Bioinformatics Identification of Regulatory Genes and Mechanism Related to Hypoxia-Induced PD-L1 Inhibitor Resistance in Hepatocellular Carcinoma
Abstract
1. Introduction
2. Results
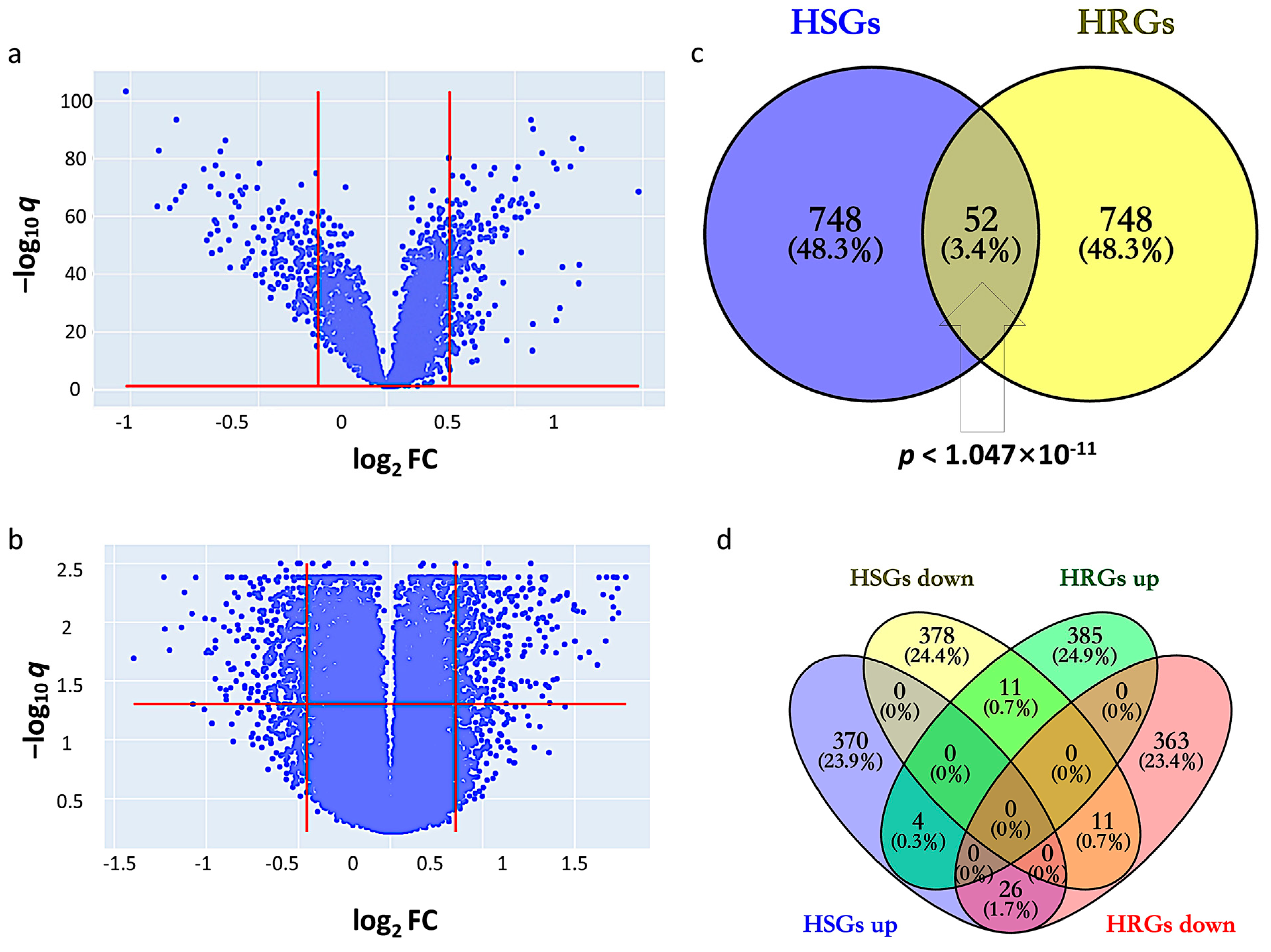
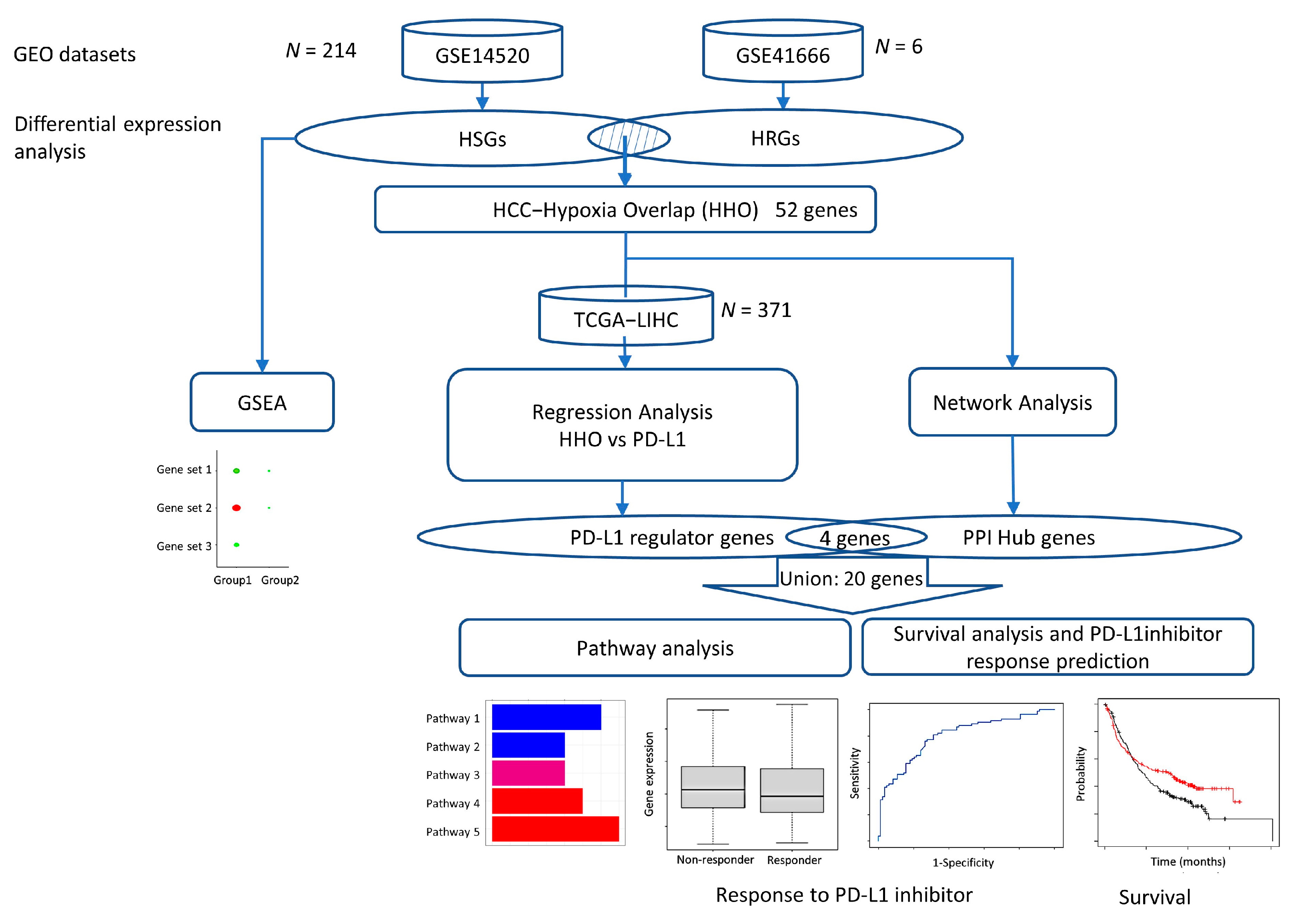
2.1. Identification of HSGs and HRGs in HCC
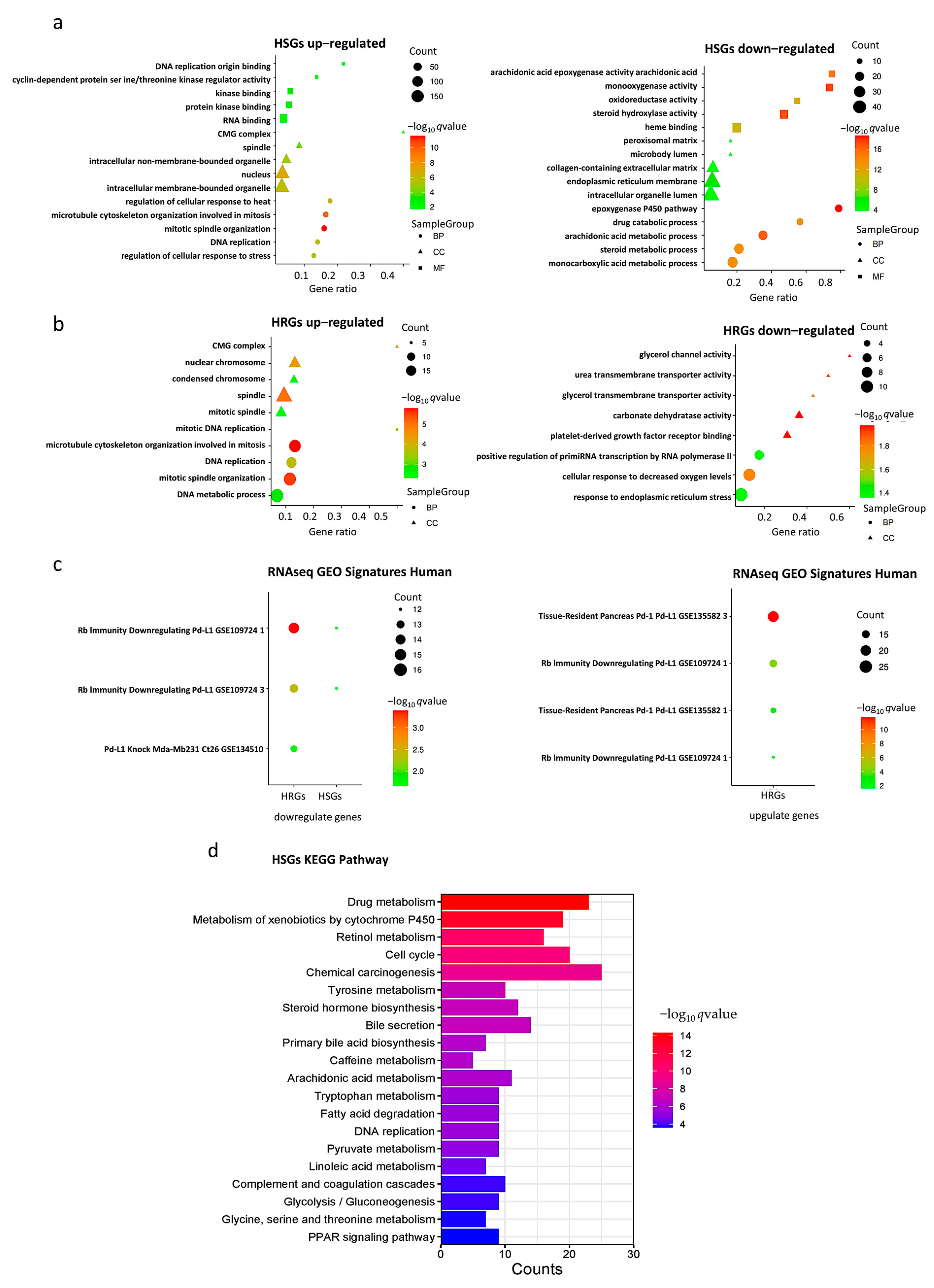
2.2. Gene Set Enrichment Analyses of HSGs and HRGs
2.3. Evaluation of the Effect of HHOs on PD-L1 Expression in the TCGA-LIHC Dataset
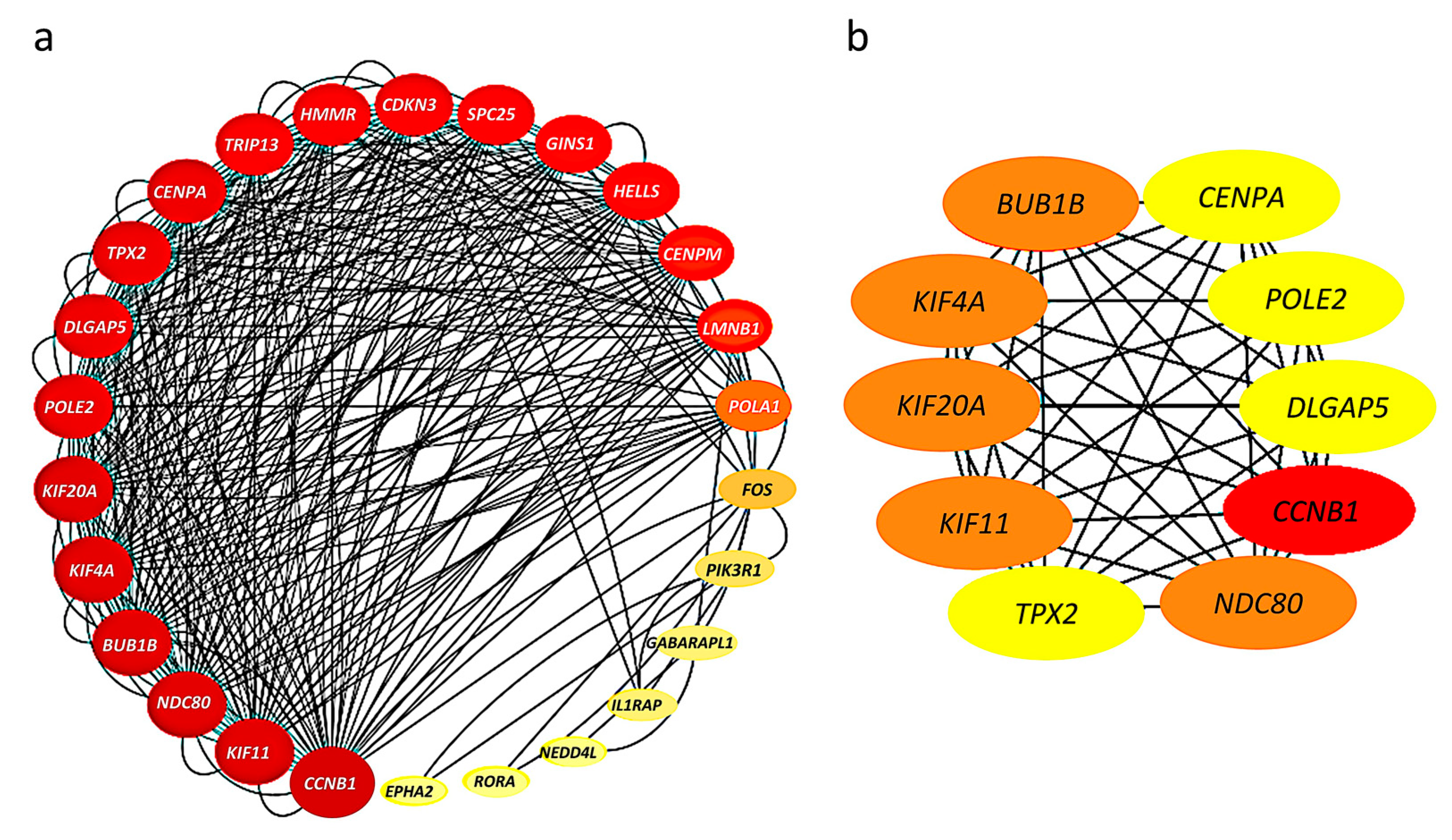
2.4. Construction of Protein–Protein Interaction Network and Identification of Hub Genes
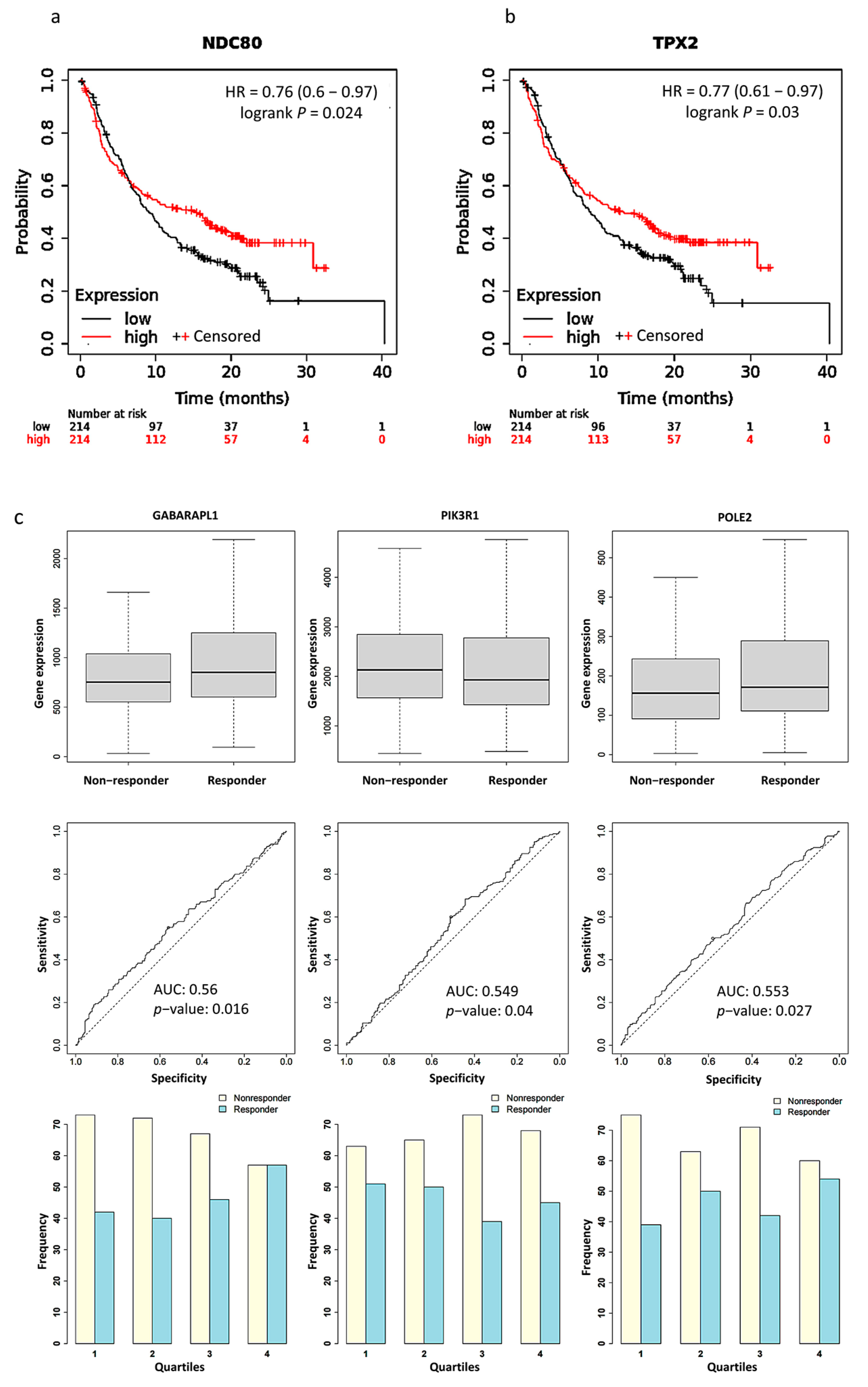
2.5. Survival Analysis and PD-L1 Inhibitor Response Prediction
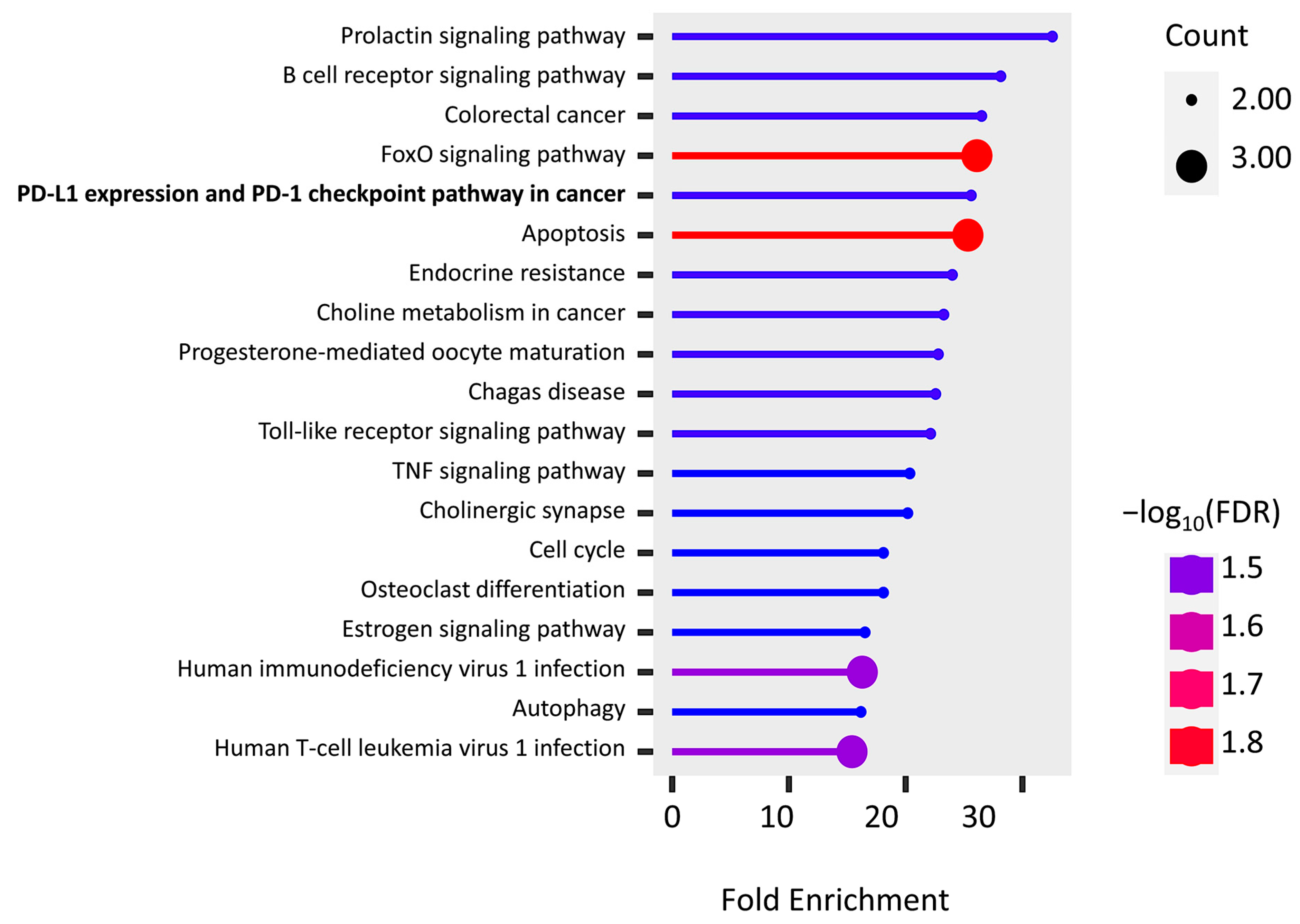
2.6. KEGG Pathway Enrichment
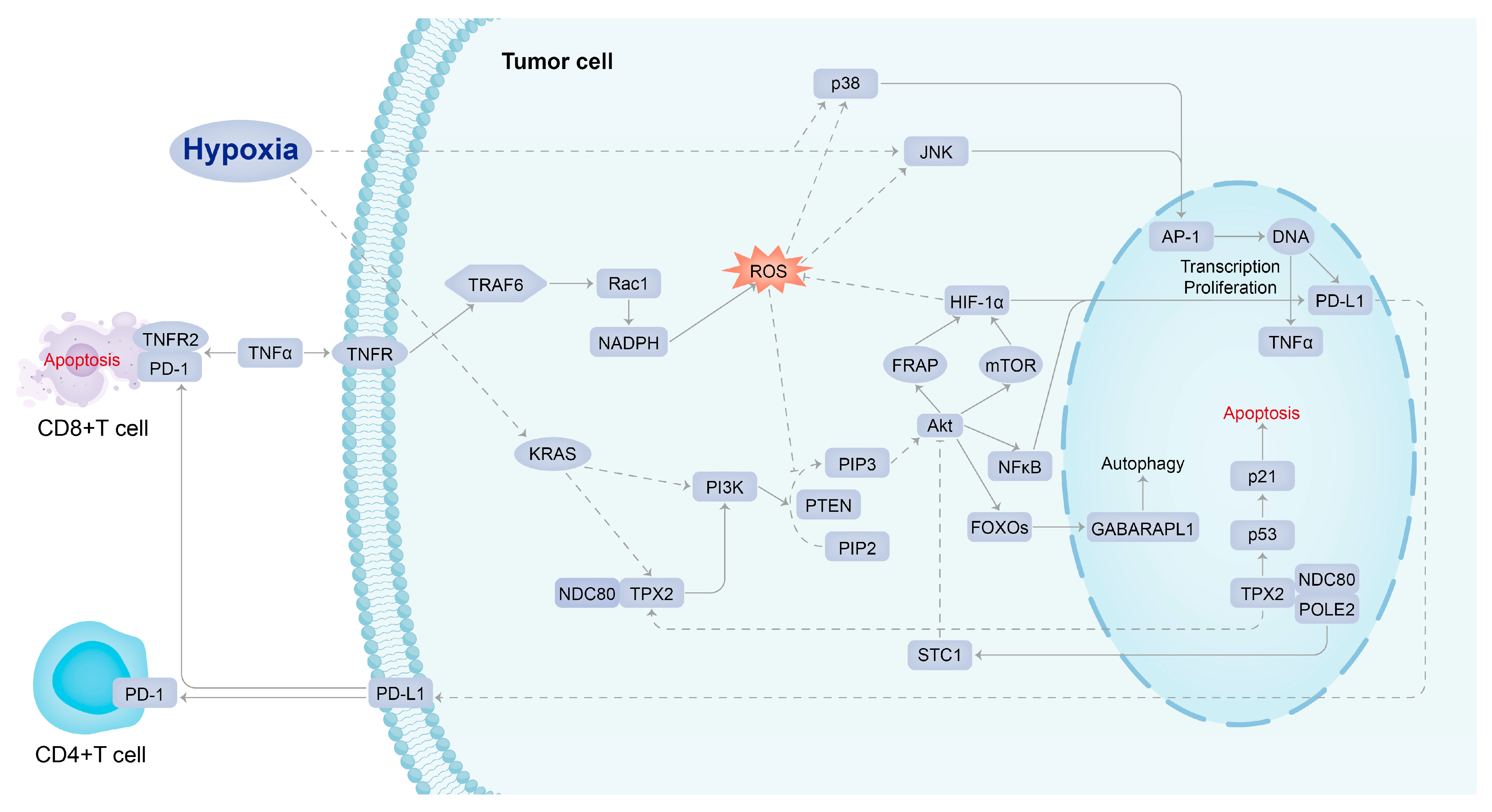
3. Discussion
4. Materials and Methods
4.1. Microarray Data Collection and Processing
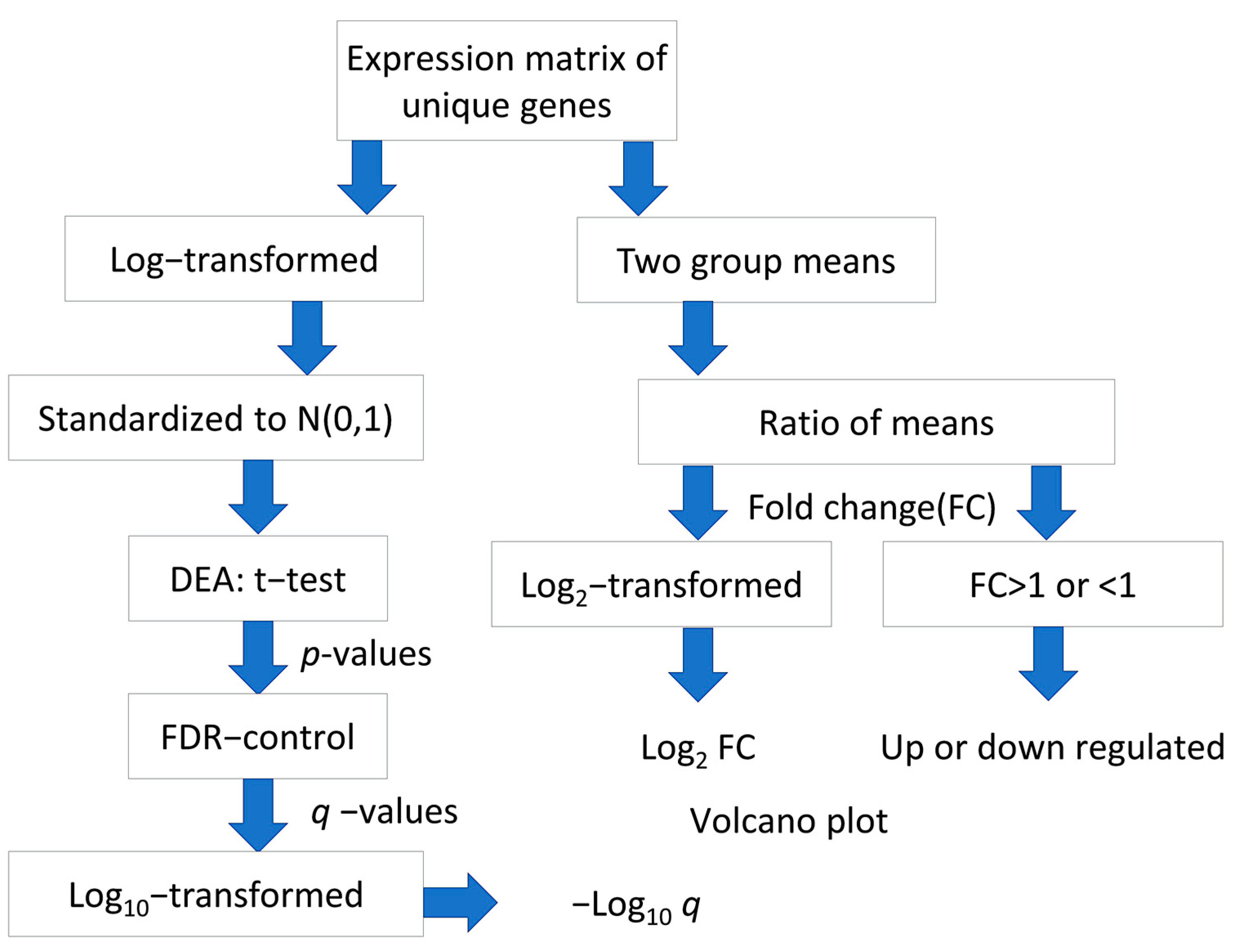
4.2. Identification of Differentially Expressed Genes
4.3. GO Function and KEGG Pathway Enrichment Analysis of HSGs and HRGs
4.4. Gene Set Enrichment Analysis (GSEA) of HSGs and HRGs
4.5. PPI Network Construction and Identification of Hub Genes
4.6. Multiple Regression Analysis of the Effect of HHOs on PD-L1
4.7. Survival Analysis and PD-L1 Inhibitor Response Prediction
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Yau, T.; Tai, D.; Chan, S.L.; Huang, Y.-H.; Choo, S.P.; Hsu, C.; Cheung, T.T.; Lin, S.-M.; Yong, W.P.; Lee, J.; et al. Systemic Treatment of Advanced Unresectable Hepatocellular Carcinoma after First-Line Therapy: Expert Recommendations from Hong Kong, Singapore, and Taiwan. Liver Cancer 2022, 11, 426–439. [Google Scholar] [CrossRef]
- European Association for the Study of the Liver. European Association for the Study of the Liver. EASL Clinical Practice Guidelines: Management of hepatocellular carcinoma. J. Hepatol. 2018, 69, 182–236. [Google Scholar] [CrossRef] [PubMed]
- Shek, D.; Read, S.A.; Nagrial, A.; Carlino, M.S.; Gao, B.; George, J.; Ahlenstiel, G. Immune-Checkpoint Inhibitors for Advanced Hepatocellular Carcinoma: A Synopsis of Response Rates. Oncol. 2021, 26, e1216–e1225. [Google Scholar] [CrossRef] [PubMed]
- Shurin, M.R.; Umansky, V. Cross-talk between HIF and PD-1/PD-L1 pathways in carcinogenesis and therapy. J. Clin. Invest. 2022, 132, e159473. [Google Scholar] [CrossRef] [PubMed]
- Guo, Y.; Xiao, Z.; Yang, L.; Gao, Y.; Zhu, Q.; Hu, L.; Huang, D.; Xu, Q. Hypoxia-inducible factors in hepatocellular carcinoma (Review). Oncol. Rep. 2020, 43, 3–15. [Google Scholar] [CrossRef]
- Mo, Z.; Liu, D.; Rong, D.; Zhang, S. Hypoxic Characteristic in the Immunosuppressive Microenvironment of Hepatocellular Carcinoma. Front. Immunol. 2021, 12, 611058. [Google Scholar] [CrossRef]
- Sadeghi, F.; Kardar, G.A.; Bolouri, M.R.; Nasri, F.; Sadri, M.; Falak, R. Overexpression of bHLH domain of HIF-1 failed to inhibit the HIF-1 transcriptional activity in hypoxia. Biol. Res. 2020, 53, 25. [Google Scholar] [CrossRef]
- Sun, Y.; Tan, J.; Miao, Y.; Zhang, Q. The role of PD-L1 in the immune dysfunction that mediates hypoxia-induced multiple organ injury. Cell Commun. Signal. 2021, 19, 76. [Google Scholar] [CrossRef] [PubMed]
- Ding, X.-C.; Wang, L.-L.; Zhang, X.-D.; Xu, J.-L.; Li, P.-F.; Liang, H.; Zhang, X.-B.; Xie, L.; Zhou, Z.-H.; Yang, J.; et al. The relationship between expression of PD-L1 and HIF-1α in glioma cells under hypoxia. J. Hematol. Oncol. 2021, 14, 1–5. [Google Scholar] [CrossRef] [PubMed]
- Lee, C.K.; Chan, S.L.; Chon, H.J. Could We Predict the Response of Immune Checkpoint Inhibitor Treatment in Hepatocellular Carcinoma? Cancers 2022, 14, 3213. [Google Scholar] [CrossRef]
- Bailey, C.M.; Liu, Y.; Liu, M.; Du, X.; Devenport, M.; Zheng, P.; Liu, Y.; Wang, Y. Targeting HIF-1α abrogates PD-L1–mediated immune evasion in tumor microenvironment but promotes tolerance in normal tissues. J. Clin. Investig. 2022, 132, e150846. [Google Scholar] [CrossRef] [PubMed]
- Jin, X.; Ding, D.; Yan, Y.; Li, H.; Wang, B.; Ma, L.; Ye, Z.; Ma, T.; Wu, Q.; Rodrigues, D.N.; et al. Phosphorylated RB Promotes Cancer Immunity by Inhibiting NF-kappaB Activation and PD-L1 Expression. Mol. Cell. 2019, 73, 22–35.e6. [Google Scholar] [CrossRef] [PubMed]
- Taniguchi, C.M.; Emanuelli, B.; Kahn, C.R. Critical nodes in signalling pathways: Insights into insulin action. Nat. Rev. Mol. Cell Biol. 2006, 7, 85–96. [Google Scholar] [CrossRef]
- Taniguchi, C.M.; Winnay, J.; Kondo, T.; Bronson, R.T.; Guimaraes, A.R.; Aleman, J.O.; Luo, J.; Stephanopoulos, G.; Weissleder, R.; Cantley, L.C.; et al. The phosphoinositide 3-kinase regulatory subunit p85alpha can exert tumor suppressor properties through negative regulation of growth factor signaling. Cancer Res. 2010, 70, 5305–5315. [Google Scholar] [CrossRef] [PubMed]
- Ai, X.; Xiang, L.; Huang, Z.; Zhou, S.; Zhang, S.; Zhang, T.; Jiang, T. Overexpression of PIK3R1 promotes hepatocellular carcinoma progression. Biol. Res. 2018, 51, 52. [Google Scholar] [CrossRef]
- Fojtík, P.; Beckerová, D.; Holomková, K.; Šenfluk, M.; Rotrekl, V. Both Hypoxia-Inducible Factor 1 and MAPK Signaling Pathway Attenuate PI3K/AKT via Suppression of Reactive Oxygen Species in Human Pluripotent Stem Cells. Front. Cell Dev. Biol. 2021, 8, 607444. [Google Scholar] [CrossRef]
- Chen, M.; Zhang, H.; Zhang, G.; Zhong, A.; Ma, Q.; Kai, J.; Tong, Y.; Xie, S.; Wang, Y.; Zheng, H.; et al. Targeting TPX2 suppresses proliferation and promotes apoptosis via repression of the PI3k/AKT/P21 signaling pathway and activation of p53 pathway in breast cancer. Biochem. Biophys. Res. Commun. 2018, 507, 74–82. [Google Scholar] [CrossRef]
- Huang, D.H.; Jian, J.; Li, S.; Zhang, Y.; Liu, L.Z. TPX2 silencing exerts anti-tumor effects on hepatocellular carcinoma by regulating the PI3K/AKT signaling pathway. Int. J. Mol. Med. 2019, 44, 2113–2122. [Google Scholar] [CrossRef]
- Scheiter, A.; Keil, F.; Lüke, F.; Grosse, J.; Verloh, N.; Opitz, S.; Schlosser, S.; Kandulski, A.; Pukrop, T.; Dietmaier, W.; et al. Identification and In-Depth Analysis of the Novel FGFR2-NDC80 Fusion in a Cholangiocarcinoma Patient: Implication for Therapy. Curr. Oncol. 2021, 28, 1161–1169. [Google Scholar] [CrossRef]
- Rizvi, S.; Gores, G.J. Emerging molecular therapeutic targets for cholangiocarcinoma. J. Hepatol. 2017, 67, 632–644. [Google Scholar] [CrossRef]
- Ju, L.-L.; Chen, L.; Li, J.-H.; Wang, Y.-F.; Lu, R.-J.; Bian, Z.-L.; Shao, J.-G. Effect of NDC80 in human hepatocellular carcinoma. World J. Gastroenterol. 2017, 23, 3675–3683. [Google Scholar] [CrossRef]
- Zhou, J.; Pei, Y.; Chen, G.; Cao, C.; Liu, J.; Ding, C.; Wang, D.; Sun, L.; Xu, P.; Niu, G. SPC24 Regulates breast cancer progression by PI3K/AKT signaling. Gene 2018, 675, 272–277. [Google Scholar] [CrossRef] [PubMed]
- Zhang, C.; Shen, Y.; Gao, L.; Wang, X.; Huang, D.; Xie, X.; Xu, D.; He, H. Targeting POLE2 Creates a Novel Vulnerability in Renal Cell Carcinoma via Modulating Stanniocalcin 1. Front. Cell Dev. Biol. 2021, 9, 622344. [Google Scholar] [CrossRef] [PubMed]
- Su, W.; Li, S.; Chen, X.; Yin, L.; Ma, P.; Ma, Y.; Su, B. GABARAPL1 suppresses metastasis by counteracting PI3K/Akt pathway in prostate cancer. Oncotarget 2017, 8, 4449–4459. [Google Scholar] [CrossRef] [PubMed]
- Jacquet, M.; Guittaut, M.; Fraichard, A.; Despouy, G. The functions of Atg8-family proteins in autophagy and cancer: Linked or unrelated? Autophagy 2021, 17, 599–611. [Google Scholar] [CrossRef] [PubMed]
- Di-Luoffo, M.; Schmitter, C.; Barrere, E.C.; Therville, N.; Delarue, M.; Guillermet-Guibert, J. The biomechanical context influences the PI3K output signaling in breast and pancreatic cancer cells. bioRxiv 2022. bioRxiv:2021.10.18.464825. [Google Scholar]
- Zhuang, H.; Wang, S.; Chen, B.; Zhang, Z.; Ma, Z.; Li, Z.; Liu, C.; Zhou, Z.; Gong, Y.; Huang, S.; et al. Prognostic Stratification Based on HIF-1 Signaling for Evaluating Hypoxic Status and Immune Infiltration in Pancreatic Ductal Adenocarcinomas. Front. Immunol. 2021, 12, 790661. [Google Scholar] [CrossRef]
- Meng, L.; Liu, B.; Ji, R.; Jiang, X.; Yan, X.; Xin, Y. Targeting the BDNF/TrkB pathway for the treatment of tumors. Oncol. Lett. 2018, 17, 2031–2039. [Google Scholar] [CrossRef]
- Barsoum, I.B.; Smallwood, C.A.; Siemens, D.R.; Graham, C.H. A Mechanism of Hypoxia-Mediated Escape from Adaptive Immunity in Cancer Cells. Cancer Res. 2014, 74, 665–674. [Google Scholar] [CrossRef]
- Lei, Q.; Wang, D.; Sun, K.; Wang, L.; Zhang, Y. Resistance Mechanisms of Anti-PD1/PDL1 Therapy in Solid Tumors. Front. Cell Dev. Biol. 2020, 8, 672. [Google Scholar] [CrossRef]
- Ahn, A.; Rodger, E.J.; Motwani, J.; Gimenez, G.; Stockwell, P.A.; Parry, M.; Hersey, P.; Chatterjee, A.; Eccles, M.R. Transcriptional Reprogramming and Constitutive PD-L1 Expression in Melanoma Are Associated with Dedifferentiation and Activation of Interferon and Tumour Necrosis Factor Signalling Pathways. Cancers 2021, 13, 4250. [Google Scholar] [CrossRef] [PubMed]
- Zalpoor, H.; Bakhtiyari, M.; Liaghat, M.; Nabi-Afjadi, M.; Ganjalikhani-Hakemi, M. Quercetin potential effects against SARS-CoV-2 infection and COVID-19-associated cancer progression by inhibiting mTOR and hypoxia-inducible factor-1alpha (HIF-1alpha). Phytother Res. 2022, 36, 2679–2682. [Google Scholar] [CrossRef] [PubMed]
- Ye, L.-L.; Wei, X.-S.; Zhang, M.; Niu, Y.-R.; Zhou, Q. The Significance of Tumor Necrosis Factor Receptor Type II in CD8(+) Regulatory T Cells and CD8(+) Effector T Cells. Front. Immunol. 2018, 9, 583. [Google Scholar] [CrossRef] [PubMed]
- Bulua, A.C.; Simon, A.; Maddipati, R.; Pelletier, M.; Park, H.; Kim, K.-Y.; Sack, M.N.; Kastner, D.L.; Siegel, R.M. Mitochondrial reactive oxygen species promote production of proinflammatory cytokines and are elevated in TNFR1-associated periodic syndrome (TRAPS). J. Exp. Med. 2011, 208, 519–533. [Google Scholar] [CrossRef]
- Sebestyén, A.; Kopper, L.; Dankó, T.; Tímár, J. Hypoxia Signaling in Cancer: From Basics to Clinical Practice. Pathol. Oncol. Res. 2021, 27, 1609802. [Google Scholar] [CrossRef]
- Schumacker, P.T. SIRT3 Controls Cancer Metabolic Reprogramming by Regulating ROS and HIF. Cancer Cell 2011, 19, 299–300. [Google Scholar] [CrossRef]
- Hu, Z.Z.; Dong, N.; Lu, D.; Jiang, X.Q.; Xu, J.J.; Wu, Z.W.; Zheng, D.T.; Wechsler, D.S. A positive feedback loop between ROS and Mxi1-0 promotes hypoxia-induced VEGF expression in human hepatocellular carcinoma cells. Cell. Signal. 2017, 31, 79–86. [Google Scholar] [CrossRef]
- Rohr-Udilova, N.; Sieghart, W.; Eferl, R.; Stoiber, D.; Björkhem-Bergman, L.; Eriksson, L.C.; Stolze, K.; Hayden, H.; Keppler, B.; Sagmeister, S.; et al. Antagonistic effects of selenium and lipid peroxides on growth control in early hepatocellular carcinoma. Hepatology 2011, 55, 1112–1121. [Google Scholar] [CrossRef]
- Séhédic, D.; Roncali, L.; Djoudi, A.; Buchtova, N.; Avril, S.; Chérel, M.; Boury, F.; Lacoeuille, F.; Hindré, F.; Garcion, E. Rapamycin-Loaded Lipid Nanocapsules Induce Selective Inhibition of the mTORC1-Signaling Pathway in Glioblastoma Cells. Front. Bioeng. Biotechnol. 2020, 8, 602998. [Google Scholar] [CrossRef]
- Salman, S.; Meyers, D.J.; Wicks, E.E.; Lee, S.N.; Datan, E.; Thomas, A.M.; Anders, N.M.; Hwang, Y.; Lyu, Y.; Yang, Y.; et al. HIF inhibitor 32-134D eradicates murine hepatocellular carcinoma in combination with anti-PD1 therapy. J. Clin. Invest. 2022, 132, e156774. [Google Scholar] [CrossRef]
- Storey, J.D.; Tibshirani, R. Statistical significance for genomewide studies. Proc. Natl. Acad. Sci. USA 2003, 100, 9440–9445. [Google Scholar] [CrossRef] [PubMed]
- Bozhilova, L.V.; Whitmore, A.V.; Wray, J.; Reinert, G.; Deane, C.M. Measuring rank robustness in scored protein interaction networks. BMC Bioinform. 2019, 20, 446. [Google Scholar] [CrossRef] [PubMed]
- Wu, Q.; Ying, X.; Yu, W.; Li, H.; Wei, W.; Lin, X.; Zhang, X. Identification of ferroptosis-related genes in syncytiotrophoblast-derived extracellular vesicles of preeclampsia. Medicine 2022, 101, e31583. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Huang, M.; Yang, S.; Tai, W.C.S.; Zhang, L.; Zhou, Y.; Cho, W.C.S.; Chan, L.W.C.; Wong, S.C.C. Bioinformatics Identification of Regulatory Genes and Mechanism Related to Hypoxia-Induced PD-L1 Inhibitor Resistance in Hepatocellular Carcinoma. Int. J. Mol. Sci. 2023, 24, 8720. https://doi.org/10.3390/ijms24108720
Huang M, Yang S, Tai WCS, Zhang L, Zhou Y, Cho WCS, Chan LWC, Wong SCC. Bioinformatics Identification of Regulatory Genes and Mechanism Related to Hypoxia-Induced PD-L1 Inhibitor Resistance in Hepatocellular Carcinoma. International Journal of Molecular Sciences. 2023; 24(10):8720. https://doi.org/10.3390/ijms24108720
Chicago/Turabian StyleHuang, Mohan, Sijun Yang, William Chi Shing Tai, Lingfeng Zhang, Yinuo Zhou, William Chi Shing Cho, Lawrence Wing Chi Chan, and Sze Chuen Cesar Wong. 2023. "Bioinformatics Identification of Regulatory Genes and Mechanism Related to Hypoxia-Induced PD-L1 Inhibitor Resistance in Hepatocellular Carcinoma" International Journal of Molecular Sciences 24, no. 10: 8720. https://doi.org/10.3390/ijms24108720
APA StyleHuang, M., Yang, S., Tai, W. C. S., Zhang, L., Zhou, Y., Cho, W. C. S., Chan, L. W. C., & Wong, S. C. C. (2023). Bioinformatics Identification of Regulatory Genes and Mechanism Related to Hypoxia-Induced PD-L1 Inhibitor Resistance in Hepatocellular Carcinoma. International Journal of Molecular Sciences, 24(10), 8720. https://doi.org/10.3390/ijms24108720







