Abstract
Antimicrobial resistance (AMR) is one of the biggest threats to human health worldwide. The World Health Organization (WHO, Geneva, Switzerland) has launched the “One-Health” approach, which encourages assessment of antibiotic-resistant genes (ARGs) within environments shared by human-animals-plants-microbes to constrain and alleviate the development of AMR. Aerosols as a medium to disseminate ARGs, have received minimal attention. In the present study, we investigated the distribution and abundance of ARGs in indoor and outdoor aerosols collected from an urban location in Kuwait and the interior of three hospitals. The high throughput quantitative polymerase chain reaction (HT-qPCR) approach was used for this purpose. The results demonstrate the presence of aminoglycoside, beta-lactam, fluoroquinolone, tetracycline, macrolide-lincosamide-streptogramin B (MLSB), multidrug-resistant (MDR) and vancomycin-resistant genes in the aerosols. The most dominant drug class was beta-lactam and the genes were IMP-2-group (0.85), Per-2 group (0.65), OXA-54 (0.57), QnrS (0.50) and OXA-55 (0.55) in the urban non-clinical settings. The indoor aerosols possessed a richer diversity (Observed, Chao1, Shannon’s and Pielou’s evenness) of ARGs compared to the outdoors. Seasonal variations (autumn vs. winter) in relative abundances and types of ARGs were also recorded (R2 of 0.132 at p < 0.08). The presence of ARGs was found in both the inhalable (2.1 µm, 1.1 µm, 0.7 µm and < 0.3 µm) and respirable (>9.0 µm, 5.8 µm, 4.7 µm and 3.3 µm) size fractions within hospital aerosols. All the ARGs are of pathogenic bacterial origin and are hosted by pathogenic forms. The findings present baseline data and underpin the need for detailed investigations looking at aerosol as a vehicle for ARG dissemination among human and non-human terrestrial biota.
1. Introduction
Antibiotics are crucial medications for treating bacterial infections. With prolonged and indiscriminate usage of antibiotics, antimicrobial resistance (AMR) has developed, which is not only compromising the efficacy of these antibiotics in treating diseases but is also spreading to non-targeted organisms in the environment. Antimicrobial-resistant bacteria (ARBs) and antibiotic-resistance genes (ARGs) have consequently emerged and lingered in the environment [1]. Due to the persistent nature of pharmaceutical residues, the problem is further exacerbated; owing to the mechanism of horizontal gene transfer, the AMR, ARGs and ARBs are spatially reaching regions where no pharmaceutical residues exist [2]. The United Nations Environment Program (UNEP) and World Health Organization (WHO) have listed AMR as one of the major public health risks and a priority environmental issue that needs our immediate attention [3,4].
Antibiotic resistance genes have been reported from differential environmental matrixes such as soil [5,6,7], marine sediments [8,9,10], coastal waters [11,12,13] and river systems [14,15]. Air as a medium for ARG dissemination is less explored [16]. Aerosols are known carriers of many pathogenic microbes [17,18,19,20,21]; it is, therefore, legitimate to speculate that they can be transmitters of ARGs. A study in China has reported the rate of ARG inhalation ranging from 103 to 104 copies per day by an adult [22]. Inhalable ARGs have been reported to occur in urban hospital aerosols and adjacent urban and suburban communities [23] as well as ambient air particulate matter surrounding a municipal solid waste treatment system [24]. In addition, this long- and short-range transport of PM2.5 has been reported as a potential AMR distributor [25]. This further strengthens the need to look at ARGs in the inhalable size fraction of air.
It is also prudent to investigate the ARGs in ambient settings [26]. Increased urbanization, population densification, climate change, improper disposal of municipal solid waste and poor sanitization are some factors that are realized as AMR contributors in urban outdoor environments [27,28]. Some examples have indicated aerosols above an estuary [29], soil dust [30], livestock excreta from farms [31,32], wastewater treatment plants [33], hospitals [23,34] and solid waste treatment plants [24] as sources of ARGs in ambient air. Selective pressure in the form of antibiotics, metals and biocides is less expected in ambient air [26]; rather, they are likely to evolve under physicochemical pressures [35] subjected to a higher degree of dispersion and face higher oxidation particularly in the urban atmosphere [36].
The classic methods of ARG detection have been used to look at only a few targeted and cultivable forms since these methods are time and labor-intensive [37]. The culture-independent molecular approach, on the other hand, has emerged as a novel tool to assess ARGs rapidly, precisely and less expensively [38,39,40]. More recently, high throughput quantitative polymerase chain reaction (HT-qPCR) is gaining popularity owing to its capability to identify hundreds of genes in a single run [41,42,43,44]. To underpin the theory of ARG spread through aerosols, we have used a genome-centric approach to assess ARGs associated with size-fractionated aerosols in indoor and ambient air [16]. In the present investigation, we, therefore, employed the HT-qPCR method with the primary objective of identifying clinically relevant ARGs in aerosols collected from an outdoor and indoor location in an urban venue. ARGs were also identified from indoor sites within three hospitals. Spatial and seasonal variations of ARGs were correspondingly studied.
2. Results
The qPCR assays positively amplified 52 ARGs in indoor and outdoor aerosols. The Shapiro-Wilk test stipulated our experimental design was balanced with a normal distribution of residuals. Hereafter, we describe the number, types, abundance and spatiotemporal variations of ARGs. Bacterial DNA was found in all the samples. The values from the standard curve predicted bacterial cell counts in the range of 104–105 m−3 of aerosols sampled in this study. The month of Oct-21 was exceptional and showed lower bacterial cell counts (102) (Indoor—1.1 × 102 cells m−3 air; Outdoor—2.0 × 102 cells m−3 air). The mean number of cells m−3 air is shown in Figure 1a. Comparisons of average bacterial counts (cells m−3 air) between indoor and outdoor sites through ANOVA returned non-significant p-values (Tukey’s HSD p > 0.05).
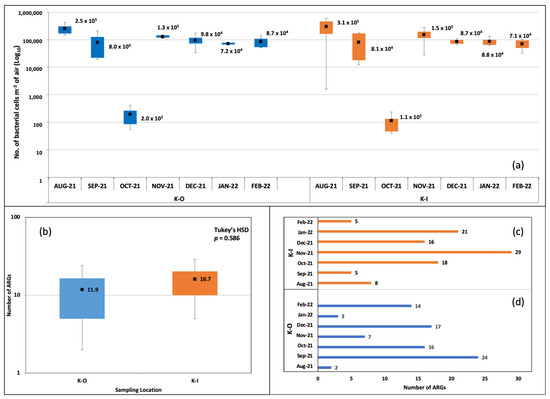
Figure 1.
Bacterial cell counts and number of ARGs detected in indoor and outdoor bioaerosols. (a) Box plot showing the number of bacterial cells per m−3 of air. (b) Box plot comparing the mean number of ARGs. (c) Number of ARGs in indoor aerosols at the different months of sampling. (d) Number of ARGs in outdoor aerosols at the different months of sampling.
2.1. Number of ARGs
The number of ARGs in indoor and outdoor samples from KISR was calculated. The numbers were higher in indoor aerosols (mean = 17) compared to outdoor (mean = 12) (Figure 1b). We employed the Kruskal–Wallis H test to compare the mean number of ARGs and recorded a non-significant difference between the two groups, χ2(4) = 2.95, p > 0.566. Similar observations were recorded through the ANOVA and Tukey’s HSD test (Figure 1b). The temporal variation in ARGs was observed. In indoor aerosols, the highest number of ARGs was recorded in Nov-21 (n = 29) > Jan-22 (n = 21) > Oct-21 (n = 18) > Dec-21 (n = 16) > Aug-21 (n = 8) > Sep-21 (n = 5) and Feb-22 (n = 5) (Figure 1c). In outdoor aerosols, the monthly ARG abundances were Sep-21 (n = 24) > Dec-21 (n = 17) > Oct-21 (n = 16) > Feb-22 (n = 14) > Nov-21 (n = 7) > Jan-22 (n = 3) > Aug-21 (n = 2) (Figure 1d).
2.2. Drug Classes and Gene IDs
The microbial DNA qPCR assay captured genes belonging to the drug classes such as aminoglycoside, beta-lactamases, fluoroquinolones, MLSB, MDR, tetracycline, and vancomycin. The absence of the erythromycin drug class was observed in the samples analyzed. The highest number of genes belonged to the beta-lactamase group both indoors (n = 58 ± 3.0) as well as outdoors (n = 50 ± 5.1). This class contributed almost 50% of ARGs in the aerosol community. Macrolides (indoor 16 ± 0.7; outdoor 9 ± 0.5) were the second highest in order (Figure 2a). We also looked at the ARG categories present in the corresponding month of sampling and discovered beta-lactamase genes to be present all the time in indoor and outdoor aerosols. It was the only category present in outdoor aerosol in the Aug-21 samples. Two more drug categories, MLSB and fluoroquinolone, were observed in Nov-21 and Jan-22, K-O samples, respectively. In Sep-21, Oct-21, Dec-21 and Feb-22, four or more drug types were found in K-O samples. Similar drug classes were recorded every month in indoor aerosols as well. At least two types were recorded monthly, and six drug classes including aminoglycoside, beta-lactam, fluoroquinolone, MLSB, MDR, and tetracycline, were documented in the Jan-22, K-I sample.
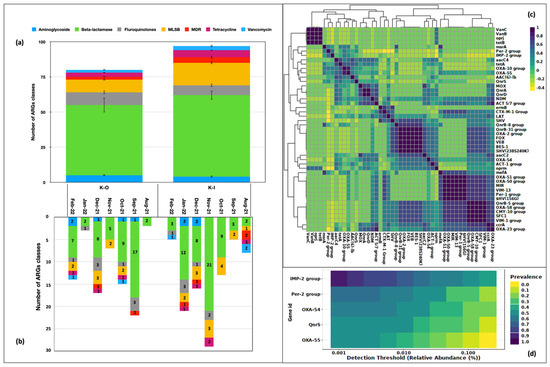
Figure 2.
Drug classes identified in bioaerosols. (a) Number of ARG of each drug type in indoor and outdoor locations. (b) Category of ARGs in the respective month of sampling. (c) Types of ARGs. (d) Core resistome of bioaerosols.
Fifty-two genes were associated with these drug classes and 47 of them were present at least twice. These genes exhibited a correlation ranging between 0.4–1.0 (Pearson’s r2; p < 0.05) (Figure 2c). We further explored the occurrence of highly prevalent genes in our samples. Five of them exhibited a prevalence > 50% and were considered as part of the core resistome. These were, namely, IMP-2-group (0.85), Per-2- group (0.65), OXA-54 (0.57), QnrS (0.50) and OXA-55 (0.55) (Figure 2d). These genes belonging to the beta-lactamase class B (IMP-2-group), class A (Per-2- group), class D (OXA 54, OXA 55), and fluoroquinolones (QnrS) that are of known clinical significance and pose a significant risk to human health.
2.3. Spatio-Temporal Variations in the Distribution of ARGs
We studied the RA of drug classes and the 47 ARGs in indoor versus outdoor aerosols in two seasons, i.e., autumn (August–November) and winter (December–February). Seasonality had a marked effect on drug classes and ARG abundance, probably because September–October is a transition period with the highest incidences of common flu and allergies in the region [45]. The indoor and outdoor samples in the two seasons had differences in the distribution of ARG classes (Figure 3a). Except for MDR being highly prevalent in the Aug-21, beta-lactams (RA 0.50-1.00) were the pre-dominant class in autumn in both indoor and outdoor samples, whereas their abundance was reduced in winter (RA 0.31–0.81). Aminoglycoside (0.09–0.30) and fluoroquinolones (0.08–0.87) were other gene classes present during winter. Among the ARG distribution in the indoor and outdoor samples, there was significant diversity and variation; almost every sample had a unique ARG profile (Figure 3b). The prevalence and abundance of all 47 ARGs differed between outdoor and indoor aerosols. The hierarchical clustering grouped them into two clusters (Figure 3c).
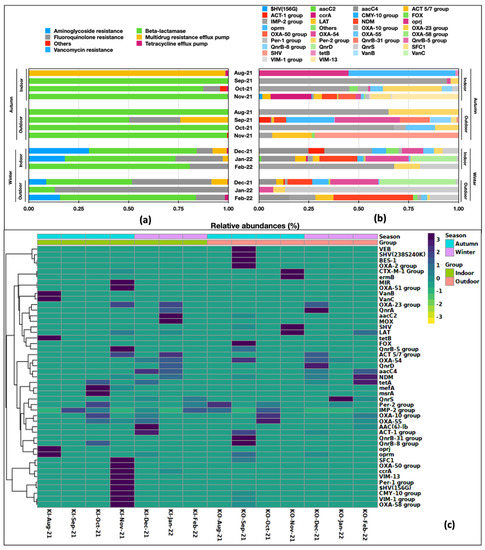
Figure 3.
Spatio-temporal variation in relative abundances of: (a) drug classes and (b) ARGs present in aerosols. (c) Hierarchical clustering on Bray Curtis distances.
2.4. ARG Richness, Evenness and Ordination Analysis
The alpha diversity indices on observed features (richness) revealed the indices to be higher in indoor aerosols (Figure 4a). The Chao1 index representing the feature evenness inclusive of rare events was also higher in indoor aerosols (Figure 4b). Similarly, the case with Shannon’s diversity index, indicated both richness and evenness greater in indoor aerosols (Figure 4c). However, Pielou’s evenness was comparable in indoor and outdoor aerosols (Figure 4d). Our results suggest the diversity of ARGs was slightly higher in indoor aerosols. This depends on the point source of ARGs in the indoor atmosphere, which is most likely a higher human footfall. Principal coordinate analysis (PCoA) distributed the indoor and outdoor ARG-laden aerosols into overlapping clusters. The F-statistic value was recorded as 0.312 with an R2 coefficient of 0.025 at p > 0.99. Variations across the first and second axes were 36.8% and 15%, respectively (Figure 4e). The non-significant p values obtained during the alpha and beta diversity analysis are attributed to a small sample size. We further employed the DeSeq2 (fitZig model) to look at differentially abundant genes specifically. The zero-inflated Gaussian fit predicted five genes to differ in abundance at a false discovery rate (FDR q-value) < 0.05. These genes were OXA-54, QnrD, VIM-1 group, AAC (6) lb and OXA-58 group (Figure 4f).
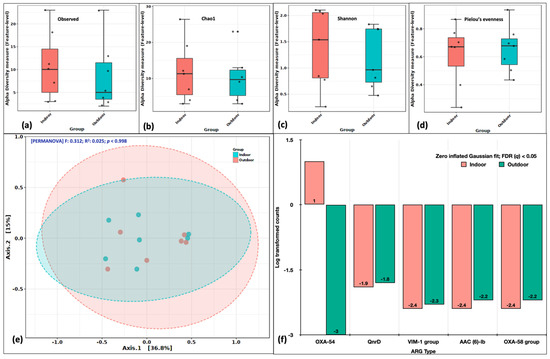
Figure 4.
Variations in ARG abundance between indoor and outdoor aerosols. Alpha diversity analysis: (a) Observed features, (b) Chao1 indices, (c) Shannon’s evenness and richness, (d) Pielou’s evenness index. Beta diversity analysis: (e) Principal coordinate analysis (PCoA) on Bray Curtis distances through Ward algorithm. Differential abundance testing: (f) fitZig model (DESeq2). The green colour in each figure represents the indoor samples, whereas the red colour represents outdoor aerosols.
A similar analysis was performed, keeping seasonality as an experimental factor. The alpha diversity indices (Observed, Chao1, Shannon and Pielou’s) were consistently higher in the winter season suggesting winter to be favorable for pathogenic forms to proliferate (Figure 5a–d). The PCoA distributed the samples into two partially overlapping clusters (Figure 5e). The F statistics was 1.82, R2 of 0.132 at p < 0.08 (Axes 1–36.8%; Axes 2–15%). The DeSeq2 identified five genes (aacC4, QnrS, QnrD, NDM and mefA) significantly abundant at an FDR (q) < 0.05. Seasonality was a better driver of variability and diversity among the identified ARGs. These results contrasted with our observations of mean gene numbers, the average number of drug classes and bacterial cell counts. However, it is the type of ARGs and drug classes, more specifically their relative abundances, that vary in space and time rather than their counts.
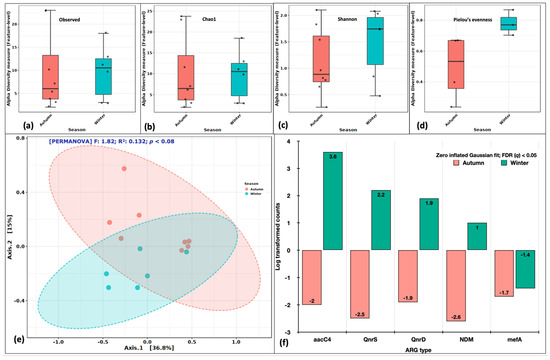
Figure 5.
Variations in ARG aerosol abundance between autumn and winter seasons. Alpha diversity analysis: (a) Observed features, (b) Chao1 indices, (c) Shannon’s evenness and richness, (d) Pielou’s evenness index. Beta diversity analysis: (e) Principal coordinate analysis (PCoA) on Bray Curtis distances through Ward algorithm. Differential abundance testing: (f) fitZig model (DESeq2). The green colour in each figure represents the indoor samples, whereas the red colour represents outdoor aerosols.
We compared the alpha diversity indices of indoor aerosols in autumn and winter. The richness (Observed, Chao1, Shannon) and evenness (Shannon, Pielou’s) were higher in the winter season (Figure 6a–d). The PCoA analysis distributed them into a single cluster. The variations among the first and second axis were 43% and 24.6%, respectively (Figure 6e). The R2 coefficient was 0.133 at p = 0.64. The genes that differed significantly in indoor aerosols between autumn and winter were oprJ, QnrD, VIM-13 and tetB at false discovery rate (q) < 0.05 (Figure 6f). Likewise, in outdoor aerosols, the alpha diversity (Figure 6g–j) was higher in the winter season. The single cluster in the PCoA plot distributed the samples at 37% at the first axis and 12.6% at the second axis (Figure 6k). The genes that significantly varied (FDR q < 0.001) in outdoor aerosols between autumn and winter were QnrD, QnrB-31, QnrB-8, SFC1 and QnrS (Figure 6l). Our results suggested temporal/seasonal variations within the aerosols collected from indoor and outdoor locations.
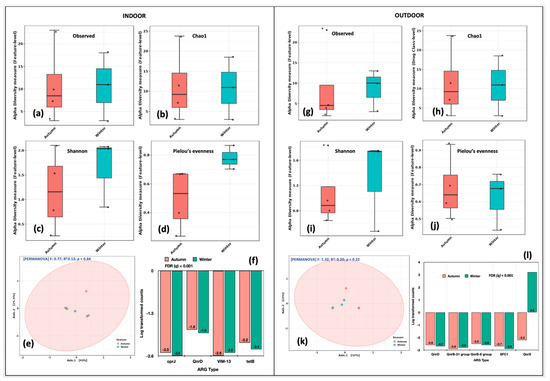
Figure 6.
Intra-spatial variations in ARG abundance between autumn and winter seasons. Indoor aerosol alpha diversity analysis: (a) Observed features, (b) Chao1 indices, (c) Shannon’s evenness and richness, (d) Pielou’s evenness index. Beta diversity analysis: (e) Principal coordinate analysis (PCoA) on Bray Curtis distances through Ward algorithm. Differential abundance testing: (f) fitZig model (DESeq2). Outdoor aerosols: (g) Observed features, (h) Chao1 indices, (i) Shannon’s evenness and richness, (j) Pielou’s evenness index. Beta diversity analysis: (k) Principal coordinate analysis (PCoA) on Bray Curtis distances through Ward algorithm. Differential abundance testing: (l) fitZig model (DESeq2). The green colour in each figure represents the indoor samples, whereas the red colour represents outdoor aerosols.
2.5. Evidence of ARGs in Indoor Aerosols of Clinical Settings
We detected the presence of ARGs in indoor aerosols collected from three major hospitals in Kuwait. The volume of air, sampling period and methodical modifications prohibit us from comparing these sites with a non-clinical location. However, hospitals are considered as hot-spots for ARG dissemination and, therefore, it was important to look at the resistomes prevailing at these sites. The drug classes and the corresponding gene identities found at each hospital are mentioned in Table 1. The three hospitals registered different types and numbers of ARGs, with highest (n = 27) observed in HI-1 hospital with beta-lactams as the dominant class. Fluoroquinolones were also found in considerable proportions. Aminoglycoside and sulfonamide resistance was completely absent in HI-1 hospital. Interestingly, MDR gene oprJ was found at HI-1, raising further concerns about its resistance against multiple drugs. Some other genes, to name a few, were tetA, vanB, and ermB, C, and A, offering resistance to tetracycline, vancomycin and MLSB drug classes, respectively. Although HI-1 and HI-2 hospitals were sampled simultaneously, the numbers were far less (n = 15) in the latter. However, HI-2 hospital recorded the presence of a single aminoglycoside gene (aadA1) and multidrug resistant oprJ. Contrary to HI-1 and HI-2, although a higher volume of air was collected from HI-3 hospital, the numbers of ARGs were less (n = 15). It is important to note that samples were collected at different time points and spatial locations; the human footfall at each location is a major contributor to the resistomes of a site. Surprisingly, beta-lactamase, a common aerosol drug class, was completely absent in HI-3 hospital. Chances of primers missing the low copy number genes are quite possible in HT-qPCR assays. Significant numbers of genes showed late amplification with a CT above the set threshold and were, therefore, omitted from the analysis. The source of ARGs in indoor hospital aerosols is most likely the patients and health care staff.

Table 1.
ARGs and their drug classes detected in aerosols collected from clinical settings of Kuwait.
We also looked at the distribution of ARGs in six size fractions. As the Anderson cascade impactor was deployed to collect aerosols from HI-3 hospital, samples of inhalable (2.1 µm, 1.1 µm, 0.7 µm and <0.3 µm) and respirable (>9.0 µm, 5.8 µm, 4.7 µm and 3.3 µm) fractions were analyzed. Intriguingly, ARGs were found in all the size fractions originating from the drug classes of aminoglycoside (0.07–0.40), MLSB (0.09–0.21), quinolones (0.00–0.06), tetracycline (0.35–1.0), MDR (0.20–0.25), sulfonamide (0.00–0.11) and other (0.00–0.11) (Figure 7a). The identified genes were aadA-1 (0.06–0.15), cefa_qacelta (0.00–0.20), qacE∆1_3 (0.00–0.11), strB (0.00–0.31), sul1_1 (0.00–0.11), aadA2_3 (0.00–0.20), ermX_1 (0.09–0.25), qepA (0.00–0.06), sugE (0.00–0.25) and tetPA (0.35–1.0) (Figure 7b). Among the identified genes, tetPA was omnipresent in all the size fractions.
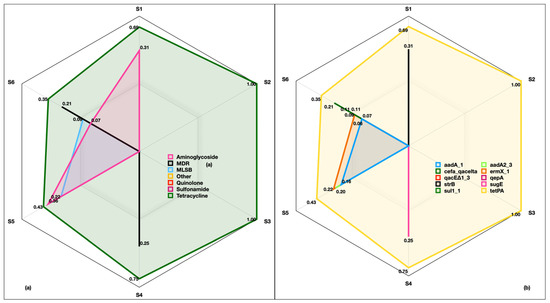
Figure 7.
Antibiotic resistance genes in inhalable and respirable size fractions of indoor aerosols collected from HI-3 hospital in Kuwait. (a) Relative abundance of drug classes in six size fractions, (b) relative abundance of corresponding genes. S1 and S6 represent the six stages of Anderson cascade impactor with a pore size of 0.30 to 0.69 µm (base filter), >0.69 to 1.3 µm (Stage 5), >1.3 to 2.1 µm (Stage 4), >2.1 to 4.2 µm (Stage 3), >4.2 to 10.2 µm (Stage 2) and >10.2 µm (Stage 1).
3. Discussion
The harmful effects of ARGs in aerosols have been realized and initial steps for their monitoring have dawned [26]. The majority of the researchers focus on the characterization of pathogenic microbes, considering them as the only important biological contaminants and often ignore these pernicious pollutants (i.e., ARGs). In this context, the present investigation adds valuable knowledge on the presence and abundance of ARGs in indoor and outdoor aerosols. The HT-qPCR assays proved a technically sound approach to map hundreds of pertinent genes that pose a significant risk to humans [46]. We report their presence in indoor aerosols (n = 52) of a research institution’s location in an urban area as well as in ambient air outside the premises. The interiors of three hospitals also had significant numbers of ARGs (n = 46). These numbers have also been reported previously in aerosols of urban wastewater treatment plants [22,33].
Antibiotic resistance genes are integrated within the bacterial genomes. Bacterial DNA was found in all the tested samples and the total load was estimated at an average of 105 cells per m−3 of air. Slightly lesser bacterial cells (104 m−3 air) were recorded in the shower aerosols of a stem cell transplant unit [47] whereas similar cell counts were recorded in eight indoor locations (a classroom, a daycare center, a dining facility, a health center, three houses, an office) in Blacksburg, Virginia [48]. The presence of a comparable number of bacterial cells in outdoor air was in congruence with our previous findings [18,19,49,50]. Similar to our studies, higher numbers of bacteria-like particles have been reported previously in an outdoor location in a university campus in the United States [48]. Temporal seasonal variations in bacterial counts were reported [51], similar to the observations made in this study. In addition to this, the positive amplification of Pan1 and Pan3 bacterial DNA in our samples suggests the presence of bacterial families, Actinobacteria, Bacteroidetes, Euryarchaeota, Firmicutes, Fusobacteria, Proteobacteria, Tenericutes and Spirochaetes. We have previously reported both pathogenic and non-pathogenic bacteria from analogous phyla in aerosol particulate fractions collected from urban, rural and clinical places [17,21,52,53,54,55]. A cocktail of microorganisms was reported in air particulate matter during a smog event in China [56].
The number of ARGs in indoor aerosols (n = 97) was 1.2-fold higher than the outdoor aerosols (n = 80). In addition, the richness and evenness of ARGs were higher indoors as compared to outdoors (greater alpha diversity parameters). This is expected because the source of ARGs in the indoor air is most probably exhaled breadth [57]. Human footfall is always higher indoors in a country such as Kuwait, due to extreme outdoor weather. Similar to our results, aerosol-associated ARGs were reportedly higher indoors at a university in Tianjin [58]. Some researchers have pointed out that the antibiotic resistome in indoor environments is shaped by the human occupants and not only exhaled breadth. Activities such as skin shedding, feces, saliva and sneezing release ARGs in close vicinity [59,60,61]. In addition, confined animal feeding operations inside a swine, cattle, layer and broiler farm in China exposed the human workers to the aerosolized ARGs in an indoor environment [62]. A couple of studies have also indicated outdoor aerosols as a major contributor to microbes and ARGs indoors [59,61].
The likely sources of ARGs in outdoor aerosols are wave action and wastewater as the area is in close proximity to a marine outfall with hospital waste discharge. This site has maximum concentrations of antibiotics and ARGs in marine sediments and coastal waters [8,9,63]. Increasingly, enriched ARGs were observed along the coastal–urban wastewater treatment plant gradient in ambient air collected in Hong Kong [33]. Submicron aerosols were reported to share antibiotic resistomes (4–17%) with wastewater and sludge in yet another study [64]. KISR is located in close proximity to Sabah Hospital complex and health facilities; therefore, the emanating hospital bioaerosol might be a contributor to ARGs in ambient outdoor aerosols [65].
The most dominant gene classes were beta-lactams, which are often considered the last choice of drug to treat severe bacterial infections such as urinary tract, bloodstream, wound and pneumonia [66,67]. More concerning is the 85% prevalence of IMP-2 group (Imipenemase) encoded by the blaIMP genes and commonly transferred between Gram-negative bacteria through horizontal gene transfer (class 1 or 3 integrons) [68]. A review from a Japanese group revealed about 88 variants of IMP-type metallo-ß-lactamase circulating worldwide, with 25% from Japan, 17% from China and 7% from France [68]. PER-2-group [69] and OXA group [70] are two other notorious beta-lactams perpetrating globally. These genes are inhabited by the ESKAPEE (Enterococcus, Staphylococcus, Klebsiella, Acinetobacter, Pseudomonas, Enterobacter and Escherichia) pathogens on the WHO list for which novel antibiotics are needed [4]. Our ARG-microbial host analysis was in complete agreement with this (Tables S1–S4).
Most of the assumptions regarding health implications are made on PM2.5 and below. Presently, we recorded ARGs in a size fraction as low as 0.30 µm both in indoor and outdoor conditions. A study conducted in Harbin; China recorded the presence of ARGs in PM2.5 in an urban atmosphere [51]. Further, we discovered ARGs in six size fractions in FW, with higher numbers in the smaller size segment of 0.30 to 0.69 µm. ARGs hosted by ARBs in this size fraction are prone to accumulation in the human airways and might express in the form of illnesses causing hospitalizations. Alternatively, these ARGs carried by the general population in an urban atmosphere pose an elevated risk of disease outbreaks under conducive meteorological conditions [26]. ARGs identified as hospital indoor aerosols pose a significant threat to healthcare professionals, patients and visitors. Hospitals are considered hotspots of resistome transmission [71,72]. Healthcare professionals are at high risk and also act as carriers of these undesirable elements [73,74,75]. Nosocomial outbreaks warrant initiation of continual biomonitoring programs in the region to tackle this serious issue.
We further believe that the stochastic elements comprising organic and inorganic contaminants often interact with inherent microbial communities making the situation even more complex [50,55,76,77,78,79,80,81]. It is also noteworthy to take into consideration the dose metrics and the chemistry of the aerosols in addition to the types and characteristics of ARGs, for a comprehensive risk assessment [26]. Most of the vulnerable microbes and ARGs are present in very low concentrations in aerosols. This study was limited in capturing the mobile genetic elements associated with these ARGs, that play a key role in the dissemination of ARGs via horizontal gene transfer. The alternative approach of next-generation sequencing might overcome the issue for its benefit in capturing the whole genome and the low-abundance genes [82,83].
Seasonal variations in abundance and types of ARGs were observed and were attributed to the differences in bacterial taxonomies. Spatio-temporal variations in microbial profiles of aerosols have been well documented in other regions as well [51,52,84,85]. Ma and group demonstrated variances in bioaerosol-associated ARGs in summer, winter, autumn and spring seasons [51]. We recorded a higher diversity, richness and evenness of ARGs in winter than in autumn in indoor aerosols. This is due to a high peak observed in the number of genes in 21 November coinciding with end of the flu season in Kuwait [45]. Higher pharmaceutical concentrations (including antibiotics) were also recorded in seawater samples collected near storm outlets along the coast of Kuwait. The authors attributed this to increased medical prescriptions during this period [86]. Temperature and humidity are major environmental parameters that fluctuate according to the season, and these are most likely the drivers of resistome variations. To add further, the fluctuations were more pronounced with respect to the types of genes and their relative abundances rather than numbers [87]. Variations in RA of microbial communities in air particulate matter have been reported previously [53].
4. Materials and Methods
4.1. Sampling
Air samples for the present investigation were collected using a customized device described in detail elsewhere [49,52]. Briefly, a sampler pumped air at the rate of 30 L min−1 for 360 min. The sampler consisted of a glass funnel housing a 0.30 µm Anodisc filter (Whatman®, Darmstadt, Germany). Aerosols < 0.30 µm were retained on the filter paper and air mass > 0.30 µm was bubbled through three gas washed glass bottles containing sterile phosphate-buffered saline (PBS) (Sigma Aldrich, St. Louis, MO, USA) [52]. The sampler was placed within the Kuwait Institute for Scientific Research (KISR) every week for 360 min from August 2021 until February 2022 covering autumn and winter seasons in Kuwait. The location in KISR was strategically chosen in a corridor that has maximum footfall. Another sampler was co-deployed at an outdoor site in KISR near an AC vent and a coastal walkway. In addition to KISR samples, permission from the Ministry of Health (MOH), Kuwait was granted to collect indoor aerosol samples for a limited duration within three hospital sites (Hospital 1—HI-1, Hospital 2—HI-2 and Hospital 3—HI-3). These hospitals cater to large populations in three major governorates of Kuwait City. The sampling details are provided in Table 2.

Table 2.
Sampling locations and types of samples collected for ARG detection.
4.2. Isolation of Nucleic Acids
The indoor and outdoor aerosol in PBS (K-O and K-I) was concentrated by adding 12% beef extract (Sigma, Oslo, Norway) and centrifugation at 10,000× g for 30 min. The pellet was treated with 600 µL of lysis buffer (RAV1) at 70 °C for 5 min from the NucleoSpin Mini kit (MachereyNagel, Cedex, France). The lysate was subsequently purified and washed following the manufacturer’s instructions. The Anderson discs collected from Farwaniya Hospital were swabbed several times with sterile PBS to collect the microbial load. The swabs were then processed similarly to the above-mentioned protocol. Aerosols were processed for RNA isolation from HI-1and HI-2 through the standard Trizol® procedure [21]. Nucleic acid quantification was performed on a Qubit fluorometer employing the Qubit HS DNA/RNA assay kits (Thermo Scientific, Waltham, MA, USA). All the RNA samples (HI-1 and HI-2), were converted to complementary DNA (cDNA) before conducting the PCR [20,21]. K-O and K-I samples collected in a month (n = 4; one sample per week) were pooled due to their low biological yields.
4.3. Quantitative Estimation of Bacterial Cells
Total bacterial counts of K-O and K-I samples were estimated through quantitative polymerase chain reaction (qPCR). Purified DNA (2 μL) was added to 2× iQTM SYBR® Green Supermix (BioRad, Mississauga, ON, Canada) along with universal 16S rRNA bacterial primers (300 nM Forward 5′-CCTACGGGNBGCASCAG-3′; Reverse 5′-GACTACNVGGGTATCTAATCC-3′). The total reaction volume was made up to 20 μL with nuclease-free water and the qPCR was accomplished on the CFX96TM deep well thermal cycler (BioRad, Mississauga, ON, Canada). Standard Escherichia coli DNA (2.4 × 106 cells/mL) and nuclease-free water were used as a positive control (PC) and negative controls (NTC), respectively. Serial dilutions (1:10 × 6) of PC were also amplified along with the samples to obtain a standard curve (R2 = 0.650; slope = −3.123; y-int = 38.971). The cycling conditions were set for initial denaturation at 95 °C (3 min); followed by 40 rounds of denaturation at 95 °C (20 s); annealing and extension at 60 °C (45 s). A PCR efficiency of 100.0% was achieved. The melt curve analysis was carried out at 60 °C. The cycle threshold (CT) values of samples were plotted on the standard curve, to quantify the target gene (16S rRNA gene). This was performed in CFX MaestroTM software (BioRad). The copy numbers of the target gene were then converted to bacterial cells per m−3 of air [18].
4.4. Microbial qPCR Arrays for ARG Detection
The microbial DNA qPCR assays (Qiagen, Germantown, MD, USA) were used for the detection of 87 ARGs originating from drug classes such as aminoglycoside, beta-lactams (class A, B, C and D), fluoroquinolone/quinolone, erythromycin, macrolide-lincosamide-streptogramin B (MLSB), multidrug resistance (MDR), tetracycline, and vancomycin from the indoor and outdoor aerosols (K-O, K-I, HI-1 and HI-2) collected in the present study. The 25 µL PCR reaction mix comprised 12.5 µL of 1× Master mix, 5 ng of template DNA, and nuclease-free water was loaded on a pre-coated 96-well plate containing the primer and probe sets unique to each ARG [88]. Bacterial DNA (Escherichia coli) was included (in triplicate) not only as a reference gene (in wells designated as PPC) but also as a PC to test for the presence of PCR inhibitors and to check the PCR efficiency. In addition, primers and probes associated with PAN bacteria 1 and 3 in the plate served as a second positive control to detect a broad range of bacterial species and confirm the presence of bacterial DNA. A plate with autoclaved nuclease-free water was run as NTC. The cycling conditions were set as initial PCR activation for 10 min at 95 °C followed by 40 cycles of denaturation at 95 °C for 15 s, annealing and extension at 60 °C for 2 min. The PCR was performed on the CFX96TM Deep Well thermal cycler (BioRad). The 2ΔCT method (where ΔCT = CT detected gene − CT 16S rRNA gene) was used to calculate the relative abundances of the detected gene in proportion to the 16S rRNA gene in each sample [44]. Samples exhibiting a CT above 35 and non-specific peaks in the dissociation curve were discarded.
4.5. SmartChipTM HT-qPCR Analysis
The DNA isolated from HI-3 hospital (n = 2 × 6) was lyophilized and shipped to Resistomap Oy, (Helsinki, Finland) for the HT-qPCR analysis through the SmartChip qPCR assay. The DNA samples were resuspended and 2 ng/µL was added to the PCR reaction mix containing 1 × SmartChipTM green gene expression master mix, 300 nM of primers and nuclease-free water. The primers (n = 296) comprised 268 oligos for ARGs (all the above-mentioned classes) and 20 other genes [43]. A total reaction volume of 100 nl was loaded on a SmartChipTM (n = 5184 wells) employing an automated SmartChipTM MultiSample NanoDispenser (Takara Bio, San Jose, CA, USA). PCR was conducted on a SmartChipTM Real-Time PCR System (Takara Bio, San Jose, CA, USA). The cycling conditions included initial enzyme activation at 95 °C for 10 min followed by 40 cycles of denaturation at 95 °C for 30 s and annealing at 60 °C for 30 s. A melt curve analysis was performed using the SmartChipTM qPCR software to exclude false positive data (amplicons with unspecific melting curves or multiple peaks). Samples exhibiting a CT above 27 were discarded. RA of amplified genes was calculated by the 2ΔCT method [44].
4.6. Statistical Analysis
The Kruskal–Wallis H test was employed to calculate the effect size, test power, outliers and R syntax. One-way ANOVA and Tukey’s HSD were also performed to compare the mean number of ARGs in indoor and outdoor aerosols. The gene list, drug classes and their relative abundances (RA) were exported into the web-based ResistoXplorer [89]. The software’s graphical user interface that implements packages in R (v4.1.0) was used [90]. The ggplot2 (3.3.0), gplots (3.03) igraph (1.2.5), virdis (0.5.1) and RColorBrewer (1.1–2) software packages were used for generating the graphs and box plots. The heat maps were created in the pheatmap (1.2.5) program. The Pearson algorithm (p < 0.05) was employed for the correlation pattern search of ARGs [91]. Four alpha diversity parameters (Observed, Chao1, Shannons and Pielou’s indices) accounting for the richness and evenness of genes were estimated [92] and compared by ANOVA. The hierarchical clustering on RA of ARGs was achieved through the WARD algorithm. Ordination analysis (principal coordinate analysis) was performed on Bray Curtis distances between the RA of ARGs applying the permutational analysis of variance (PERMANOVA) [93]. The core resistome analysis was executed on the identified ARGs. The DESeq2 (v1.26.0) was used to predict the differential abundant genes between the experimental parameters [94].
5. Conclusions
To summarize the findings of this study, ARGs were found in indoor and outdoor aerosols and exhibited spatial and seasonal variations. Beta-lactams were the most dominant ARG classes that need immediate attention. ARGs were present in hospital aerosols, hence serious thought needs to be given to regular ARG monitoring as humans are at a great risk owing to inhalation of these ARGs. In addition to biomonitoring, stewardship programs to educate the general people, livestock managers and health care consultants are also advisable to minimize the use of antibiotics to tackle this ever-increasing challenge of ARGs and address the One Health Approach proposed by WHO.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/ijms24076756/s1.
Author Contributions
Conceptualization, N.H. and S.U.; Writing, reviewing and editing, N.H. and S.U.; Resources and sampling, M.B. and S.U.; Laboratory experiments and formal analysis, A.S., F.Z., N.A.R. and M.K.; Software and visualization, N.H. All authors have read and agreed to the published version of the manuscript.
Funding
We thank Kuwait Institute for Scientific Research (KISR) (grants FB160C, FB157C) and Kuwait Foundation for Advancement of Science (KFAS grants PN20-43BO-01 and PR18-14SE-01) for supporting this research.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
All the data related to this article are presented within the manuscript or provided as Supplementary Files.
Acknowledgments
We thank MOH, Kuwait to grant access to the hospital premises during the COVID-19 pandemic.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Kim, D.-W.; Cha, C.-J. Antibiotic resistome from the One-Health perspective: Understanding and controlling antimicrobial resistance transmission. Exp. Mol. Med. 2021, 53, 301–309. [Google Scholar] [CrossRef]
- Zhou, Z.-C.; Shuai, X.-Y.; Lin, Z.-J.; Liu, Y.; Zhu, L.; Chen, H. Prevalence of multi-resistant plasmids in hospital inhalable particulate matter (PM) and its impact on horizontal gene transfer. Environ. Pollut. 2021, 270, 116296. [Google Scholar] [CrossRef]
- Desai, B.H. 14. United Nations Environment Program (UNEP). Yearb. Int. Environ. Law 2017, 28, 498–505. [Google Scholar] [CrossRef]
- World Health Organization. Antimicrobial Resistance: Global Report on Surveillance; World Health Organization: Geneva, Switzerland, 2014. [Google Scholar]
- Xu, H.; Chen, Z.; Huang, R.; Cui, Y.; Li, Q.; Zhao, Y.; Wang, X.; Mao, D.; Luo, Y.; Ren, H. Antibiotic resistance gene-carrying plasmid spreads into the plant endophytic bacteria using soil bacteria as carriers. Environ. Sci. Technol. 2021, 55, 10462–10470. [Google Scholar] [CrossRef] [PubMed]
- Xu, Y.; Li, H.; Shao, Z.; Li, X.; Zheng, X.; Xu, J. Fate of antibiotic resistance genes in farmland soil applied with three different fertilizers during the growth cycle of pakchoi and after harvesting. J. Environ. Manag. 2021, 289, 112576. [Google Scholar] [CrossRef]
- Wu, J.; Wang, J.; Li, Z.; Guo, S.; Li, K.; Xu, P.; Ok, Y.S.; Jones, D.L.; Zou, J. Antibiotics and antibiotic resistance genes in agricultural soils: A systematic analysis. Crit. Rev. Environ. Sci. Technol. 2023, 53, 847–864. [Google Scholar] [CrossRef]
- Habibi, N.; Uddin, S.; Lyons, B.; Al-Sarawi, H.A.; Behbehani, M.; Shajan, A.; Razzack, N.A.; Zakir, F.; Alam, F. Antibiotic resistance genes associated with marine surface sediments: A baseline from the shores of Kuwait. Sustainability 2022, 14, 8029. [Google Scholar] [CrossRef]
- Habibi, N.; Uddin, S.; Al-Sarawi, H.; Aldhameer, A.; Shajan, A.; Zakir, F.; Abdul Razzack, N.; Alam, F. Metagenomes from coastal sediments of Kuwait: Insights into the microbiome, metabolic functions and resistome. Microorganisms 2023, 11, 531. [Google Scholar] [CrossRef] [PubMed]
- Al-Sarawi, H.A.; Najem, A.B.; Lyons, B.P.; Uddin, S.; Al-Sarawi, M.A. Antimicrobial resistance in Escherichia coli isolated from marine sediment samples from Kuwait bay. Sustainability 2022, 14, 11325. [Google Scholar] [CrossRef]
- Amarasiri, M.; Sano, D.; Suzuki, S. Understanding human health risks caused by antibiotic resistant bacteria (ARB) and antibiotic resistance genes (ARG) in water environments: Current knowledge and questions to be answered. Crit. Rev. Environ. Sci. Technol. 2020, 50, 2016–2059. [Google Scholar] [CrossRef]
- Al-Sarawi, H.A.; Jha, A.N.; Al-Sarawi, M.A.; Lyons, B.P. Historic and contemporary contamination in the marine environment of Kuwait: An overview. Mar. Pollut. Bull. 2015, 100, 621–628. [Google Scholar] [CrossRef]
- Al-Sarawi, H.A.; Jha, A.N.; Baker-Austin, C.; Al-Sarawi, M.A.; Lyons, B.P. Baseline screening for the presence of antimicrobial resistance in E. coli isolated from Kuwait’s marine environment. Mar. Pollut. Bull. 2018, 129, 893–898. [Google Scholar] [CrossRef] [PubMed]
- Chakraborty, J.; Sapkale, V.; Rajput, V.; Shah, M.; Kamble, S.; Dharne, M. Shotgun metagenome guided exploration of anthropogenically driven resistomic hotspots within Lonar soda lake of India. Ecotoxicol. Environ. Saf. 2020, 194, 110443. [Google Scholar] [CrossRef] [PubMed]
- Yang, X.; Yan, L.; Yang, Y.; Zhou, H.; Cao, Y.; Wang, S.; Xue, B.; Li, C.; Zhao, C.; Zhang, X. The occurrence and distribution pattern of antibiotic resistance genes and bacterial community in the Ili river. Front. Environ. Sci. 2022, 212, 840428. [Google Scholar] [CrossRef]
- Chen, P.; Guo, X.; Li, F. Antibiotic resistance genes in bioaerosols: Emerging, non-ignorable and pernicious pollutants. J. Clean. Prod. 2022, 348, 131094. [Google Scholar] [CrossRef]
- Habibi, N.; Uddin, S.; Al Salameen, F.; Behbehani, M.; Shirshikhar, F.; Abdul Razzack, N.; Shajan, A.; Hussain, F.Z. Collection of bacterial community associated with size fractionated aerosols from Kuwait. Data 2021, 6, 123. [Google Scholar] [CrossRef]
- Habibi, N.; Uddin, S.; Al Salameen, F.; Al Amad, S.; Kumar, V.; Al Otaibi, M. Identification and Characterization of Novel Corona and Associated Respiratory Viruses in Aerosols; Final Report FB157C; Kuwait Institute for Scientific Research: Safat, Kuwait, 2021. [Google Scholar]
- Habibi, N.; Uddin, S.; Al-Salameen, F.; Al-Amad, S.; Kumar, V.; Al-Otaibi, M.; Razzack, N.A.; Shajan, A.; Shirshikar, F. SARS-CoV-2, other respiratory viruses and bacteria in aerosols: Report from Kuwait’s hospitals. Indoor Air 2021, 31, 1815–1825. [Google Scholar] [CrossRef]
- Habibi, N.; Uddin, S.; Behbehani, M.; Al Salameen, F.; Razzack, N.A.; Zakir, F.; Shajan, A.; Alam, F. Bacterial and fungal communities in indoor aerosols from two Kuwaiti hospitals. Front. Microbiol. 2022, 13, 955913. [Google Scholar] [CrossRef]
- Habibi, N.; Uddin, S.; Behbehani, M.; Abdul Razzack, N.; Hussain, F.Z.; Shajan, A. SARS-CoV-2 in hospital air as revealed by comprehensive respiratory viral panel sequencing. Infect. Prev. Pract. 2022, 4, 100199. [Google Scholar] [CrossRef]
- Xie, J.; Jin, L.; He, T.; Chen, B.; Luo, X.; Feng, B.; Huang, W.; Li, J.; Fu, P.; Li, X. Bacteria and antibiotic resistance genes (ARGs) in PM2. 5 from China: Implications for human exposure. Environ. Sci. Technol. 2018, 53, 963–972. [Google Scholar] [CrossRef]
- He, P.; Wu, Y.; Huang, W.; Wu, X.; Lv, J.; Liu, P.; Bu, L.; Bai, Z.; Chen, S.; Feng, W. Characteristics of and variation in airborne ARGs among urban hospitals and adjacent urban and suburban communities: A metagenomic approach. Environ. Int. 2020, 139, 105625. [Google Scholar] [CrossRef] [PubMed]
- Li, L.; Wang, Q.; Bi, W.; Hou, J.; Xue, Y.; Mao, D.; Das, R.; Luo, Y.; Li, X. Municipal solid waste treatment system increases ambient airborne bacteria and antibiotic resistance genes. Environ. Sci. Technol. 2020, 54, 3900–3908. [Google Scholar] [CrossRef] [PubMed]
- McEachran, A.D.; Blackwell, B.R.; Hanson, J.D.; Wooten, K.J.; Mayer, G.D.; Cox, S.B.; Smith, P.N. Antibiotics, bacteria, and antibiotic resistance genes: Aerial transport from cattle feed yards via particulate matter. Environ. Health Perspect. 2015, 123, 337–343. [Google Scholar] [CrossRef] [PubMed]
- Jin, L.; Xie, J.; He, T.; Wu, D.; Li, X. Airborne transmission as an integral environmental dimension of antimicrobial resistance through the “One Health” lens. Crit. Rev. Environ. Sci. Technol. 2022, 52, 4172–4193. [Google Scholar] [CrossRef]
- Ginn, O.; Lowry, S.; Brown, J. A systematic review of enteric pathogens and antibiotic resistance genes in outdoor urban aerosols. Environ. Res. 2022, 212, 113097. [Google Scholar] [CrossRef]
- Rocha-Melogno, L.; Crank, K.C.; Ginn, O.; Bergin, M.H.; Brown, J.; Gray, G.C.; Hamilton, K.A.; Bibby, K.; Deshusses, M.A. Quantitative microbial risk assessment of outdoor aerosolized pathogens in cities with poor sanitation. Sci. Total Environ. 2022, 827, 154233. [Google Scholar] [CrossRef]
- Liang, Z.; Yu, Y.; Ye, Z.; Li, G.; Wang, W.; An, T. Pollution profiles of antibiotic resistance genes associated with airborne opportunistic pathogens from typical area, Pearl River Estuary and their exposure risk to human. Environ. Int. 2020, 143, 105934. [Google Scholar] [CrossRef]
- Gat, D.; Mazar, Y.; Cytryn, E.; Rudich, Y. Origin-dependent variations in the atmospheric microbiome community in Eastern Mediterranean dust storms. Environ. Sci. Technol. 2017, 51, 6709–6718. [Google Scholar] [CrossRef]
- Li, N.; Liu, C.; Zhang, Z.; Li, H.; Song, T.; Liang, T.; Li, B.; Li, L.; Feng, S.; Su, Q. Research and technological advances regarding the study of the spread of antimicrobial resistance genes and antimicrobial-resistant bacteria related to animal husbandry. Int. J. Environ. Res. Public Health 2019, 16, 4896. [Google Scholar] [CrossRef]
- Brooks, J.; McLaughlin, M.; Scheffler, B.; Miles, D. Microbial and antibiotic resistant constituents associated with biological aerosols and poultry litter within a commercial poultry house. Sci. Total Environ. 2010, 408, 4770–4777. [Google Scholar] [CrossRef]
- Xie, J.; Jin, L.; Wu, D.; Pruden, A.; Li, X. Inhalable antibiotic resistome from wastewater treatment plants to urban areas: Bacterial hosts, dissemination risks, and source contributions. Environ. Sci. Technol. 2022, 56, 7040–7051. [Google Scholar] [CrossRef] [PubMed]
- Zhou, Z.-C.; Liu, Y.; Lin, Z.-J.; Shuai, X.-Y.; Zhu, L.; Xu, L.; Meng, L.-X.; Sun, Y.-J.; Chen, H. Spread of antibiotic resistance genes and microbiota in airborne particulate matter, dust, and human airways in the urban hospital. Environ. Int. 2021, 153, 106501. [Google Scholar] [CrossRef] [PubMed]
- Zhang, T.; Li, X.; Wang, M.; Chen, H.; Yang, Y.; Chen, Q.-l.; Yao, M. Time-resolved spread of antibiotic resistance genes in highly polluted air. Environ. Int. 2019, 127, 333–339. [Google Scholar] [CrossRef] [PubMed]
- Zhen, Q.; Deng, Y.; Wang, Y.; Wang, X.; Zhang, H.; Sun, X.; Ouyang, Z. Meteorological factors had more impact on airborne bacterial communities than air pollutants. Sci. Total Environ. 2017, 601, 703–712. [Google Scholar] [CrossRef] [PubMed]
- Lee, G.; Yoo, K. A review of the emergence of antibiotic resistance in bioaerosols and its monitoring methods. Rev. Environ. Sci. Bio/Technol. 2022, 21, 799–827. [Google Scholar] [CrossRef]
- Franklin, A.; Brinkman, N.; Jahne, M.; Keely, S. Twenty-first century molecular methods for analyzing antimicrobial resistance in surface waters to support One Health assessments. J. Microbiol. Methods 2021, 184, 106174. [Google Scholar] [CrossRef]
- Yadav, B.; Tyagi, R. Development of molecular methods to detect and control emerging drug-resistance pathogens. In Current Developments in Biotechnology and Bioengineering; Elsevier: Amsterdam, The Netherlands, 2020; pp. 377–419. [Google Scholar]
- Luby, E.; Ibekwe, A.M.; Zilles, J.; Pruden, A. Molecular methods for assessment of antibiotic resistance in agricultural ecosystems: Prospects and challenges. J. Environ. Qual. 2016, 45, 441–453. [Google Scholar] [CrossRef]
- Waseem, H.; Jameel, S.; Ali, J.; Saleem Ur Rehman, H.; Tauseef, I.; Farooq, U.; Jamal, A.; Ali, M.I. Contributions and challenges of high throughput qPCR for determining antimicrobial resistance in the environment: A critical review. Molecules 2019, 24, 163. [Google Scholar] [CrossRef]
- Waseem, H.; ur Rehman, H.S.; Ali, J.; Iqbal, M.J.; Ali, M.I. Global trends in ARGs measured by HT-qPCR platforms. In Antibiotics and Antimicrobial Resistance Genes in the Environment; Elsevier: Amsterdam, The Netherlands, 2020; pp. 206–222. [Google Scholar]
- Lai, F.Y.; Muziasari, W.; Virta, M.; Wiberg, K.; Ahrens, L. Profiles of environmental antibiotic resistomes in the urban aquatic recipients of Sweden using high-throughput quantitative PCR analysis. Environ. Pollut. 2021, 287, 117651. [Google Scholar] [CrossRef]
- Muziasari, W.I.; Pitkänen, L.K.; Sørum, H.; Stedtfeld, R.D.; Tiedje, J.M.; Virta, M. Corrigendum: The resistome of farmed fish feces contributes to the enrichment of antibiotic resistance genes in sediments below Baltic Sea fish farms. Front. Microbiol. 2017, 8, 1491. [Google Scholar] [CrossRef]
- Owayed, A.F.; Husain, E.H.; Al-Khabaz, A.; Al-Qattan, H.Y.; Al-Shammari, N. Epidemiology and clinical presentation of pandemic influenza A (H1N1) among hospitalized children in Kuwait. Med. Princ. Pract. 2012, 21, 254–258. [Google Scholar] [CrossRef] [PubMed]
- Gupta, M.; Lee, S.; Bisesi, M.; Lee, J. Indoor microbiome and antibiotic resistance on floor surfaces: An exploratory study in three different building types. Int. J. Environ. Res. Public Health 2019, 16, 4160. [Google Scholar] [CrossRef]
- Perkins, S.D.; Mayfield, J.; Fraser, V.; Angenent, L.T. Potentially pathogenic bacteria in shower water and air of a stem cell transplant unit. Appl. Environ. Microbiol. 2009, 75, 5363–5372. [Google Scholar] [CrossRef]
- Prussin, A.J.; Garcia, E.B.; Marr, L.C. Total concentrations of virus and bacteria in indoor and outdoor air. Environ. Sci. Technol. Lett. 2015, 2, 84–88. [Google Scholar] [CrossRef] [PubMed]
- Habibi, N.; Behbehani, M.; Uddin, S.; AL Salameen, F.; Shajan, A.; Zakir, F. A safe and effective sample collection method for assessment of SARS-CoV-2 in aerosol samples. In Environmental Resilience and Transformation in Times of COVID-19; Ramanathan, A.L., Chidambaram, S., Jonathan, M.P., Munoz-Arriola, F., Prasanna, M.V., Kumar, P., Eds.; Elsevier: Amsterdam, The Netherlands, 2021. [Google Scholar]
- Uddin, S.; Habibi, N.; Fowler, S.W.; Behbehani, M.; Gevao, B.; Faizuddin, M.; Gorgun, A.U. Aerosols as vectors for contaminants: A perspective based on outdoor aerosol data from Kuwait. Atmosphere 2023, 14, 470. [Google Scholar] [CrossRef]
- Ma, L.; Yabo, S.D.; Lu, L.; Jiang, J.; Meng, F.; Qi, H. Seasonal variation characteristics of inhalable bacteria in bioaerosols and antibiotic resistance genes in Harbin. J. Hazard. Mater. 2023, 446, 130597. [Google Scholar] [CrossRef]
- Behbehani, M.; Uddin, S.; Habibi, N.; Al Salameen, F.; Sajid, S.; Abdulrazzack, N.; Zakir, F.; Shirshikhar, F. 210Po in Ultrafine Aerosol Particles and Its Likelihood to Mutate the Microbial Community.; Final Report FB160C; Kuwait Institute for Scientific Research: Safat, Kuwait, 2021. [Google Scholar]
- Al Salameen, F.; Habibi, N.; Uddin, S.; Al Mataqi, K.; Kumar, V.; Al Doaij, B.; Al Amad, S.; Al Ali, E.; Shirshikhar, F. Spatio-temporal variations in bacterial and fungal community associated with dust aerosol in Kuwait. PLoS ONE 2020, 15, e0241283. [Google Scholar] [CrossRef]
- Al-Salameen, F.; Habibi, N.; Uddin, S.; Al-Mataqi, K.; Al-Doaij, B.; Al-Amad, S.; Al-Ali, E. Characterization and Identification of Microorganisms Associated with Airborne Dust in Kuwait; Final Report EM075C; Kuwait Institute for Scientific Research: Safat, Kuwait, 2021. [Google Scholar]
- Behbehani, M.; Carvalho, F.P.; Uddin, S.; Habibi, N. Enhanced polonium concentrations in aerosols from the Gulf oil producing region and the role of microorganisms. Int. J. Environ. Res. Public Health 2021, 18, 13309. [Google Scholar] [CrossRef]
- Cao, C.; Jiang, W.; Wang, B.; Fang, J.; Lang, J.; Tian, G.; Jiang, J.; Zhu, T.F. Inhalable microorganisms in Beijing’s PM2. 5 and PM10 pollutants during a severe smog event. Environ. Sci. Technol. 2014, 48, 1499–1507. [Google Scholar] [CrossRef]
- Prussin, A.J.; Marr, L.C. Sources of airborne microorganisms in the built environment. Microbiome 2015, 3, 78. [Google Scholar] [CrossRef]
- Zhao, Y.; Wang, Q.; Chen, Z.; Mao, D.; Luo, Y. Significant higher airborne antibiotic resistance genes and the associated inhalation risk in the indoor than the outdoor. Environ. Pollut. 2021, 268, 115620. [Google Scholar] [CrossRef] [PubMed]
- Adams, R.I.; Bateman, A.C.; Bik, H.M.; Meadow, J.F. Microbiota of the indoor environment: A meta-analysis. Microbiome 2015, 3, 49. [Google Scholar] [CrossRef] [PubMed]
- Gilbert, Y.; Veillette, M.; Duchaine, C. Airborne bacteria and antibiotic resistance genes in hospital rooms. Aerobiologia 2010, 26, 185–194. [Google Scholar] [CrossRef]
- Miletto, M.; Lindow, S.E. Relative and contextual contribution of different sources to the composition and abundance of indoor air bacteria in residences. Microbiome 2015, 3, 61. [Google Scholar] [CrossRef]
- Chen, M.; Qiu, T.; Sun, Y.; Song, Y.; Wang, X.; Gao, M. Diversity of tetracycline-and erythromycin-resistant bacteria in aerosols and manures from four types of animal farms in China. Environ. Sci. Pollut. Res. 2019, 26, 24213–24222. [Google Scholar] [CrossRef] [PubMed]
- Gevao, B.; Uddin, S.; Krishnan, D.; Rajagopalan, S.; Habibi, N. Antibiotics in wastewater: Baseline of the influent and effluent Streams in Kuwait. Toxics 2022, 10, 174. [Google Scholar] [CrossRef]
- Yang, T.; Jiang, L.; Bi, X.; Cheng, L.; Zheng, X.; Wang, X.; Zhou, X. Submicron aerosols share potential pathogens and antibiotic resistomes with wastewater or sludge. Sci. Total Environ. 2022, 821, 153521. [Google Scholar] [CrossRef] [PubMed]
- Wei, K.; Zou, Z.; Zheng, Y.; Li, J.; Shen, F.; Wu, C.-Y.; Wu, Y.; Hu, M.; Yao, M. Ambient bioaerosol particle dynamics observed during haze and sunny days in Beijing. Sci. Total Environ. 2016, 550, 751–759. [Google Scholar] [CrossRef]
- Waśko, I.; Kozińska, A.; Kotlarska, E.; Baraniak, A. Clinically Relevant β-Lactam Resistance Genes in Wastewater Treatment Plants. Int. J. Environ. Res. Public Health 2022, 19, 13829. [Google Scholar] [CrossRef]
- Tooke, C.L.; Hinchliffe, P.; Bragginton, E.C.; Colenso, C.K.; Hirvonen, V.H.; Takebayashi, Y.; Spencer, J. β-Lactamases and β-Lactamase Inhibitors in the 21st Century. J. Mol. Biol. 2019, 431, 3472–3500. [Google Scholar] [CrossRef]
- Pongchaikul, P.; Mongkolsuk, P. Comprehensive Analysis of Imipenemase (IMP)-Type Metallo-β-Lactamase: A global distribution threatening Asia. Antibiotics 2022, 11, 236. [Google Scholar] [CrossRef] [PubMed]
- Philippon, A.; Slama, P.; Dény, P.; Labia, R. A structure-based classification of class A β-lactamases, a broadly diverse family of enzymes. Clin. Microbiol. Rev. 2016, 29, 29–57. [Google Scholar] [CrossRef] [PubMed]
- Evans, B.A.; Amyes, S.G. OXA β-lactamases. Clin. Microbiol. Rev. 2014, 27, 241–263. [Google Scholar] [CrossRef] [PubMed]
- Kunhikannan, S.; Thomas, C.J.; Franks, A.E.; Mahadevaiah, S.; Kumar, S.; Petrovski, S. Environmental hotspots for antibiotic resistance genes. Microbiologyopen 2021, 10, e1197. [Google Scholar] [CrossRef] [PubMed]
- Lautenbach, E.; Marsicano, R.; Tolomeo, P.; Heard, M.; Serrano, S.; Stieritz, D.D. Epidemiology of Gram-negative antimicrobial resistance in a multi-state network of long-term care facilities. Infect. Control Hosp. Epidemiol. Off. J. Soc. Hosp. Epidemiol. Am. 2009, 30, 790. [Google Scholar] [CrossRef]
- Habibi, N.; Mustafa, A.S.; Khan, M.W. Composition of nasal bacterial community and its seasonal variation in health care workers stationed in a clinical research laboratory. PLoS ONE 2021, 16, e0260314. [Google Scholar] [CrossRef]
- Verhoeven, P.O.; Gagnaire, J.; Botelho-Nevers, E.; Grattard, F.; Carricajo, A.; Lucht, F.; Pozzetto, B.; Berthelot, P. Detection and clinical relevance of Staphylococcus aureus nasal carriage: An update. Expert Rev. Anti-Infect. Ther. 2014, 12, 75–89. [Google Scholar] [CrossRef]
- Reyes, N.; Montes, O.; Figueroa, S.; Tiwari, R.; Sollecito, C.C.; Emmerich, R.; Usyk, M.; Geliebter, J.; Burk, R.D. Staphylococcus aureus nasal carriage and microbiome composition among medical students from Colombia: A cross-sectional study. F1000Research 2020, 9, 78. [Google Scholar] [CrossRef]
- Habibi, N.; Uddin, S.; Fowler, S.W.; Behbehani, M. Microplastics in the atmosphere: A review. J. Environ. Expo. Assess. 2022, 1, 6. [Google Scholar] [CrossRef]
- Habibi, N.; Uddin, S.; Al-Salameen, F.; Al-Amad, S.; Abdul Razzack, N.; Shajan, A. Evidences of airborne spread of SARS CoV2 in indoor air. In Proceedings of the Scientific Poster Day 2020–2021; Kuwait University: Kuwait City, Kuwait, 2021. [Google Scholar]
- Uddin, S.; Al-Ghadban, A.; Gevao, B.; Al-Shamroukh, D.; Al-Khabbaz, A. Estimation of suspended particulate matter in Gulf using MODIS data. Aquat. Ecosyst. Health Manag. 2012, 15, 41–44. [Google Scholar] [CrossRef]
- Uddin, S.; Fowler, S.W.; Habibi, N.; Sajid, S.; Dupont, S.; Behbehani, M. A preliminary assessment of size-fractionated microplastics in indoor aerosol—Kuwait’s baseline. Toxics 2022, 10, 71. [Google Scholar] [CrossRef] [PubMed]
- Gevao, B.; Al-Bahloul, M.; Zafar, J.; Al-Matrouk, K.; Helaleh, M. Polycyclic aromatic hydrocarbons in indoor air and dust in Kuwait: Implications for sources and nondietary human exposure. Arch. Environ. Contam. Toxicol. 2007, 53, 503–512. [Google Scholar] [CrossRef] [PubMed]
- Gevao, B.; Uddin, S.; Al-Bahloul, M.; Al-Mutairi, A. Persistent organic pollutants on human and sheep hair and comparison with POPs in indoor and outdoor air. J. Environ. Expo. Assess. 2022, 1, 5. [Google Scholar] [CrossRef]
- Madi, N.; Al-Nakib, W.; Mustafa, A.S.; Habibi, N. Metagenomic analysis of viral diversity in respiratory samples from patients with respiratory tract infections in Kuwait. J. Med. Virol. 2018, 90, 412–420. [Google Scholar] [CrossRef] [PubMed]
- Zaheer, R.; Noyes, N.; Ortega Polo, R.; Cook, S.R.; Marinier, E.; Van Domselaar, G.; Belk, K.E.; Morley, P.S.; McAllister, T.A. Impact of sequencing depth on the characterization of the microbiome and resistome. Sci. Rep. 2018, 8, 5890. [Google Scholar] [CrossRef] [PubMed]
- Núñez, A.; de Paz, G.A.; Rastrojo, A.; Ferencova, Z.; Gutiérrez-Bustillo, A.M.; Alcamí, A.; Moreno, D.A.; Guantes, R. Temporal patterns of variability for prokaryotic and eukaryotic diversity in the urban air of Madrid (Spain). Atmos. Environ. 2019, 217, 116972. [Google Scholar] [CrossRef]
- Tignat-Perrier, R.; Dommergue, A.; Thollot, A.; Magand, O.; Amato, P.; Joly, M.; Sellegri, K.; Vogel, T.M.; Larose, C. Seasonal shift in airborne microbial communities. Sci. Total Environ. 2020, 716, 137129. [Google Scholar] [CrossRef]
- Gevao, B.; Uddin, S.; Dupont, S. Baseline concentrations of pharmaceuticals in Kuwait’s coastal marine environment. Mar. Pollut. Bull. 2021, 173, 113040. [Google Scholar] [CrossRef]
- Alper, E.; Elkilani, A.; Bouhamra, W. Estimation of air exchange rates in Kuwaiti residential buildings. In Proceedings of the 20th AIVC and Indoor Air 99 Conference “Ventilation and Indoor Air Quality in Buildings, Edinburgh, Scotland, 9–13 August 1999. [Google Scholar]
- Gaviria-Figueroa, A.; Preisner, E.C.; Hoque, S.; Feigley, C.E.; Norman, R.S. Emission and dispersal of antibiotic resistance genes through bioaerosols generated during the treatment of municipal sewage. Sci. Total Environ. 2019, 686, 402–412. [Google Scholar] [CrossRef]
- Dhariwal, A.; Junges, R.; Chen, T.; Petersen, F.C. ResistoXplorer: A web-based tool for visual, statistical and exploratory data analysis of resistome data. NAR Genom. Bioinform. 2021, 3, lqab018. [Google Scholar] [CrossRef]
- Schauberger, P.; Walker, A.; Braglia, L.; Sturm, J.; Garbuszus, J.M.; Barbone, J.M. Openxlsx: Read, Write and Edit XLSX Files. R Package Rersion 4.1.0. 2018. Available online: https://CRAN.R-project.org/package=openxlsx (accessed on 25 March 2023).
- Cohen, I.; Huang, Y.; Chen, J.; Benesty, J. Pearson correlation coefficient. In Noise Reduction in Speech Processing; Springer: Berlin/Heidelberg, Germany, 2009; pp. 1–4. [Google Scholar]
- Bengtsson-Palme, J. The diversity of uncharacterized antibiotic resistance genes can be predicted from known gene variants—But not always. Microbiome 2018, 6, 125. [Google Scholar] [CrossRef] [PubMed]
- Anderson, M.J.; Walsh, D.C. PERMANOVA, ANOSIM, and the Mantel test in the face of heterogeneous dispersions: What null hypothesis are you testing? Ecol. Monogr. 2013, 83, 557–574. [Google Scholar] [CrossRef]
- Love, M.I.; Huber, W.; Anders, S. Moderated estimation of fold change and dispersion for RNA-seq data with DESeq2. Genome Biol. 2014, 15, 550. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).