New Insight into Mechanisms of Cardiovascular Diseases: An Integrative Analysis Approach to Identify TheranoMiRNAs
Abstract
1. Introduction
2. Results
2.1. miRBase Tool Analysis
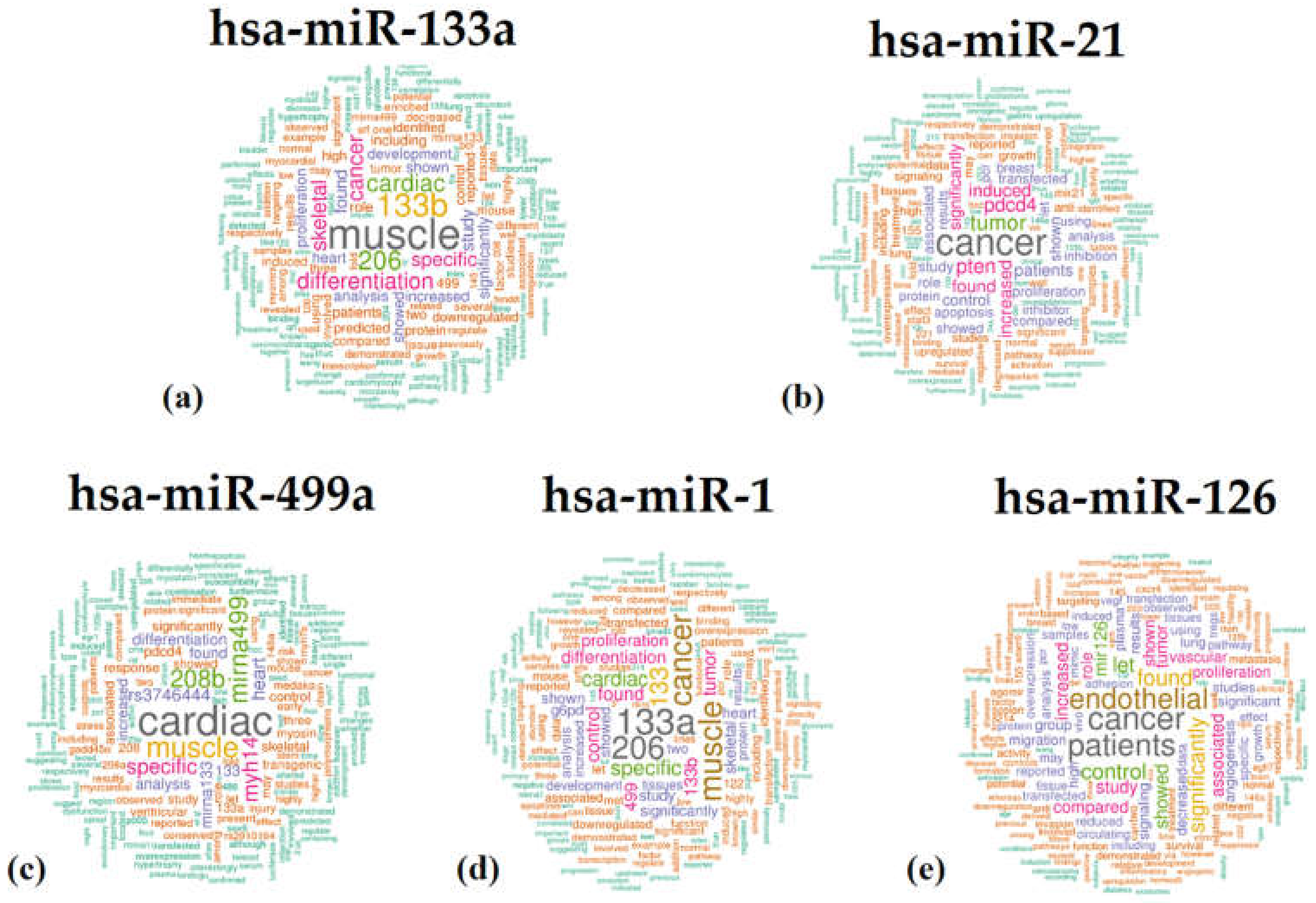
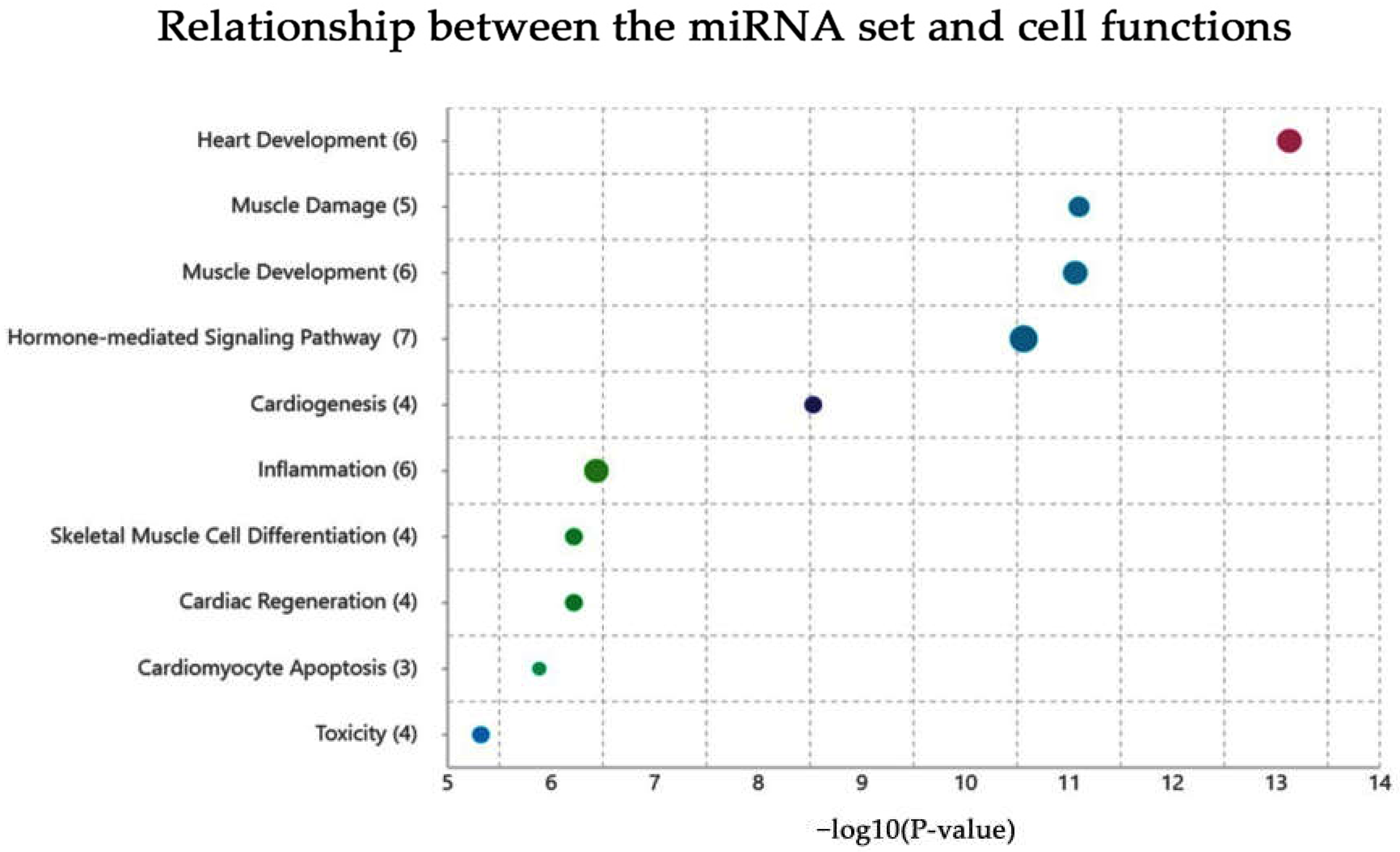
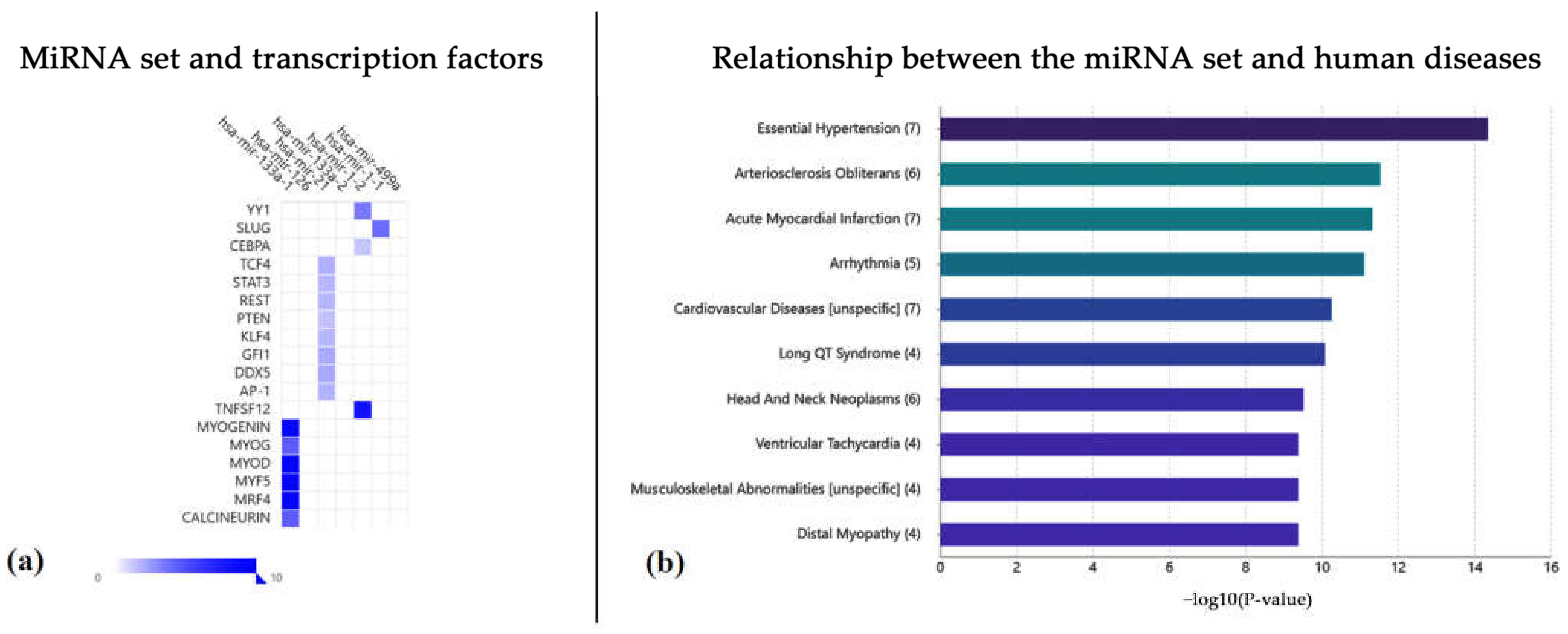
2.2. Functional Annotation Analysis via the TAM 2.0 Tool
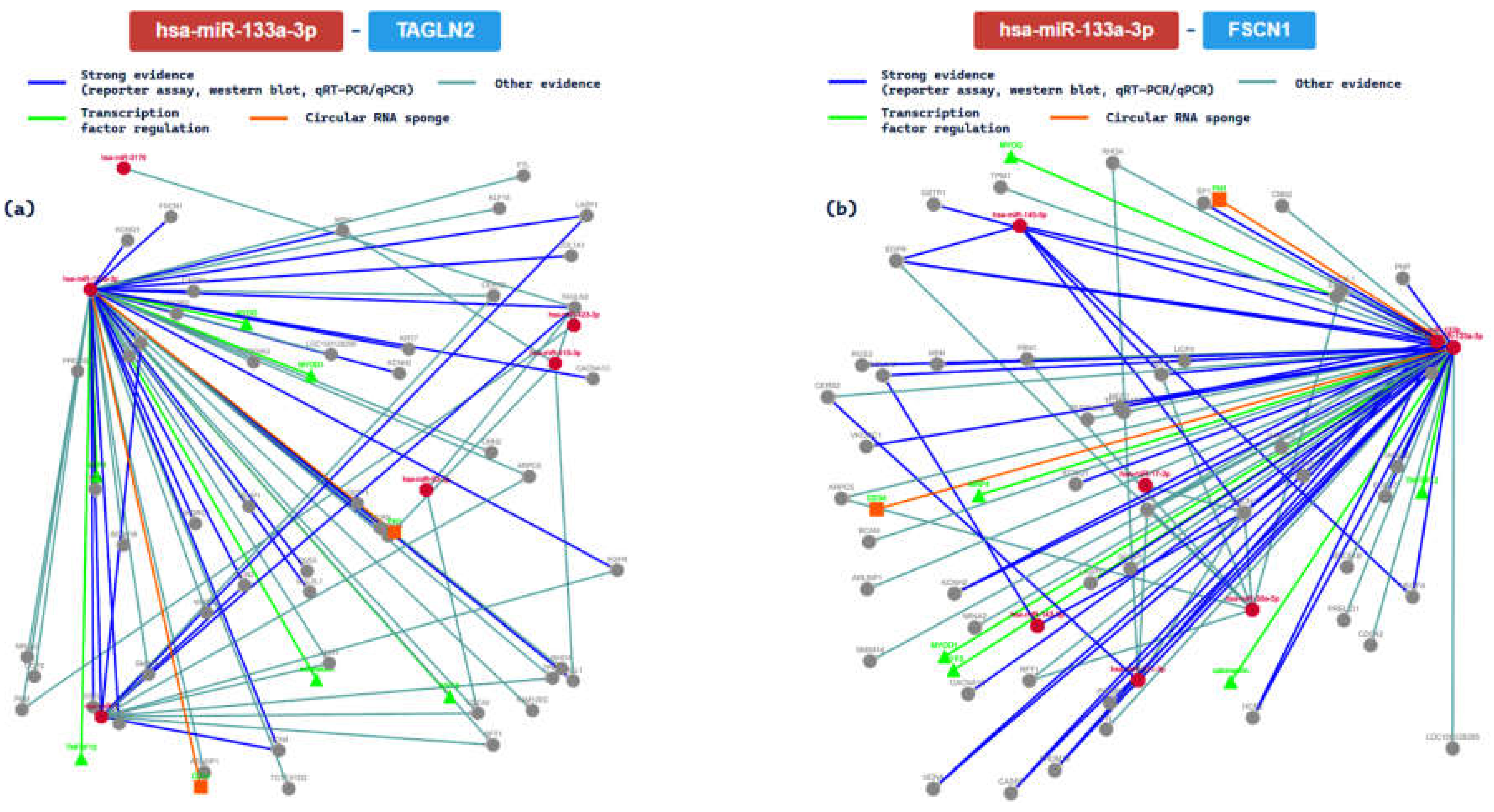
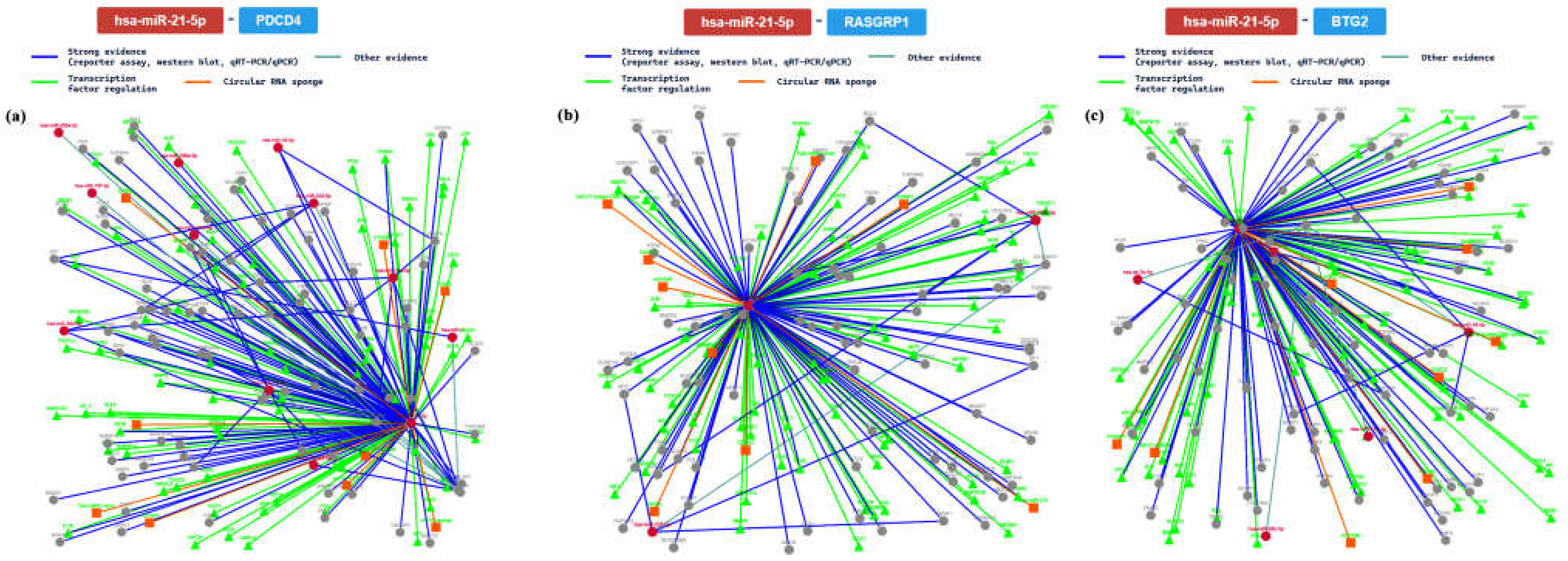
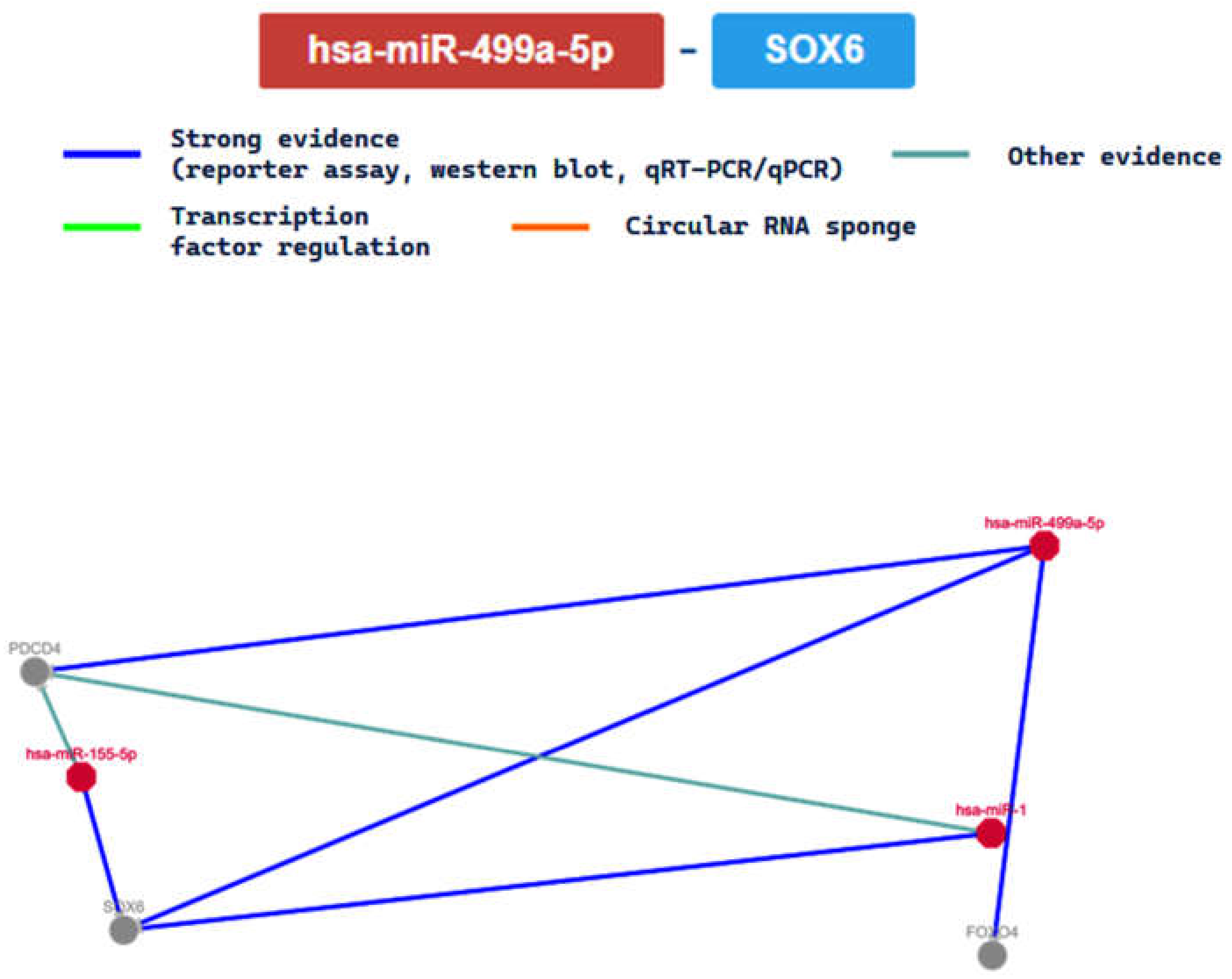
2.3. miRTarBase Tool: Report about Experimentally Validated miRNA—Target Interactions of the Selected miRNAs
3. Discussion
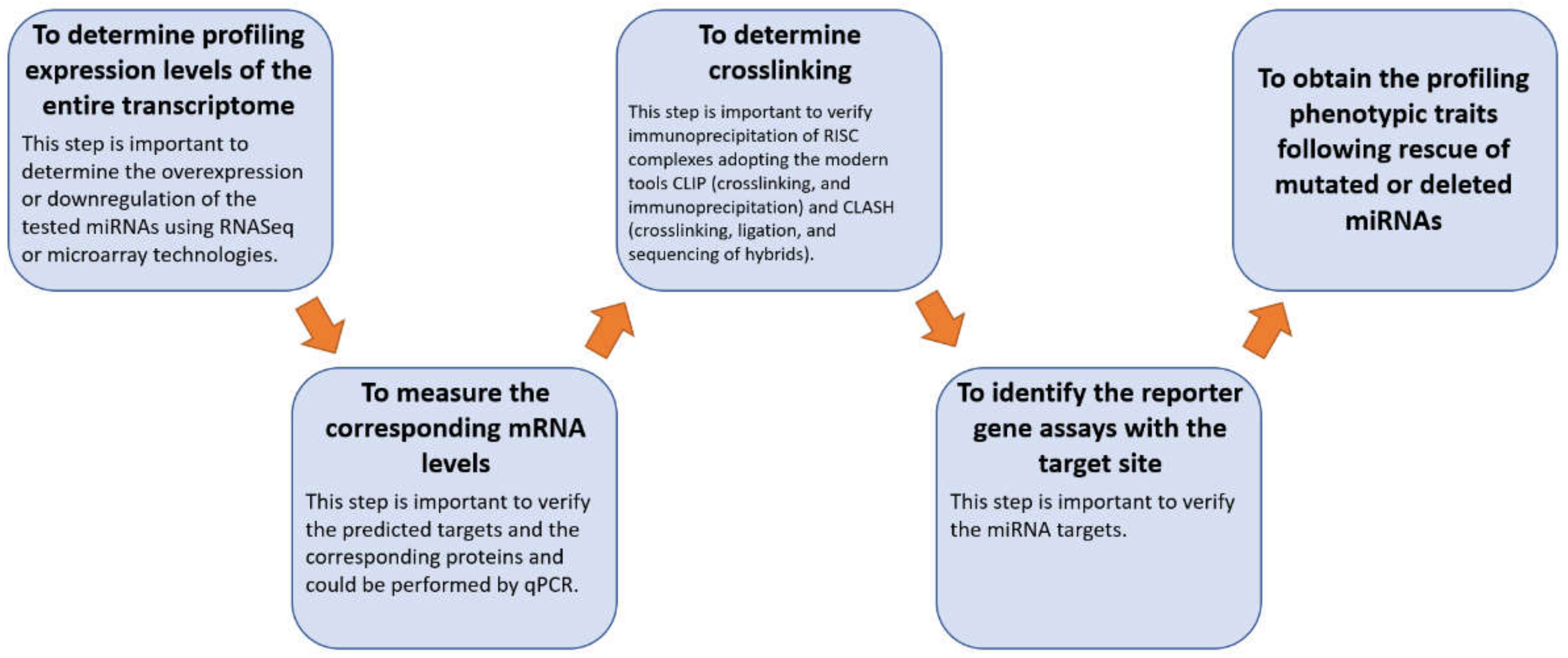
4. Materials and Methods
4.1. miRNA Selection
4.2. miRBase Tool
4.3. TAM 2.0 Tool
4.4. miRTarBase Tool
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Cheng, Y.; Dong, L.; Zhang, J.; Zhao, Y.; Li, Z. Recent advances in microRNA detection. Analyst 2018, 143, 1758–1774. [Google Scholar] [CrossRef]
- Yuan, Z.; Zhou, Y.; Gao, S.; Cheng, Y.; Li, Z. Homogeneous and Sensitive Detection of microRNA with Ligase Chain Reaction and Lambda Exonuclease-Assisted Cationic Conjugated Polymer Biosensing. ACS Appl. Mater. Interfaces 2014, 6, 6181–6185. [Google Scholar] [CrossRef] [PubMed]
- Sumaiya, K.; Ponnusamy, T.; Natarajaseenivasan, K.; Shanmughapriya, S. Cardiac Metabolism and MiRNA Interference. Int. J. Mol. Sci. 2023, 24, 50. [Google Scholar] [CrossRef] [PubMed]
- Bronze-Da-Rocha, E. MicroRNAs Expression Profiles in Cardiovascular Diseases. BioMed Res. Int. 2014, 2014, 985408. [Google Scholar] [CrossRef] [PubMed]
- Sessa, F.; Salerno, M.; Di Mizio, G.; Bertozzi, G.; Messina, G.; Tomaiuolo, B.; Pisanelli, D.; Maglietta, F.; Ricci, P.; Pomara, C. Anabolic Androgenic Steroids: Searching New Molecular Biomarkers. Front. Pharmacol. 2018, 9, 1321. [Google Scholar] [CrossRef]
- Rops, M.A.; Smit, D.L.; de Ronde, W. Bias Among Health Care Professionals: How Prejudice Leads to Diagnostic Delay in Patients Using Anabolic–Androgenic Steroids. Androg. Clin. Res. Ther. 2022, 3, 203–207. [Google Scholar] [CrossRef]
- Sessa, F.; Salerno, M.; Esposito, M.; Cocimano, G.; Pomara, C. miRNA Dysregulation in Cardiovascular Diseases: Current Opinion and Future Perspectives. Int. J. Mol. Sci. 2023, 24, 5192. [Google Scholar] [CrossRef]
- Nazarov, P.V.; Kreis, S. Integrative approaches for analysis of mRNA and microRNA high-throughput data. Comput. Struct. Biotechnol. J. 2021, 19, 1154–1162. [Google Scholar] [CrossRef]
- Annaratone, L.; De Palma, G.; Bonizzi, G.; Sapino, A.; Botti, G.; Berrino, E.; Mannelli, C.; Arcella, P.; Di Martino, S.; Steffan, A.; et al. Basic principles of biobanking: From biological samples to precision medicine for patients. Virchows Arch. 2021, 479, 233–246. [Google Scholar] [CrossRef]
- Speirs, V.; Cox, A.; Chelala, C.; James, J.A.; Adams, R.A.; Jones, J.L. A biobank perspective on use of tissue samples donated by trial participants. Lancet Oncol. 2022, 23, e205. [Google Scholar] [CrossRef]
- Sessa, F.; Salerno, M.; Bertozzi, G.; Cipolloni, L.; Messina, G.; Aromatario, M.; Polo, L.; Turillazzi, E.; Pomara, C. miRNAs as Novel Biomarkers of Chronic Kidney Injury in Anabolic-Androgenic Steroid Users: An Experimental Study. Front. Pharmacol. 2020, 11, 563756. [Google Scholar] [CrossRef]
- Guo, D.; Fan, Y.; Yue, J.-R.; Lin, T. A regulatory miRNA–mRNA network is associated with transplantation response in acute kidney injury. Hum. Genom. 2021, 15, 69. [Google Scholar] [CrossRef] [PubMed]
- Zhou, Q.; Yin, J.; Wang, Y.; Zhuang, X.; He, Z.; Chen, Z.; Yang, X. MicroRNAs as potential biomarkers for the diagnosis of Traumatic Brain Injury: A systematic review and meta-analysis. Int. J. Med. Sci. 2021, 18, 128–136. [Google Scholar] [CrossRef] [PubMed]
- Sessa, F.; Salerno, M.; Cipolloni, L.; Bertozzi, G.; Messina, G.; Di Mizio, G.; Asmundo, A.; Pomara, C. Anabolic-androgenic steroids and brain injury: miRNA evaluation in users compared to cocaine abusers and elderly people. Aging 2020, 12, 15314–15327. [Google Scholar] [CrossRef] [PubMed]
- Sessa, F.; Maglietta, F.; Bertozzi, G.; Salerno, M.; Di Mizio, G.; Messina, G.; Montana, A.; Ricci, P.; Pomara, C. Human Brain Injury and miRNAs: An Experimental Study. Int. J. Mol. Sci. 2019, 20, 1546. [Google Scholar] [CrossRef]
- Li, J.; Xu, J.; Zhong, L.; Zhang, Q.; Wang, H.; Tian, J.; Lu, X. An orthogonal direction iterative algorithm of the transport-of-intensity equation. Opt. Lasers Eng. 2019, 120, 6–12. [Google Scholar] [CrossRef]
- Riffo-Campos, Á.L.; Riquelme, I.; Brebi-Mieville, P. Tools for Sequence-Based miRNA Target Prediction: What to Choose? Int. J. Mol. Sci. 2016, 17, 1987. [Google Scholar] [CrossRef]
- Pasquinelli, A.E. MicroRNAs and their targets: Recognition, regulation and an emerging reciprocal relationship. Nat. Rev. Genet. 2012, 13, 271–282. [Google Scholar] [CrossRef]
- Mahjoubin-Tehran, M.; Aghaee-Bakhtiari, S.H.; Sahebkar, A.; Butler, A.E.; Oskuee, R.K.; Jalili, A. In silico and in vitro analysis of microRNAs with therapeutic potential in atherosclerosis. Sci. Rep. 2022, 12, 20334. [Google Scholar] [CrossRef]
- Ahmed, M.M.; Ishrat, R.; Tazyeen, S.; Alam, A.; Farooqui, A.; Ali, R.; Imam, N.; Tamkeen, N.; Ali, S.; Malik, Z.; et al. In Silico Integrative Approach Revealed Key MicroRNAs and Associated Target Genes in Cardiorenal Syndrome. Bioinform. Biol. Insights 2021, 15, 11779322211027396. [Google Scholar] [CrossRef]
- Ben Or, G.; Veksler-Lublinsky, I. Comprehensive machine-learning-based analysis of microRNA–target interactions reveals variable transferability of interaction rules across species. BMC Bioinform. 2021, 22, 264. [Google Scholar] [CrossRef] [PubMed]
- McGeary, S.E.; Lin, K.S.; Shi, C.Y.; Pham, T.M.; Bisaria, N.; Kelley, G.M.; Bartel, D.P. The biochemical basis of microRNA targeting efficacy. Science 2019, 366, eaav1741. [Google Scholar] [CrossRef] [PubMed]
- Kozomara, A.; Birgaoanu, M.; Griffiths-Jones, S. miRBase: From microRNA sequences to function. Nucleic Acids Res. 2019, 47, D155–D162. [Google Scholar] [CrossRef] [PubMed]
- Ohanian, M.; Humphreys, D.T.; Anderson, E.; Preiss, T.; Fatkin, D. A heterozygous variant in the human cardiac miR-133 gene, MIR133A2, alters miRNA duplex processing and strand abundance. BMC Genet. 2013, 14, 18. [Google Scholar] [CrossRef]
- Enielsen, S.; Ehvid, T.; Ekelly, M.; Elindegaard, B.; Edethlefsen, C.; Ewinding, K.; Emathur, N.; Escheele, C.; Pedersen, B.K.; Laye, M.J. Muscle specific miRNAs are induced by testosterone and independently upregulated by age. Front. Physiol. 2014, 4, 394. [Google Scholar] [CrossRef]
- Pisano, F.; Altomare, C.; Cervio, E.; Barile, L.; Rocchetti, M.; Ciuffreda, M.C.; Malpasso, G.; Copes, F.; Mura, M.; Danieli, P.; et al. Combination of miRNA499 and miRNA133 Exerts a Synergic Effect on Cardiac Differentiation. Stem Cells 2015, 33, 1187–1199. [Google Scholar] [CrossRef] [PubMed]
- Deng, Z.; Chen, J.-F.; Wang, D.-Z. Transgenic overexpression of miR-133a in skeletal muscle. BMC Musculoskelet. Disord. 2011, 12, 115. [Google Scholar] [CrossRef]
- Pahl, M.C.; Derr, K.; Gäbel, G.; Hinterseher, I.; Elmore, J.R.; Schworer, C.M.; Peeler, T.C.; Franklin, D.P.; Gray, J.L.; Carey, D.J.; et al. MicroRNA expression signature in human abdominal aortic aneurysms. BMC Med. Genom. 2012, 5, 25. [Google Scholar] [CrossRef]
- Chen, L.; Zhou, J.-P.; Kuang, D.-B.; Tang, J.; Li, Y.-J.; Chen, X.-P. 4-HNE Increases Intracellular ADMA Levels in Cultured HUVECs: Evidence for miR-21-Dependent Mechanisms. PLoS ONE 2013, 8, e64148. [Google Scholar] [CrossRef]
- Liu, L.-Z.; Li, C.; Chen, Q.; Jing, Y.; Carpenter, R.; Jiang, Y.; Kung, H.-F.; Lai, L.; Jiang, B.-H. MiR-21 Induced Angiogenesis through AKT and ERK Activation and HIF-1α Expression. PLoS ONE 2011, 6, e19139. [Google Scholar] [CrossRef]
- Song, J.T.; Hu, B.; Qu, H.Y.; Bi, C.L.; Huang, X.Z.; Zhang, M. Mechanical Stretch Modulates MicroRNA 21 Expression, Participating in Proliferation and Apoptosis in Cultured Human Aortic Smooth Muscle Cells. PLoS ONE 2012, 7, e47657. [Google Scholar] [CrossRef] [PubMed]
- Zhu, H.; Li, C.; Bai, W.; Su, L.-L.; Liu, J.-Q.; Li, Y.; Shi, J.-H.; Cai, W.-X.; Bai, X.-Z.; Jia, Y.-H.; et al. MicroRNA-21 Regulates hTERT via PTEN in Hypertrophic Scar Fibroblasts. PLoS ONE 2014, 9, e97114. [Google Scholar] [CrossRef] [PubMed]
- Green, D.E.; Murphy, T.C.; Kang, B.-Y.; Searles, C.D.; Hart, C.M. PPARγ Ligands Attenuate Hypoxia-Induced Proliferation in Human Pulmonary Artery Smooth Muscle Cells through Modulation of MicroRNA-21. PLoS ONE 2015, 10, e0133391. [Google Scholar] [CrossRef] [PubMed]
- Shieh, J.T.C.; Huang, Y.; Gilmore, J.; Srivastava, D. Elevated miR-499 Levels Blunt the Cardiac Stress Response. PLoS ONE 2011, 6, e19481. [Google Scholar] [CrossRef]
- Bhuiyan, S.S.; Kinoshita, S.; Wongwarangkana, C.; Asaduzzaman, M.; Asakawa, S.; Watabe, S. Evolution of the myosin heavy chain gene MYH14 and its intronic microRNA miR-499: Muscle-specific miR-499 expression persists in the absence of the ancestral host gene. BMC Evol. Biol. 2013, 13, 142. [Google Scholar] [CrossRef]
- Fu, J.-D.; Rushing, S.N.; Lieu, D.K.; Chan, C.W.; Kong, C.-W.; Geng, L.; Wilson, K.D.; Chiamvimonvat, N.; Boheler, K.R.; Wu, J.C.; et al. Distinct Roles of MicroRNA-1 and -499 in Ventricular Specification and Functional Maturation of Human Embryonic Stem Cell-Derived Cardiomyocytes. PLoS ONE 2011, 6, e27417. [Google Scholar] [CrossRef]
- Wang, N.; Yang, C.; Xie, F.; Sun, L.; Su, X.; Wang, Y.; Wei, R.; Zhang, R.; Li, X.; Yang, B.; et al. Gadd45α: A Novel Diabetes-Associated Gene Potentially Linking Diabetic Cardiomyopathy and Baroreflex Dysfunction. PLoS ONE 2012, 7, e49077. [Google Scholar] [CrossRef]
- Zhang, X.; Azhar, G.; Helms, S.A.; Wei, J.Y. Regulation of cardiac microRNAs by serum response factor. J. Biomed. Sci. 2011, 18, 15. [Google Scholar] [CrossRef]
- Li, Q.; Guo, J.; Lin, X.; Yang, X.; Ma, Y.; Fan, G.-C.; Chang, J. An Intragenic SRF-Dependent Regulatory Motif Directs Cardiac-Specific microRNA-1-1/133a-2 Expression. PLoS ONE 2013, 8, e75470. [Google Scholar] [CrossRef]
- Ceci, M.; Carlantoni, C.; Missinato, M.A.; Bonvissuto, D.; Di Giacomo, B.; Contu, R.; Romano, N. Micro RNAs are involved in activation of epicardium during zebrafish heart regeneration. Cell Death Discov. 2018, 4, 41. [Google Scholar] [CrossRef]
- Koutsoulidou, A.; Mastroyiannopoulos, N.P.; Furling, D.; Uney, J.B.; Phylactou, L.A. Expression of miR-1, miR-133a, miR-133b and miR-206 increases during development of human skeletal muscle. BMC Dev. Biol. 2011, 11, 34. [Google Scholar] [CrossRef] [PubMed]
- Witkowski, M.; Weithauser, A.; Tabaraie, T.; Steffens, D.; Kränkel, N.; Stratmann, B.; Tschoepe, D.; Landmesser, U.; Rauch-Kroehnert, U. Micro–RNA-126 Reduces the Blood Thrombogenicity in Diabetes Mellitus via Targeting of Tissue Factor. Arter. Thromb. Vasc. Biol. 2016, 36, 1263–1271. [Google Scholar] [CrossRef] [PubMed]
- Qin, A.; Wen, Z.; Zhou, Y.; Li, Y.; Li, Y.; Luo, J.; Ren, T.; Xu, L. MicroRNA-126 regulates the induction and function of CD4+ Foxp3+ regulatory T cells through PI3K/AKT pathway. J. Cell. Mol. Med. 2013, 17, 252–264. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.; Lee, A.Y.W.; Wigg, J.P.; Peshavariya, H.; Liu, P.; Zhang, H. miR-126 Regulation of Angiogenesis in Age-Related Macular Degeneration in CNV Mouse Model. Int. J. Mol. Sci. 2016, 17, 895. [Google Scholar] [CrossRef] [PubMed]
- Dos Santos, A.M.; Metzinger, L.; Haddad, O.; M’Baya-Moutoula, E.; Taïbi, F.; Charnaux, N.; Massy, Z.A.; Hlawaty, H.; Meuth, V.M.-L. miR-126 Is Involved in Vascular Remodeling under Laminar Shear Stress. BioMed Res. Int. 2015, 2015, 497280. [Google Scholar] [CrossRef]
- Izuhara, M.; Kuwabara, Y.; Saito, N.; Yamamoto, E.; Hakuno, D.; Nakashima, Y.; Horie, T.; Baba, O.; Nishiga, M.; Nakao, T.; et al. Prevention of neointimal formation using miRNA-126-containing nanoparticle-conjugated stents in a rabbit model. PLoS ONE 2017, 12, e0172798. [Google Scholar] [CrossRef]
- Hasty, P.; Bradley, A.; Morris, J.H.; Edmondson, D.G.; Venuti, J.M.; Olson, E.N.; Klein, W.H. Muscle deficiency and neonatal death in mice with a targeted mutation in the myogenin gene. Nature 1993, 364, 501–506. [Google Scholar] [CrossRef]
- Long, K.; Su, D.; Li, X.; Li, H.; Zeng, S.; Zhang, Y.; Zhong, Z.; Lin, Y.; Li, X.; Lu, L.; et al. Identification of enhancers responsible for the coordinated expression of myosin heavy chain isoforms in skeletal muscle. BMC Genom. 2022, 23, 519. [Google Scholar] [CrossRef]
- Lei, S.; Li, C.; She, Y.; Zhou, S.; Shi, H.; Chen, R. Roles of super enhancers and enhancer RNAs in skeletal muscle development and disease. Cell Cycle 2023, 22, 495–505. [Google Scholar] [CrossRef]
- Lazure, F.; Blackburn, D.M.; Corchado, A.H.; Sahinyan, K.; Karam, N.; Sharanek, A.; Nguyen, D.; Lepper, C.; Najafabadi, H.S.; Perkins, T.J.; et al. Myf6/MRF4 is a myogenic niche regulator required for the maintenance of the muscle stem cell pool. EMBO Rep. 2020, 21, e49499. [Google Scholar] [CrossRef]
- Jo, S.; Kim, H.-R.; Mun, Y.; Jun, C.-D. Transgelin-2 in immunity: Its implication in cell therapy. J. Leukoc. Biol. 2018, 104, 903–910. [Google Scholar] [CrossRef] [PubMed]
- The GeneCards Suite Project Team. TAGLN2 Gene—Transgelin 2. Available online: https://www.genecards.org/cgi-bin/carddisp.pl?gene=TAGLN2 (accessed on 28 March 2023).
- The GeneCards Suite Project Team. FSCN1 Gene—Fascin Actin-Bundling Protein 1. Available online: https://www.genecards.org/cgi-bin/carddisp.pl?gene=FSCN1 (accessed on 28 March 2023).
- The GeneCards Suite Project Team. PDCD4 Gene—Programmed Cell Death 4. Available online: https://www.genecards.org/cgi-bin/carddisp.pl?gene=PDCD4 (accessed on 28 March 2023).
- The GeneCards Suite Project Team. RASGRP1 Gene—RAS Guanyl Releasing Protein 1. Available online: https://www.genecards.org/cgi-bin/carddisp.pl?gene=RASGRP1 (accessed on 28 March 2023).
- The GeneCards Suite Project Team. BTG2 Gene—BTG Anti-Proliferation Factor 2. Available online: https://www.genecards.org/cgi-bin/carddisp.pl?gene=BTG2 (accessed on 28 March 2023).
- The GeneCards Suite Project Team. SOX6 Gene—SRY-Box Transcription Factor 6. Available online: https://www.genecards.org/cgi-bin/carddisp.pl?gene=SOX6 (accessed on 28 March 2023).
- The GeneCards Suite Project Team PTMA Gene—Prothymosin Alpha. Available online: https://www.genecards.org/cgi-bin/carddisp.pl?gene=PTMA (accessed on 28 March 2023).
- The GeneCards Suite Project Team. SERP1 Gene—Stress Associated Endoplasmic Reticulum Protein 1. Available online: https://www.genecards.org/cgi-bin/carddisp.pl?gene=SERP1 (accessed on 28 March 2023).
- The GeneCards Suite Project Team. SRSF9 Gene—Serine and Arginine Rich Splicing Factor 9. Available online: https://www.genecards.org/cgi-bin/carddisp.pl?gene=SRSF9 (accessed on 28 March 2023).
- The GeneCards Suite Project Team. VEGFA Gene—Vascular Endothelial Growth Factor A. Available online: https://www.genecards.org/cgi-bin/carddisp.pl?gene=VEGFA (accessed on 28 March 2023).
- The GeneCards Suite Project Team. SOX2 Gene—SRY-Box Transcription Factor 2. Available online: https://www.genecards.org/cgi-bin/carddisp.pl?gene=SOX2 (accessed on 28 March 2023).
- Mukushkina, D.; Aisina, D.; Pyrkova, A.; Ryskulova, A.; Labeit, S.; Ivashchenko, A. In silico Prediction of miRNA Interactions with Candidate Atherosclerosis Gene mRNAs. Front. Genet. 2020, 11, 605054. [Google Scholar] [CrossRef] [PubMed]
- Kern, F.; Backes, C.; Hirsch, P.; Fehlmann, T.; Hart, M.; Meese, E.; Keller, A. What’s the target: Understanding two decades of in silico microRNA-target prediction. Briefings Bioinform. 2020, 21, 1999–2010. [Google Scholar] [CrossRef] [PubMed]
- O’Sullivan, J.W.; Raghavan, S.; Marquez-Luna, C.; Luzum, J.A.; Damrauer, S.M.; Ashley, E.A.; O’Donnell, C.J.; Willer, C.J.; Natarajan, P. Polygenic Risk Scores for Cardiovascular Disease: A Scientific Statement from the American Heart Association. Circulation 2022, 146, E93–E118. [Google Scholar] [CrossRef]
- O’Sullivan, J.W.; Ashley, E.A.; Elliott, P.M. Polygenic risk scores for the prediction of cardiometabolic disease. Eur. Heart J. 2023, 44, 89–99. [Google Scholar] [CrossRef]
- Hanna, J.; Hossain, G.S.; Kocerha, J. The Potential for microRNA Therapeutics and Clinical Research. Front. Genet. 2019, 10, 478. [Google Scholar] [CrossRef]
- Ahmad, N.; Haider, S.; Jagannathan, S.; Anaissie, E.; Driscoll, J.J. MicroRNA theragnostics for the clinical management of multiple myeloma. Leukemia 2014, 28, 732–738. [Google Scholar] [CrossRef]
- Allegra, A.; Cicero, N.; Tonacci, A.; Musolino, C.; Gangemi, S. Circular RNA as a Novel Biomarker for Diagnosis and Prognosis and Potential Therapeutic Targets in Multiple Myeloma. Cancers 2022, 14, 1700. [Google Scholar] [CrossRef]
- Sessa, F.; Magdy, T.; Sukasem, C.; Patrinos, G.P. Brief research reports in pharmacogenetics and pharmacogenomics: 2022. Front. Pharmacol. 2023, 14, 608. [Google Scholar] [CrossRef]
- Akhtar, M.M.; Micolucci, L.; Islam, S.; Olivieri, F.; Procopio, A.D. Bioinformatic tools for microRNA dissection. Nucleic Acids Res. 2016, 44, 24–44. [Google Scholar] [CrossRef]
- Shukla, V.; Varghese, V.K.; Kabekkodu, S.P.; Mallya, S.; Satyamoorthy, K. A compilation of Web-based research tools for miRNA analysis. Briefings Funct. Genom. 2017, 16, 249–273. [Google Scholar] [CrossRef] [PubMed]
- Singh, N.K. miRNAs target databases: Developmental methods and target identification techniques with functional annotations. Cell. Mol. Life Sci. 2017, 74, 2239–2261. [Google Scholar] [CrossRef] [PubMed]
- Polito, R.; Nigro, E.; Elce, A.; Monaco, M.L.; Iacotucci, P.; Carnovale, V.; Comegna, M.; Gelzo, M.; Zarrilli, F.; Corso, G.; et al. Adiponectin Expression Is Modulated by Long-Term Physical Activity in Adult Patients Affected by Cystic Fibrosis. Mediat. Inflamm. 2019, 2019, 2153934. [Google Scholar] [CrossRef] [PubMed]
- Zhu, Y.-F.; Wang, R.; Chen, W.; Cao, Y.-D.; Li, L.-P.; Chen, X. miR-133a-3p attenuates cardiomyocyte hypertrophy through inhibiting pyroptosis activation by targeting IKKε. Acta Histochem. 2021, 123, 151653. [Google Scholar] [CrossRef] [PubMed]
- Escate, R.; Padró, T.; Suades, R.; Camino, S.; Muñiz, O.; Diaz-Diaz, J.L.; Sionis, A.; Mata, P.; Badimon, L. High miR-133a levels in the circulation anticipates presentation of clinical events in familial hypercholesterolaemia patients. Cardiovasc. Res. 2021, 117, 109–122. [Google Scholar] [CrossRef]
- Colpaert, R.M.; Calore, M. MicroRNAs in Cardiac Diseases. Cells 2019, 8, 737. [Google Scholar] [CrossRef]
- Dai, B.; Wang, F.; Nie, X.; Du, H.; Zhao, Y.; Yin, Z.; Li, H.; Fan, J.; Wen, Z.; Wang, D.W.; et al. The Cell Type–Specific Functions of miR-21 in Cardiovascular Diseases. Front. Genet. 2020, 11, 563166. [Google Scholar] [CrossRef]
- Surina, S.; Fontanella, R.A.; Scisciola, L.; Marfella, R.; Paolisso, G.; Barbieri, M. miR-21 in Human Cardiomyopathies. Front. Cardiovasc. Med. 2021, 8, 767064. [Google Scholar] [CrossRef]
- Wan, Q.; Xu, T.; Ding, W.; Zhang, X.; Ji, X.; Yu, T.; Yu, W.; Lin, Z.; Wang, J. miR-499-5p Attenuates Mitochondrial Fission and Cell Apoptosis via p21 in Doxorubicin Cardiotoxicity. Front. Genet. 2019, 9, 734. [Google Scholar] [CrossRef]
- Nukala, S.B.; Jousma, J.; Cho, Y.; Lee, W.H.; Ong, S.G. Long non-coding RNAs and microRNAs as crucial regulators in cardio-oncology. Cell Biosci. 2022, 12, 24. [Google Scholar] [CrossRef]
- Shi, Y.; Han, Y.; Niu, L.; Li, J.; Chen, Y. MiR-499 inhibited hypoxia/reoxygenation induced cardiomyocytes injury by targeting SOX6. Biotechnol. Lett. 2019, 41, 837–847. [Google Scholar] [CrossRef] [PubMed]
- Seok, H.; Lee, H.; Lee, S.; Ahn, S.H.; Lee, H.-S.; Kim, G.-W.D.; Peak, J.; Park, J.; Cho, Y.K.; Jeong, Y.; et al. Position-specific oxidation of miR-1 encodes cardiac hypertrophy. Nature 2020, 584, 279–285. [Google Scholar] [CrossRef] [PubMed]
- Li, M.; Chen, X.; Chen, L.; Chen, K.; Zhou, J.; Song, J. MiR-1-3p that correlates with left ventricular function of HCM can serve as a potential target and differentiate HCM from DCM. J. Transl. Med. 2018, 16, 161. [Google Scholar] [CrossRef] [PubMed]
- Yamada, H.; Suzuki, K.; Fujii, R.; Kawado, M.; Hashimoto, S.; Watanabe, Y.; Iso, H.; Fujino, Y.; Wakai, K.; Tamakoshi, A. Circulating miR-21, miR-29a, and miR-126 are associated with premature death risk due to cancer and cardiovascular disease: The JACC Study. Sci. Rep. 2021, 11, 5298. [Google Scholar] [CrossRef]
- Zhelankin, A.; Stonogina, D.; Vasiliev, S.; Babalyan, K.; Sharova, E.; Doludin, Y.; Shchekochikhin, D.; Generozov, E.; Akselrod, A. Circulating Extracellular miRNA Analysis in Patients with Stable CAD and Acute Coronary Syndromes. Biomolecules 2021, 11, 962. [Google Scholar] [CrossRef]
- Birgaoanu, M.; Sachse, M.; Gatsiou, A. RNA Editing Therapeutics: Advances, Challenges and Perspectives on Combating Heart Disease. Cardiovasc. Drugs Ther. 2023, 37, 401–411. [Google Scholar] [CrossRef]
- Lu, M.; Shi, B.; Wang, J.; Cao, Q.; Cui, Q. TAM: A method for enrichment and depletion analysis of a microRNA category in a list of microRNAs. BMC Bioinform. 2010, 11, 419. [Google Scholar] [CrossRef]
- Huang, H.-Y.; Lin, Y.-C.; Cui, S.; Huang, Y.; Tang, Y.; Xu, J.; Bao, J.; Li, Y.; Wen, J.; Zuo, H.; et al. miRTarBase update 2022: An informative resource for experimentally validated miRNA–target interactions. Nucleic Acids Res. 2022, 50, D222–D230. [Google Scholar] [CrossRef]



| miRNA (Mature Sequence) | Accession Number | Sequence | Genomic Localization |
|---|---|---|---|
| hsa-miR-133a-3p | MIMAT0000427 | UUUGGUCCCCUUCAACCAGCUG | chr18: 21825698-21825785 [−] |
| hsa-miR-21-5p | MIMAT0000076 | UAGCUUAUCAGACUGAUGUUGA | chr17: 59841266-59841337 [+] |
| hsa-miR-499a-5p | MIMAT0002870 | UUAAGACUUGCAGUGAUGUUU | chr20: 34990376-34990497 [+] |
| hsa-miR-1-3p | MIMAT0000416 | UGGAAUGUAAAGAAGUAUGUAU | chr18: 21829004-21829088 [−] |
| hsa-miR-126-3p | MIMAT0000445 | UCGUACCGUGAGUAAUAAUGCG | chr9: 136670602-136670686 [+] |
| Rank, First Authors, Year | Article Title | N° of Sentences | Other Human miRNAs | |
|---|---|---|---|---|
| hsa-miR-133a- | n° 1, Ohanian et al., 2013 [24] | A heterozygous variant in the human cardiac miR-133 gene, MIR133A2, alters miRNA duplex processing and strand abundance. | 85 | miR-96, miR-1-2, miR-133a-2, miR-206, miR-1-1, miR-133b, miR-499a, miR-499b |
| n° 3, Nielsen et al., 2013 [25] | Muscle specific miRNAs are induced by testosterone and independently upregulated by age. | 48 | miR-1-2, miR-133a-2, miR-206, miR-1-1, miR-133b | |
| n° 4, Pisano et al., 2015 [26] | Combination of miRNA499 and miRNA133 exerts a synergic effect on cardiac differentiation. | 60 | miR-1-2, miR-133a-2, miR-1-1, miR-133b, miR-449a, miR-499a, miR-449b, miR-449c, miR-499b | |
| n° 5, Deng et al., 2011 [27] | Transgenic overexpression of miR-133a in skeletal muscle. | 53 | miR-208a, miR-214, miR-221, miR-222, miR-1-2, miR-133a-2, miR-206, miR-1-1, miR-155, miR-133b, miR-146b, miR-499a, miR-208b, miR-499b | |
| n° 6, Pahl et al., 2012 [28] | MicroRNA expression signature in human abdominal aortic aneurysms. | 27 | miR-21, miR-29b-1, miR-29b-2, miR-30c-2, miR-181a-2, miR-204, miR-211, miR-181a-1, miR-133a-2, miR-146a, miR-331, miR-133b, miR-146b | |
| hsa-miR-21 | n° 7, Chen et al., 2013 [29] | 4-HNE increases intracellular ADMA levels in cultured HUVECs: evidence for miR-21-dependent mechanisms. | 97 | / |
| n° 8, Liu et al., 2011 [30] | MiR-21 induced angiogenesis through AKT and ERK activation and HIF-1α expression. | 81 | let-7a-1, let-7a-2, let-7a-3, let-7b, let-7c, let-7d, let-7e, let-7f-1, let-7f-2, let-7g, let-7i, miR-27b, miR-130a, miR-126, miR-296, miR-378a, miR-378d-2, miR-378b, miR-378c, miR-378d-1, miR-378e, miR-378f, miR-378g, miR-378h, miR-378i, miR-378j | |
| n° 9, Song et al., 2012 [31] | Mechanical stretch modulates microRNA 21 expression, participating in proliferation and apoptosis in cultured human aortic smooth muscle cells. | 93 | miR-19a, miR-26a-1, miR-23b, miR-26a-2 | |
| n° 12, Zhu et al., 2014 [32] | MicroRNA-21 regulates hTERT via PTEN in hypertrophic scar fibroblasts. | 103 | / | |
| n° 60, Green et al., 2014 [33] | PPARγ Ligands Attenuate Hypoxia-Induced Proliferation in Human Pulmonary Artery Smooth Muscle Cells through Modulation of MicroRNA-21. | 71 | miR-204 | |
| hsa-miR-499a | n° 1, Shieh et al., 2011 [34] | Elevated miR-499 levels blunt the cardiac stress response. | 107 | miR-16-1, miR-16-2, miR-208a, miR-1-2, miR-133a-1, miR-133a-2, miR-206, miR-1-1, miR-133b, miR-208b, miR-499b |
| n° 2, Pisano et al., 2015 [26] | Combination of miRNA499 and miRNA133 exerts a synergic effect on cardiac differentiation. | 60 | miR-1-2, miR-133a-2, mirR-1-1, miR-133b, miR-449a, miR-499a, miR-449b, miR-449c, miR-499b | |
| n° 3, Bhuiyan et al., 2013 [35] | Evolution of the myosin heavy chain gene MYH14 and its intronic microRNA miR-499: muscle-specific miR-499 expression persists in the absence of the ancestral host gene. | 95 | miR-499b | |
| n° 6, Fu et al., 2013 [36] | Distinct roles of microRNA-1 and -499 in ventricular specification and functional maturation of human embryonic stem cell-derived cardiomyocytes. | 42 | let-7a-1, let-7a-2, let-7a-3, let-7b, let-7c, let-7d, let-7e, let-7f-1, let-7f-2, miR-21, miR-26b, miR-27a, miR-30a, miR-208a, let-7i, miR-1-2, miR-27b, miR-30b, miR-133a-1, miR-133a-2, miR-143, miR-125a, miR-126, miR-188, miR-1-1, miR-302a, miR-296, miR-302b, miR-302c, miR-302d, miR-371a, miR-133b, miR-208b, miR-302e, miR-302f, miR-371b, miR-499b | |
| n° 7, Wang et al., 2012 [37] | Gadd45α: a novel diabetes-associated gene potentially linking diabetic cardiomyopathy and baroreflex dysfunction. | 29 | miR-1-2, miR-133a-1, miR-133a-2, miR-320a, miR-1-1, miR-320b-1, miR-320c-1, miR-320b-2, miR-320d-1, miR-320c-2, miR-320d-2, miR-320e, miR-499b | |
| hsa-miR-1 | n° 5, Fu et al., 2013 [36] | Distinct roles of microRNA-1 and -499 in ventricular specification and functional maturation of human embryonic stem cell-derived cardiomyocytes. | 42 | let-7a-1, let-7a-2, let-7a-3, let-7b, let-7c, let-7d, let-7e, let-7f-1, let-7f-2, miR-21, miR-26b, miR-27a, miR-30a, miR-208a, let-7i, miR-1-2, miR-27b, miR-30b, miR-133a-1, miR-133a-2, miR-143, miR-125a, miR-126, miR-188, mir-1-1, miR-302a, miR-296, miR-302b, miR-302c, miR-302d, miR-371a, miR-133b, miR-208b, miR-302e, miR-302f, miR-371b, miR-499b |
| n° 7, Zhang et al., 2011 [38] | Regulation of cardiac microRNAs by serum response factor. | 31 | miR-21, miR-199a-1, miR-199a-2, miR-1-2, miR-133a-1, miR-133a-2, miR-381, miR-499a, miR-499b | |
| n° 11, Li et al., 2013 [39] | An intragenic SRF-dependent regulatory motif directs cardiac-specific microRNA-1-1/133a-2 expression. | 37 | miR-1-2, miR-133a-1, miR-133a-2, miR-133b | |
| n° 12, Ceci et al., 2018 [40] | Micro RNAs are involved in activation of epicardium during zebrafish heart regeneration. | 24 | miR-1-2, miR-133a-1, miR-133a-2, miR-133b | |
| n° 13, Koutsoulidou et al., 2018 [41] | Expression of miR-1, miR-133a, miR-133b and miR-206 increases during development of human skeletal muscle. | 28 | miR-1-2, miR-133a-1, miR-133a-2, miR-206, miR-133b | |
| hsa-miR-126 | n° 2, Witkowski et al., 2016 [42] | Micro-RNA-126 Reduces the Blood Thrombogenicity in Diabetes Mellitus via Targeting of Tissue Factor. | 105 | miR-19a, miR-19b-1, miR-19b-2 |
| n° 5, Qin et al., 2013 [43] | MicroRNA-126 regulates the induction and function of CD4+ Foxp3+ regulatory T cells through PI3K/AKT pathway | 97 | miR-15a, miR-15b, miR-146a, miR-155 | |
| n° 10, Wang et al., 2016 [44] | miR-126 Regulation of Angiogenesis in Age-Related Macular Degeneration in CNV Mouse Model. | 70 | miR-23a, miR-31, miR-23b, miR-122, miR-145, miR-146a, miR-150, miR-184, miR-23c | |
| n° 11, Mondadori et al., 2015 [45] | miR-126 Is Involved in Vascular Remodeling under Laminar Shear Stress. | 59 | miR-222 | |
| n° 12, Izuhara et al., 2015 [46] | Prevention of neointimal formation using miRNA-126-containing nanoparticle-conjugated stents in a rabbit model. | 89 | / |
| miRTarBase ID | Target | Validation Methods | |||||||
|---|---|---|---|---|---|---|---|---|---|
| Strong Evidence | Less-Strong Evidence | ||||||||
| Reported Assay | Western Blot | qPCR | Microarray | NGS | pSILAC | Other | CLIP-Seq | ||
| MIRT005604 | TAGLN2 | ||||||||
| MIRT003542 | FSCN1 | ||||||||
| MIRT000327 | CDC42 | ||||||||
| MIRT001204 | HCN2 | ||||||||
| MIRT002925 | KCNQ1 | ||||||||
| MIRT004831 | KCNH2 | ||||||||
| MIRT006571 | PNP | ||||||||
| MIRT021705 | ARPC5 | ||||||||
| MIRT021714 | COL1A1 | ||||||||
| MIRT052647 | BCL2L1 | ||||||||
| MIRT052648 | MCL1 | ||||||||
| MIRT053333 | RFFL | ||||||||
| MIRT081957 | UBA2 | ||||||||
| MIRT437953 | ANXA2 | ||||||||
| MIRT437954 | SNX30 | ||||||||
| MIRT437955 | SGMS2 | ||||||||
| MIRT737550 | TGFB1 | ||||||||
| miRTarBase ID | Target | Validation Methods | |||||||
|---|---|---|---|---|---|---|---|---|---|
| Strong Evidence | Less-Strong Evidence | ||||||||
| Reported Assay | Western Blot | qPCR | Microarray | NGS | pSILAC | Other | CLIP-Seq | ||
| MIRT003054 | PDCD4 | ||||||||
| MIRT000019 | RASGRP1 | ||||||||
| MIRT002416 | BTG2 | ||||||||
| MIRT000157 | CDC25A | ||||||||
| MIRT000159 | BCL2 | ||||||||
| MIRT000168 | RPS7 | ||||||||
| MIRT000954 | TIMP3 | ||||||||
| MIRT000960 | SOX5 | ||||||||
| MIRT000969 | RECK | ||||||||
| MIRT001189 | TGFBR2 | ||||||||
| MIRT001190 | PTEN | ||||||||
| MIRT001980 | TPM1 | ||||||||
| MIRT002406 | CDK6 | ||||||||
| MIRT002410 | APAF1 | ||||||||
| MIRT003567 | SERPINB5 | ||||||||
| MIRT003837 | BMPR2 | ||||||||
| MIRT005429 | MSH2 | ||||||||
| MIRT005807 | EGFR | ||||||||
| miRTarBase ID | Target | Validation Methods | |||||||
|---|---|---|---|---|---|---|---|---|---|
| Strong Evidence | Less-Strong Evidence | ||||||||
| Reported Assay | Western Blot | qPCR | Microarray | NGS | pSILAC | Other | CLIP-Seq | ||
| MIRT004658 | SOX6 | ||||||||
| MIRT006698 | FOXO4 | ||||||||
| MIRT006699 | PDCD4 | ||||||||
| MIRT054151 | ETS1 | ||||||||
| MIRT734866 | VAV3 | ||||||||
| miRTarBase ID | Target | Validation Methods | |||||||
|---|---|---|---|---|---|---|---|---|---|
| Strong Evidence | Less-Strong Evidence | ||||||||
| Reported Assay | Western Blot | qPCR | Microarray | NGS | pSILAC | Other | CLIP-Seq | ||
| MIRT001343 | PTMA | ||||||||
| MIRT002806 | SERP1 | ||||||||
| MIRT005072 | SRSF9 | ||||||||
| MIRT001357 | MET | ||||||||
| MIRT002788 | TAGLN2 | ||||||||
| MIRT002956 | G6PD | ||||||||
| MIRT004670 | PAX3 | ||||||||
| MIRT005089 | TWF1 | ||||||||
| MIRT001364 | GPD2 | ||||||||
| MIRT000933 | HCN4 | ||||||||
| MIRT001375 | CORO1C | ||||||||
| MIRT001388 | ANXA2 | ||||||||
| MIRT001983 | KCNJ2 | ||||||||
| MIRT002751 | XPO6 | ||||||||
| MIRT002808 | LASP1 | ||||||||
| MIRT003765 | PNP | ||||||||
| miRTarBase ID | Target | Validation Methods | |||||||
|---|---|---|---|---|---|---|---|---|---|
| Strong Evidence | Less-Strong Evidence | ||||||||
| Reported Assay | Western Blot | qPCR | Microarray | NGS | pSILAC | Other | CLIP-Seq | ||
| MIRT003428 | VEGFA | ||||||||
| MIRT005370 | SOX2 | ||||||||
| MIRT000967 | TOM1 | ||||||||
| MIRT002993 | CRK | ||||||||
| MIRT005538 | TWF1 | ||||||||
| MIRT006679 | SLC7A5 | ||||||||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sessa, F.; Salerno, M.; Esposito, M.; Cocimano, G.; Pisanelli, D.; Malik, A.; Khan, A.A.; Pomara, C. New Insight into Mechanisms of Cardiovascular Diseases: An Integrative Analysis Approach to Identify TheranoMiRNAs. Int. J. Mol. Sci. 2023, 24, 6781. https://doi.org/10.3390/ijms24076781
Sessa F, Salerno M, Esposito M, Cocimano G, Pisanelli D, Malik A, Khan AA, Pomara C. New Insight into Mechanisms of Cardiovascular Diseases: An Integrative Analysis Approach to Identify TheranoMiRNAs. International Journal of Molecular Sciences. 2023; 24(7):6781. https://doi.org/10.3390/ijms24076781
Chicago/Turabian StyleSessa, Francesco, Monica Salerno, Massimiliano Esposito, Giuseppe Cocimano, Daniela Pisanelli, Abdul Malik, Azmat Ali Khan, and Cristoforo Pomara. 2023. "New Insight into Mechanisms of Cardiovascular Diseases: An Integrative Analysis Approach to Identify TheranoMiRNAs" International Journal of Molecular Sciences 24, no. 7: 6781. https://doi.org/10.3390/ijms24076781
APA StyleSessa, F., Salerno, M., Esposito, M., Cocimano, G., Pisanelli, D., Malik, A., Khan, A. A., & Pomara, C. (2023). New Insight into Mechanisms of Cardiovascular Diseases: An Integrative Analysis Approach to Identify TheranoMiRNAs. International Journal of Molecular Sciences, 24(7), 6781. https://doi.org/10.3390/ijms24076781











