The Attenuation of Insulin/IGF-1 Signaling Pathway Plays a Crucial Role in the Myo-Inositol-Alleviated Aging in Caenorhabditis elegans
Abstract
1. Introduction
2. Results
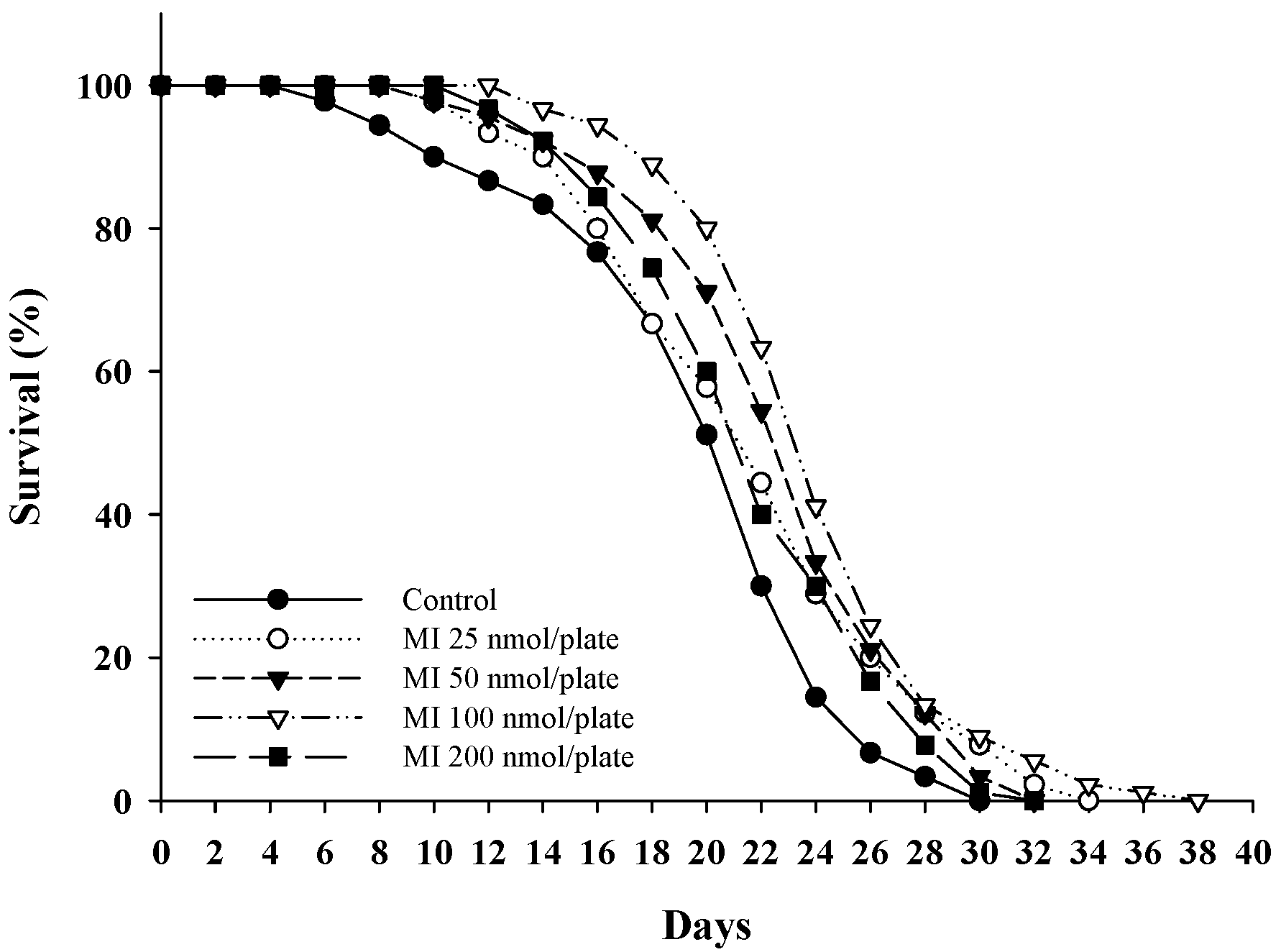
2.1. Effects of MI on the Lifespan in the C. elegans When the Osmotic Pressure Interference Was Excluded
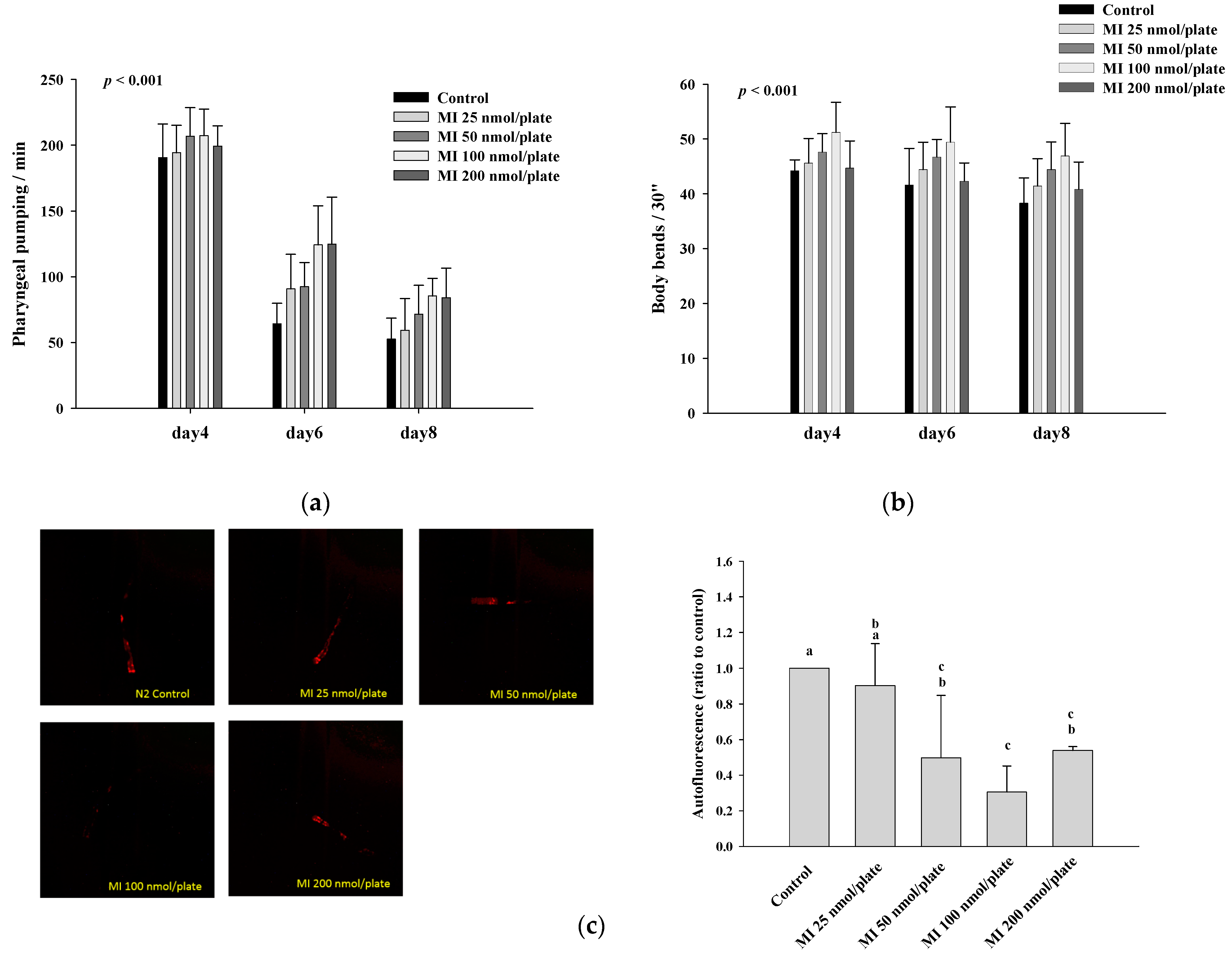
2.2. Effects of MI on the Pharyngeal Pumping, Body Bend and Autofluorescence in the C. elegans When the Osmotic Pressure Interference Was Excluded
2.3. Effects of MI on the Lifespan, Health Indexes and Autofluorescence of the AKT-1 Mutants
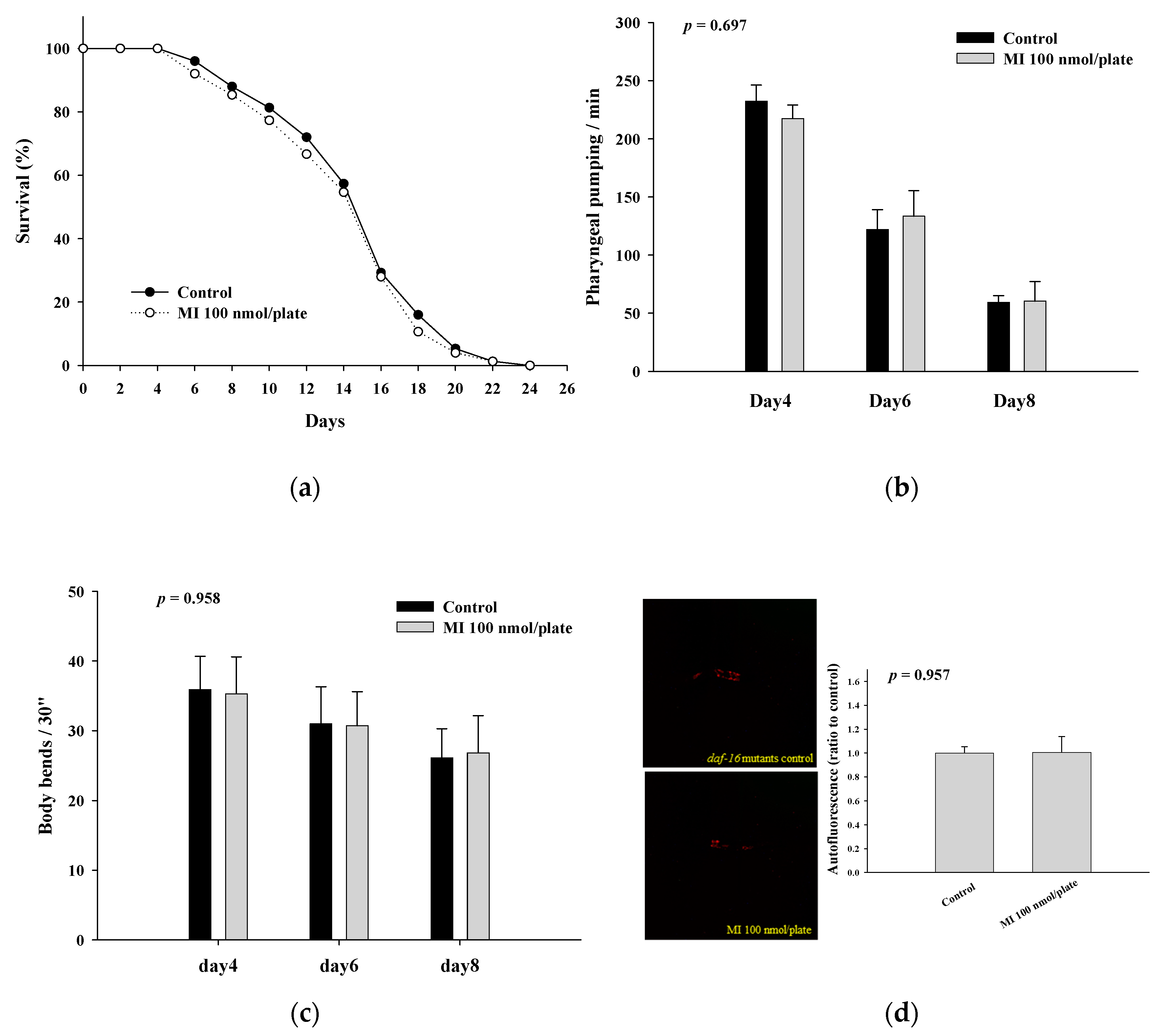
2.4. Effects of MI on the Lifespan, Health Indexes and Autofluorescence of the DAF-16 Mutants
2.5. Effects of MI on the Lifespan, Health Indexes and Autofluorescence of the DAF-18 Mutants When the Osmotic Pressure Interference Was Excluded
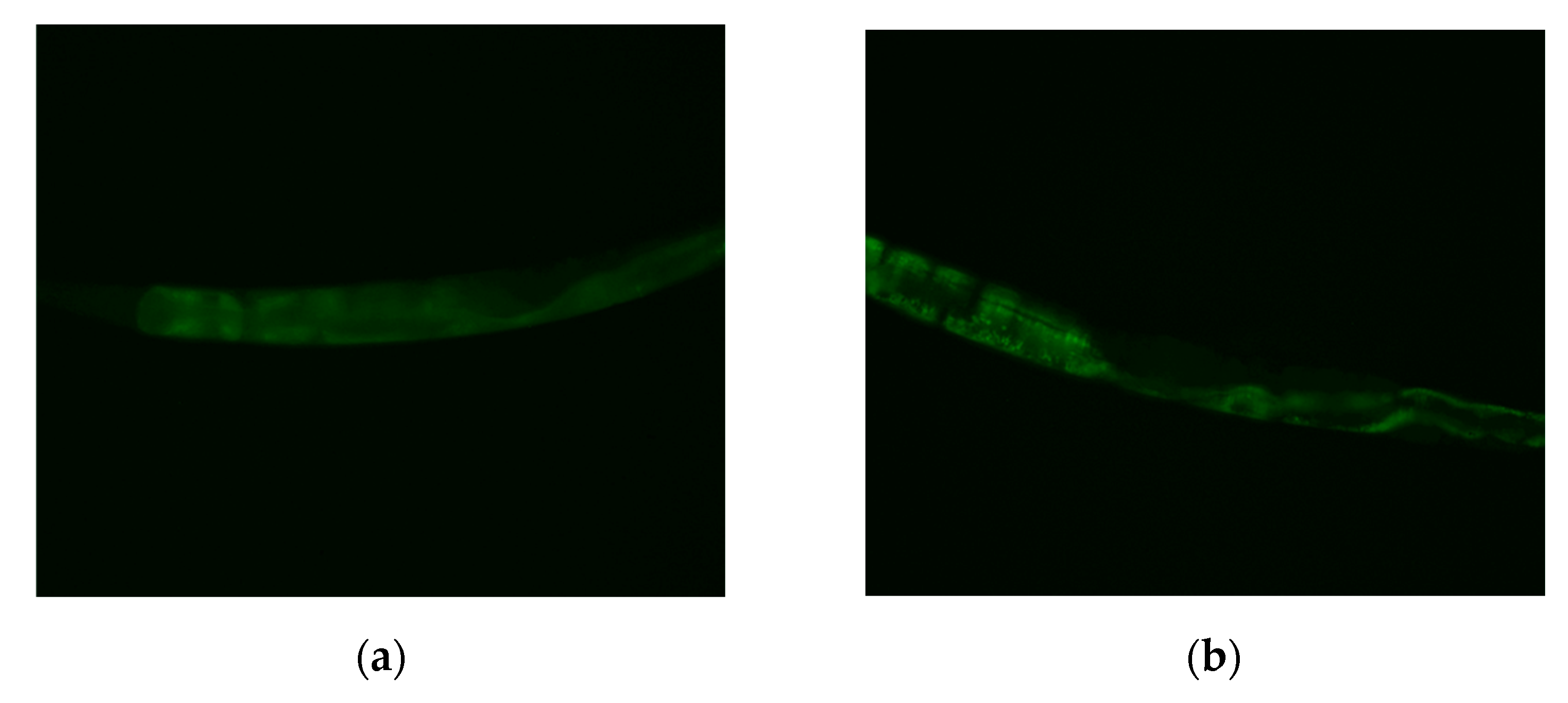
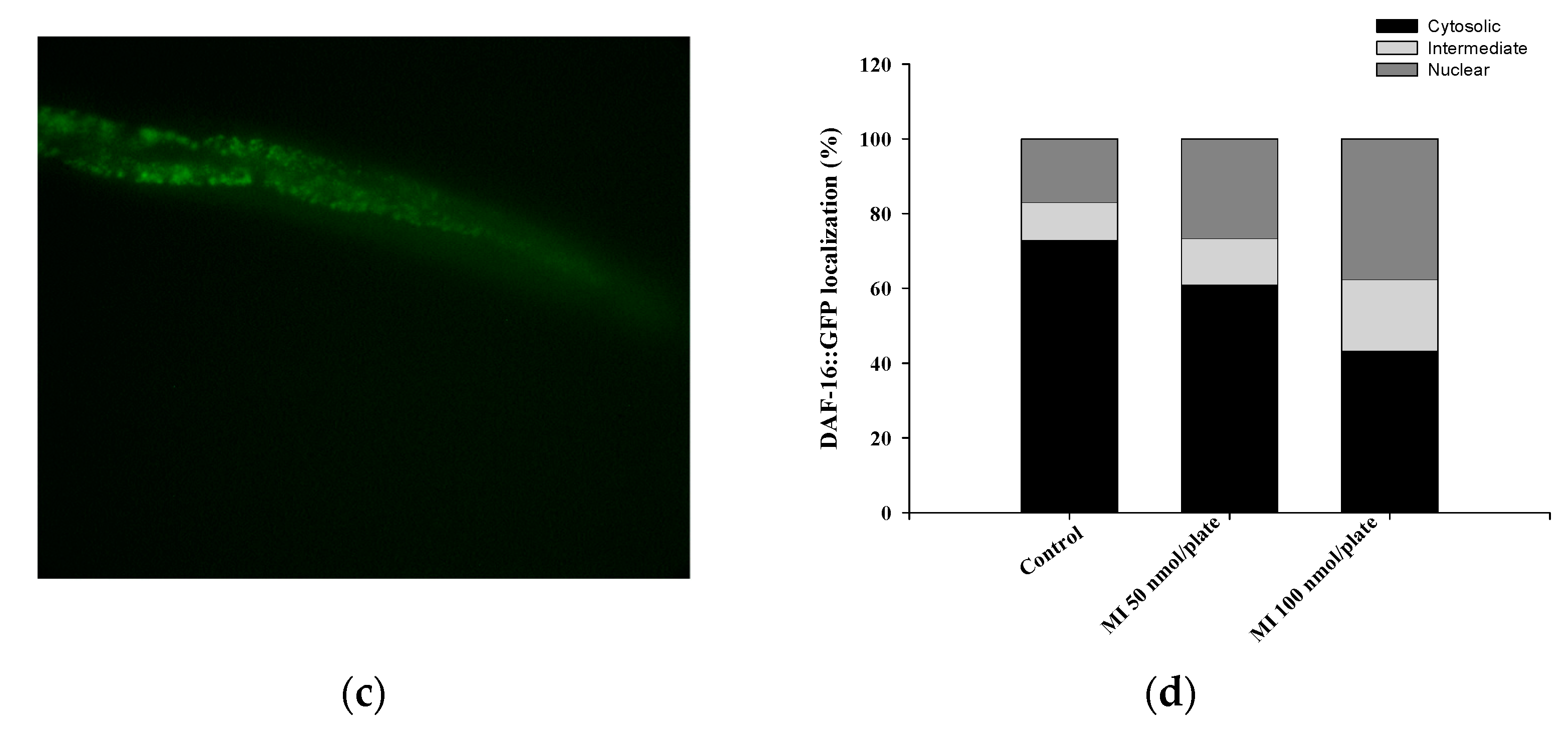
2.6. Effects of MI on the Nuclear Localization of the DAF-16
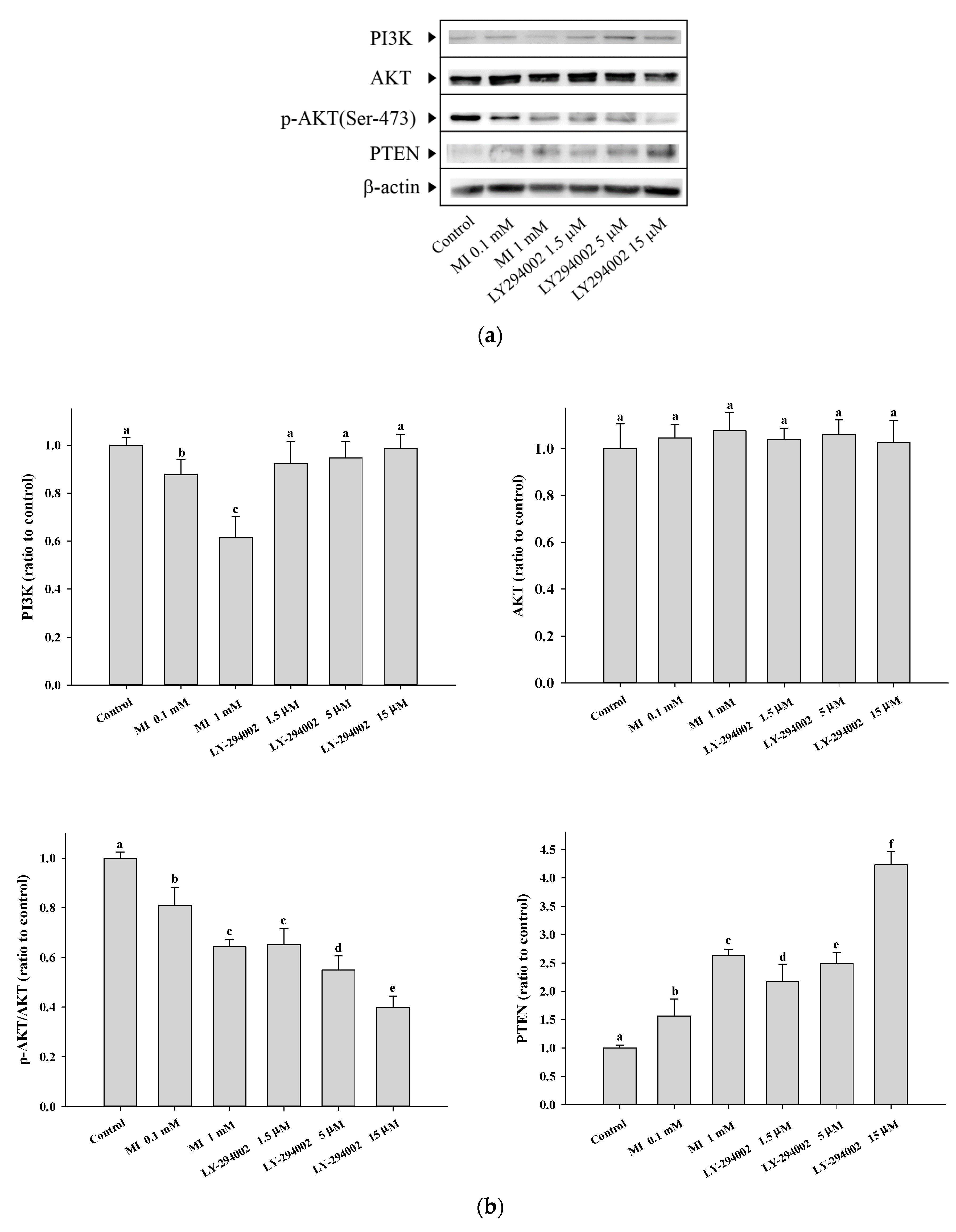
2.7. Effects of MI on the Expression of PI3K and Phosphorylation of AKT in the C. elegans and the Hs68 Cells
2.8. Effects of MI on the PTEN Expressions in the Hs68 Cells
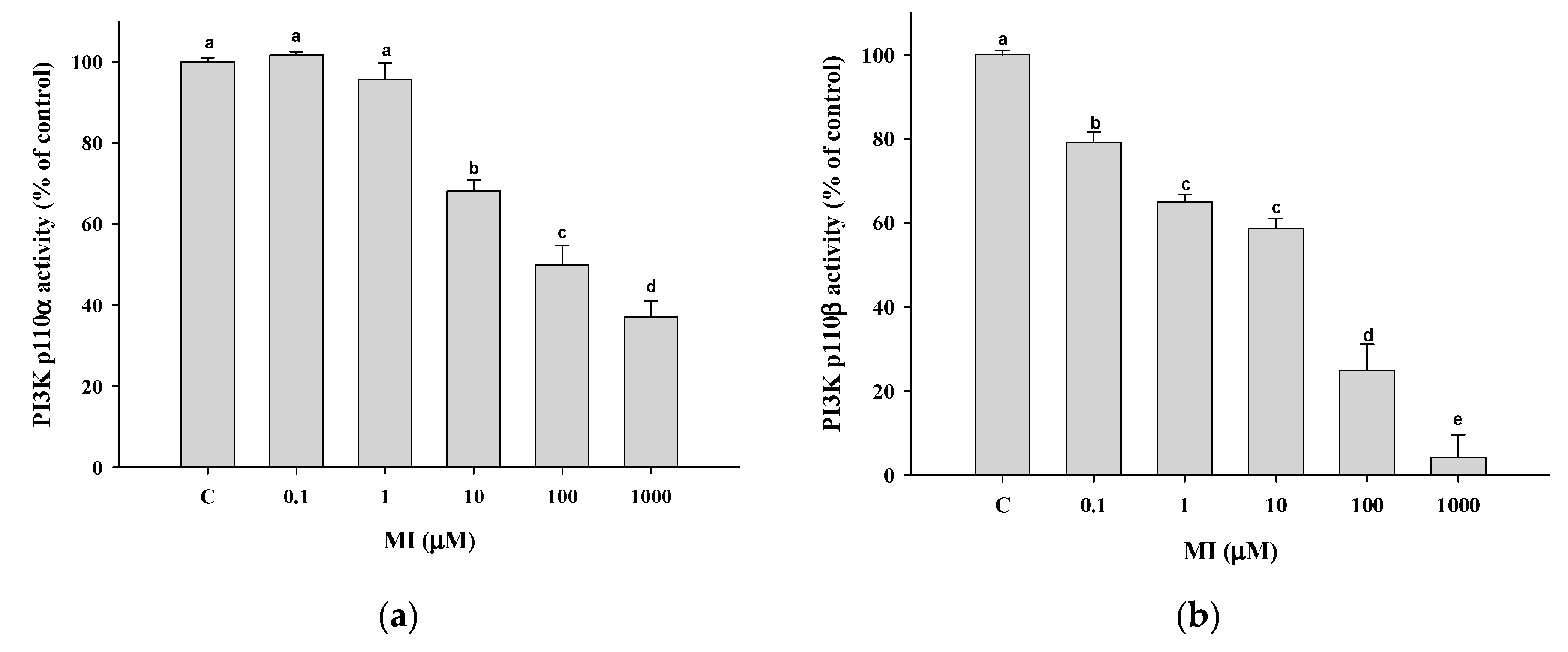
2.9. Effects of MI on the PI3K Activity In Vitro
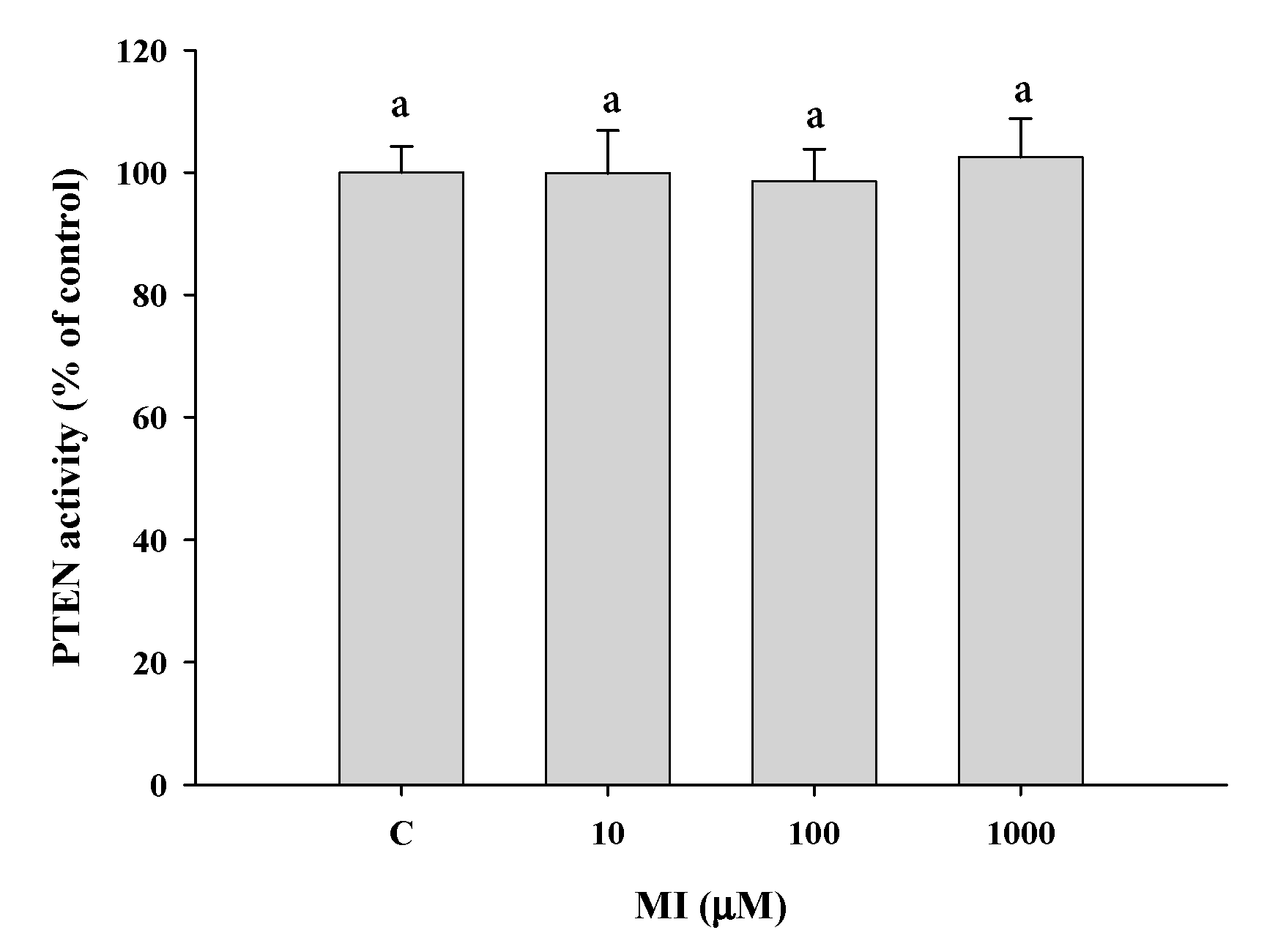
2.10. Effects of MI on the PTEN Activities In Vitro
3. Discussion
4. Materials and Methods
4.1. Materials
4.2. Handling Procedures for the C. elegans
4.3. Synchronization of the C. elegans
4.4. Assay of the Lifespan of the C. elegans
4.5. Assay of the Pharyngeal Pumping in the C. elegans
4.6. Assay of the Body Bends in the C. elegans
4.7. Determination of the Autofluorescence in the C. elegans
4.8. Distinguishing the Loss-of-Function Mutants
4.9. DAF-16 Nuclear Localization Assay in the C. elegans
4.10. Western Blotting in the C. elegans and Hs68 Cells
4.11. Measurement of the Inhibition of MI on the PI3K Activity
4.12. In Vitro PTEN Activity Assay
4.13. Statistical Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Altintas, O.; Park, S.; Lee, S.J. The role of insulin/IGF-1 signaling in the longevity of model invertebrates, C. elegans and D. melanogaster. BMB Rep. 2016, 49, 81–92. [Google Scholar] [CrossRef] [PubMed]
- Murphy, C.T.; Hu, P.J. Insulin/insulin-like growth factor signaling in C. elegans. WormBook 2013, 26, 1–43. [Google Scholar] [CrossRef] [PubMed]
- Hoshino, S.; Kobayashi, M.; Higami, Y. Mechanisms of the anti-aging and prolongevity effects of caloric restriction: Evidence from studies of genetically modified animals. Aging 2018, 10, 2243–2251. [Google Scholar] [CrossRef] [PubMed]
- Santos, J.; Leitão-Correia, F.; Sousa, M.J.; Leão, C. Dietary restriction and nutrient balance in aging. Oxid. Med. Cell Longev. 2016, 2016, 4010357. [Google Scholar] [CrossRef] [PubMed]
- Sun, X.; Chen, W.D.; Wang, Y.D. DAF-16/FOXO transcription factor in aging and longevity. Front. Pharmacol. 2017, 8, 548. [Google Scholar] [CrossRef]
- Hwangbo, D.S.; Lee, H.Y.; Abozaid, L.S.; Min, K.J. Mechanisms of lifespan regulation by calorie restriction and intermittent fasting in model organisms. Nutrients 2020, 12, 1194. [Google Scholar] [CrossRef]
- Ingram, D.K.; Roth, G.S. Calorie restriction mimetics: Can you have your cake and eat it, too? Ageing Res. Rev. 2015, 20, 46–62. [Google Scholar] [CrossRef]
- Testa, G.; Biasi, F.; Poli, G.; Chiarpotto, E. Calorie restriction and dietary restriction mimetics: A strategy for improving healthy aging and longevity. Curr. Pharm. Des. 2014, 20, 2950–2977. [Google Scholar] [CrossRef]
- Calvert, S.; Tacutu, R.; Sharifi, S.; Teixeira, R.; Ghosh, P.; de Magalhães, J.P. A network pharmacology approach reveals new candidate caloric restriction mimetics in C. elegans. Aging Cell 2016, 15, 256–266. [Google Scholar] [CrossRef]
- Bizzarri, M.; Dinicola, S.; Bevilacqua, A.; Cucina, A. Broad spectrum anticancer activity of myo-inositol and inositol hexakisphosphate. Int. J. Endocrinol. 2016, 2016, 5616807. [Google Scholar] [CrossRef]
- Dinicola, S.; Fabrizi, G.; Masiello, M.G.; Proietti, S.; Palombo, A.; Minini, M.; Harrath, A.H.; Alwasel, S.H.; Ricci, G.; Catizone, A.; et al. Inositol induces mesenchymal-epithelial reversion in breast cancer cells through cytoskeleton rearrangement. Exp. Cell Res. 2016, 345, 37–50. [Google Scholar] [CrossRef]
- Han, W.; Gills, J.J.; Memmott, R.M.; Lam, S.; Dennis, P.A. The chemopreventive agent myoinositol inhibits Akt and extracellular signal-regulated kinase in bronchial lesions from heavy smokers. Cancer Prev. Res. 2009, 2, 370–376. [Google Scholar] [CrossRef]
- Gonzalez-Uarquin, F.; Rodehutscord, M.; Huber, K. Myo-inositol: Its metabolism and potential implications for poultry nutrition-a review. Poult. Sci. 2020, 99, 893–905. [Google Scholar] [CrossRef] [PubMed]
- López-Gambero, A.J.; Sanjuan, C.; Serrano-Castro, P.J.; Suárez, J.; Rodríguez de Fonseca, F. The Biomedical uses of inositols: A nutraceutical approach to metabolic dysfunction in aging and neurodegenerative diseases. Biomedicines 2020, 8, 295. [Google Scholar] [CrossRef] [PubMed]
- Chhetri, D.R. myo-Inositol and its derivatives: Their emerging role in the treatment of human diseases. Front. Pharmacol. 2019, 10, 1172. [Google Scholar] [CrossRef] [PubMed]
- Thomas, M.P.; Mills, S.J.; Potter, B.V. The “other” inositols and their phosphates: Synthesis, biology, and medicine (with recent advances in myo-inositol chemistry). Angew. Chem. Int. Ed. Engl. 2016, 55, 1614–1650. [Google Scholar] [CrossRef] [PubMed]
- Schellack, G.; Harirari, P.; Schellack, N. B-complex vitamin deficiency and supplementation. S. Afr. Pharm. J. 2015, 83, 4. [Google Scholar]
- Bizzarri, M.; Fuso, A.; Dinicola, S.; Cucina, A.; Bevilacqua, A. Pharmacodynamics and pharmacokinetics of inositol(s) in health and disease. Expert. Opin. Drug. Metab. Toxicol. 2016, 12, 1181–1196. [Google Scholar] [CrossRef]
- Croze, M.L.; Soulage, C.O. Potential role and therapeutic interests of myo-inositol in metabolic diseases. Biochimie 2013, 95, 1811–1827. [Google Scholar] [CrossRef]
- Vucenik, I. Anticancer properties of inositol hexaphosphate and inositol: An overview. J. Nutr. Sci. Vitaminol. 2019, 65, S18–S22. [Google Scholar] [CrossRef]
- Nishino, H.; Murakoshi, M.; Masuda, M.; Tokuda, H.; Satomi, Y.; Onozuka, M.; Yamaguchi, S.; Bu, P.; Tsuruta, A.; Nosaka, K.; et al. Suppression of lung and liver carcinogenesis in mice by oral administration of myo-inositol. Anticancer Res. 1999, 19, 3663–3664. [Google Scholar] [PubMed]
- Hada, B.; Yoo, M.R.; Seong, K.M.; Jin, Y.W.; Myeong, H.K.; Min, K.J. D-chiro-inositol and pinitol extend the life span of Drosophila melanogaster. J Gerontol. A Biol. Sci. Med. Sci. 2013, 68, 226–234. [Google Scholar] [CrossRef] [PubMed]
- Shi, D.; Xia, X.; Cui, A.; Xiong, Z.; Yan, Y.; Luo, J.; Chen, G.; Zeng, Y.; Cai, D.; Hou, L.; et al. The precursor of PI(3,4,5)P3 alleviates aging by activating DAF-18(Pten) and independent of DAF-16. Nat. Commun. 2020, 11, 4496. [Google Scholar] [CrossRef] [PubMed]
- Chandler-Brown, D.; Choi, H.; Park, S.; Ocampo, B.R.; Chen, S.; Le, A.; Sutphin, G.L.; Shamieh, L.S.; Smith, E.D.; Kaeberlein, M. Sorbitol treatment extends lifespan and induces the osmotic stress response in Caenorhabditis elegans. Front. Genet. 2015, 6, 316. [Google Scholar] [CrossRef] [PubMed]
- Yaguchi, Y.; Komura, T.; Kashima, N.; Tamura, M.; Kage-Nakadai, E.; Saeki, S.; Terao, K.; Nishikawa, Y. Influence of oral supplementation with sesamin on longevity of Caenorhabditis elegans and the host defense. Eur. J. Nutr. 2014, 53, 1659–1668. [Google Scholar] [CrossRef]
- Yang, N.C.; Cho, Y.H.; Lee, I. The lifespan extension ability of nicotinic acid depends on whether the intracellular NAD(+) level is lower than the sirtuin-saturating concentrations. Int. J. Mol. Sci. 2020, 21, 142. [Google Scholar] [CrossRef]
- Tao, L.; Xie, Q.; Ding, Y.H.; Li, S.T.; Peng, S.; Zhang, Y.P.; Tan, D.; Yuan, Z.; Dong, M.Q. CAMKII and calcineurin regulate the lifespan of Caenorhabditis elegans through the FOXO transcription factor DAF-16. Elife 2013, 2, e00518. [Google Scholar] [CrossRef]
- Luo, H.; Yang, Y.; Duan, J.; Wu, P.; Jiang, Q.; Xu, C. PTEN-regulated AKT/FoxO3a/Bim signaling contributes to reactive oxygen species-mediated apoptosis in selenite-treated colorectal cancer cells. Cell Death Dis. 2013, 4, e481. [Google Scholar] [CrossRef]
- Estevez, A.O.; Morgan, K.L.; Szewczyk, N.J.; Gems, D.; Estevez, M. The neurodegenerative effects of selenium are inhibited by FOXO and PINK1/PTEN regulation of insulin/insulin-like growth factor signaling in Caenorhabditis elegans. Neurotoxicology 2014, 41, 28–43. [Google Scholar] [CrossRef]
- Vanhaesebroeck, B.; Whitehead, M.A.; Piñeiro, R. Molecules in medicine mini-review: Isoforms of PI3K in biology and disease. J. Mol. Med. 2016, 94, 5–11. [Google Scholar] [CrossRef]
- Jean, S.; Kiger, A.A. Classes of phosphoinositide 3-kinases at a glance. J. Cell Sci. 2014, 127, 923–928. [Google Scholar] [CrossRef] [PubMed]
- Unoki, M.; Nakamura, Y. Growth-suppressive effects of BPOZ and EGR2, two genes involved in the PTEN signaling pathway. Oncogene 2001, 20, 4457–4465. [Google Scholar] [CrossRef] [PubMed]
- Mei, Y.; Zhang, Y.; Yamamoto, K.; Xie, W.; Mak, T.W.; You, H. FOXO3a-dependent regulation of Pink1 (Park6) mediates survival signaling in response to cytokine deprivation. Proc. Natl. Acad. Sci. USA 2009, 106, 5153–5158. [Google Scholar] [CrossRef]
- O’Flanagan, C.H.; O’Neill, C. PINK1 signalling in cancer biology. Biochim. Biophys. Acta. 2014, 1846, 590–598. [Google Scholar] [CrossRef] [PubMed]
- Sutphin, G.L.; Kaeberlein, M. Measuring Caenorhabditis elegans life span on solid media. JoVE 2009, 27, 1152. [Google Scholar]
- Iwasa, H.; Yu, S.; Xue, J.; Driscoll, M. Novel EGF pathway regulators modulate C. elegans healthspan and lifespan via EGF receptor, PLC-gamma, and IP3R activation. Aging Cell 2010, 9, 490–505. [Google Scholar] [CrossRef]
- Zhao, Y.; Gilliat, A.F.; Ziehm, M.; Turmaine, M.; Wang, H.; Ezcurra, M.; Yang, C.; Phillips, G.; McBay, D.; Zhang, W.B.; et al. Two forms of death in ageing Caenorhabditis elegans. Nat. Commun. 2017, 8, 15458. [Google Scholar] [CrossRef]
- Pincus, Z.; Mazer, T.C.; Slack, F.J. Autofluorescence as a measure of senescence in C. elegans: Look to red, not blue or green. Aging 2016, 8, 889–898. [Google Scholar] [CrossRef]
- Oh, S.W.; Mukhopadhyay, A.; Svrzikapa, N.; Jiang, F.; Davis, R.J.; Tissenbaum, H.A. JNK regulates lifespan in Caenorhabditis elegans by modulating nuclear translocation of forkhead transcription factor/DAF-16. Proc. Natl. Acad. Sci. USA 2005, 102, 4494–4499. [Google Scholar] [CrossRef]
- Song, B.; Zheng, B.; Li, T.; Liu, R.H. Raspberry extract promoted longevity and stress tolerance via the insulin/IGF signaling pathway and DAF-16 in Caenorhabditis elegans. Food Funct. 2020, 11, 3598–3609. [Google Scholar] [CrossRef]
- Jeong, D.E.; Lee, Y.; Lee, S.V. Western Blot Analysis of C. elegans Proteins. Methods Mol. Biol. 2018, 1742, 213–225. [Google Scholar] [PubMed]
- Dai, W.; Choubey, M.; Patel, S.; Singer, H.A.; Ozcan, L. Adipocyte CAMK2 deficiency improves obesity-associated glucose intolerance. Mol. Metab. 2021, 53, 101300. [Google Scholar] [CrossRef] [PubMed]
- Tan, Y.H.; Shudo, T.; Yoshida, T.; Sugiyama, Y.; Si, J.Y.; Tsukano, C.; Takemoto, Y.; Kakizuka, A. Ellagic acid, extracted from Sanguisorba officinalis, induces G1 arrest by modulating PTEN activity in B16F10 melanoma cells. Genes Cells 2019, 24, 688–704. [Google Scholar] [CrossRef] [PubMed]

 as: “The inhibition step of MI or the inactivation step in the signaling pathway”, the symbol ⇩ as “The detected downregulation of protein expressions or protein phosphorylation by MI”, and ⇧ as “The detected upregulation of protein expression by MI” in this study. NB: the references are indicated in square brackets.
as: “The inhibition step of MI or the inactivation step in the signaling pathway”, the symbol ⇩ as “The detected downregulation of protein expressions or protein phosphorylation by MI”, and ⇧ as “The detected upregulation of protein expression by MI” in this study. NB: the references are indicated in square brackets.
 as: “The inhibition step of MI or the inactivation step in the signaling pathway”, the symbol ⇩ as “The detected downregulation of protein expressions or protein phosphorylation by MI”, and ⇧ as “The detected upregulation of protein expression by MI” in this study. NB: the references are indicated in square brackets.
as: “The inhibition step of MI or the inactivation step in the signaling pathway”, the symbol ⇩ as “The detected downregulation of protein expressions or protein phosphorylation by MI”, and ⇧ as “The detected upregulation of protein expression by MI” in this study. NB: the references are indicated in square brackets.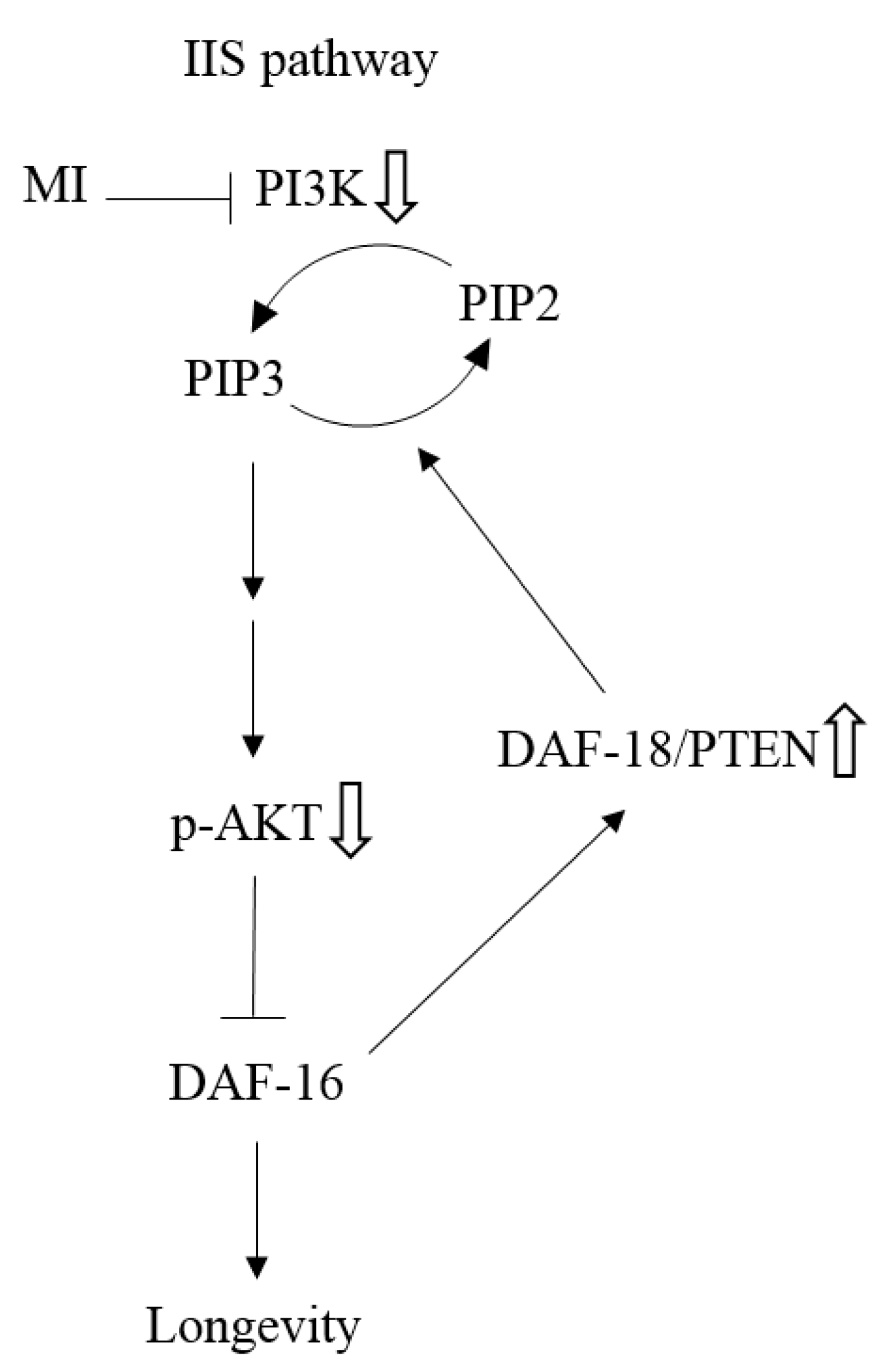
| Mean Lifespan (Day) | Median Lifespan (Day) | Maximum Lifespan (Day) | p Value * | |
|---|---|---|---|---|
| Control | 20.0 ± 5.6 | 22 | 30 | |
| MI 25 nmol/plate | 22.0 ± 5.7 | 22 | 34 | 0.028 |
| MI 50 nmol/plate | 23.0 ± 5.0 | 24 | 32 | <0.001 |
| MI 100 nmol/plate | 24.4 ± 4.7 | 24 | 38 | <0.001 |
| MI 200 nmol/plate | 22.1 ± 4.7 | 22 | 32 | 0.076 |
| Mutants | Mean Lifespan (Day) | Median Lifespan (Day) | Maximum Lifespan (Day) | p Value * |
|---|---|---|---|---|
| AKT-1 Control MI | 21.9 ± 5.4 | 22 | 34 | |
| 22.0 ± 5.3 | 22 | 36 | 0.921 | |
| DAF-16 Control MI | 14.9 ± 4.2 | 16 | 24 | |
| 14.4 ± 4.3 | 16 | 24 | 0.517 | |
| DAF-18 Control MI | 14.2 ± 3.4 | 14 | 22 | |
| 13.4 ± 3.4 | 14 | 20 | 0.121 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yang, N.-C.; Chin, C.-Y.; Zheng, Y.-X.; Lee, I. The Attenuation of Insulin/IGF-1 Signaling Pathway Plays a Crucial Role in the Myo-Inositol-Alleviated Aging in Caenorhabditis elegans. Int. J. Mol. Sci. 2023, 24, 6194. https://doi.org/10.3390/ijms24076194
Yang N-C, Chin C-Y, Zheng Y-X, Lee I. The Attenuation of Insulin/IGF-1 Signaling Pathway Plays a Crucial Role in the Myo-Inositol-Alleviated Aging in Caenorhabditis elegans. International Journal of Molecular Sciences. 2023; 24(7):6194. https://doi.org/10.3390/ijms24076194
Chicago/Turabian StyleYang, Nae-Cherng, Chia-Yu Chin, Ya-Xin Zheng, and Inn Lee. 2023. "The Attenuation of Insulin/IGF-1 Signaling Pathway Plays a Crucial Role in the Myo-Inositol-Alleviated Aging in Caenorhabditis elegans" International Journal of Molecular Sciences 24, no. 7: 6194. https://doi.org/10.3390/ijms24076194
APA StyleYang, N.-C., Chin, C.-Y., Zheng, Y.-X., & Lee, I. (2023). The Attenuation of Insulin/IGF-1 Signaling Pathway Plays a Crucial Role in the Myo-Inositol-Alleviated Aging in Caenorhabditis elegans. International Journal of Molecular Sciences, 24(7), 6194. https://doi.org/10.3390/ijms24076194





