ding, G.L.; El-Prince, M.; Gao, Q.; Shi, B.W.; Pan, H.H.; Huang, Y.T.; Jin, M.; et al. A molecular mechanism underlying ovarian dysfunction of polycystic ovary syndrome: Hyperandrogenism induces epigenetic alterations in the granulosa cells. J. Mol. Med. (Berl) 2012, 90, 911–923. [Google Scholar] [CrossRef] [PubMed]
- Combs, J.C.; Hill, M.J.; Decherney, A.H. Polycystic Ovarian Syndrome Genetics and Epigenetics. Clin. Obstet. Gynecol. 2021, 64, 20–25. [Google Scholar] [CrossRef] [PubMed]
- Stener-Victorin, E.; Deng, Q. Epigenetic inheritance of polycystic ovary syndrome-challenges and opportunities for treatment. Nat. Rev. Endocrinol. 2021, 17, 521–533. [Google Scholar] [CrossRef]
- Chen, J.; Shen, S.; Tan, Y.; Xia, D.; Xia, Y.; Cao, Y.; Wang, W.; Wu, X.; Wang, H.; Yi, L.; et al. The correlation of aromatase activity and obesity in women with or without polycystic ovary syndrome. J. Ovarian Res. 2015, 8, 11. [Google Scholar] [CrossRef]
- Eldar-Geva, T.; Spitz, I.M.; Groome, N.P.; Margalioth, E.J.; Homburg, R. Follistatin and activin A serum concentrations in obese and non-obese patients with polycystic ovary syndrome. Hum. Reprod. 2001, 16, 2552–2556. [Google Scholar] [CrossRef]
- Reyes-Muñoz, E.; Ortega-Gonzalez, C.; Martínez-Cruz, N.; Arce-Sánchez, L.; Estrada-Gutierrez, G.; Morán, C.; Sánchez-Serrano, A.P.; Higareda-Sánchez, R.; de la Jara-Díaz, J.F. Association of obesity and overweight with the prevalence of insulin resistance, pre-diabetes and clinical-biochemical characteristics among infertile Mexican women with polycystic ovary syndrome: A cross-sectional study. BMJ Open. 2016, 6, e012107. [Google Scholar] [CrossRef]
- Glueck, C.J.; Pranikoff, J.; Aregawi, D.; Wang, P. Prevention of gestational diabetes by metformin plus diet in patients with polycystic ovary syndrome. Fertil. Steril. 2008, 89, 625–634. [Google Scholar] [CrossRef]
- García-Hernández, S.C.; Porchia, L.M.; Pacheco-Soto, B.T.; López-Bayghen, E.; González-Mejía, M.E. Metformin does not improve insulin sensitivity over hypocaloric diets in women with polycystic ovary syndrome: A systematic review of 12 studies. Gynecol. Endocrinol. 2021, 37, 968–976. [Google Scholar] [CrossRef]
- Frary, J.M.; Bjerre, K.P.; Glintborg, D.; Ravn, P. The effect of dietary carbohydrates in women with polycystic ovary syndrome: A systematic review. Minerva Endocrinol. 2016, 41, 57–69. [Google Scholar]
- Oppelt, P.G.; Mueller, A.; Jentsch, K.; Kronawitter, D.; Reissmann, C.; Dittrich, R.; Beckmann, M.W.; Cupisti, S. The effect of metformin treatment for 2 years without caloric restriction on endocrine and metabolic parameters in women with polycystic ovary syndrome. Exp. Clin. Endocrinol. Diabetes 2010, 118, 633–637. [Google Scholar] [CrossRef]
- Zhou, W.; Santos, L.; Dimitriadis, E. Characterization of the role for cadherin 6 in the regulation of human endometrial receptivity. Reprod. Biol. Endocrinol. 2020, 18, 66. [Google Scholar] [CrossRef] [PubMed]
- Lai, T.H.; Chang, F.W.; Lin, J.J.; Ling, Q.D. Gene expression of human endometrial L-selectin ligand in relation to the phases of the natural menstrual cycle. Sci. Rep. 2018, 8, 1443. [Google Scholar] [CrossRef] [PubMed]
- Casals, G.; Ordi, J.; Creus, M.; Fabregues, F.; Casamitjana, R.; Quinto, L.; Campo, E.; Balasch, J. Osteopontin and alphavbeta3 integrin expression in the endometrium of infertile and fertile women. Reprod. Biomed. Online 2008, 16, 808–816. [Google Scholar] [CrossRef] [PubMed]
- Kimber, S.J. Leukaemia inhibitory factor in implantation and uterine biology. Reproduction 2005, 130, 131–145. [Google Scholar] [CrossRef]
- Gregory, C.W.; Wilson, E.M.; Apparao, K.B.; Lininger, R.A.; Meyer, W.R.; Kowalik, A.; Fritz, M.A.; Lessey, B.A. Steroid receptor coactivator expression throughout the menstrual cycle in normal and abnormal endometrium. J. Clin. Endocrinol. Metab. 2002, 87, 2960–2966. [Google Scholar] [CrossRef]
- Quezada, S.; Avellaira, C.; Johnson, M.C.; Gabler, F.; Fuentes, A.; Vega, M. Evaluation of steroid receptors, coregulators, and molecules associated with uterine receptivity in secretory endometria from untreated women with polycystic ovary syndrome. Fertil. Steril. 2006, 85, 1017–1026. [Google Scholar] [CrossRef]
- Uchikawa, J.; Shiozawa, T.; Shih, H.C.; Miyamoto, T.; Feng, Y.Z.; Kashima, H.; Oka, K.; Konishi, I. Expression of steroid receptor coactivators and corepressors in human endometrial hyperplasia and carcinoma with relevance to steroid receptors and Ki-67 expression. Cancer 2003, 98, 2207–2213. [Google Scholar] [CrossRef]
- Bircan, S.; Ensari, A.; Ozturk, S.; Erdogan, N.; Dundar, I.; Ortac, F. Immunohistochemical analysis of c-myc, c-jun and estrogen receptor in normal, hyperplastic and neoplastic endometrium. Pathol. Oncol. Res. 2005, 11, 32–39. [Google Scholar] [CrossRef]
- Chen, S.U.; Chou, C.H.; Chen, M.J.; Chen, T.H.; Yang, Y.S.; Yang, J.H. Apoptotic effects of high estradiol concentrations on endometrial glandular cells. J. Clin. Endocrinol. Metab. 2014, 99, E971–E980. [Google Scholar] [CrossRef]
- Bulun, S.E.; Monsavais, D.; Pavone, M.E.; Dyson, M.; Xue, Q.; Attar, E.; Tokunaga, H.; Su, E.J. Role of estrogen receptor-beta in endometriosis. Semin. Reprod. Med. 2012, 30, 39–45. [Google Scholar] [PubMed]
- Wang, A.; Ji, L.; Shang, W.; Li, M.; Chen, L.; White, R.E.; Han, G. Expression of GPR30, ERalpha and ERbeta in endometrium during window of implantation in patients with polycystic ovary syndrome: A pilot study. Gynecol. Endocrinol. 2011, 27, 251–255. [Google Scholar] [CrossRef] [PubMed]
- Fornes, R.; Ormazabal, P.; Rosas, C.; Gabler, F.; Vantman, D.; Romero, C.; Vega, M. Changes in the expression of insulin signaling pathway molecules in endometria from polycystic ovary syndrome women with or without hyperinsulinemia. Mol. Med. 2010, 16, 129–136. [Google Scholar] [CrossRef] [PubMed]
- Krikun, G.; Schatz, F.; Taylor, R.; Critchley, H.O.; Rogers, P.A.; Huang, J.; Lockwood, C.J. Endometrial endothelial cell steroid receptor expression and steroid effects on gene expression. J. Clin. Endocrinol. Metab. 2005, 90, 1812–1818. [Google Scholar] [CrossRef]
- Li, X.; Cui, P.; Jiang, H.Y.; Guo, Y.R.; Pishdari, B.; Hu, M.; Feng, Y.; Billig, H.; Shao, R. Reversing the reduced level of endometrial GLUT4 expression in polycystic ovary syndrome: A mechanistic study of metformin action. Am. J. Transl. Res. 2015, 7, 574–586. [Google Scholar]
- Liu, J.; Zhao, Y.; Chen, L.; Li, R.; Ning, Y.; Zhu, X. Role of metformin in functional endometrial hyperplasia and polycystic ovary syndrome involves the regulation of MEG3/miR223/GLUT4 and SNHG20/miR4486/GLUT4 signaling. Mol. Med. Rep. 2022, 26, 218. [Google Scholar] [CrossRef]
- Sang, Q.; Zhang, S.; Zou, S.; Wang, H.; Feng, R.; Li, Q.; Jin, L.; He, L.; Xing, Q.; Wang, L. Quantitative analysis of follistatin (FST) promoter methylation in peripheral blood of patients with polycystic ovary syndrome. Reprod. Biomed. Online 2013, 26, 157–163. [Google Scholar] [CrossRef]
- Sagvekar, P.; Mangoli, V.; Desai, S.; Patil, A.; Mukherjee, S. LINE1 CpG-DNA Hypomethylation in Granulosa Cells and Blood Leukocytes Is Associated With PCOS and Related Traits. J. Clin. Endocrinol. Metab. 2017, 102, 1396–1405. [Google Scholar] [CrossRef]
- Pruksananonda, K.; Wasinarom, A.; Sereepapong, W.; Sirayapiwat, P.; Rattanatanyong, P.; Mutirangura, A. Epigenetic modification of long interspersed elements-1 in cumulus cells of mature and immature oocytes from patients with polycystic ovary syndrome. Clin. Exp. Reprod. Med. 2016, 43, 82–89. [Google Scholar] [CrossRef]
- Jiao, J.; Sagnelli, M.; Shi, B.; Fang, Y.; Shen, Z.; Tang, T.; Dong, B.; Li, D.; Wang, X. Genetic and epigenetic characteristics in ovarian tissues from polycystic ovary syndrome patients with irregular menstruation resemble those of ovarian cancer. BMC Endocr. Disord. 2019, 19, 30. [Google Scholar] [CrossRef]
- Wu, Y.; Halverson, G.; Basir, Z.; Strawn, E.; Yan, P.; Guo, S.W. Aberrant methylation at HOXA10 may be responsible for its aberrant expression in the endometrium of patients with endometriosis. Am. J. Obstet. Gynecol. 2005, 193, 371–380. [Google Scholar] [CrossRef] [PubMed]
- Yu, M.; Tang, J.; Huang, Y.; Guo, C.; Du, P.; Li, N.; Quan, Q. HOXA10 Regulates the Synthesis of Cholesterol in Endometrial Stromal Cells. Front. Endocrinol. (Lausanne) 2022, 13, 852671. [Google Scholar] [CrossRef] [PubMed]
- Lee, B.; Du, H.; Taylor, H.S. Experimental murine endometriosis induces DNA methylation and altered gene expression in eutopic endometrium. Biol. Reprod. 2009, 80, 79–85. [Google Scholar] [CrossRef]
- Kim, J.J.; Taylor, H.S.; Lu, Z.; Ladhani, O.; Hastings, J.M.; Jackson, K.S.; Wu, Y.; Guo, S.W.; Fazleabas, A.T. Altered expression of HOXA10 in endometriosis: Potential role in decidualization. Mol. Hum. Reprod. 2007, 13, 323–332. [Google Scholar] [CrossRef]
- Andersson, K.L.; Bussani, C.; Fambrini, M.; Polverino, V.; Taddei, G.L.; Gemzell-Danielsson, K.; Scarselli, G. DNA methylation of HOXA10 in eutopic and ectopic endometrium. Hum. Reprod. 2014, 29, 1906–1911. [Google Scholar] [CrossRef] [PubMed]
- Fambrini, M.; Sorbi, F.; Bussani, C.; Cioni, R.; Sisti, G.; Andersson, K.L. Hypermethylation of HOXA10 gene in mid-luteal endometrium from women with ovarian endometriomas. Acta Obstet. Gynecol. Scand. 2013, 92, 1331–1334. [Google Scholar] [CrossRef]
- Kulp, J.L.; Mamillapalli, R.; Taylor, H.S. Aberrant HOXA10 Methylation in Patients with Common Gynecologic Disorders: Implications for Reproductive Outcomes. Reprod. Sci. 2016, 23, 455–463. [Google Scholar] [CrossRef]
- Kokosar, M.; Benrick, A.; Perfilyev, A.; Nilsson, E.; Kallman, T.; Ohlsson, C.; Ling, C.; Stener-Victorin, E. A Single Bout of Electroacupuncture Remodels Epigenetic and Transcriptional Changes in Adipose Tissue in Polycystic Ovary Syndrome. Sci. Rep. 2018, 8, 1878. [Google Scholar] [CrossRef]
- Kokosar, M.; Benrick, A.; Perfilyev, A.; Fornes, R.; Nilsson, E.; Maliqueo, M.; Behre, C.J.; Sazonova, A.; Ohlsson, C.; Ling, C.; et al. Epigenetic and Transcriptional Alterations in Human Adipose Tissue of Polycystic Ovary Syndrome. Sci. Rep. 2016, 6, 22883. [Google Scholar] [CrossRef]
- Amani Abkenari, S.; Safdarian, L.; Amidi, F.; Hosseini, A.; Aryanpour, R.; Salahi, E.; Sobhani, A. Metformin improves epigenetic modification involved in oocyte growth and embryo development in polycystic ovary syndrome mice model. Mol. Reprod. Dev. 2021, 88, 817–829. [Google Scholar] [CrossRef]
- Mifflin, M.D.; St Jeor, S.T.; Hill, L.A.; Scott, B.J.; Daugherty, S.A.; Koh, Y.O. A new predictive equation for resting energy expenditure in healthy individuals. Am. J. Clin. Nutr. 1990, 51, 241–247. [Google Scholar] [CrossRef] [PubMed]
- Fox, R.; Corrigan, E.; Thomas, P.A.; Hull, M.G. The diagnosis of polycystic ovaries in women with oligo-amenorrhoea: Predictive power of endocrine tests. Clin. Endocrinol. (Oxf) 1991, 34, 127–131. [Google Scholar] [CrossRef] [PubMed]
- Sawada, M.; Masuyama, H.; Hayata, K.; Kamada, Y.; Nakamura, K.; Hiramatsu, Y. Pregnancy complications and glucose intolerance in women with polycystic ovary syndrome. Endocr. J. 2015, 62, 1017–1023. [Google Scholar] [CrossRef] [PubMed]
- Livak, K.J.; Schmittgen, T.D. Analysis of relative gene expression data using real-time quantitative PCR and the 2(-Delta Delta C(T)) Method. Methods 2001, 25, 402–408. [Google Scholar] [CrossRef] [PubMed]
- Vázquez-Martínez, E.R.; Camacho-Arroyo, I.; Zarain-Herzberg, A.; Rodríguez, M.C.; Mendoza-Garcés, L.; Ostrosky-Wegman, P.; Cerbón, M. Estradiol differentially induces progesterone receptor isoforms expression through alternative promoter regulation in a mouse embryonic hypothalamic cell line. Endocrine 2016, 52, 618–631. [Google Scholar] [CrossRef]

| Variable | CONTROL (n = 8) | PCOS (n = 15) |
|---|---|---|
| Age (years old) | 27.80 ± 5.1 | 27.70 ± 4.5 |
| BMI (kg/m2) | 25.50 ± 3.2 | 27.11 ± 4.7 |
| Androstenedione (ng/mL) | 2.08 ± 0.60 | 3.57 ± 1.45 * |
| DHEA (µg/dL) | 150.80 ± 79.92 | 161.00 ± 94.98 |
| Estradiol (pg/mL) | 39.30 ± 15.76 | 62.67 ± 12.75 * |
| FSH (mIU/mL) | 5.59 ± 1.58 | 5.40 ± 1.25 |
| LH (mIU/mL) | 4.42 ± 2.11 | 7.01 ± 3.26 |
| LH/FSH | 0.80 ± 0.41 | 1.28 ± 0.40 * |
| Progesterone (ng/mL) | 0.28 ± 0.07 | 0.25 ± 0.06 |
| Prolactin (ng/mL) | 11.53 ± 4.56 | 10.17 ± 3.53 |
| SHBG (nmol/L) | 44.8 ± 27.32 | 28.57 ± 12.34 |
| Testosterone (nmol/mL) | 0.81 ± 0.13 | 1.49 ± 0.40 * |
| FAI index | 2.21 ± 0.93 | 6.04 ± 2.96 * |
| Glucose (mg/dL) | 82.17 ± 8.19 | 89.54 ± 11.10 |
| Insulin (µIU/mL) | 5.17 ± 3.10 | 15.67 ± 11.37 |
| HOMA-IR index | 1.04 ± 0.56 | 3.51 ± 2.62 * |
| Variable | PCOS (n = 8) | PCOS + MET (n = 8) |
|---|---|---|
| BMI (kg/m2) | 28.51 ± 4.68 | 27.08 ± 5.06 * |
| Androstenedione (ng/mL) | 4.46 ± 1.60 | 3.41 ± 0.98 * |
| Estradiol (pg/mL) | 69.73 ± 10.42 | 59.80 ± 10.16 * |
| Insulin (µIU/mL) | 21.45 ± 11.07 | 11.32 ± 7.13 * |
| HOMA-IR index | 4.90 ± 2.74 | 2.39 ± 1.43 * |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
García-Gómez, E.; Gómez-Viais, Y.I.; Cruz-Aranda, M.M.; Martínez-Razo, L.D.; Reyes-Mayoral, C.; Ibarra-González, L.; Montoya-Estrada, A.; Osorio-Caballero, M.; Perichart-Perera, O.; Camacho-Arroyo, I.; et al. The Effect of Metformin and Carbohydrate-Controlled Diet on DNA Methylation and Gene Expression in the Endometrium of Women with Polycystic Ovary Syndrome. Int. J. Mol. Sci. 2023, 24, 6857. https://doi.org/10.3390/ijms24076857
García-Gómez E, Gómez-Viais YI, Cruz-Aranda MM, Martínez-Razo LD, Reyes-Mayoral C, Ibarra-González L, Montoya-Estrada A, Osorio-Caballero M, Perichart-Perera O, Camacho-Arroyo I, et al. The Effect of Metformin and Carbohydrate-Controlled Diet on DNA Methylation and Gene Expression in the Endometrium of Women with Polycystic Ovary Syndrome. International Journal of Molecular Sciences. 2023; 24(7):6857. https://doi.org/10.3390/ijms24076857
Chicago/Turabian StyleGarcía-Gómez, Elizabeth, Yadira Inés Gómez-Viais, Martin Mizael Cruz-Aranda, Luis Daniel Martínez-Razo, Christian Reyes-Mayoral, Lizeth Ibarra-González, Araceli Montoya-Estrada, Mauricio Osorio-Caballero, Otilia Perichart-Perera, Ignacio Camacho-Arroyo, and et al. 2023. "The Effect of Metformin and Carbohydrate-Controlled Diet on DNA Methylation and Gene Expression in the Endometrium of Women with Polycystic Ovary Syndrome" International Journal of Molecular Sciences 24, no. 7: 6857. https://doi.org/10.3390/ijms24076857
APA StyleGarcía-Gómez, E., Gómez-Viais, Y. I., Cruz-Aranda, M. M., Martínez-Razo, L. D., Reyes-Mayoral, C., Ibarra-González, L., Montoya-Estrada, A., Osorio-Caballero, M., Perichart-Perera, O., Camacho-Arroyo, I., Cerbón, M., Reyes-Muñoz, E., & Vázquez-Martínez, E. R. (2023). The Effect of Metformin and Carbohydrate-Controlled Diet on DNA Methylation and Gene Expression in the Endometrium of Women with Polycystic Ovary Syndrome. International Journal of Molecular Sciences, 24(7), 6857. https://doi.org/10.3390/ijms24076857






