Hydroxy-Selenomethionine Mitigated Chronic Heat Stress-Induced Porcine Splenic Damage via Activation of Nrf2/Keap1 Signal and Suppression of NFκb and STAT Signal
Abstract
1. Introduction
2. Results
2.1. Serum Immunoglobulin and Inflammatory Level
2.2. Spleen Mass, Se Deposition, HSP70 mRNA and Protein Expression
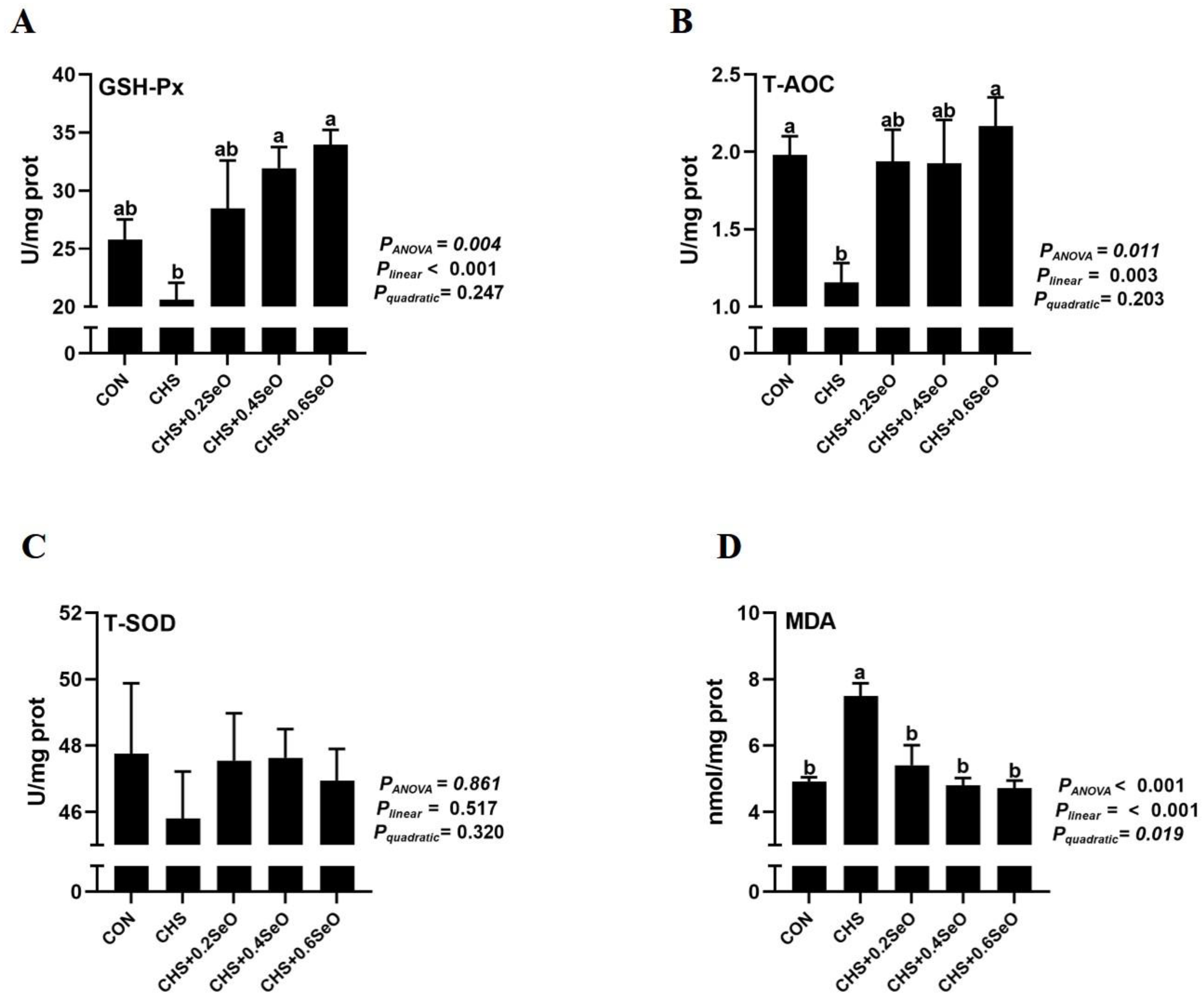
2.3. Splenic Antioxidant Capacity
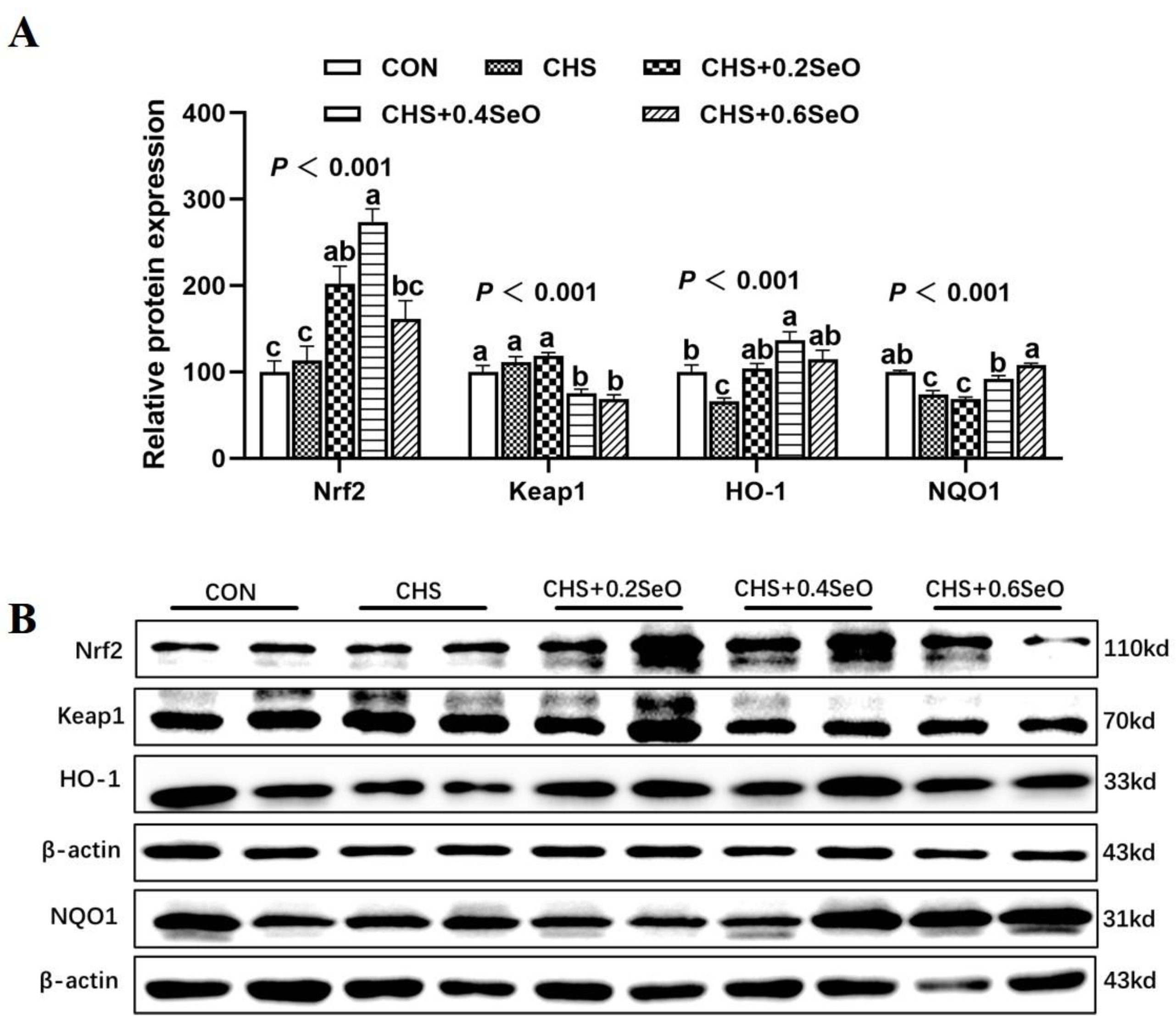
2.4. The Protein Abundance Related to the NRF2 Signal
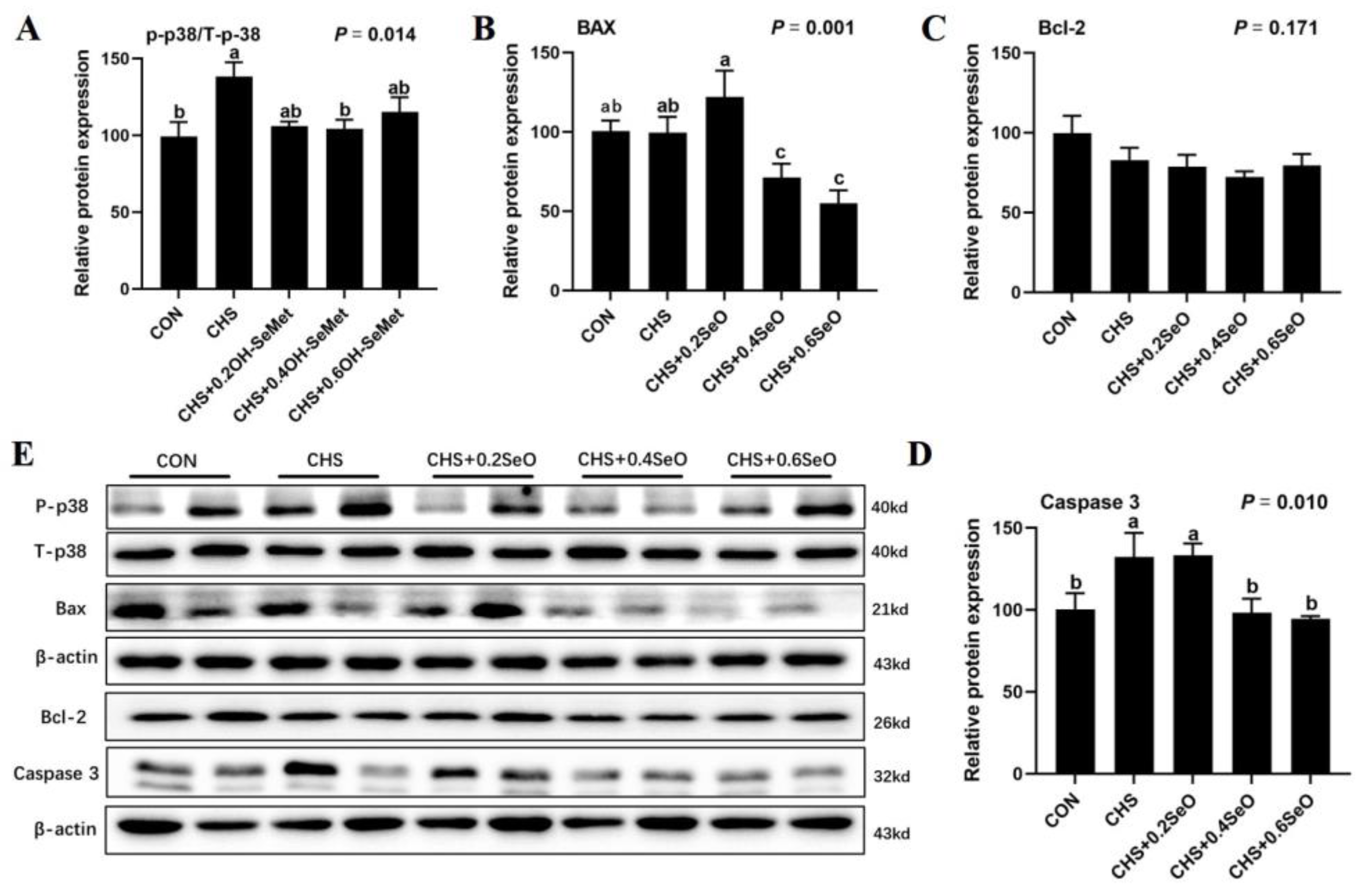
2.5. The Protein Expression of Apoptosis-Related Proteins
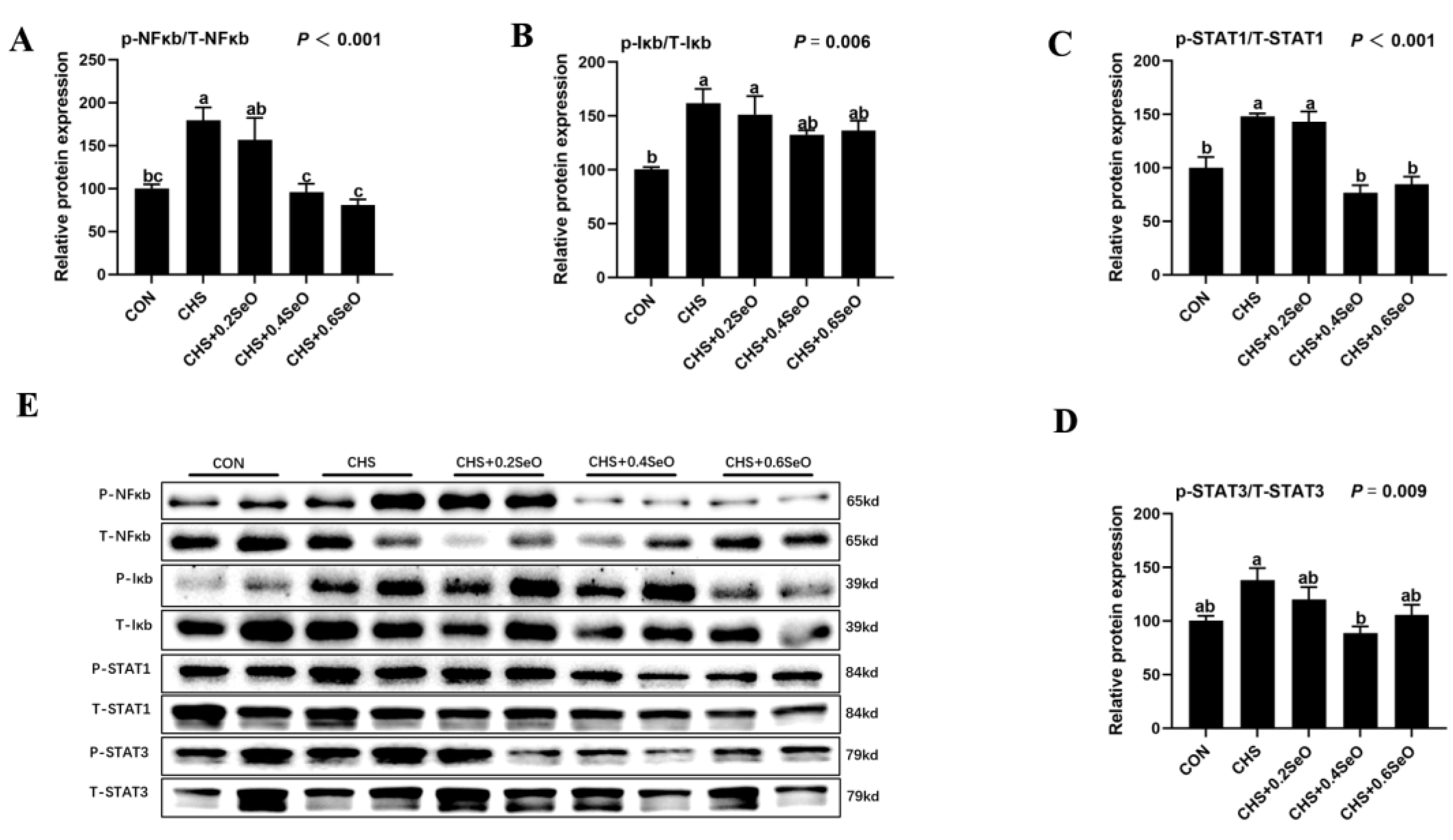
2.6. The Phosphorylation Levels of NFκb and STAT Signal
2.7. The mRNA Expression of Inflammatory Response-Related Genes
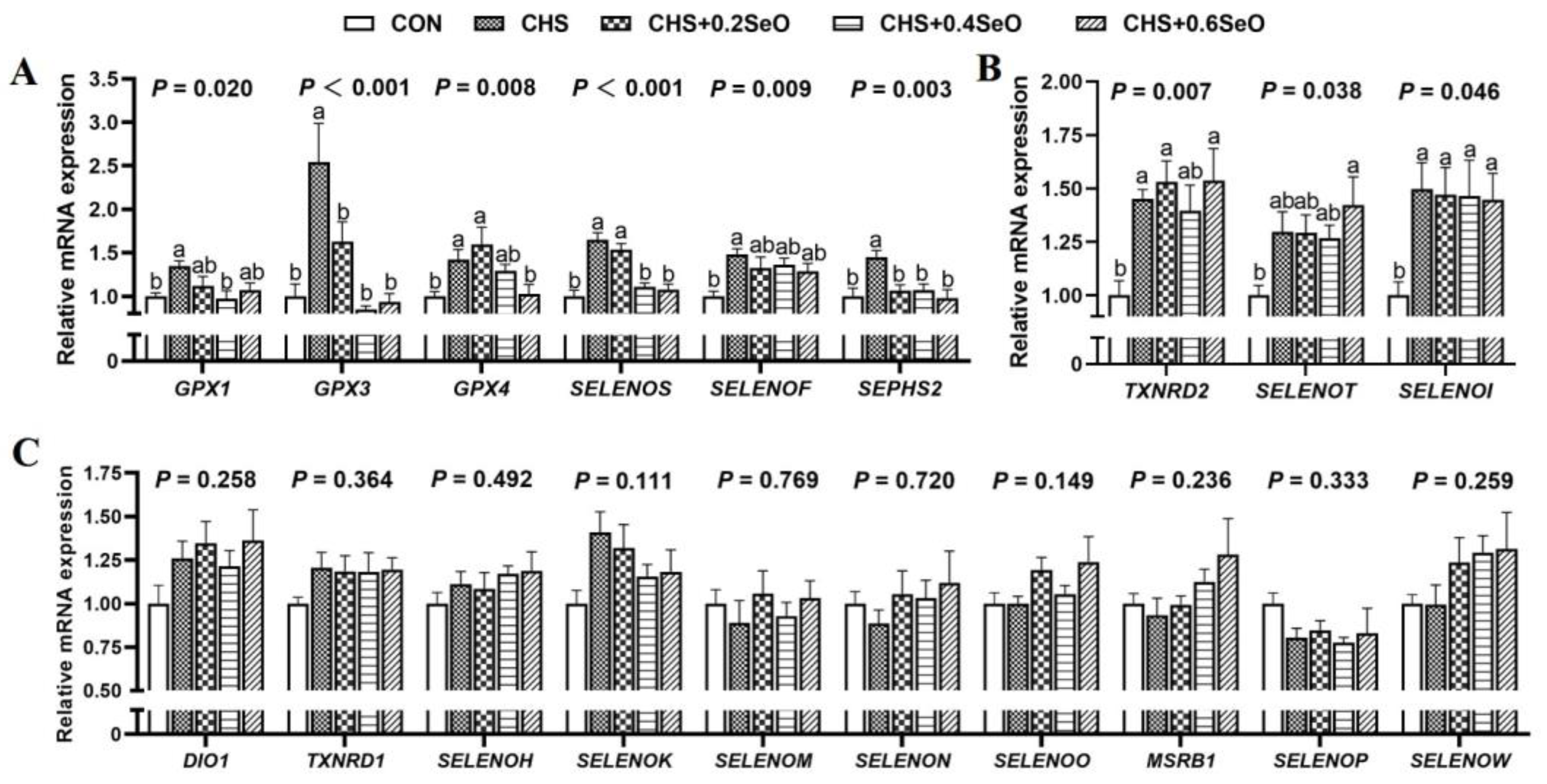
2.8. The mRNA Expression of Splenic Selenoproteins
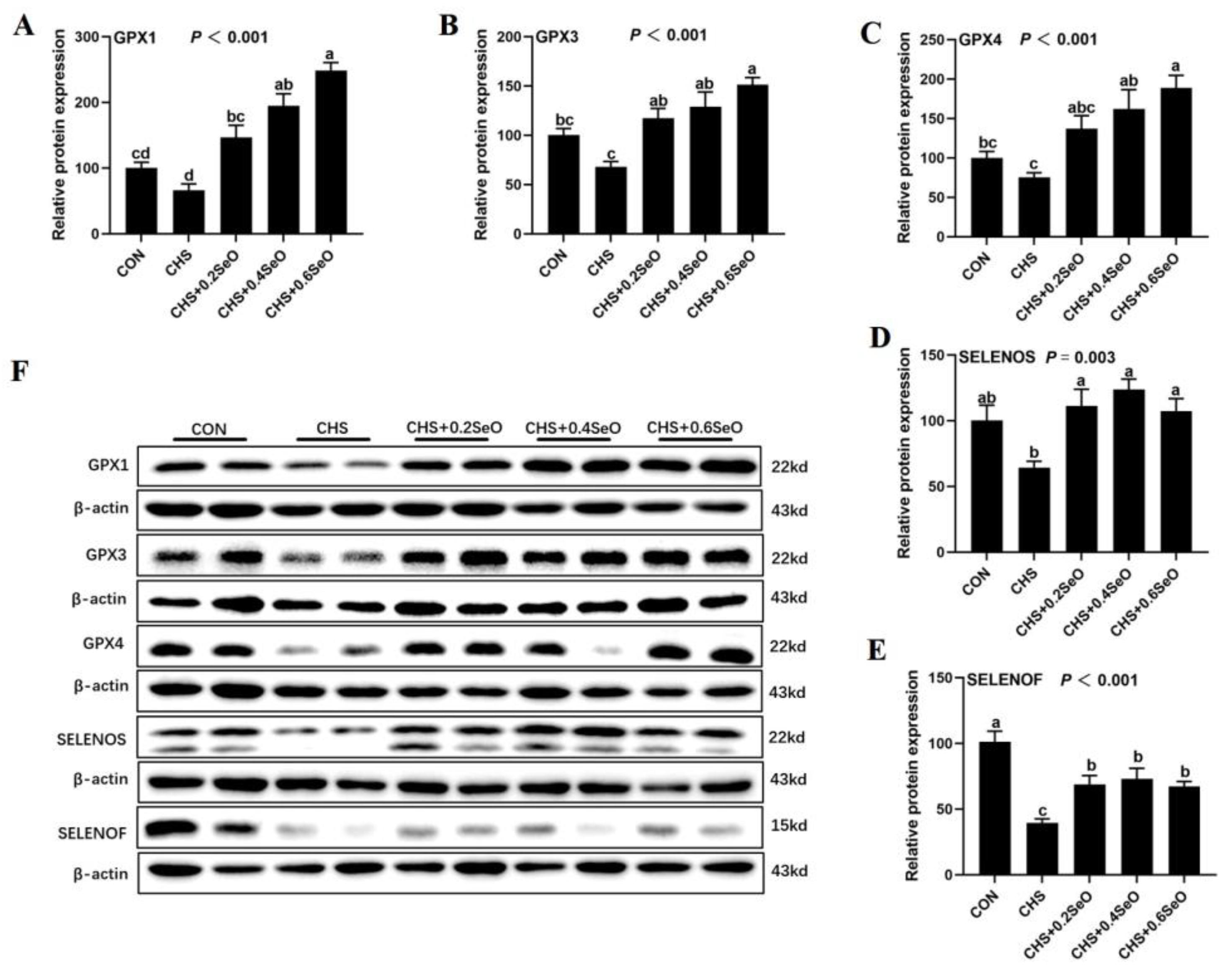
2.9. The Proteins Expression of Key Response Splenic Selenoproteins
3. Discussion
4. Materials and Methods
4.1. Animal, Experiment Design and Management
4.2. Blood and Tissue Collection
4.3. Blood Inflammatory Factor and Immunoglobulin
4.4. Selenium Concentration in Spleen Tissue
4.5. Splenic Antioxidant Capacity Analyses
4.6. Real-Time PCR Analyses
4.7. Western Blot Analyses
4.8. Statistical Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Shields, S.; Orme-Evans, G. The Impacts of Climate Change Mitigation Strategies on Animal Welfare. Animals 2015, 5, 361–394. [Google Scholar] [CrossRef]
- Huo, C.; Xiao, C.; She, R.; Liu, T.; Tian, J.; Dong, H.; Tian, H.; Hu, Y. Chronic heat stress negatively affects the immune functions of both spleens and intestinal mucosal system in pigs through the inhibition of apoptosis. Microb. Pathog. 2019, 136, 103672. [Google Scholar] [CrossRef]
- Bagath, M.; Krishnan, G.; Devaraj, C.; Rashamol, V.P.; Pragna, P.; Lees, A.M.; Sejian, V. The impact of heat stress on the immune system in dairy cattle: A review. Res. Vet. Sci. 2019, 126, 94–102. [Google Scholar] [CrossRef] [PubMed]
- Collin, A.; Lebreton, Y.; Fillaut, M.; Vincent, A.; Thomas, F.; Herpin, P. Effects of exposure to high temperature and feeding level on regional blood flow and oxidative capacity of tissues in piglets. Exp. Physiol. 2001, 86, 83–91. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Tang, J.; He, Y.; Jia, G.; Liu, G.; Tian, G.; Chen, X.; Cai, J.; Kang, B.; Zhao, H. Selenogenome and AMPK signal insight into the protective effect of dietary selenium on chronic heat stress-induced hepatic metabolic disorder in growing pigs. J. Anim. Sci. Biotechnol. 2021, 12, 68. [Google Scholar] [CrossRef]
- Pearce, S.C.; Gabler, N.K.; Ross, J.W.; Escobar, J.; Patience, J.F.; Rhoads, R.P.; Baumgard, L.H. The effects of heat stress and plane of nutrition on metabolism in growing pigs. J. Anim. Sci. 2013, 91, 2108–2118. [Google Scholar] [CrossRef] [PubMed]
- Bronte, V.; Pittet, M.J. The Spleen in Local and Systemic Regulation of Immunity. Immunity 2013, 39, 806–818. [Google Scholar] [CrossRef]
- Zhao, N.; Mu, L.; Chang, X.; Zhu, L.; Geng, Y.; Li, G. Effects of varying intensities of heat stress on neuropeptide Y and proopiomelanocortin mRNA expression in rats. Biomed. Rep. 2020, 13, 39. [Google Scholar] [PubMed]
- Jlali, M.; Briens, M.; Rouffineau, F.; Geraert, P.-A.; Mercier, Y. Evaluation of the efficacy of 2-hydroxy-4-methylselenobutanoic acid on growth performance and tissue selenium retention in growing pigs. J. Anim. Sci. 2014, 92, 182–188. [Google Scholar] [CrossRef]
- Briens, M.; Mercier, Y.; Rouffineau, F.; Vacchina, V.; Geraert, P.-A. Comparative study of a new organic selenium source v. seleno-yeast and mineral selenium sources on muscle selenium enrichment and selenium digestibility in broiler chickens. Br. J. Nutr. 2013, 110, 617–624. [Google Scholar] [CrossRef]
- Chen, X.-D.; Zhao, Z.-P.; Zhou, J.-C.; Lei, X.G. Evolution, regulation, and function of porcine selenogenome. Free. Radic. Biol. Med. 2018, 127, 116–123. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Jiang, L.; Li, Y.; Luo, X.; He, J. Effect of Different Selenium Supplementation Levels on Oxidative Stress, Cytokines, and Immunotoxicity in Chicken Thymus. Biol. Trace Element Res. 2016, 172, 488–495. [Google Scholar] [CrossRef] [PubMed]
- Liu, F.; Cottrell, J.J.; Furness, J.B.; Rivera, L.R.; Kelly, F.W.; Wijesiriwardana, U.; Pustovit, R.V.; Fothergill, L.J.; Bravo, D.M.; Celi, P.; et al. Selenium and vitamin E together improve intestinal epithelial barrier function and alleviate oxidative stress in heat-stressed pigs. Exp. Physiol. 2016, 101, 801–810. [Google Scholar] [CrossRef]
- Huang, Z.; Rose, A.H.; Hoffmann, P.R. The Role of Selenium in Inflammation and Immunity: From Molecular Mechanisms to Therapeutic Opportunities. Antioxidants Redox Signal. 2012, 16, 705–743. [Google Scholar] [CrossRef]
- Zheng, Y.; Xie, T.; Li, S.; Wang, W.; Wang, Y.; Cao, Z.; Yang, H. Effects of Selenium as a Dietary Source on Performance, Inflammation, Cell Damage, and Reproduction of Livestock Induced by Heat Stress: A Review. Front. Immunol. 2021, 12, 6002. [Google Scholar] [CrossRef] [PubMed]
- Ma, Q. Role of nrf2 in oxidative stress and toxicity. Annu. Rev. Pharmacol. Toxicol. 2013, 53, 401–426. [Google Scholar] [CrossRef]
- Hayes, J.D.; McMahon, M.; Chowdhry, S.; Dinkova-Kostova, A.T.; Turpaev, K.; Welsh, N.; Díaz-Alonso, J.; Paraíso-Luna, J.; Navarrete, C.; del Río, C.; et al. Cancer Chemoprevention Mechanisms Mediated Through the Keap1–Nrf2 Pathway. Antioxidants Redox Signal. 2010, 13, 1713–1748. [Google Scholar] [CrossRef] [PubMed]
- Han, X.; Song, X.; Yu, F.; Chen, L. A ratiometric fluorescent probe for imaging and quantifying anti-apoptotic effects of GSH under temperature stress. Chem. Sci. 2017, 8, 6991–7002. [Google Scholar] [CrossRef]
- Song, J.-H.; Kim, K.-J.; Choi, S.-Y.; Koh, E.-J.; Park, J.; Lee, B.-Y. Korean ginseng extract ameliorates abnormal immune response through the regulation of inflammatory constituents in Sprague Dawley rat subjected to environmental heat stress. J. Ginseng Res. 2018, 43, 252–260. [Google Scholar] [CrossRef]
- Seo, H.Y.; Kim, M.K.; Lee, S.H.; Hwang, J.S.; Park, K.G.; Jang, B.K. Kahweol Ameliorates the Liver Inflammation through the Inhibition of NF-κB and STAT3 Activation in Primary Kupffer Cells and Primary Hepatocytes. Nutrients 2018, 10, 863. [Google Scholar] [CrossRef]
- Liu, T.; Zhang, L.; Joo, D.; Sun, S.C. NF-κB signaling in inflammation. Signal Transduct. Target. Ther. 2017, 2, 17023. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; He, A.; Tang, J.; Shah, A.M.; Jia, G.; Liu, G.; Tian, G.; Chen, X.; Cai, J.; Kang, B.; et al. Selenium alleviates the negative effect of heat stress on myogenic differentiation of C2C12 cells with the response of selenogenome. J. Therm. Biol. 2021, 97, 102874. [Google Scholar] [CrossRef] [PubMed]
- Tang, J.; He, A.; Yan, H.; Jia, G.; Liu, G.; Chen, X.; Cai, J.; Tian, G.; Shang, H.; Zhao, H. Damage to the myogenic differentiation of C2C12 cells by heat stress is associated with up-regulation of several selenoproteins. Sci. Rep. 2018, 8, 10601. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.; Jing, J.; Yan, H.; Tang, J.; Jia, G.; Liu, G.; Chen, X.; Tian, G.; Cai, J.; Shang, H.; et al. Selenium Pretreatment Alleviated LPS-Induced Immunological Stress Via Upregulation of Several Selenoprotein Encoding Genes in Murine RAW264.7 Cells. Biol. Trace Element Res. 2018, 186, 505–513. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Yin, S.; Tang, J.; Liu, Y.; Jia, G.; Liu, G.; Tian, G.; Chen, X.; Cai, J.; Kang, B.; et al. Hydroxy Selenomethionine Improves Meat Quality through Optimal Skeletal Metabolism and Functions of Selenoproteins of Pigs under Chronic Heat Stress. Antioxidants 2021, 10, 1558. [Google Scholar] [CrossRef]
- Li, D.; Tong, Q.; Shi, Z.; Li, H.; Wang, Y.; Li, B.; Yan, G.; Chen, H.; Zheng, W. Effects of chronic heat stress and ammonia concentration on blood parameters of laying hens. Poult. Sci. 2020, 99, 3784–3792. [Google Scholar] [CrossRef]
- Safdari-Rostamabad, M.; Hosseini-Vashan, S.J.; Perai, A.H.; Sarir, H. Nanoselenium Supplementation of Heat-Stressed Broilers: Effects on Performance, Carcass Characteristics, Blood Metabolites, Immune Response, Antioxidant Status, and Jejunal Morphology. Biol. Trace Elem. Res. 2017, 178, 105–116. [Google Scholar] [CrossRef]
- Hoffmann, P.R.; Jourdan-Le Saux, C.; Hoffmann, F.W.; Chang, P.S.; Bollt, O.; He, Q.; Tam, E.K.; Berry, M.J. A Role for Dietary Selenium and Selenoproteins in Allergic Airway Inflammation. J. Immunol. 2007, 179, 3258–3267. [Google Scholar] [CrossRef]
- Cui, Y.; Hao, Y.; Li, J.; Bao, W.; Li, G.; Gao, Y.; Gu, X. Chronic Heat Stress Induces Immune Response, Oxidative Stress Response, and Apoptosis of Finishing Pig Liver: A Proteomic Approach. Int. J. Mol. Sci. 2016, 17, 393. [Google Scholar] [CrossRef]
- Akbarian, A.; Michiels, J.; DeGroote, J.; Majdeddin, M.; Golian, A.; De Smet, S. Association between heat stress and oxidative stress in poultry; mitochondrial dysfunction and dietary interventions with phytochemicals. J. Anim. Sci. Biotechnol. 2016, 7, 37. [Google Scholar] [CrossRef]
- Tang, J.-Y.; Wang, L.-Q.; Jia, G.; Liu, G.-M.; Chen, X.-L.; Tian, G.; Cai, J.-Y.; Shang, H.-Y.; Zhao, H. The hydroxy-analogue of selenomethionine alleviated lipopolysaccharide-induced inflammatory responses is associated with recover expression of several selenoprotein encoding genes in the spleens of Kunming mice. RSC Adv. 2019, 9, 40462–40470. [Google Scholar] [CrossRef] [PubMed]
- Yates, M.S.; Tran, Q.T.; Dolan, P.M.; Osburn, W.O.; Shin, S.; McCulloch, C.C.; Silkworth, J.B.; Taguchi, K.; Yamamoto, M.; Williams, C.R.; et al. Genetic versus chemoprotective activation of Nrf2 signaling: Overlapping yet distinct gene expression profiles between Keap1 knockout and triterpenoid-treated mice. Carcinogenesis 2009, 30, 1024–1031. [Google Scholar] [CrossRef] [PubMed]
- Reisman, S.A.; Buckley, D.B.; Tanaka, Y.; Klaassen, C.D. CDDO-Im protects from acetaminophen hepatotoxicity through induction of Nrf2-dependent genes. Toxicol. Appl. Pharmacol. 2009, 236, 109–114. [Google Scholar] [CrossRef] [PubMed]
- Kay, H.Y.; Won Yang, J.; Kim, T.H.; Lee, D.Y.; Kang, B.; Ryu, J.-H.; Jeon, R.; Kim, S.G. Ajoene, a Stable Garlic By-Product, Has an Antioxidant Effect through Nrf2-Mediated Glutamate-Cysteine Ligase Induction in HepG2 Cells and Primary Hepatocytes. J. Nutr. 2010, 140, 1211–1219. [Google Scholar] [CrossRef]
- Shin, D.-H.; Park, H.-M.; Jung, K.-A.; Choi, H.-G.; Kim, J.-A.; Kim, D.-D.; Kim, S.G.; Kang, K.W.; Ku, S.K.; Kensler, T.W.; et al. The NRF2–heme oxygenase-1 system modulates cyclosporin A-induced epithelial–mesenchymal transition and renal fibrosis. Free. Radic. Biol. Med. 2010, 48, 1051–1063. [Google Scholar] [CrossRef]
- Reszka, E.; Wieczorek, E.; Jablonska, E.; Janasik, B.; Fendler, W.; Wasowicz, W. Association between plasma selenium level and NRF2 target genes expression in humans. J. Trace Elem. Med. Biol. Organ Soc. Miner. Trace Elem. (GMS) 2015, 30, 102–106. [Google Scholar] [CrossRef]
- Zhang, J.; Wang, H.; Peng, D.; Taylor, E.W. Further insight into the impact of sodium selenite on selenoenzymes: High-dose selenite enhances hepatic thioredoxin reductase 1 activity as a consequence of liver injury. Toxicol. Lett. 2008, 176, 223–229. [Google Scholar] [CrossRef]
- Schwarz, M.; Lossow, K.; Kopp, J.F.; Schwerdtle, T.; Kipp, A.P. Crosstalk of Nrf2 with the Trace Elements Selenium, Iron, Zinc, and Copper. Nutrients 2019, 11, 2112. [Google Scholar] [CrossRef]
- Yao, H.-D.; Wu, Q.; Zhang, Z.-W.; Li, S.; Wang, X.-L.; Lei, X.-G.; Xu, S.-W. Selenoprotein W serves as an antioxidant in chicken myoblasts. Biochim. Biophys. Acta (BBA) 2013, 1830, 3112–3120. [Google Scholar] [CrossRef]
- Yao, H.-D.; Wu, Q.; Zhang, Z.-W.; Zhang, J.-L.; Li, S.; Huang, J.-Q.; Ren, F.-Z.; Xu, S.-W.; Wang, X.-L.; Lei, X.G. Gene Expression of Endoplasmic Reticulum Resident Selenoproteins Correlates with Apoptosis in Various Muscles of Se-Deficient Chicks. J. Nutr. 2013, 143, 613–619. [Google Scholar] [CrossRef]
- Kanter, M.; Aktas, C.; Erboga, M. Heat stress decreases testicular germ cell proliferation and increases apoptosis in short term: An immunohistochemical and ultrastructural study. Toxicol. Ind. Health 2013, 29, 99–113. [Google Scholar] [CrossRef] [PubMed]
- Hamanaka, R.B.; Chandel, N.S. Mitochondrial reactive oxygen species regulate cellular signaling and dictate biological outcomes. Trends Biochem. Sci. 2010, 35, 505–513. [Google Scholar] [CrossRef] [PubMed]
- Porter, A.G.; Jänicke, R.U. Emerging roles of caspase-3 in apoptosis. Cell Death Differ. 1999, 6, 99–104. [Google Scholar] [CrossRef] [PubMed]
- Xu, D.; Li, B.; Cao, N.; Li, W.; Tian, Y.; Huang, Y. The protective effects of polysaccharide of Atractylodes macrocephala Koidz (PAMK) on the chicken spleen under heat stress via antagonizing apoptosis and restoring the immune function. Oncotarget 2017, 8, 70394–70405. [Google Scholar] [CrossRef]
- Chen, M.; Li, X.; Fan, R.; Cao, C.; Yao, H.; Xu, S. Selenium antagonizes cadmium-induced apoptosis in chicken spleen but not involving Nrf2-regulated antioxidant response. Ecotoxicol. Environ. Saf. 2017, 145, 503–510. [Google Scholar] [CrossRef]
- Fernandes-Alnemri, T.; Yu, J.-W.; Datta, P.; Wu, J.; Alnemri, E.S. AIM2 activates the inflammasome and cell death in response to cytoplasmic DNA. Nature 2009, 458, 509–513. [Google Scholar] [CrossRef]
- Shih, V.F.-S.; Tsui, R.; Caldwell, A.; Hoffmann, A. A single NFκB system for both canonical and non-canonical signaling. Cell Res. 2011, 21, 86–102. [Google Scholar] [CrossRef]
- Schieven, G.L. The Biology of p38 Kinase: A Central Role in Inflammation. Curr. Top. Med. Chem. 2005, 5, 921–928. [Google Scholar] [CrossRef]
- Mello, A.S.; de Oliveira, D.C.; Bizzarro, B.; Sá-Nunes, A.; Hastreiter, A.A.; Beltran, J.S.D.O.; Xavier, J.G.; Borelli, P.; Fock, R.A. Protein Malnutrition Alters Spleen Cell Proliferation and IL-2 and IL-10 Production by Affecting the STAT-1 and STAT-3 Balance. Inflammation 2014, 37, 2125–2138. [Google Scholar] [CrossRef]
- He, S.; Yu, Q.; He, Y.; Hu, R.; Xia, S.; He, J. Dietary resveratrol supplementation inhibits heat stress-induced high-activated innate immunity and inflammatory response in spleen of yellow-feather broilers. Poult. Sci. 2019, 98, 6378–6387. [Google Scholar] [CrossRef]
- Wang, Y.; Li, C.; Pan, C.; Liu, E.; Zhao, X.; Ling, Q. Alterations to transcriptomic profile, histopathology, and oxidative stress in liver of pikeperch (Sander lucioperca) under heat stress. Fish Shellfish. Immunol. 2019, 95, 659–669. [Google Scholar] [CrossRef] [PubMed]
- Zhuang, C.; Gao, J.; Liu, G.; Zhou, M.; Yang, J.; Wang, D.; Kastelic, J.P.; Han, B. Selenomethionine activates selenoprotein S, suppresses Fas/FasL and the mitochondrial pathway, and reduces Escherichia coli-induced apoptosis of bovine mammary epithelial cells. J. Dairy Sci. 2021, 104, 10171–10182. [Google Scholar] [CrossRef] [PubMed]
- Conrad, M.; Jakupoglu, C.; Moreno, S.G.; Lippl, S.; Banjac, A.; Schneider, M.; Beck, H.; Hatzopoulos, A.K.; Just, U.; Sinowatz, F.; et al. Essential Role for Mitochondrial Thioredoxin Reductase in Hematopoiesis, Heart Development, and Heart Function. Mol. Cell. Biol. 2004, 24, 9414–9423. [Google Scholar] [CrossRef]
- Dikiy, A.; Novoselov, S.V.; Fomenko, D.E.; Sengupta, A.; Carlson, B.A.; Cerny, R.L.; Ginalski, K.; Grishin, N.V.; Hatfield, D.L.; Gladyshev, V.N. SelT, SelW, SelH, and Rdx12: Genomics and Molecular Insights into the Functions of Selenoproteins of a Novel Thioredoxin-like Family. Biochemistry 2007, 46, 6871–6882. [Google Scholar] [CrossRef] [PubMed]
- Yim, S.H.; Everley, R.A.; Schildberg, F.A.; Lee, S.-G.; Orsi, A.; Barbati, Z.R.; Karatepe, K.; Fomenko, D.E.; Tsuji, P.A.; Luo, H.R.; et al. Role of Selenof as a Gatekeeper of Secreted Disulfide-Rich Glycoproteins. Cell Rep. 2018, 23, 1387–1398. [Google Scholar] [CrossRef]
- Wu, R.T.; Cao, L.; Chen, B.P.; Cheng, W.-H. Selenoprotein H Suppresses Cellular Senescence through Genome Maintenance and Redox Regulation. J. Biol. Chem. 2014, 289, 34378–34388. [Google Scholar] [CrossRef]
- He, Y.; Liu, Y.; Tang, J.; Jia, G.; Liu, G.; Tian, G.; Chen, X.; Cai, J.; Kang, B.; Zhao, H. Selenium exerts protective effects against heat stress-induced barrier disruption and inflammation response in jejunum of growing pigs. J. Sci. Food Agric. 2021, 102, 496–504. [Google Scholar] [CrossRef] [PubMed]
- Huang, X.; Tang, J.; Xu, J.; Jia, G.; Liu, G.; Chen, X.; Cai, J.; Shang, H.; Zhao, H. Supranutritional dietary selenium induced hyperinsulinemia and dyslipidemia via affected expression of selenoprotein genes and insulin signal-related genes in broiler. RSC Adv. 2016, 6, 84990–84998. [Google Scholar] [CrossRef]
- Saxton, A.M. A macro for converting mean separation output to letter groupings in Proc Mixed. In Proceedings of the 23rd SAS Users Group International, Nashville, TN, USA, 22–25 March 1998; pp. 1243–1246. [Google Scholar]



| Item | Con | High-Temperature Conditions | SEM * | ANOVA p-Value | Linear | Quadratic | |||
|---|---|---|---|---|---|---|---|---|---|
| CHS | 0.2SeO | 0.4SeO | 0.6SeO | ||||||
| IgA (mg/L) | 49.54 | 46.67 | 46.66 | 42.23 | 43.16 | 0.97 | 0.103 | 0.133 | 0.827 |
| IgG (g/L) | 3.88 ab | 4.38a | 4.32 a | 3.57 ab | 3.03 b | 0.14 | 0.002 | <0.001 | 0.296 |
| IgM (mg/L) | 0.40 | 0.41 | 0. 43 | 0.41 | 0.45 | 0.01 | 0.432 | 0.198 | 0.488 |
| TNF-α (ng/L) | 0.41 | 0.43 | 0.36 | 0.45 | 0.45 | 0.01 | 0.115 | 0.233 | 0.228 |
| IL-6 (ng/L) | 1.10 a | 0.94 ab | 1.08 a | 0.82 b | 0.97 ab | 0.27 | 0.009 | 0.963 | 0.358 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Liu, Y.; Yin, S.; He, Y.; Tang, J.; Pu, J.; Jia, G.; Liu, G.; Tian, G.; Chen, X.; Cai, J.; et al. Hydroxy-Selenomethionine Mitigated Chronic Heat Stress-Induced Porcine Splenic Damage via Activation of Nrf2/Keap1 Signal and Suppression of NFκb and STAT Signal. Int. J. Mol. Sci. 2023, 24, 6461. https://doi.org/10.3390/ijms24076461
Liu Y, Yin S, He Y, Tang J, Pu J, Jia G, Liu G, Tian G, Chen X, Cai J, et al. Hydroxy-Selenomethionine Mitigated Chronic Heat Stress-Induced Porcine Splenic Damage via Activation of Nrf2/Keap1 Signal and Suppression of NFκb and STAT Signal. International Journal of Molecular Sciences. 2023; 24(7):6461. https://doi.org/10.3390/ijms24076461
Chicago/Turabian StyleLiu, Yan, Shenggang Yin, Ying He, Jiayong Tang, Junning Pu, Gang Jia, Guangmang Liu, Gang Tian, Xiaoling Chen, Jingyi Cai, and et al. 2023. "Hydroxy-Selenomethionine Mitigated Chronic Heat Stress-Induced Porcine Splenic Damage via Activation of Nrf2/Keap1 Signal and Suppression of NFκb and STAT Signal" International Journal of Molecular Sciences 24, no. 7: 6461. https://doi.org/10.3390/ijms24076461
APA StyleLiu, Y., Yin, S., He, Y., Tang, J., Pu, J., Jia, G., Liu, G., Tian, G., Chen, X., Cai, J., Kang, B., Che, L., & Zhao, H. (2023). Hydroxy-Selenomethionine Mitigated Chronic Heat Stress-Induced Porcine Splenic Damage via Activation of Nrf2/Keap1 Signal and Suppression of NFκb and STAT Signal. International Journal of Molecular Sciences, 24(7), 6461. https://doi.org/10.3390/ijms24076461







