Partners in Postmortem Interval Estimation: X-ray Diffraction and Fourier Transform Spectroscopy
Abstract
1. Introduction
2. Results
2.1. Analysis of Crystallographic Indices in Teeth by XRD to Determine PMI
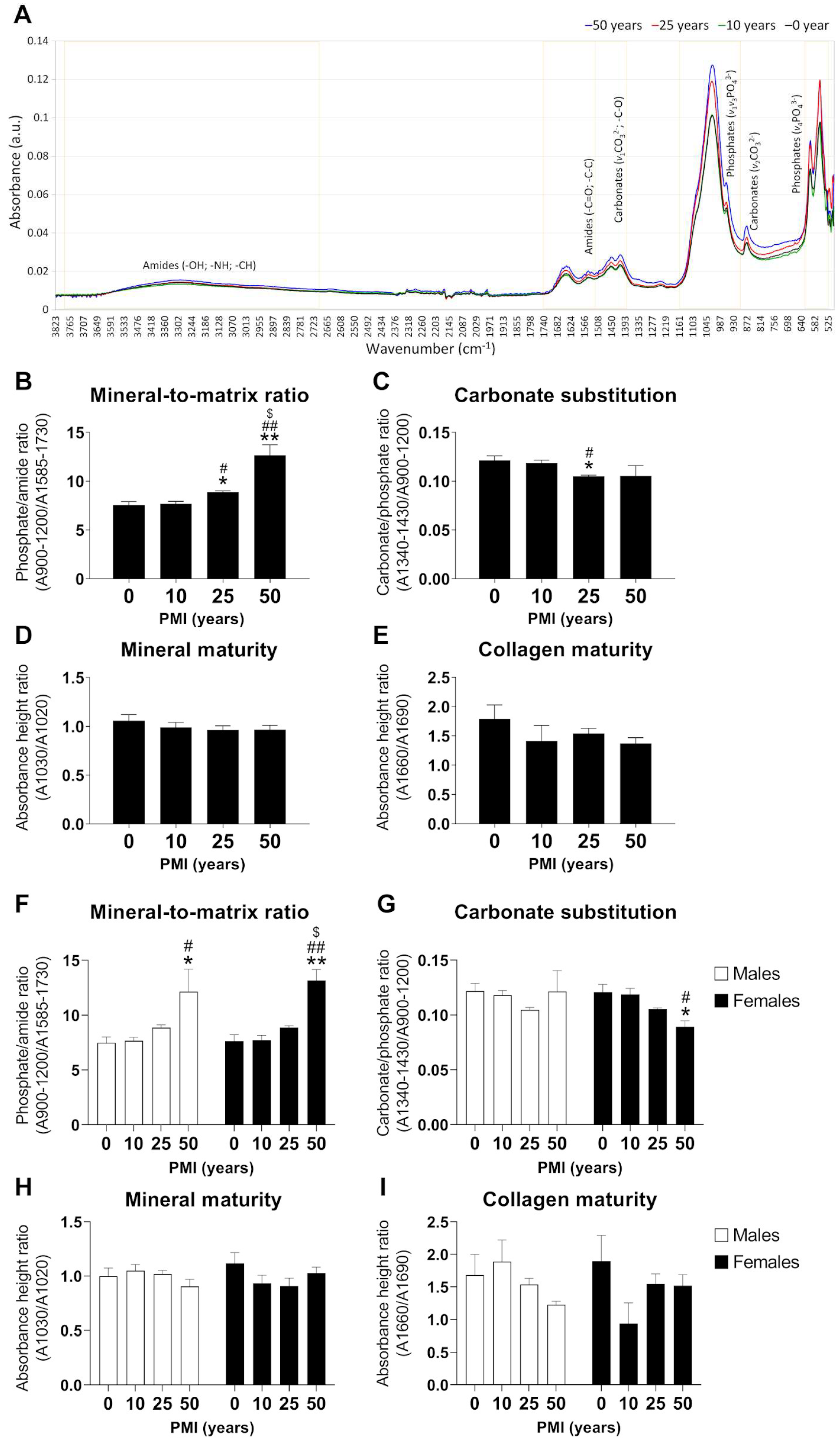
2.2. Analysis of Tooth Composition by ATR-FTIR to Determine PMI
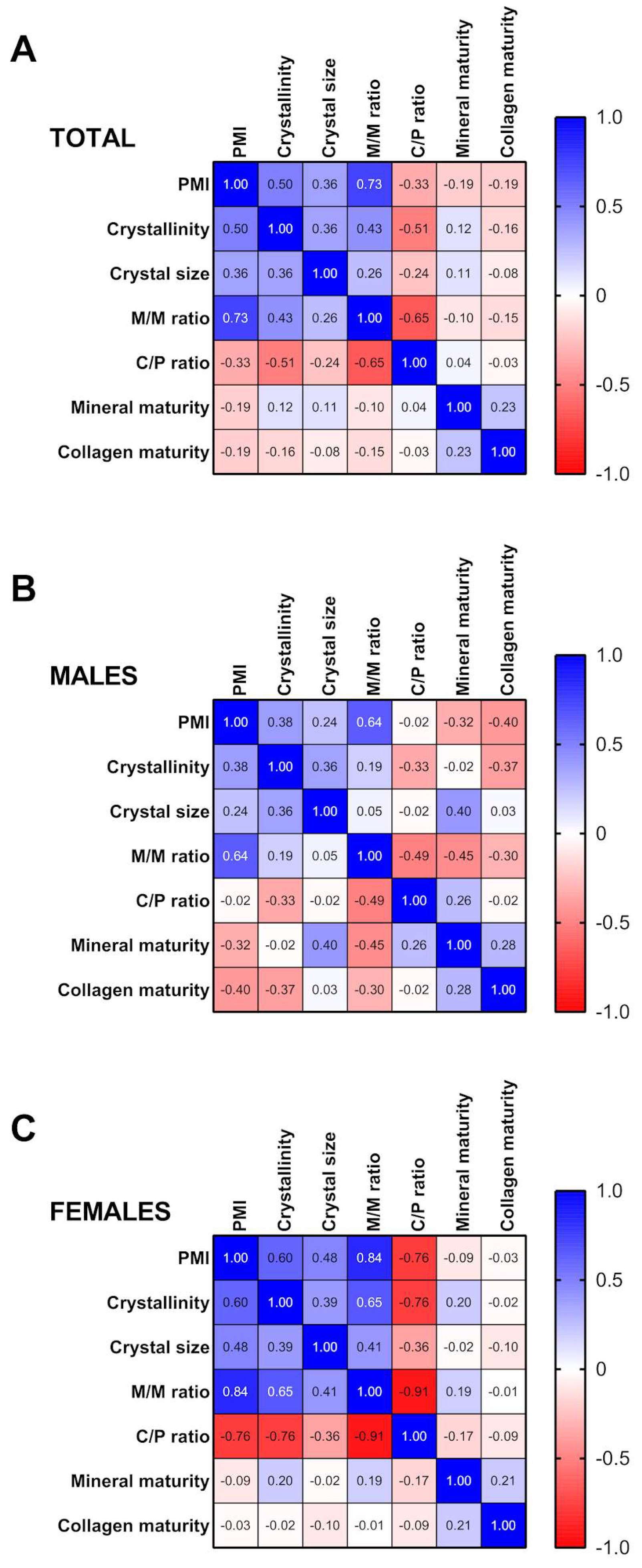
2.3. Correlation Analysis between PMI, Crystallographic Indices and Tooth Compounds
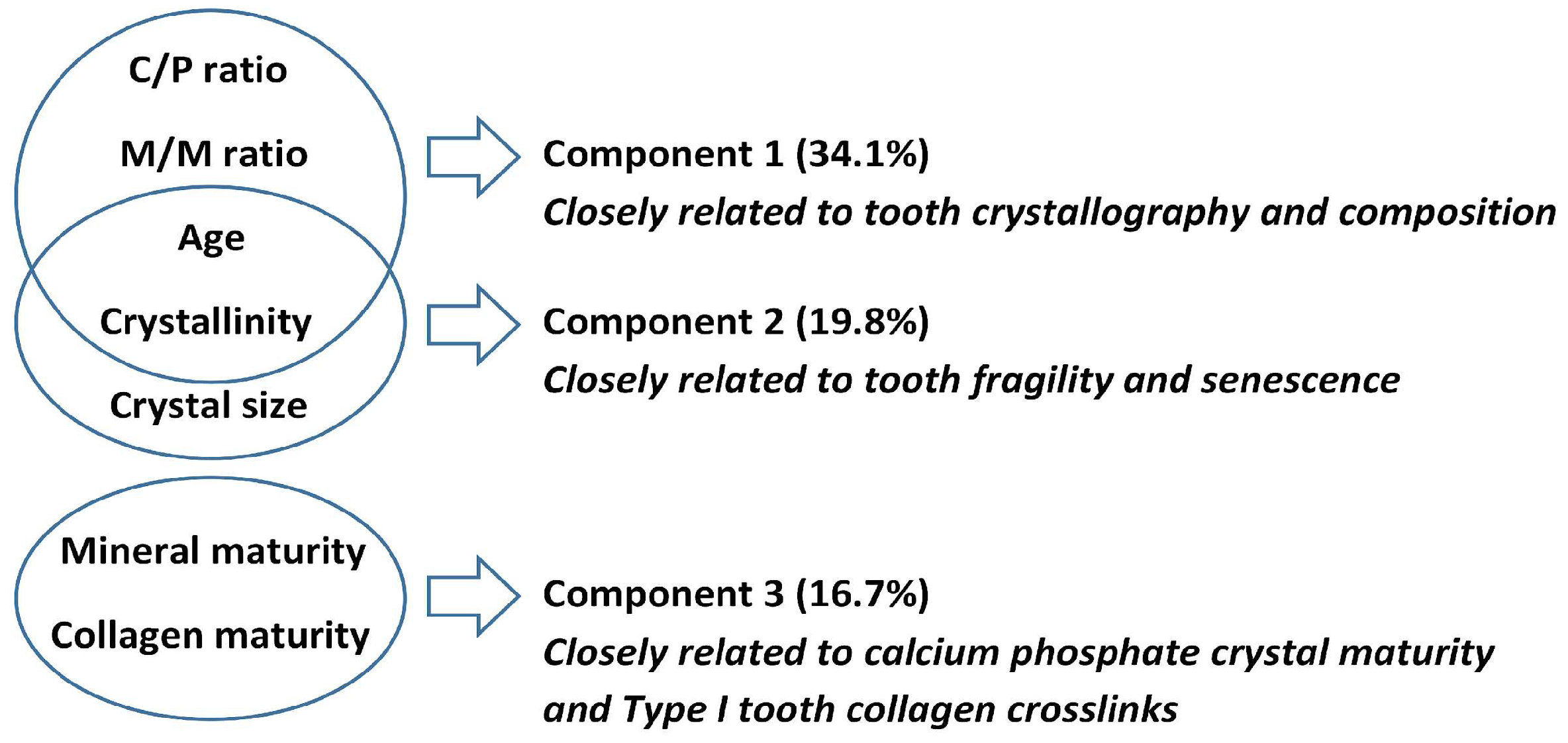
2.4. Changes in Crystallographic Indices and Tooth Compound Parameters Explain PMI
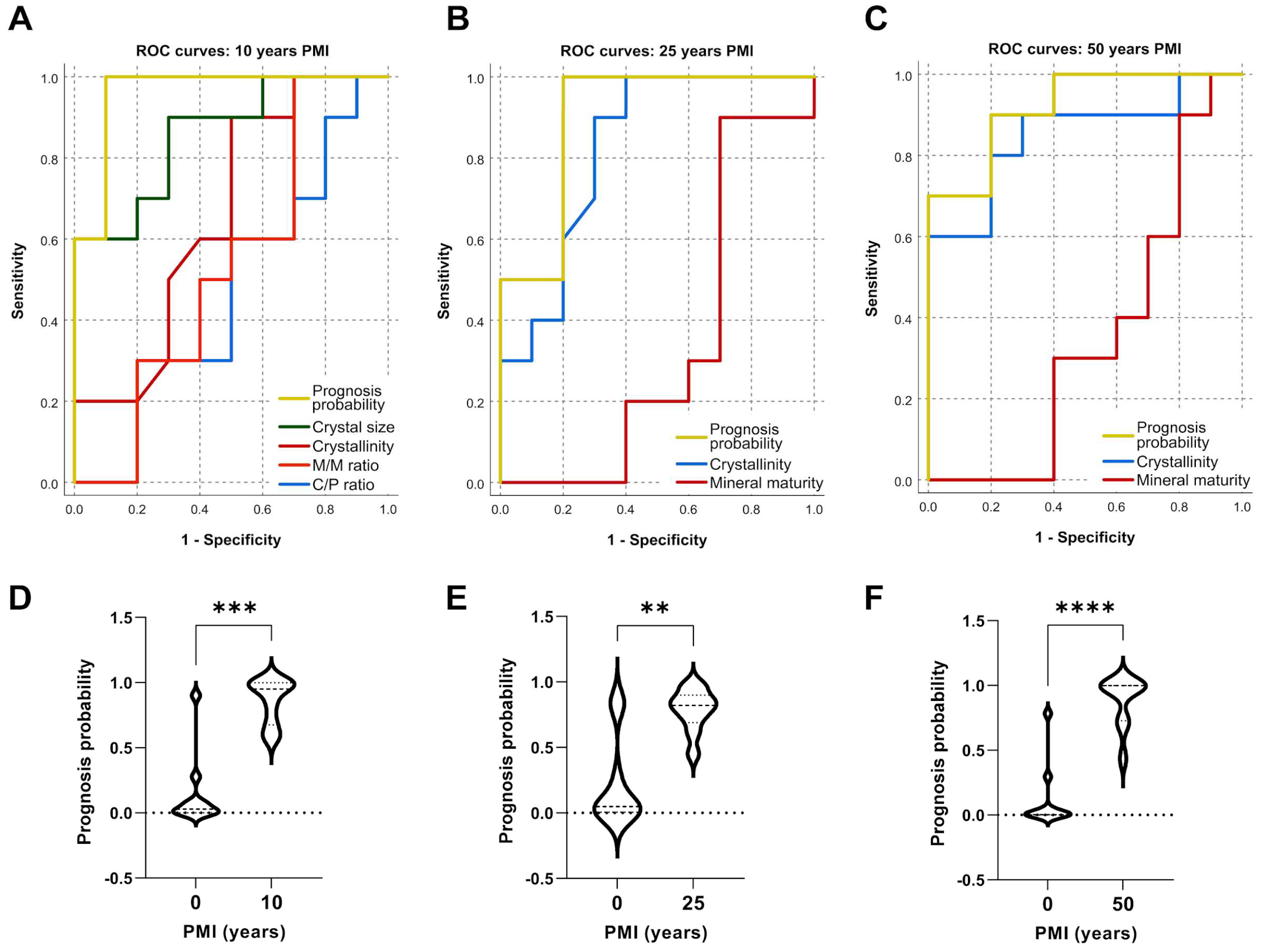
2.5. Predicting PMI from Crystallographic Indices and Tooth Compound Parameters
3. Discussion
4. Materials and Methods
4.1. Samples
4.2. Sample Preparation
4.3. X-ray Powder Diffraction
4.4. ATR-FTIR Spectroscopy
4.5. Statistical Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Hunter, M.C.; Pozhitkov, A.E.; Noble, P.A. Accurate predictions of postmortem interval using linear regression analyses of gene meter expression data. Forensic Sci. Int. 2017, 275, 90–101. [Google Scholar] [CrossRef] [PubMed]
- Ferreira, M.T.; Cunha, E. Será credível estimar o PMI em restos cadavéricos em avançado estado de decomposição? Cad. GEEvH 2012, 1, 7–20. [Google Scholar]
- Shrestha, R.; Kanchan, T.; Krishan, K. Methods of Estimation of Time Since Death. [Updated 15 May 2022]. In StatPearls; StatPearls Publishing: Treasure Island, FL, USA, 2023. Available online: https://www.ncbi.nlm.nih.gov/books/NBK549867/ (accessed on 30 March 2023).
- Zhang, J.; Wei, X.; Huang, J.; Lin, H.; Deng, K.; Li, Z.; Shao, Y.; Zou, D.; Chen, Y.; Huang, P.; et al. Attenuated total reflectance Fourier transform infrared (ATR-FTIR) spectral prediction of postmortem interval from vitreous humor samples. Anal. Bioanal. Chem. 2018, 410, 7611–7620. [Google Scholar] [CrossRef] [PubMed]
- Kumar, S.; Verma, A.K. Estimation of postmortem interval using the data of insulin level in the cadaver’s blood. Data Brief. 2016, 7, 354–356. [Google Scholar] [CrossRef] [PubMed]
- Abo El-Noor, M.M.; Elhosary, N.M.; Khedr, N.F.; El-Desouky, K.I. Estimation of Early Postmortem Interval Through Biochemical and Pathological Changes in Rat Heart and Kidney. Am. J. Forensic Med. Pathol. 2016, 37, 40–46. [Google Scholar] [CrossRef]
- Wang, Q.; Zhang, Y.; Lin, H.; Zha, S.; Fang, R.; Wei, X.; Fan, S.; Wang, Z. Estimation of the late postmortem interval using FTIR spectroscopy and chemometrics in human skeletal remains. Forensic Sci Int. 2017, 28, 113–120. [Google Scholar] [CrossRef]
- Villanueva, P.E.; Girela, F.; Castellanos, M. The application of differential thermal analysis and thermogravimetric analysis to dating bone remains. J Forensic Sci. 1976, 21, 822–830. [Google Scholar] [CrossRef]
- Risoluti, R.; Gullifa, G.; Fineschi, V.; Frati, P.; Materazzi, S. Application of Innovative TGA/Chemometric Approach for Forensic Purposes: The Estimation of the Time since Death in Contaminated Specimens. Diagnostics 2021, 11, 121. [Google Scholar] [CrossRef]
- Akbulut, N.; Çetin, S.; Bilecenoğlu, B.; Altan, A.; Akbulut, S.; Ocak, M.; Orhan, K. The micro-CT evaluation of enamel-cement thickness, abrasion, and mineral density in teeth in the postmortem interval (PMI): New parameters for the determination of PMI. Int J Legal Med. 2020, 134, 645–653. [Google Scholar] [CrossRef]
- Schmidt, V.M.; Zelger, P.; Wöss, C.; Huck, C.W.; Arora, R.; Bechtel, E.; Stahl, A.; Brunner, A.; Zelger, B.; Schirmer, M.; et al. Post-Mortem Interval of Human Skeletal Remains Estimated with Handheld NIR Spectrometry. Biology 2022, 11, 1020. [Google Scholar] [CrossRef]
- Prieto-Bonete, G.; Pérez-Cárceles, M.D.; Maurandi-López, A.; Pérez-Martínez, C.; Luna, A. Association between protein profile and postmortem interval in human bone remains. J. Proteomics. 2019, 192, 54–63. [Google Scholar] [CrossRef]
- Peng, D.; Lv, M.; Li, Z.; Tian, H.; Qu, S.; Jin, B.; Long, B.; Liang, W.; Zhang, L. Postmortem interval determination using mRNA markers and DNA normalization. Int. J. Leg. Med. 2020, 134, 149–157. [Google Scholar] [CrossRef]
- Martínez, P.N.; Menéndez, S.T.; Villaronga, M.L.Á.; Ubelaker, D.H.; García-Pedrero, J.M.; Zapico, S.C. The big sleep: Elucidating the sequence of events in the first hours of death to determine the postmortem interval. Sci. Justice 2019, 59, 418–424. [Google Scholar] [CrossRef]
- Hu, L.; Xing, Y.; Jiang, P.; Gan, L.; Zhao, F.; Peng, W.; Li, W.; Tong, Y.; Deng, S. Predicting the postmortem interval using human intestinal microbiome data and random forest algorithm. Sci. Justice 2021, 61, 516–527. [Google Scholar] [CrossRef]
- Prieto-Castelló, M.J.; Hernández del Rincón, P.; Pérez-Sirvent, C.; Alvarez-Jiménez, P.; Pérez-Cárceles, M.D.; Osuna, E.; Luna, A. Application of biochemical and X-ray diffraction analyses to establish the postmortem interval. Forensic Sci. Int. 2007, 172, 112–118. [Google Scholar] [CrossRef]
- Ortiz-Herrero, L.; Uribe, B.; Armas, L.H.; Alonso, M.L.; Sarmiento, A.; Irurita, J.; Alonso, R.M.; Maguregui, M.I.; Etxeberria, F.; Bartolomé, L. Estimation of the post-mortem interval of human skeletal remains using Raman spectroscopy and chemometrics. Forensic Sci. Int. 2021, 329, 111087. [Google Scholar] [CrossRef]
- Patonai, Z.; Maasz, G.; Avar, P.; Schmidt, J.; Lorand, T.; Bajnoczky, I.; Mark, L. Novel dating method to distinguish between forensic and archeological human skeletal remains by bone mineralization indexes. Int. J. Leg. Med. 2013, 127, 529–533. [Google Scholar] [CrossRef]
- Damann, F.E.; Williams, D.E.; Layton, A.C. Potential Use of Bacterial Community Succession in Decaying Human Bone for Estimating Postmortem Interval. J. Forensic Sci. 2015, 60, 844–850. [Google Scholar] [CrossRef]
- Wang, Q.; He, H.; Li, B.; Lin, H.; Zhang, Y.; Zhang, J.; Wang, Z. UV-Vis and ATR-FTIR spectroscopic investigations of postmortem interval based on the changes in rabbit plasma. PLoS ONE 2017, 12, e0182161. [Google Scholar] [CrossRef]
- Lin, H.; Zhang, Y.; Wang, Q.; Li, B.; Fan, S.; Wang, Z. Species identification of bloodstains by ATR-FTIR spectroscopy: The effects of bloodstain age and the deposition environment. Int. J. Leg. Med. 2018, 132, 667–674. [Google Scholar] [CrossRef]
- Zhang, J.; Li, B.; Wang, Q.; Li, C.; Zhang, Y.; Lin, H.; Wang, Z. Characterization of postmortem biochemical changes in rabbit plasma using ATR-FTIR combined with chemometrics: A preliminary study. Spectrochim. Acta A Mol. Biomol. Spectrosc. 2017, 173, 733–739. [Google Scholar] [CrossRef] [PubMed]
- Baptista, A.; Pedrosa, M.; Curate, F.; Ferreira, M.T.; Marques, M.P.M. Estimation of the post-mortem interval in human bones by infrared spectroscopy. Int. J. Leg. Med. 2022, 136, 309–317. [Google Scholar] [CrossRef] [PubMed]
- Reyes-Gasga, J.; Martínez-Piñeiro, E.L.; Rodríguez-Álvarez, G.; Tiznado-Orozco, G.E.; García-García, R.; Brès, E.F. XRD and FTIR crystallinity indices in sound human tooth enamel and synthetic hydroxyapatite. Mater. Sci. Eng. C Mater. Biol. Appl. 2013, 33, 4568–4574. [Google Scholar] [CrossRef] [PubMed]
- Ortiz-Ruiz, A.J.; Teruel-Fernández, J.D.; Alcolea-Rubio, L.A.; Hernández-Fernández, A.; Martínez-Beneyto, Y.; Gispert-Guirado, F. Structural differences in enamel and dentin in human, bovine, porcine, and ovine teeth. Ann. Anat. 2018, 218, 7–17. [Google Scholar] [CrossRef]
- Lopes, C.C.A.; Limirio, P.H.J.o.; Novais, V.R.; Dechichi, P. Fourier transform infrared spectroscopy (FTIR) application chemical characterization of enamel, dentin and bone. Appl. Spectrosc. Rev. 2018, 53, 747–769. [Google Scholar] [CrossRef]
- Wei, X.; Yu, K.; Wu, H.; Shen, C.; Li, H.; Liu, R.; Sun, Q.; Wang, Z. Species identification of teeth of human and non-human. Forensic Sci. Int. 2022, 333, 111205. [Google Scholar] [CrossRef]
- Pate, M.L.; Aguilar-Caballos, M.P.; Beltrán-Aroca, C.M.; Pérez-Vicente, C.; Lozano-Molina, M.; Girela-López, E. Use of XRD and SEM/EDX to predict age and sex from fire-affected dental remains. Forensic Sci. Med. Pathol. 2018, 14, 432–441. [Google Scholar] [CrossRef]
- Shah, P.; Velani, P.R.; Lakade, L.; Dukle, S. Teeth in forensics: A review. Indian J. Dent. Res. 2019, 30, 291–299. [Google Scholar] [CrossRef]
- Thurzo, A.; Jančovičová, V.; Hain, M.; Thurz, O.M.; Novák, B.; Kosnáčová, H.; Lehotská, V.; Varga, I.; Kováč, P.; Moravanský, N. Human Remains Identification Using Micro-CT, Chemometric and AI Methods in Forensic Experimental Reconstruction of Dental Patterns after Concentrated Sulphuric Acid Significant Impact. Molecules 2022, 27, 4035. [Google Scholar] [CrossRef]
- Dobberstein, R.C.; Huppertz, J.; von Wurmb-Schwark, N.; Ritz-Timme, S. Degradation of biomolecules in artificially and naturally aged teeth: Implications for age estimation based on aspartic acid racemization and DNA analysis. Forensic Sci. Int. 2008, 179, 181–191. [Google Scholar] [CrossRef]
- Zapico, S.C.; Gauthier, Q.; Antevska, A.; McCord, B.R. Identifying Methylation Patterns in Dental Pulp Aging: Application to Age-at-Death Estimation in Forensic Anthropology. Int. J. Mol. Sci. 2021, 22, 3717. [Google Scholar] [CrossRef] [PubMed]
- Garriga, J.A.; Ubelaker, D.H.; Zapico, S.C. Evaluation of macroscopic changes and the efficiency of DNA profiling from burnt teeth. Sci. Justice 2016, 56, 437–442. [Google Scholar] [CrossRef] [PubMed]
- Higgins, D.; Austin, J.J. Teeth as a source of DNA for forensic identification of human remains: A review. Sci. Justice 2013, 53, 433–441. [Google Scholar] [CrossRef] [PubMed]
- Swets, J.A. Measuring the accuracy of diagnostic systems. Science 1998, 240, 1285–1293. [Google Scholar] [CrossRef] [PubMed]
- Cazalbou, S.; Eichert, D.; Crouet, C.; Combes, C.; Rey, C. Minéralisations biologiques à base de phosphate de calcium. C. R. Palevol. 2004, 3, 563–572. [Google Scholar] [CrossRef]
- Lebon, M.; Zazzo, A.; Reiche, I. Screening in situ bone and teeth preservation by ATR-FTIR mapping. Palaeogeogr. Palaeoclimatol. Palaeoecol. 2014, 416, 110–119. [Google Scholar] [CrossRef]
- Figueiredo, M.; Fernando, A.; Martins, G.; Freitas, J.; Judas, F.; Figueiredo, H. Effect of the calcination temperature on the composition and microstructure of hydroxyapatite derived from human and animal bone. Ceram. Int. 2010, 36, 2383–2393. [Google Scholar] [CrossRef]
- Grynpas, M. The crystallinity of bone mineral. J. Miner. Sci. 1976, 11, 1691–1696. [Google Scholar] [CrossRef]
- Farlay, F.D.; Panczer, G.; Rey, C.; Delmas, P.; Boivin, G. Mineral maturity and crystallinity index are distinct characteristics of bone mineral. J. Bone Miner. Metab. 2010, 28, 433–445. [Google Scholar] [CrossRef]
- Sa, Y.; Guo, Y.; Feng, X.; Wang, M.; Li, P.; Gao, Y.; Yang, X.; Jiang, T. Are different crystallinity-index-calculating methods of hydroxyapatite efficient and consistent? New J. Chem. 2017, 41, 5723–5731. [Google Scholar] [CrossRef]
- Rubio, L.; Vargas, A.; Rivera, P.; López-Gambero, A.J.; Tovar, R.; Christians, J.K.; Martín-de-Las-Heras, S.; Rodríguez de Fonseca, F.; Chowen, J.A.; Argente, J.; et al. Recombinant IGF-1 Induces Sex-Specific Changes in Bone Composition and Remodeling in Adult Mice with Pappa2 Deficiency. Int. J. Mol. Sci. 2021, 22, 4048. [Google Scholar] [CrossRef]
- Surovell, T.A.; Stiner, M.C. Standardizing infra-red measures of bone mineral crystallinity: An experimental approach. J. Arch. Sci. 2000, 28, 633–642. [Google Scholar] [CrossRef]
- Dal Sasso, G.; Asscher, Y.; Angelini, I.; Nodari, L.; Artioli, G. A universal curve of apatite crystallinity for the assessment of bone integrity and preservation. Sci. Rep. 2018, 8, 12025. [Google Scholar] [CrossRef]
- Querido, W.; Ailavajhala, R.; Padalkar, M.; Pleshko, N. Validated Approaches for Quantification of Bone Mineral Crystallinity Using Transmission Fourier Transform Infrared (FT-IR), Attenuated Total Reflection (ATR) FT-IR, and Raman Spectroscopy. Appl. Spectrosc. 2018, 72, 1581–1593. [Google Scholar] [CrossRef]
- Paschalis, E.P.; Mendelsohn, R.; Boskey, A.L. Infrared assessment of bone quality: A review. Clin. Orthop. Relat. Res. 2011, 469, 2170–2178. [Google Scholar] [CrossRef]
- Paschalis, E.P. Fourier Transform Infrared Imaging of Bone. Methods Mol. Biol. 2019, 1914, 641–649. [Google Scholar] [CrossRef]
- Taylor, E.A.; Lloyd, A.A.; Salazar-Lara, C.; Donnelly, E. Raman and Fourier Transform Infrared (FT-IR) Mineral to Matrix Ratios Correlate with Physical Chemical Properties of Model Compounds and Native Bone Tissue. Appl. Spectrosc. 2017, 71, 2404–2410. [Google Scholar] [CrossRef]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Rubio, L.; Suárez, J.; Martin-de-las-Heras, S.; Zapico, S.C. Partners in Postmortem Interval Estimation: X-ray Diffraction and Fourier Transform Spectroscopy. Int. J. Mol. Sci. 2023, 24, 6793. https://doi.org/10.3390/ijms24076793
Rubio L, Suárez J, Martin-de-las-Heras S, Zapico SC. Partners in Postmortem Interval Estimation: X-ray Diffraction and Fourier Transform Spectroscopy. International Journal of Molecular Sciences. 2023; 24(7):6793. https://doi.org/10.3390/ijms24076793
Chicago/Turabian StyleRubio, Leticia, Juan Suárez, Stella Martin-de-las-Heras, and Sara C. Zapico. 2023. "Partners in Postmortem Interval Estimation: X-ray Diffraction and Fourier Transform Spectroscopy" International Journal of Molecular Sciences 24, no. 7: 6793. https://doi.org/10.3390/ijms24076793
APA StyleRubio, L., Suárez, J., Martin-de-las-Heras, S., & Zapico, S. C. (2023). Partners in Postmortem Interval Estimation: X-ray Diffraction and Fourier Transform Spectroscopy. International Journal of Molecular Sciences, 24(7), 6793. https://doi.org/10.3390/ijms24076793









