Multi-Targeting Anticancer Activity of a New 4-Thiazolidinone Derivative with Anti-HER2 Antibodies in Human AGS Gastric Cancer Cells
Abstract
1. Introduction
2. Results
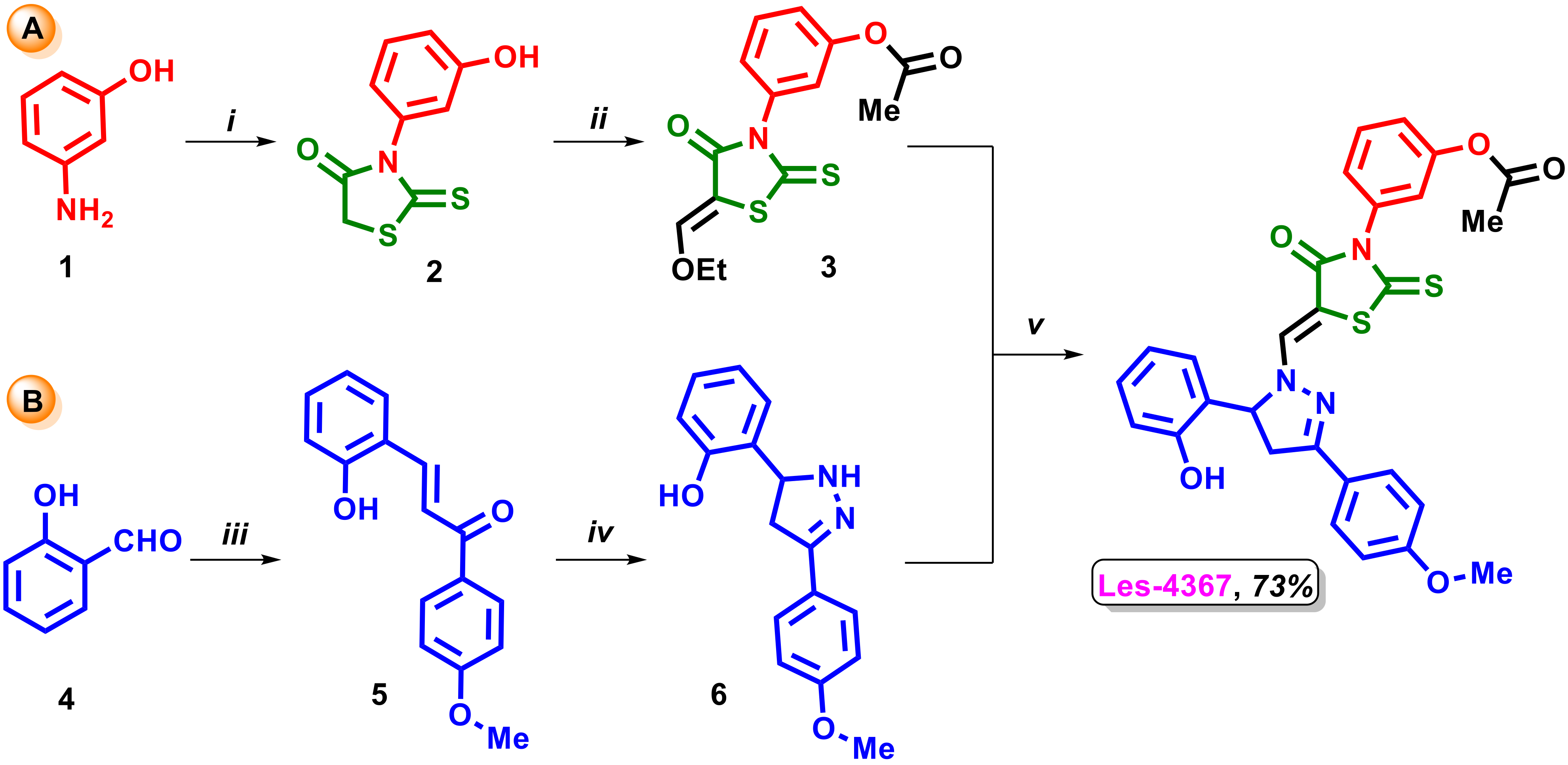
2.1. Synthesis of 4-Thiazolidinone-Based Derivative Les-4367
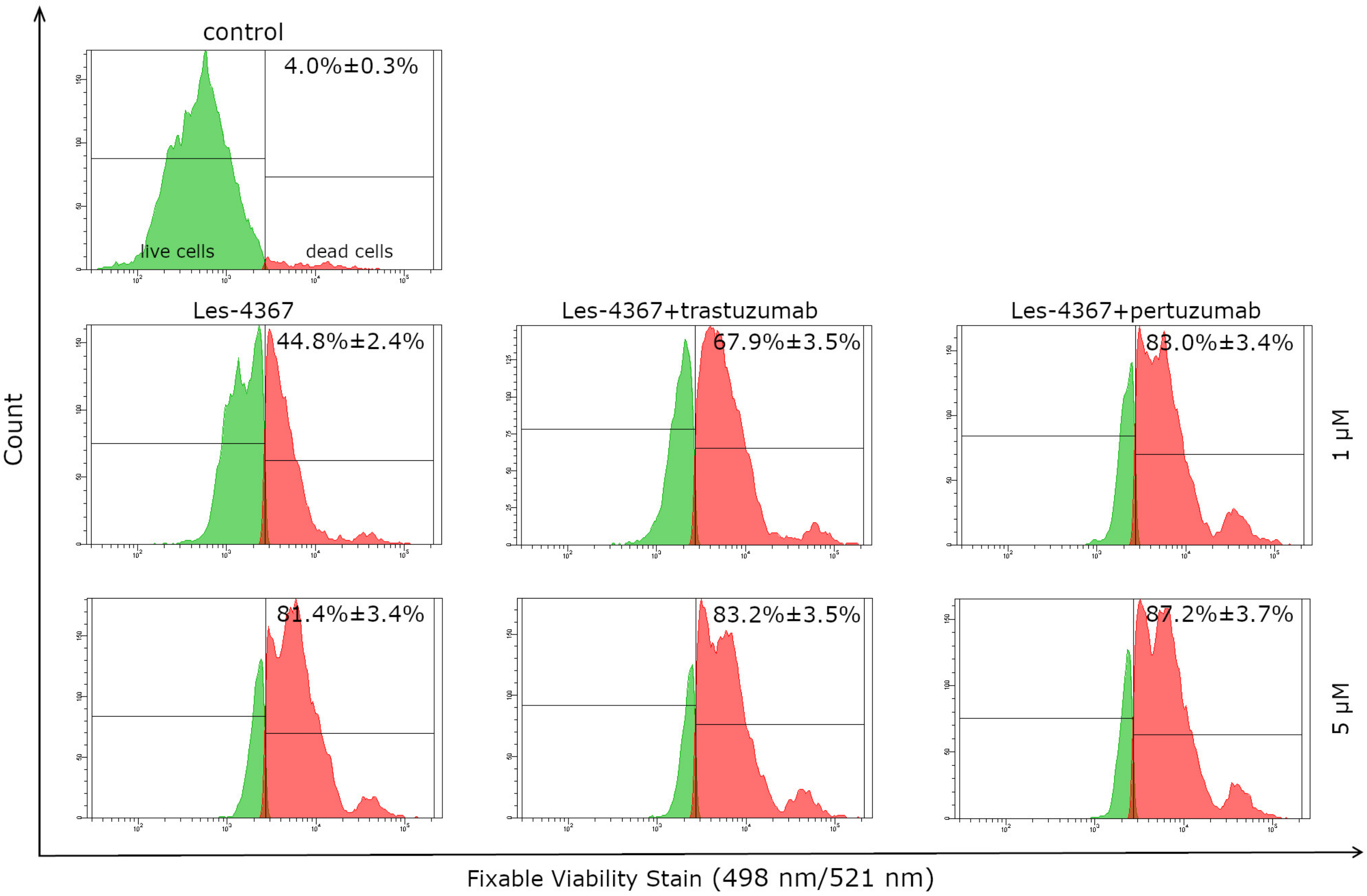
2.2. Viability
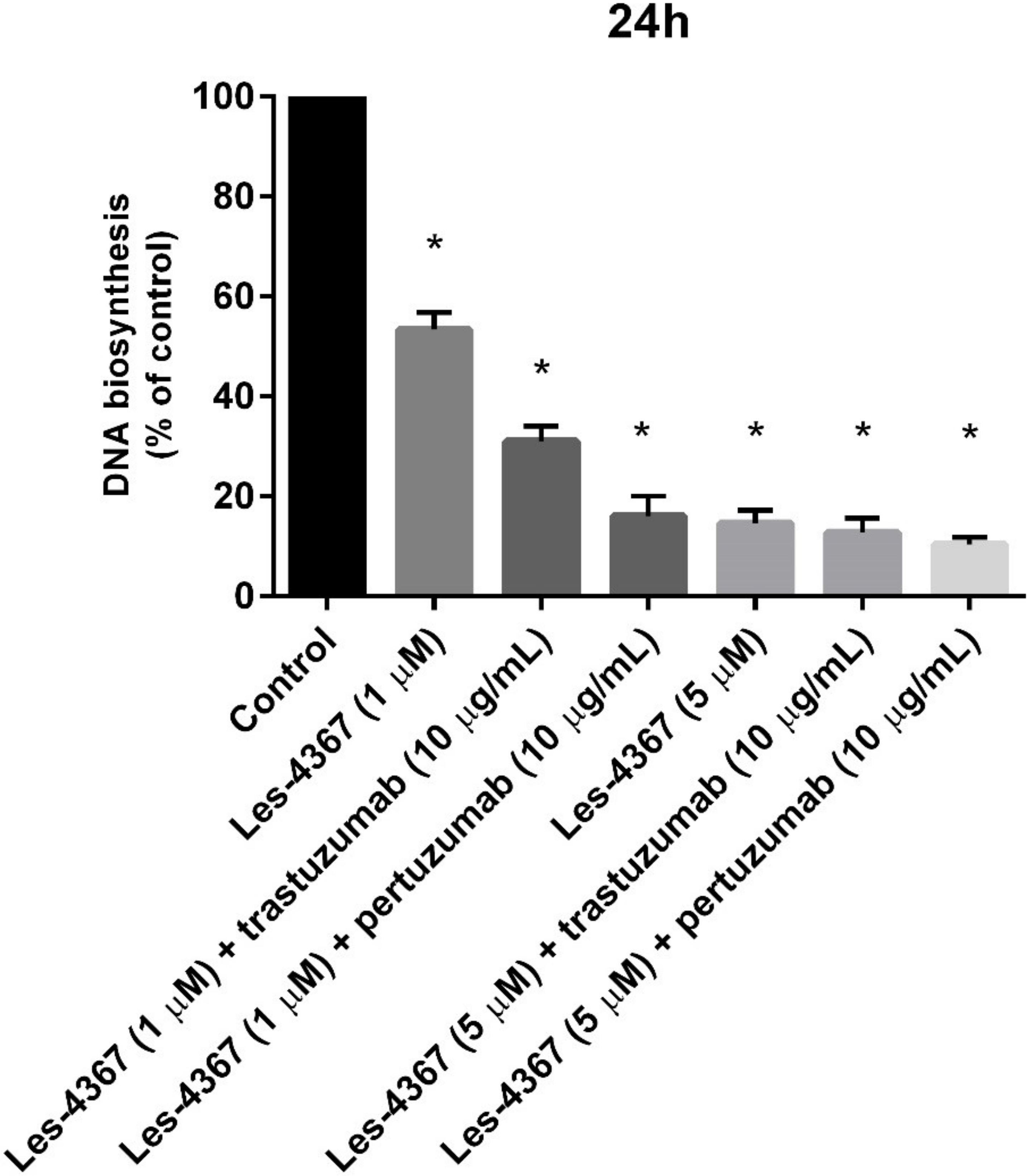
2.3. Anti-HER2 Antibodies (Trastuzumab, Pertuzumab) Increase the Anti-Proliferative Effect of a Novel 4-Thiazolidinone Derivative (Les-4367) in AGS Gastric Cancer Cells Compared with Agents Used Alone
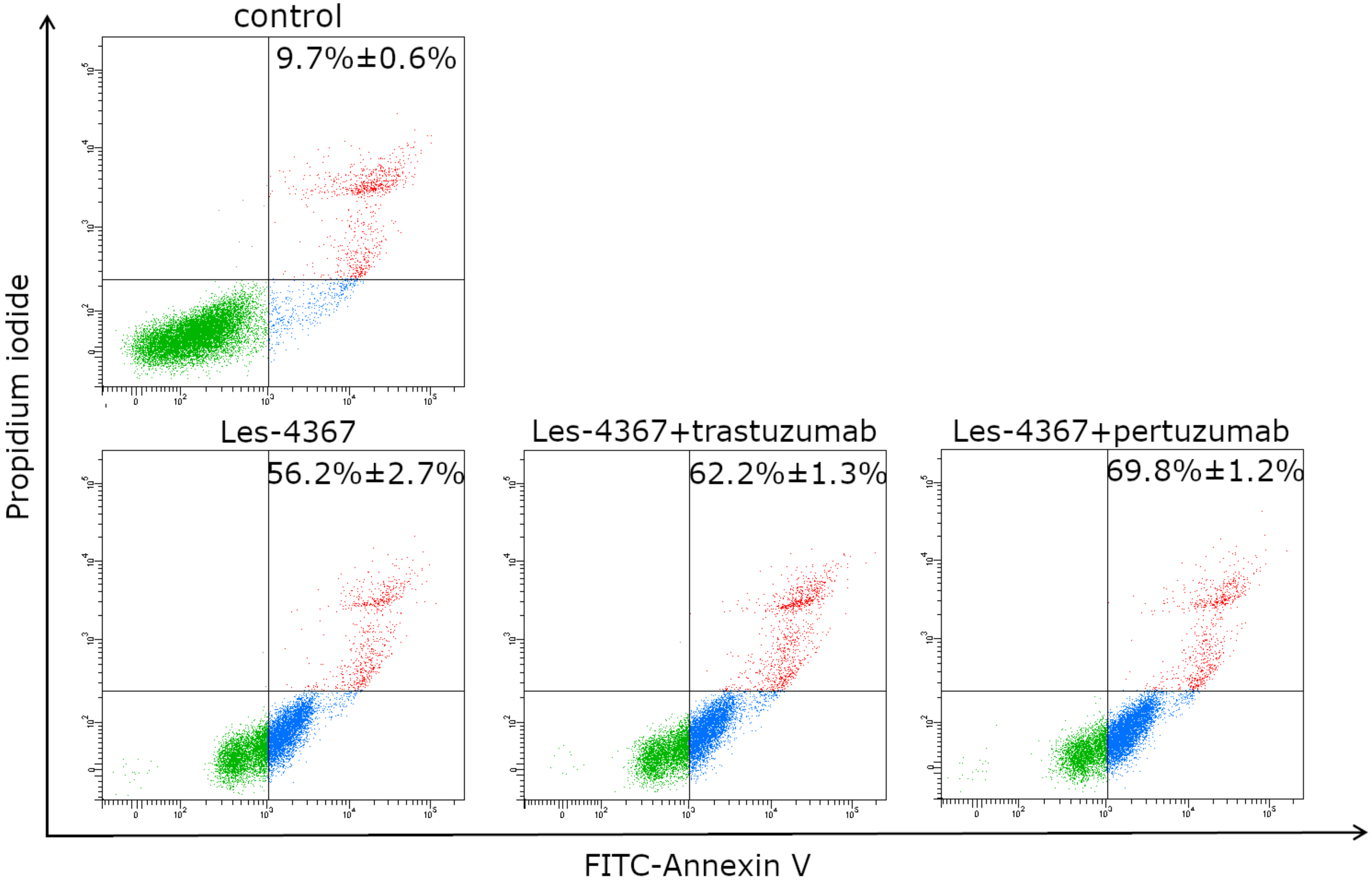
2.4. Induction of Apoptosis
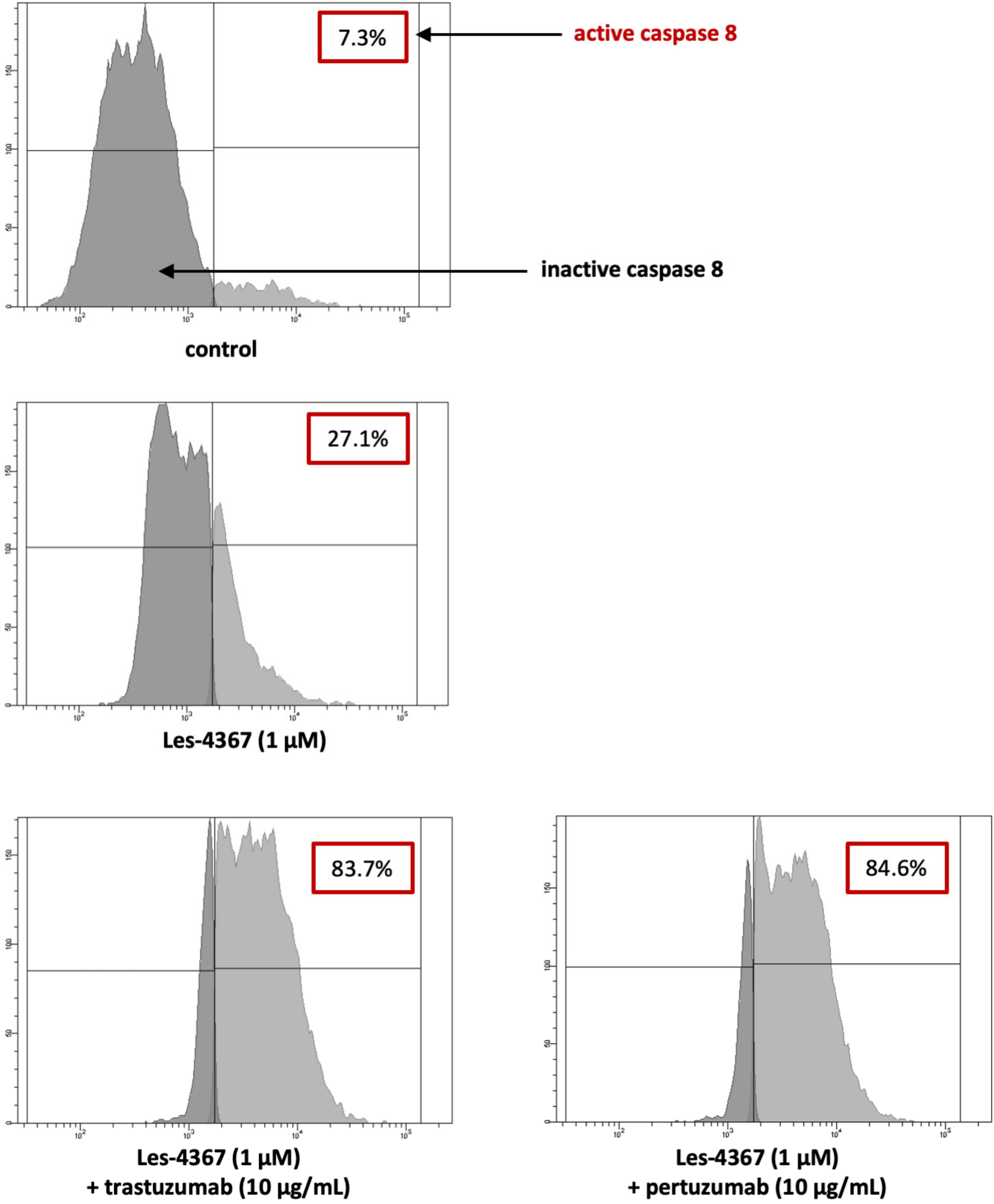
2.5. Trastuzumab or Pertuzumab Combined with Les-4367 Increase Caspase-8 Initiator Activity in AGS Gastric Cancer Cells Compared with the Agent Used Alone
2.6. Trastuzumab or Pertuzumab Combined with Les-4367 Increase Caspase-9 Initiator Activity in AGS Gastric Cancer Cells Compared with the Agent Alone
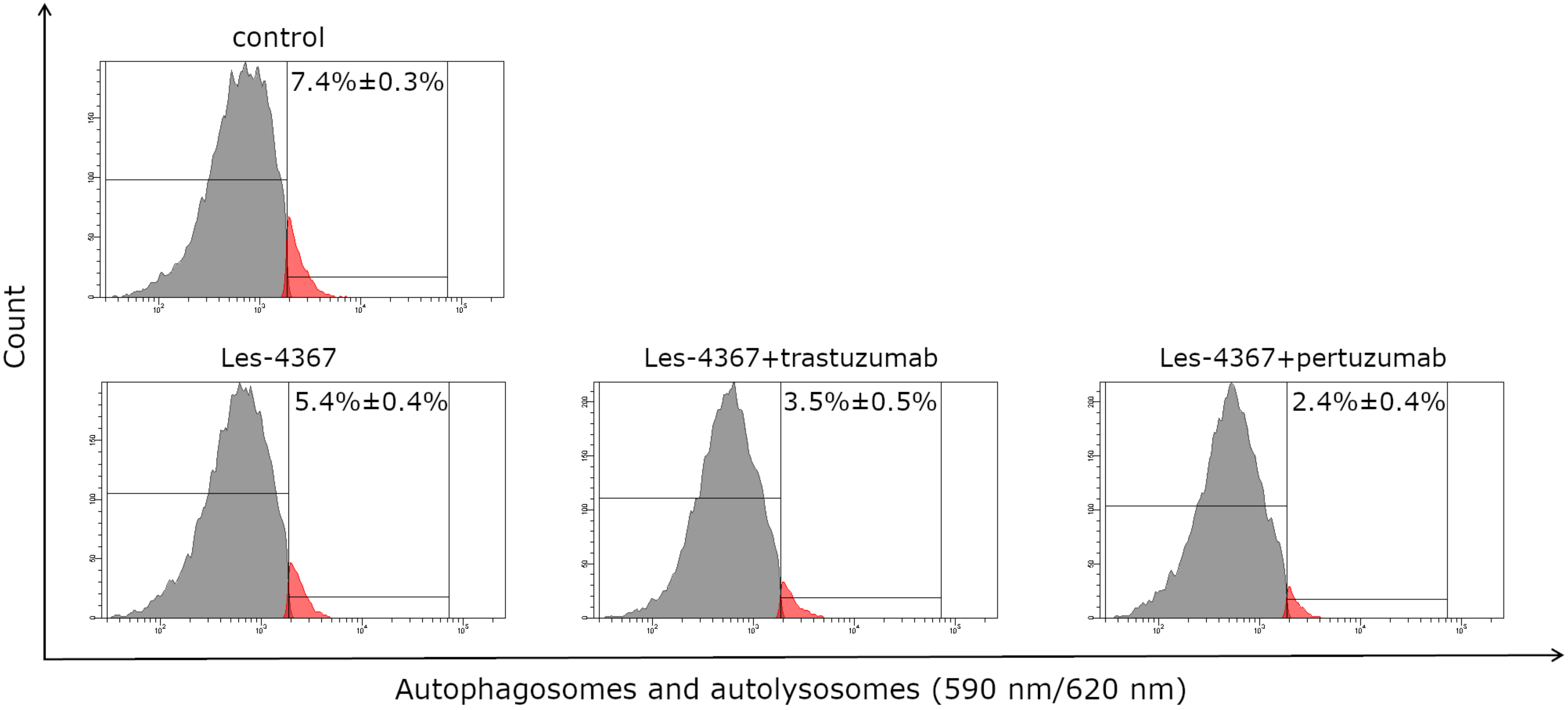
2.7. Autophagy
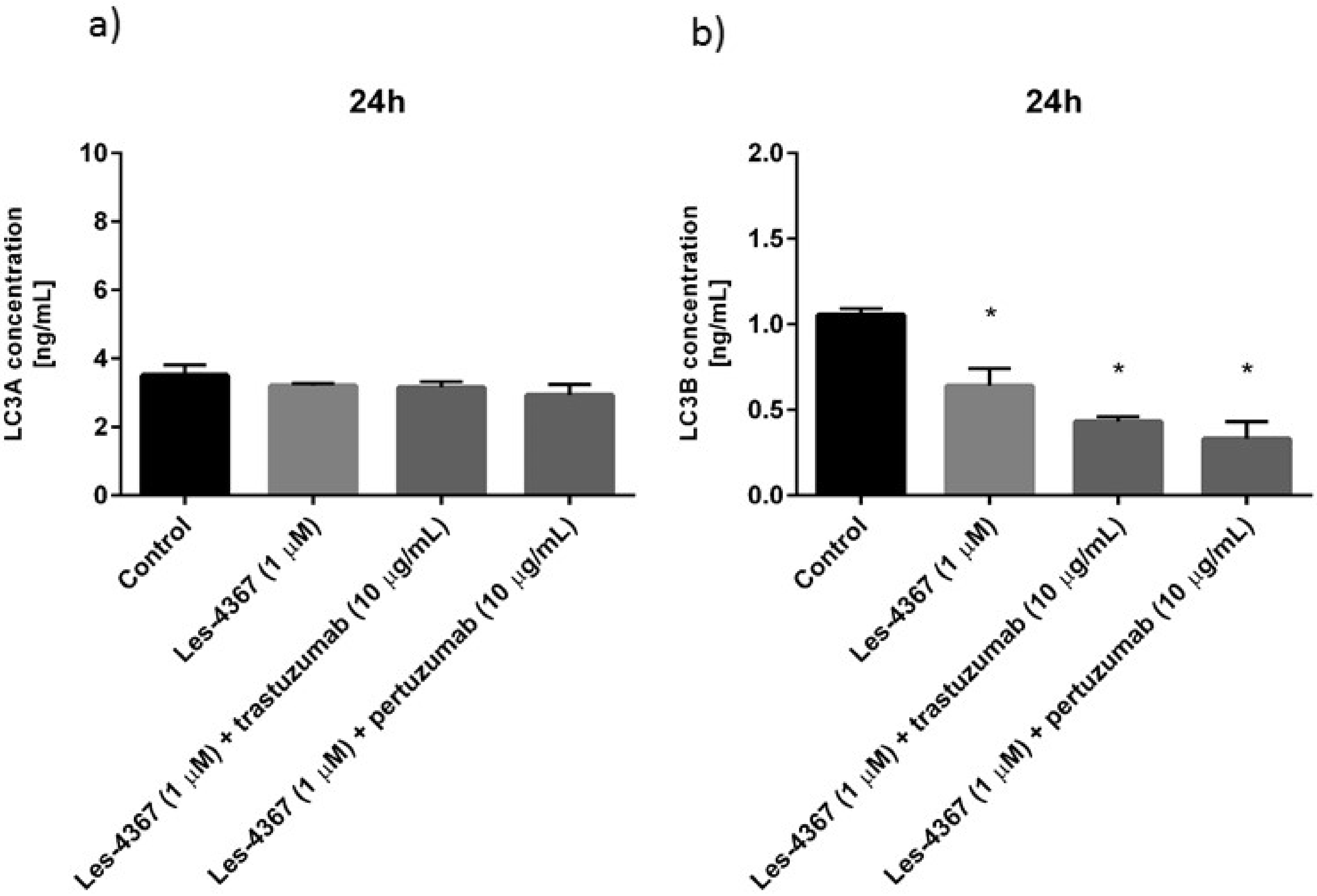
2.8. Trastuzumab or Pertuzumab Combined with Les-4367 Decrease Beclin-1, LC3A, and LC3B Concentrations in AGS Gastric Cancer Cells
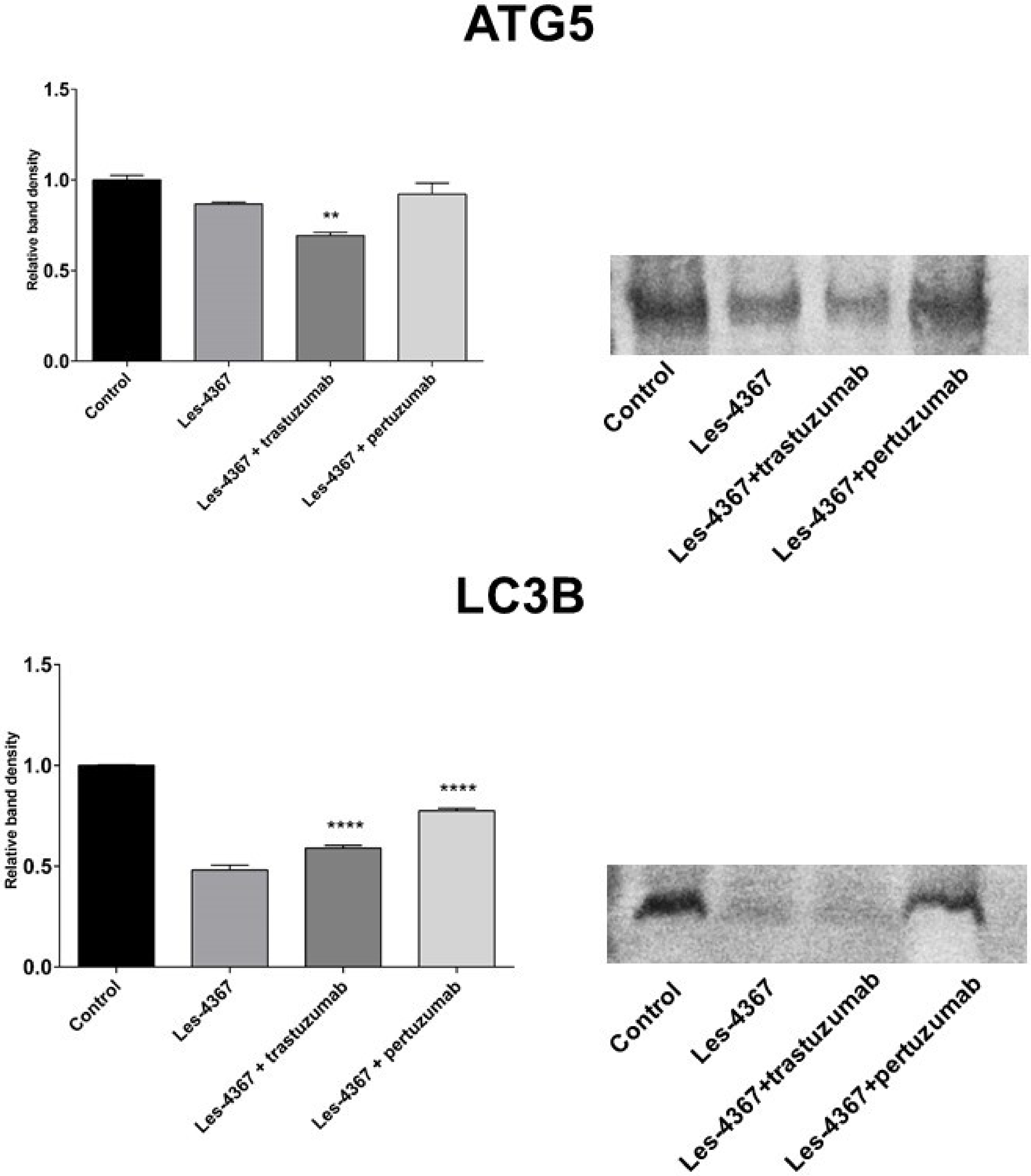
2.9. Trastuzumab or Pertuzumab Combined with Les-4367 Decrease ATG5 and LC3B Expression in AGS Gastric Cancer Cells
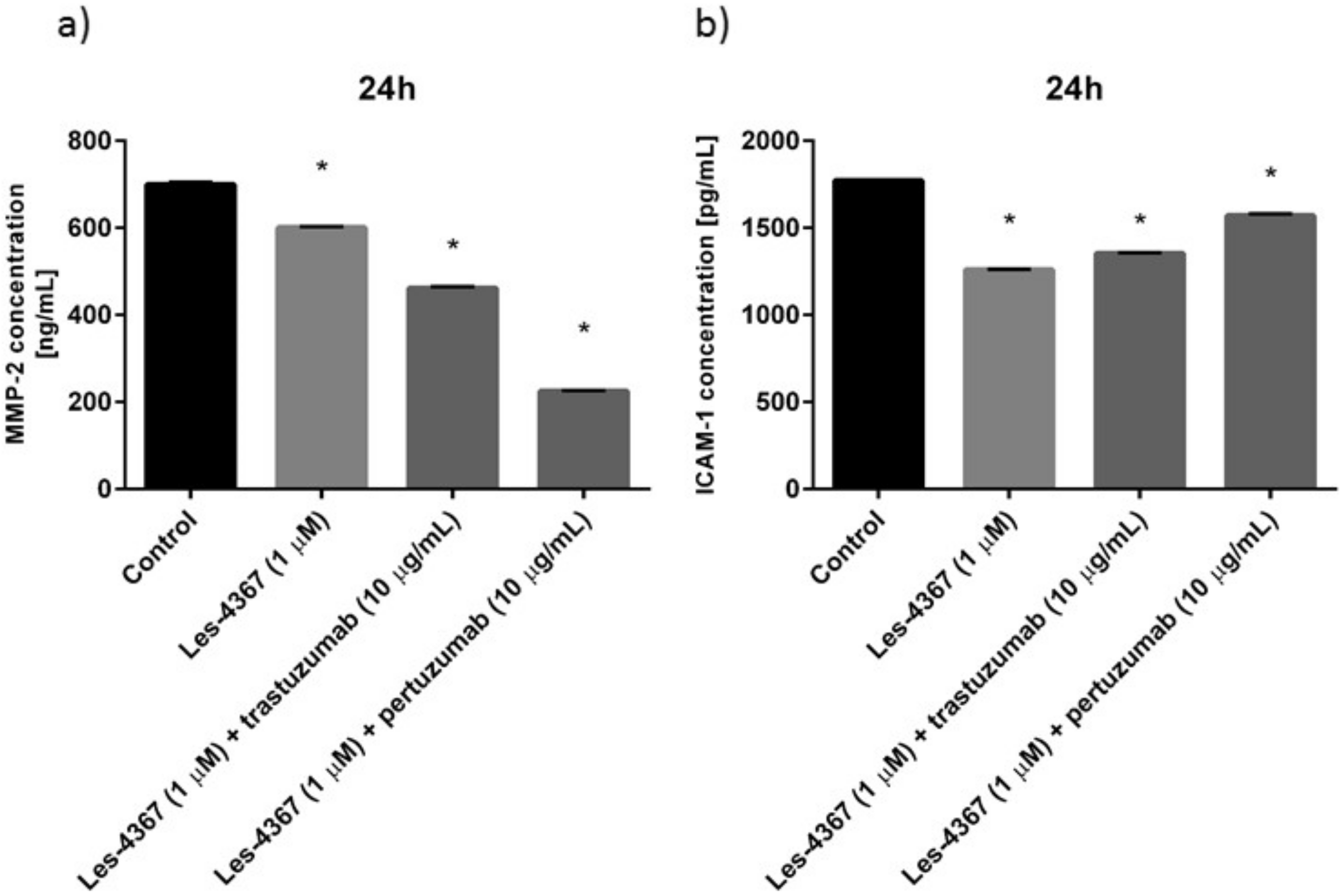
2.10. Trastuzumab or Pertuzumab Combined with Les-4367 Decrease MMP-2 and ICAM-1 Concentrations in AGS Gastric Cancer Cells
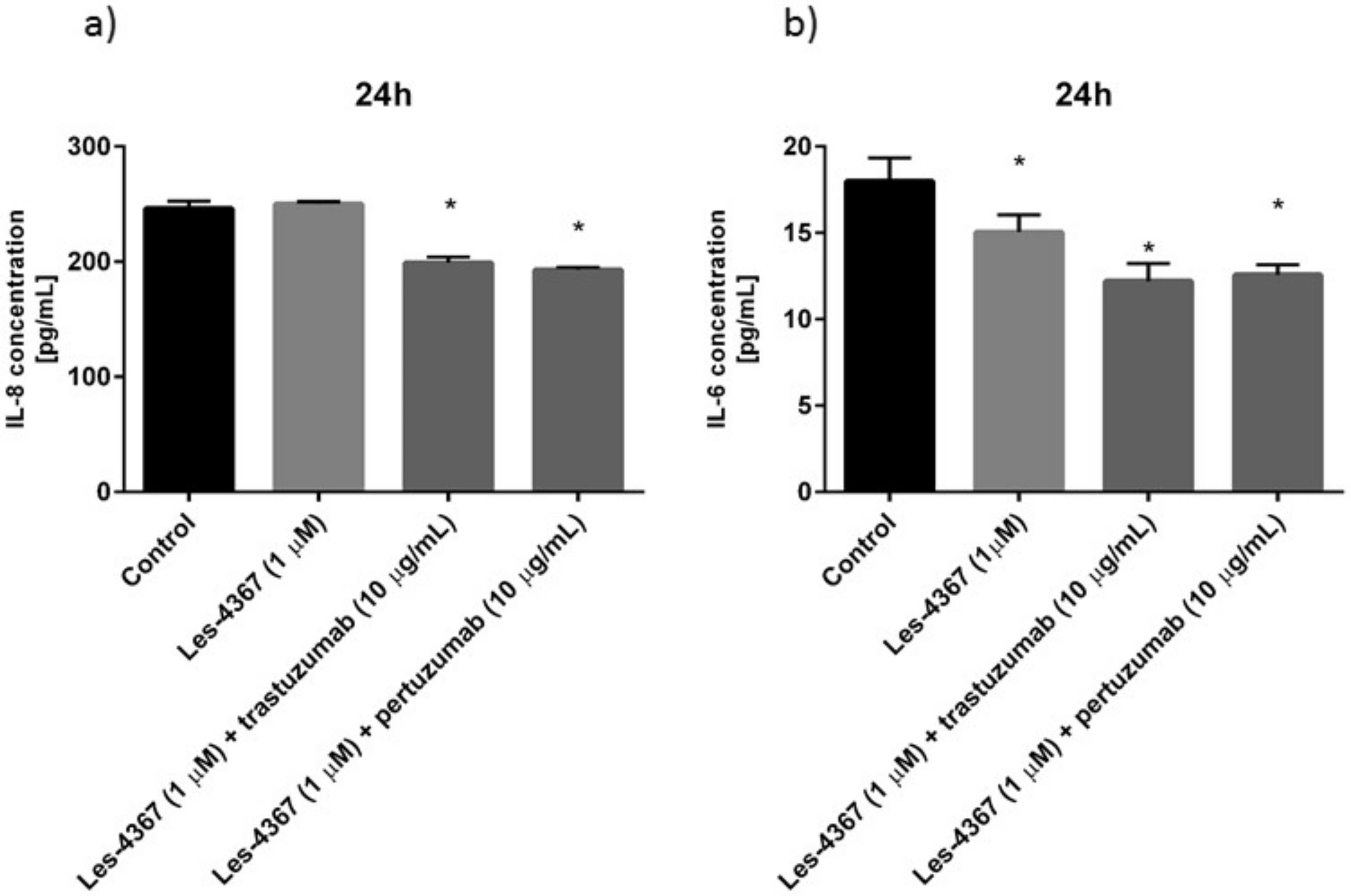
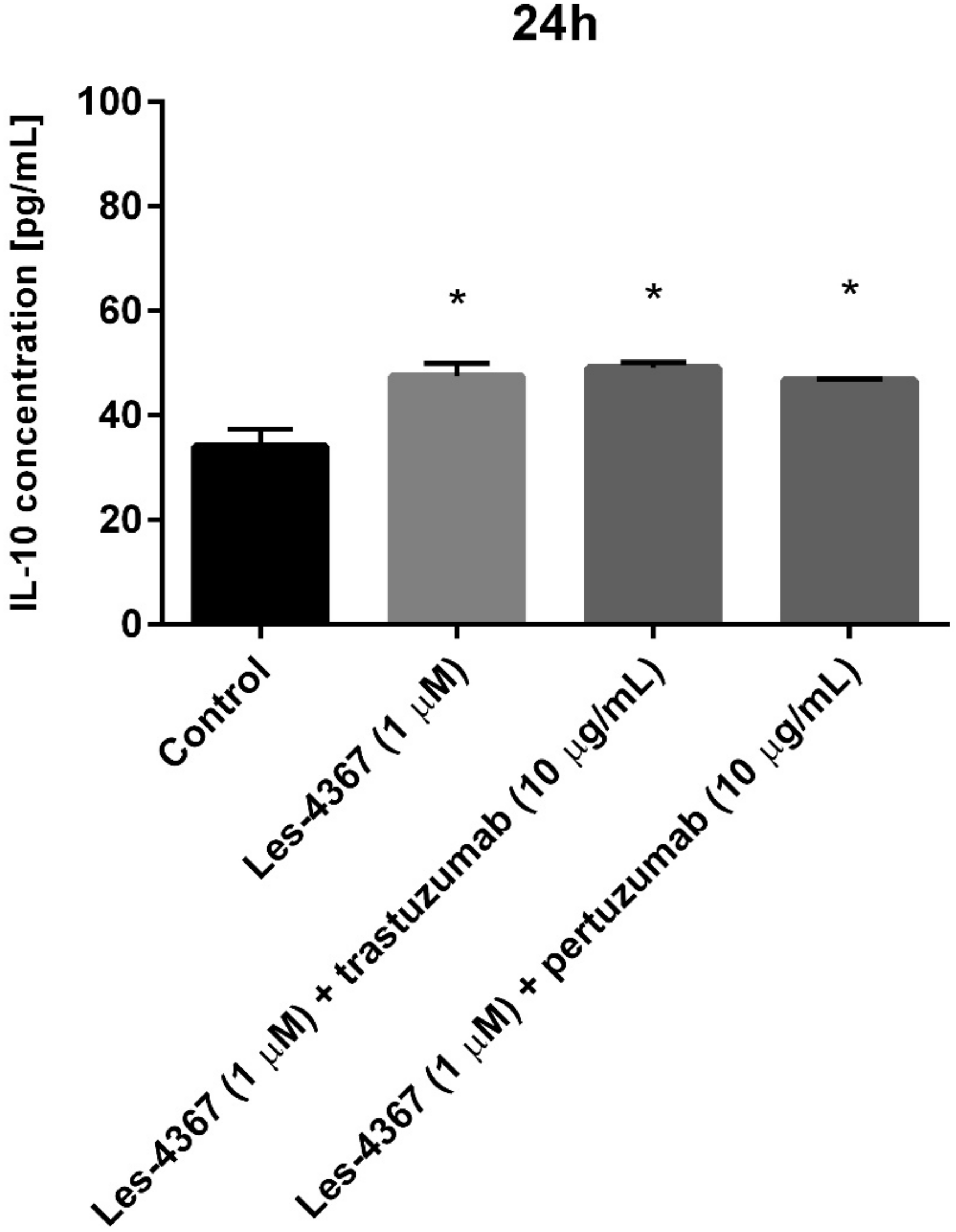
2.11. Trastuzumab or Pertuzumab Combined with Les-4367 Exert Anti-Inflammatory Potential in AGS Gastric Cancer Cells
3. Discussion
4. Materials and Methods
4.1. Compounds
4.2. Cell Culture
4.3. Viability
4.4. [3H]thymidine Incorporation Assay
4.5. Apoptosis
4.6. Caspase-8 Enzymatic Activity Assay
4.7. Caspase-9 Enzymatic Activity Assay
4.8. Autophagy
4.9. Concentrations of Beclin-1, IL-8 and IL-6, IL-10 and ICAM-1
4.10. ATG5 and LC3B Expression
4.11. Concentrations of LC3A, LC3B, and MMP-2
4.12. Statistical Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| AGS-CRL-1739 | human gastric cancer cell line |
| Akt | protein kinase B |
| ANOVA | analysis of variance |
| ATCC | American Type Culture Collection |
| ATG5 | autophagy related 5 |
| AV/PI | annexin V/propidium iodide |
| BCIP/NBT | 5-chromo-4-chloro-3-indolyl-phosphate/nitro blue tetrazolium |
| BMI | body mass index |
| COX-2 | cyclooxygenase-2 |
| DLD-1 | colorectal adenocarcinoma cell line |
| DMEM | Dulbecco’s Modified Eagle’s Medium |
| DMSO | dimethyl sulfoxide |
| DNA | deoxyribonucleic acid |
| EDTA | ethylenediaminetetraacetic acid |
| ELISA | enzyme-linked immunosorbent assay |
| ERBB2 | Erb-B2 receptor tyrosine kinase 2 |
| FAM-FLICA | fluorochrome-labeled inhibitors of caspases |
| FBS | fetal bovine serum |
| FDA | Food and Drug Administration |
| FITC | fluorescein isothiocyanate |
| FV-429 | flavonoid |
| GC | gastric cancer |
| HCC 1954 | epithelial breast cancer cell |
| HER-2 | human epidermal growth factor receptor 2 |
| HGF/MET | hepatocyte growth factor/proto- oncogenic receptor tyrosine kinase |
| HRP | horseradish peroxidase |
| HT-29 | human adenocarcinoma colorectal cell line |
| ICAM-1 | intercellular adhesion molecule 1 |
| ICT | intramolecular charge transfer |
| IL-1β | interleukin 1β |
| IL-6 | interleukin 6 |
| IL-8 | interleukin 8 |
| IL-10 | interleukin 10 |
| LC3A | microtubule-associated protein 1A/1B light chain 3A |
| LC3B | microtubule-associated protein 1A/1B light chain 3B |
| LC-MS | liquid chromatography tandem mass spectrometry |
| MAPK | mitogen-activated protein kinase |
| MCF-7 | human breast cancer cell line |
| MDA-MB-231 | triple-negative human breast cancer cell line |
| MMP-2 | Metalloproteinase-2 |
| 13C NMR | carbon-13 nuclear magnetic resonance |
| 1H NMR | proton nuclear magnetic resonance |
| NSCLC | non-small cell lung cancer |
| O.D. | optical density |
| PARP | poly (ADP-ribose) polymerase |
| PBS | Phosphate-buffered saline |
| PD-1 | programmed cell death protein 1 |
| PDL-2 | program cell death ligand 2 |
| PI | propidium iodide |
| PI3K | phosphatidylinositol-3 kinase |
| ROS | reactive oxygen species |
| SD | standard deviation |
| SDS-PAGE | sodium dodecyl-sulfate polyacrylamide gel electrophoresis |
| SK-BR-3 | human breast cancer cell line that overexpresses the HER2 gene product |
| STAT3 | signal transducer and activator of transcription 3 |
| TBS-T | tris-buffered saline with tween |
| TKI | tyrosine kinase inhibitor |
| TLC | thin layer chromatography |
| TMB | tetramethylbenzidine |
| mTOR | mammalian target of rapamycin |
| TRIS | tris(hydroxymethyl)aminomethane |
| VEGF | vascular endothelial growth factor |
References
- Aggarwal, B.B.; Sung, B.; Gupta, S.C. Advances in Experimental Medicine and Biology; Springer: Basel, Switzerland, 2014. [Google Scholar]
- Hoyo, C.; Cook, M.B.; Kamangar, F.; Freedman, N.D.; Whiteman, D.C.; Bernstein, L.; Brown, L.M.; Risch, H.A.; Ye, W.; Sharp, L.; et al. Body mass index in relation to oesophageal and oesophagogastric junction adenocarcinomas: A pooled analysis from the International BEACON Consortium. Int. J. Epidemiol. 2012, 41, 1706–1718. [Google Scholar] [CrossRef] [PubMed]
- Bernini, M.; Barbi, S.; Roviello, F.; Scarpa, A.; Moore, P.; Pedrazzani, C.; Beghelli, S.; Marrelli, D.; De Manzoni, G. Family history of gastric cancer: A correlation between epidemiologic findings and clinical data. Gastric. Cancer 2006, 9, 9–13. [Google Scholar] [CrossRef] [PubMed]
- Rubenstein, J.H.; Taylor, J.B. Meta-analysis: The association of oesophageal adenocarcinoma with symptoms of gastro-oesophageal reflux. Aliment. Pharmacol. Ther. 2010, 32, 1222–1227. [Google Scholar] [CrossRef]
- Preston, D.L.; Ron, E.; Tokuoka, S.; Funamoto, S.; Nishi, N.; Soda, M.; Mabuchi, K.; Kodama, K. Solid cancer incidence in atomic bomb survivors: 1958–1998. Radiat. Res. 2007, 168, 1–64. [Google Scholar] [CrossRef] [PubMed]
- Abnet, C.C.; Kamangar, F.; Dawsey, S.M.; Stolzenberg-Solomon, R.Z.; Albanes, D.; Pietinen, P.; Virtamo, J.; Taylor, P.R. Tooth loss is associated with increased risk of gastric non-cardia adenocarcinoma in a cohort of Finnish smokers. Scand. J. Gastroenterol. 2005, 40, 681–687. [Google Scholar] [CrossRef] [PubMed]
- Abnet, C.C.; Qiao, Y.L.; Mark, S.D.; Dong, Z.W.; Taylor, P.R.; Dawsey, S.M. Prospective study of tooth loss and incident esophageal and gastric cancers in China. Cancer Causes Control 2001, 12, 847–854. [Google Scholar] [CrossRef] [PubMed]
- Ren, J.S.; Kamangar, F.; Forman, D.; Islami, F. Pickled food and risk of gastric cancer—A systematic review and meta-analysis of English and Chinese literature. Cancer Epidemiol. Biomarkers Prev. 2012, 21, 905–915. [Google Scholar] [CrossRef] [PubMed]
- Fock, K.M.; Talley, N.; Moayyedi, P.; Hunt, R.; Azuma, T.; Sugano, K.; Xiao, S.D.; Lam, S.K.; Goh, K.L.; Chiba, T.; et al. Asia-Pacific consensus guidelines on gastric cancer prevention. J. Gastroenterol. Hepatol. 2008, 23, 351–365. [Google Scholar] [CrossRef]
- Karimi, P.; Islami, F.; Anandasabapathy, S.; Freedman, N.D.; Kamangar, F. Gastric cancer: Descriptive epidemiology, risk factors, screening, and prevention. Cancer Epidemiol. Biomarkers Prev. 2014, 23, 700–713. [Google Scholar] [CrossRef]
- Meric-Bernstam, F.; Johnson, A.M.; Dumbrava, E.E.I.; Raghav, K.; Balaji, K.; Bhatt, M.; Murthy, R.K.; Rodon, J.; Piha-Paul, S.A. Advances in HER2-Targeted Therapy: Novel Agents and Opportunities Beyond Breast and Gastric Cancer. Clin. Cancer Res. 2019, 25, 2033–2041. [Google Scholar] [CrossRef]
- Moasser, M.M. The oncogene HER2: Its signaling and transforming functions and its role in human cancer pathogenesis. Oncogene 2007, 26, 6469–6487. [Google Scholar] [CrossRef]
- Bang, Y.J.; Van Cutsem, E.; Feyereislova, A.; Chung, H.C.; Shen, L.; Sawaki, A.; Lordick, F.; Ohtsu, A.; Omuro, Y.; Satoh, T.; et al. Trastuzumab in combination with chemotherapy versus chemotherapy alone for treatment of HER2-positive advanced gastric or gastro-oesophageal junction cancer (ToGA): A phase 3, open-label, randomised controlled trial. Lancet 2010, 376, 687–697. [Google Scholar] [CrossRef] [PubMed]
- Chung, H.C.; Bang, Y.J.; Fuchs, C.; Qin, S.K.; Satoh, T.; Shitara, K.; Tabernero, J.; Van Cutsem, E.; Alsina, M.; Cao, Z.A.; et al. First-line pembrolizumab/placebo plus trastuzumab and chemotherapy in HER2-positive advanced gastric cancer: KEYNOTE-811. Future Oncol. 2021, 17, 491–501. [Google Scholar] [CrossRef]
- Li, Y.; Zhang, X.; Liu, D.; Gong, J.; Wang, D.D.; Li, S.; Peng, Z.; Li, Y.; Wang, X.; Lin, P.P.; et al. Evolutionary expression of HER2 conferred by chromosome aneuploidy on circulating gastric cancer cells contributes to developing targeted and chemotherapeutic resistance. Clin. Cancer Res. 2018, 24, 5261–5271. [Google Scholar] [CrossRef] [PubMed]
- Wang, D.S.; Liu, Z.X.; Lu, Y.X.; Bao, H.; Wu, X.; Zeng, Z.L.; Liu, Z.; Zhao, Q.; He, C.-Y.; Lu, J.-H.; et al. Liquid biopsies to track trastuzumab resistance in metastatic HER2-positive gastric cancer. Gut 2019, 68, 1152–1161. [Google Scholar] [CrossRef]
- Roszczenko, P.; Holota, S.; Szewczyk, O.K.; Dudchak, R.; Bielawski, K.; Bielawska, A.; Lesyk, R. 4-Thiazolidinone-Bearing Hybrid Molecules in Anticancer Drug Design. Int. J. Mol. Sci. 2022, 23, 13135. [Google Scholar] [CrossRef]
- Buzun, K.; Gornowicz, A.; Lesyk, R.; Kryshchyshyn-Dylevych, A.; Gzella, A.; Czarnomysy, R.; Latacz, G.; Olejarz-Maciej, A.; Handzlik, J.; Bielawski, K.; et al. 2-{5-[(Z,2Z)-2-Chloro-3-(4-nitrophenyl)-2-propenylidene]-4-oxo-2-thioxothiazolidin-3-yl}-3-methylbutanoic Acid as a Potential Anti-Breast Cancer Molecule. Int. J. Mol. Sci. 2022, 23, 4091. [Google Scholar] [CrossRef] [PubMed]
- Havrylyuk, D.; Roman, O.; Lesyk, R. Synthetic approaches, structure activity relationship and biological applications for pharmacologically attractive pyrazole/pyrazoline-thiazolidine-based hybrids. Eur. J. Med. Chem. 2016, 113, 145–166. [Google Scholar] [CrossRef]
- Senkiv, J.; Finiuk, N.; Kaminskyy, D.; Havrylyuk, D.; Wojtyra, M.; Kril, I.; Gzella, A.; Stoika, R.; Lesyk, R. 5-Ene-4-thiazolidinones induce apoptosis in mammalian leukemia cells. Eur. J. Med. Chem. 2016, 117, 33–46. [Google Scholar] [CrossRef] [PubMed]
- Maruo, Y.; Gochi, A.; Kaihara, A.; Shimamura, H.; Yamada, T.; Tanaka, N.; Orita, K. ICAM-1 expression and the soluble ICAM-1 level for evaluating the metastatic potential of gastric cancer. Int. J. Cancer 2002, 100, 486–490. [Google Scholar] [CrossRef] [PubMed]
- Jung, W.C.; Jang, Y.J.; Kim, J.H.; Park, S.S.; Park, S.H.; Kim, S.J.; Mok, Y.J.; Kim, C.S. Expression of intercellular adhesion molecule-1 and e-selectin in gastric cancer and their clinical significance. J. Gastric. Cancer 2012, 12, 140–148. [Google Scholar] [CrossRef]
- Patel, T.H.; Cecchini, M. Targeted Therapies in Advanced Gastric Cancer Current Treatment Options. Oncology 2020, 21, 70. [Google Scholar] [CrossRef] [PubMed]
- Kang, Y.K.; Rha, S.Y.; Tassone, P.; Barriuso, J.; Yu, R.; Szado, T.; Garg, A.; Bang, Y.-J. A phase IIa dose-finding and safety study of firstline pertuzumab in combination with trastuzumab, capecitabine and cisplatin in patients with HER2- positive advanced gastric cancer. Br. J. Cancer 2014, 111, 660–666. [Google Scholar] [CrossRef] [PubMed]
- Tabernero, J.; Hoff, P.M.; Shen, L.; Ohtsu, A.; Shah, M.A.; Cheng, K.; Song, C.; Wu, H.; Eng-Wong, J.; Kim, K.; et al. Pertuzumab plus trastuzumab and chemotherapy for HER2-positive metastatic gastric or gastro-oesophageal junction cancer (JACOB): Final analysis of a double-blind, randomised, placebocontrolled phase 3 study. Lancet Oncol. 2018, 19, 1372–1384. [Google Scholar] [CrossRef]
- Gornowicz, A.; Szymanowski, W.; Czarnomysy, R.; Bielawski, K.; Bielawska, A. Anti-HER2 monoclonal antibodies intensify the susceptibility of human gastric cancer cells to etoposide by promoting apoptosis, but not autophagy. PLoS ONE 2021, 16, e0255585. [Google Scholar] [CrossRef]
- Frejlich, E.; Rudno-Rudzińska, J.; Janiszewski, K.; Salomon, L.; Kotulski, K.; Pelzer, O.; Grzebieniak, Z.; Tarnawa, R.; Kielan, W. Caspases and their role in gastric cancer. Adv. Clin. Exp. Med. 2013, 22, 593–602. [Google Scholar] [PubMed]
- Buzun, K.; Gornowicz, A.; Lesyk, R.; Bielawski, K.; Bielawska, A. Autophagy Modulators in Cancer Therapy. Int. J. Mol. Sci. 2021, 22, 5804. [Google Scholar] [CrossRef]
- Hu, P.; Wang, J.; Qing, Y.; Li, H.; Sun, W.; Yu, X.; Hui, H.; Guo, Q.; Xu, J. FV-429 induces autophagy blockage and lysosome-dependent cell death of T-cell malignancies via lysosomal dysregulation. Cell Death Dis. 2021, 12, 80. [Google Scholar] [CrossRef]
- Zhou, Y.; Wei, L.; Zhang, H.; Dai, Q.; Li, Z.; Yu, B.; Guo, Q.; Lu, N. FV-429 induced apoptosis through ROS-mediated ERK2 nuclear translocation and p53 activation in gastric cancer cells. J. Cell. Biochem. 2015, 116, 1624–1637. [Google Scholar] [CrossRef] [PubMed]
- Gornowicz, A.; Szymanowska, A.; Mojzych, M.; Bielawski, K.; Bielawska, A. The effect of novel 7-methyl-5-phenyl-pyrazolo[4,3-e]tetrazolo[4,5-b][1,2,4]triazine sulfonamide derivatives on apoptosis and autophagy in DLD-1 and HT-29 colon cancer cells. Int. J. Mol. Sci. 2020, 21, 5221. [Google Scholar] [CrossRef]
- Giraud, A.S.; Menheniott, T.R.; Judd, L.M. Targeting STAT3 in gastric cancer. Expert Opin. Ther. Targets 2012, 16, 889–901. [Google Scholar] [CrossRef] [PubMed]
- Isomoto, H.; Matsushima, K.; Inoue, N.; Hayashi, T.; Nakayama, T.; Kunizaki, M.; Hidaka, S.; Nakayama, M.; Hisatsune, J.; Nakashima, M.; et al. Interweaving microRNAs and proinflammatory cytokines in gastric mucosa with reference to H. pylori infection. J. Clin. Immunol. 2012, 32, 290–299. [Google Scholar] [CrossRef] [PubMed]
- Noh, S.; Jung, J.J.; Jung, M.; Kim, T.S.; Park, C.H.; Lim, S.J.; Jeung, H.C.; Cheol, H.; Chung, H.C.; Rha, S.Y. MMP-2 as a putative biomarker for carcinomatosis in gastric cancer. Hepatogastroenterology 2011, 58, 2015–2019. [Google Scholar] [CrossRef]
- Mrena, J.; Wiksten, J.P.; Nordling, S.; Kokkola, A.; Ristimäki, A.; Haglund, C. MMP-2 but not MMP-9 associated with COX-2 and survival in gastric cancer. J. Clin. Pathol. 2006, 59, 618–623. [Google Scholar] [CrossRef] [PubMed]
- Tsujisaki, M.; Imai, K.; Hirata, H.; Hanzawa, Y.; Masuya, J.; Nakano, T.; Sugiyama, T.; Matsui, M.; Hinoda, Y.; Yachi, A. Detection of circulating intercellular adhesion molecule-1 antigen in malignant diseases. Clin. Exp. Immunol. 1991, 85, 3–8. [Google Scholar] [CrossRef]
- Natali, P.; Nicotra, M.R.; Cavaliere, R.; Bigotti, A.; Romano, G.; Temponi, M.; Ferrone, S. Differential expression of intercellular adhesion molecule 1 in primary and metastatic melanoma lesions. Cancer Res. 1990, 50, 1271–1278. [Google Scholar] [PubMed]
- Koyama, S.; Ebihara, T.; Fukao, K. Expression of intercellular adhesion molecule 1 (ICAM-1) during the development of invasion and/or metastasis of gastric carcinoma. J. Cancer Res. Clin. Oncol. 1992, 118, 609–614. [Google Scholar] [CrossRef]
- Kang, L.; Kim, M.; Lee, Y.M. Expression of ICAM-1 in Blood Vascular Endothelium and Tissues in Human Premalignant Lesion and Gastric/Hepatocellular Carcinomas. Korean J. Gastroenterol. 2022, 79, 170–176. [Google Scholar] [CrossRef]
- Gornowicz, A.; Szymanowski, W.; Bielawska, A.; Szymanowska, A.; Czarnomysy, R.; Kałuża, Z.; Bielawski, K. Monoclonal anti-MUC1 antibody with novel octahydropyrazino[2,1-a:5,4-a’]diisoquinoline derivative as a potential multi-targeted strategy in MCF-7 breast cancer cells. Oncol. Rep. 2019, 42, 1391–1403. [Google Scholar] [CrossRef]


| Cancer Cell Lines | IC50 |
|---|---|
| AGS | 1.5 µM |
| SK-BR-3 | 9.05 µM |
| HCC1954 | >10 µM |
| MDA-MB-231 | 3.33 µM |
| HT-29 | 8.73 µM |
| DLD-1 | 7.0 µM |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gornowicz, A.; Lesyk, R.; Czarnomysy, R.; Holota, S.; Shepeta, Y.; Popławska, B.; Podolak, M.; Szymanowski, W.; Bielawski, K.; Bielawska, A. Multi-Targeting Anticancer Activity of a New 4-Thiazolidinone Derivative with Anti-HER2 Antibodies in Human AGS Gastric Cancer Cells. Int. J. Mol. Sci. 2023, 24, 6791. https://doi.org/10.3390/ijms24076791
Gornowicz A, Lesyk R, Czarnomysy R, Holota S, Shepeta Y, Popławska B, Podolak M, Szymanowski W, Bielawski K, Bielawska A. Multi-Targeting Anticancer Activity of a New 4-Thiazolidinone Derivative with Anti-HER2 Antibodies in Human AGS Gastric Cancer Cells. International Journal of Molecular Sciences. 2023; 24(7):6791. https://doi.org/10.3390/ijms24076791
Chicago/Turabian StyleGornowicz, Agnieszka, Roman Lesyk, Robert Czarnomysy, Serhii Holota, Yulia Shepeta, Bożena Popławska, Magdalena Podolak, Wojciech Szymanowski, Krzysztof Bielawski, and Anna Bielawska. 2023. "Multi-Targeting Anticancer Activity of a New 4-Thiazolidinone Derivative with Anti-HER2 Antibodies in Human AGS Gastric Cancer Cells" International Journal of Molecular Sciences 24, no. 7: 6791. https://doi.org/10.3390/ijms24076791
APA StyleGornowicz, A., Lesyk, R., Czarnomysy, R., Holota, S., Shepeta, Y., Popławska, B., Podolak, M., Szymanowski, W., Bielawski, K., & Bielawska, A. (2023). Multi-Targeting Anticancer Activity of a New 4-Thiazolidinone Derivative with Anti-HER2 Antibodies in Human AGS Gastric Cancer Cells. International Journal of Molecular Sciences, 24(7), 6791. https://doi.org/10.3390/ijms24076791







