STEAP1 Knockdown Decreases the Sensitivity of Prostate Cancer Cells to Paclitaxel, Docetaxel and Cabazitaxel
Abstract
1. Introduction
2. Results
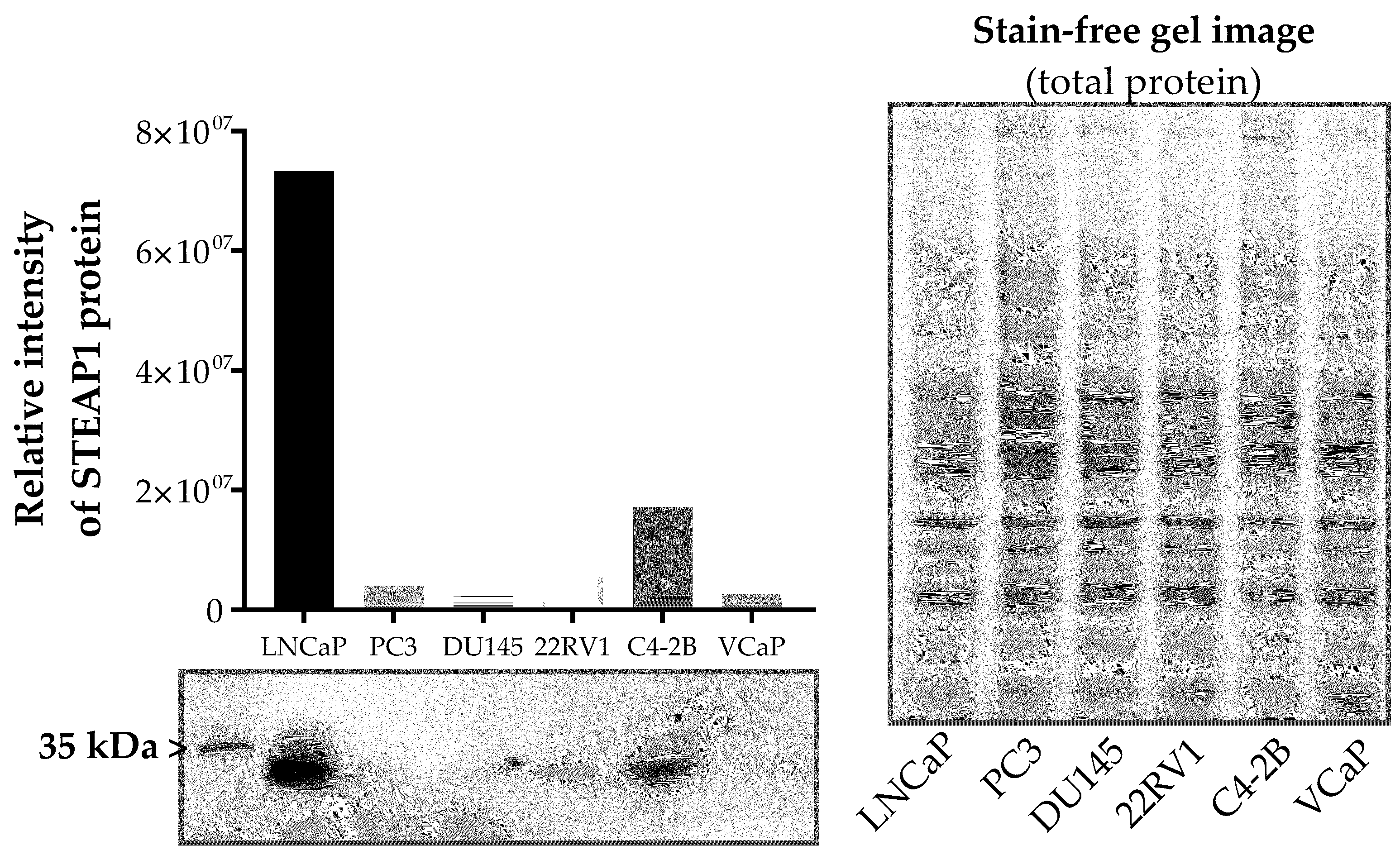
2.1. Effect of Paclitaxel, Docetaxel and Cabazitaxel on STEAP1 Expression in PCa Cells
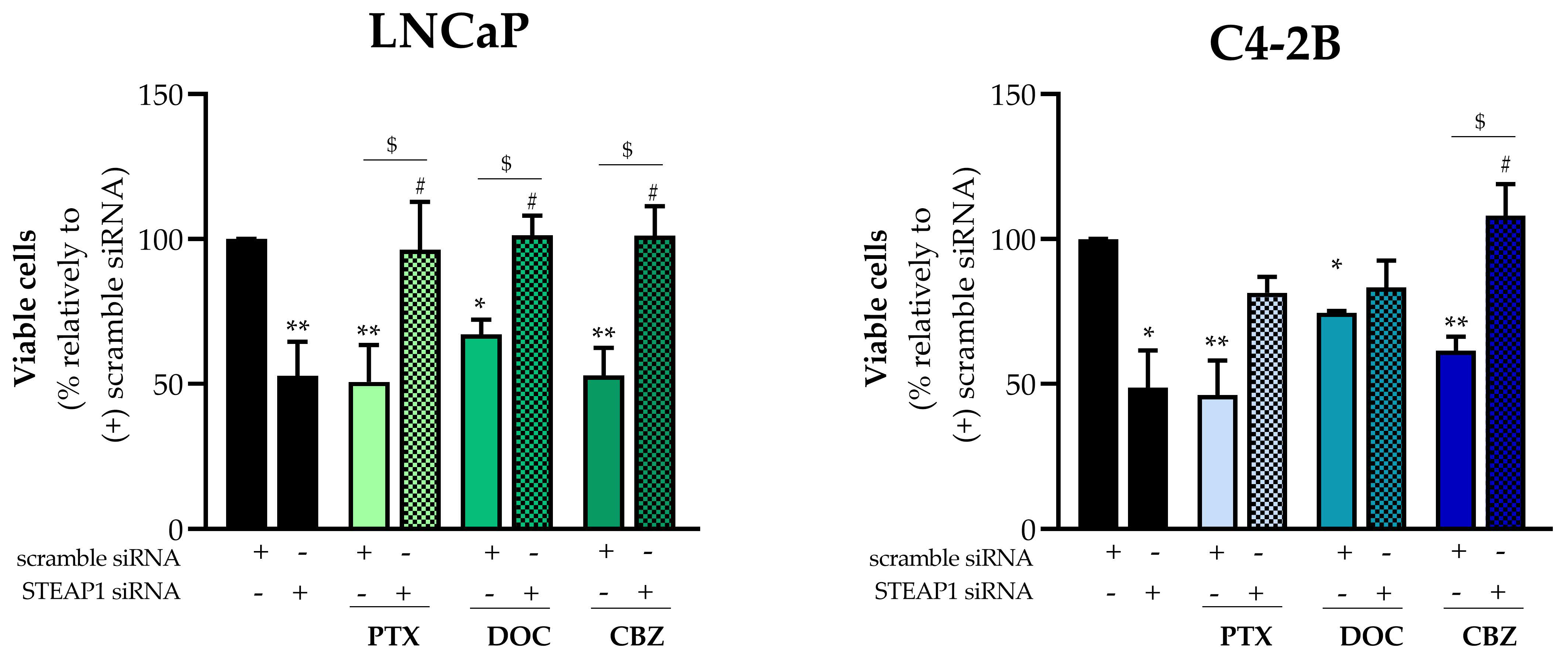
2.2. Effect of STEAP1 Gene Knockdown Associated with Taxane-Based Drugs on PCa Cells Viability
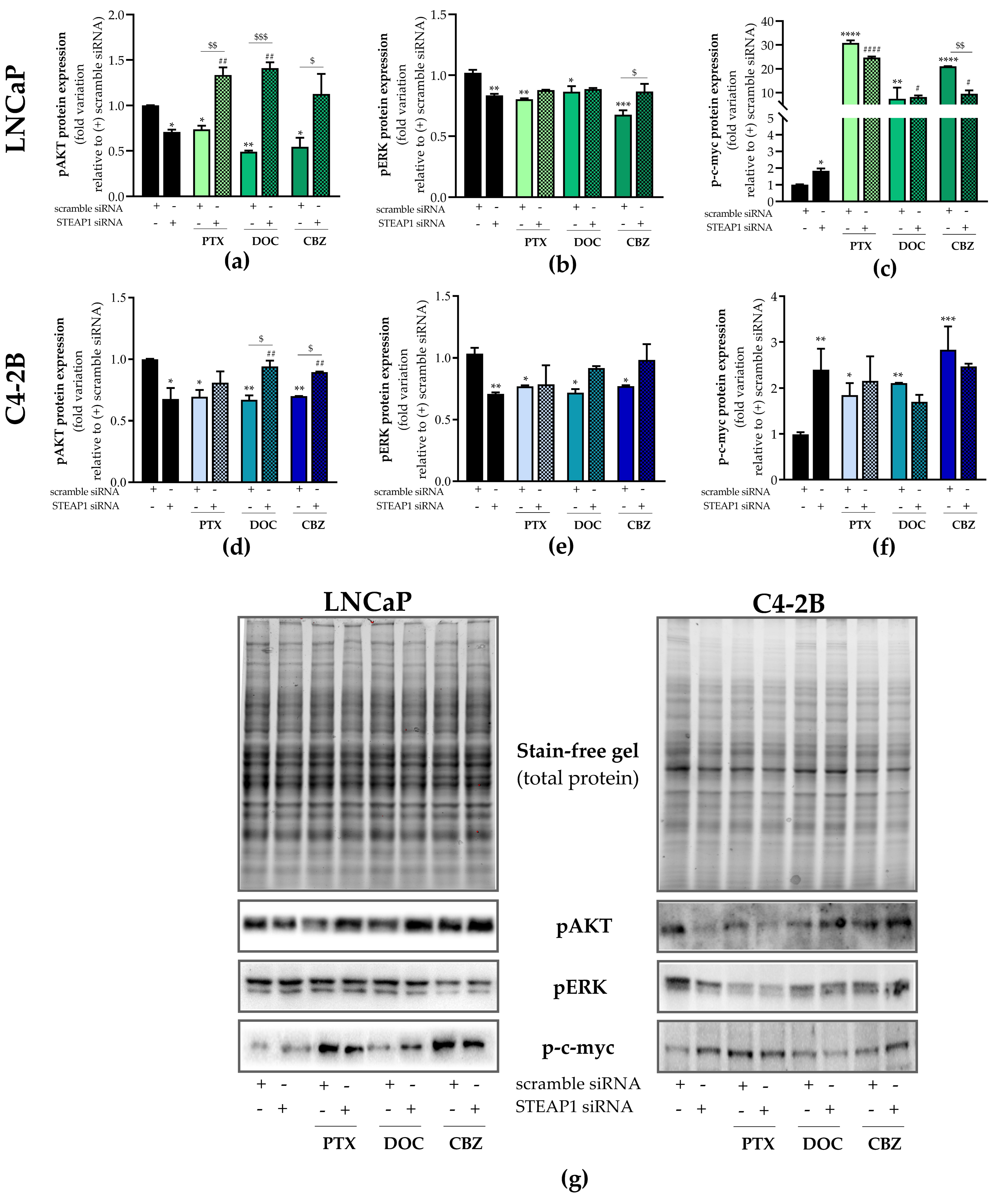
2.3. Effect of STEAP1 Knockdown and Chemotherapeutic Drugs in Survival Pathways
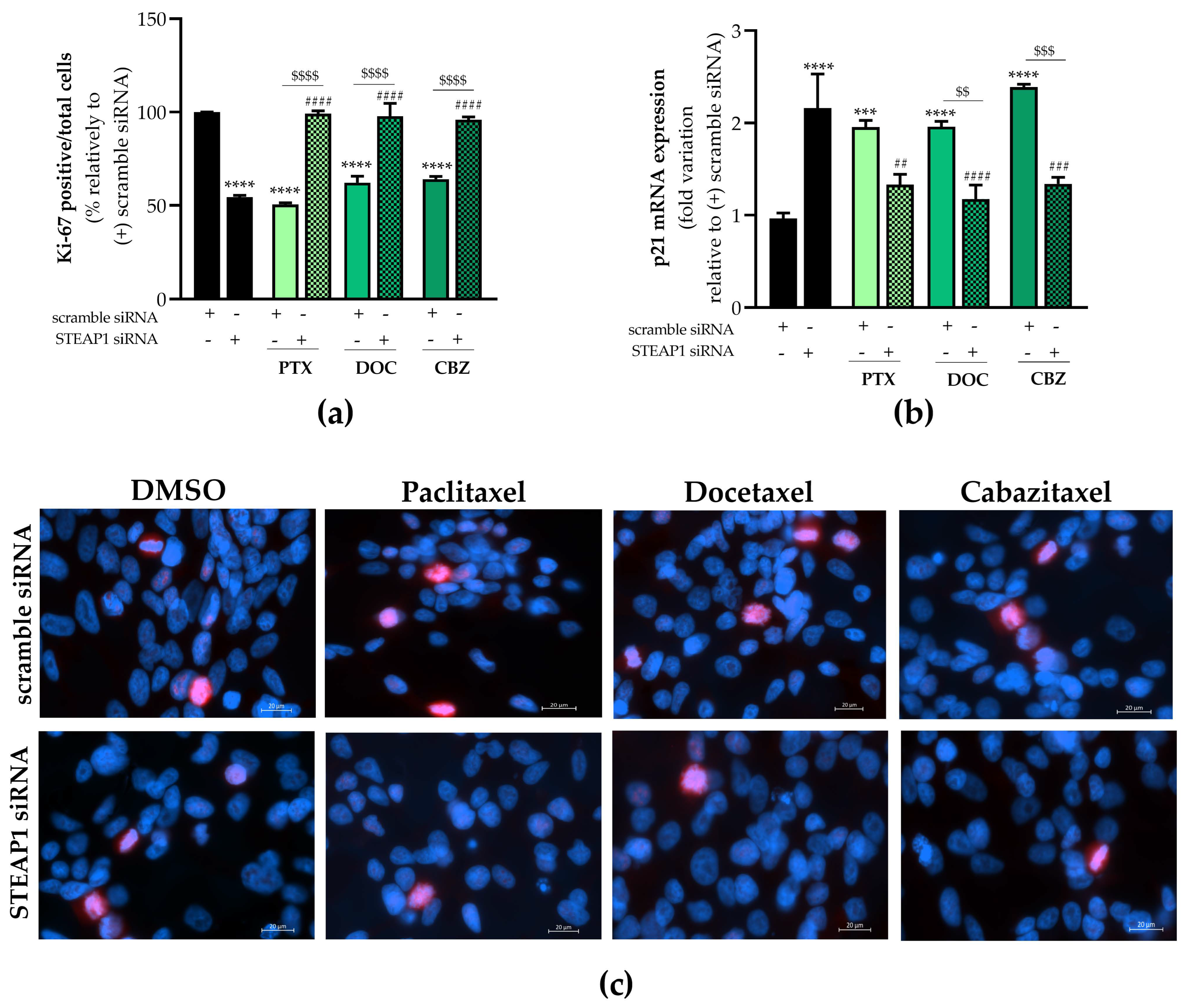
2.4. Effect of STEAP1 Knockdown and Chemotherapeutic Drugs in Proliferative Activity
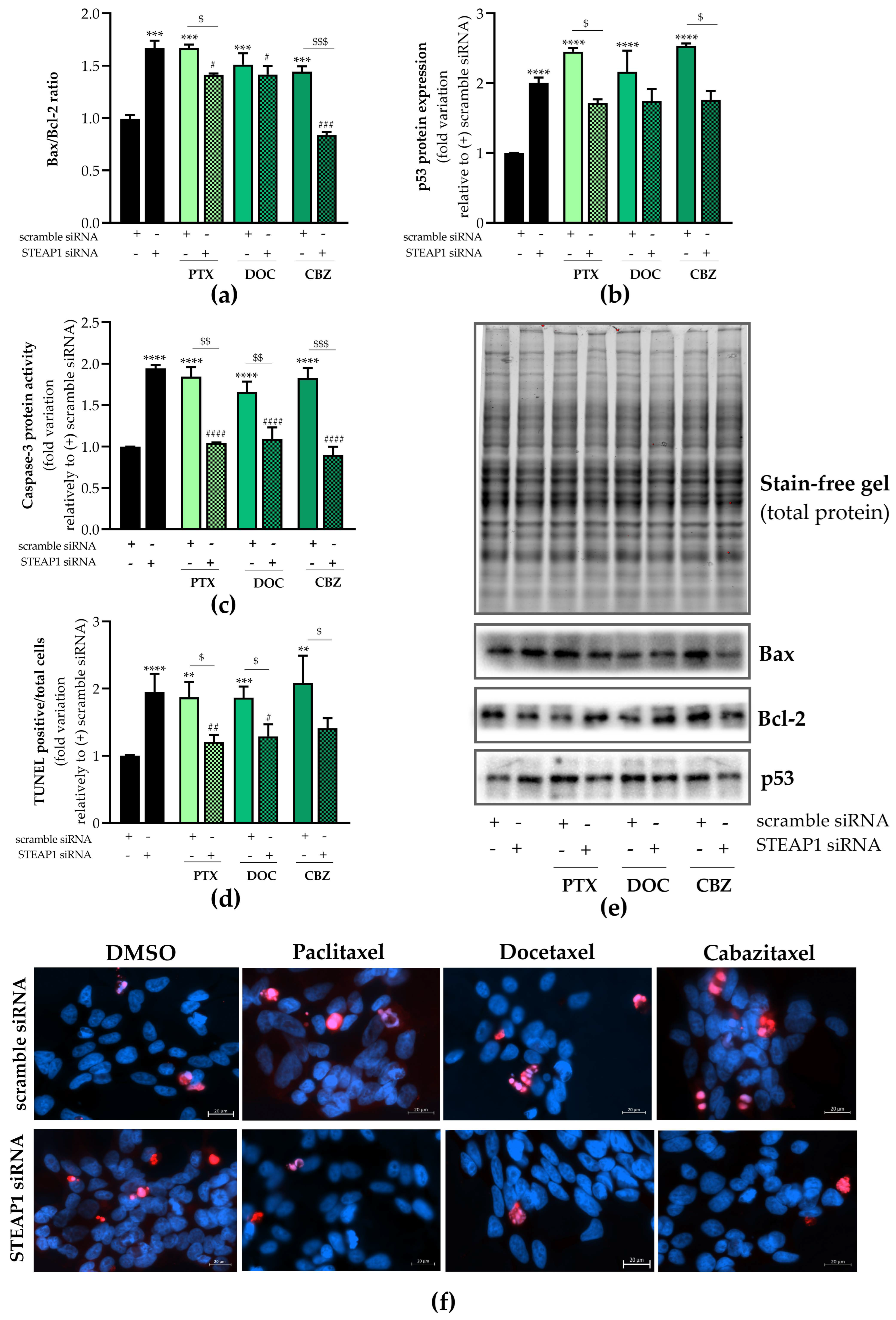
2.5. Effect of STEAP1 Knockdown and Chemotherapeutic Drugs in Apoptosis
3. Discussion
4. Materials and Methods
4.1. Cell line and Culture Conditions
4.2. Small-Interfering RNA Transfection and Treatments
4.3. Reverse Transcription Real-Time Quantitative Polymerase Chain Reaction (RT-qPCR)
4.4. Protein Extraction and Western Blot
4.5. Cell Viability Assay
4.6. Ki-67 Fluorescent Immunocytochemistry
4.7. Terminal Deoxynucleotidyl Transferase-Mediated dUTP Nick End Labeling (TUNEL) Assay
4.8. Caspase-3-like Activity Assay
4.9. Statistical Analysis
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Hubert, R.S.; Vivanco, I.; Chen, E.; Rastegar, S.; Leong, K.; Mitchell, S.C.; Madraswala, R.; Zhou, Y.; Kuo, J.; Raitano, A.B.; et al. STEAP: A prostate-specific cell-surface antigen highly expressed in human prostate tumors. Proc. Natl. Acad. Sci. USA 1999, 96, 14523. [Google Scholar] [CrossRef]
- Challita-Eid, P.; Morrison, K.J.; Etessami, S.; An, Z.; Morrison, K.J.; Perez-Villar, J.J.; Raitano, A.B.; Jia, X.-C.; Gudas, J.M.; Kanner, S.B.; et al. Monoclonal Antibodies to Six-Transmembrane Epithelial Antigen of the Prostate-1 Inhibit Intercellular Communication In vitro and Growth of Human Tumor Xenografts In vivo. Cancer Res. 2007, 67, 5798–5805. [Google Scholar] [CrossRef]
- Ihlaseh-Catalano, S.M.; Drigo, S.A.; de Jesus, C.M.; Domingues, M.A.; Trindade Filho, J.C.; de Camargo, J.L.; Rogatto, S.R. STEAP1 protein overexpression is an independent marker for biochemical recurrence in prostate carcinoma. Histopathology 2013, 63, 678–685. [Google Scholar] [CrossRef] [PubMed]
- Gomes, I.M.; Arinto, P.; Lopes, C.; Santos, C.R.; Maia, C.J. STEAP1 is overexpressed in prostate cancer and prostatic intraepithelial neoplasia lesions, and it is positively associated with Gleason scor. Urol. Oncol. Semin. Orig. Investig. 2014, 32, 53.e23–53.e29. [Google Scholar]
- Gomes, I.M.; Maia, C.J.; Santos, C.R. STEAP proteins: From structure to applications in cancer therapy. Mol. Cancer Res. 2012, 10, 573–587. [Google Scholar] [CrossRef]
- Chen, W.-J.; Wu, H.-T.; Li, C.-L.; Lin, Y.-K.; Fang, Z.-X.; Lin, W.-T.; Liu, J. Regulatory Roles of Six-Transmembrane Epithelial Antigen of the Prostate Family Members in the Occurrence and Development of Malignant Tumors. Front. Cell Dev. Biol. 2021, 9, 752426. [Google Scholar] [CrossRef]
- Ohgami, R.S.; Campagna, D.R.; McDonald, A.; Fleming, M.D. The Steap proteins are metalloreductases. Blood 2006, 108, 1388–1394. [Google Scholar] [CrossRef]
- Liang, Y.; Xing, X.; Beamer, M.A.; Swindell, W.R.; Sarkar, M.K.; Roberts, L.W.; Voorhees, J.J.; Kahlenberg, J.M.; Harms, P.W.; Johnston, A.; et al. Six-transmembrane epithelial antigens of the prostate comprise a novel inflammatory nexus in patients with pustular skin disorders. J. Allergy Clin. Immunol. 2017, 139, 1217–1227. [Google Scholar] [CrossRef] [PubMed]
- Liao, Y.; Zhao, J.; Bulek, K.; Tang, F.; Chen, X.; Cai, G.; Jia, S.; Fox, P.L.; Huang, E.; Pizarro, T.T.; et al. Inflammation mobilizes copper metabolism to promote colon tumorigenesis via an IL-17-STEAP4-XIAP axis. Nat. Commun. 2020, 11, 900. [Google Scholar] [CrossRef]
- Grunewald, T.G.P.; Diebold, I.; Esposito, I.; Plehm, S.; Hauer, K.; Thiel, U.; da Silva-Buttkus, P.; Neff, F.; Unland, R.; Müller-Tidow, C.; et al. STEAP1 is associated with the invasive and oxidative stress phenotype of Ewing tumors. Mol. Cancer Res. 2012, 10, 52–65. [Google Scholar] [CrossRef]
- Gomes, I.M.; Rocha, S.M.; Gaspar, C.; Alvelos, M.I.; Santos, C.R.; Socorro, S.; Maia, C.J. Knockdown of STEAP1 inhibits cell growth and induces apoptosis in LNCaP prostate cancer cells counteracting the effect of androgens. Med. Oncol. 2018, 35, 40. [Google Scholar] [CrossRef]
- Iijima, K.; Nakamura, H.; Takada, K.; Hayasaka, N.; Kubo, T.; Umeyama, Y.; Iyama, S.; Miyanishi, K.; Kobune, M.; Kato, J. Six-transmembrane epithelial antigen of the prostate 1 accelerates cell proliferation by targeting c-Myc in liver cancer cells. Oncol. Lett. 2021, 22, 546. [Google Scholar] [CrossRef] [PubMed]
- Jiang, J.N.; Wu, Y.Y.; Fang, X.D.; Ji, F.J. EIF4E regulates STEAP1 expression in peritoneal metastasis. J. Cancer 2020, 11, 990–996. [Google Scholar] [CrossRef] [PubMed]
- Jiao, Z.; Huang, L.; Sun, J.; Xie, J.; Wang, T.; Yin, X.; Zhang, H.; Chen, J. Six-transmembrane epithelial antigen of the prostate 1 expression promotes ovarian cancer metastasis by aiding progression of epithelial-to-mesenchymal transition. Histochem. Cell Biol. 2020, 154, 215–230. [Google Scholar] [CrossRef]
- Huo, S.-F.; Shang, W.-L.; Yu, M.; Ren, X.-P.; Wen, H.-X.; Chai, C.-Y.; Sun, L.; Hui, K.; Liu, L.-H.; Wei, S.-H. STEAP1 facilitates metastasis and epithelial-mesenchymal transition of lung adenocarcinoma via the JAK2/STAT3 signaling pathway. Biosci. Rep. 2020, 40, BSR20193169. [Google Scholar] [CrossRef]
- Zhang, Z.; Hou, W.B.; Zhang, C.; Tan, Y.E.; Zhang, D.D.; An, W.; Pan, S.W.; Wu, W.D.; Chen, Q.C.; Xu, H.M. A research of STEAP1 regulated gastric cancer cell proliferation, migration and invasion in vitro and in vivos. J. Cell. Mol. Med. 2020, 24, 14217–14230. [Google Scholar] [CrossRef]
- Barroca-Ferreira, J.; Pais, J.P.; Santos, M.M.; Goncalves, A.M.; Gomes, I.M.; Sousa, I.; Rocha, S.M.; Passarinha, L.A.; Maia, C.J. Targeting STEAP1 Protein in Human Cancer: Current Trends and Future Challenges. Curr. Cancer Drug Targets 2018, 18, 222–230. [Google Scholar] [CrossRef] [PubMed]
- Rocha, S.M.; Socorro, S.; Passarinha, L.A.; Maia, C.J. Comprehensive Landscape of STEAP Family Members Expression in Human Cancers: Unraveling the Potential Usefulness in Clinical Practice Using Integrated Bioinformatics Analysis. Data 2022, 7, 64. [Google Scholar] [CrossRef]
- Nakamura, H.; Maeda, H. Cancer Chemotherapy. Fundam. Pharm. Nanosci. 2022, 401–427. Available online: https://www.ncbi.nlm.nih.gov/books/NBK564367/ (accessed on 24 June 2022).
- Yadav, A.; Singh, S.; Sohi, H.; Dang, S. Advances in Delivery of Chemotherapeutic Agents for Cancer Treatment. AAPS PharmSciTech 2021, 23, 25. [Google Scholar] [CrossRef]
- Huebner, N.A.; Shariat, S.F.; Resch, I.; Gust, K.; Kramer, G. The role of taxane-based chemotherapy in the treatment of prostate cancer. Curr. Opin. Urol. 2020, 30, 527–533. [Google Scholar] [CrossRef]
- Škubník, J.; Pavlíčková, V.; Ruml, T.; Rimpelová, S. Current Perspectives on Taxanes: Focus on Their Bioactivity, Delivery and Combination Therapy. Plants 2021, 10, 569. [Google Scholar] [CrossRef] [PubMed]
- Long, H.J. Paclitaxel (Taxol): A novel anticancer chemotherapeutic drug. Mayo Clin. Proc. 1994, 69, 341–345. [Google Scholar] [CrossRef] [PubMed]
- Pienta, K.J. Preclinical mechanisms of action of docetaxel and docetaxel combinations in prostate cancer. Semin. Oncol. 2001, 28 (Suppl. 15), 3–7. [Google Scholar] [CrossRef] [PubMed]
- Watanabe, H.; Kawakami, A.; Sato, R.; Watanabe, K.; Matsushita, Y.; Miyake, H. Molecular Mechanism Mediating Cytotoxic Activity of Cabazitaxel in Docetaxel-resistant Human Prostate Cancer Cells. Anticancer Res. 2021, 41, 3753–3758. [Google Scholar] [CrossRef] [PubMed]
- Cevik, O.; Acidereli, H.; Turut, F.A.; Yildirim, S.; Acilan, C. Cabazitaxel exhibits more favorable molecular changes compared to other taxanes in androgen-independent prostate cancer cells. J. Biochem. Mol. Toxicol. 2020, 34, e22542. [Google Scholar] [CrossRef] [PubMed]
- Takai, M.; Kato, S.; Nakano, M.; Fujimoto, S.; Iinuma, K.; Ishida, T.; Taniguchi, M.; Tamaki, M.; Uno, M.; Takahashi, Y.; et al. Efficacy of cabazitaxel and the influence of clinical factors on the overall survival of patients with castration-resistant prostate cancer: A local experience of a multicenter retrospective study. Asia. Pac. J. Clin. Oncol. 2021, 17, 238–244. [Google Scholar] [CrossRef]
- Miyake, H.; Sato, R.; Watanabe, K.; Matsushita, Y.; Watanabe, H.; Motoyama, D.; Ito, T.; Sugiyama, T.; Otsuka, T. Prognostic significance of third-line treatment for patients with metastatic castration-resistant prostate cancer: Comparative assessments between cabazitaxel and other agents. Int. J. Clin. Oncol. 2021, 26, 1745–1751. [Google Scholar] [CrossRef]
- Rouyer, M.; Oudard, S.; Joly, F.; Fizazi, K.; Tubach, F.; Jove, J.; Lacueille, C.; Lamarque, S.; Guiard, E.; Balestra, A.; et al. Overall and progression-free survival with cabazitaxel in metastatic castration-resistant prostate cancer in routine clinical practice: The FUJI cohort. Br. J. Cancer 2019, 121, 1001–1008. [Google Scholar] [CrossRef]
- Kreis, K.; Horenkamp-Sonntag, D.; Schneider, U.; Zeidler, J.; Glaeske, G.; Weissbach, L. Safety and survival of docetaxel and cabazitaxel in metastatic castration-resistant prostate cancer. BJU Int. 2022, 129, 470–479. [Google Scholar] [CrossRef]
- Harper, J.W.; Adami, G.R.; Wei, N.; Keyomarsi, K.; Elledge, S.J. The p21 Cdk-interacting protein Cip1 is a potent inhibitor of G1 cyclin-dependent kinases. Cell 1993, 75, 805–816. [Google Scholar] [CrossRef]
- Green, D.R. Caspases and Their Substrates. Cold Spring Harb. Perspect. Biol. 2022, 14, a041012. [Google Scholar] [CrossRef]
- Mansoori, B.; Mohammadi, A.; Davudian, S.; Shirjang, S.; Baradaran, B. The different mechanisms of cancer drug resistance: A brief review. Adv. Pharm. Bull. 2017, 7, 339. [Google Scholar] [CrossRef]
- Rocha, S.M.; Nascimento, D.; Cardoso, A.M.; Passarinha, L.; Socorro, S.; Maia, C.J. STEAP1 regulation and its influence modulating the response of LNCaP prostate cancer cells to bicalutamide, enzalutamide and apalutamide. Mol. Med. Rep. 2023, 27, 52. [Google Scholar] [CrossRef]
- Aghajani, M.; Mokhtarzadeh, A.; Aghebati-Maleki, L.; Mansoori, B.; Mohammadi, A.; Safaei, S.; Asadzadeh, Z.; Hajiasgharzadeh, K.; Khaze Shahgoli, V.; Baradaran, B. CD133 suppression increases the sensitivity of prostate cancer cells to paclitaxel. Mol. Biol. Rep. 2020, 47, 3691–3703. [Google Scholar] [CrossRef]
- Lu, X.; Yang, F.; Chen, D.; Zhao, Q.; Chen, D.; Ping, H.; Xing, N. Quercetin reverses docetaxel resistance in prostate cancer via androgen receptor and PI3K/Akt signaling pathways. Int. J. Biol. Sci. 2020, 16, 1121–1134. [Google Scholar] [CrossRef] [PubMed]
- Kosaka, T.; Hongo, H.; Miyazaki, Y.; Nishimoto, K.; Miyajima, A.; Oya, M.; Kosaka, T.; Hongo, H.; Miyazaki, Y.; Nishimoto, K.; et al. Reactive oxygen species induction by cabazitaxel through inhibiting Sestrin-3 in castration resistant prostate cancer. Oncotarget 2017, 8, 87675–87683. [Google Scholar] [CrossRef] [PubMed]
- Wu, Y.Y.; Jiang, J.N.; Fang, X.D.; Ji, F.J. STEAP1 Regulates Tumorigenesis and Chemoresistance During Peritoneal Metastasis of Gastric Cancer. Front. Physiol. 2018, 9, 1132. [Google Scholar] [CrossRef]
- Yang, F.; Cai, J.; Zhan, H.; Situ, J.; Li, W.; Mao, Y.; Luo, Y. Suppression of TRPM7 Inhibited Hypoxia-Induced Migration and Invasion of Androgen-Independent Prostate Cancer Cells by Enhancing RACK1-Mediated Degradation of HIF-1 α. Oxid. Med. Cell. Longev. 2020, 2020, 6724810. [Google Scholar] [CrossRef]
- Chen, L.; Cao, R.; Wang, G.; Yuan, L.; Qian, G.; Guo, Z.; Wu, C.L.; Wang, X.; Xiao, Y. Downregulation of TRPM7 suppressed migration and invasion by regulating epithelial-mesenchymal transition in prostate cancer cells. Med Oncol. 2017, 34, 127. [Google Scholar] [CrossRef] [PubMed]
- Sun, Y.; Selvaraj, S.; Varma, A.; Derry, S.; Sahmoun, A.E.; Singh, B.B. Increase in serum Ca2+/Mg2+ ratio promotes proliferation of prostate cancer cells by activating TRPM7 channels. J. Biol. Chem. 2013, 288, 255–263. [Google Scholar] [CrossRef] [PubMed]
- Castiglioni, S.; Cazzaniga, A.; Trapani, V.; Cappadone, C.; Farruggia, G.; Merolle, L.; Wolf, F.I.; Iotti, S.; Maier, J.A.M. Magnesium homeostasis in colon carcinoma LoVo cells sensitive or resistant to doxorubicin. Sci. Rep. 2015, 5, 16538. [Google Scholar] [CrossRef] [PubMed]
- Martini, M.; De Santis, M.C.; Braccini, L.; Gulluni, F.; Hirsch, E. PI3K/AKT signaling pathway and cancer: An updated review. Ann. Med. 2014, 46, 372–383. [Google Scholar] [CrossRef]
- Cao, Z.; Liao, Q.; Su, M.; Huang, K.; Jin, J.; Cao, D. AKT and ERK dual inhibitors: The way forward? Cancer Lett. 2019, 459, 30–40. [Google Scholar] [CrossRef] [PubMed]
- Fulda, S. Modulation of mitochondrial apoptosis by PI3K inhibitors. Mitochondrion 2013, 13, 195–198. [Google Scholar] [CrossRef]
- Zhu, L.; Han, M.B.; Gao, Y.; Wang, H.; Dai, L.; Wen, Y.; Na, L.X. Curcumin triggers apoptosis via upregulation of Bax/Bcl-2 ratio and caspase activation in SW872 human adipocytes. Mol. Med. Rep. 2015, 12, 1151–1156. [Google Scholar] [CrossRef]
- Gottlieb, T.M.; Leal, J.F.M.; Seger, R.; Taya, Y.; Oren, M. Cross-talk between Akt, p53 and Mdm2: Possible implications for the regulation of apoptosis. Oncogene 2002, 21, 1299–1303. [Google Scholar] [CrossRef] [PubMed]
- He, S.L.; Wang, W.P.; Yang, Y.S.; Li, E.M.; Xu, L.Y.; Chen, L.Q. FAM3B promotes progression of oesophageal carcinoma via regulating the AKT-MDM2-p53 signalling axis and the epithelial-mesenchymal transition. J. Cell. Mol. Med. 2019, 23, 1375–1385. [Google Scholar] [CrossRef]
- He, G.; Siddik, Z.H.; Huang, Z.; Wang, R.; Koomen, J.; Kobayashi, R.; Khokhar, A.R.; Kuang, J. Induction of p21 by p53 following DNA damage inhibits both Cdk4 and Cdk2 activities. Oncogene 2005, 24, 2929–2943. [Google Scholar] [CrossRef]
- Li, B.; Lin, Z.; Liang, Q.; Hu, Y.; Xu, W.F. PAQR6 Expression Enhancement Suggests a Worse Prognosis in Prostate Cancer Patients. Open life Sci. 2018, 13, 511–517. [Google Scholar] [CrossRef]
- Hawsawi, O.; Henderson, V.; Burton, L.J.; Dougan, J.; Nagappan, P.; Odero-Marah, V. High mobility group A2 (HMGA2) promotes EMT via MAPK pathway in prostate cancer. Biochem. Biophys. Res. Commun. 2018, 504, 196–202. [Google Scholar] [CrossRef]
- Mang, J.; Merkle, K.; Heller, M.; Schüler, J.; Tolstov, Y.; Li, J.; Hohenfellner, M.; Duensing, S. Molecular complexity of taxane-induced cytotoxicity in prostate cancer cells. Urol. Oncol. 2017, 35, 32.e9–32.e16. [Google Scholar] [CrossRef]
- Park, C.H.; Han, S.E.; Nam-Goong, I.S.; Kim, Y.I.; Kim, E.S. Combined Effects of Baicalein and Docetaxel on Apoptosis in 8505c Anaplastic Thyroid Cancer Cells via Downregulation of the ERK and Akt/mTOR Pathways. Endocrinol. Metab. 2018, 33, 121. [Google Scholar] [CrossRef] [PubMed]
- Faskhoudi, M.A.; Molaei, P.; Sadrkhanloo, M.; Orouei, S.; Hashemi, M.; Bokaie, S.; Rashidi, M.; Entezari, M.; Zarrabi, A.; Hushmandi, K.; et al. Molecular landscape of c-Myc signaling in prostate cancer: A roadmap to clinical translation. Pathol. Res. Pract. 2022, 233, 153851. [Google Scholar] [CrossRef]
- McMahon, S.B. MYC and the Control of Apoptosis. Cold Spring Harb. Perspect. Med. 2014, 4, a014407. [Google Scholar] [CrossRef] [PubMed]
- Junttila, M.R.; Westermarck, J. Mechanisms of MYC stabilization in human malignancies. Cell Cycle 2008, 7, 592–596. [Google Scholar] [CrossRef]
- Wang, Y.; Huang, Z.; Chen, C.Z.; Liu, C.; Evans, C.P.; Gao, A.C.; Zhou, F.; Chen, H.W. Therapeutic targeting of MDR1 expression by RORG antagonists resensitizes cross-resistant CRPC to taxane via coordinated induction of cell death programs. Mol. Cancer Ther. 2020, 19, 364–374. [Google Scholar] [CrossRef] [PubMed]
- Tolba, M.F.; Esmat, A.; Al-Abd, A.M.; Azab, S.S.; Khalifa, A.E.; Mosli, H.A.; Abdel-Rahman, S.Z.; Abdel-Naim, A.B. Caffeic acid phenethyl ester synergistically enhances docetaxel and paclitaxel cytotoxicity in prostate cancer cells. IUBMB Life 2013, 65, 716–729. [Google Scholar] [CrossRef] [PubMed]
- Pettersson, A.; Gerke, T.; Penney, K.L.; Lis, R.T.; Stack, E.C.; Pertega-Gomes, N.; Zadra, G.; Tyekucheva, S.; Giovannucci, E.L.; Mucci, L.A.; et al. MYC Overexpression at the Protein and mRNA Level and Cancer Outcomes among Men Treated with Radical Prostatectomy for Prostate Cancer. Cancer Epidemiol. Biomarkers Prev. 2018, 27, 201–207. [Google Scholar] [CrossRef] [PubMed]
- Hatano, K.; Yamaguchi, S.; Nimura, K.; Murakami, K.; Nagahara, A.; Fujita, K.; Uemura, M.; Nakai, Y.; Tsuchiya, M.; Nakayama, M.; et al. Residual prostate cancer cells after docetaxel therapy increase the tumorigenic potential via constitutive signaling of CXCR4, ERK1/2 and c-Myc. Mol. Cancer Res. 2013, 11, 1088–1100. [Google Scholar] [CrossRef]
- Lei, X.; Hu, X.; Zhang, T.; Zhang, J.; Wu, C.; Hong, W.; Jiang, Y.; Wang, Q.; Xie, Y.; Zhao, Y.; et al. HMGB1 release promotes paclitaxel resistance in castration-resistant prostate cancer cells via activating c-Myc expression. Cell. Signal. 2020, 72, 109631. [Google Scholar] [CrossRef] [PubMed]
- Erdogan, S.; Doganlar, O.; Doganlar, Z.B.; Turkekul, K. Naringin sensitizes human prostate cancer cells to paclitaxel therapy. Prostate Int. 2018, 6, 126–135. [Google Scholar] [CrossRef]
- Lima, T.S.; Iglesias-Gato, D.; Souza, L.D.O.; Stenvang, J.; Lima, D.S.; Roder, M.A.; Brasso, K.; Moreira, J.M.A. Molecular Profiling of Docetaxel-Resistant Prostate Cancer Cells Identifies Multiple Mechanisms of Therapeutic Resistance. Cancers 2021, 13, 1290. [Google Scholar] [CrossRef] [PubMed]
- Eskiler, G.G.; Ozkan, A.D.; Eryilmaz, I.E.; Egeli, U.; Cecener, G. Association between the anticancer efficacy of cabazitaxel and toll-like receptor 4 mediating signaling pathways in metastatic castration-resistant prostate cancer cells. Hum. Exp. Toxicol. 2021, 40, 1122–1129. [Google Scholar] [CrossRef] [PubMed]
- Pfaffl, M.W. Quantification strategies in real-time PCR. AZ Quant. PCR 2004, 1, 89–113. [Google Scholar]
- Neris, R.L.S.; Dobles, A.M.C.; Gomes, A.V. Western Blotting Using In-Gel Protein Labeling as a Normalization Control: Advantages of Stain-Free Technology. Methods Mol. Biol. 2021, 2261, 443–456. [Google Scholar]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Rocha, S.M.; Nascimento, D.; Coelho, R.S.; Cardoso, A.M.; Passarinha, L.A.; Socorro, S.; Maia, C.J. STEAP1 Knockdown Decreases the Sensitivity of Prostate Cancer Cells to Paclitaxel, Docetaxel and Cabazitaxel. Int. J. Mol. Sci. 2023, 24, 6643. https://doi.org/10.3390/ijms24076643
Rocha SM, Nascimento D, Coelho RS, Cardoso AM, Passarinha LA, Socorro S, Maia CJ. STEAP1 Knockdown Decreases the Sensitivity of Prostate Cancer Cells to Paclitaxel, Docetaxel and Cabazitaxel. International Journal of Molecular Sciences. 2023; 24(7):6643. https://doi.org/10.3390/ijms24076643
Chicago/Turabian StyleRocha, Sandra M., Daniel Nascimento, Rafaella S. Coelho, Ana Margarida Cardoso, Luís A. Passarinha, Sílvia Socorro, and Cláudio J. Maia. 2023. "STEAP1 Knockdown Decreases the Sensitivity of Prostate Cancer Cells to Paclitaxel, Docetaxel and Cabazitaxel" International Journal of Molecular Sciences 24, no. 7: 6643. https://doi.org/10.3390/ijms24076643
APA StyleRocha, S. M., Nascimento, D., Coelho, R. S., Cardoso, A. M., Passarinha, L. A., Socorro, S., & Maia, C. J. (2023). STEAP1 Knockdown Decreases the Sensitivity of Prostate Cancer Cells to Paclitaxel, Docetaxel and Cabazitaxel. International Journal of Molecular Sciences, 24(7), 6643. https://doi.org/10.3390/ijms24076643









