Direct Electrochemistry of Glucose Dehydrogenase-Functionalized Polymers on a Modified Glassy Carbon Electrode and Its Molecular Recognition of Glucose
Abstract
1. Introduction
2. Results and Discussion
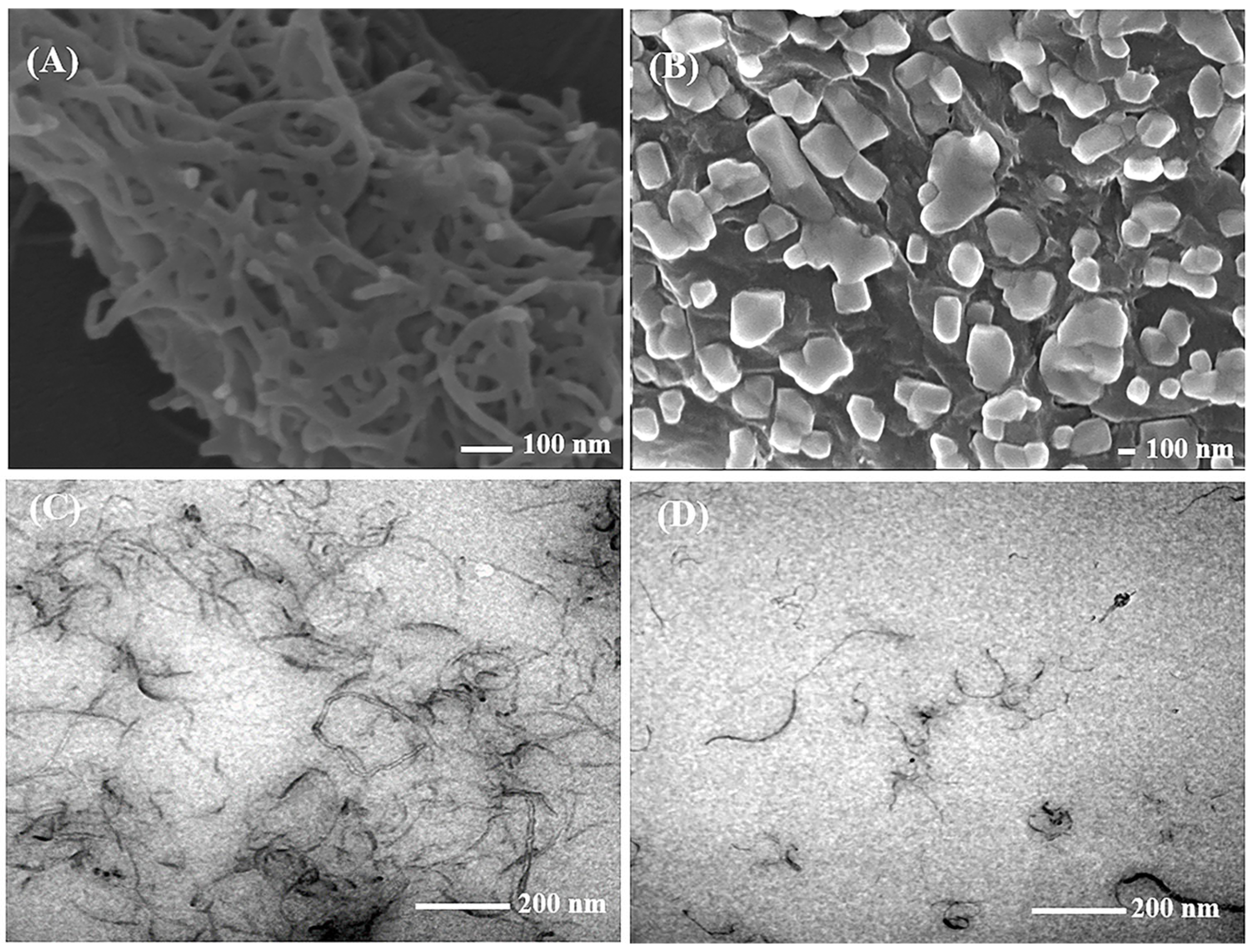
2.1. Characteristics of the Nanomaterials
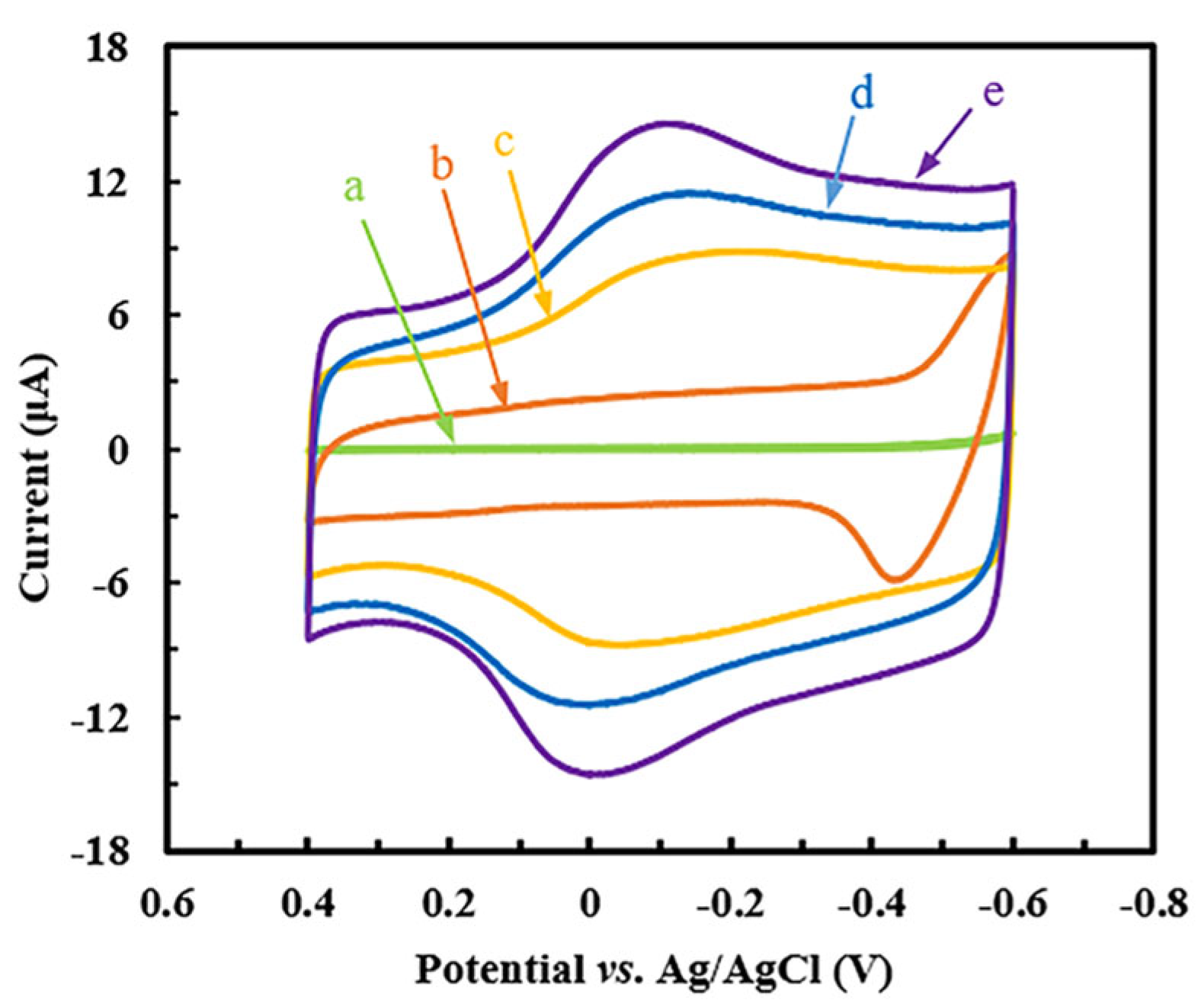
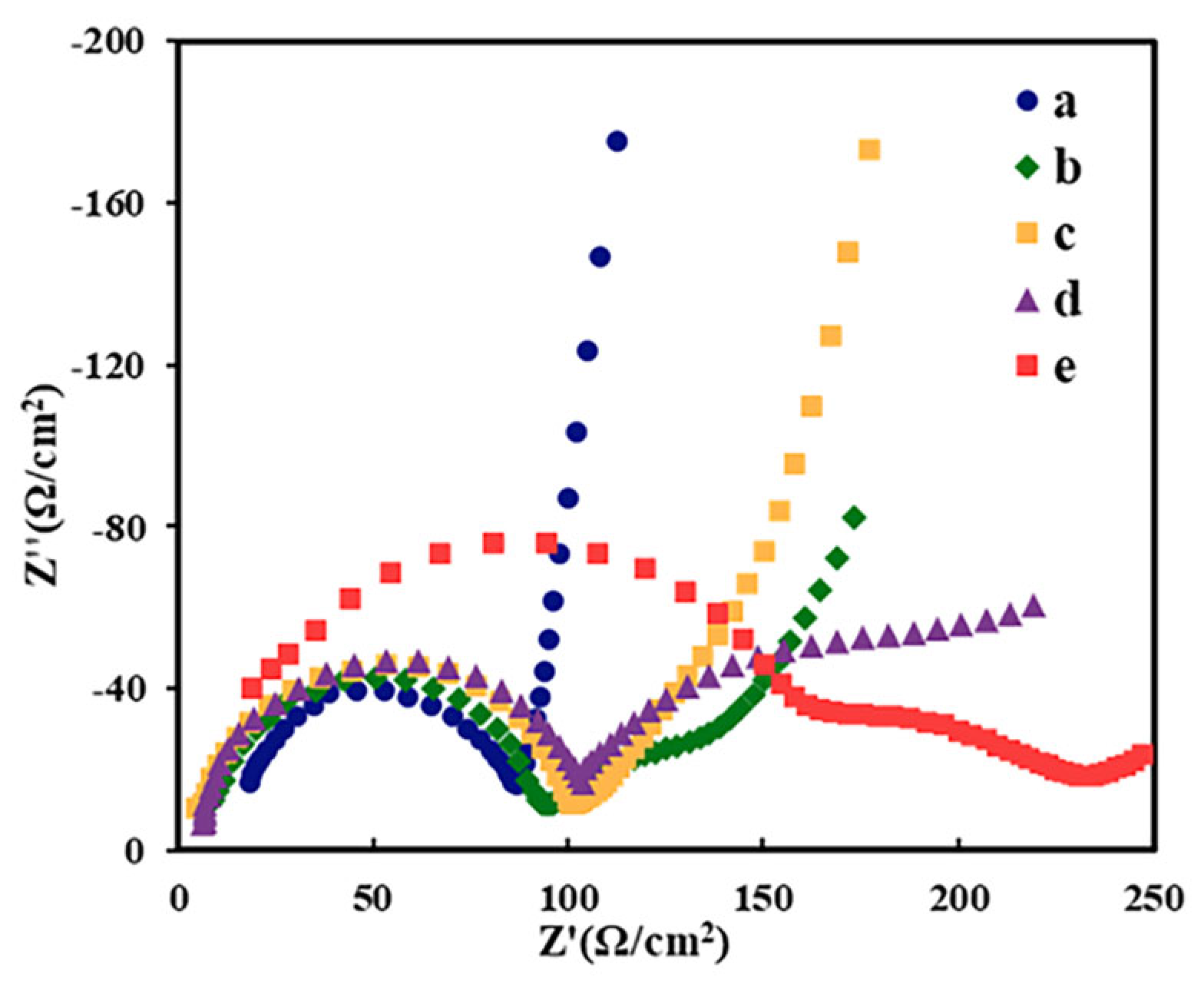
2.2. Electrochemical Behavior of Different Modified GCEs
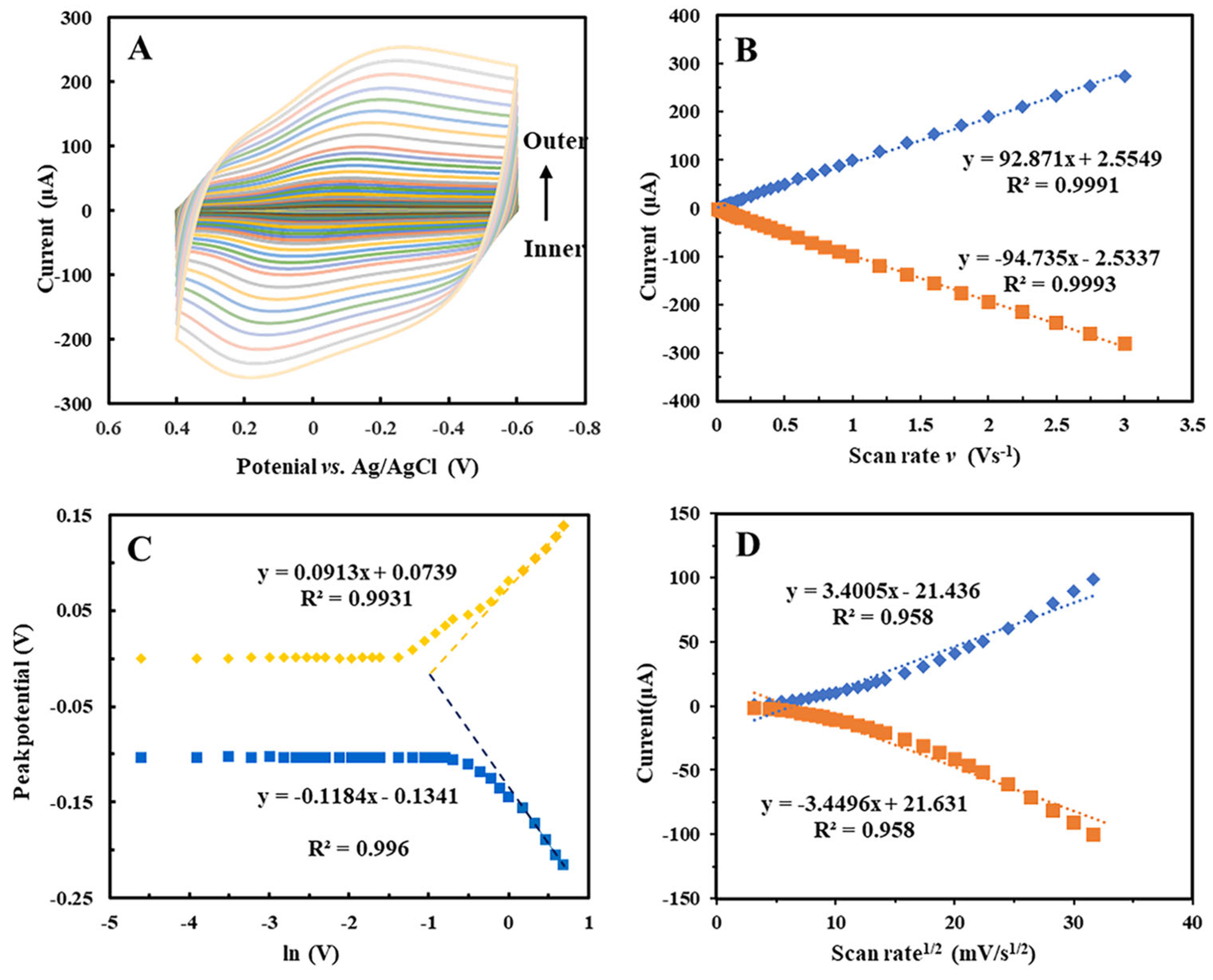
2.3. Effects of Scan Rates
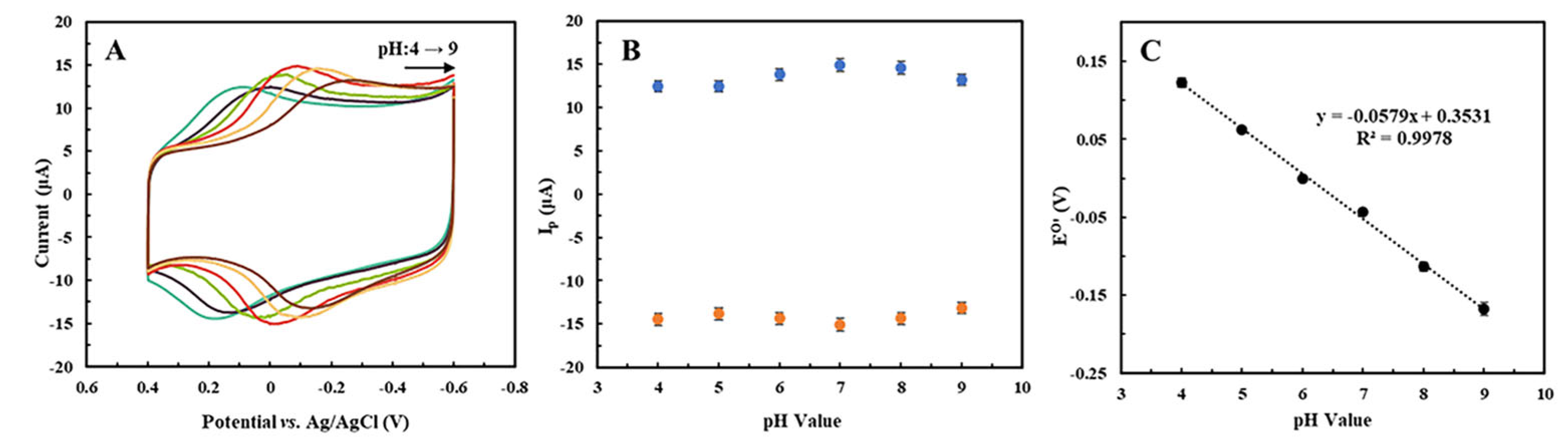
2.4. Effect of pH on the Peak Potential
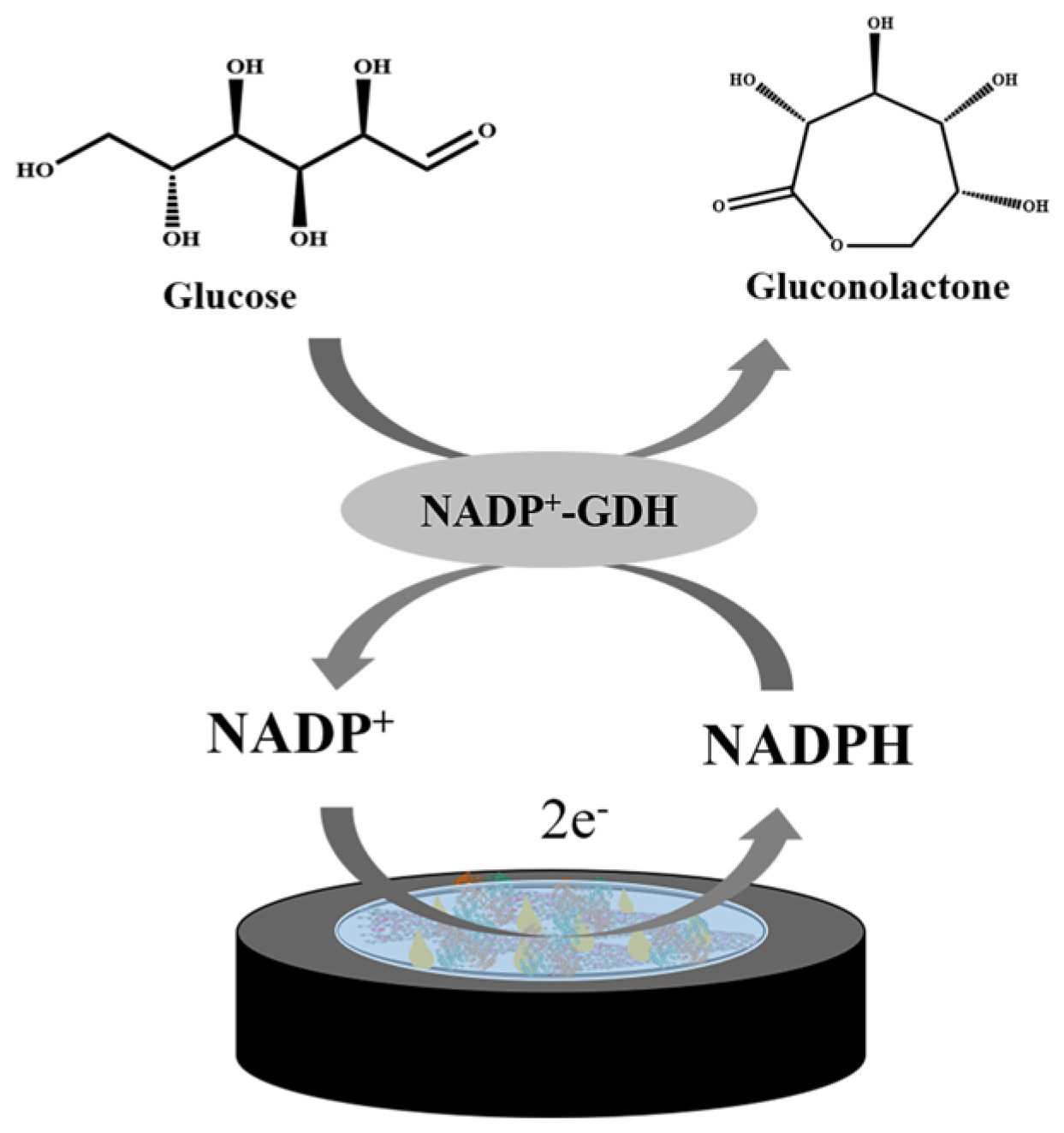
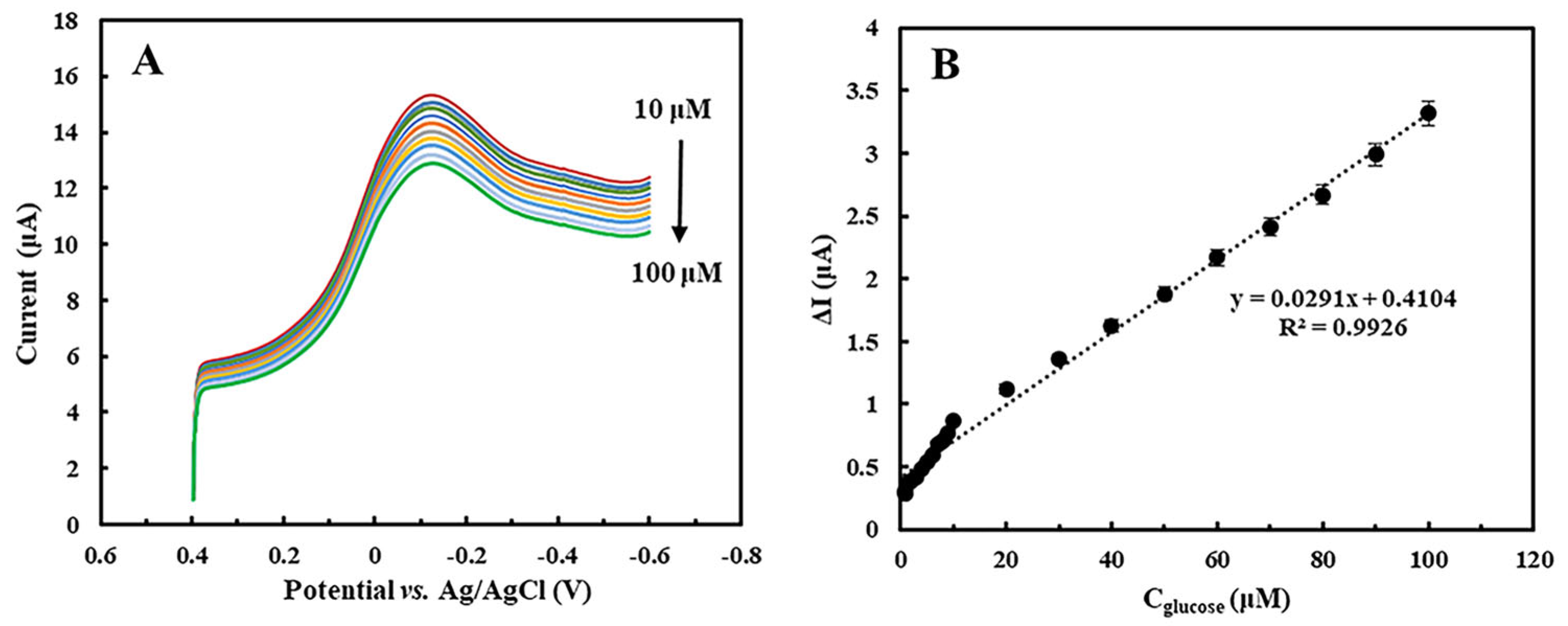
2.5. Detection of Glucose Concentration in Samples
2.6. Comparison of Sensor and HPLC Methods for the Detection of Glucose
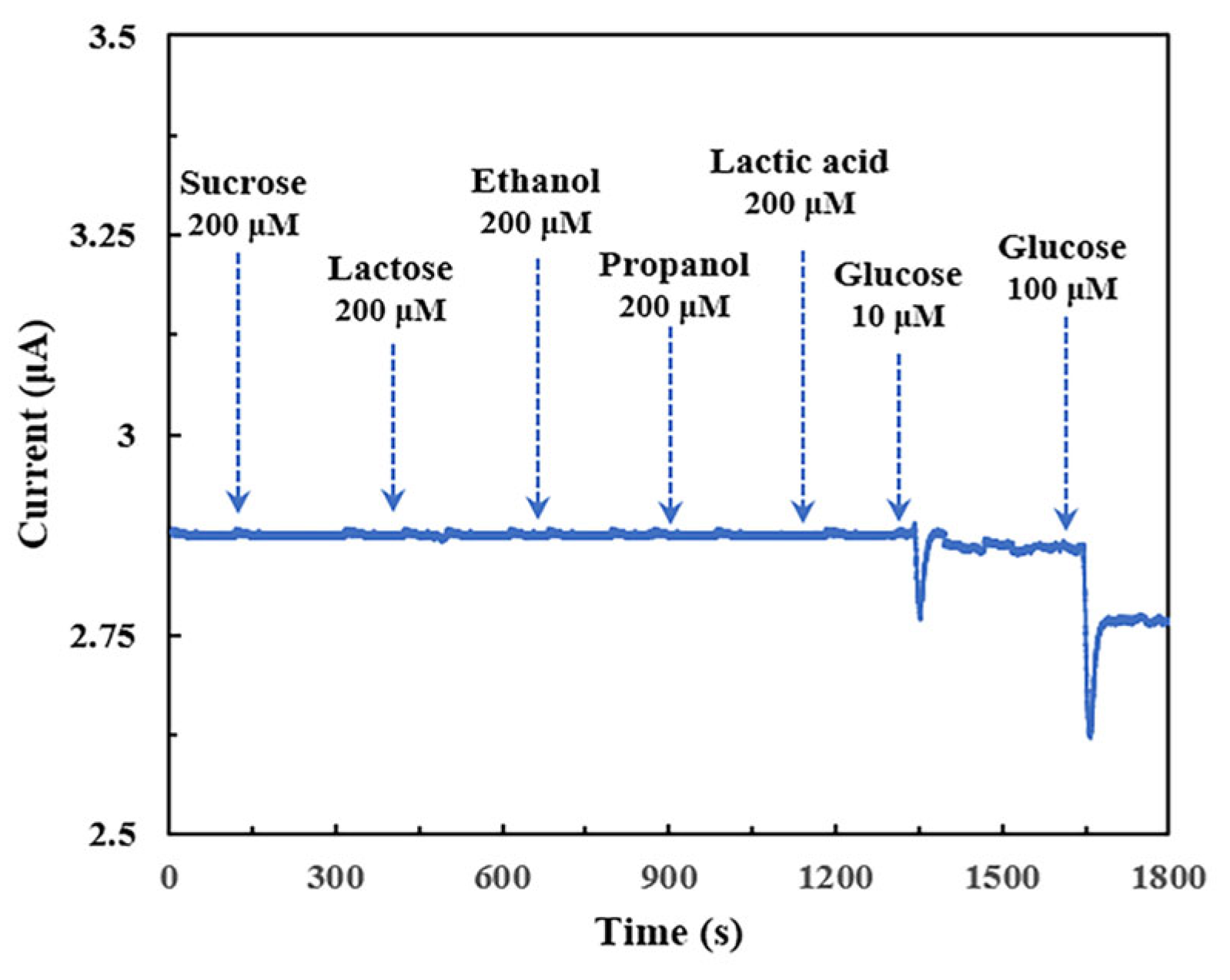
2.7. Anti-Interference Performance and Selectivity of Modified Electrode
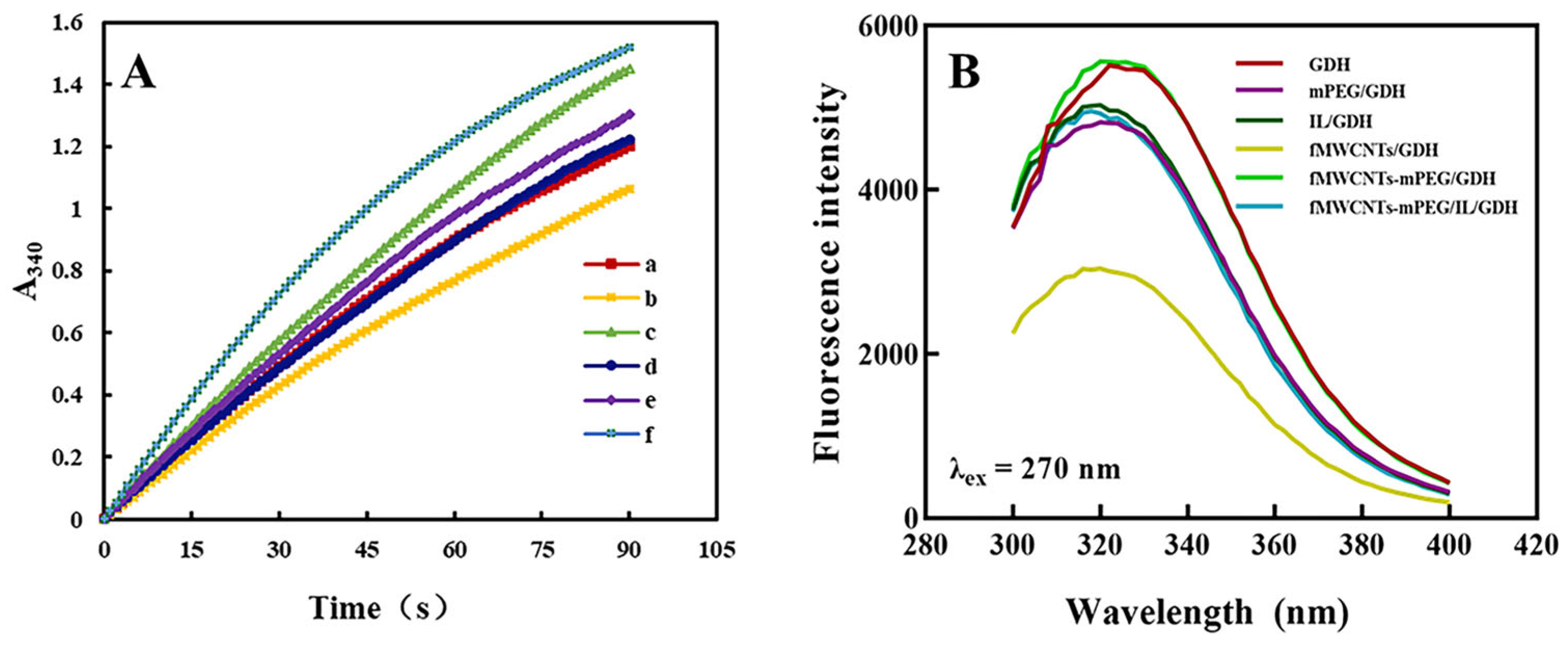
2.8. Characterization of Composite Functional Polymer
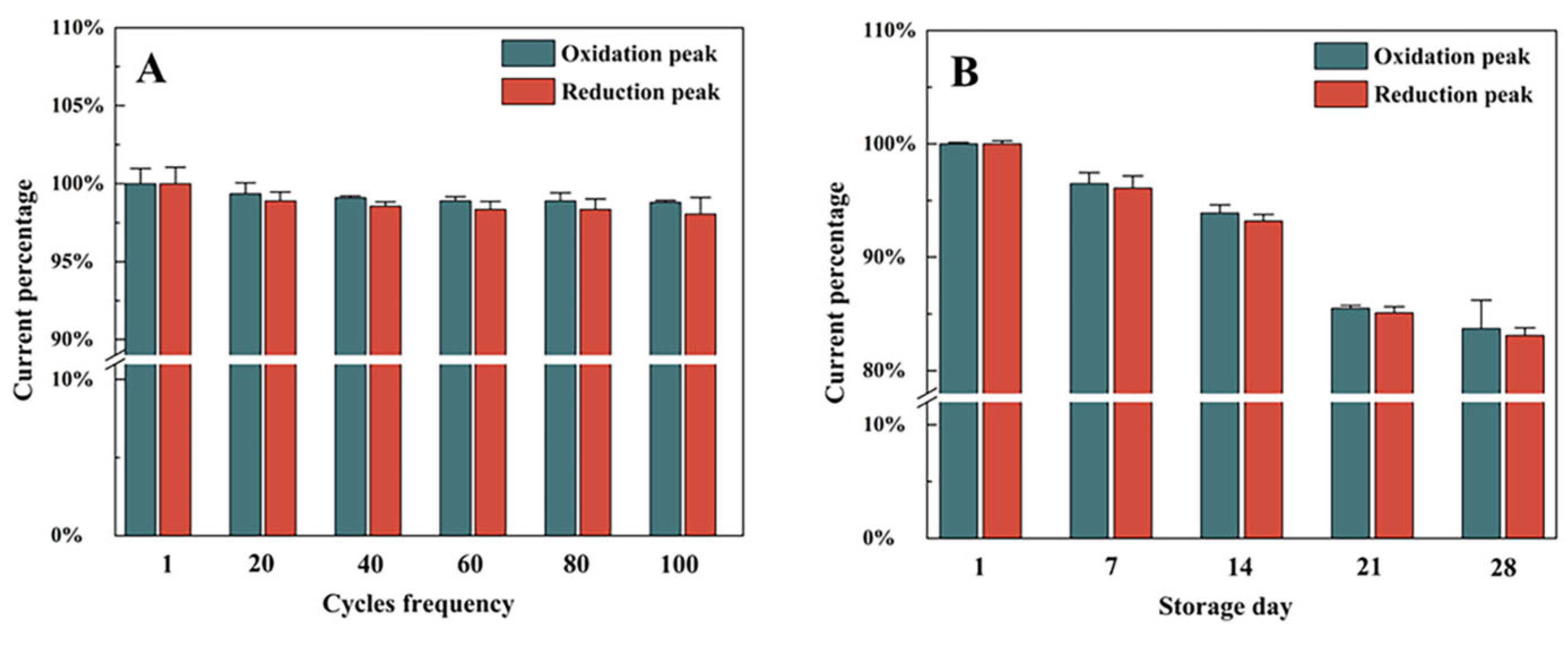
2.9. Stability of NF/IL/GDH/mPEG-fMWCNTs/GCE
3. Materials and Methods
3.1. Reagents and Materials
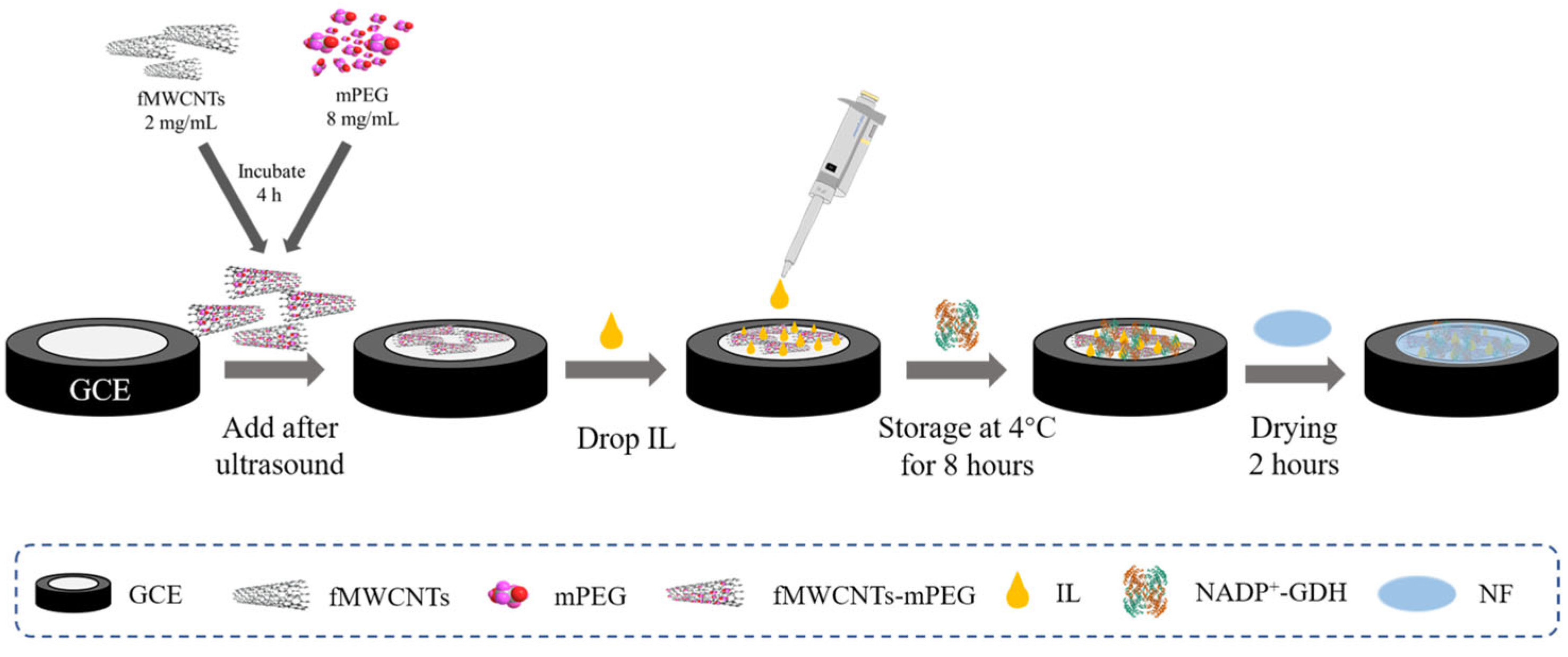
3.2. Preparation of Functional Polymer-Modified GCE
3.3. Biosensor Construction and Measurement
3.4. Apparatus and Measurements
3.5. Nanomaterials and Glucose Dehydrogenation Enzyme Compatibility
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Li, M.; Yan, D.; Hao, M.; Huang, X.; Xu, Y.; Li, W.; Liu, W. Quantification of Glucose, fructose and 1,5-Anhydroglucitol in plasma of diabetic patients by ultra performance liquid chromatography tandem mass spectrometry. J. Chromatogr. B Analyt. Technol. Biomed. Life Sci. 2022, 1200, 123277. [Google Scholar] [CrossRef] [PubMed]
- Mao, X.; Zhang, C. A microfluidic cloth-based photoelectrochemical analytical device for the detection of glucose in saliva. Talanta 2022, 238 Pt 2, 123052. [Google Scholar] [CrossRef] [PubMed]
- Jeon, H.J.; Kim, H.S.; Chung, E.; Lee, D.Y. Nanozyme-based colorimetric biosensor with a systemic quantification algorithm for noninvasive glucose monitoring. Theranostics 2022, 12, 6308–6338. [Google Scholar] [CrossRef] [PubMed]
- Jayathilake, N.M.; Koley, D. Glucose Microsensor with Covalently Immobilized Glucose Oxidase for Probing Bacterial Glucose Uptake by Scanning Electrochemical Microscopy. Anal. Chem. 2020, 92, 3589–3597. [Google Scholar] [CrossRef]
- Kadam, U.S.; Cho, Y.; Park, T.Y.; Hong, J.C. Aptamer-based CRISPR-Cas powered diagnostics of diverse biomarkers and small molecule targets. Appl. Biol. Chem. 2023, 66, 13. [Google Scholar] [CrossRef]
- Kadam, U.S.; Hong, J.C. Advances in aptameric biosensors designed to detect toxic contaminants from food, water, human fluids, and the environment. Trends Environ. Anal. Chem. 2022, 36, e00184. [Google Scholar] [CrossRef]
- Li, G.; Qi, X.; Wu, J.; Xu, L.; Wan, X.; Liu, Y.; Chen, Y.; Li, Q. Ultrasensitive, label-free voltammetric determination of norfloxacin based on molecularly imprinted polymers and Au nanoparticle-functionalized black phosphorus nanosheet nanocomposite. J. Hazard Mater. 2022, 436, 129107. [Google Scholar] [CrossRef]
- Li, G.; Wu, J.; Qi, X.; Wan, X.; Liu, Y.; Chen, Y.; Xu, L. Molecularly imprinted polypyrrole film-coated poly(3,4-ethylenedioxythiophene):polystyrene sulfonate-functionalized black phosphorene for the selective and robust detection of norfloxacin. Mater. Today Chem. 2022, 26, 101043. [Google Scholar] [CrossRef]
- Valdes-Ramirez, G.; Galicia, L. Biosensing Membrane Base on Ferulic Acid and Glucose Oxidase for an Amperometric Glucose Biosensor. Molecules 2021, 26, 3757. [Google Scholar] [CrossRef]
- Hossain, M.M.; Morshed, J.; Tsujimura, S. Designing a cross-linked redox network for a mediated enzyme-based electrode. Chem. Commun. 2021, 57, 6999–7002. [Google Scholar] [CrossRef]
- Morshed, J.; Nakagawa, R.; Hossain, M.M.; Nishina, Y.; Tsujimura, S. Disposable electrochemical glucose sensor based on water-soluble quinone-based mediators with flavin adenine dinucleotide-dependent glucose dehydrogenase. Biosens. Bioelectron. 2021, 189, 113357. [Google Scholar] [CrossRef]
- Lisdat, F. PQQ-GDH—Structure, function and application in bioelectrochemistry. Bioelectrochemistry 2020, 134, 107496. [Google Scholar] [CrossRef]
- Tsuruoka, N.; Soto, S.S.; Tahar, A.B.; Zebda, A.; Tsujimura, S. Mediated electrochemical oxidation of glucose via poly(methylene green) grafted on the carbon surface catalyzed by flavin adenine dinucleotide-dependent glucose dehydrogenase. Colloids Surf. B Biointerfaces 2020, 192, 111065. [Google Scholar] [CrossRef]
- Hyun, J.; Abigail, M.; Choo, J.W.; Ryu, J.; Kim, H.K. Effects of N-/C-Terminal Extra Tags on the Optimal Reaction Conditions, Activity, and Quaternary Structure of Bacillus thuringiensis Glucose 1-Dehydrogenase. J. Microbiol. Biotechnol. 2016, 26, 1708–1716. [Google Scholar] [CrossRef]
- Stolarczyk, K.; Rogalski, J.; Bilewicz, R. NAD(P)-dependent glucose dehydrogenase: Applications for biosensors, bioelectrodes, and biofuel cells. Bioelectrochemistry 2020, 135, 107574. [Google Scholar] [CrossRef] [PubMed]
- Nishioka, T.; Yasutake, Y.; Nishiya, Y.; Tamura, T. Structure-guided mutagenesis for the improvement of substrate specificity of Bacillus megaterium glucose 1-dehydrogenase IV. FEBS J. 2012, 279, 3264–3275. [Google Scholar] [CrossRef]
- Ding, H.T.; Du, Y.Q.; Liu, D.F.; Li, Z.L.; Chen, X.J.; Zhao, Y.H. Cloning and expression in E. coli of an organic solvent-tolerant and alkali-resistant glucose 1-dehydrogenase from Lysinibacillus sphaericus G10. Bioresour. Technol. 2011, 102, 1528–1536. [Google Scholar] [CrossRef]
- Ertek, B.; Dilgin, Y. Photoamperometric flow injection analysis of glucose based on dehydrogenase modified quantum dots-carbon nanotube nanocomposite electrode. Bioelectrochemistry 2016, 112, 138–144. [Google Scholar] [CrossRef]
- Tahar, A.B.; Szymczyk, A.; Tingry, S.; Vadgama, P.; Zelsmanne, M.; Tsujumura, S.; Cinquin, P.; Martin, D.; Zebda, A. One-year stability of glucose dehydrogenase confined in a 3D carbon nanotube electrode with coated poly-methylene green: Application as bioanode for a glucose biofuel cell. J. Electroanal. Chem. 2019, 847, 113069. [Google Scholar] [CrossRef]
- Ding, H.; Gao, F.; Yu, Y.; Chen, B. Biochemical and Computational Insights on a Novel Acid-Resistant and Thermal-Stable Glucose 1-Dehydrogenase. Int. J. Mol. Sci. 2017, 18, 1198. [Google Scholar] [CrossRef]
- Qian, W.Z.; Ou, L.; Li, C.X.; Pan, J.; Xu, J.H.; Chen, Q.; Zheng, G.W. Evolution of Glucose Dehydrogenase for Cofactor Regeneration in Bioredox Processes with Denaturing Agents. Chembiochem 2020, 21, 2680–2688. [Google Scholar] [CrossRef] [PubMed]
- Viehauser, M.C.; Breslmayr, E.; Scheiblbrandner, S.; Schachinger, F.; Ma, S.; Ludwig, R. A cytochrome b-glucose dehydrogenase chimeric enzyme capable of direct electron transfer. Biosens. Bioelectron. 2022, 196, 113704. [Google Scholar] [CrossRef]
- Xie, W.; Bülow, L.; Xie, B. Pyrroloquinoline quinone glucose dehydrogenase adopted in thermometric analysis for enhancement of glucose determination. J. Therm. Anal. Calorim. 2018, 134, 1913–1919. [Google Scholar] [CrossRef]
- Guo, Z.; Murphy, L.; Stein, V.; Johnston, W.A.; Alcala-Perez, S.; Alexandrov, K. Engineered PQQ-Glucose Dehydrogenase as a Universal Biosensor Platform. J. Am. Chem. Soc. 2016, 138, 10108–10111. [Google Scholar] [CrossRef]
- Rahimi, P.; Rafiee-Pour, H.A.; Ghourchian, H.; Norouzi, P.; Ganjali, M.R. Ionic-liquid/NH2-MWCNTs as a highly sensitive nano-composite for catalase direct electrochemistry. Biosens. Bioelectron. 2010, 25, 1301–1306. [Google Scholar] [CrossRef]
- Tsujimura, S.; Takeuchi, S. Toward an ideal platform structure based on MgO-templated carbon for flavin adenine dinucleotide-dependent glucose dehydrogenase-Os polymer-hydrogel electrodes. Electrochim. Acta 2020, 343, 136110. [Google Scholar] [CrossRef]
- Ma, B.; Gao, F.; Yu, N.; Zhao, C.; Li, S.; She, Z.; Guo, L.; Jin, C.; Zhao, Y.; Gao, M. Long-term impacts of carboxyl functionalized multi-walled carbon nanotubes on the performance, microbial enzymatic activity and microbial community of sequencing batch reactor. Bioresour. Technol. 2019, 286, 121382. [Google Scholar] [CrossRef]
- Wepasnick, K.A.; Smith, B.A.; Schrote, K.E.; Wilson, H.K.; Diegelmann, S.R.; Fairbrother, D.H. Surface and structural characterization of multi-walled carbon nanotubes following different oxidative treatments. Carbon 2011, 49, 24–36. [Google Scholar] [CrossRef]
- Ören, T.; Anık, Ü. Carboxylic acid functionalized multi-walled carbon nanotube assisted centri-voltammetry as a new approach for caffeine detection. New J. Chem. 2017, 41, 11800–11806. [Google Scholar] [CrossRef]
- Wang, C.; Li, J.; Tan, R.; Wang, Q.; Zhang, Z. Colorimetric method for glucose detection with enhanced signal intensity using ZnFe2O4-carbon nanotube-glucose oxidase composite material. Analyst 2019, 144, 1831–1839. [Google Scholar] [CrossRef]
- Perez, R.L.; Ayala, C.E.; Opiri, M.M.; Ezzir, A.; Li, G.; Warner, I.M. Recycling Thermoset Epoxy Resin Using Alkyl-Methyl-Imidazolium Ionic Liquids as Green Solvents. ACS Appl. Polym. Mater. 2021, 3, 5588–5595. [Google Scholar] [CrossRef] [PubMed]
- Yang, T.; Yang, X.L.; Zhang, Y.S.; Xiao, B.; Hong, J. Glucose biosensing using glassy carbon electrode modified with polyhydroxy-C60, glucose oxidase and ionic-liquid. Biomed. Mater. Eng. 2014, 24, 2197–2202. [Google Scholar] [CrossRef] [PubMed]
- Kumar, S.; Tripathy, S.; Singh, O.K.; Singh, S.G. Cerium oxide nanofiber based electroanalytical sensor for TNF-alpha detection: Improved interfacial stability with Nafion. Bioelectrochemistry 2021, 138, 107725. [Google Scholar] [CrossRef]
- Chen, S.; Shang, K.; Gao, X.; Wang, X. The development of NAD(+)-dependent dehydrogenase screen-printed biosensor based on enzyme and nanoporous gold co-catalytic strategy. Biosens. Bioelectron. 2022, 211, 114376. [Google Scholar] [CrossRef]
- Bai, L.; Wen, D.; Yin, J.; Deng, L.; Zhu, C.; Dong, S. Carbon nanotubes-ionic liquid nanocomposites sensing platform for NADH oxidation and oxygen, glucose detection in blood. Talanta 2012, 91, 110–115. [Google Scholar] [CrossRef]
- Song, X.-Y.; Meng, X.; Xiao, B.-L.; Li, Y.-Y.; Ma, X.-X.; Moosavi-Movahedi, A.A.; Hong, J. MWCNTs-CTAB and HFs-Lac Nanocomposite-Modified Glassy Carbon Electrode for Rutin Determination. Biosensors 2022, 12, 632. [Google Scholar] [CrossRef]
- Laviron, E. General expression of the linear potential sweep voltammogram in the case of diffusionless electrochemical systems. J. Electroanal. Chem. Interfacial Electrochem. 1979, 101, 19–28. [Google Scholar] [CrossRef]
- Hong, J.; Yang, W.-Y.; Zhao, Y.-X.; Xiao, B.-L.; Gao, Y.-F.; Yang, T.; Ghourchian, H.; Moosavi-Movahedi, Z.; Sheibani, N.; Li, J.-G.; et al. Catalase immobilized on a functionalized multi-walled carbon nanotubes–gold nanocomposite as a highly sensitive bio-sensing system for detection of hydrogen peroxide. Electrochim. Acta 2013, 89, 317–325. [Google Scholar] [CrossRef]
- Zhao, W.-J.; Xiao, B.-L.; Song, X.-Y.; Meng, X.; Ma, X.-X.; Li, Y.-Y.; Hong, J.; Moosavi-Movahedi, A.A. A Highly Sensitive Electrochemical Sensor Based on β-cyclodextrin Functionalized Multi-Wall Carbon Nanotubes and Fe3O4 Nanoparticles for Rutin Detection. J. Electrochem. Soc. 2022, 169, 047509. [Google Scholar] [CrossRef]
- Keerthi, M.; Kumar Panda, A.; Wang, Y.H.; Liu, X.; He, J.H.; Chung, R.J. Titanium nanoparticle anchored functionalized MWCNTs for electrochemical detection of ractopamine in porcine samples with ultrahigh sensitivity. Food Chem. 2022, 378, 132083. [Google Scholar] [CrossRef]
- Ning, Y.N.; Xiao, B.L.; Niu, N.N.; Moosavi-Movahedi, A.A.; Hong, J. Glucose Oxidase Immobilized on a Functional Polymer Modified Glassy Carbon Electrode and Its Molecule Recognition of Glucose. Polymers 2019, 11, 115. [Google Scholar] [CrossRef] [PubMed]
- Lee, H.; Lee, Y.S.; Reginald, S.S.; Baek, S.; Lee, E.M.; Choi, I.G.; Chang, I.S. Biosensing and electrochemical properties of flavin adenine dinucleotide (FAD)-Dependent glucose dehydrogenase (GDH) fused to a gold binding peptide. Biosens. Bioelectron. 2020, 165, 112427. [Google Scholar] [CrossRef] [PubMed]
- Han, Q.; Gong, W.; Zhang, Z.; Wang, L.; Wang, B.; Cai, L.; Meng, Q.; Li, Y.; Liu, Q.; Yang, Y.; et al. Orientated Immobilization of FAD-Dependent Glucose Dehydrogenase on Electrode by Carbohydrate-Binding Module Fusion for Efficient Glucose Assay. Int. J. Mol. Sci. 2021, 22, 5529. [Google Scholar] [CrossRef] [PubMed]
- Hoshino, T.; Sekiguchi, S.; Muguruma, H. Amperometric biosensor based on multilayer containing carbon nanotube, plasma-polymerized film, electron transfer mediator phenothiazine, and glucose dehydrogenase. Bioelectrochemistry 2012, 84, 1–5. [Google Scholar] [CrossRef]
- Zhou, H.; Zhang, Z.; Yu, P.; Su, L.; Ohsaka, T.; Mao, L. Noncovalent attachment of NAD+ cofactor onto carbon nanotubes for preparation of integrated dehydrogenase-based electrochemical biosensors. Langmuir 2010, 26, 6028–6032. [Google Scholar] [CrossRef]
- Kim, D.-M.; Kim, M.-Y.; Reddy, S.S.; Cho, J.; Cho, C.-H.; Jung, S.; Shim, Y.-B. Electron-Transfer Mediator for a NAD-Glucose Dehydrogenase-Based Glucose Sensor. Anal. Chem. 2013, 85, 11643–11649. [Google Scholar] [CrossRef]
- Jeon, W.Y.; Kim, H.H.; Choi, Y.B. Development of a Glucose Sensor Based on Glucose Dehydrogenase Using Polydopamine-Functionalized Nanotubes. Membranes 2021, 11, 384. [Google Scholar] [CrossRef]
- Mahadevan, A.; Fernando, S. Inorganic iron-sulfur clusters enhance electron transport when used for wiring the NAD-glucose dehydrogenase based redox system. Mikrochim. Acta 2018, 185, 337. [Google Scholar] [CrossRef]
- Hua, L.; Qianqian, B.; Jianfeng, Z.; Yinbiao, X.; Shengyu, Y.; Weishi, X.; Yang, S.; Yupeng, L. Directed evolution engineering to improve activity of glucose dehydrogenase by increasing pocket hydrophobicity. Front. Microbiol. 2022, 13, 1044226. [Google Scholar] [CrossRef]
- Brett, C.M.A. Electrochemical Impedance Spectroscopy in the Characterisation and Application of Modified Electrodes for Electrochemical Sensors and Biosensors. Molecules 2022, 27, 1497. [Google Scholar] [CrossRef]
- Saglam, O.; Kizilkaya, B.; Uysal, H.; Dilgin, Y. Biosensing of glucose in flow injection analysis system based on glucose oxidase-quantum dot modified pencil graphite electrode. Talanta 2016, 147, 315–321. [Google Scholar] [CrossRef]
- Veskoukis, A.S.; Margaritelis, N.V.; Kyparos, A.; Paschalis, V.; Nikolaidis, M.G. Spectrophotometric assays for measuring redox biomarkers in blood and tissues: The NADPH network. Redox Rep. 2018, 23, 47–56. [Google Scholar] [CrossRef] [PubMed]
- Liang, B.; Li, L.; Tang, X.; Lang, Q.; Wang, H.; Li, F.; Shi, J.; Shen, W.; Palchetti, I.; Mascini, M.; et al. Microbial surface display of glucose dehydrogenase for amperometric glucose biosensor. Biosens. Bioelectron. 2013, 45, 19–24. [Google Scholar] [CrossRef]
| Modified Electrode | Linear Range | LOD | Ref. | |
|---|---|---|---|---|
| (mM) | (μM) | (μM) | ||
| GDH-CS/CNTs/IL/GCE | 1.767 | 20–1 × 103 | 9 | [35] |
| GDH-GBP/Au/SPE | 26.9 | 3 × 103–3 × 104 | 3.41 × 104 | [42] |
| NF/GDH-NL-CBM2/SMWNT/GCE | 12.3 | 1.2 × 102–4.07 × 104 | 51 | [43] |
| PPF/sSWNT/GDH/GCE | 8.2 | 2.5 × 102–2.5 × 103 | 7.1 | [44] |
| GDH/NAD+/MWCNT/GCE | - | 10–300 | 4.81 | [45] |
| NF/NAD-GDH/FePhenTPy-RGO | - | 1.6 × 103–3.3 × 104 | 6.6 × 104 | [46] |
| Ru(dmo-bpy)2Cl2/GDH/PDA-MWCNT/SPCEs | - | 0–10 × 103 | 5.8 × 102 | [47] |
| Au/FeS/GDH/GA | - | 10 × 102–10 × 104 | 7.7 × 102 | [48] |
| NF/GDH/IL/mPEG-fMWCNTs/GCE | 0.21 | 0.8–100 | 0.46 | This work |
| Concentration (μM) | HPLC (μM) | Sensor (μM) | RSD (%) |
|---|---|---|---|
| 10 | 9.74 | 9.41 ± 0.03 | 2.53 |
| 20 | 19.70 | 19.73 ± 0.07 | 1.27 |
| 50 | 52.24 | 50.62 ± 0.24 | 1.85 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sun, Y.; Xue, W.; Zhao, J.; Bao, Q.; Zhang, K.; Liu, Y.; Li, H. Direct Electrochemistry of Glucose Dehydrogenase-Functionalized Polymers on a Modified Glassy Carbon Electrode and Its Molecular Recognition of Glucose. Int. J. Mol. Sci. 2023, 24, 6152. https://doi.org/10.3390/ijms24076152
Sun Y, Xue W, Zhao J, Bao Q, Zhang K, Liu Y, Li H. Direct Electrochemistry of Glucose Dehydrogenase-Functionalized Polymers on a Modified Glassy Carbon Electrode and Its Molecular Recognition of Glucose. International Journal of Molecular Sciences. 2023; 24(7):6152. https://doi.org/10.3390/ijms24076152
Chicago/Turabian StyleSun, Yang, Weishi Xue, Jianfeng Zhao, Qianqian Bao, Kailiang Zhang, Yupeng Liu, and Hua Li. 2023. "Direct Electrochemistry of Glucose Dehydrogenase-Functionalized Polymers on a Modified Glassy Carbon Electrode and Its Molecular Recognition of Glucose" International Journal of Molecular Sciences 24, no. 7: 6152. https://doi.org/10.3390/ijms24076152
APA StyleSun, Y., Xue, W., Zhao, J., Bao, Q., Zhang, K., Liu, Y., & Li, H. (2023). Direct Electrochemistry of Glucose Dehydrogenase-Functionalized Polymers on a Modified Glassy Carbon Electrode and Its Molecular Recognition of Glucose. International Journal of Molecular Sciences, 24(7), 6152. https://doi.org/10.3390/ijms24076152




