Malic Enzyme 1 (ME1) Promotes Adiposity and Hepatic Steatosis and Induces Circulating Insulin and Leptin in Obese Female Mice
Abstract
1. Introduction
2. Results
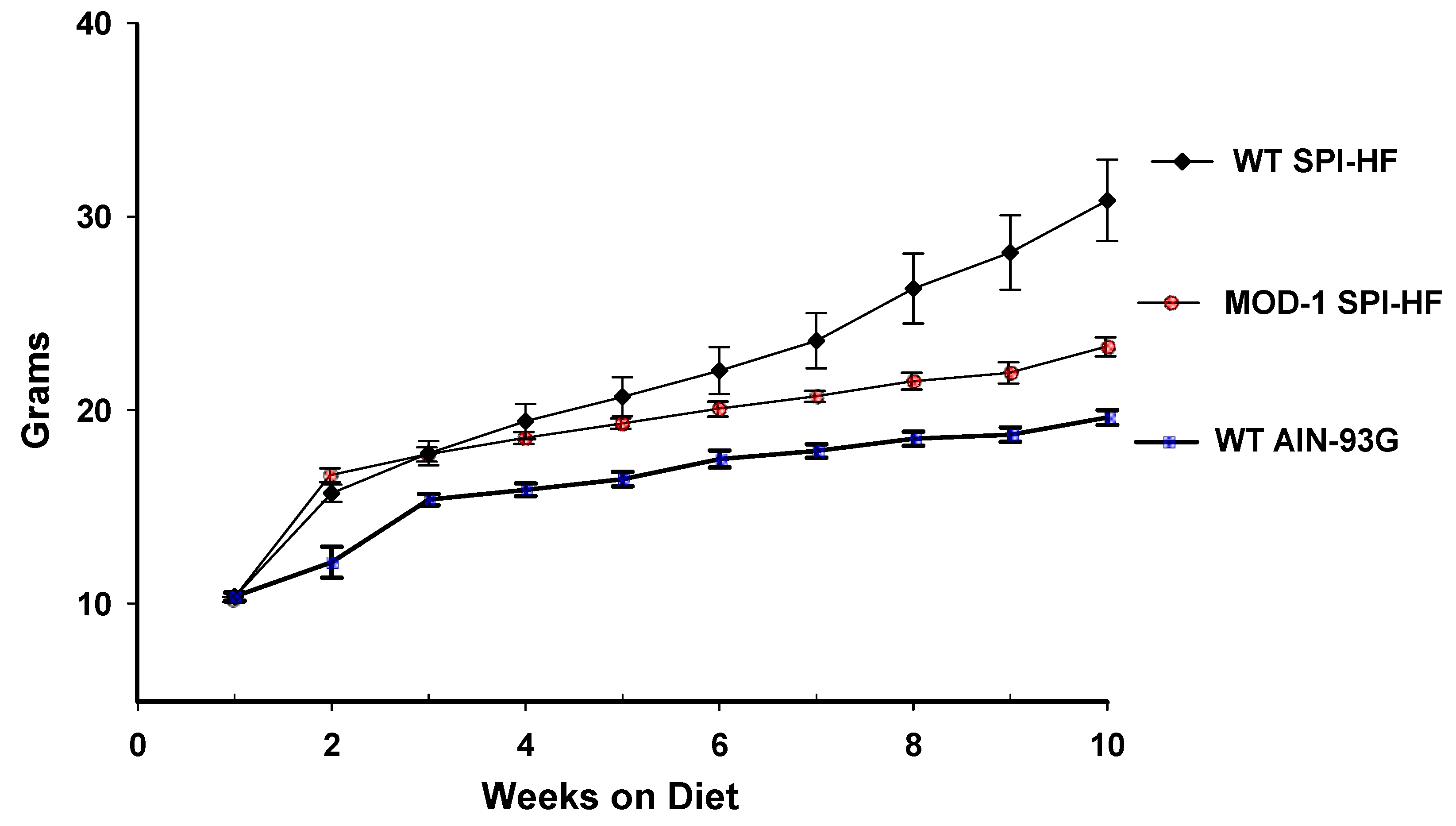
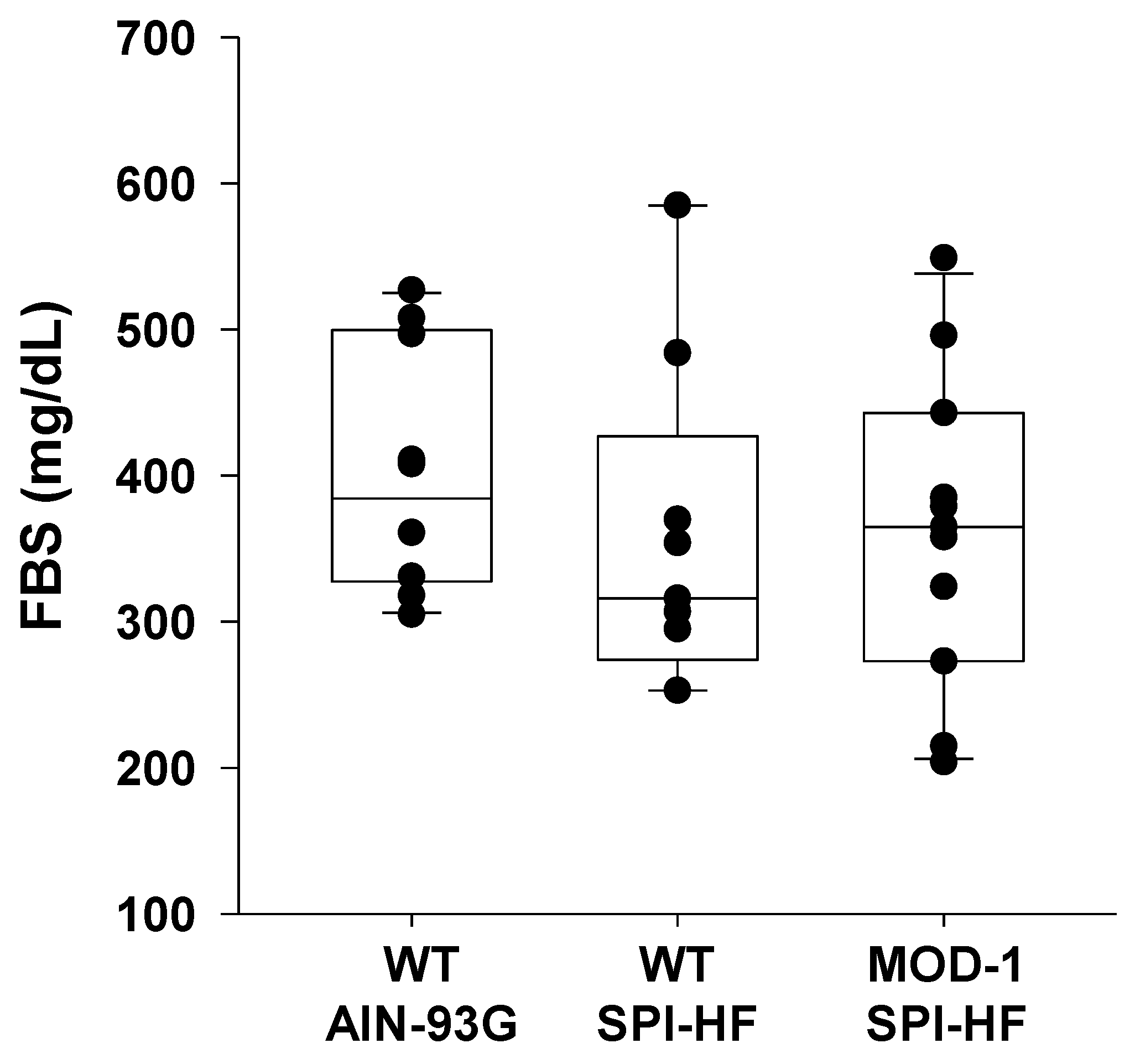
2.1. A High-Fat Containing, Soy Protein-Based Diet Is Obesogenic for Female C57BL/6 Mice, and a Mutant Me1 Allele Counters This Phenotype
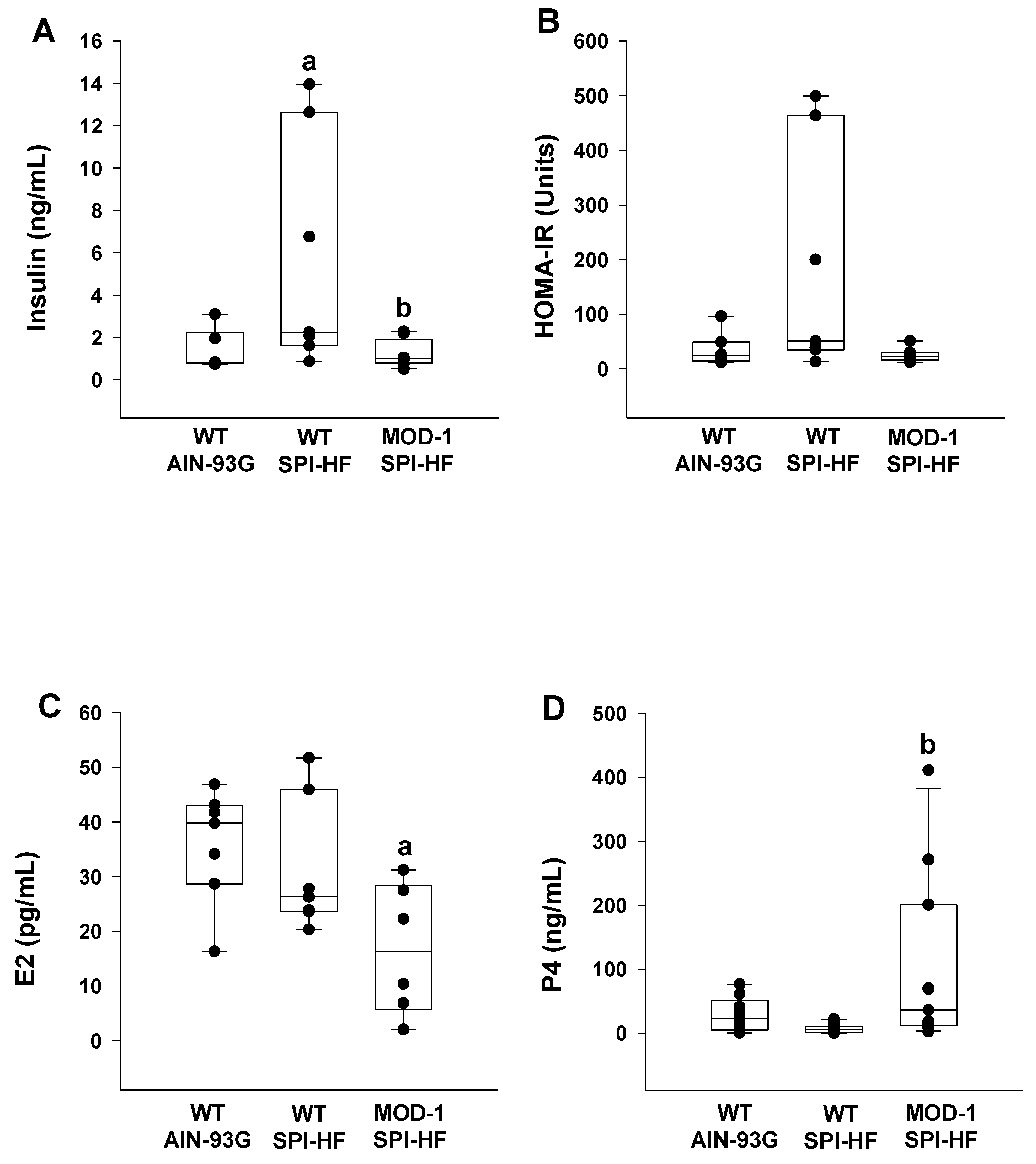
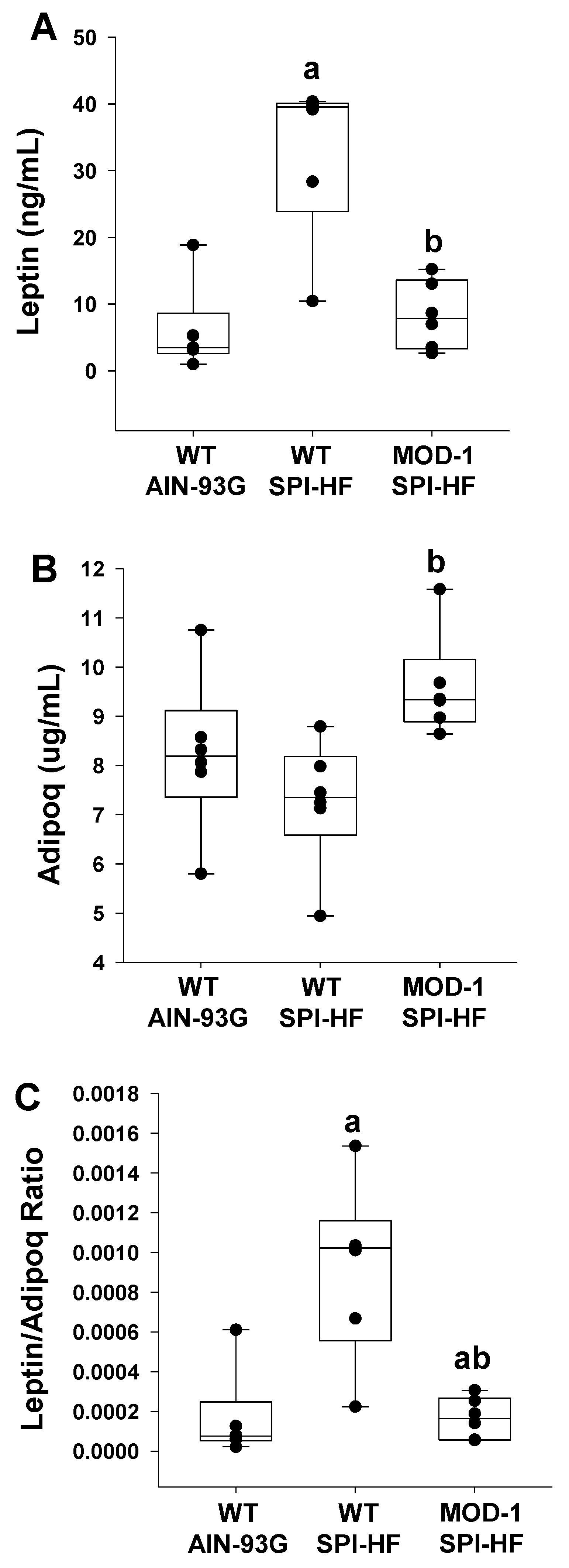
2.2. Effects of High-Fat Soy Protein Diet and Me1 Mutation on Hormonal Profiles
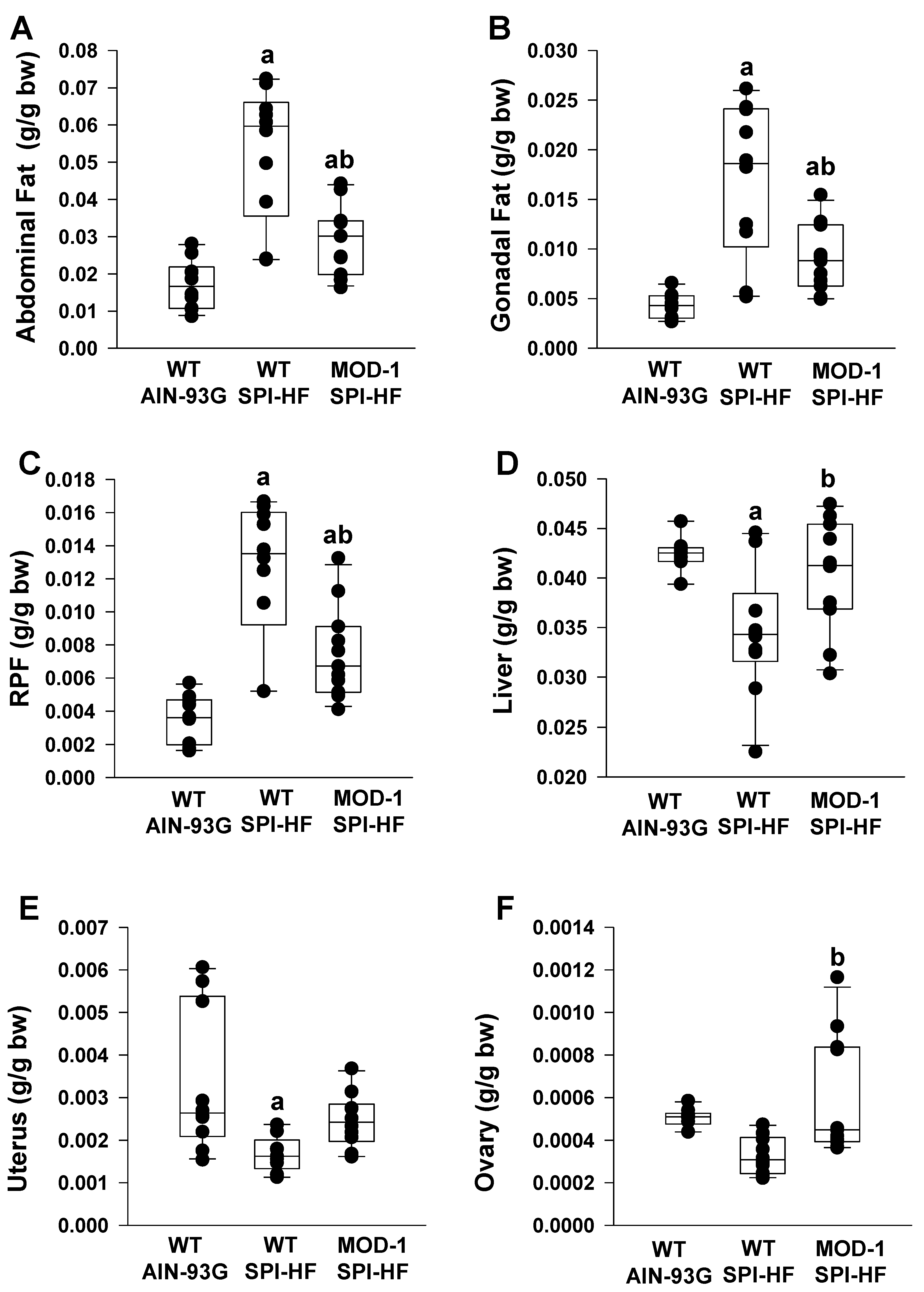
2.3. Effects of Me1 Mutation on Adipose Tissue Indices
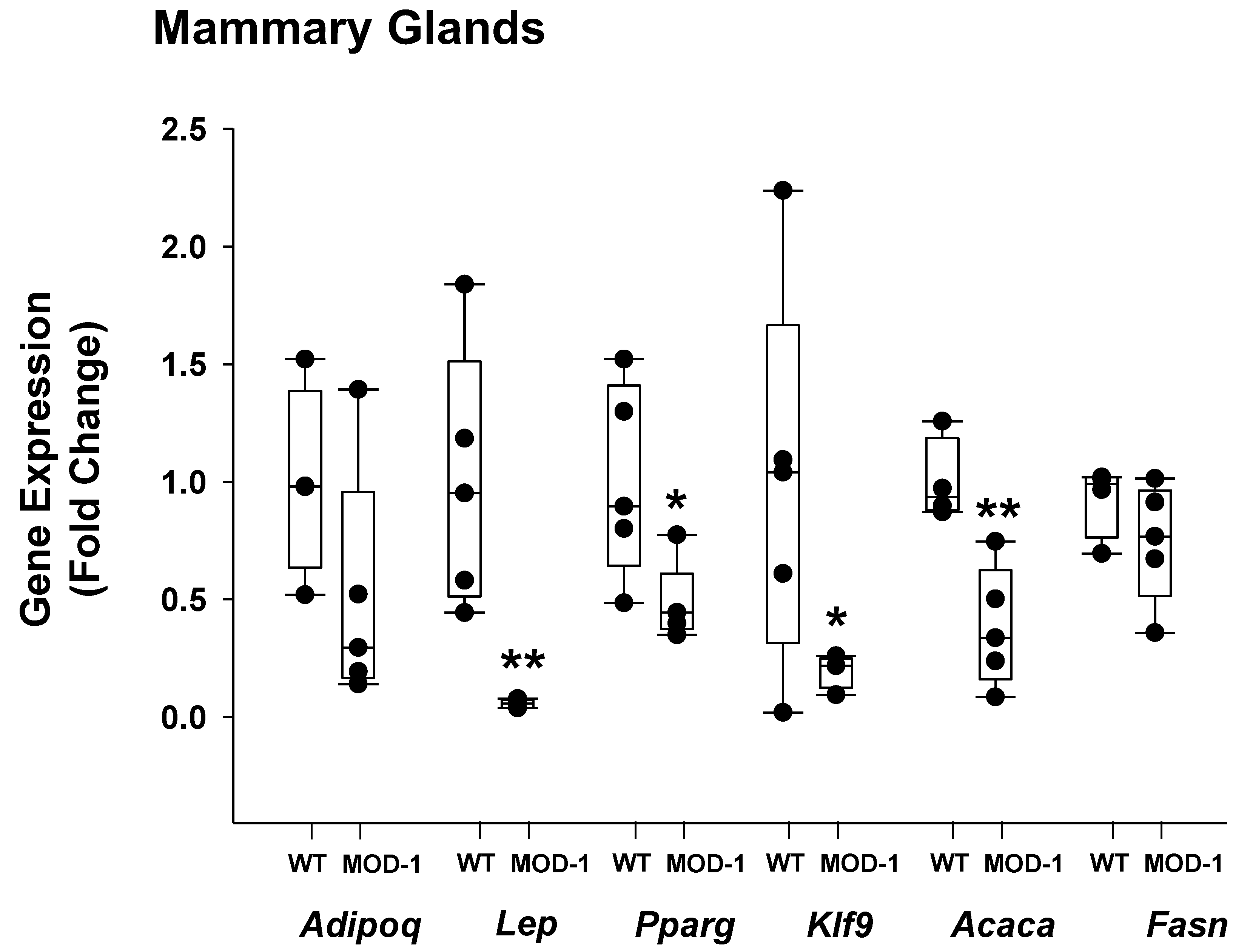
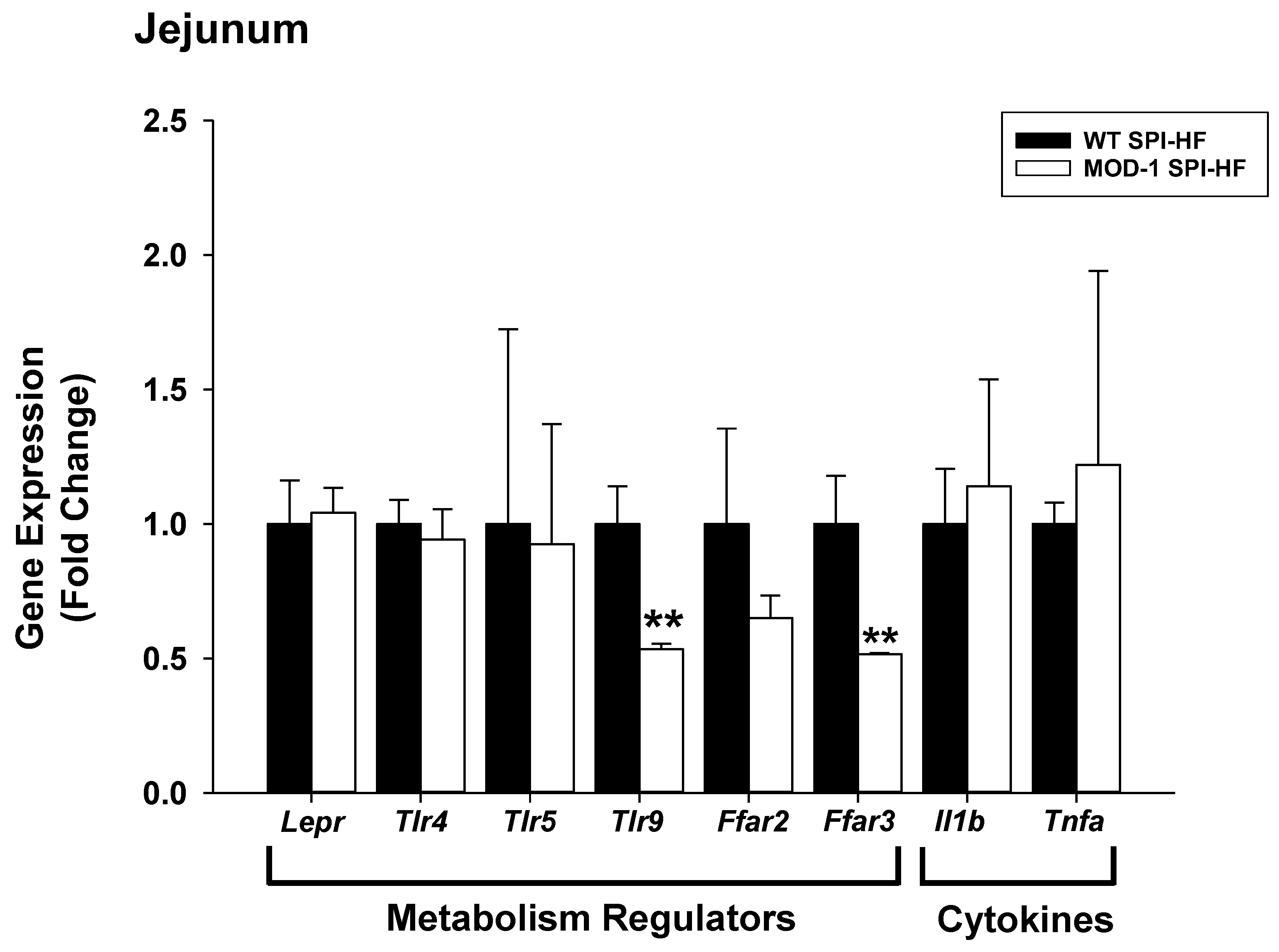
2.4. Effects of Me1 Mutation on Select Mammary Gland Genes
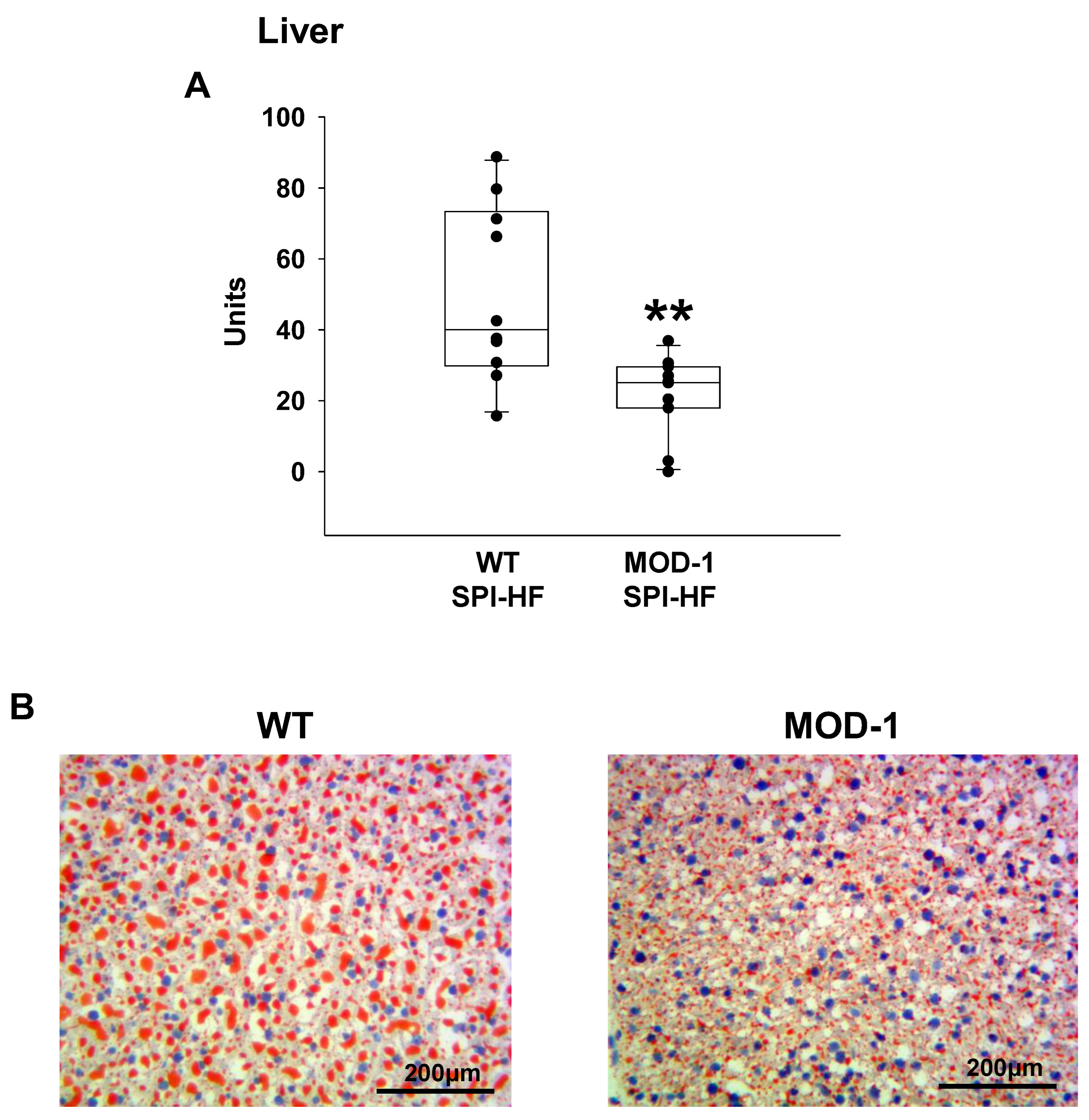
2.5. Effects of Me1 Mutation on Liver Lipid Content
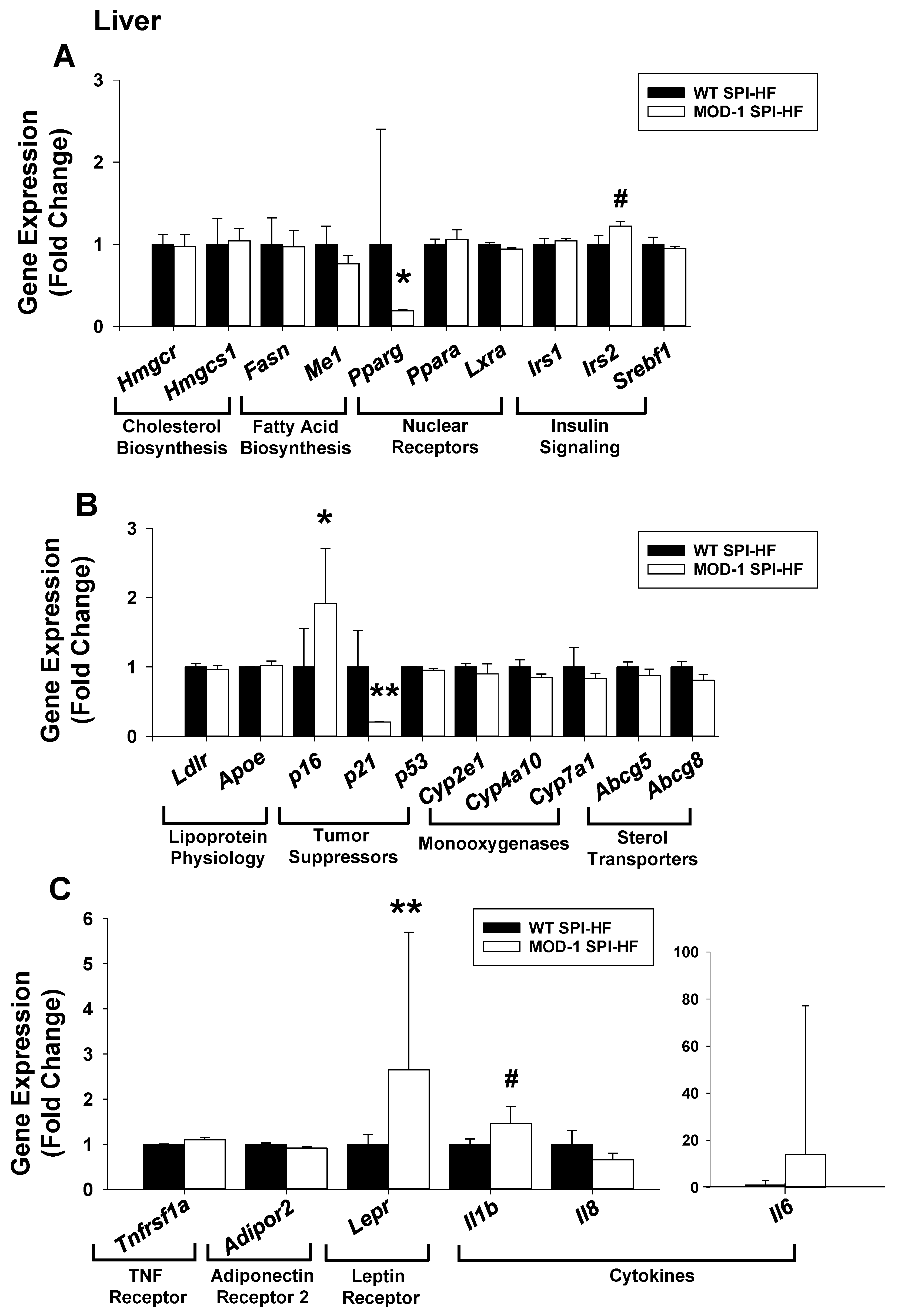
2.6. Effects of Me1 Mutation on Liver Gene Expression Patterns
3. Discussion
4. Materials and Methods
4.1. Experimental Animals and Diets
4.2. Serum Glucose, Insulin, Leptin, Adiponectin, Estrogen, and Progesterone
4.3. RNA Isolation and Quantitative RT-PCR (qPCR)
4.4. Histology
4.5. Statistical Analysis
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- NCD Risk Factor Collaboration (NCD-RisC). Worldwide trends in body-mass index, underweight, overweight, and obesity from 1975 to 2016: A pooled analysis of 2416 population-based measurement studies in 128·9 million children, adolescents, and adults. Lancet 2017, 390, 2627–2642. [Google Scholar] [CrossRef] [PubMed]
- Loos, R.J.F.; Yeo, G.S.H. The genetics of obesity: From discovery to biology. Nat. Rev. Genet. 2021, 23, 120–133. [Google Scholar] [CrossRef] [PubMed]
- Ahima, R.S.; Prabakaran, D.; Mantzoros, C.; Qu, D.; Lowell, B.; Maratos-Flier, E.; Flier, J.S. Role of leptin in the neuroendocrine response to fasting. Nature 1996, 382, 250–252. [Google Scholar] [CrossRef] [PubMed]
- Ke, W.; Reed, J.N.; Yang, C.; Higgason, N.; Rayyan, L.; Wählby, C.; Carpenter, A.E.; Civelek, M.; O’Rourke, E.J. Genes in human obesity loci are causal obesity genes in C. elegans. PLOS Genet. 2021, 17, e1009736. [Google Scholar] [CrossRef] [PubMed]
- Yang, X.; Deignan, J.L.; Qi, H.; Zhu, J.; Qian, S.; Zhong, J.; Torosyan, G.; Majid, S.; Falkard, B.; Kleinhanz, R.R.; et al. Validation of candidate causal genes for obesity that affect shared metabolic pathways and networks. Nat. Genet. 2009, 41, 415–423. [Google Scholar] [CrossRef]
- Al-Dwairi, A.; Pabona, J.M.P.; Simmen, R.C.M.; Simmen, F.A. Cytosolic malic enzyme 1 (ME1) mediates high fat diet-induced adiposity, endocrine profile, and gastrointestinal tract proliferation-associated biomarkers in male mice. PLoS ONE 2012, 7, e46716. [Google Scholar] [CrossRef]
- Zhong, H.; Beaulaurier, J.; Lum, P.Y.; Molony, C.; Yang, X.; MacNeil, D.J.; Weingarth, D.T.; Zhang, B.; Greenawalt, D.; Dobrin, R.; et al. Liver and adipose expression associated SNPs are enriched for association to type 2 diabetes. PLoS Genet. 2010, 6, e1000932. [Google Scholar] [CrossRef]
- Ramírez, O.; Quintanilla, R.; Varona, L.; Gallardo, D.; Díaz, I.; Pena, R.; Amills, M. DECR1 and ME1 genotypes are associated with lipid composition traits in Duroc pigs. J. Anim. Breed. Genet. 2013, 131, 46–52. [Google Scholar] [CrossRef]
- Bartz, M.; Kociucka, B.; Mankowska, M.; Switonski, M.; Szydlowski, M. Transcript level of the porcine ME1 gene is affected by SNP in its 3’UTR, which is also associated with subcutaneous fat thickness. J. Anim. Breed. Genet. 2013, 131, 271–278. [Google Scholar] [CrossRef]
- Xing, K.; Zhu, F.; Zhai, L.; Chen, S.; Tan, Z.; Sun, Y.; Hou, Z.; Wang, C. Identification of genes for controlling swine adipose deposition by integrating transcriptome, whole-genome resequencing, and quantitative trait loci data. Sci. Rep. 2016, 6, 23219. [Google Scholar] [CrossRef]
- Simmen, F.A.; Alhallak, I.; Simmen, R.C.M. Malic enzyme 1 (ME1) in the biology of cancer: It is not just intermediary metabolism. J. Mol. Endocrinol. 2020, 65, R77–R90. [Google Scholar] [CrossRef] [PubMed]
- López, I.P.; Marti, A.; Milagro, F.I.; Zulet, M.D.L.A.; Moreno-Aliaga, M.J.; Martinez, J.A.; De Miguel, C. DNA microarray analysis of genes differentially expressed in diet-induced (cafeteria) obese rats. Obes. Res. 2003, 11, 188–194. [Google Scholar] [CrossRef] [PubMed]
- Kondo, H.; Minegishi, Y.; Komine, Y.; Mori, T.; Matsumoto, I.; Abe, K.; Tokimitsu, I.; Hase, T.; Murase, T. Differential regulation of intestinal lipid metabolism-related genes in obesity-resistant A/J vs. obesity-prone C57BL/6J mice. Am. J. Physiol. Metab. 2006, 291, E1092–E1099. [Google Scholar] [CrossRef]
- Desmarchelier, C.; Dahlhoff, C.; Keller, S.; Sailer, M.; Jahreis, G.; Daniel, H. C57Bl/6 N mice on a western diet display reduced intestinal and hepatic cholesterol levels despite a plasma hypercholesterolemia. BMC Genom. 2012, 13, 84. [Google Scholar] [CrossRef]
- Del Rio, D.; Stucchi, P.; Hernández-Nuño, F.; Cano, V.; Morales, L.; Chowen, J.A.; Del Olmo, N.; Ruiz-Gayo, M. Free-choice high-fat diet alters circadian oscillation of energy intake in adolescent mice: Role of prefrontal cortex. Eur. J. Nutr. 2016, 56, 1833–1844. [Google Scholar] [CrossRef] [PubMed]
- Russo, B.; Menduni, M.; Borboni, P.; Picconi, F.; Frontoni, S. Autonomic Nervous System in Obesity and Insulin-Resistance-The Complex Interplay between Leptin and Central Nervous System. Int. J. Mol. Sci. 2021, 22, 5187. [Google Scholar] [CrossRef] [PubMed]
- Kilpeläinen, T.O.; Carli, J.F.M.; Skowronski, A.A.; Sun, Q.; Kriebel, J.; Feitosa, M.F.; Hedman, K.; Drong, A.W.; Hayes, J.E.; Zhao, J.; et al. Genome-wide meta-analysis uncovers novel loci influencing circulating leptin levels. Nat. Commun. 2016, 7, 10494. [Google Scholar] [CrossRef]
- Yaghootkar, H.; Zhang, Y.; Spracklen, C.N.; Karaderi, T.; Huang, L.O.; Bradfield, J.; Schurmann, C.; Fine, R.S.; Preuss, M.H.; Kutalik, Z.; et al. Genetic Studies of Leptin Concentrations Implicate Leptin in the Regulation of Early Adiposity. Diabetes 2020, 69, 2806–2818. [Google Scholar] [CrossRef]
- Sánchez-Jiménez, F.; Pérez, A.P.; de la Cruz-Merino, L.; Sánchez-Margalet, V. Obesity and Breast Cancer: Role of Leptin. Front. Oncol. 2019, 9, 596. [Google Scholar] [CrossRef]
- Buonaiuto, R.; Napolitano, F.; Parola, S.; De Placido, P.; Forestieri, V.; Pecoraro, G.; Servetto, A.; Formisano, L.; Formisano, P.; Giuliano, M.; et al. Insight on the Role of Leptin: A Bridge from Obesity to Breast Cancer. Biomolecules. iomolecules 2022, 12, 1394. [Google Scholar] [CrossRef]
- Słabuszewska-Jóźwiak, A.; Lukaszuk, A.; Janicka-Kośnik, M.; Wdowiak, A.; Jakiel, G. Role of Leptin and Adiponectin in Endometrial Cancer. Int. J. Mol. Sci. 2022, 23, 5307. [Google Scholar] [CrossRef] [PubMed]
- Socol, C.T.; Chira, A.; Martinez-Sanchez, M.A.; Nuñez-Sanchez, M.A.; Maerescu, C.M.; Mierlita, D.; Rusu, A.V.; Ruiz-Alcaraz, A.J.; Trif, M.; Ramos-Molina, B. Leptin Signaling in Obesity and Colorectal Cancer. Int. J. Mol. Sci. 2022, 23, 4713. [Google Scholar] [CrossRef] [PubMed]
- Sul, H.S.; Wise, L.S.; Brown, M.L.; Rubin, C.S. Cloning of cDNA sequences for murine malic enzyme and the identification of aberrantly large malic enzyme mRNA in MOD-1 null mice. J. Biol. Chem. 1984, 259, 555–559. [Google Scholar] [CrossRef] [PubMed]
- Brown, M.L.; Wise, L.S.; Rubin, C.S. The molecular basis for a cytosolic malic enzyme null mutation. Malic enzyme mRNA from MOD-1 null mice contains an internal in-frame duplication that extends the coding sequence by 522 nucleotides. J. Biol. Chem. 1988, 263, 4494–4499. [Google Scholar] [CrossRef]
- Vandesompele, J.; De Preter, K.; Pattyn, F.; Poppe, B.; Van Roy, N.; De Paepe, A.; Speleman, F. Accurate normalization of real-time quantitative RT-PCR data by geometric averaging of multiple internal control genes. Genome Biol. 2002, 3, RESEARCH0034. [Google Scholar] [CrossRef]
- Al-Dwairi, A.; Brown, A.R.; Pabona, J.M.P.; Van, T.H.; Hamdan, H.; Mercado, C.P.; Quick, C.M.; Wight, P.A.; Simmen, R.C.M.; Simmen, F.A. Enhanced gastrointestinal expression of cytosolic malic enzyme (ME1) induces intestinal and liver lipogenic gene expression and intestinal cell proliferation in mice. PLoS ONE 2014, 9, e113058. [Google Scholar] [CrossRef]
- Penza, M.; Montani, C.; Romani, A.; Vignolini, P.; Pampaloni, B.; Tanini, A.; Brandi, M.L.; Magdalena, P.A.; Nadal, A.; Ottobrini, L.; et al. Genistein affects adipose tissue deposition in a dose-dependent and gender-specific manner. Endocrinology 2006, 147, 5740–5751. [Google Scholar] [CrossRef]
- Hakkak, R.; Shaaf, S.; Jo, C.H.; Macleod, S.; Korourian, S. Effects of high-isoflavone soy diet vs. casein protein diet and obesity on DMBA-induced mammary tumor development. Oncol. Lett. 2010, 2, 29–36. [Google Scholar] [CrossRef]
- Strakovsky, R.S.; Lezmi, S.; Flaws, J.A.; Schantz, S.L.; Pan, Y.-X.; Helferich, W.G. Genistein exposure during the early postnatal period favors the development of obesity in female, but not male rats. Toxicol. Sci. 2013, 138, 161–174. [Google Scholar] [CrossRef]
- Zanella, I.; Marrazzo, E.; Biasiotto, G.; Penza, M.; Romani, A.; Vignolini, P.; Caimi, L.; Di Lorenzo, D. Soy and the soy isoflavone genistein promote adipose tissue development in male mice on a low-fat diet. Eur. J. Nutr. 2014, 54, 1095–1107. [Google Scholar] [CrossRef] [PubMed]
- Marraudino, M.; Ponti, G.; Moussu, C.; Farinetti, A.; Macchi, E.; Accornero, P.; Gotti, S.; Collado, P.; Keller, M.; Panzica, G. Early Postnatal Genistein Administration Affects Mice Metabolism and Reproduction in a Sexually Dimorphic Way. Metabolites 2021, 11, 449. [Google Scholar] [CrossRef] [PubMed]
- Rockwood, S.; Mason, D.; Lord, R.; Lamar, P.; Prozialeck, W.; Al-Nakkash, L. Genistein diet improves body weight, serum glucose and triglyceride levels in both male and female ob/ob mice. Diabetes, Metab. Syndr. Obesity Targets Ther. 2019, 12, 2011–2021. [Google Scholar] [CrossRef] [PubMed]
- Stubbins, R.E.; Najjar, K.; Holcomb, V.B.; Hong, J.; Núñez, N.P. Oestrogen alters adipocyte biology and protects female mice from adipocyte inflammation and insulin resistance. Diabetes, Obes. Metab. 2011, 14, 58–66. [Google Scholar] [CrossRef] [PubMed]
- Litwak, S.A.; Wilson, J.L.; Chen, W.; Garcia-Rudaz, C.; Khaksari, M.; Cowley, M.A.; Enriori, P.J. Estradiol prevents fat accumulation and overcomes leptin resistance in female high-fat diet mice. Endocrinology 2014, 155, 4447–4460. [Google Scholar] [CrossRef]
- Erlandsson, M.C.; Islander, U.; Moverare, S.; Ohlsson, C.; Carlsten, H. Estrogenic agonism and antagonism of the soy isoflavone genistein in uterus, bone and lymphopoiesis in mice. Apmis 2005, 113, 317–323. [Google Scholar] [CrossRef] [PubMed]
- Penza, M.; Montani, C.; Romani, A.; Vignolini, P.; Ciana, P.; Maggi, A.; Pampaloni, B.; Caimi, L.; Di Lorenzo, D. Genistein accumulates in body depots and is mobilized during fasting, reaching estrogenic levels in serum that counter the hormonal actions of estradiol and organochlorines. Toxicol. Sci. 2007, 97, 299–307. [Google Scholar] [CrossRef] [PubMed]
- Cochary, E.F.; Kikinis, Z.; E Paulson, K. Positional and temporal regulation of lipogenic gene expression in mouse liver. Gene Expr. 1993, 3, 265–278. [Google Scholar]
- Garcia, C.; Benito, B.; Jolin, T.; Santisteban, P. Insulin regulation of malic enzyme gene expression in rat liver: Evidence for nuclear proteins that bind to two putative insulin response elements. Mol. Endocrinol. 1994, 8, 1361–1369. [Google Scholar] [CrossRef]
- Streeper, R.S.; Chapman, S.C.; Ayala, J.E.; Svitek, C.A.; Goldman, J.K.; Cave, A.; O’Brien, R.M. A phorbol ester-insensitive AP-1 motif mediates the stimulatory effect of insulin on rat malic enzyme gene transcription. Mol. Endocrinol. 1998, 12, 1778–1791. [Google Scholar] [CrossRef] [PubMed]
- Luong, X.G.; Stevens, S.K.; Jekle, A.; Lin, T.-I.; Gupta, K.; Misner, D.; Chanda, S.; Mukherjee, S.; Williams, C.; Stoycheva, A.; et al. Regulation of gene transcription by thyroid hormone receptor β agonists in clinical development for the treatment of non-alcoholic steatohepatitis (NASH). PLoS ONE 2020, 15, e0240338. [Google Scholar] [CrossRef]
- Yadav, A.; Kataria, M.A.; Saini, V.; Yadav, A. Role of leptin and adiponectin in insulin resistance. Clin. Chim. Acta 2013, 417, 80–84. [Google Scholar] [CrossRef] [PubMed]
- Xu, H.; Hirosumi, J.; Uysal, K.T.; Guler, A.D.; Hotamisligil, G.S. Exclusive action of transmembrane TNF alpha in adipose tissue leads to reduced adipose mass and local but not systemic insulin resistance. Endocrinology 2002, 143, 1502–1511. [Google Scholar] [CrossRef] [PubMed]
- Smith, U.; Axelsen, M.; Carvalho, E.; Eliasson, B.; Jansson, P.-A.; Wesslau, C. Insulin signaling and action in fat cells: Associations with insulin resistance and type 2 diabetes. Ann. N. Y. Acad. Sci. 1999, 892, 119–126. [Google Scholar] [CrossRef] [PubMed]
- Petersen, K.F.; Shulman, G. Etiology of insulin resistance. Am. J. Med. 2006, 119 (Suppl. 1), S10–S16. [Google Scholar] [CrossRef] [PubMed]
- Emamgholipour, S.; Ebrahimi, R.; Bahiraee, A.; Niazpour, F.; Meshkani, R. Acetylation and insulin resistance: A focus on metabolic and mitogenic cascades of insulin signaling. Crit. Rev. Clin. Lab. Sci. 2020, 57, 196–214. [Google Scholar] [CrossRef] [PubMed]
- E Lenert, M.; Chaparro, M.M.; Burton, M.D. Homeostatic Regulation of Estrus Cycle of Young Female Mice on Western Diet. J. Endocr. Soc. 2021, 5, bvab010. [Google Scholar] [CrossRef]
- Yu, H.; Duan, C.; Yang, Z.; Wang, Y.; Yue, Z.; Guo, B. Malic enzyme 1 is important for uterine decidualization in response to progesterone/cAMP/PKA/HB-EGF pathway. FASEB J. 2020, 34, 3820–3837. [Google Scholar] [CrossRef]
- Wafer, R.; Tandon, P.; Minchin, J.E.N. The Role of Peroxisome Proliferator-Activated Receptor Gamma (PPARG) in Adipogenesis: Applying Knowledge from the Fish Aquaculture Industry to Biomedical Research. Front. Endocrinol. 2017, 8, 102. [Google Scholar] [CrossRef]
- Pei, H.; Yao, Y.; Yang, Y.; Liao, K.; Wu, J.-R. Krüppel-like factor KLF9 regulates PPARγ transactivation at the middle stage of adipogenesis. Cell Death Differ. 2010, 18, 315–327. [Google Scholar] [CrossRef]
- Alhallak, I.; Wolter, K.G.; Munoz, A.C.; A Simmen, F.; Ward, R.J.; A Petty, S.; Li, L.-X.; Simmen, R.C. Breast adipose regulation of premenopausal breast epithelial phenotype involves interleukin 10. J. Mol. Endocrinol. 2021, 67, 173–188. [Google Scholar] [CrossRef]
- Lee, S.M.; Muratalla, J.; Karimi, S.; Diaz-Ruiz, A.; Frutos, M.D.; Guzman, G.; Ramos-Molina, B.; Cordoba-Chacon, J. Hepatocyte PPARγ contributes to the progression of non-alcoholic steatohepatitis in male and female obese mice. Cell. Mol. Life Sci. 2023, 80, 39. [Google Scholar] [CrossRef] [PubMed]
- Lee, Y.K.; Park, J.E.; Lee, M.; Hardwick, J.P. Hepatic lipid homeostasis by peroxisome proliferator-activated receptor gamma 2. Liver Res. 2018, 2, 209–215. [Google Scholar] [CrossRef] [PubMed]
- Li, L.; Fu, J.; Liu, D.; Sun, J.; Hou, Y.; Chen, C.; Shao, J.; Wang, L.; Wang, X.; Zhao, R.; et al. Hepatocyte-specific Nrf2 deficiency mitigates high-fat diet-induced hepatic steatosis: Involvement of reduced PPARγ expression. Redox Biol. 2019, 30, 101412. [Google Scholar] [CrossRef] [PubMed]
- Chartoumpekis, D.V.; Ziros, P.G.; Zaravinos, A.; Iskrenova, R.P.; Psyrogiannis, A.I.; Kyriazopoulou, V.E.; Sykiotis, G.P.; Habeos, I.G. Hepatic gene expression profiling in Nrf2 knockout mice after long-term high-fat diet-induced obesity. xidative Med. Cell. Longev. 2013, 2013, 340731. [Google Scholar] [CrossRef]
- Hoffler, U.; Hobbie, K.; Wilson, R.; Bai, R.; Rahman, A.; Malarkey, D.; Travlos, G.; Ghanayem, B.I. Diet-induced obesity is associated with hyperleptinemia, hyperinsulinemia, hepatic steatosis, and glomerulopathy in C57Bl/6J mice. Endocrine 2009, 36, 311–325. [Google Scholar] [CrossRef]
- Tang, X.; Li, J.; Xiang, W.; Cui, Y.; Xie, B.; Wang, X.; Xu, Z.; Gan, L. Metformin increases hepatic leptin receptor and decreases steatosis in mice. J. Endocrinol. 2016, 230, 227–237. [Google Scholar] [CrossRef]
- Saki, K.; Mansouri, V.; Abdi, S.; Fathi, M.; Razzaghi, Z.; Haghazali, M. Assessment of common and differentially expressed proteins between diabetes mellitus and fatty liver disease: A network analysis. Gastroenterol. Hepatol. Bed Bench. 2021, 14 (Suppl. 1), S94–S101. [Google Scholar] [PubMed]
- Ritschka, B.; Knauer-Meyer, T.; Gonçalves, D.S.; Mas, A.; Plassat, J.-L.; Durik, M.; Jacobs, H.; Pedone, E.; Di Vicino, U.; Cosma, M.P.; et al. The senotherapeutic drug ABT-737 disrupts aberrant p21 expression to restore liver regeneration in adult mice. Genes Dev. 2020, 34, 489–494. [Google Scholar] [CrossRef]
- Zhang, Z.; Wen, H.; Peng, B.; Weng, J.; Zeng, F. CDKN2A deregulation in fatty liver disease and its accelerative role in the process of lipogenesis. FASEB J. 2021, 35, e21230. [Google Scholar] [CrossRef]
- Rumio, C.; Sommariva, M.; Sfondrini, L.; Palazzo, M.; Morelli, D.; Viganò, L.; de Cecco, L.; Tagliabue, E.; Balsari, A. Induction of Paneth cell degranulation by orally administered Toll-like receptor ligands. J. Cell. Physiol. 2011, 227, 1107–1113. [Google Scholar] [CrossRef]
- Sardi, C.; Luchini, P.; Emanuelli, A.; Giannoni, A.; Martini, E.; Manara, L.M.; Sfondrini, L.; Kallikourdis, M.; Sommariva, M.; Rumio, C. Three months of Western diet induces small intestinal mucosa alteration in TLR KO mice. Microsc. Res. Tech. 2017, 80, 563–569. [Google Scholar] [CrossRef] [PubMed]
- Nøhr, M.K.; Pedersen, M.H.; Gille, A.; Egerod, K.L.; Engelstoft, M.S.; Husted, A.S.; Sichlau, R.M.; Grunddal, K.V.; Seier Poulsen, S.; Han, S.; et al. Offermanns S, Schwartz TW. GPR41/FFAR3 and GPR43/FFAR2 as cosensors for short-chain fatty acids in enteroendocrine cells vs FFAR3 in enteric neurons and FFAR2 in enteric leukocytes. Endocrinology 2013, 154, 3552–3564. [Google Scholar] [CrossRef] [PubMed]
- Kennedy, R.L.; Vangaveti, V.; Jarrod, G.; Shashidhar, V.; Baune, B.T. Free fatty acid receptors: Emerging targets for treatment of diabetes and its complications. Ther. Adv. Endocrinol. Metab. 2010, 1, 165–175. [Google Scholar] [CrossRef] [PubMed]
- Xiong, Y.; Miyamoto, N.; Shibata, K.; Valasek, M.A.; Motoike, T.; Kedzierski, R.M.; Yanagisawa, M. Short-chain fatty acids stimulate leptin production in adipocytes through the G protein-coupled receptor GPR41. Proc. Natl. Acad. Sci. USA 2004, 101, 1045–1050. [Google Scholar] [CrossRef] [PubMed]
- Zaibi, M.S.; Stocker, C.J.; O’Dowd, J.; Davies, A.; Bellahcene, M.; Cawthorne, M.A.; Brown, A.J.; Smith, D.M.; Arch, J.R. Roles of GPR41 and GPR43 in leptin secretory responses of murine adipocytes to short chain fatty acids. FEBS Lett. 2010, 584, 2381–2386. [Google Scholar] [CrossRef] [PubMed]
- Chambers, E.S.; Morrison, D.J.; Frost, G. Control of appetite and energy intake by SCFA: What are the potential underlying mechanisms? Proc. Nutr. Soc. 2015, 74, 328–336. [Google Scholar] [CrossRef] [PubMed]
- Cook, T.M.; Gavini, C.K.; Jesse, J.; Aubert, G.; Gornick, E.; Bonomo, R.; Gautron, L.; Layden, B.T.; Mansuy-Aubert, V. Vagal neuron expression of the microbiota-derived metabolite receptor, free fatty acid receptor (FFAR3), is necessary for normal feeding behavior. Mol. Metab. 2021, 54, 101350. [Google Scholar] [CrossRef]
- Li, W.; Kou, J.; Qin, J.; Li, L.; Zhang, Z.; Pan, Y.; Xue, Y.; Du, W. NADPH levels affect cellular epigenetic state by inhibiting HDAC3-Ncor complex. Nat. Metab. 2021, 3, 75–89. [Google Scholar] [CrossRef]
- Zhang, Y.J.; Wang, Z.; Sprous, D.; Nabioullin, R. In silico design and synthesis of piperazine-1-pyrrolidine-2,5-dione scaffold-based novel malic enzyme inhibitors. Bioorganic Med. Chem. Lett. 2006, 16, 525–528. [Google Scholar] [CrossRef]
- Yoshida, T.; Kawabe, T.; Cantley, L.C.; Lyssiotis, C.A. Discovery and Characterization of a Novel Allosteric Small-Molecule Inhibitor of NADP+-Dependent Malic Enzyme 1. Biochemistry 2022, 61, 1548–1553. [Google Scholar] [CrossRef]
- Montales, M.T.E.; Melnyk, S.B.; Simmen, F.A.; Simmen, R.C. Maternal metabolic perturbations elicited by high-fat diet promote Wnt-1-induced mammary tumor risk in adult female offspring via long-term effects on mammary and systemic phenotypes. Carcinogenesis 2014, 35, 2102–2112. [Google Scholar] [CrossRef] [PubMed]
- BBrown, A.R.; Alhallak, I.; Simmen, R.C.M.; Melnyk, S.B.; Heard-Lipsmeyer, M.E.; Montales, M.T.E.; Habenicht, D.; Van, T.T.; Simmen, F.A. Krüppel-like Factor 9 (KLF9) Suppresses Hepatocellular Carcinoma (HCC)-Promoting Oxidative Stress and Inflammation in Mice Fed High-Fat Diet. Cancers 2022, 14, 1737. [Google Scholar] [CrossRef] [PubMed]


| Component | AIN-93G (TD.95092, Harlan) (g/Kg) | SPI-HF (TD.110630, Harlan) (g/Kg) |
|---|---|---|
| Isolated Soy Protein | 0 | 210.0 |
| Casein | 200.0 | 0 |
| L-Cystine | 3.0 | 1.2 |
| L-Methionine | 0 | 2.5 |
| Corn starch | 397.486 | 130.0 |
| Maltodextrin | 132.0 | 180.0 |
| Sucrose | 100.0 | 150.0 |
| Lard | 0 | 210.0 |
| Corn oil | 70.0 | 20.0 |
| Cellulose | 50.0 | 28.26 |
| Mineral mix | 35.0 | 50.0 |
| Vitamin mix | 10.0 | 15.0 |
| Choline bitartrate | 2.5 | 3.0 |
| TBHQ, antioxidant | 0.014 | 0.04 |
| Gene | Forward Primer (5′-3′) | Reverse Primer (5′-3′) |
|---|---|---|
| Abcg5 | CCTGAACATTCCAATCCCTTTG | ACGTTTCTATTTCCCGCTCTC |
| Abcg8 | GGCAAAGGAACTCAACACAAG | TCCCGGAAGTCATTGGAAATC |
| Acaca | AAGGCTATGTGAAGGATGTGG | CTGTCTGAAGAGGTTAGGGAAG |
| Actb | ACCTTCTACAATGAGCTGCG | CTGGATGGCTACGTACATGG |
| Adipoq | TGTCTGTACGATTGTCAGTGG | GCAGGATTAAGAGGAACAGGAG |
| Adipor2 | AGGAAGATGAAGGGTTTATGGG | AATCCGGTAGCACATCGTG |
| Apoe | CAATTGCGAAGATGAAGGCTC | TAATCCCAGAAGCGGTTCAG |
| Cyp2e1 | TCACTGGACATCAACTGCC | TGGTCTCTGTTCCTGCAAAG |
| Cyp4a10 | CCCAAGTGCCTTTCCTAGATG | GCAAACCATACCCAATCCAAG |
| Cyp7a1 | AACGATACACTCTCCACCTTTG | CTGCTTTCATTGCTTCAGGG |
| Fasn | CCCCTCTGTTAATTGGCTCC | TTGTGGAAGTGCAGGTTAGG |
| Ffar2 | CTATGTAGCCCAGACCAGC | CACCTGCCAGAACTCCTTG |
| Ffar3 | CTTCTTTCTTGGCAATTACTGGC | CCGAAATGGTCAGGTTTAGCAA |
| Hmgcr | AGTCAGTGGGAACTATTGCAC | TTACGTCAACCATAGCTTCCG |
| Hmgcs1 | TGTTCTCTTACGGTTCTGGC | AAGTTCTCGAGTCAAGCCTTG |
| Il1b | ACGGACCCCAAAAGATGAAG | TTCTCCACAGCCACAATGAG |
| Il6 | CAAAGCCAGAGTCCTTCAGAG | GTCCTTAGCCACTCCTTCTG |
| Il8 | TGCTAGTAGAAGGGTGTTGTGCGA | TCCCACACATGTCCTCACCCTAAT |
| Irs1 | ATAGCGTAACTGGACATCACAG | GCATCGTACCATCTACTGAAGAG |
| Irs2 | GTCCAGGCACTGGAGCTTT | GCTGGTAGCGCTTCACTCTT |
| Klf9 | GGCTGTGGGAAAGTCTATGG | AGTGTGGGTCCGGTAGTG |
| Ldlr | ACCCGCCAAGATCAAGAAAG | GCTGGAGATAGAGTGGAGTTTG |
| Lep | AGCCTCACTCTACTCCACAG | CCTCTACATGATTCTTGGGAGC |
| Lepr | ATTTCCTCTTGTGTCCTACTGC | AAGATGCTCAAATGTTTCAGGC |
| Lxra | CCTACGTCTCCATCAACCAC | ACACTTGCTCTGAATGGACG |
| Me1 | AGTATCCATGACAAAGGGCAC | ATCCCATTACAGCCAAGGTC |
| P16 | GTGCGATATTTGCGTTCCG | TCTGCTCTTGGGATTGGC |
| P21 | CAGATCCACAGCGATATCCAG | AGAGACAACGGCACACTTTG |
| P53 | ATGTTCCGGGAGCTGAATG | CCCCACTTTCTTGACCATTG |
| Ppara | CATTTCCCTGTTTGTGGCTG | ATCTGGATGGTTGCTCTGC |
| Pparg | TGTTATGGGTGAAACTCTGGG | AGAGCTGATTCCGAAGTTGG |
| Ppia | GCAGACAAAGTTCCAAAGACAG | CATTATGGCGTGTAAAGTCACC |
| Srebf1 | CCATCGACTACATCCGCTTC | GCCCTCCATAGACACATCTG |
| Tbp | AAGAAAGGGAGAATCATGGACC | GAGTAAGTCCTGTGCCGTAAG |
| Tlr4 | TTCAGAACTTCAGTGGCTGG | TGTTAGTCCAGAGAAACTTCCTG |
| Tlr5 | CATCTGTGAGACACCCCTTG | CAGGGAGATATTACCAACACGG |
| Tlr9 | AACCGCCACTTCTATAACCAG | GTAAGACAGAGCAAGGCAGG |
| Tnfa | CTTCTGTCTACTGAACTTCGGG | CAGGCTTGTCACTCGAATTTTG |
| Tnfrsf1a | CTCTGCTCTACGAATCACTCTG | CACAGCATACAGAATCGCAAG |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Simmen, F.A.; Pabona, J.M.P.; Al-Dwairi, A.; Alhallak, I.; Montales, M.T.E.; Simmen, R.C.M. Malic Enzyme 1 (ME1) Promotes Adiposity and Hepatic Steatosis and Induces Circulating Insulin and Leptin in Obese Female Mice. Int. J. Mol. Sci. 2023, 24, 6613. https://doi.org/10.3390/ijms24076613
Simmen FA, Pabona JMP, Al-Dwairi A, Alhallak I, Montales MTE, Simmen RCM. Malic Enzyme 1 (ME1) Promotes Adiposity and Hepatic Steatosis and Induces Circulating Insulin and Leptin in Obese Female Mice. International Journal of Molecular Sciences. 2023; 24(7):6613. https://doi.org/10.3390/ijms24076613
Chicago/Turabian StyleSimmen, Frank A., John Mark P. Pabona, Ahmed Al-Dwairi, Iad Alhallak, Maria Theresa E. Montales, and Rosalia C. M. Simmen. 2023. "Malic Enzyme 1 (ME1) Promotes Adiposity and Hepatic Steatosis and Induces Circulating Insulin and Leptin in Obese Female Mice" International Journal of Molecular Sciences 24, no. 7: 6613. https://doi.org/10.3390/ijms24076613
APA StyleSimmen, F. A., Pabona, J. M. P., Al-Dwairi, A., Alhallak, I., Montales, M. T. E., & Simmen, R. C. M. (2023). Malic Enzyme 1 (ME1) Promotes Adiposity and Hepatic Steatosis and Induces Circulating Insulin and Leptin in Obese Female Mice. International Journal of Molecular Sciences, 24(7), 6613. https://doi.org/10.3390/ijms24076613









