Starvation Induces Extracellular Accumulation of Polyphosphate in Dictyostelium discoideum to Inhibit Macropinocytosis, Phagocytosis, and Exocytosis
Abstract
1. Introduction
2. Results
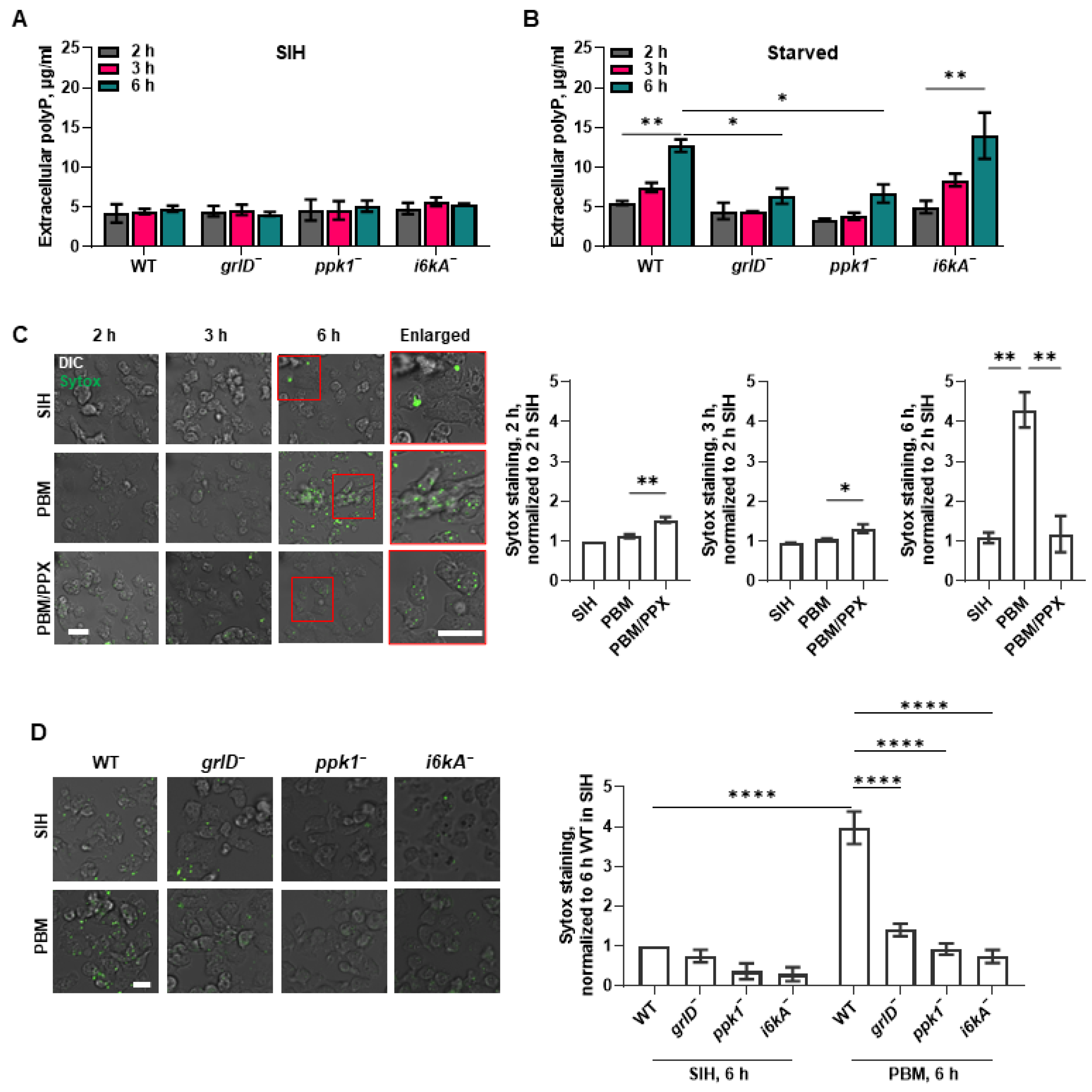
2.1. Starving D. discoideum Cells Require GrlD and Ppk1 to Accumulate Normal Levels of Extracellular and Cell Surface polyP, and I6kA to Accumulate Normal Levels of Cell Surface polyP
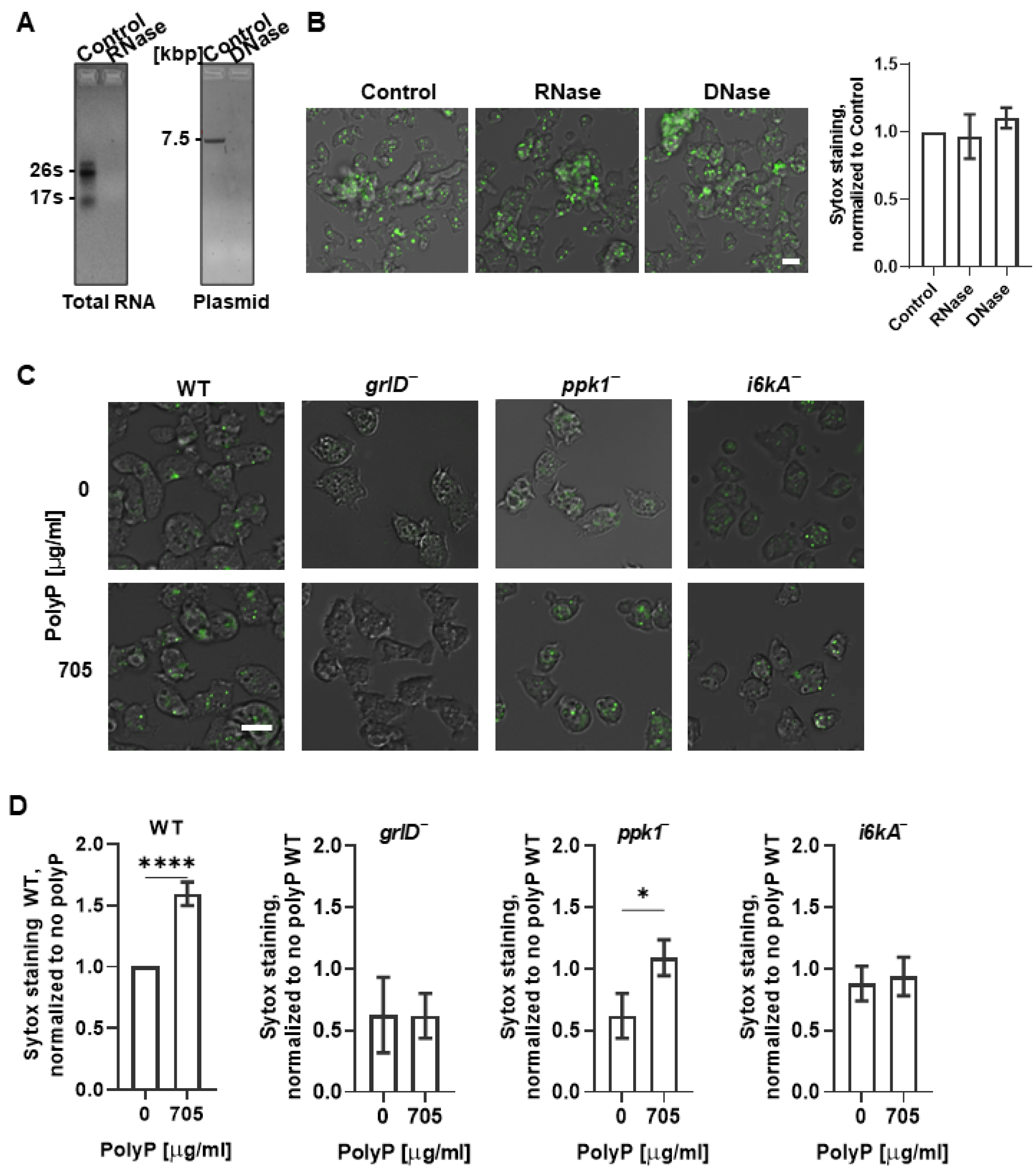
2.2. Cells Require GrlD and I6kA to Bind Exogenous polyP
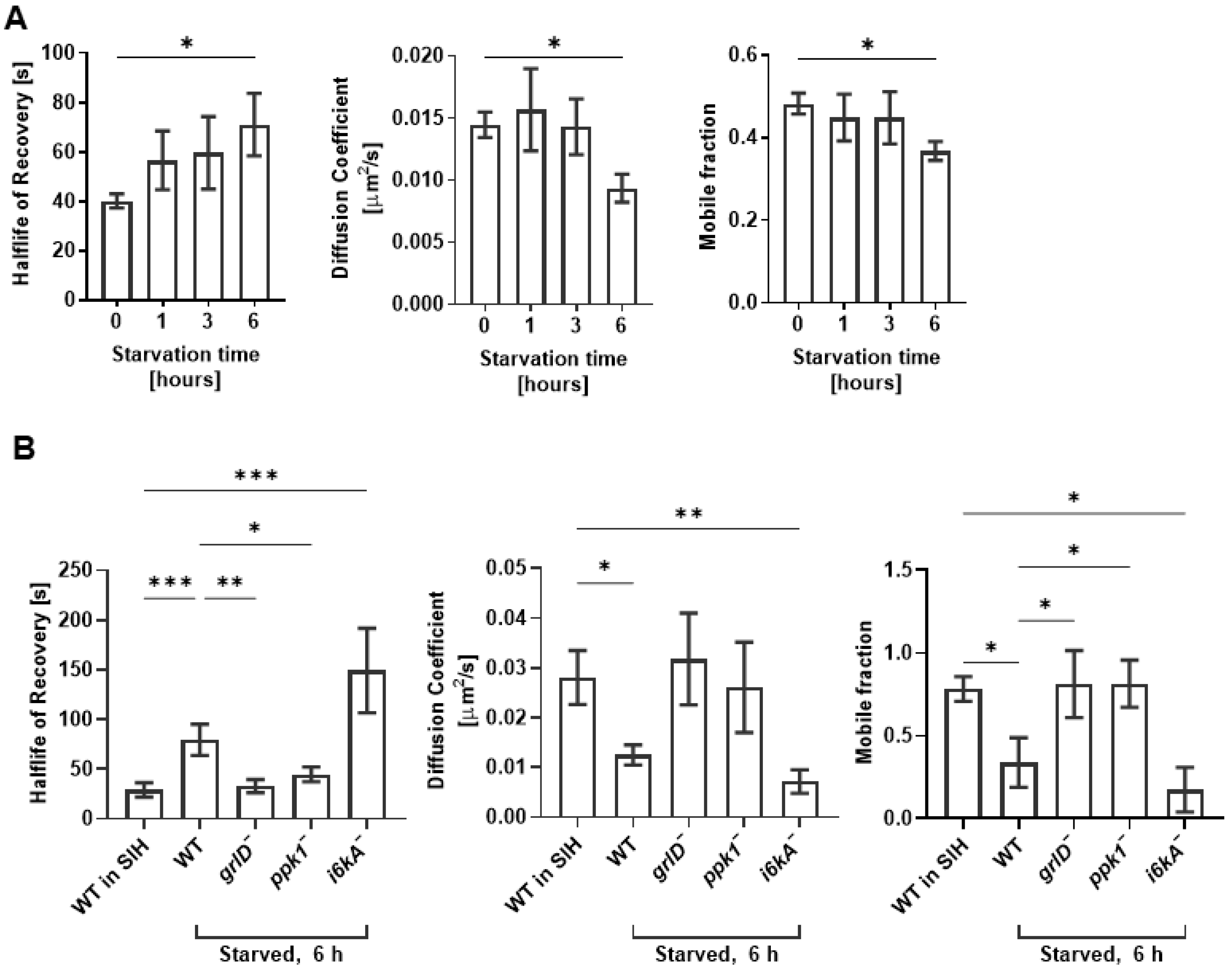
2.3. Starvation Reduces the Cell Membrane Fluidity of D. discoideum Cells, and This Requires GrlD and Ppk1
2.4. Starving D. discoideum Cells Require GrlD, Ppk1, and I6kA to Reduce Macropinocytosis and Nutrient Retention
2.5. Starving D. discoideum Cells Require GrlD, Ppk1, and I6kA to Reduce Phagocytosis
2.6. Cells Lacking GrlD or Ppk1 Are Abnormally Large
3. Discussion
4. Materials and Methods
4.1. D. discoideum Cell Culture
4.2. Recombinant Polyphosphatase Purification and polyP Concentration Measurement
4.3. PolyP Binding Assay and SYTOX Staining of Membrane-Bound polyP
4.4. DNase and RNase Treatments
4.5. Fluorescence Recovery after Photobleaching (FRAP)
4.6. Macropinocytosis and Exocytosis Assays
4.7. Phagocytosis Assay
4.8. Cell Size, Mass, and Protein Determination
4.9. Statistical Analysis
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Cardelli, J. Phagocytosis and macropinocytosis in Dictyostelium: Phosphoinositide-based processes, biochemically distinct. Traffic 2001, 2, 311–320. [Google Scholar] [CrossRef]
- Vuillemin, P. Une Acrasiee bacteriophage. C. R. S Eances L’acad. Emie Sci. Paris 1903, 137, 387–389. [Google Scholar]
- Cosson, P.; Soldati, T. Eat, kill or die: When amoeba meets bacteria. Curr. Opin. Microbiol. 2008, 11, 271–276. [Google Scholar] [CrossRef] [PubMed]
- Watts, D.; Ashworth, J. Growth of myxamoebae of the cellular slime mould Dictyostelium discoideum in axenic culture. Biochem. J. 1970, 119, 171. [Google Scholar] [CrossRef] [PubMed]
- Franke, J.; Kessin, R. A defined minimal medium for axenic strains of Dictyostelium discoideum. Proc. Natl. Acad. Sci. USA 1977, 74, 2157–2161. [Google Scholar] [CrossRef] [PubMed]
- Watts, D. Vitamin requirements for growth of Myxamoebae of Dictyostelium discoideum in a defined medium. Microbiology 1977, 98, 355–361. [Google Scholar] [CrossRef]
- Sussman, R.; Sussman, M. Cultivation of Dictyostelium discoideum in axenic medium. Biochem. Biophys. Res. Commun. 1967, 29, 53–55. [Google Scholar] [CrossRef] [PubMed]
- Hacker, U.; Albrecht, R.; Maniak, M. Fluid-phase uptake by macropinocytosis in Dictyostelium. J. Cell Sci. 1997, 110 Pt 2, 105–112. [Google Scholar] [CrossRef] [PubMed]
- Loomis, W.F., Jr. Sensitivity of Dictyostelium discoideum to nucleic acid analogues. Exp. Cell Res. 1971, 64, 484–486. [Google Scholar] [CrossRef]
- Bloomfield, G.; Traynor, D.; Sander, S.P.; Veltman, D.M.; Pachebat, J.A.; Kay, R.R. Neurofibromin controls macropinocytosis and phagocytosis in Dictyostelium. Elife 2015, 4, e04940. [Google Scholar] [CrossRef]
- Maniak, M. Fusion and fission events in the endocytic pathway of Dictyostelium. Traffic 2003, 4, 1–5. [Google Scholar] [CrossRef] [PubMed]
- Neuhaus, E.M.; Almers, W.; Soldati, T. Morphology and dynamics of the endocytic pathway in Dictyostelium discoideum. Mol. Biol. Cell 2002, 13, 1390–1407. [Google Scholar] [CrossRef]
- Raper, K.B. Dictyostelium discoideum, a new species of. J. Agric. Res. 1935, 50, 135. [Google Scholar]
- Kessin, R.H. Dictyostelium: Evolution, Cell Biology, and the Development of Multicellularity; Cambridge University Press: Cambridge, UK, 2001; Volume 38. [Google Scholar]
- Smith, E.W.; Lima, W.C.; Charette, S.J.; Cosson, P. Effect of starvation on the endocytic pathway in Dictyostelium cells. Eukaryot. Cell 2010, 9, 387–392. [Google Scholar] [CrossRef]
- Katoh, M.; Chen, G.; Roberge, E.; Shaulsky, G.; Kuspa, A. Developmental commitment in Dictyostelium discoideum. Eukaryot. Cell 2007, 6, 2038–2045. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Zhuchenko, O.; Kuspa, A.; Soldati, T. Social amoebae trap and kill bacteria by casting DNA nets. Nat. Commun. 2016, 7, 10938. [Google Scholar] [CrossRef]
- Chen, G.; Zhuchenko, O.; Kuspa, A. Immune-like phagocyte activity in the social amoeba. Science 2007, 317, 678–681. [Google Scholar] [CrossRef]
- Rao, N.N.; Gomez-Garcia, M.R.; Kornberg, A. Inorganic polyphosphate: Essential for growth and survival. Annu. Rev. Biochem. 2009, 78, 605–647. [Google Scholar] [CrossRef]
- Kornberg, A.; Rao, N.N.; Ault-Riche, D. Inorganic polyphosphate: A molecule of many functions. Annu. Rev. Biochem. 1999, 68, 89–125. [Google Scholar] [CrossRef]
- Crooke, E.; Akiyama, M.; Rao, N.N.; Kornberg, A. Genetically altered levels of inorganic polyphosphate in Escherichia coli. J. Biol. Chem. 1994, 269, 6290–6295. [Google Scholar] [CrossRef]
- Rashid, M.H.; Rumbaugh, K.; Passador, L.; Davies, D.G.; Hamood, A.N.; Iglewski, B.H.; Kornberg, A. Polyphosphate kinase is essential for biofilm development, quorum sensing, and virulence of Pseudomonas aeruginosa. Proc. Natl. Acad. Sci. USA 2000, 97, 9636–9641. [Google Scholar] [CrossRef]
- Schroder, H.C.; Kurz, L.; Muller, W.E.; Lorenz, B. Polyphosphate in bone. Biochem. C/C Biokhimiia 2000, 65, 296–303. [Google Scholar]
- Suess, P.M.; Watson, J.; Chen, W.; Gomer, R.H. Extracellular polyphosphate signals through Ras and Akt to prime Dictyostelium discoideum cells for development. J. Cell Sci. 2017, 130, 2394–2404. [Google Scholar] [CrossRef]
- Hassanian, S.M.; Avan, A.; Ardeshirylajimi, A. Inorganic polyphosphate: A key modulator of inflammation. J. Thromb. Haemost. 2017, 15, 213–218. [Google Scholar] [CrossRef] [PubMed]
- Smith, S.A.; Mutch, N.J.; Baskar, D.; Rohloff, P.; Docampo, R.; Morrissey, J.H. Polyphosphate modulates blood coagulation and fibrinolysis. Proc. Natl. Acad. Sci. USA 2006, 103, 903–908. [Google Scholar] [CrossRef] [PubMed]
- Suess, P.M.; Gomer, R.H. Extracellular Polyphosphate Inhibits Proliferation in an Autocrine Negative Feedback Loop in Dictyostelium discoideum. J. Biol. Chem. 2016, 291, 20260–20269. [Google Scholar] [CrossRef] [PubMed]
- Rijal, R.; Kirolos, S.A.; Rahman, R.J.; Gomer, R.H. Dictyostelium discoideum cells retain nutrients when the cells are about to outgrow their food source. J. Cell Sci. 2022, 135, jcs260107. [Google Scholar] [CrossRef]
- Zhang, H.; Gomez-Garcia, M.R.; Shi, X.; Rao, N.N.; Kornberg, A. Polyphosphate kinase 1, a conserved bacterial enzyme, in a eukaryote, Dictyostelium discoideum, with a role in cytokinesis. Proc. Natl. Acad. Sci. USA 2007, 104, 16486–16491. [Google Scholar] [CrossRef]
- Livermore, T.M.; Chubb, J.R.; Saiardi, A. Developmental accumulation of inorganic polyphosphate affects germination and energetic metabolism in Dictyostelium discoideum. Proc. Natl. Acad. Sci. USA 2016, 113, 996–1001. [Google Scholar] [CrossRef]
- Saiardi, A.; Erdjument-Bromage, H.; Snowman, A.M.; Tempst, P.; Snyder, S.H. Synthesis of diphosphoinositol pentakisphosphate by a newly identified family of higher inositol polyphosphate kinases. Curr. Biol. 1999, 9, 1323–1326. [Google Scholar] [CrossRef]
- Suess, P.M.; Tang, Y.; Gomer, R.H. The putative G protein-coupled receptor GrlD mediates extracellular polyphosphate sensing in Dictyostelium discoideum. Mol. Biol. Cell 2019, 30, 1118–1128. [Google Scholar] [CrossRef]
- Klein, G.; Cotter, D.A.; Martin, J.B.; Bof, M.; Satre, M. Germination of Dictyostelium discoideum spores. A phosphorus-31 NMR analysis. Biochemistry 1988, 27, 8199–8203. [Google Scholar] [CrossRef]
- Aschar-Sobbi, R.; Abramov, A.Y.; Diao, C.; Kargacin, M.E.; Kargacin, G.J.; French, R.J.; Pavlov, E. High sensitivity, quantitative measurements of polyphosphate using a new DAPI-based approach. J. Fluoresc. 2008, 18, 859–866. [Google Scholar] [CrossRef] [PubMed]
- Verhoef, J.J.; Barendrecht, A.D.; Nickel, K.F.; Dijkxhoorn, K.; Kenne, E.; Labberton, L.; McCarty, O.J.; Schiffelers, R.; Heijnen, H.F.; Hendrickx, A.P.; et al. Polyphosphate nanoparticles on the platelet surface trigger contact system activation. Blood 2017, 129, 1707–1717. [Google Scholar] [CrossRef]
- Wurst, H.; Kornberg, A. A soluble exopolyphosphatase of Saccharomyces cerevisiae. Purification and characterization. J. Biol. Chem. 1994, 269, 10996–11001. [Google Scholar] [CrossRef] [PubMed]
- Ben-Dov, N.; Korenstein, R. Proton-induced endocytosis is dependent on cell membrane fluidity, lipid-phase order and the membrane resting potential. Biochim. Biophys. Acta 2013, 1828, 2672–2681. [Google Scholar] [CrossRef]
- Ge, S.; White, J.G.; Haynes, C.L. Critical role of membrane cholesterol in exocytosis revealed by single platelet study. ACS Chem. Biol. 2010, 5, 819–828. [Google Scholar] [CrossRef] [PubMed]
- Tang, Y.; Wu, Y.; Herlihy, S.E.; Brito-Aleman, F.J.; Ting, J.H.; Janetopoulos, C.; Gomer, R.H. An Autocrine Proliferation Repressor Regulates Dictyostelium discoideum Proliferation and Chemorepulsion Using the G Protein-Coupled Receptor GrlH. mBio 2018, 9, e02443-17. [Google Scholar] [CrossRef] [PubMed]
- Luo, H.R.; Huang, Y.E.; Chen, J.C.; Saiardi, A.; Iijima, M.; Ye, K.; Huang, Y.; Nagata, E.; Devreotes, P.; Snyder, S.H. Inositol pyrophosphates mediate chemotaxis in Dictyostelium via pleckstrin homology domain-PtdIns(3,4,5)P3 interactions. Cell 2003, 114, 559–572. [Google Scholar] [CrossRef] [PubMed]
- Fey, P.; Dodson, R.J.; Basu, S.; Chisholm, R.L. One Stop Shop for Everything Dictyostelium: Dicty Base and the Dicty Stock Center in 2012. In Dictyostelium Discoideum Protocols; Eichinger, L., Rivero, F., Eds.; Humana Press: Totowa, NJ, USA, 2013; pp. 59–92. [Google Scholar] [CrossRef]
- Sussman, M. Chapter 14 Biochemical and Genetic Methods in the Study of Cellular Slime Mold Development. In Methods in Cell Biology; Prescott, D.M., Ed.; Academic Press: Cambridge, MA, USA, 1966; Volume 2, pp. 397–410. [Google Scholar]
- Brock, D.A.; Gomer, R.H. A cell-counting factor regulating structure size in Dictyostelium. Genes Dev. 1999, 13, 1960–1969. [Google Scholar] [CrossRef]
- Gray, M.J.; Wholey, W.Y.; Wagner, N.O.; Cremers, C.M.; Mueller-Schickert, A.; Hock, N.T.; Krieger, A.G.; Smith, E.M.; Bender, R.A.; Bardwell, J.C.; et al. Polyphosphate is a primordial chaperone. Mol. Cell 2014, 53, 689–699. [Google Scholar] [CrossRef] [PubMed]
- Brock, D.A.; Gomer, R.H. A secreted factor represses cell proliferation in Dictyostelium. Development 2005, 132, 4553–4562. [Google Scholar] [CrossRef] [PubMed]
- Rijal, R.; Cadena, L.A.; Smith, M.R.; Carr, J.F.; Gomer, R.H. Polyphosphate is an extracellular signal that can facilitate bacterial survival in eukaryotic cells. Proc. Natl. Acad. Sci. USA 2020, 117, 31923–31934. [Google Scholar] [CrossRef]
- Laasmaa, M.; Vendelin, M.; Peterson, P. Application of regularized Richardson-Lucy algorithm for deconvolution of confocal microscopy images. J. Microsc. 2011, 243, 124–140. [Google Scholar] [CrossRef] [PubMed]
- Shihan, M.H.; Novo, S.G.; Le Marchand, S.J.; Wang, Y.; Duncan, M.K. A simple method for quantitating confocal fluorescent images. Biochem. Biophys. Rep. 2021, 25, 100916. [Google Scholar] [CrossRef] [PubMed]
- Rijal, R.; Consalvo, K.M.; Lindsey, C.K.; Gomer, R.H. An endogenous chemorepellent directs cell movement by inhibiting pseudopods at one side of cells. Mol. Biol. Cell 2019, 30, 242–255. [Google Scholar] [CrossRef] [PubMed]
- Veltman, D.M.; Akar, G.; Bosgraaf, L.; Van Haastert, P.J. A new set of small, extrachromosomal expression vectors for Dictyostelium discoideum. Plasmid 2009, 61, 110–118. [Google Scholar] [CrossRef]
- Aaij, C.; Borst, P. The gel electrophoresis of DNA. Biochim. Biophys. Acta 1972, 269, 192–200. [Google Scholar] [CrossRef]
- Tanaka, M.; Kikuchi, T.; Uno, H.; Okita, K.; Kitanishi-Yumura, T.; Yumura, S. Turnover and flow of the cell membrane for cell migration. Sci. Rep. 2017, 7, 12970. [Google Scholar] [CrossRef]
- Rivero, F.; Maniak, M. Quantitative and Microscopic Methods for Studying the Endocytic Pathway. In Dictyostelium Discoideum Protocols; Eichinger, L., Rivero, F., Eds.; Humana Press: Totowa, NJ, USA, 2006; pp. 423–438. [Google Scholar] [CrossRef]
- Phillips, J.E.; Gomer, R.H. The ROCO kinase QkgA is necessary for proliferation inhibition by autocrine signals in Dictyostelium discoideum. Eukaryot. Cell 2010, 9, 1557–1565. [Google Scholar] [CrossRef]


Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Rijal, R.; Ismail, I.; Jing, S.; Gomer, R.H. Starvation Induces Extracellular Accumulation of Polyphosphate in Dictyostelium discoideum to Inhibit Macropinocytosis, Phagocytosis, and Exocytosis. Int. J. Mol. Sci. 2023, 24, 5923. https://doi.org/10.3390/ijms24065923
Rijal R, Ismail I, Jing S, Gomer RH. Starvation Induces Extracellular Accumulation of Polyphosphate in Dictyostelium discoideum to Inhibit Macropinocytosis, Phagocytosis, and Exocytosis. International Journal of Molecular Sciences. 2023; 24(6):5923. https://doi.org/10.3390/ijms24065923
Chicago/Turabian StyleRijal, Ramesh, Issam Ismail, Shiyu Jing, and Richard H. Gomer. 2023. "Starvation Induces Extracellular Accumulation of Polyphosphate in Dictyostelium discoideum to Inhibit Macropinocytosis, Phagocytosis, and Exocytosis" International Journal of Molecular Sciences 24, no. 6: 5923. https://doi.org/10.3390/ijms24065923
APA StyleRijal, R., Ismail, I., Jing, S., & Gomer, R. H. (2023). Starvation Induces Extracellular Accumulation of Polyphosphate in Dictyostelium discoideum to Inhibit Macropinocytosis, Phagocytosis, and Exocytosis. International Journal of Molecular Sciences, 24(6), 5923. https://doi.org/10.3390/ijms24065923





