Short Photoperiod-Dependent Enrichment of Akkermansia spec. as the Major Change in the Intestinal Microbiome of Djungarian Hamsters (Phodopus sungorus)
Abstract
1. Introduction
2. Results
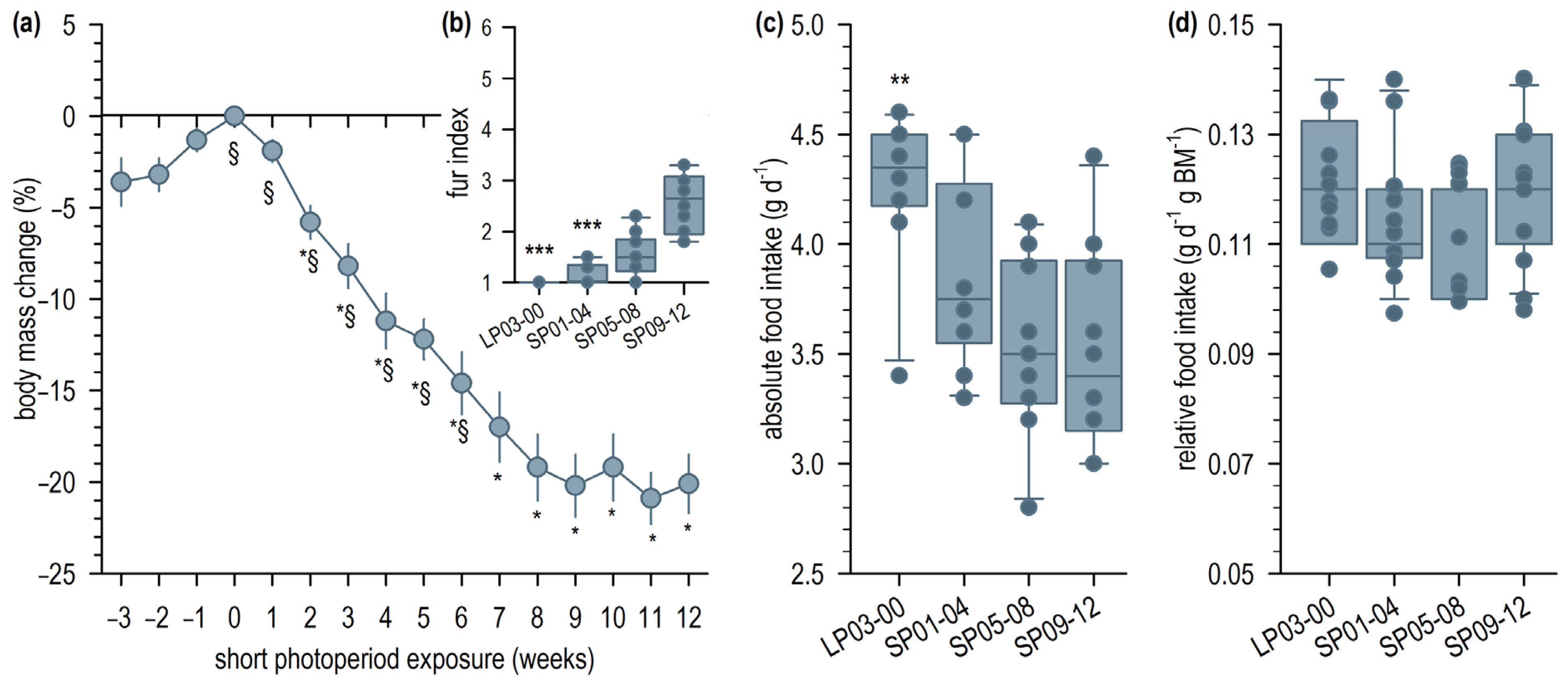
2.1. Short Photoperiod Acclimation
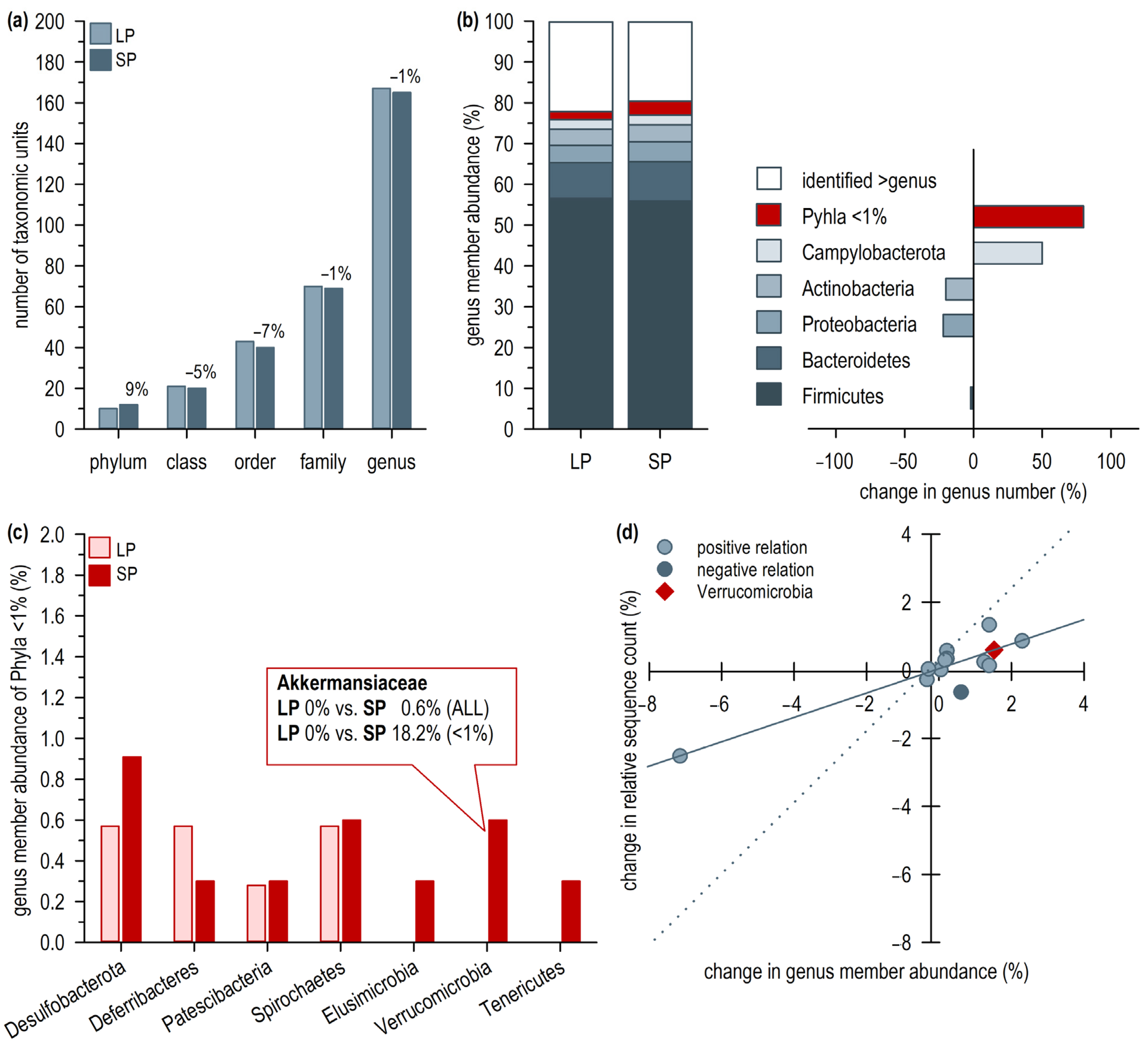
2.2. Short Photoperiod-Induced Change in Intestinal Microbiome
3. Discussion
4. Materials and Methods
4.1. Animals and Housing
4.2. Short Photoperiod Acclimation and Sampling
4.3. 16S-rRNA Next Generation Sequencing
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Raval, U.; Harary, J.M.; Zeng, E.; Pasinetti, G.M. The Dichotomous Role of the Gut Microbiome in Exacerbating and Ameliorating Neurodegenerative Disorders. Expert Rev. Neurother. 2020, 20, 673–686. [Google Scholar] [CrossRef]
- Vallianou, N.; Stratigou, T.; Christodoulatos, G.S.; Dalamaga, M. Understanding the Role of the Gut Microbiome and Microbial Metabolites in Obesity and Obesity-Associated Metabolic Disorders: Current Evidence and Perspectives. Curr. Obes. Rep. 2019, 8, 317–332. [Google Scholar] [CrossRef]
- Foster, J.A.; Lyte, M.; Meyer, E.; Cryan, J.F. Gut Microbiota and Brain Function: An Evolving Field in Neuroscience. Int. J. Neuropsychopharmacol. 2016, 19, pyv114. [Google Scholar] [CrossRef] [PubMed]
- Cubuk, C.; Bank, J.H.H.; Herwig, A. The Chemistry of Cold: Mechanisms of Torpor Regulation in the Siberian Hamster. Physiology 2016, 31, 51–59. [Google Scholar] [CrossRef] [PubMed]
- Scherbarth, F.; Steinlechner, S. Endocrine Mechanisms of Seasonal Adaptation in Small Mammals: From Early Results to Present Understanding. J. Comp. Physiol. B 2010, 180, 935–952. [Google Scholar] [CrossRef]
- Diedrich, V.; Haugg, E.; Dreier, C.; Herwig, A. What Can Seasonal Models Teach Us about Energy Balance? J. Endocrinol. 2020, 244, R17–R32. [Google Scholar] [CrossRef] [PubMed]
- Hoffmann, K. The Critical Photoperiod in the Djungarian Hamster Phodopus sungorus. In Vertebrate Circadian Systems: Structure and Physiology; Aschoff, J., Daan, S., Groos, G., Eds.; Springer: Berlin/Heidelberg, Germany, 1982; pp. 297–304. [Google Scholar]
- Heldmaier, G.; Steinlechner, S. Seasonal Control of Energy Requirements for Thermoregulation in the Djungarian Hamster (Phodopus sungorus), Living in Natural Photoperiod. J. Comp. Physiol. B 1981, 142, 429–437. [Google Scholar] [CrossRef]
- Heldmaier, G.; Steinlechner, S. Seasonal Pattern and Energetics of Short Daily Torpor in the Djungarian Hamster, Phodopus sungorus. Oecologia 1981, 48, 265–270. [Google Scholar] [CrossRef]
- Piscitiello, E.; Herwig, A.; Haugg, E.; Schröder, B.; Breves, G.; Steinlechner, S.; Diedrich, V. Acclimation of Intestinal Morphology and Function in Djungarian Hamsters (Phodopus sungorus) Related to Seasonal and Acute Energy Balance. J. Exp. Biol. 2020, 224, jeb232876. [Google Scholar] [CrossRef]
- Bäckhed, F.; Ding, H.; Wang, T.; Hooper, L.V.; Gou, Y.K.; Nagy, A.; Semenkovich, C.F.; Gordon, J.I. The Gut Microbiota as an Environmental Factor That Regulates Fat Storage. Proc. Natl. Acad. Sci. USA 2004, 101, 15718–15723. [Google Scholar] [CrossRef]
- Bailey, M.T.; Walton, J.C.; Dowd, S.E.; Weil, Z.M.; Nelson, R.J. Photoperiod Modulates Gut Bacteria Composition in Male Siberian Hamsters (Phodopus sungorus). Brain Behav. Immun. 2010, 24, 577–584. [Google Scholar] [CrossRef] [PubMed]
- Fine, J.B.; Bartness, T.J. Daylength and Body Mass Affect Diet Self-Selection by Siberian Hamsters. Physiol. Behav. 1996, 59, 1039–1050. [Google Scholar] [CrossRef] [PubMed]
- Shor, E.K.; Brown, S.P.; Freeman, D.A. A Novel Role for the Pineal Gland: Regulating Seasonal Shifts in the Gut Microbiota of Siberian Hamsters. J. Pineal Res. 2020, 69, 12696. [Google Scholar] [CrossRef] [PubMed]
- Ren, C.C.; Sylvia, K.E.; Munley, K.M.; Deyoe, J.E.; Henderson, S.G.; Vu, M.P.; Demas, G.E. Photoperiod Modulates the Gut Microbiome and Aggressive Behavior in Siberian Hamsters. J. Exp. Biol. 2020, 223 Pt 3, jeb212548. [Google Scholar] [CrossRef]
- Cusick, J.A.; Wellman, C.L.; Demas, G.E. Maternal Stress and the Maternal Microbiome Have Sex-Specific Effects on Offspring Development and Aggressive Behavior in Siberian Hamsters (Phodopus sungorus). Horm. Behav. 2022, 141, 105146. [Google Scholar] [CrossRef]
- Haugg, E.; Herwig, A.; Diedrich, V. Body Temperature and Activity Adaptation of Short Photoperiod Exposed Djungarian Hamsters (Phodopus sungorus): Timing, Traits, and Torpor. Front. Physiol. 2021, 12, 626779. [Google Scholar] [CrossRef]
- Sylvia, K.E.; Jewell, C.P.; Rendon, N.M.; St John, E.A.; Demas, G.E. Sex-Specific Modulation of the Gut Microbiome and Behavior in Siberian Hamsters. Brain Behav. Immun. 2017, 60, 51–62. [Google Scholar] [CrossRef]
- Zhu, H.; Li, G.; Liu, J.; Xu, X.; Zhang, Z. Gut Microbiota Is Associated with the Effect of Photoperiod on Seasonal Breeding in Male Brandt’s Voles (Lasiopodomys brandtii). Microbiome 2022, 10, 194. [Google Scholar] [CrossRef]
- Maurice, C.F.; Cl Knowles, S.; Ladau, J.; Pollard, K.S.; Fenton, A.; Pedersen, A.B.; Turnbaugh, P.J. Marked Seasonal Variation in the Wild Mouse Gut Microbiota. ISME J. 2015, 9, 2423–2434. [Google Scholar] [CrossRef]
- Stevenson, T.J.; Duddleston, K.N.; Buck, C.L. Effects of Season and Host Physiological State on the Diversity, Density, and Activity of the Arctic Ground Squirrel Cecal Microbiota. Appl. Environ. Microbiol. 2014, 80, 5611–5622. [Google Scholar] [CrossRef]
- Greene, L.K.; Andriambeloson, J.; Rasoanaivo, H.A.; Yoder, A.D. Variation in Gut Microbiome Structure across the Annual Hibernation Cycle in a Wild Primate. FEMS Microbiol. Ecol. 2022, 98, fiac070. [Google Scholar] [CrossRef] [PubMed]
- Ley, R.E.; Hamady, M.; Lozupone, C.; Turnbaugh, P.J.; Ramey, R.R.; Bircher, J.S.; Schlegel, M.L.; Tucker, T.A.; Schrenzel, M.D.; Knight, R.; et al. Evolution of Mammals and Their Gut Microbes. Science 2008, 320, 1647–1651. [Google Scholar] [CrossRef] [PubMed]
- Sonoyama, K.; Fujiwara, R.; Takemura, N.; Ogasawara, T.; Watanabe, J.; Ito, H.; Morita, T. Response of Gut Microbiota to Fasting and Hibernation in Syrian Hamsters. Appl. Environ. Microbiol. 2009, 75, 6451–6456. [Google Scholar] [CrossRef] [PubMed]
- Cui, M.; Xiao, H.; Luo, D.; Zhang, X.; Zhao, S.; Zheng, Q.; Li, Y.; Zhao, Y.; Dong, J.; Li, H.; et al. Circadian Rhythm Shapes the Gut Microbiota Affecting Host Radiosensitivity. Int. J. Mol. Sci. 2016, 17, 1786. [Google Scholar] [CrossRef] [PubMed]
- Ley, R.E.; Bäckhed, F.; Turnbaugh, P.; Lozupone, C.A.; Knight, R.D.; Gordon, J.I. Obesity Alters Gut Microbial Ecology. Proc. Natl. Acad. Sci. USA 2005, 102, 11070–11075. [Google Scholar] [CrossRef]
- Turnbaugh, P.J.; Ley, R.E.; Mahowald, M.A.; Magrini, V.; Mardis, E.R.; Gordon, J.I. An Obesity-Associated Gut Microbiome with Increased Capacity for Energy Harvest. Nature 2006, 444, 1027–1031. [Google Scholar] [CrossRef]
- Korhonen, T.; Mustonen, A.M.; Nieminen, P.; Saarela, S. Effects of Cold Exposure, Exogenous Melatonin and Short-Day Treatment on the Weight-Regulation and Body Temperature of the Siberian Hamster (Phodopus sungorus). Regul. Pept. 2008, 149, 60–66. [Google Scholar] [CrossRef]
- Klurfeld, D.M.; Davis, C.D.; Karp, R.W.; Allen-Vercoe, E.; Chang, E.B.; Chassaing, B.; Fahey, G.C.; Hamaker, B.R.; Holscher, H.D.; Lampe, J.W.; et al. Considerations for Best Practices in Studies of Fiber or Other Dietary Components and the Intestinal Microbiome. Am. J. Physiol. Endocrinol. Metab. 2018, 315, E1087–E1097. [Google Scholar] [CrossRef]
- Hildebrandt, M.A.; Hoffmann, C.; Sherrill-Mix, S.A.; Keilbaugh, S.A.; Hamady, M.; Chen, Y.Y.; Knight, R.; Ahima, R.S.; Bushman, F.; Wu, G.D. High-Fat Diet Determines the Composition of the Murine Gut Microbiome Independently of Obesity. Gastroenterology 2009, 137, 1716–1724.e2. [Google Scholar] [CrossRef]
- Shor, E.K.; Brown, S.P.; Freeman, D.A. Bacteria and Bellicosity: Photoperiodic Shifts in Gut Microbiota Drive Seasonal Aggressive Behavior in Male Siberian Hamsters. J. Biol. Rhythm. 2022, 37, 296–309. [Google Scholar] [CrossRef]
- Ebling, F.J.P.; Barrett, P. The Regulation of Seasonal Changes in Food Intake and Body Weight. J. Neuroendocrinol. 2008, 20, 827–833. [Google Scholar] [CrossRef] [PubMed]
- Derrien, M.; Vaughan, E.E.; Plugge, C.M.; de Vos, W.M. Akkermansia municiphila gen. nov., sp. nov., a Human Intestinal Mucin-Degrading Bacterium. Int. J. Syst. Evol. Microbiol. 2004, 54, 1469–1476. [Google Scholar] [CrossRef] [PubMed]
- Plovier, H.; Everard, A.; Druart, C.; Depommier, C.; van Hul, M.; Geurts, L.; Chilloux, J.; Ottman, N.; Duparc, T.; Lichtenstein, L.; et al. A Purified Membrane Protein from Akkermansia muciniphila or the Pasteurized Bacterium Improves Metabolism in Obese and Diabetic Mice. Nat. Med. 2016, 23, 107–113. [Google Scholar] [CrossRef] [PubMed]
- Hasani, A.; Ebrahimzadeh, S.; Hemmati, F.; Khabbaz, A.; Hasani, A.; Gholizadeh, P. The Role of Akkermansia muciniphila in Obesity, Diabetes and Atherosclerosis. J. Med. Microbiol. 2021, 70, 001435. [Google Scholar] [CrossRef] [PubMed]
- Zhang, T.; Li, Q.; Cheng, L.; Buch, H.; Zhang, F. Akkermansia muciniphila Is a Promising Probiotic. Microb. Biotechnol. 2019, 12, 1109–1125. [Google Scholar] [CrossRef] [PubMed]
- Zhai, Q.; Feng, S.; Arjan, N.; Chen, W. A next Generation Probiotic, Akkermansia muciniphila. Crit. Rev. Food Sci. Nutr. 2018, 59, 3227–3236. [Google Scholar] [CrossRef] [PubMed]
- Abuqwider, J.N.; Mauriello, G.; Altamimi, M. Akkermansia muciniphila, a New Generation of Beneficial Microbiota in Modulating Obesity: A Systematic Review. Microorganisms 2021, 9, 1098. [Google Scholar] [CrossRef]
- Depommier, C.; Everard, A.; Druart, C.; Plovier, H.; van Hul, M.; Vieira-Silva, S.; Falony, G.; Raes, J.; Maiter, D.; Delzenne, N.M.; et al. Supplementation with Akkermansia muciniphila in Overweight and Obese Human Volunteers: A Proof-of-Concept Exploratory Study. Nat. Med. 2019, 25, 1096–1103. [Google Scholar] [CrossRef]
- Depommier, C.; van Hul, M.; Everard, A.; Delzenne, N.M.; de Vos, W.M.; Cani, P.D. Pasteurized Akkermansia muciniphila Increases Whole-Body Energy Expenditure and Fecal Energy Excretion in Diet-Induced Obese Mice. Gut Microbes 2020, 11, 1231–1245. [Google Scholar] [CrossRef]
- Muegge, B.D.; Kuczynski, J.; Knights, D.; Clemente, J.C.; González, A.; Fontana, L.; Henrissat, B.; Knight, R.; Gordon, J.I. Diet Drives Convergence in Gut Microbiome Functions across Mammalian Phylogeny and within Humans. Science 2011, 332, 970–974. [Google Scholar] [CrossRef]
- Geiser, F. Ecological Physiology of Daily Torpor and Hibernation; Springer: Berlin, Germany, 2021. [Google Scholar]
- Florant, G.L.; Healy, J.E. The Regulation of Food Intake in Mammalian Hibernators: A Review. J. Comp. Physiol. B 2011, 182, 451–467. [Google Scholar] [CrossRef] [PubMed]
- Humphries, M.M.; Thomas, D.W.; Kramer, D.L. The Role of Energy Availability in Mammalian Hibernation: A Cost-benefit Approach. Physiol. Biochem. Zool. 2003, 76, 165–179. [Google Scholar] [CrossRef]
- Carey, H.V.; Walters, W.A.; Knight, R. Seasonal Restructuring of the Ground Squirrel Gut Microbiota over the Annual Hibernation Cycle. Am. J. Physiol. Regul. Integr. Comp. Physiol. 2013, 304, R33–R42. [Google Scholar] [CrossRef]
- Sommer, F.; Ståhlman, M.; Ilkayeva, O.; Arnemo, J.M.; Kindberg, J.; Josefsson, J.; Newgard, C.B.; Fröbert, O.; Bäckhed, F. The Gut Microbiota Modulates Energy Metabolism in the Hibernating Brown Bear Ursus arctos. Cell Rep. 2016, 14, 1655–1661. [Google Scholar] [CrossRef] [PubMed]
- Munley, K.M.; Han, Y.; Lansing, M.X.; Demas, G.E. Winter Madness: Melatonin as a Neuroendocrine Regulator of Seasonal Aggression. J. Exp. Zool. Part A Ecol. Integr. Physiol. 2022, 337, 873–889. [Google Scholar] [CrossRef] [PubMed]
- Figala, J.; Hoffmann, K.; Goldau, G. Zur Jahresperiodik Beim Dsungarischen Zwerghamster Phodopus sungorus Pallas. Oecologia 1973, 12, 89–118. [Google Scholar] [CrossRef]
- Lilja, S.; Stoll, C.; Krammer, U.; Hippe, B.; Duszka, K.; Debebe, T.; Höfinger, I.; König, J.; Pointner, A.; Haslberger, A. Five Days Periodic Fasting Elevates Levels of Longevity Related Christensenella and Sirtuin Expression in Humans. Int. J. Mol. Sci. 2021, 22, 2331. [Google Scholar] [CrossRef]
- Huse, S.M.; Dethlefsen, L.; Huber, J.A.; Welch, D.M.; Relman, D.A.; Sogin, M.L. Exploring Microbial Diversity and Taxonomy Using SSU RRNA Hypervariable Tag Sequencing. PLoS Genet. 2008, 4, e1000255. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kissmann, A.-K.; Rosenau, F.; Herwig, A.; Diedrich, V. Short Photoperiod-Dependent Enrichment of Akkermansia spec. as the Major Change in the Intestinal Microbiome of Djungarian Hamsters (Phodopus sungorus). Int. J. Mol. Sci. 2023, 24, 6605. https://doi.org/10.3390/ijms24076605
Kissmann A-K, Rosenau F, Herwig A, Diedrich V. Short Photoperiod-Dependent Enrichment of Akkermansia spec. as the Major Change in the Intestinal Microbiome of Djungarian Hamsters (Phodopus sungorus). International Journal of Molecular Sciences. 2023; 24(7):6605. https://doi.org/10.3390/ijms24076605
Chicago/Turabian StyleKissmann, Ann-Kathrin, Frank Rosenau, Annika Herwig, and Victoria Diedrich. 2023. "Short Photoperiod-Dependent Enrichment of Akkermansia spec. as the Major Change in the Intestinal Microbiome of Djungarian Hamsters (Phodopus sungorus)" International Journal of Molecular Sciences 24, no. 7: 6605. https://doi.org/10.3390/ijms24076605
APA StyleKissmann, A.-K., Rosenau, F., Herwig, A., & Diedrich, V. (2023). Short Photoperiod-Dependent Enrichment of Akkermansia spec. as the Major Change in the Intestinal Microbiome of Djungarian Hamsters (Phodopus sungorus). International Journal of Molecular Sciences, 24(7), 6605. https://doi.org/10.3390/ijms24076605




