Theophylline-Induced Relaxation Is Enhanced after Testosterone Treatment via Increased KV1.2 and KV1.5 Protein Expression in Guinea Pig Tracheal Smooth Muscle
Abstract
1. Introduction
2. Results
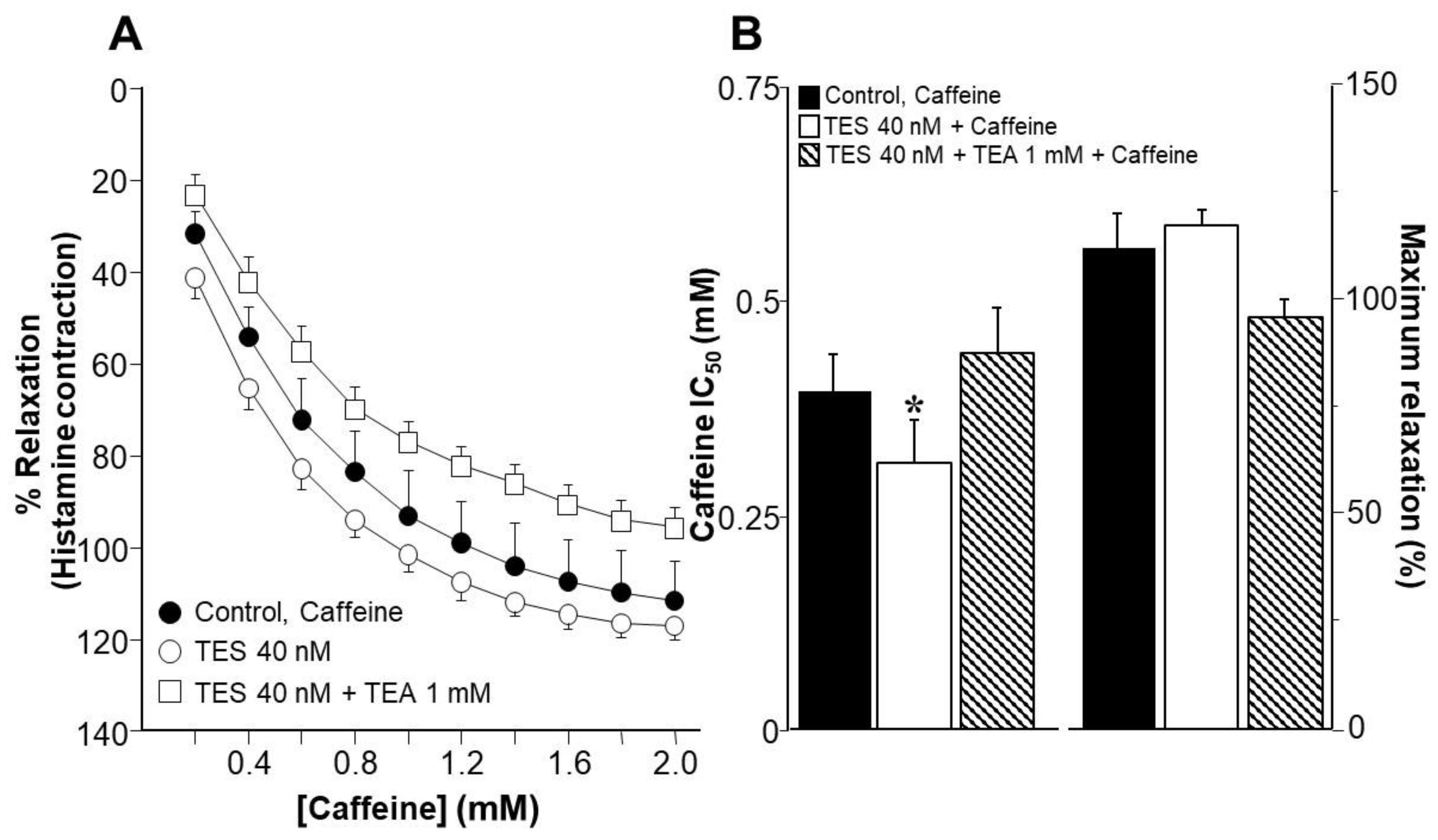
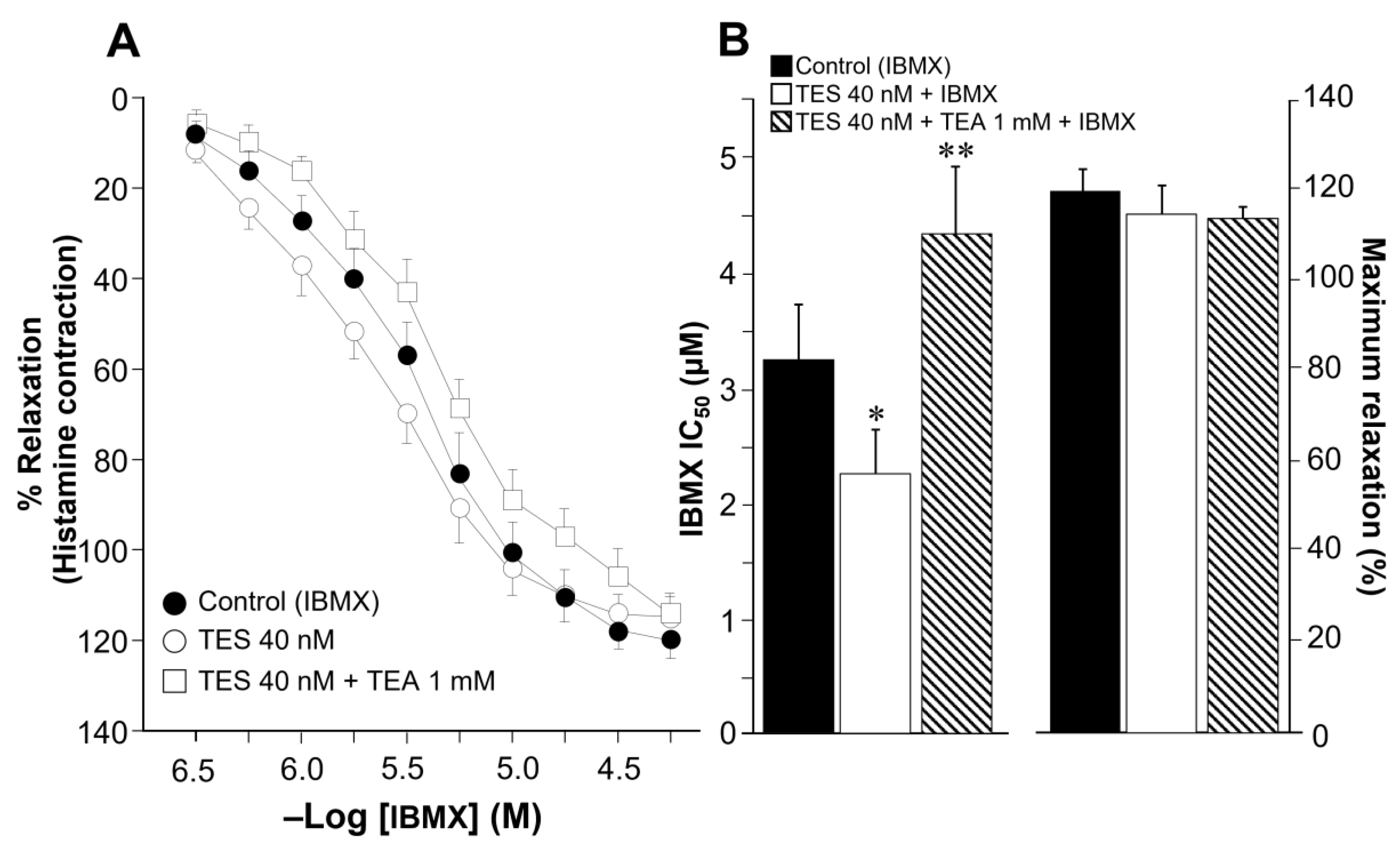
2.1. Testosterone Enhances Tracheal Smooth Muscle Relaxation Induced by the Methylxanthines Caffeine and Isobutylmethylxanthine in Association with K+ Channels
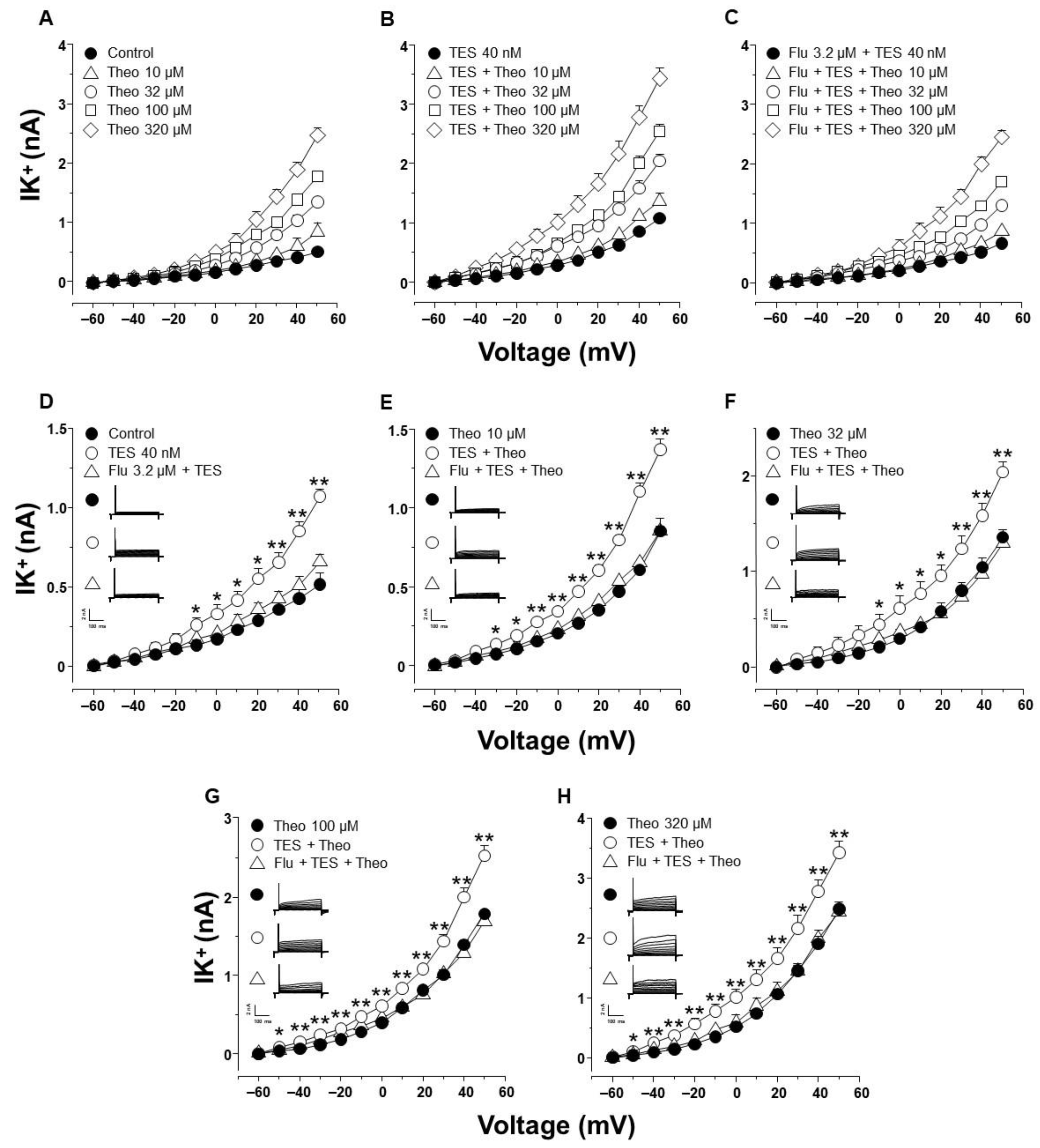
2.2. Theophylline-Induced Relaxation of Tracheal Smooth Muscle Is also Enhanced by Testosterone, and K+ Channels Are Involved in This Androgen’s Action
2.3. Testosterone Increases Theophylline-Induced K+ Currents via the Androgen Receptor Signaling Pathway in Tracheal Myocytes
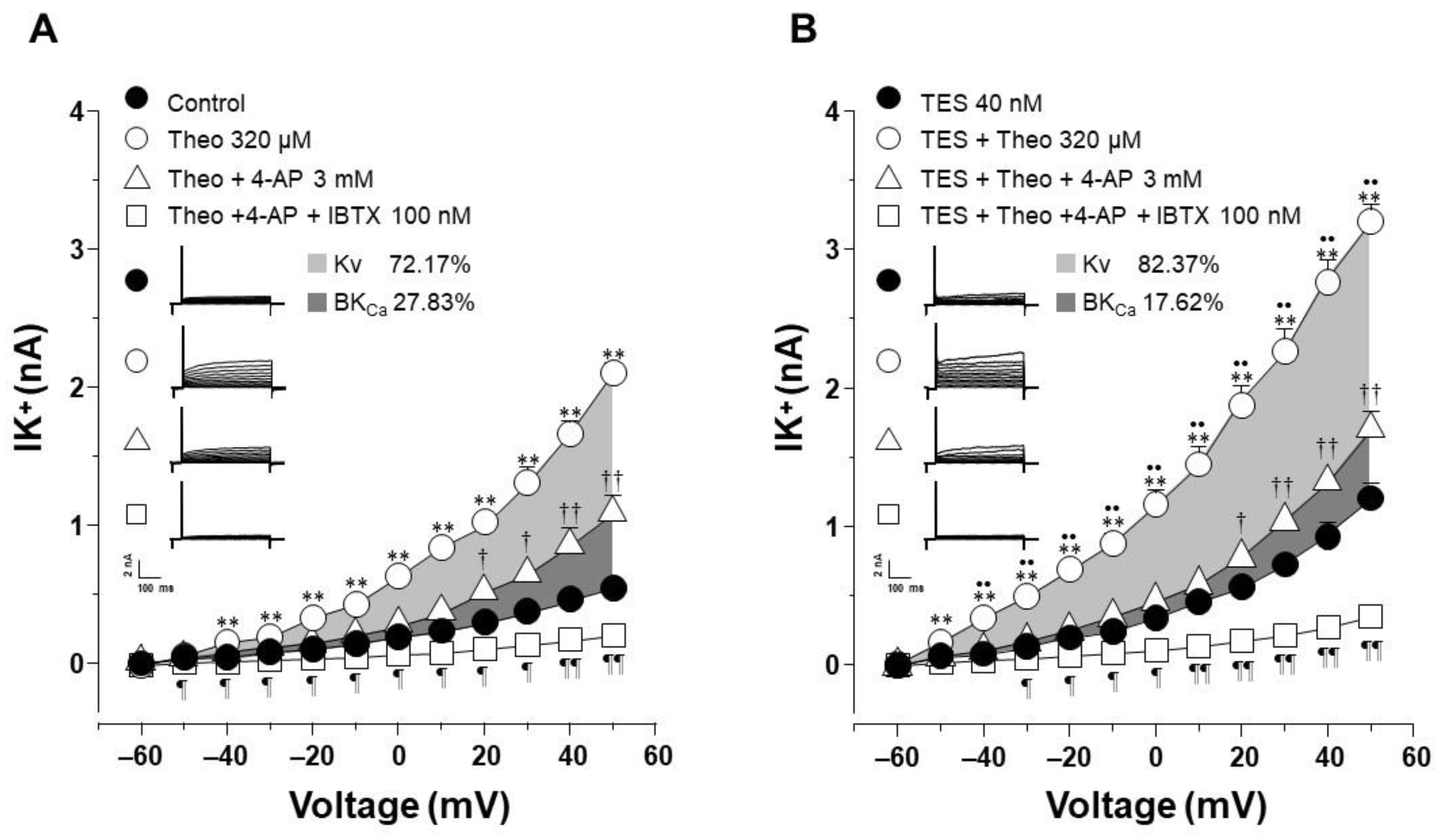
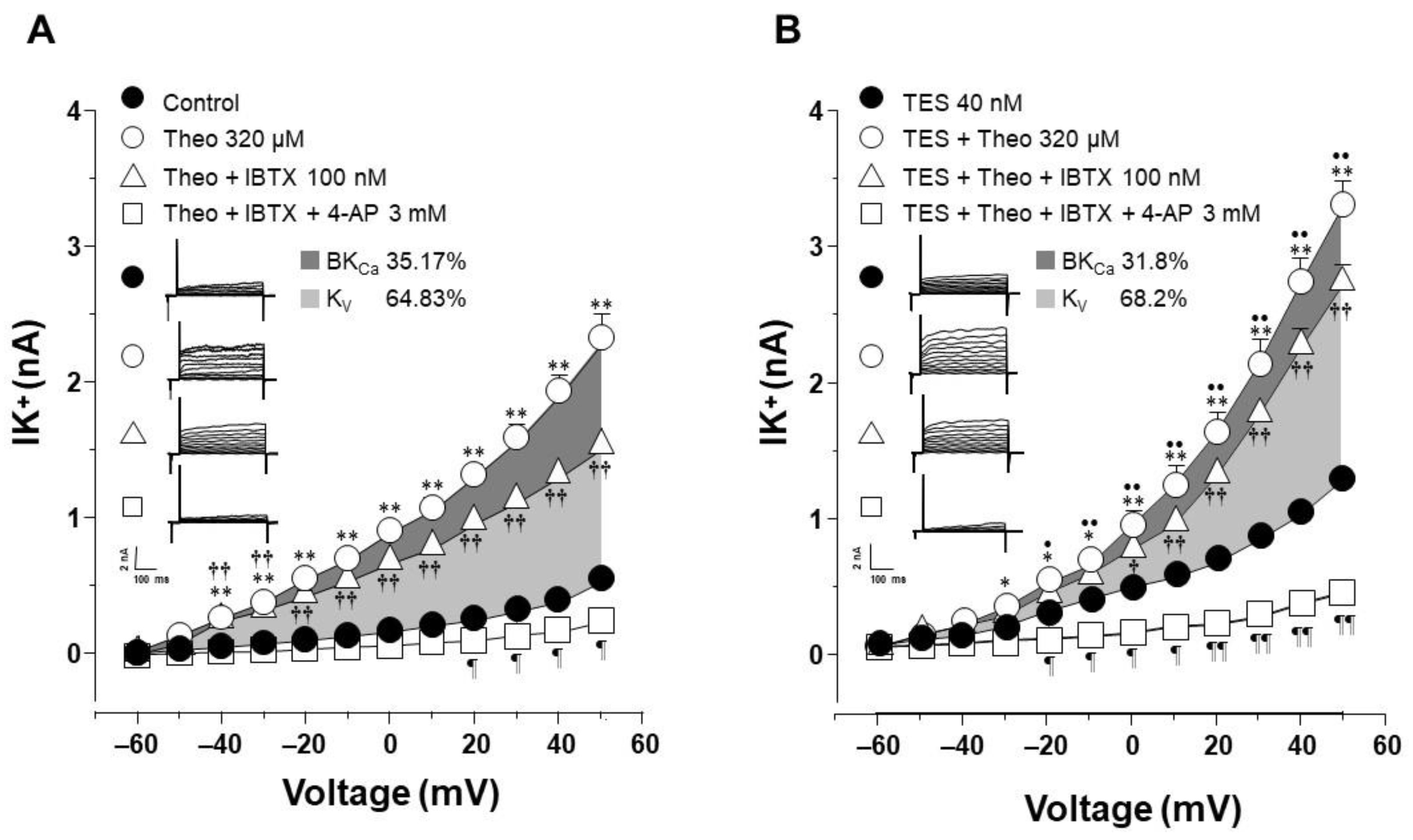
2.4. The Effect of Testosterone Is Mediated Mainly by the Upregulation of KV Channels
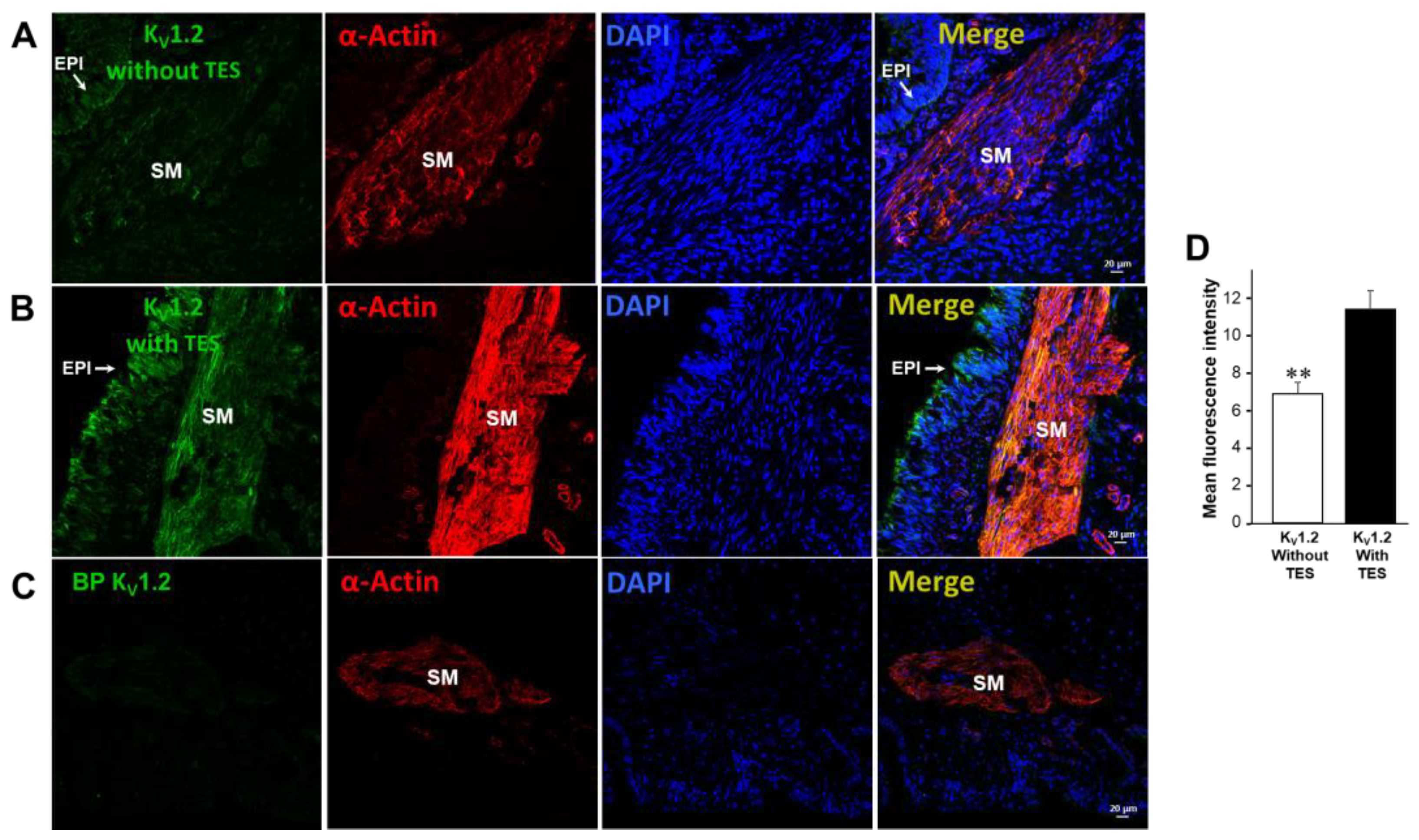
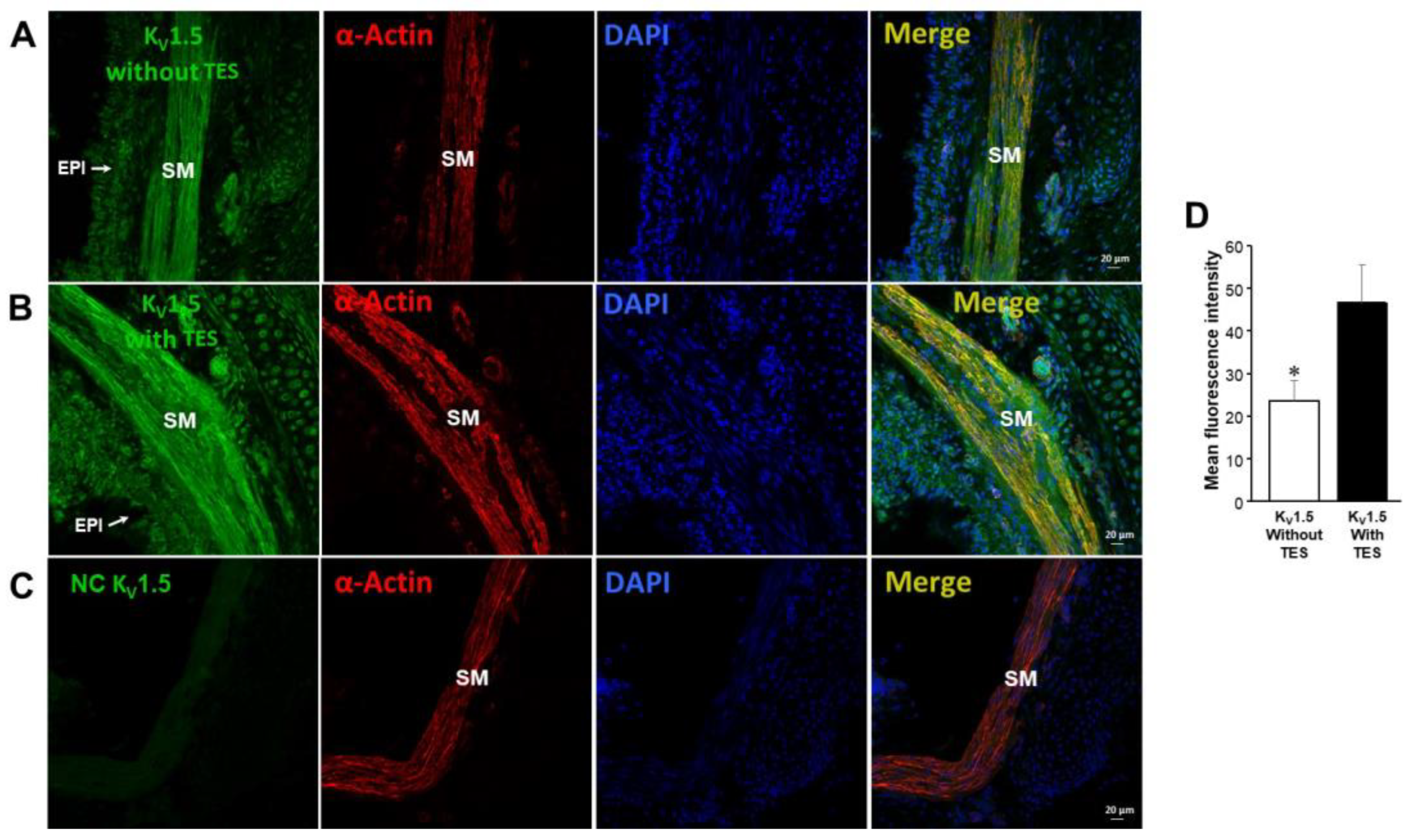
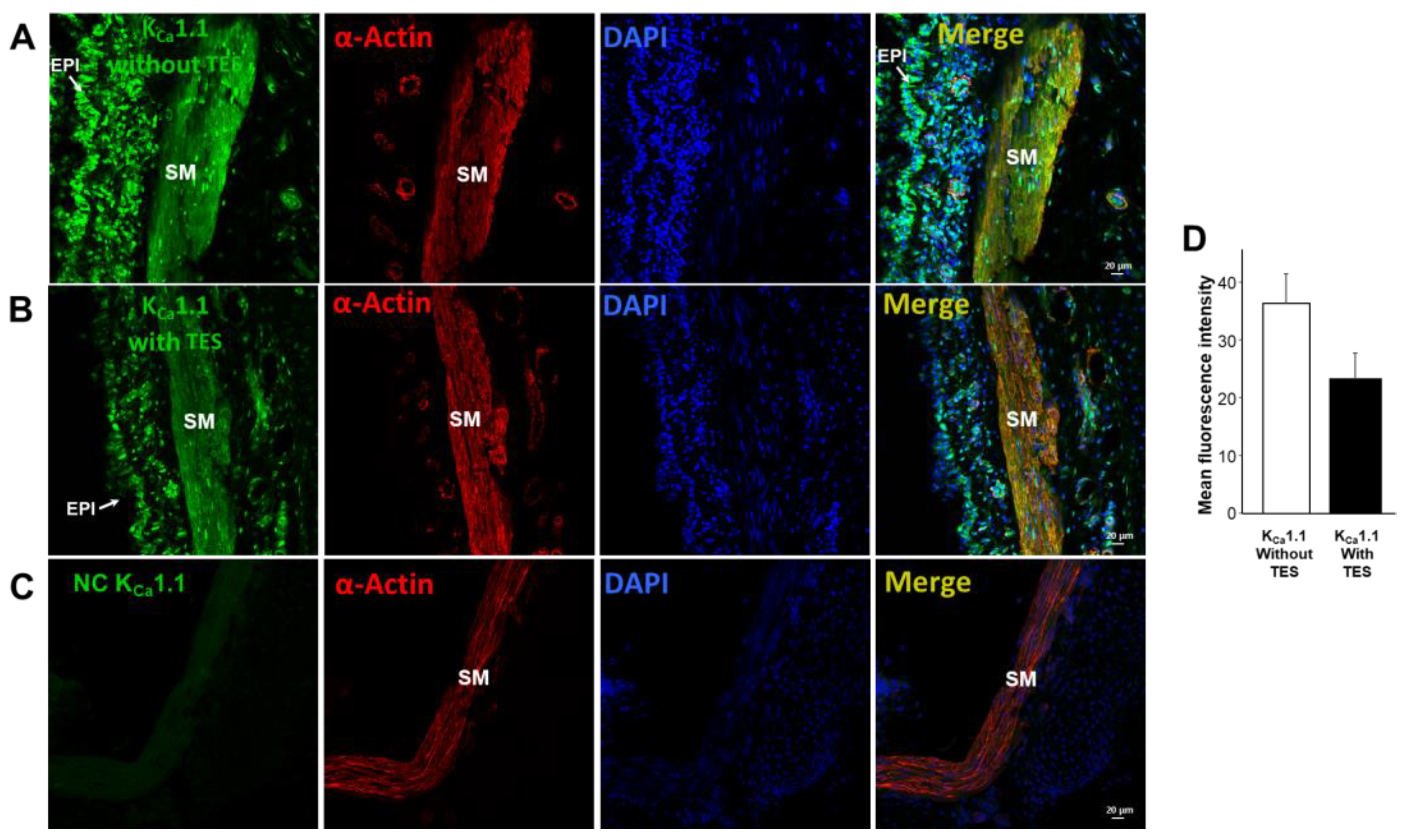
2.5. KV1.2, KV1.5, and BKCa Subtypes Are Expressed in Guinea Pig Airway Smooth Muscle and Testosterone Only Increases the Expression of KV Channels
3. Discussion
4. Materials and Methods
4.1. Experimental Animals
4.2. Organ Baths
4.3. Patch-Clamp Studies
4.4. Double Immunofluorescence
4.5. Drugs and Chemicals
4.6. Statistical Analysis
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Lo Coco, F.; Lanuzza, F.; Micali, G.; Cappellano, G. Determination of theobromine, theophylline, and caffeine in by-products of cupuacu and cacao seeds by high-performance liquid chromatography. J. Chromatogr. Sci. 2007, 45, 273–275. [Google Scholar] [CrossRef] [PubMed]
- Barnes, P.J. Theophylline. Pharmaceuticals 2010, 3, 725–747. [Google Scholar] [CrossRef] [PubMed]
- Barnes, P.J. Theophylline: New perspectives for an old drug. Am. J. Respir. Crit. Care Med. 2003, 167, 813–818. [Google Scholar] [CrossRef] [PubMed]
- Ford, P.A.; Durham, A.L.; Russell, R.E.; Gordon, F.; Adcock, I.M.; Barnes, P.J. Treatment effects of low-dose theophylline combined with an inhaled corticosteroid in COPD. Chest 2010, 137, 1338–1344. [Google Scholar] [CrossRef]
- Yamauchi, K.; Kobayashi, H.; Tanifuji, Y.; Yoshida, T.; Pian, H.D.; Inoue, H. Efficacy and safety of intravenous theophylline administration for treatment of mild acute exacerbation of bronchial asthma. Respirology 2005, 10, 491–496. [Google Scholar] [CrossRef]
- Macht, D.; Ting, G.-C. A study of antispasmodic drugs on the bronchus. J. Pharmacol. Exp. Ther. 1921, 18, 373–398. [Google Scholar]
- Tilley, S.L. Methylxanthines in asthma. Handb. Exp. Pharmacol. 2011, 200, 439–456. [Google Scholar] [CrossRef]
- Herrmann, G.; Aynesworth, M.B. Successful treatment of persistent extreme dyspnea “status asthmaticus”. Use of theophylline ethylene diamine (aminophylline, U.S.P.) intravenously. 1938. J. Lab. Clin. Med. 1990, 115, 512–525. [Google Scholar]
- Somerville, L.L.; National Heart, L.; Blood, I. Theophylline revisited. Allergy Asthma Proc. 2001, 22, 347–351. [Google Scholar]
- Mitenko, P.A.; Ogilvie, R.I. Rational intravenous doses of theophylline. N. Engl. J. Med. 1973, 289, 600–603. [Google Scholar] [CrossRef]
- Turner-Warwick, M. Study of theophylline plasma levels after oral administration of new theophylline compounds. Br. Med. J. 1957, 2, 67–69. [Google Scholar] [CrossRef] [PubMed]
- Dasta, J.; Mirtallo, J.M.; Altman, M. Comparison of standard- and sustained-release theophylline tablets in patients with chronic obstructive pulmonary disease. Am. J. Hosp. Pharm. 1979, 36, 613–617. [Google Scholar] [CrossRef] [PubMed]
- Hayashi, T.; Kanbe, H.; Okada, M.; Suzuki, M.; Ikeda, Y.; Onuki, Y.; Kaneko, T.; Sonobe, T. Formulation study and drug release mechanism of a new theophylline sustained-release preparation. Int. J. Pharm. 2005, 304, 91–101. [Google Scholar] [CrossRef] [PubMed]
- Simons, F.E.; Becker, A.B.; Simons, K.J.; Gillespie, C.A. The bronchodilator effect and pharmacokinetics of theobromine in young patients with asthma. J. Allergy Clin. Immunol. 1985, 76, 703–707. [Google Scholar] [CrossRef]
- Welsh, E.J.; Bara, A.; Barley, E.; Cates, C.J. Caffeine for asthma. Cochrane Database Syst. Rev. 2010, 1, CD001112. [Google Scholar] [CrossRef]
- Zuo, H.; Cattani-Cavalieri, I.; Musheshe, N.; Nikolaev, V.O.; Schmidt, M. Phosphodiesterases as therapeutic targets for respiratory diseases. Pharmacol. Ther. 2019, 197, 225–242. [Google Scholar] [CrossRef]
- Wang, S.; Xie, Y.; Huo, Y.W.; Li, Y.; Abel, P.W.; Jiang, H.; Zou, X.; Jiao, H.Z.; Kuang, X.; Wolff, D.W.; et al. Airway relaxation mechanisms and structural basis of osthole for improving lung function in asthma. Sci. Signal. 2020, 13, eaax0273. [Google Scholar] [CrossRef]
- Rabe, K.F.; Magnussen, H.; Dent, G. Theophylline and selective PDE inhibitors as bronchodilators and smooth muscle relaxants. Eur. Respir. J. 1995, 8, 637–642. [Google Scholar] [CrossRef]
- Rabe, K.F.; Tenor, H.; Dent, G.; Schudt, C.; Liebig, S.; Magnussen, H. Phosphodiesterase isozymes modulating inherent tone in human airways: Identification and characterization. Am. J. Physiol. 1993, 264, L458–L464. [Google Scholar] [CrossRef]
- Billington, C.K.; Joseph, S.K.; Swan, C.; Scott, M.G.; Jobson, T.M.; Hall, I.P. Modulation of human airway smooth muscle proliferation by type 3 phosphodiesterase inhibition. Am. J. Physiol. 1999, 276, L412–L419. [Google Scholar] [CrossRef]
- Le Jeune, I.R.; Shepherd, M.; Van Heeke, G.; Houslay, M.D.; Hall, I.P. Cyclic AMP-dependent transcriptional up-regulation of phosphodiesterase 4D5 in human airway smooth muscle cells. Identification and characterization of a novel PDE4D5 promoter. J. Biol. Chem. 2002, 277, 35980–35989. [Google Scholar] [CrossRef] [PubMed]
- Barnes, P.J. Theophylline. Am. J. Respir. Crit. Care Med. 2013, 188, 901–906. [Google Scholar] [CrossRef] [PubMed]
- Billington, C.K.; Penn, R.B. Signaling and regulation of G protein-coupled receptors in airway smooth muscle. Respir. Res. 2003, 4, 4. [Google Scholar] [CrossRef]
- Yue, C.; Dodge, K.L.; Weber, G.; Sanborn, B.M. Phosphorylation of serine 1105 by protein kinase A inhibits phospholipase Cβ3 stimulation by Gαq. J. Biol. Chem. 1998, 273, 18023–18027. [Google Scholar] [CrossRef] [PubMed]
- Yang, C.M.; Hsu, M.C.; Tsao, H.L.; Chiu, C.T.; Ong, R.; Hsieh, J.T.; Fan, L.W. Effect of cAMP elevating agents on carbachol-induced phosphoinositide hydrolysis and calcium mobilization in cultured canine tracheal smooth muscle cells. Cell Calcium 1996, 19, 243–254. [Google Scholar] [CrossRef] [PubMed]
- Bai, Y.; Sanderson, M.J. Airway smooth muscle relaxation results from a reduction in the frequency of Ca2+ oscillations induced by a cAMP-mediated inhibition of the IP3 receptor. Respir. Res. 2006, 7, 34. [Google Scholar] [CrossRef]
- Wellman, G.C.; Santana, L.F.; Bonev, A.D.; Nelson, M.T. Role of phospholamban in the modulation of arterial Ca2+ sparks and Ca2+-activated K+ channels by cAMP. Am. J. Physiol. Cell Physiol. 2001, 281, C1029–C1037. [Google Scholar] [CrossRef]
- Wang, Z.W.; Kotlikoff, M.I. Activation of KCa channels in airway smooth muscle cells by endogenous protein kinase A. Am. J. Physiol. 1996, 271, L100–L105. [Google Scholar] [CrossRef]
- Kume, H.; Hall, I.P.; Washabau, R.J.; Takagi, K.; Kotlikoff, M.I. β-adrenergic agonists regulate KCa channels in airway smooth muscle by cAMP-dependent and -independent mechanisms. J. Clin. Investig. 1994, 93, 371–379. [Google Scholar] [CrossRef]
- Mason, H.S.; Latten, M.J.; Godoy, L.D.; Horowitz, B.; Kenyon, J.L. Modulation of Kv1.5 currents by protein kinase A, tyrosine kinase, and protein tyrosine phosphatase requires an intact cytoskeleton. Mol. Pharmacol. 2002, 61, 285–293. [Google Scholar] [CrossRef]
- Jonas, E.A.; Kaczmarek, L.K. Regulation of potassium channels by protein kinases. Curr. Opin. Neurobiol. 1996, 6, 318–323. [Google Scholar] [CrossRef]
- Brueggemann, L.I.; Cribbs, L.L.; Schwartz, J.; Wang, M.; Kouta, A.; Byron, K.L. Mechanisms of PKA-Dependent Potentiation of Kv7.5 Channel Activity in Human Airway Smooth Muscle Cells. Int. J. Mol. Sci. 2018, 19, 2223. [Google Scholar] [CrossRef] [PubMed]
- Adda, S.; Fleischmann, B.K.; Freedman, B.D.; Yu, M.; Hay, D.W.; Kotlikoff, M.I. Expression and function of voltage-dependent potassium channel genes in human airway smooth muscle. J. Biol. Chem. 1996, 271, 13239–13243. [Google Scholar] [CrossRef] [PubMed]
- Boyle, J.P.; Tomasic, M.; Kotlikoff, M.I. Delayed rectifier potassium channels in canine and porcine airway smooth muscle cells. J. Physiol. 1992, 447, 329–350. [Google Scholar] [CrossRef] [PubMed]
- Brueggemann, L.I.; Haick, J.M.; Neuburg, S.; Tate, S.; Randhawa, D.; Cribbs, L.L.; Byron, K.L. KCNQ (Kv7) potassium channel activators as bronchodilators: Combination with a β2-adrenergic agonist enhances relaxation of rat airways. Am. J. Physiol. Lung Cell Mol. Physiol. 2014, 306, L476–L486. [Google Scholar] [CrossRef]
- Campos-Bedolla, P.; Vargas, M.H.; Segura, P.; Carbajal, V.; Calixto, E.; Figueroa, A.; Flores-Soto, E.; Barajas-López, C.; Mendoza-Patiño, N.; Montaño, L.M. Airway smooth muscle relaxation induced by 5-HT2A receptors: Role of Na+/K+-ATPase pump and Ca2+-activated K+ channels. Life Sci. 2008, 83, 438–446. [Google Scholar] [CrossRef] [PubMed]
- Carbajal-García, A.; Reyes-García, J.; Casas-Hernández, M.F.; Flores-Soto, E.; Díaz-Hernández, V.; Solis-Chagoyán, H.; Sommer, B.; Montaño, L.M. Testosterone augments β2 adrenergic receptor genomic transcription increasing salbutamol relaxation in airway smooth muscle. Mol. Cell Endocrinol. 2020, 510, 110801. [Google Scholar] [CrossRef]
- Montaño, L.M.; Cruz-Valderrama, J.E.; Figueroa, A.; Flores-Soto, E.; García-Hernández, L.M.; Carbajal, V.; Segura, P.; Méndez, C.; Díaz, V.; Barajas-López, C. Characterization of P2Y receptors mediating ATP induced relaxation in guinea pig airway smooth muscle: Involvement of prostaglandins and K+ channels. Pflugers Arch. 2011, 462, 573–585. [Google Scholar] [CrossRef]
- Miura, M.; Belvisi, M.G.; Stretton, C.D.; Yacoub, M.H.; Barnes, P.J. Role of potassium channels in bronchodilator responses in human airways. Am. Rev. Respir. Dis. 1992, 146, 132–136. [Google Scholar] [CrossRef]
- Yung, J.A.; Fuseini, H.; Newcomb, D.C. Hormones, sex, and asthma. Ann. Allergy Asthma Immunol. 2018, 120, 488–494. [Google Scholar] [CrossRef]
- Reyes-García, J.; Montaño, L.M.; Carbajal-García, A.; Wang, Y.X. Sex Hormones and Lung Inflammation. Adv. Exp. Med. Biol. 2021, 1304, 259–321. [Google Scholar] [CrossRef] [PubMed]
- Carbajal-García, A.; Reyes-García, J.; Montaño, L.M. Androgen Effects on the Adrenergic System of the Vascular, Airway, and Cardiac Myocytes and Their Relevance in Pathological Processes. Int. J. Endocrinol. 2020, 2020, 8849641. [Google Scholar] [CrossRef] [PubMed]
- de Marco, R.; Locatelli, F.; Sunyer, J.; Burney, P. Differences in incidence of reported asthma related to age in men and women. A retrospective analysis of the data of the European Respiratory Health Survey. Am. J. Respir. Crit. Care Med. 2000, 162, 68–74. [Google Scholar] [CrossRef] [PubMed]
- Kjellman, B.; Gustafsson, P.M. Asthma from childhood to adulthood: Asthma severity, allergies, sensitization, living conditions, gender influence and social consequences. Respir. Med. 2000, 94, 454–465. [Google Scholar] [CrossRef] [PubMed]
- Muller, D.C.; Giles, G.G.; Bassett, J.; Morris, H.A.; Manning, J.T.; Hopper, J.L.; English, D.R.; Severi, G. Second to fourth digit ratio (2D:4D) and concentrations of circulating sex hormones in adulthood. Reprod. Biol. Endocrinol. 2011, 9, 57. [Google Scholar] [CrossRef] [PubMed]
- Townsend, E.A.; Miller, V.M.; Prakash, Y.S. Sex differences and sex steroids in lung health and disease. Endocr. Rev. 2012, 33, 1–47. [Google Scholar] [CrossRef]
- Lucas-Herald, A.K.; Alves-Lopes, R.; Montezano, A.C.; Ahmed, S.F.; Touyz, R.M. Genomic and non-genomic effects of androgens in the cardiovascular system: Clinical implications. Clin. Sci. 2017, 131, 1405–1418. [Google Scholar] [CrossRef]
- Espinoza, J.; Montaño, L.M.; Perusquia, M. Nongenomic bronchodilating action elicited by dehydroepiandrosterone (DHEA) in a guinea pig asthma model. J. Steroid BioChem. Mol. Biol. 2013, 138, 174–182. [Google Scholar] [CrossRef]
- Perusquía, M.; Navarrete, E.; Jasso-Kamel, J.; Montaño, L.M. Androgens induce relaxation of contractile activity in pregnant human myometrium at term: A nongenomic action on L-type calcium channels. Biol. Reprod 2005, 73, 214–221. [Google Scholar] [CrossRef]
- Montaño, L.M.; Flores-Soto, E.; Sommer, B.; Solis-Chagoyán, H.; Perusquía, M. Androgens are effective bronchodilators with anti-inflammatory properties: A potential alternative for asthma therapy. Steroids 2020, 153, 108509. [Google Scholar] [CrossRef]
- Perusquía, M.; Flores-Soto, E.; Sommer, B.; Campuzano-González, E.; Martínez-Villa, I.; Martínez-Banderas, A.I.; Montaño, L.M. Testosterone-induced relaxation involves L-type and store-operated Ca2+ channels blockade, and PGE2 in guinea pig airway smooth muscle. Pflugers Arch. 2015, 467, 767–777. [Google Scholar] [CrossRef] [PubMed]
- Montaño, L.M.; Espinoza, J.; Flores-Soto, E.; Chávez, J.; Perusquía, M. Androgens are bronchoactive drugs that act by relaxing airway smooth muscle and preventing bronchospasm. J. Endocrinol. 2014, 222, 1–13. [Google Scholar] [CrossRef] [PubMed]
- Flores-Soto, E.; Reyes-García, J.; Carbajal-García, A.; Campuzano-González, E.; Perusquía, M.; Sommer, B.; Montaño, L.M. Sex steroids effects on guinea pig airway smooth muscle tone and intracellular Ca2+ basal levels. Mol. Cell Endocrinol. 2017, 439, 444–456. [Google Scholar] [CrossRef]
- Montaño, L.M.; Flores-Soto, E.; Reyes-García, J.; Díaz-Hernández, V.; Carbajal-García, A.; Campuzano-González, E.; Ramírez-Salinas, G.L.; Velasco-Velázquez, M.A.; Sommer, B. Testosterone induces hyporesponsiveness by interfering with IP3 receptors in guinea pig airway smooth muscle. Mol. Cell Endocrinol. 2018, 473, 17–30. [Google Scholar] [CrossRef] [PubMed]
- Bordallo, J.; de Boto, M.J.; Meana, C.; Velasco, L.; Bordallo, C.; Suarez, L.; Cantabrana, B.; Sanchez, M. Modulatory role of endogenous androgens on airway smooth muscle tone in isolated guinea-pig and bovine trachea; involvement of β2-adrenoceptors, the polyamine system and external calcium. Eur. J. Pharmacol. 2008, 601, 154–162. [Google Scholar] [CrossRef] [PubMed]
- Foster, P.S.; Goldie, R.G.; Paterson, J.W. Effect of steroids on β-adrenoceptor-mediated relaxation of pig bronchus. Br. J. Pharmacol. 1983, 78, 441–445. [Google Scholar] [CrossRef] [PubMed]
- Salt, P.J.; Iverson, L.L. Inhibition of the extraneuronal uptake of catecholamine in the isolated rat heart by cholesterol. Nat. New Biol. 1972, 238, 91–92. [Google Scholar] [CrossRef]
- Fuseini, H.; Yung, J.A.; Cephus, J.Y.; Zhang, J.; Goleniewska, K.; Polosukhin, V.V.; Peebles, R.S., Jr.; Newcomb, D.C. Testosterone Decreases House Dust Mite-Induced Type 2 and IL-17A-Mediated Airway Inflammation. J. Immunol. 2018, 201, 1843–1854. [Google Scholar] [CrossRef]
- Laffont, S.; Blanquart, E.; Savignac, M.; Cenac, C.; Laverny, G.; Metzger, D.; Girard, J.P.; Belz, G.T.; Pelletier, L.; Seillet, C.; et al. Androgen signaling negatively controls group 2 innate lymphoid cells. J. Exp. Med. 2017, 214, 1581–1592. [Google Scholar] [CrossRef]
- Deenadayalu, V.P.; White, R.E.; Stallone, J.N.; Gao, X.; Garcia, A.J. Testosterone relaxes coronary arteries by opening the large-conductance, calcium-activated potassium channel. Am. J. Physiol. Heart Circ. Physiol. 2001, 281, H1720–H1727. [Google Scholar] [CrossRef]
- Han, D.H.; Chae, M.R.; Jung, J.H.; So, I.; Park, J.K.; Lee, S.W. Effect of testosterone on potassium channel opening in human corporal smooth muscle cells. J. Sex. Med. 2008, 5, 822–832. [Google Scholar] [CrossRef]
- Masuda, K.; Takanari, H.; Morishima, M.; Ma, F.; Wang, Y.; Takahashi, N.; Ono, K. Testosterone-mediated upregulation of delayed rectifier potassium channel in cardiomyocytes causes abbreviation of QT intervals in rats. J. Physiol. Sci. 2018, 68, 759–767. [Google Scholar] [CrossRef] [PubMed]
- Bazán-Perkins, B.; Sánchez-Guerrero, E.; Carbajal, V.; Barajas-López, C.; Montaño, L.M. Sarcoplasmic reticulum Ca2+ depletion by caffeine and changes of [Ca2+]i during refilling in bovine airway smooth muscle cells. Arch. Med. Res. 2000, 31, 558–563. [Google Scholar] [CrossRef] [PubMed]
- Shahid, M.; van Amsterdam, R.G.; de Boer, J.; ten Berge, R.E.; Nicholson, C.D.; Zaagsma, J. The presence of five cyclic nucleotide phosphodiesterase isoenzyme activities in bovine tracheal smooth muscle and the functional effects of selective inhibitors. Br. J. Pharmacol. 1991, 104, 471–477. [Google Scholar] [CrossRef]
- Bryson, S.E.; Rodger, I.W. Effects of phosphodiesterase inhibitors on normal and chemically-skinned isolated airway smooth muscle. Br. J. Pharmacol. 1987, 92, 673–681. [Google Scholar] [CrossRef]
- Goseva, Z.; Gjorcev, A.; Kaeva, B.J.; Janeva, E.J.; Angelovska, I. Analysis of Plasma Concentrations of Theophylline in Smoking and Nonsmoking Patients with Asthma. Open Access Maced J. Med. Sci. 2015, 3, 672–675. [Google Scholar] [CrossRef]
- Weinberger, M.; Hendeles, L. Theophylline in asthma. N. Engl. J. Med. 1996, 334, 1380–1388. [Google Scholar] [CrossRef]
- Ramey, O.L.; Silva Almodovar, A.; Nahata, M.C. Medication adherence in Medicare-enrolled older adults with asthma before and during the coronavirus disease 2019 pandemic. Ann. Allergy Asthma Immunol. 2022, 128, 561–567. [Google Scholar] [CrossRef]
- Cazzola, M.; Matera, M.G. The effect of doxofylline in asthma and COPD. Respir. Med. 2020, 164, 105904. [Google Scholar] [CrossRef]
- Kurata, Y.; Muraki, S.; Kashihara, Y.; Hirota, T.; Araki, H.; Ieiri, I. Differences in Theophylline Clearance Between Patients With Chronic Hepatitis and Those With Liver Cirrhosis. Ther. Drug Monit. 2020, 42, 829–834. [Google Scholar] [CrossRef]
- Cazzola, M.; Page, C.P.; Calzetta, L.; Matera, M.G. Pharmacology and therapeutics of bronchodilators. Pharmacol. Rev. 2012, 64, 450–504. [Google Scholar] [CrossRef]
- Monteiro, J.P.; Alves, M.G.; Oliveira, P.F.; Silva, B.M. Structure-Bioactivity Relationships of Methylxanthines: Trying to Make Sense of All the Promises and the Drawbacks. Molecules 2016, 21, 974. [Google Scholar] [CrossRef]
- Guillot, C.; Fornaris, M.; Badier, M.; Orehek, J. Spontaneous and provoked resistance to isoproterenol in isolated human bronchi. J. Allergy Clin. Immunol. 1984, 74, 713–718. [Google Scholar] [CrossRef]
- Billington, C.K.; Le Jeune, I.R.; Young, K.W.; Hall, I.P. A major functional role for phosphodiesterase 4D5 in human airway smooth muscle cells. Am. J. Respir. Cell Mol. Biol. 2008, 38, 1–7. [Google Scholar] [CrossRef]
- Mehats, C.; Jin, S.L.; Wahlstrom, J.; Law, E.; Umetsu, D.T.; Conti, M. PDE4D plays a critical role in the control of airway smooth muscle contraction. FASEB J. 2003, 17, 1831–1841. [Google Scholar] [CrossRef]
- Hansen, G.; Jin, S.; Umetsu, D.T.; Conti, M. Absence of muscarinic cholinergic airway responses in mice deficient in the cyclic nucleotide phosphodiesterase PDE4D. Proc. Natl. Acad. Sci. USA 2000, 97, 6751–6756. [Google Scholar] [CrossRef]
- Billington, C.K.; Ojo, O.O.; Penn, R.B.; Ito, S. cAMP regulation of airway smooth muscle function. Pulm. Pharmacol. Ther. 2013, 26, 112–120. [Google Scholar] [CrossRef]
- Morgan, S.J.; Deshpande, D.A.; Tiegs, B.C.; Misior, A.M.; Yan, H.; Hershfeld, A.V.; Rich, T.C.; Panettieri, R.A.; An, S.S.; Penn, R.B. β-Agonist-mediated relaxation of airway smooth muscle is protein kinase A-dependent. J. Biol. Chem. 2014, 289, 23065–23074. [Google Scholar] [CrossRef]
- Nielsen-Kudsk, J.E. Potassium channel modulation: A new drug principle for regulation of smooth muscle contractility. Studies on isolated airways and arteries. Dan. Med. Bull. 1996, 43, 429–447. [Google Scholar]
- Huang, J.C.; Garcia, M.L.; Reuben, J.P.; Kacsorowski, G.J. Inhibition of β-adrenoceptor agonist relaxation of airway smooth muscle by Ca2+-activated K+ channel blockers. Eur. J. Pharmacol. 1993, 235, 37–43. [Google Scholar] [CrossRef]
- Longmore, J.; Bray, K.M.; Weston, A.H. The contribution of Rb-permeable potassium channels to the relaxant and membrane hyperpolarizing actions of cromakalim, RP49356 and diazoxide in bovine tracheal smooth muscle. Br. J. Pharmacol. 1991, 102, 979–985. [Google Scholar] [CrossRef]
- Liu, B.; Freyer, A.M.; Hall, I.P. Bradykinin activates calcium-dependent potassium channels in cultured human airway smooth muscle cells. Am. J. Physiol. Lung Cell Mol. Physiol. 2007, 292, L898–L907. [Google Scholar] [CrossRef]
- Bi, D.; Toyama, K.; Lemaitre, V.; Takai, J.; Fan, F.; Jenkins, D.P.; Wulff, H.; Gutterman, D.D.; Park, F.; Miura, H. The intermediate conductance calcium-activated potassium channel KCa3.1 regulates vascular smooth muscle cell proliferation via controlling calcium-dependent signaling. J. Biol. Chem. 2013, 288, 15843–15853. [Google Scholar] [CrossRef]
- Yu, M.; Liu, S.L.; Sun, P.B.; Pan, H.; Tian, C.L.; Zhang, L.H. Peptide toxins and small-molecule blockers of BK channels. Acta Pharmacol. Sin. 2016, 37, 56–66. [Google Scholar] [CrossRef]
- Imaizumi, Y.; Watanabe, M. The effect of tetraethylammonium chloride on potassium permeability in the smooth muscle cell membrane of canine trachea. J. Physiol. 1981, 316, 33–46. [Google Scholar] [CrossRef]
- Kotlikoff, M.I. Potassium channels in airway smooth muscle: A tale of two channels. Pharmacol. Ther. 1993, 58, 1–12. [Google Scholar] [CrossRef]
- Cairrao, E.; Alvarez, E.; Santos-Silva, A.J.; Verde, I. Potassium channels are involved in testosterone-induced vasorelaxation of human umbilical artery. Naunyn Schmiedebergs Arch. Pharmacol. 2008, 376, 375–383. [Google Scholar] [CrossRef]
- Huang, X.Y.; Morielli, A.D.; Peralta, E.G. Molecular basis of cardiac potassium channel stimulation by protein kinase A. Proc. Natl. Acad. Sci. USA 1994, 91, 624–628. [Google Scholar] [CrossRef]
- Chowdhury, N.U.; Guntur, V.P.; Newcomb, D.C.; Wechsler, M.E. Sex and gender in asthma. Eur. Respir. Rev. 2021, 30, 210067. [Google Scholar] [CrossRef]
- Canguven, O.; Albayrak, S. Do low testosterone levels contribute to the pathogenesis of asthma? Med. Hypotheses. 2011, 76, 585–588. [Google Scholar] [CrossRef]
- Fahy, J.V. Type 2 inflammation in asthma--present in most, absent in many. Nat. Rev. Immunol. 2015, 15, 57–65. [Google Scholar] [CrossRef]
- Evasovic, J.M.; Singer, C.A. Regulation of IL-17A and implications for TGF-β1 comodulation of airway smooth muscle remodeling in severe asthma. Am. J. Physiol. Lung Cell Mol. Physiol. 2019, 316, L843–L868. [Google Scholar] [CrossRef]
- Montaño, L.M.; Sommer, B.; Gómez-Verjan, J.C.; Morales-Paoli, G.S.; Ramírez-Salinas, G.L.; Solis-Chagoyán, H.; Sánchez-Florentino, Z.A.; Calixto, E.; Pérez-Figueroa, G.E.; Carter, R.; et al. Theophylline: Old Drug in a New Light, Application in COVID-19 through Computational Studies. Int. J. Mol. Sci. 2022, 23, 4167. [Google Scholar] [CrossRef]
- Raker, V.K.; Becker, C.; Steinbrink, K. The cAMP Pathway as Therapeutic Target in Autoimmune and Inflammatory Diseases. Front. Immunol. 2016, 7, 123. [Google Scholar] [CrossRef]
- Koga, K.; Takaesu, G.; Yoshida, R.; Nakaya, M.; Kobayashi, T.; Kinjyo, I.; Yoshimura, A. Cyclic adenosine monophosphate suppresses the transcription of proinflammatory cytokines via the phosphorylated c-Fos protein. Immunity 2009, 30, 372–383. [Google Scholar] [CrossRef]
- Johnson, P.R.; Roth, M.; Tamm, M.; Hughes, M.; Ge, Q.; King, G.; Burgess, J.K.; Black, J.L. Airway smooth muscle cell proliferation is increased in asthma. Am. J. Respir. Crit. Care Med. 2001, 164, 474–477. [Google Scholar] [CrossRef]
- Dente, F.L.; Carnevali, S.; Bartoli, M.L.; Cianchetti, S.; Bacci, E.; Di Franco, A.; Vagaggini, B.; Paggiaro, P. Profiles of proinflammatory cytokines in sputum from different groups of severe asthmatic patients. Ann. Allergy Asthma Immunol. 2006, 97, 312–320. [Google Scholar] [CrossRef]
- Pace, S.; Pergola, C.; Dehm, F.; Rossi, A.; Gerstmeier, J.; Troisi, F.; Pein, H.; Schaible, A.M.; Weinigel, C.; Rummler, S.; et al. Androgen-mediated sex bias impairs efficiency of leukotriene biosynthesis inhibitors in males. J. Clin. Investig. 2017, 127, 3167–3176. [Google Scholar] [CrossRef]
- Stapulionis, R.; Kolli, S.; Deutscher, M.P. Efficient mammalian protein synthesis requires an intact F-actin system. J. Biol. Chem. 1997, 272, 24980–24986. [Google Scholar] [CrossRef]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Reyes-García, J.; Díaz-Hernández, V.; Carbajal-García, A.; Casas-Hernández, M.F.; Sommer, B.; Montaño, L.M. Theophylline-Induced Relaxation Is Enhanced after Testosterone Treatment via Increased KV1.2 and KV1.5 Protein Expression in Guinea Pig Tracheal Smooth Muscle. Int. J. Mol. Sci. 2023, 24, 5884. https://doi.org/10.3390/ijms24065884
Reyes-García J, Díaz-Hernández V, Carbajal-García A, Casas-Hernández MF, Sommer B, Montaño LM. Theophylline-Induced Relaxation Is Enhanced after Testosterone Treatment via Increased KV1.2 and KV1.5 Protein Expression in Guinea Pig Tracheal Smooth Muscle. International Journal of Molecular Sciences. 2023; 24(6):5884. https://doi.org/10.3390/ijms24065884
Chicago/Turabian StyleReyes-García, Jorge, Verónica Díaz-Hernández, Abril Carbajal-García, María F. Casas-Hernández, Bettina Sommer, and Luis M. Montaño. 2023. "Theophylline-Induced Relaxation Is Enhanced after Testosterone Treatment via Increased KV1.2 and KV1.5 Protein Expression in Guinea Pig Tracheal Smooth Muscle" International Journal of Molecular Sciences 24, no. 6: 5884. https://doi.org/10.3390/ijms24065884
APA StyleReyes-García, J., Díaz-Hernández, V., Carbajal-García, A., Casas-Hernández, M. F., Sommer, B., & Montaño, L. M. (2023). Theophylline-Induced Relaxation Is Enhanced after Testosterone Treatment via Increased KV1.2 and KV1.5 Protein Expression in Guinea Pig Tracheal Smooth Muscle. International Journal of Molecular Sciences, 24(6), 5884. https://doi.org/10.3390/ijms24065884





