Treatment of Advanced Non-Small Cell Lung Cancer with RET Fusions: Reality and Hopes
Abstract
1. Introduction
2. Canonical and Aberrant RET Signaling: Pathogenesis and Epidemiology in Advanced Non-Small Cell Lung Cancer
3. Early Attempts at Target Therapy for RET-Rearranged Advanced Non-Small Cell Lung Cancer: Multikinase Inhibitors
| Authors | Type of Trial/Phase | Drug | Patients | Efficacy Results | Safety Profile |
|---|---|---|---|---|---|
| Gautschi et al. [47] | Retrospective | Cabozantinib | 19 | DCR: 63% mPFS: 3.6 months mOS: 4.9 months | Not Available (N/A) |
| Drilon et al. [51] | Prospective Phase II | Cabozantinib | 25 | ORR: 28% DCR: 100% mPFS: 5.5 months mOS: 9.9 months | TRAEs leading to dose reductions: 73% TRAEs leading to drug discontinuation: 8% |
| Nokihara et al. [52] | Prospective Phase I | Cabozantinib | 2 | ORR: 50% | N/A |
| Gautschi et al. [47] | Retrospective | Vandetanib | 11 | DCR: 45% mPFS: 2.9 months mOS: 10.2 months | N/A |
| Platt et al. [48] | Retrospective | Vandetanib | 3 | DCR: 0% | N/A |
| Lee et al. [54] | Prospective Phase II | Vandetanib | 17 | ORR: 18% DCR: 65% mPFS: 4.5 months mOS: 11.6 months | Grade 3 TRAEs: 29.4% TRAEs leading to dose reductions: 23.5% |
| Yoh et al. [55] | Prospective Phase II | Vandetanib | 19 | ORR: 47% DCR: 90% mPFS: 4.7 months | TRAEs leading to dose reductions: 53% TRAEs leading to drug discontinuation: 21% |
| Gautschi et al. [47] | Retrospective | Sunitinib | 9 | DCR: 55% mPFS: 2.2 months mOS: 6.8 months | N/A |
| Gautschi et al. [47] | Retrospective | Sorafenib | 2 | DCR: 100% | N/A |
| Horiike et al. [53] | Prospective Phase II | Sorafenib | 3 | ORR: 0% DCR: 33.3% | TRAEs leading to dose reductions: 73% |
| Lin et al. [49] | Retrospective | Alectinib | 4 | DCR: 100% | N/A |
| Ribeiro et al. [50] | Retrospective | Alectinib | 4 | DCR: 50% | N/A |
| Gautschi et al. [47] | Retrospective | Alectinib | 2 | DCR: 0% | N/A |
| Gautschi et al. [47] | Retrospective | Lenvatinib | 2 | DCR: 50% | N/A |
| Hida et al. [56] | Prospective Phase II | Lenvatinib | 25 | ORR: 16% DCR: 76% mPFS: 7.3 months | ≥ grade 3 TRAES: 92% TRAEs leading to dose reductions: 64% TRAEs leading to drug interruption: 76% TRAEs leading to drug discontinuation: 24% |
| Gautschi et al. [47] | Retrospective | Nintedanib | 2 | DCR: 100% | N/A |
| Gautschi et al. [47] | Retrospective | Ponatinib | 2 | DCR: 100% | N/A |
| Gautschi et al. [47] | Retrospective | Regorafenib | 1 | DCR: 0% | N/A |
4. Current Standard of Care for RET-Rearranged Advanced Non-Small Cell Lung Cancer: RET-Selective Tyrosine Kinase Inhibitors
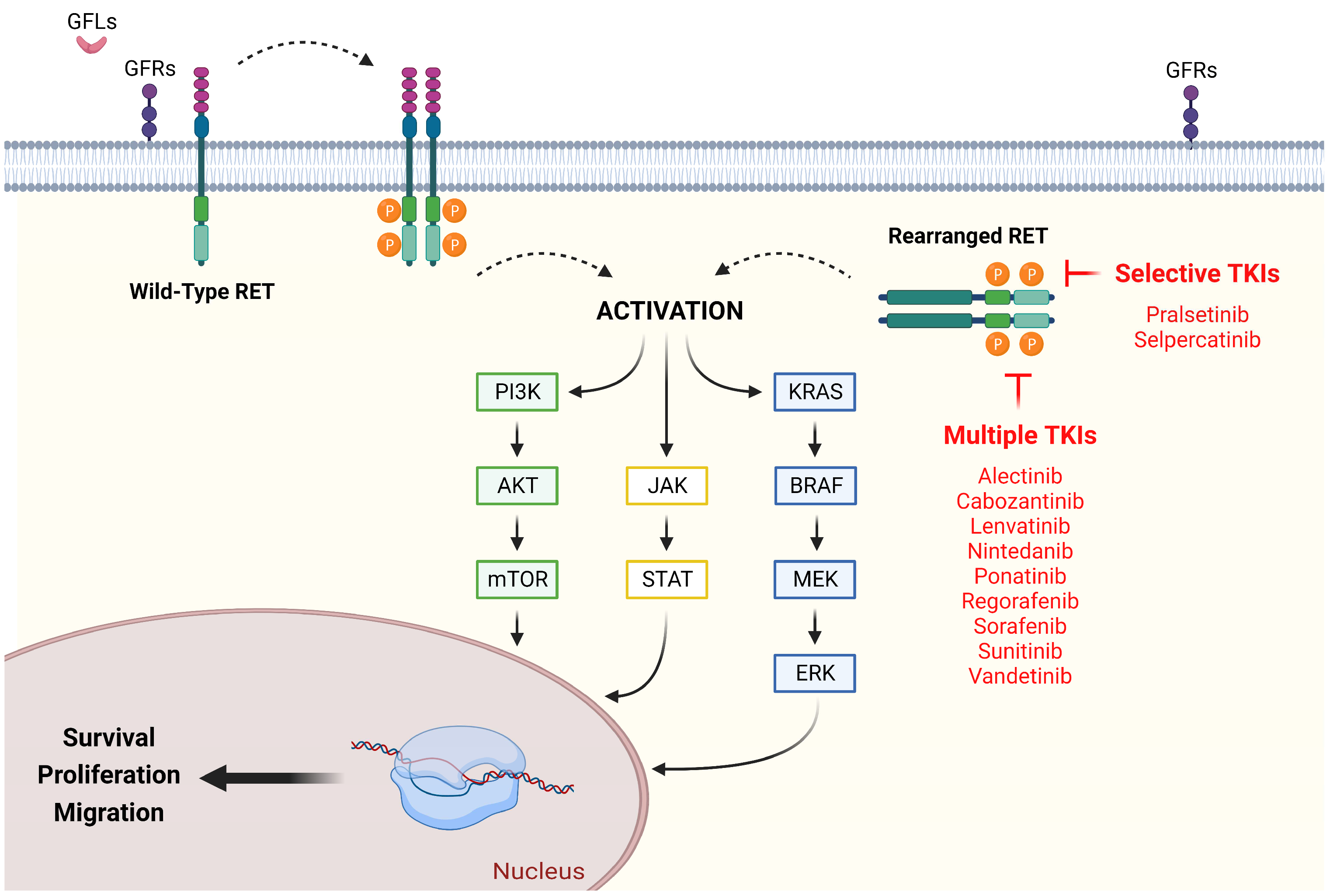
5. Resistance Mechanisms to RET-Selective Tyrosine Kinase Inhibitors and Potential Strategies to Overcome Tumor Adaptation
6. Additional Molecular Insight on Tyrosine Kinase Inhibitors Resistance in RET-Positive NSCLC
7. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Sung, H.; Ferlay, J.; Siegel, R.L.; Laversanne, M.; Soerjomataram, I.; Jemal, A.; Bray, F. Global Cancer Statistics 2020: GLOBOCAN Estimates of Incidence and Mortality Worldwide for 36 Cancers in 185 Countries. CA Cancer J. Clin. 2021, 71, 209–249. [Google Scholar] [CrossRef] [PubMed]
- Siegel, R.L.; Miller, K.D.; Fuchs, H.E.; Jemal, A. Cancer statistics, 2022. CA Cancer J. Clin. 2022, 72, 7–33. [Google Scholar] [CrossRef] [PubMed]
- Oliver, A.L. Lung Cancer: Epidemiology and Screening. Surg. Clin. N. Am. 2022, 102, 335–344. [Google Scholar] [CrossRef]
- Thai, A.A.; Solomon, B.J.; Sequist, L.V.; Gainor, J.F.; Heist, R.S. Lung cancer. Lancet 2021, 398, 535–554. [Google Scholar] [CrossRef]
- Thandra, K.C.; Barsouk, A.; Saginala, K.; Aluru, J.S.; Barsouk, A. Epidemiology of lung cancer. Contemp. Oncol. 2021, 25, 45–52. [Google Scholar]
- Zappa, C.; Mousa, S.A. Non-small cell lung cancer: Current treatment and future advances. Transl. Lung Cancer Res. 2016, 5, 288–300. [Google Scholar] [CrossRef]
- Sher, T.; Dy, G.K.; Adjei, A.A. Small cell lung cancer. Mayo Clin. Proc. 2008, 83, 355–367. [Google Scholar] [CrossRef]
- Kenfield, S.A.; Wei, E.K.; Stampfer, M.J.; Rosner, B.A.; Colditz, G.A. Comparison of aspects of smoking among the four histological types of lung cancer. Tob. Control. 2008, 17, 198–204. [Google Scholar] [CrossRef]
- Noguchi, M.; Morikawa, A.; Kawasaki, M.; Matsuno, Y.; Yamada, T.; Hirohashi, S.; Kondo, H.; Shimosato, Y. Small adenocarcinoma of the lung. Histologic characteristics and prognosis. Cancer 1995, 75, 2844–2852. [Google Scholar] [CrossRef]
- Socinski, M.A.; Obasaju, C.; Gandara, D.; Hirsch, F.R.; Bonomi, P.; Bunn, P.; Kim, E.S.; Langer, C.J.; Natale, R.B.; Novello, S.; et al. Clinicopathologic Features of Advanced Squamous NSCLC. J. Thorac. Oncol. 2016, 11, 1411–1422. [Google Scholar] [CrossRef]
- Molina, J.R.; Yang, P.; Cassivi, S.D.; Schild, S.E.; Adjei, A.A. Non-small cell lung cancer: Epidemiology, risk factors, treatment, and survivorship. Mayo Clin. Proc. 2008, 83, 584–594. [Google Scholar] [CrossRef]
- Ganti, A.K.; Klein, A.B.; Cotarla, I.; Seal, B.; Chou, E. Update of Incidence, Prevalence, Survival, and Initial Treatment in Patients With Non-Small Cell Lung Cancer in the US. JAMA Oncol. 2021, 7, 1824–1832. [Google Scholar] [CrossRef] [PubMed]
- Inamura, K. Lung cancer: Understanding its molecular pathology and the 2015 WHO classification. Front. Oncol. 2017, 7, 193. [Google Scholar] [CrossRef]
- Chan, B.A.; Hughes, B.G. Targeted therapy for non-small cell lung cancer: Current standards and the promise of the future. Transl. Lung Cancer Res. 2015, 4, 36–54. [Google Scholar] [CrossRef] [PubMed]
- Wang, M.; Herbst, R.S.; Boshoff, C. Toward personalized treatment approaches for non-small-cell lung cancer. Nat. Med. 2021, 27, 1345–1356. [Google Scholar] [CrossRef] [PubMed]
- Ferrara, M.G.; Di Noia, V.; D’Argento, E.; Vita, E.; Damiano, P.; Cannella, A.; Ribelli, M.; Pilotto, S.; Milella, M.; Tortora, G.; et al. Oncogene-Addicted Non-Small-Cell Lung Cancer: Treatment Opportunities and Future Perspectives. Cancers 2020, 12, 1196. [Google Scholar] [CrossRef]
- Zhang, Y.-L.; Yuan, J.-Q.; Wang, K.-F.; Fu, X.-H.; Han, X.-R.; Threapleton, D.; Yang, Z.-Y.; Mao, C.; Tang, J.-L. The prevalence of EGFR mutation in patients with non-small cell lung cancer: A systematic review and meta-analysis. Oncotarget 2016, 7, 78985–78993. [Google Scholar] [CrossRef]
- Skoulidis, F.; Heymach, J.V. Co-occurring genomic alterations in non-small-cell lung cancer biology and therapy. Nat. Rev. Cancer 2019, 19, 495–509. [Google Scholar] [CrossRef]
- Hirsch, F.R.; Scagliotti, G.V.; Mulshine, J.L.; Kwon, R.; Curran, W.J., Jr.; Wu, Y.-L.; Paz-Ares, L. Lung cancer: Current therapies and new targeted treatments. Lancet 2017, 389, 299–311. [Google Scholar] [CrossRef]
- Chevallier, M.; Borgeaud, M.; Addeo, A.; Friedlaender, A. Oncogenic driver mutations in non-small cell lung cancer: Past, present and future. World J. Clin. Oncol. 2021, 12, 217–237. [Google Scholar] [CrossRef]
- Zhu, Q.-G.; Zhang, S.-M.; Ding, X.-X.; He, B.; Zhang, H.-Q. Driver genes in non-small cell lung cancer: Characteristics, detection methods, and targeted therapies. Oncotarget 2017, 8, 57680–57692. [Google Scholar] [CrossRef] [PubMed]
- Yang, S.-R.; Schultheis, A.M.; Yu, H.; Mandelker, D.; Ladanyi, M.; Büttner, R. Precision medicine in non-small cell lung cancer: Current applications and future directions. Semin. Cancer Biol. 2020, 84, 184–198. [Google Scholar] [CrossRef] [PubMed]
- Yuan, M.; Huang, L.-L.; Chen, J.-H.; Wu, J.; Xu, Q. The emerging treatment landscape of targeted therapy in non-small-cell lung cancer. Signal Transduct. Target. Ther. 2019, 4, 61. [Google Scholar] [CrossRef] [PubMed]
- Herbst, R.S.; Morgensztern, D.; Boshoff, C. The biology and management of non-small cell lung cancer. Nature 2018, 553, 446–454. [Google Scholar] [CrossRef] [PubMed]
- Chen, R.; Manochakian, R.; James, L.; Azzouqa, A.-G.; Shi, H.; Zhang, Y.; Zhao, Y.; Zhou, K.; Lou, Y. Emerging therapeutic agents for advanced non-small cell lung cancer. J. Hematol. Oncol. 2020, 13, 58. [Google Scholar] [CrossRef]
- Ye, Z.; Huang, Y.; Ke, J.; Zhu, X.; Leng, S.; Luo, H. Breakthrough in targeted therapy for non-small cell lung cancer. Biomed. Pharmacother. 2020, 133, 111079. [Google Scholar] [CrossRef]
- Reck, M.; Rodríguez-Abreu, D.; Robinson, A.G.; Hui, R.; Csőszi, T.; Fülöp, A.; Gottfried, M.; Peled, N.; Tafreshi, A.; Cuffe, S.; et al. Pembrolizumab versus Chemotherapy for PD-L1–Positive Non–Small-Cell Lung Cancer. N. Engl. J. Med. 2016, 375, 1823–1833. [Google Scholar] [CrossRef]
- Meador, C.B.; Hata, A.N. Acquired resistance to targeted therapies in NSCLC: Updates and evolving insights. Pharmacol. Ther. 2020, 210, 107522. [Google Scholar] [CrossRef]
- Frisone, D.; Friedlaender, A.; Addeo, A.; Tsantoulis, P. The Landscape of Immunotherapy Resistance in NSCLC. Front. Oncol. 2022, 12, 817548. [Google Scholar] [CrossRef]
- Ibáñez, C.F. Structure and Physiology of the RET Receptor Tyrosine Kinase. Cold Spring Harb. Perspect. Biol. 2013, 5, a009134. [Google Scholar] [CrossRef]
- Li, J.; Shang, G.; Chen, Y.-J.; Brautigam, C.A.; Liou, J.; Zhang, X.; Bai, X.-C. Cryo-EM analyses reveal the common mechanism and diversification in the activation of RET by different ligands. eLife 2019, 8, e47650. [Google Scholar] [CrossRef]
- Taraviras, S.; Marcos-Gutierrez, C.V.; Durbec, P.; Jani, H.; Grigoriou, M.; Sukumaran, M.; Wang, L.C.; Hynes, M.; Raisman, G.; Pachnis, V. Signalling by the RET receptor tyrosine kinase and its role in the development of the mammalian enteric nervous system. Development 1999, 126, 2785–2797. [Google Scholar] [CrossRef] [PubMed]
- Michels, S.; Scheel, A.H.; Scheffler, M.; Schultheis, A.M.; Gautschi, O.; Aebersold, F.; Diebold, J.; Pall, G.; Rothschild, S.; Bubendorf, L.; et al. Clinicopathological Characteristics of RET Rearranged Lung Cancer in European Patients. J. Thorac. Oncol. 2016, 11, 122–127. [Google Scholar] [CrossRef]
- Zhang, K.; Chen, H.; Wang, Y.; Yang, L.; Zhou, C.; Yin, W.; Wang, G.; Mao, X.; Xiang, J.; Li, B.; et al. Clinical Characteristics and Molecular Patterns of RET-Rearranged Lung Cancer in Chinese Patients. Oncol. Res. 2019, 27, 575–582. [Google Scholar] [CrossRef]
- Drilon, A.; Lin, J.J.; Filleron, T.; Ni, A.; Milia, J.; Bergagnini, I.; Hatzoglou, V.; Velcheti, V.; Offin, M.; Li, B.; et al. Frequency of Brain Metastases and Multikinase Inhibitor Outcomes in Patients With RET–Rearranged Lung Cancers. J. Thorac. Oncol. 2018, 13, 1595–1601. [Google Scholar] [CrossRef] [PubMed]
- Shi, M.; Wang, W.; Zhang, J.; Li, B.; Lv, D.; Wang, D.; Wang, S.; Cheng, D.; Ma, T. Identification of RET fusions in a Chinese multicancer retrospective analysis by next-generation sequencing. Cancer Sci. 2022, 113, 308–318. [Google Scholar] [CrossRef]
- Qiu, Z.; Ye, B.; Wang, K.; Zhou, P.; Zhao, S.; Li, W.; Tian, P. Unique Genetic Characteristics and Clinical Prognosis of Female Patients with Lung Cancer Harboring RET Fusion Gene. Sci. Rep. 2020, 10, 10387. [Google Scholar] [CrossRef]
- Drusbosky, L.M.; Rodriguez, E.; Dawar, R.; Ikpeazu, C.V. Therapeutic strategies in RET gene rearranged non-small cell lung cancer. J. Hematol. Oncol. 2021, 14, 50. [Google Scholar] [CrossRef] [PubMed]
- Offin, M.; Guo, R.; Wu, S.L.; Sabari, J.; Land, J.D.; Ni, A.; Montecalvo, J.; Halpenny, D.F.; Buie, L.W.; Pak, T.; et al. Immunophenotype and Response to Immunotherapy of RET-Rearranged Lung Cancers. JCO Precis. Oncol. 2019, 3, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Bhandari, N.R.; Hess, L.M.; Han, Y.; Zhu, Y.E.; Sireci, A.N. Efficacy of immune checkpoint inhibitor therapy in patients with RET fusion-positive non-small-cell lung cancer. Immunotherapy 2021, 13, 893–904. [Google Scholar] [CrossRef]
- Seegobin, K.; Majeed, U.; Wiest, N.; Manochakian, R.; Lou, Y.; Zhao, Y. Immunotherapy in Non-Small Cell Lung Cancer With Actionable Mutations Other Than EGFR. Front. Oncol. 2021, 11, 750657. [Google Scholar] [CrossRef] [PubMed]
- Meng, Y.; Yang, Y.; Fang, Y.; Lin, X.; Xie, X.; Deng, H.; Wu, J.; Zhou, M.; Sun, N.; Xie, Z.; et al. The Treatment Status of Patients in NSCLC With RET Fusion Under the Prelude of Selective RET-TKI Application in China: A Multicenter Retrospective Research. Front. Oncol. 2022, 12, 864367. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.; Hu, X.; Shen, T.; Li, Q.; Mooers, B.H.M.; Wu, J. RET kinase alterations in targeted cancer therapy. Cancer Drug Resist. 2020, 3, 472–481. [Google Scholar] [CrossRef]
- Ackermann, C.J.; Stock, G.; Tay, R.; Dawod, M.; Gomes, F.; Califano, R. Targeted Therapy For RET-Rearranged Non-Small Cell Lung Cancer: Clinical Development And Future Directions. OncoTargets Ther. 2019, 12, 7857–7864. [Google Scholar] [CrossRef] [PubMed]
- Stinchcombe, T.E. Current management of RET rearranged non-small cell lung cancer. Ther. Adv. Med. Oncol. 2020, 12, 28634. [Google Scholar] [CrossRef]
- Thein, K.Z.; Velcheti, V.; Mooers, B.H.; Wu, J.; Subbiah, V. Precision therapy for RET-altered cancers with RET inhibitors. Trends Cancer 2021, 7, 1074–1088. [Google Scholar] [CrossRef]
- Gautschi, O.; Milia, J.; Filleron, T.; Wolf, J.; Carbone, D.P.; Owen, D.; Camidge, R.; Narayanan, V.; Doebele, R.C.; Besse, B.; et al. Targeting RET in Patients With RET-Rearranged Lung Cancers: Results From the Global, Multicenter RET Registry. J. Clin. Oncol. 2017, 35, 1403–1410. [Google Scholar] [CrossRef]
- Platt, A.; Morten, J.; Ji, Q.; Elvin, P.; Womack, C.; Su, X.; Donald, E.; Gray, N.; Read, J.; Bigley, G.; et al. A retrospective analysis of RET translocation, gene copy number gain and expression in NSCLC patients treated with vandetanib in four randomized Phase III studies. BMC Cancer 2015, 15, 171. [Google Scholar] [CrossRef]
- Lin, J.J.; Kennedy, E.; Sequist, L.V.; Brastianos, P.K.; Goodwin, K.E.; Stevens, S.; Wanat, A.C.; Stober, L.L.; Digumarthy, S.R.; Engelman, J.A.; et al. Clinical Activity of Alectinib in Advanced RET -Rearranged Non–Small Cell Lung Cancer. J. Thorac. Oncol. 2016, 11, 2027–2032. [Google Scholar] [CrossRef]
- Ribeiro, M.F.S.A.; Alessi, J.V.M.; Oliveira, L.J.C.; Gongora, A.B.L.; Sacardo, K.P.; Zucchetti, B.M.; Shimada, A.K.; Barbosa, F.D.G.; Feher, O.; Katz, A. Alectinib activity in chemotherapy-refractory metastatic RET-rearranged non-small cell lung carcinomas: A case series. Lung Cancer 2020, 139, 9–12. [Google Scholar] [CrossRef]
- Drilon, A.; Rekhtman, N.; Arcila, M.; Wang, L.; Ni, A.; Albano, M.; Van Voorthuysen, M.; Somwar, R.; Smith, R.S.; Montecalvo, J.; et al. Cabozantinib in patients with advanced RET-rearranged non-small-cell lung cancer: An open-label, single-centre, phase 2, single-arm trial. Lancet Oncol. 2016, 17, 1653–1660. [Google Scholar] [CrossRef] [PubMed]
- Nokihara, H.; Nishio, M.; Yamamoto, N.; Fujiwara, Y.; Horinouchi, H.; Kanda, S.; Horiike, A.; Ohyanagi, F.; Yanagitani, N.; Nguyen, L.; et al. Phase 1 Study of Cabozantinib in Japanese Patients With Expansion Cohorts in Non–Small-Cell Lung Cancer. Clin. Lung Cancer 2019, 20, e317–e328. [Google Scholar] [CrossRef] [PubMed]
- Horiike, A.; Takeuchi, K.; Uenami, T.; Kawano, Y.; Tanimoto, A.; Kaburaki, K.; Tambo, Y.; Kudo, K.; Yanagitani, N.; Ohyanagi, F.; et al. Sorafenib treatment for patients with RET fusion-positive non-small cell lung cancer. Lung Cancer 2016, 93, 43–46. [Google Scholar] [CrossRef] [PubMed]
- Lee, S.-H.; Lee, J.-K.; Ahn, M.-J.; Kim, D.-W.; Sun, J.-M.; Keam, B.; Kim, T.; Heo, D.; Ahn, J.; Choi, Y.-L.; et al. Vandetanib in pretreated patients with advanced non-small cell lung cancer-harboring RET rearrangement: A phase II clinical trial. Ann. Oncol. 2017, 28, 292–297. [Google Scholar] [CrossRef]
- Yoh, K.; Seto, T.; Satouchi, M.; Nishio, M.; Yamamoto, N.; Murakami, H.; Nogami, N.; Matsumoto, S.; Kohno, T.; Tsuta, K.; et al. Vandetanib in patients with previously treated RET-rearranged advanced non-small-cell lung cancer (LURET): An open-label, multicentre phase 2 trial. Lancet Respir. Med. 2017, 5, 42–50. [Google Scholar] [CrossRef]
- Hida, T.; Velcheti, V.; Reckamp, K.L.; Nokihara, H.; Sachdev, P.; Kubota, T.; Nakada, T.; Dutcus, C.E.; Ren, M.; Tamura, T. A phase 2 study of lenvatinib in patients with RET fusion-positive lung adenocarcinoma. Lung Cancer 2019, 138, 124–130. [Google Scholar] [CrossRef]
- Cascetta, P.; Sforza, V.; Manzo, A.; Carillio, G.; Palumbo, G.; Esposito, G.; Montanino, A.; Costanzo, R.; Sandomenico, C.; De Cecio, R.; et al. RET Inhibitors in Non-Small-Cell Lung Cancer. Cancers 2021, 13, 4415. [Google Scholar] [CrossRef]
- Takamori, S.; Matsubara, T.; Haratake, N.; Toyokawa, G.; Fujishita, T.; Toyozawa, R.; Ito, K.; Yamaguchi, M.; Taguchi, K.; Okamoto, T.; et al. Targeted Therapy for RET Fusion Lung Cancer: Breakthrough and Unresolved Issue. Front. Oncol. 2021, 11, 704084. [Google Scholar] [CrossRef]
- Michelotti, A.; de Scordilli, M.; Bertoli, E.; De Carlo, E.; Del Conte, A.; Bearz, A. NSCLC as the Paradigm of Precision Medicine at Its Finest: The Rise of New Druggable Molecular Targets for Advanced Disease. Int. J. Mol. Sci. 2022, 23, 6748. [Google Scholar] [CrossRef]
- Singh, N.; Temin, S.; Baker, S., Jr.; Blanchard, E.; Brahmer, J.R.; Celano, P.; Duma, N.; Ellis, P.M.; Elkins, I.B.; Haddad, R.Y.; et al. Therapy for Stage IV Non-Small-Cell Lung Cancer With Driver Alterations: ASCO Living Guideline. J. Clin. Oncol. 2022, 40, 3310–3322. [Google Scholar] [CrossRef]
- Drilon, A.; Oxnard, G.R.; Tan, D.S.W.; Loong, H.H.F.; Johnson, M.; Gainor, J.; McCoach, C.E.; Gautschi, O.; Besse, B.; Cho, B.C.; et al. Efficacy of Selpercatinib in RET Fusion-Positive Non-Small-Cell Lung Cancer. N. Engl. J. Med. 2020, 383, 813–824. [Google Scholar] [CrossRef] [PubMed]
- Drilon, A.; Subbiah, V.; Gautschi, O.; Tomasini, P.; de Braud, F.; Solomon, B.J.; Tan, D.S.-W.; Alonso, G.; Wolf, J.; Park, K.; et al. Selpercatinib in Patients With RET Fusion–Positive Non–Small-Cell Lung Cancer: Updated Safety and Efficacy From the Registrational LIBRETTO-001 Phase I/II Trial. J. Clin. Oncol. 2022, 41, JCO-22. [Google Scholar] [CrossRef] [PubMed]
- Gainor, J.F.; Curigliano, G.; Kim, D.-W.; Lee, D.H.; Besse, B.; Baik, C.S.; Doebele, R.C.; Cassier, P.A.; Lopes, G.; Tan, D.S.W.; et al. Pralsetinib for RET fusion-positive non-small-cell lung cancer (ARROW): A multi-cohort, open-label, phase 1/2 study. Lancet Oncol. 2021, 22, 959–969. [Google Scholar] [CrossRef]
- Griesinger, F.; Curigliano, G.; Thomas, M.; Subbiah, V.; Baik, C.; Tan, D.; Lee, D.; Misch, D.; Garralda, E.; Kim, D.-W.; et al. Safety and efficacy of pralsetinib in RET fusion–positive non-small-cell lung cancer including as first-line therapy: Update from the ARROW trial. Ann. Oncol. 2022, 33, 1168–1178. [Google Scholar] [CrossRef]
- Fancelli, S.; Caliman, E.; Mazzoni, F.; Brugia, M.; Castiglione, F.; Voltolini, L.; Pillozzi, S.; Antonuzzo, L. Chasing the Target: New Phenomena of Resistance to Novel Selective RET Inhibitors in Lung Cancer. Updated Evidence and Future Perspectives. Cancers 2021, 13, 1091. [Google Scholar] [CrossRef] [PubMed]
- Choudhury, N.J.; Drilon, A. Decade in review: A new era for RET-rearranged lung cancers. Transl. Lung Cancer Res. 2020, 9, 2571–2580. [Google Scholar] [CrossRef]
- Osta, B.E.; Ramalingam, S.S. RET Fusion: Joining the Ranks of Targetable Molecular Drivers in NSCLC. JTO Clin. Res. Rep. 2020, 1, 100050. [Google Scholar] [CrossRef]
- Solomon, B.J.; Tan, L.; Lin, J.J.; Wong, S.Q.; Hollizeck, S.; Ebata, K.; Tuch, B.B.; Yoda, S.; Gainor, J.F.; Sequist, L.V.; et al. RET Solvent Front Mutations Mediate Acquired Resistance to Selective RET Inhibition in RET-Driven Malignancies. J. Thorac. Oncol. 2020, 15, 541–549. [Google Scholar] [CrossRef]
- Lin, J.; Liu, S.; McCoach, C.; Zhu, V.; Tan, A.; Yoda, S.; Peterson, J.; Do, A.; Prutisto-Chang, K.; Dagogo-Jack, I.; et al. Mechanisms of resistance to selective RET tyrosine kinase inhibitors in RET fusion-positive non-small-cell lung cancer. Ann. Oncol. 2020, 31, 1725–1733. [Google Scholar] [CrossRef]
- Lin, J.J.; Gainor, J.F. An early look at selective RET inhibitor resistance: New challenges and opportunities. Br. J. Cancer 2021, 124, 1757–1758. [Google Scholar] [CrossRef]
- Rosen, E.Y.; Won, H.H.; Zheng, Y.; Cocco, E.; Selcuklu, D.; Gong, Y.; Friedman, N.D.; de Bruijn, I.; Sumer, O.; Bielski, C.M.; et al. The evolution of RET inhibitor resistance in RET-driven lung and thyroid cancers. Nat. Commun. 2022, 13, 1450. [Google Scholar] [CrossRef]
- Drilon, A.E.; Zhai, D.; Rogers, E.; Deng, W.; Zhang, X.; Ung, J.; Lee, D.; Rodon, L.; Graber, A.; Zimmerman, Z.F.; et al. The next-generation RET inhibitor TPX-0046 is active in drug-resistant and naïve RET-driven cancer models. J. Clin. Oncol. 2020, 38, 3616. [Google Scholar] [CrossRef]
- Rosen, E.Y.; Johnson, M.L.; Clifford, S.E.; Somwar, R.; Kherani, J.F.; Son, J.; Bertram, A.A.; Davare, M.A.; Gladstone, E.G.; Ivanova, E.V.; et al. Overcoming MET-Dependent Resistance to Selective RET Inhibition in Patients with RET Fusion–Positive Lung Cancer by Combining Selpercatinib with Crizotinib. Clin. Cancer Res. 2021, 27, 34–42. [Google Scholar] [CrossRef] [PubMed]
- Nelson-Taylor, S.K.; Le, A.T.; Yoo, M.; Schubert, L.; Mishall, K.M.; Doak, A.; Varella-Garcia, M.; Tan, A.-C.; Doebele, R.C. Resistance to RET-Inhibition in RET-Rearranged NSCLC Is Mediated By Reactivation of RAS/MAPK Signaling. Mol. Cancer Ther. 2017, 16, 1623–1633. [Google Scholar] [CrossRef]
- Chang, H.; Sung, J.H.; Moon, S.U.; Kim, H.S.; Kim, J.W.; Lee, J.S. EGF Induced RET Inhibitor Resistance in CCDC6-RET Lung Cancer Cells. Yonsei Med. J. 2017, 58, 9–18. [Google Scholar] [CrossRef] [PubMed]
- Raman, R.; Villefranc, J.A.; Ullmann, T.M.; Thiesmeyer, J.; Anelli, V.; Yao, J.; Hurley, J.R.; Pauli, C.; Bareja, R.; Eng, K.W.; et al. Inhibition of FGF receptor blocks adaptive resistance to RET inhibition in CCDC6-RET–rearranged thyroid cancer. J. Exp. Med. 2022, 219, e20210390. [Google Scholar] [CrossRef]
- Schubert, L.; Le, A.T.; Estrada-Bernal, A.; Doak, A.E.; Yoo, M.; Ferrara, S.E.; Goodspeed, A.; Kinose, F.; Rix, U.; Tan, A.-C.; et al. Novel Human-Derived RET Fusion NSCLC Cell Lines Have Heterogeneous Responses to RET Inhibitors and Differential Regulation of Downstream Signaling. Mol. Pharmacol. 2021, 99, 435–447. [Google Scholar] [CrossRef]
- Hayashi, T.; Odintsov, I.; Smith, R.S.; Ishizawa, K.; Liu, A.J.W.; Delasos, L.; Kurzatkowski, C.; Tai, H.; Gladstone, E.; Vojnic, M.; et al. RET inhibition in novel patient-derived models of RET fusion- positive lung adenocarcinoma reveals a role for MYC upregulation. Dis. Model. Mech. 2020, 14, dmm047779. [Google Scholar] [CrossRef]
- Fujimura, T.; Furugaki, K.; Harada, N.; Yoshimura, Y. Enhanced antitumor effect of alectinib in combination with cyclin-dependent kinase 4/6 inhibitor against RET-fusion-positive non-small cell lung cancer cells. Cancer Biol. Ther. 2020, 21, 863–870. [Google Scholar] [CrossRef]
- Repetto, M.; Crimini, E.; Ascione, L.; Bielo, L.B.; Belli, C.; Curigliano, G. The return of RET GateKeeper mutations? an in-silico exploratory analysis of potential resistance mechanisms to novel RET macrocyclic inhibitor TPX-0046. Investig. New Drugs 2022, 40, 1133–1136. [Google Scholar] [CrossRef]
- Drilon, A.; Rogers, E.; Zhai, D.; Deng, W.; Zhang, X.; Lee, D.; Ung, J.; Whitten, J.; Zhang, H.; Liu, J.; et al. TPX-0046 is a novel and potent RET/SRC inhibitor for RET-driven cancers. Ann. Oncol. 2019, 30, v190–v191. [Google Scholar] [CrossRef]
- Di Noia, V.; D’Aveni, A.; D’Argento, E.; Rossi, S.; Ghirardelli, P.; Bortolotti, L.; Vavassori, V.; Bria, E.; Ceresoli, G. Treating disease progression with osimertinib in EGFR-mutated non-small-cell lung cancer: Novel targeted agents and combination strategies. ESMO Open 2021, 6, 100280. [Google Scholar] [CrossRef] [PubMed]
- Pan, Y.; Deng, C.; Qiu, Z.; Cao, C.; Wu, F. The Resistance Mechanisms and Treatment Strategies for ALK-Rearranged Non-Small Cell Lung Cancer. Front. Oncol. 2021, 11, 713530. [Google Scholar] [CrossRef] [PubMed]
- Yu, H.; Goldberg, S.; Le, X.; Piotrowska, Z.; Smith, P.; Mensi, I.; Kirova, B.; Chmielecki, J.; Li-Sucholeicki, X.; Szekeres, P.; et al. P2.01-22 ORCHARD: A Phase II Platform Study in Patients with Advanced NSCLC Who Have Progressed on First-Line Osimertinib Therapy. J. Thorac. Oncol. 2019, 14, S647. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Rocco, D.; Sapio, L.; Della Gravara, L.; Naviglio, S.; Gridelli, C. Treatment of Advanced Non-Small Cell Lung Cancer with RET Fusions: Reality and Hopes. Int. J. Mol. Sci. 2023, 24, 2433. https://doi.org/10.3390/ijms24032433
Rocco D, Sapio L, Della Gravara L, Naviglio S, Gridelli C. Treatment of Advanced Non-Small Cell Lung Cancer with RET Fusions: Reality and Hopes. International Journal of Molecular Sciences. 2023; 24(3):2433. https://doi.org/10.3390/ijms24032433
Chicago/Turabian StyleRocco, Danilo, Luigi Sapio, Luigi Della Gravara, Silvio Naviglio, and Cesare Gridelli. 2023. "Treatment of Advanced Non-Small Cell Lung Cancer with RET Fusions: Reality and Hopes" International Journal of Molecular Sciences 24, no. 3: 2433. https://doi.org/10.3390/ijms24032433
APA StyleRocco, D., Sapio, L., Della Gravara, L., Naviglio, S., & Gridelli, C. (2023). Treatment of Advanced Non-Small Cell Lung Cancer with RET Fusions: Reality and Hopes. International Journal of Molecular Sciences, 24(3), 2433. https://doi.org/10.3390/ijms24032433






