Comparative Transcriptome and Metabolome Analyses of Broccoli Germplasms with Purple and Green Curds Reveal the Structural Genes and Transitional Regulators Regulating Color Formation
Abstract
1. Introduction
2. Results
2.1. Phenotypic Difference of Curd Color between GB767 and PB767
2.2. Transcriptome Sequencing and Data Quality
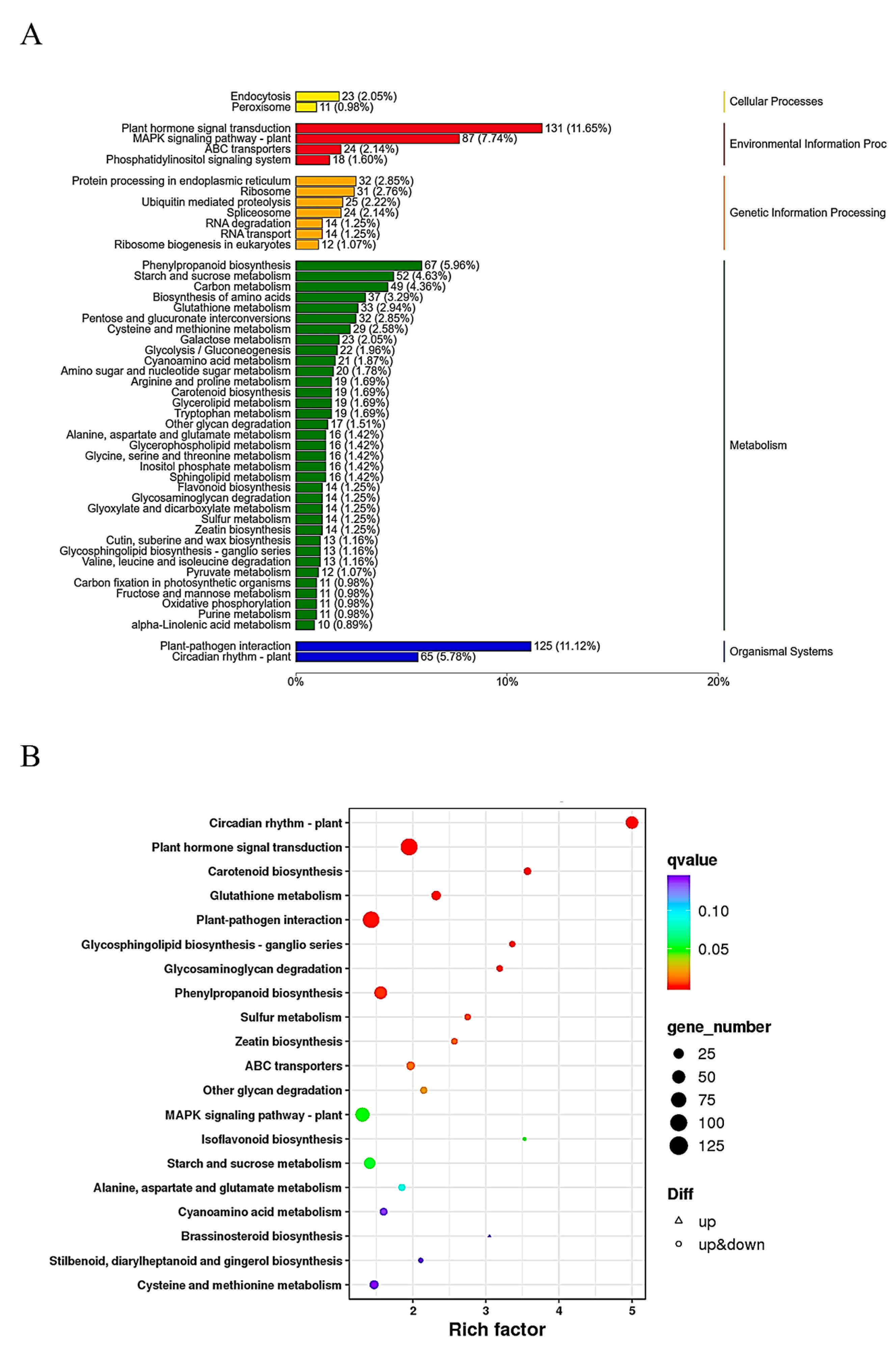
2.3. GO and KEGG Term Classification of Differentially Expressed Genes (DEGs) in GB767 vs. PB767
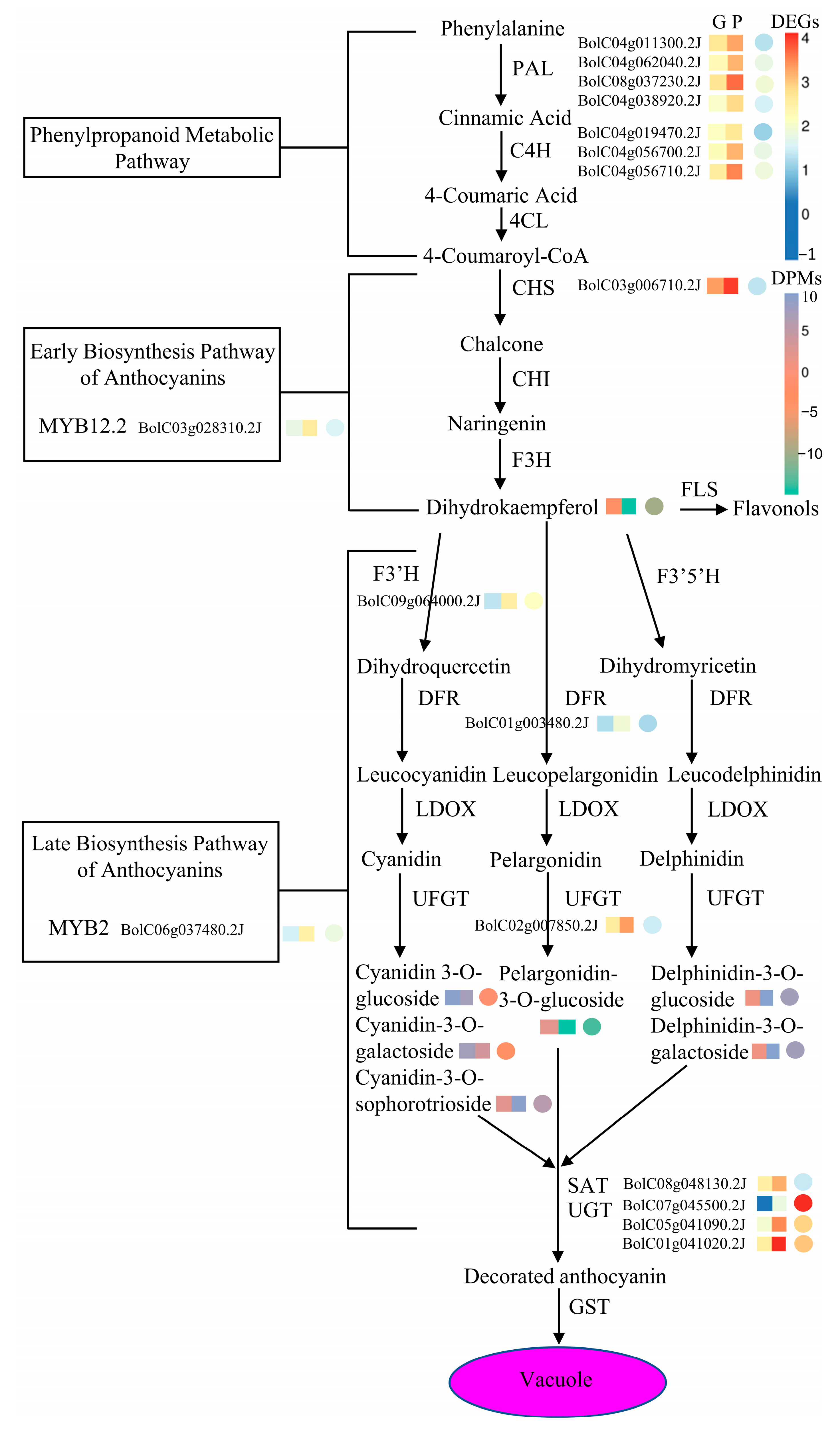
2.4. Transcription Factors and Anthocyanin-Related Genes in DEGs
2.5. Promoter Sequence Variation Analysis of 19 Differentially Expressed Anthocyanidin-Related Genes
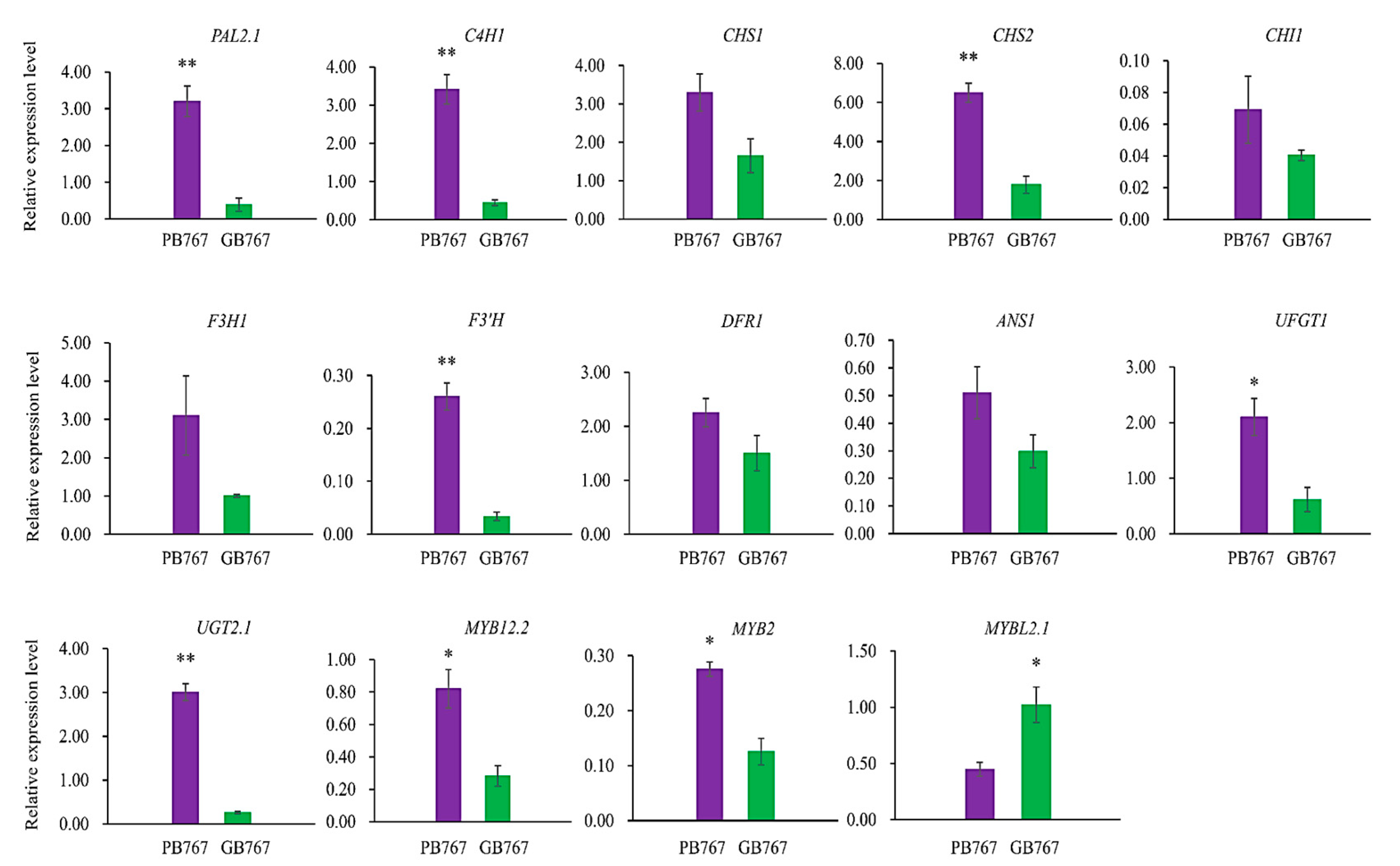
2.6. qRT-PCR Validation
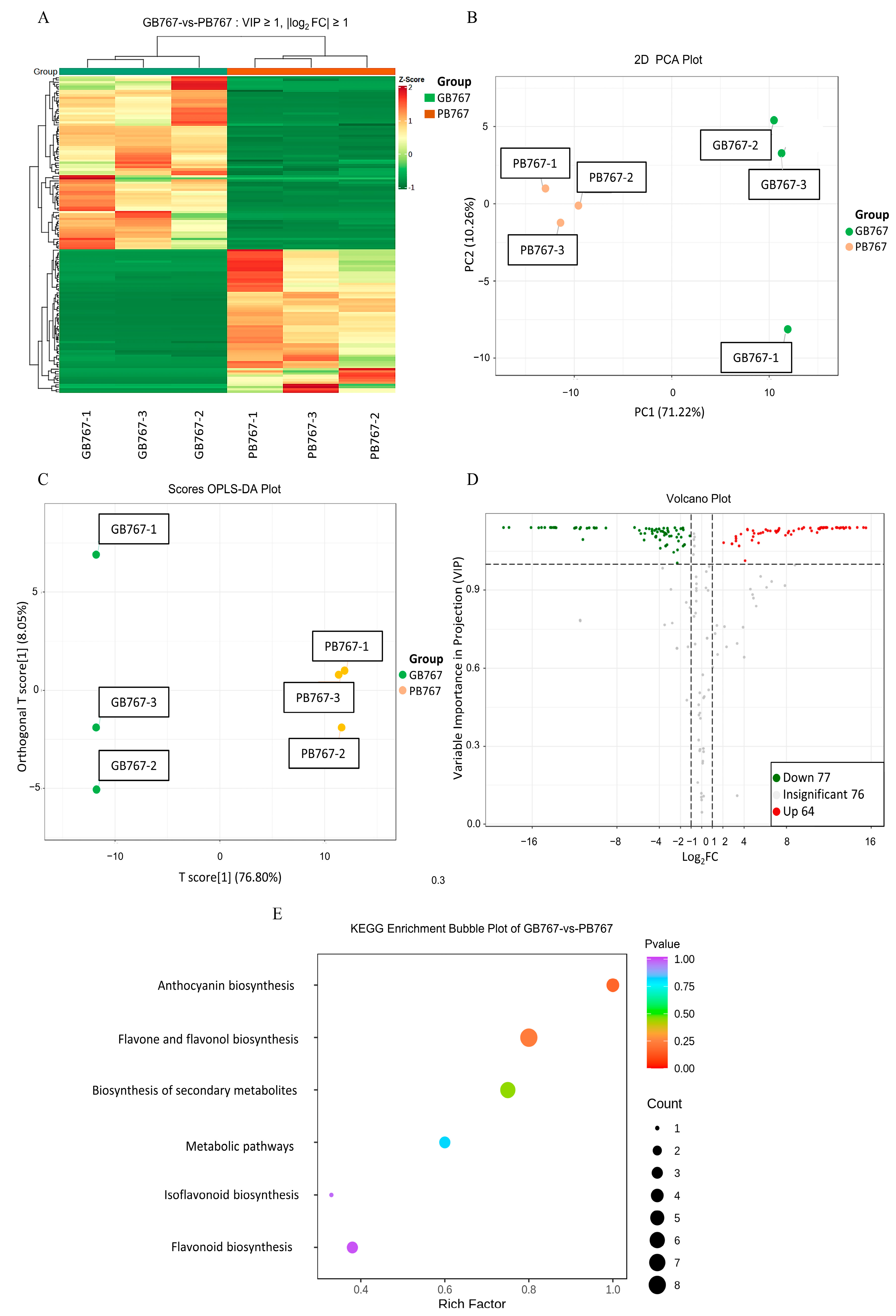
2.7. Analysis of the Widely Targeted Detection of Flavonoid Metabolome Data
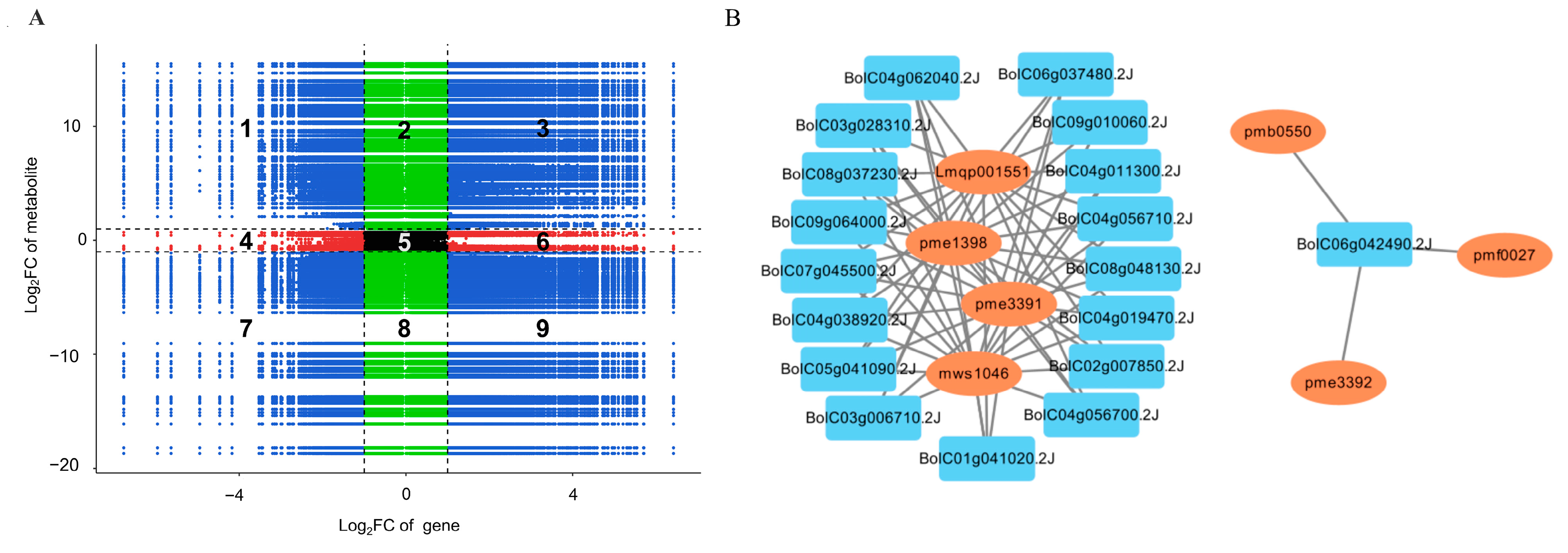
2.8. Correlation Analysis on Genes and Metabolites Involved in Anthocyanin Biosynthesis of GB767 and PB767
3. Discussion
3.1. Delphinidin-Based Anthocyanins in the Floret Sepals Are Responsible for Purplish Curds in PB767
3.2. One Broccoli CYP75B/F3′H Might Acquire Flavonoid 5′-Hydroxylase Activities, Promoting the Accumulation of Delphinidins in Purple Curds
3.3. Anthocyanin Biosynthesis-Related Genes Are Differentially Regulated in Purple Broccoli
4. Materials and Methods
4.1. Plant Materials and Sample Preparation
4.2. RNA Library Preparation and Transcriptome Sequencing Analysis
4.3. Validation of RNA-Seq Data by qRT-PCR
4.4. Metabolite Extraction and UPLC Conditions
4.5. Qualitative and Quantitative Analysis of Metabolites
4.6. Correlation Analysis between Transcriptome and Metabolome Data
4.7. Statistical Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Domínguez-Perles, R.; Martínez-Ballesta, M.C.; Carvajal, M.; García-Viguera, C.; Moreno, D.A. Broccoli-derived by-products—A promising source of bioactive ingredients. J. Food Sci. 2010, 75, C383–C392. [Google Scholar] [CrossRef] [PubMed]
- Farnham, M.W.; Kopsell, D.A. Importance of genotype on carotenoid and chlorophyll levels in broccoli heads. HortScience 2009, 44, 1248–1253. [Google Scholar] [CrossRef]
- Brown, A.F.; Yousef, G.G.; Jeffery, E.H.; Klein, B.P.; Wallig, M.A.; Kushad, M.M.; Juvik, J.A. Glucosinolate profiles in broccoli: Variation in levels and implications in breeding for cancer chemoprotection. J. Am. Soc. Hort. Sci. 2002, 127, 807–813. [Google Scholar] [CrossRef]
- Rodríguez-Hernández Mdel, C.; Moreno, D.A.; Carvajal, M.; García-Viguera, C.; Martínez-Ballesta Mdel, C. Natural antioxidants in purple sprouting broccoli under Mediterranean climate. J. Food Sci. 2012, 77, C1058–C1063. [Google Scholar] [CrossRef]
- Silva, S.; Costa, E.M.; Calhau, C.; Morais, R.M.; Pintado, M.E. Anthocyanin extraction from plant tissues: A review. Crit. Rev. Food Sci. Nutr. 2017, 57, 3072–3083. [Google Scholar] [CrossRef]
- Iwashina, T. Contribution to flower colors of flavonoids including anthocyanins: A review. Nat. Prod. Commun. 2015, 10, 529–544. [Google Scholar] [CrossRef]
- Grotewold, E. The genetics and biochemistry of floral pigments. Annu. Rev. Plant Biol. 2006, 57, 761–780. [Google Scholar] [CrossRef]
- Falcone Ferreyra, M.L.; Rius, S.P.; Casati, P. Flavonoids: Biosynthesis, biological functions, and biotechnological applications. Front. Plant Sci. 2012, 3, 222. [Google Scholar] [CrossRef]
- Mol, J.; Grotewold, E.; Koes, R. How genes paint flowers and seeds. Trends Plant Sci. 1998, 3, 212–217. [Google Scholar] [CrossRef]
- Winkel-Shirley, B. Biosynthesis of flavonoids and effects of stress. Curr. Opin. Plant Biol. 2002, 5, 218–223. [Google Scholar] [CrossRef]
- Alappat, B.; Alappat, J. Anthocyanin pigments: Beyond Aesthetics. Molecules 2020, 25, 5500. [Google Scholar] [CrossRef] [PubMed]
- Yousuf, B.; Gul, K.; Wani, A.A.; Singh, P. Health benefits of anthocyanins and their encapsulation for potential use in food systems: A review. Crit. Rev. Food Sci. Nutr. 2016, 56, 2223–2230. [Google Scholar] [CrossRef]
- Albert, N.W.; Davies, K.M.; Lewis, D.H.; Zhang, H.; Montefiori, M.; Brendolise, C.; Boase, M.R.; Ngo, H.; Jameson, P.E.; Schwinn, K.E. A conserved network of transcriptional activators and repressors regulates anthocyanin pigmentation in eudicots. Plant Cell 2014, 26, 962–980. [Google Scholar] [CrossRef] [PubMed]
- Petroni, K.; Tonelli, C. Recent advances on the regulation of anthocyanin synthesis in reproductive organs. Plant Sci. 2011, 181, 219–229. [Google Scholar] [CrossRef] [PubMed]
- Chen, L.; Hu, B.; Qin, Y.; Hu, G.; Zhao, J. Advance of the negative regulation of anthocyanin biosynthesis by MYB transcription factors. Plant Physiol. Biochem. 2019, 136, 178–187. [Google Scholar] [CrossRef]
- Yan, H.; Pei, X.; Zhang, H.; Li, X.; Zhang, X.; Zhao, M.; Chiang, V.L.; Sederoff, R.R.; Zhao, X. MYB-mediated regulation of anthocyanin biosynthesis. Int. J. Mol. Sci. 2021, 22, 3103. [Google Scholar] [CrossRef]
- Baudry, A.; Heim, M.A.; Dubreucq, B.; Caboche, M.; Weisshaar, B.; Lepiniec, L. TT2, TT8, and TTG1 synergistically specify the expression of BANYULS and proanthocyanidin biosynthesis in Arabidopsis thaliana. Plant J. 2004, 39, 366–380. [Google Scholar] [CrossRef]
- Mehrtens, F.; Kranz, H.; Bednarek, P.; Weisshaar, B. The Arabidopsis transcription factor MYB12 is a flavonol-specific regulator of phenylpropanoid biosynthesis. Plant Physiol. 2005, 138, 1083–1096. [Google Scholar] [CrossRef]
- Czemmel, S.; Stracke, R.; Weisshaar, B.; Cordon, N.; Harris, N.N.; Walker, A.R.; Robinson, S.P.; Bogs, J. The grapevine R2R3-MYB transcription factor VvMYBF1 regulates flavonol synthesis in developing grape berries. Plant Physiol. 2009, 151, 1513–1530. [Google Scholar] [CrossRef]
- Matsui, K.; Umemura, Y.; Ohme-Takagi, M. AtMYBL2, a protein with a single MYB domain, acts as a negative regulator of anthocyanin biosynthesis in Arabidopsis. Plant J. 2008, 55, 954–967. [Google Scholar] [CrossRef]
- Aharoni, A.; De Vos, C.H.; Wein, M.; Sun, Z.; Greco, R.; Kroon, A.; Mol, J.N.; O’Connell, A.P. The strawberry FaMYB1 transcription factor suppresses anthocyanin and flavonol accumulation in transgenic tobacco. Plant J. 2001, 28, 319–332. [Google Scholar] [CrossRef]
- Matus, J.T.; Aquea, F.; Arce-Johnson, P. Analysis of the grape MYB R2R3 subfamily reveals expanded wine quality-related clades and conserved gene structure organization across Vitis and Arabidopsis genomes. BMC Plant Biol. 2008, 8, 83. [Google Scholar] [CrossRef] [PubMed]
- Lin-Wang, K.; Micheletti, D.; Palmer, J.; Volz, R.; Lozano, L.; Espley, R.; Hellens, R.P.; Chagnè, D.; Rowan, D.D.; Troggio, M.; et al. High temperature reduces apple fruit colour via modulation of the anthocyanin regulatory complex. Plant Cell Environ. 2011, 34, 1176–1190. [Google Scholar] [CrossRef] [PubMed]
- He, Q.; Wu, J.; Xue, Y.; Zhao, W.; Li, R.; Zhang, L. The novel gene BrMYB2, located on chromosome A07, with a short intron 1 controls the purple-head trait of Chinese cabbage (Brassica rapa L.). Hortic. Res. 2020, 7, 97. [Google Scholar] [CrossRef]
- Ye, S.; Hua, S.; Ma, T.; Ma, X.; Chen, Y.; Wu, L.; Zhao, L.; Yi, B.; Ma, C.; Tu, J.; et al. Genetic and multi-omics analyses reveal BnaA07.PAP2In-184-317 as the key gene conferring anthocyanin-based color in Brassica napus flowers. J. Exp. Bot. 2022, 73, 6630–6645. [Google Scholar] [CrossRef] [PubMed]
- Song, H.; Yi, H.; Lee, M.; Han, C.; Lee, J.; Kim, H.; Park, J.; Nou, I.; Kim, S.; Hur, Y. Purple Brassica oleracea var. capitata F. rubra is due to the loss of BoMYBL2–1 expression. BMC Plant Biol. 2018, 18, 82. [Google Scholar] [CrossRef] [PubMed]
- Tanaka, Y.; Sasaki, N.; Ohmiya, A. Biosynthesis of plant pigments: Anthocyanins, betalains and carotenoids. Plant J. 2008, 54, 733–749. [Google Scholar] [CrossRef]
- Tanaka, Y.; Brugliera, F.; Chandler, S. Recent progress of flower colour modification by biotechnology. Int. J. Mol. Sci. 2009, 10, 5350–5369. [Google Scholar] [CrossRef]
- Yu, O.; McGonigle, B. Metabolic engineering of isoflavone biosynthesis. In Advances in Agronomy; Academic Press: Cambridge, MA, USA, 2005; Volume 86, pp. 147–190. [Google Scholar] [CrossRef]
- He, Q.; Zhang, Z.; Zhang, L. Anthocyanin accumulation, antioxidant ability and stability, and a transcriptional analysis of anthocyanin biosynthesis in purple heading Chinese cabbage (Brassica rapa L. ssp. pekinensis). J. Agric. Food Chem. 2016, 64, 132–145. [Google Scholar] [CrossRef]
- Lin, J.Y.; Li, C.Y.; Hwang, I.F. Characterisation of the pigment components in red cabbage (Brassica oleracea L. var.) juice and their anti-inflammatory effects on LPS-stimulated murine splenocytes. Food Chem. 2008, 109, 771–781. [Google Scholar] [CrossRef]
- Ren, J.; Liu, Z.; Niu, R.; Feng, H. Mapping of Re, a gene conferring the red leaf trait in ornamental kale (Brassica oleracea L. var acephala). Plant Breed. 2015, 134, 494–500. [Google Scholar] [CrossRef]
- Guo, N.; Cheng, F.; Wu, J.; Liu, B.; Zheng, S.; Liang, J.; Wang, X. Anthocyanin biosynthetic genes in Brassica rapa. BMC Genom. 2014, 15, 426. [Google Scholar] [CrossRef]
- Chen, D.; Yang, Y.; Niu, G.; Shan, X.; Zhang, X.; Jiang, H.; Liu, L.; Wen, Z.; Ge, X.; Zhao, Q.; et al. Metabolic and RNA sequencing analysis of cauliflower curds with different types of pigmentation. AoB Plants 2022, 14, plac001. [Google Scholar] [CrossRef]
- Liu, C.; Yao, X.; Li, G.; Huang, L.; Xie, Z. Transcriptomic profiling of purple broccoli reveals light-induced anthocyanin biosynthetic signaling and structural genes. PeerJ 2020, 8, e8870. [Google Scholar] [CrossRef]
- Tanaka, Y.; Brugliera, F. Flower colour and cytochromes P450. Philos. Trans. R. Soc. Lond. B Biol. Sci. 2013, 368, 20120432. [Google Scholar] [CrossRef]
- Noda, N. Recent advances in the research and development of blue flowers. Breed. Sci. 2018, 68, 79–87. [Google Scholar] [CrossRef] [PubMed]
- Katsumoto, Y.; Fukuchi-Mizutani, M.; Fukui, Y.; Brugliera, F.; Holton, T.A.; Karan, M.; Nakamura, N.; Yonekura-Sakakibara, K.; Togami, J.; Pigeaire, A.; et al. Engineering of the rose flavonoid biosynthetic pathway successfully generated blue-hued flowers accumulating delphinidin. Plant Cell Physiol. 2007, 48, 1589–1600. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Y.; Qi, X.; Liu, Z.; Zheng, W.; Guan, J.; Liu, Z.; Ren, J.; Feng, H.; Zhang, Y. Transcriptome and metabolome profiling to explore the causes of purple leaves formation in non-heading Chinese cabbage (Brassica rapa L. ssp. chinensis Makino var. mutliceps Hort.). Foods 2022, 11, 1787. [Google Scholar] [CrossRef] [PubMed]
- Dandan, X.; Gao, L.; Xiaofeng, L.; Yang, X.; Zhu, Y.; Zhu, H. Metabolome and transcriptome revealed genes associated with anthocyanin accumulation in purple Caitai (Brassica compestris. var. tsai-tai Hort.). Sci. Hortic. 2022, 303, 111171. [Google Scholar] [CrossRef]
- Seitz, C.; Ameres, S.; Schlangen, K.; Forkmann, G.; Halbwirth, H. Multiple evolution of flavonoid 3′,5′-hydroxylase. Planta 2015, 242, 561–573. [Google Scholar] [CrossRef] [PubMed]
- Mizutani, M. Impacts of diversification of cytochrome P450 on plant metabolism. Biol. Pharm. Bull. 2012, 35, 824–832. [Google Scholar] [CrossRef] [PubMed]
- Ishiguro, K.; Taniguchi, M.; Tanaka, Y. Functional analysis of Antirrhinum kelloggii flavonoid 3′-hydroxylase and flavonoid 3′,5′-hydroxylase genes; critical role in flower color and evolution in the genus Antirrhinum. J. Plant Res. 2012, 125, 451–456. [Google Scholar] [CrossRef] [PubMed]
- Seitz, C.; Ameres, S.; Forkmann, G. Identification of the molecular basis for the functional difference between flavonoid 3′-hydroxylase and flavonoid 3′,5′-hydroxylase. FEBS Lett. 2007, 581, 3429–3434. [Google Scholar] [CrossRef]
- Liu, C.; Yao, X.; Li, G.; Huang, L.; Liu, C.; Xie, Z. Development of novel markers and creation of non-anthocyanin and anthocyanin-rich broccoli (Brassica oleracea var. italica) cultivars. Appl. Sci. 2022, 12, 6267. [Google Scholar] [CrossRef]
- Allan, A.C.; Hellens, R.P.; Laing, W.A. MYB transcription factors that colour our fruit. Trends Plant Sci. 2008, 13, 99–102. [Google Scholar] [CrossRef]
- Xu, W.; Dubos, C.; Lepiniec, L. Transcriptional control of flavonoid biosynthesis by MYB-bHLH-WDR complexes. Trends Plant Sci. 2015, 20, 176–185. [Google Scholar] [CrossRef] [PubMed]
- Albert, N.W.; Davies, K.M.; Schwinn, K.E. Gene regulation networks generate diverse pigmentation patterns in plants. Plant Signal. Behav. 2014, 9, e29526. [Google Scholar] [CrossRef] [PubMed]
- Kim, D.; Langmead, B.; Salzberg, S.L. HISAT: A fast spliced aligner with low memory requirements. Nat. Methods 2015, 12, 357–360. [Google Scholar] [CrossRef] [PubMed]
- Love, M.I.; Huber, W.; Anders, S. Moderated estimation of fold change and dispersion for RNA-seq data with DESeq2. Genome Biol. 2014, 15, 550. [Google Scholar] [CrossRef]
- Young, M.D.; Wakefield, M.J.; Smyth, G.K.; Oshlack, A. Gene ontology analysis for RNA-seq: Accounting for selection bias. Genome Biol. 2010, 11, R14. [Google Scholar] [CrossRef]
- Mao, X.; Cai, T.; Olyarchuk, J.G.; Wei, L. Automated genome annotation and pathway identification using the KEGG Orthology (KO) as a controlled vocabulary. Bioinformatics 2005, 21, 3787–3793. [Google Scholar] [CrossRef] [PubMed]
- Hu, M.; Qi, Z.; Ren, Z.; Tong, J.; Wang, B.; Wu, Z.; Hao, J.; Liu, N. Genome-wide analysis of auxin response factors in lettuce (Lactuca sativa L.) reveals the positive roles of LsARF8a in thermally induced bolting. Int. J. Mol. Sci. 2022, 23, 13509. [Google Scholar] [CrossRef] [PubMed]
- Livak, K.J.; Schmittgen, T.D. Analysis of relative gene expression data using real-time quantitative PCR and the 2−ΔΔCT method. Methods 2001, 25, 402–408. [Google Scholar] [CrossRef] [PubMed]
- Fraga, C.G.; Clowers, B.H.; Moore, R.J.; Zink, E.M. Signature-discovery approach for sample matching of a nerve-agent precursor using liquid chromatography-mass spectrometry, XCMS, and chemometrics. Anal. Chem. 2010, 82, 4165–4173. [Google Scholar] [CrossRef]
- Liu, N.; Hu, M.M.; Liang, H.; Tong, J.; Xie, L.; Wang, B.J.; Ji, Y.H.; He, H.J.; Liu, M.C.; Wu, Z.H. Physiological, transcriptomic, and metabolic analyses reveal that mild salinity improves the growth, nutrition, and flavor properties of hydroponic Chinese chive (Allium tuberosum Rottler ex Spr). Front. Nutr. 2022, 9, 1000271. [Google Scholar] [CrossRef]


| Samples | Clean Reads | Clean Bases | Q30 | Rate of GC Content |
|---|---|---|---|---|
| GB767-1 | 21.11 Mb | 6.32 Gb | 93.98% | 47.73% |
| GB767-2 | 20.97 Mb | 6.36 Gb | 93.91% | 47.48% |
| GB767-3 | 20.85 Mb | 6.23 Gb | 93.96% | 47.49% |
| PB767-1 | 26.79 Mb | 8.02 Gb | 94.41% | 47.07% |
| PB767-2 | 22.29 Mb | 6.67 Gb | 94.00% | 47.34% |
| PB767-3 | 23.70 Mb | 7.08 Gb | 93.83% | 47.39% |
| Compounds | GB767 | PB767 | Log2FC | Regulated | KEGG Map |
|---|---|---|---|---|---|
| 3-O-Acetylpinobanksin | - | 2.00 × 104 | 11.12 | up | ko00941 |
| Aromadendrin | 8.85 × 103 | - | −9.94 | down | ko00941, ko01100, ko01110 |
| Gallocatechin | - | 2.71 × 104 | 11.56 | up | ko00941, ko01110 |
| Pelargonidin-3-O-glucoside | 2.59 × 105 | - | −14.82 | down | ko00942, ko01100, ko01110 |
| Delphinidin-3-O-glucoside | 1.58 × 105 | 2.28 × 107 | 7.17 | up | ko00942 |
| Cyanidin-3-O-glucoside | 1.91 × 107 | 5.74 × 106 | −1.73 | down | ko00942 |
| Petunidin-3-O-glucoside | 1.25 × 104 | 8.92 × 106 | 9.47 | up | ko00942 |
| 2,6,7,4’-Tetrahydroxyisoflavanone | 1.20 × 105 | 1.63 × 104 | −2.87 | down | ko00943, ko01110 |
| Quercetin-3-O-sophoroside | 2.64 × 105 | 3.50 × 107 | 7.05 | up | ko00944 |
| Kaempferol-3-O-sophorotrioside | 6.73 × 106 | 1.29 × 106 | −2.38 | down | ko00944 |
| Kaempferol-3-O-galactoside | 2.95 × 105 | 2.80 × 104 | −3.40 | down | ko00944 |
| Quercetin-3-O-glucoside | 7.85 × 104 | 5.69 × 105 | 2.86 | up | ko00944, ko01100, ko01110 |
| Apigenin-7-O-glucoside | 1.34 × 105 | - | −13.86 | down | ko00944 |
| Kaempferol-3-O-glucoside | 5.73 × 106 | 4.78 × 105 | −3.58 | down | ko00944, ko01110 |
| Kaempferol-3-O-sophoroside | 3.00 × 105 | 7.62 × 104 | −1.98 | down | ko00944 |
| Luteolin-7-O-glucoside | 3.85 × 106 | - | −18.71 | down | ko00944 |
| Categories | Metabolites | GB767 | PB767 | Log2FC | Regulated |
|---|---|---|---|---|---|
| Cyanidin | Cyanidin-3-O-sophorotrioside | 2.96 × 105 | 1.58 × 107 | 5.74 | up |
| Cyanidin-3-O-glucoside | 1.91 × 107 | 5.74 × 106 | −1.73 | down | |
| Cyanidin-3-O-galactoside | 5.58 × 106 | 6.28 × 105 | −3.15 | down | |
| Pelargonidin | Pelargonidin-3-O-glucoside | 2.59 × 105 | - | - | down |
| Delphinidin | Delphinidin-3-O-glucoside | 1.58 × 105 | 2.28 × 107 | 7.17 | up |
| Delphinidin-3-O-galactoside | 1.58 × 105 | 2.44 × 107 | 7.27 | up | |
| Petunidin | Petunidin-3-O-glucoside | 1.25 × 104 | 8.92 × 106 | 9.47 | up |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wen, S.; Li, N.; Song, S.; Liu, N.; Ding, Y. Comparative Transcriptome and Metabolome Analyses of Broccoli Germplasms with Purple and Green Curds Reveal the Structural Genes and Transitional Regulators Regulating Color Formation. Int. J. Mol. Sci. 2023, 24, 6115. https://doi.org/10.3390/ijms24076115
Wen S, Li N, Song S, Liu N, Ding Y. Comparative Transcriptome and Metabolome Analyses of Broccoli Germplasms with Purple and Green Curds Reveal the Structural Genes and Transitional Regulators Regulating Color Formation. International Journal of Molecular Sciences. 2023; 24(7):6115. https://doi.org/10.3390/ijms24076115
Chicago/Turabian StyleWen, Shaozhe, Ning Li, Shuhui Song, Ning Liu, and Yunhua Ding. 2023. "Comparative Transcriptome and Metabolome Analyses of Broccoli Germplasms with Purple and Green Curds Reveal the Structural Genes and Transitional Regulators Regulating Color Formation" International Journal of Molecular Sciences 24, no. 7: 6115. https://doi.org/10.3390/ijms24076115
APA StyleWen, S., Li, N., Song, S., Liu, N., & Ding, Y. (2023). Comparative Transcriptome and Metabolome Analyses of Broccoli Germplasms with Purple and Green Curds Reveal the Structural Genes and Transitional Regulators Regulating Color Formation. International Journal of Molecular Sciences, 24(7), 6115. https://doi.org/10.3390/ijms24076115






