Dioleoylphosphatidylglycerol Inhibits Heat Shock Protein B4 (HSPB4)-Induced Inflammatory Pathways In Vitro
Abstract
1. Introduction
2. Results
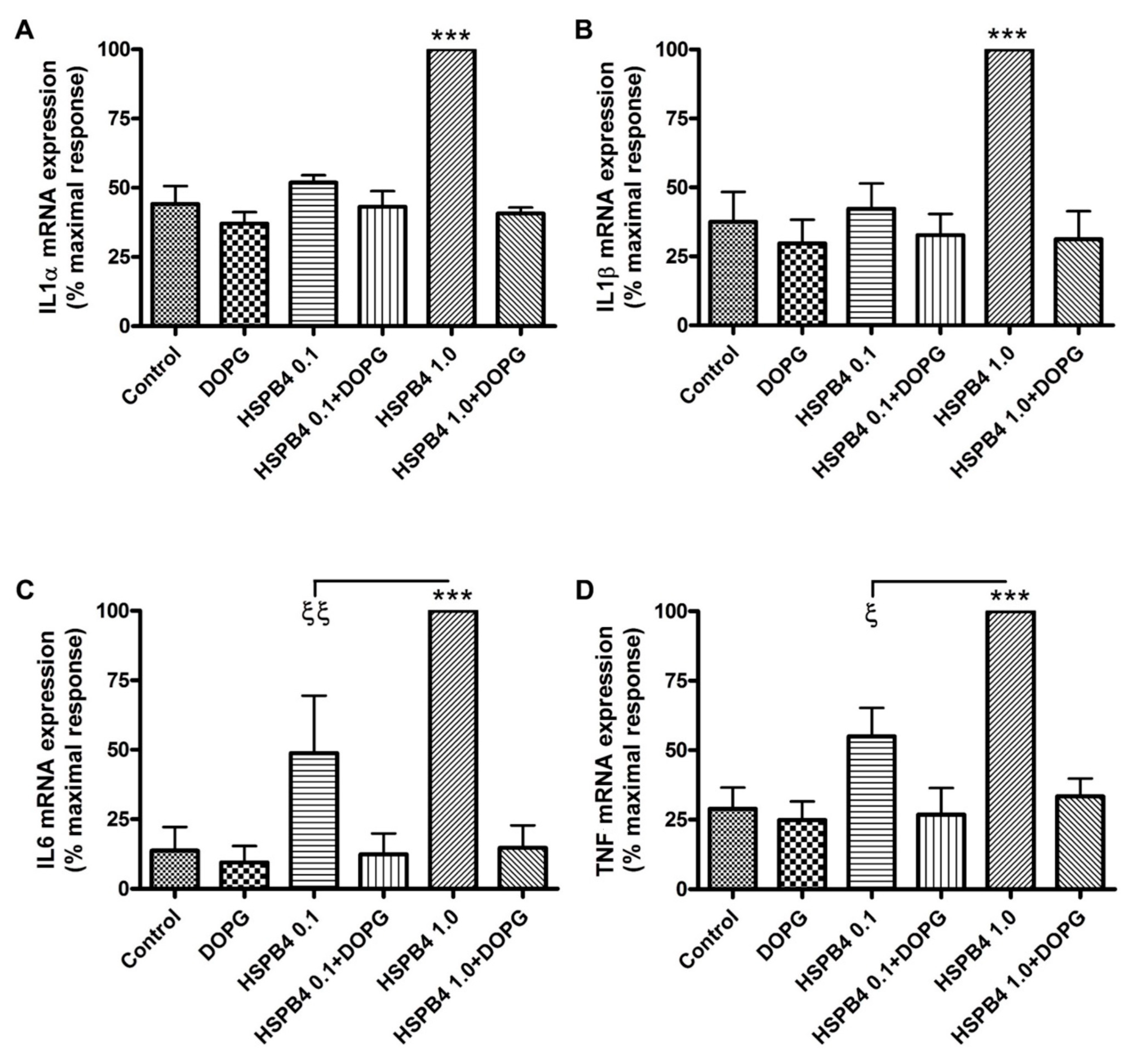
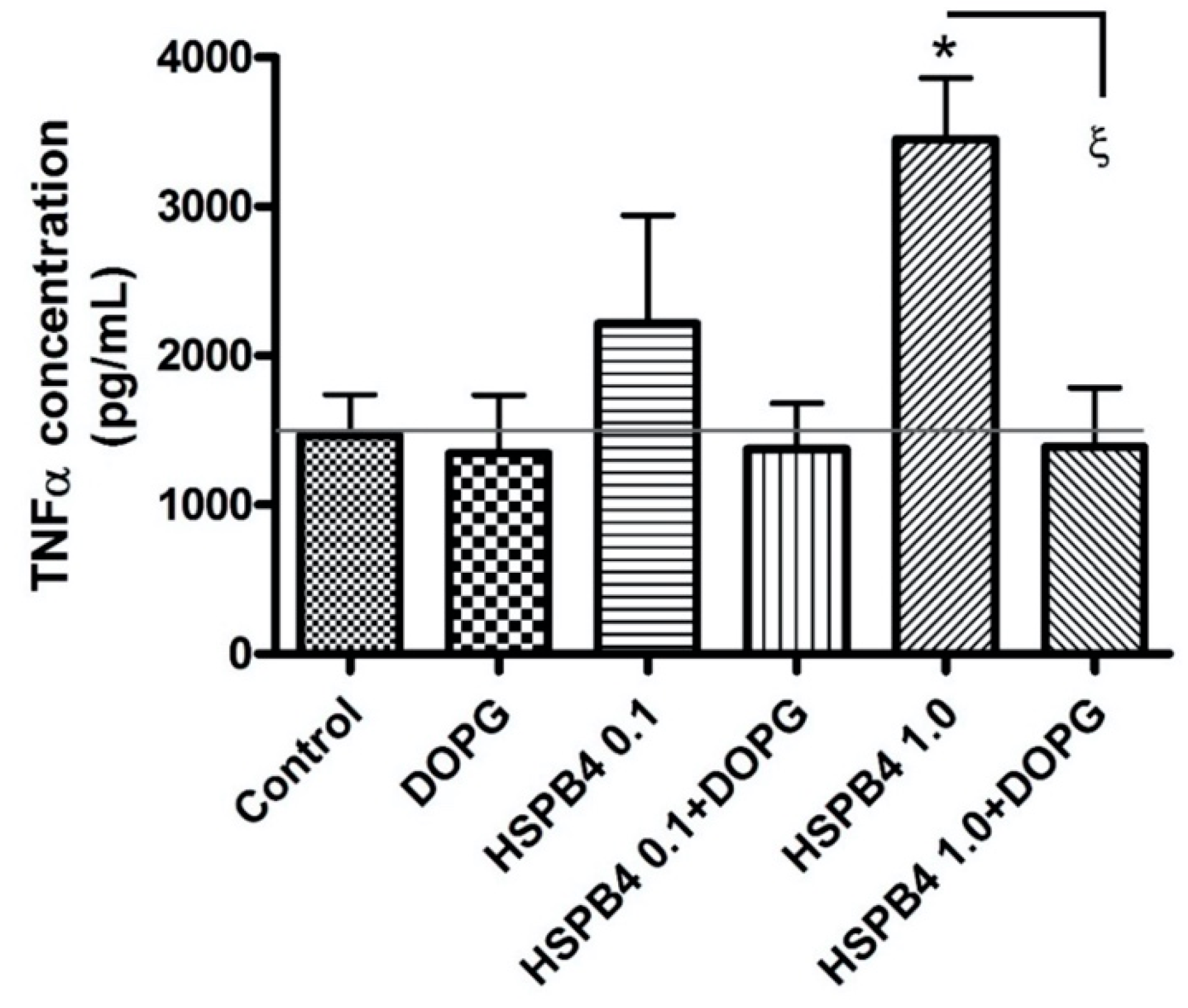
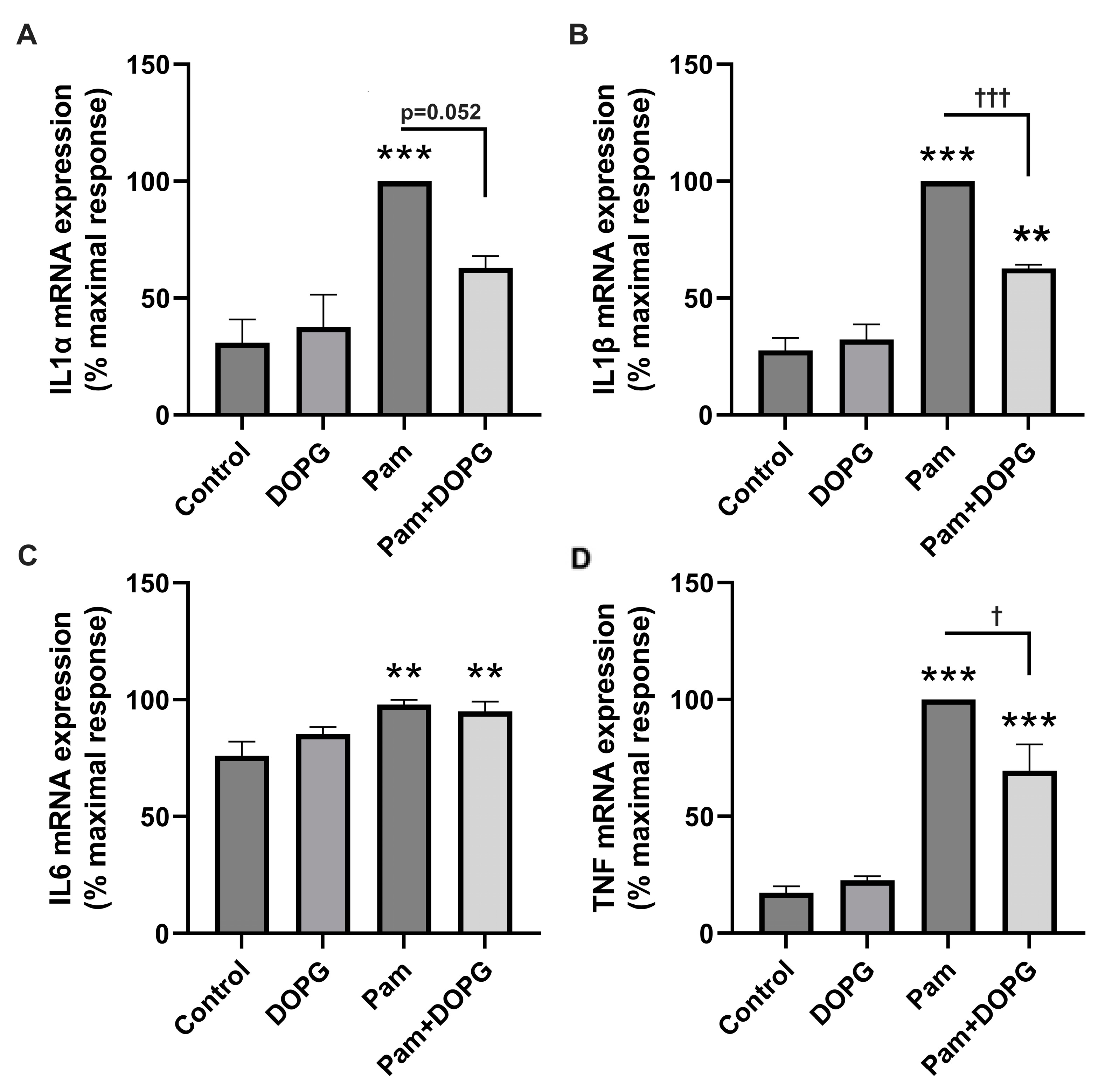
2.1. DOPG Inhibited Inflammatory Mediator Expression and Production in Mouse Macrophages Stimulated by HSPB4 and Human Corneal Epithelial Cells Exposed to a TLR2 Agonist
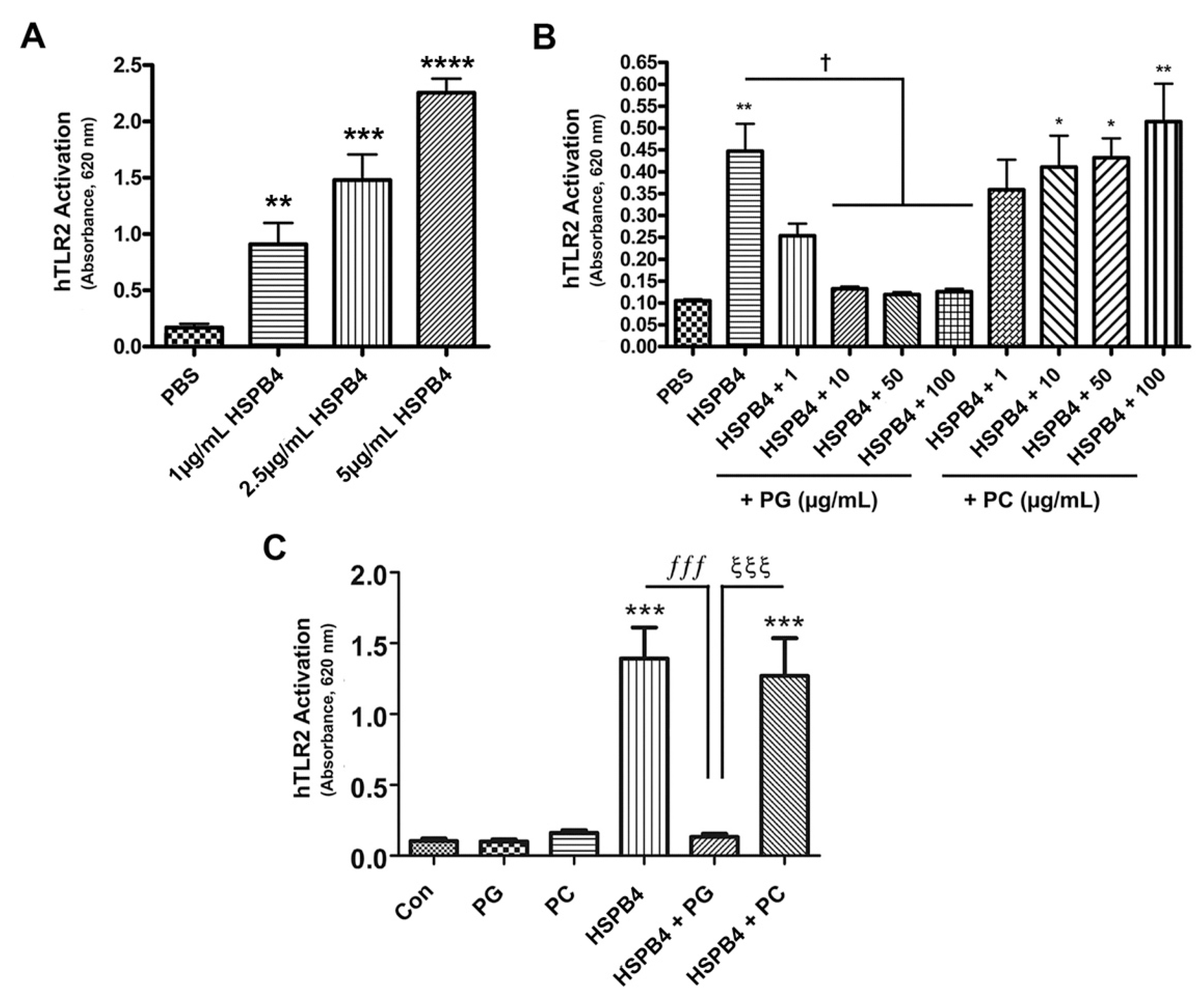
2.2. HSPB4 Dose-Dependently Induced TLR2 Activation in Human Cells, and DOPG Inhibited This Response
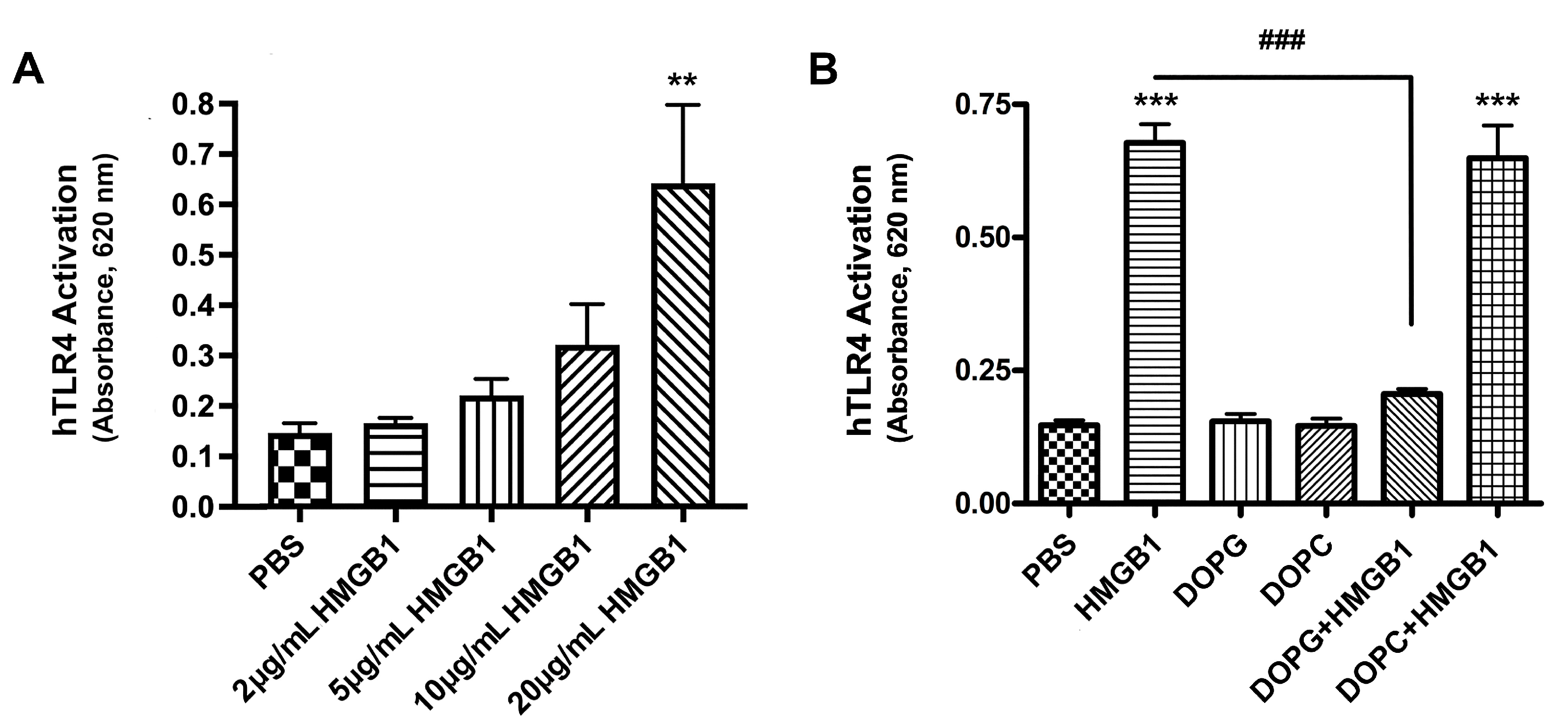
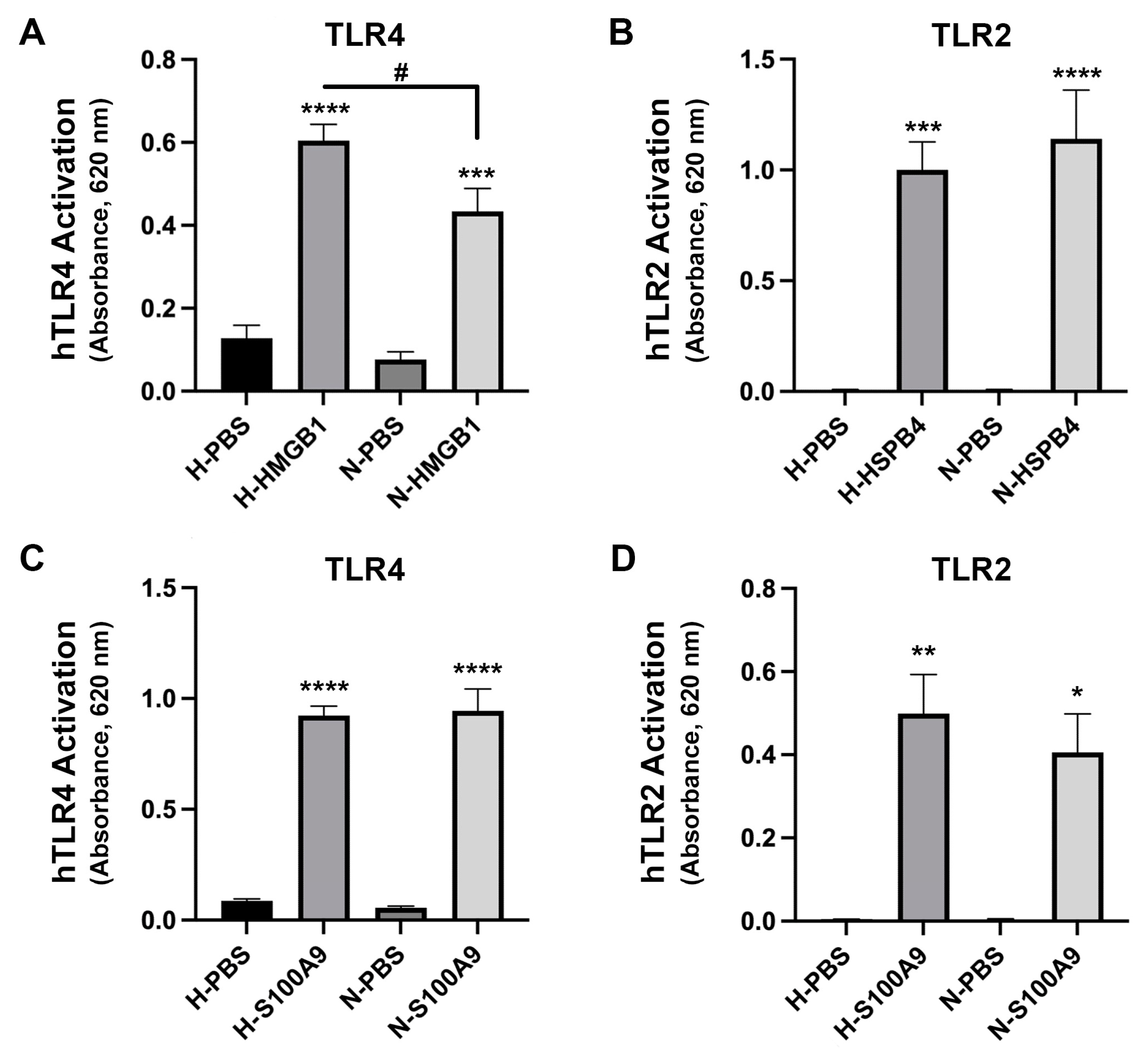
2.3. HMGB1 Activated TLR4, and DOPG, but Not DOPC, Inhibited This Activation
2.4. HMGB1-Induced TLR4 Activation Was Increased in High Glucose Conditions but Ligand-Induced TLR2 Activation Was Not
2.5. CD14 Was Required for Maximal Activation of TLR2 and TLR4 by PAMPs and DAMPs
2.5.1. Anti-CD14 Antibody and DOPG Inhibited hTLR4 Activation by LPS and S100A9
2.5.2. TLR2 Activation by a PAMP or DAMP Was Inhibited by DOPG and by an Antibody Recognizing CD14, but Effects Were Not Additive
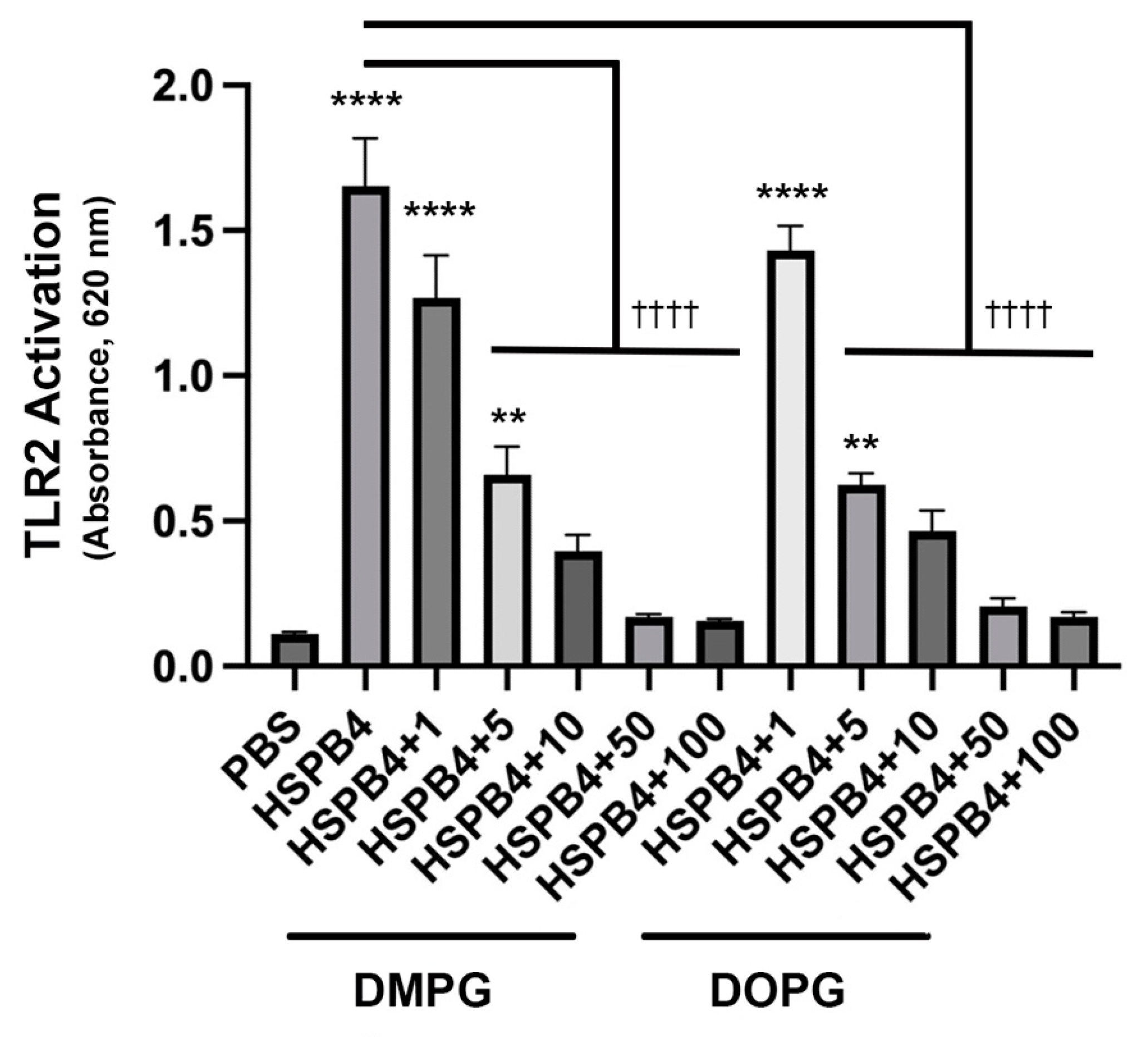
2.6. DMPG, a Phosphatidylglycerol Already Used in Commercial Eye Drops, Has a Similar Inhibitory Effect as DOPG on HSPB4-Induced TLR2 Activation
3. Discussion
4. Materials and Methods
4.1. Materials
4.2. Cell Culture
4.3. Quantitative Real-Time Polymerase Chain Reaction (qRT-PCR)
4.4. Absorbance Assays to Assess TLR2 or 4 Activity
4.5. ELISA Assay
4.6. Statistical Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Lassance, L.; Marino, G.K.; Medeiros, C.S.; Thangavadivel, S.; Wilson, S.E. Fibrocyte migration, differentiation and apoptosis during the corneal wound healing response to injury. Exp. Eye Res. 2018, 170, 177–187. [Google Scholar] [CrossRef] [PubMed]
- Barbosa, F.L.; Chaurasia, S.S.; Cutler, A.; Asosingh, K.; Kaur, H.; de Medeiros, F.W.; Agrawal, V.; Wilson, S.E. Corneal myofibroblast generation from bone marrow-derived cells. Exp. Eye Res. 2010, 91, 92–96. [Google Scholar] [CrossRef]
- Oh, J.Y.; Choi, H.; Lee, R.H.; Roddy, G.W.; Ylöstalo, J.H.; Wawrousek, E.; Prockop, D.J. Identification of the HSPB4/TLR2/NF-κB axis in macrophage as a therapeutic target for sterile inflammation of the cornea. EMBO Mol. Med. 2012, 4, 435–448. [Google Scholar] [CrossRef]
- Numata, M.; Chu, H.W.; Dakhama, A.; Voelker, D.R. Pulmonary surfactant phosphatidylglycerol inhibits respiratory syncytial virus–induced inflammation and infection. Proc. Natl. Acad. Sci. USA 2010, 107, 320–325. [Google Scholar] [CrossRef] [PubMed]
- Choudhary, V.; Uaratanawong, R.; Patel, R.R.; Patel, H.; Bao, W.; Hartney, B.; Cohen, E.; Chen, X.; Zhong, Q.; Isales, C.M. Phosphatidylglycerol inhibits toll-like receptor–mediated inflammation by danger-associated molecular patterns. J. Investig. Dermatol. 2019, 139, 868–877. [Google Scholar] [CrossRef]
- Bollag, W.B.; Olala, L.O.; Xie, D.; Lu, X.; Qin, H.; Choudhary, V.; Patel, R.; Bogorad, D.; Estes, A.; Watsky, M. Dioleoylphosphatidylglycerol accelerates corneal epithelial wound healing. Investig. Ophthalmol. Vis. Sci. 2020, 61, 29. [Google Scholar] [CrossRef]
- Kuronuma, K.; Mitsuzawa, H.; Takeda, K.; Nishitani, C.; Chan, E.D.; Kuroki, Y.; Nakamura, M.; Voelker, D.R. Anionic Pulmonary Surfactant Phospholipids Inhibit Inflammatory Responses from Alveolar Macrophages and U937 Cells by Binding the Lipopolysaccharide-interacting Proteins CD14 and MD-2. J. Biol. Chem. 2009, 284, 25488–25500. [Google Scholar] [CrossRef] [PubMed]
- Sun, H.; Saeedi, P.; Karuranga, S.; Pinkepank, M.; Ogurtsova, K.; Duncan, B.B.; Stein, C.; Basit, A.; Chan, J.C.; Mbanya, J.C. IDF Diabetes Atlas: Global, regional and country-level diabetes prevalence estimates for 2021 and projections for 2045. Diabetes Res. Clin. Pract. 2022, 183, 109119. [Google Scholar] [CrossRef] [PubMed]
- Klein, R.; Klein, B.E. Vision disorders in diabetes. Diabetes Am. 1995, 1, 293. [Google Scholar]
- Ljubimov, A.V. Diabetic complications in the cornea. Vis. Res. 2017, 139, 138–152. [Google Scholar] [CrossRef]
- Skarbez, K.; Priestley, Y.; Hoepf, M.; Koevary, S.B. Comprehensive review of the effects of diabetes on ocular health. Expert Rev. Ophthalmol. 2010, 5, 557–577. [Google Scholar] [CrossRef]
- Schultz, R.; Van Horn, D.; Peters, M.; Klewin, K.M.; Schutten, W. Diabetic keratopathy. Trans. Am. Ophthalmol. Soc. 1981, 79, 180. [Google Scholar]
- Wang, H.; Qu, H.; Deng, H. Plasma HMGB-1 levels in subjects with obesity and type 2 diabetes: A cross-sectional study in China. PLoS ONE 2015, 10, e0136564. [Google Scholar] [CrossRef] [PubMed]
- Zhou, Y.; Wang, T.; Wang, Y.; Meng, F.; Ying, M.; Han, R.; Hao, P.; Wang, L.; Li, X. Blockade of extracellular high-mobility group box 1 attenuates inflammation-mediated damage and haze grade in mice with corneal wounds. Int. Immunopharmacol. 2020, 83, 106468. [Google Scholar] [CrossRef]
- Entezari, M.; Weiss, D.J.; Sitapara, R.; Whittaker, L.; Wargo, M.J.; Li, J.; Wang, H.; Yang, H.; Sharma, L.; Phan, B.D. Inhibition of high-mobility group box 1 protein (HMGB1) enhances bacterial clearance and protects against Pseudomonas aeruginosa pneumonia in cystic fibrosis. Mol. Med. 2012, 18, 477–485. [Google Scholar] [CrossRef] [PubMed]
- McClellan, S.; Jiang, X.; Barrett, R.; Hazlett, L.D. High-mobility group box 1: A novel target for treatment of Pseudomonas aeruginosa keratitis. J. Immunol. 2015, 194, 1776–1787. [Google Scholar] [CrossRef]
- Ekanayaka, S.A.; McClellan, S.A.; Barrett, R.P.; Kharotia, S.; Hazlett, L.D. Glycyrrhizin reduces HMGB1 and bacterial load in Pseudomonas aeruginosa keratitis. Investig. Ophthalmol. Vis. Sci. 2016, 57, 5799–5809. [Google Scholar] [CrossRef] [PubMed]
- Huang, W.; Tang, Y.; Li, L. HMGB1, a potent proinflammatory cytokine in sepsis. Cytokine 2010, 51, 119–126. [Google Scholar] [CrossRef]
- Dasu, M.R.; Devaraj, S.; Park, S.; Jialal, I. Increased toll-like receptor (TLR) activation and TLR ligands in recently diagnosed type 2 diabetic subjects. Diabetes Care 2010, 33, 861–868. [Google Scholar] [CrossRef]
- Škrha Jr, J.; Kalousova, M.; Švarcová, J.; Muravska, A.; Kvasnička, J.; Landova, L.; Zima, T.; Škrha, J. Relationship of soluble RAGE and RAGE ligands HMGB1 and EN-RAGE to endothelial dysfunction in type 1 and type 2 diabetes mellitus. Exp. Clin. Endocrinol. Diabetes 2012, 120, 277–281. [Google Scholar] [CrossRef]
- Hou, Y.; Lan, J.; Zhang, F.; Wu, X. Expression profiles and potential corneal epithelial wound healing regulation targets of high-mobility group box 1 in diabetic mice. Exp. Eye Res. 2021, 202, 108364. [Google Scholar] [CrossRef]
- Xie, D.; Choudhary, V.; Seremwe, M.; Edwards, J.G.; Wang, A.; Emmons, A.C.; Bollag, K.A.; Johnson, M.H.; Bollag, W.B. Soy phosphatidylglycerol reduces inflammation in a contact irritant ear edema mouse model in vivo. J. Pharmacol. Exp. Ther. 2018, 366, 1–8. [Google Scholar] [CrossRef]
- Bollag, W.B.; Xie, D.; Zheng, X.; Zhong, X. A potential role for the phospholipase D2-aquaporin-3 signaling module in early keratinocyte differentiation: Production of a phosphatidylglycerol signaling lipid. J. Investig. Dermatol. 2007, 127, 2823–2831. [Google Scholar] [CrossRef] [PubMed]
- Choudhary, V.; Griffith, S.; Chen, X.; Bollag, W.B. Pathogen-associated molecular pattern-induced TLR2 and TLR4 activation increases keratinocyte production of inflammatory mediators and is inhibited by phosphatidylglycerol. Mol. Pharmacol. 2020, 97, 324–335. [Google Scholar] [CrossRef]
- Wang, Y.; Zhong, J.; Zhang, X.; Liu, Z.; Yang, Y.; Gong, Q.; Ren, B. The role of HMGB1 in the pathogenesis of type 2 diabetes. J. Diabetes Res. 2016, 2016, 2543268. [Google Scholar] [CrossRef]
- Tsoyi, K.; Jang, H.J.; Nizamutdinova, I.T.; Kim, Y.M.; Lee, Y.S.; Kim, H.J.; Seo, H.G.; Lee, J.H.; Chang, K.C. Metformin inhibits HMGB1 release in LPS-treated RAW 264.7 cells and increases survival rate of endotoxaemic mice. Br. J. Pharmacol. 2011, 162, 1498–1508. [Google Scholar] [CrossRef]
- Cameron, A.R.; Morrison, V.L.; Levin, D.; Mohan, M.; Forteath, C.; Beall, C.; McNeilly, A.D.; Balfour, D.J.; Savinko, T.; Wong, A.K. Anti-inflammatory effects of metformin irrespective of diabetes status. Circ. Res. 2016, 119, 652–665. [Google Scholar] [CrossRef] [PubMed]
- Morrison, D.C.; Ryan, J.L. Endotoxins and disease mechanisms. Annu. Rev. Med. 1987, 38, 417–432. [Google Scholar] [CrossRef]
- van Bergenhenegouwen, J.; Plantinga, T.S.; Joosten, L.A.; Netea, M.G.; Folkerts, G.; Kraneveld, A.D.; Garssen, J.; Vos, A.P. TLR2 & Co: A critical analysis of the complex interactions between TLR2 and coreceptors. J. Leukoc. Biol. 2013, 94, 885–902. [Google Scholar]
- Poltorak, A.; He, X.; Smirnova, I.; Liu, M.-Y.; Huffel, C.V.; Du, X.; Birdwell, D.; Alejos, E.; Silva, M.; Galanos, C. Defective LPS signaling in C3H/HeJ and C57BL/10ScCr mice: Mutations in Tlr4 gene. Science 1998, 282, 2085–2088. [Google Scholar] [CrossRef] [PubMed]
- O'brien, A.; Rosenstreich, D.L.; Scher, I.; Campbell, G.H.; Macdermott, R.P.; Formal, S.B. Genetic control of susceptibility to Salmonella typhimurium in mice: Role of the LPS gene. J. Immunol. 1980, 124, 20–24. [Google Scholar] [CrossRef]
- Kandasamy, P.; Numata, M.; Berry, K.Z.; Fickes, R.; Leslie, C.C.; Murphy, R.C.; Voelker, D.R. Structural analogs of pulmonary surfactant phosphatidylglycerol inhibit toll-like receptor 2 and 4 signaling. J. Lipid Res. 2016, 57, 993–1005. [Google Scholar] [CrossRef]
- Tong, L.; Lan, W.; Lim, R.R.; Chaurasia, S.S. S100A proteins as molecular targets in the ocular surface inflammatory diseases. Ocul. Surf. 2014, 12, 23–31. [Google Scholar] [CrossRef] [PubMed]
- Erridge, C. Endogenous ligands of TLR2 and TLR4: Agonists or assistants? J. Leukoc. Biol. 2010, 87, 989–999. [Google Scholar] [CrossRef]
- McInturff, J.E.; Modlin, R.L.; Kim, J. The role of toll-like receptors in the pathogenesis and treatment of dermatological disease. J. Investig. Dermatol. 2005, 125, 1–8. [Google Scholar] [CrossRef]
- Available online: https://systane-ca.myalcon.com/ca-en/eye-care/systane/products/systane-complete/ingredients/#:~:text=SYSTANE%20®%20COMPLETE%20Lubricant%20Eye%20Drops%20is%20a,POLYQUAD%2A%20%28polidronium%20chloride%29%200.001%25%20preservative%2C%20and%20purified%20water (accessed on 18 March 2023).
- Numata, M.; Kandasamy, P.; Nagashima, Y.; Posey, J.; Hartshorn, K.; Woodland, D.; Voelker, D.R. Phosphatidylglycerol suppresses influenza A virus infection. Am. J. Respir. Cell Mol. Biol. 2012, 46, 479–487. [Google Scholar] [CrossRef] [PubMed]
- Numata, M.; Nagashima, Y.; Moore, M.L.; Berry, K.Z.; Chan, M.; Kandasamy, P.; Peebles, R.S.; Murphy, R.C.; Voelker, D.R. Phosphatidylglycerol provides short-term prophylaxis against respiratory syncytial virus infection. J. Lipid Res. 2013, 54, 2133–2143. [Google Scholar] [CrossRef]
- Ralph, R.A. Tetracyclines and the treatment of corneal stromal ulceration: A review. Cornea 2000, 19, 274–277. [Google Scholar] [CrossRef] [PubMed]
- Su, W.; Li, Z.; Lin, M.; Li, Y.; He, Z.; Wu, C.; Liang, D. The effect of doxycycline temperature-sensitive hydrogel on inhibiting the corneal neovascularization induced by BFGF in rats. Graefe’s Arch. Clin. Exp. Ophthalmol. 2011, 249, 421–427. [Google Scholar] [CrossRef]
- Solomon, A.; Rosenblatt, M.; Li, D.-Q.; Liu, Z.; Monroy, D.; Ji, Z.; Lokeshwar, B.L.; Pflugfelder, S.C. Doxycycline inhibition of interleukin-1 in the corneal epithelium. Investig. Ophthalmol. Vis. Sci. 2000, 41, 2544–2557. [Google Scholar] [CrossRef]
- Khoshdel, A.R.; Emami Aleagha, O.; Shahriary, A.; Aghamollaei, H.; Najjar Asiabani, F. Topical Effects of N-Acetyl Cysteine and Doxycycline on Inflammatory and Angiogenic Factors in the Rat Model of Alkali-Burned Cornea. J. Interferon Cytokine Res. 2022, 42, 82–89. [Google Scholar] [CrossRef] [PubMed]
- D'Agostino, P.; Arcoleo, F.; Barbera, C.; Di Bella, G.; La Rosa, M.; Misiano, G.; Milano, S.; Brai, M.; Cammarata, G.; Feo, S. Tetracycline inhibits the nitric oxide synthase activity induced by endotoxin in cultured murine macrophages. Eur. J. Pharmacol. 1998, 346, 283–290. [Google Scholar] [CrossRef] [PubMed]
- Elisia, I.; Pae, H.B.; Lam, V.; Cederberg, R.; Hofs, E.; Krystal, G. Comparison of RAW264.7, human whole blood and PBMC assays to screen for immunomodulators. J Immunol. Methods 2018, 452, 26–31. [Google Scholar] [CrossRef] [PubMed]
- Chikama, T.-I.; Wakuta, M.; Liu, Y.; Nishida, T. Deviated mechanism of wound healing in diabetic corneas. Cornea 2007, 26, S75–S81. [Google Scholar] [CrossRef] [PubMed]
- Lutty, G.A. Effects of diabetes on the eye. Investig. Ophthalmol. Vis. Sci. 2013, 54, ORSF81–ORSF87. [Google Scholar] [CrossRef] [PubMed]
- Xu, K.-P.; Li, Y.; Ljubimov, A.V.; Yu, F.-S.X. High glucose suppresses epidermal growth factor receptor/phosphatidylinositol 3-kinase/Akt signaling pathway and attenuates corneal epithelial wound healing. Diabetes 2009, 58, 1077–1085. [Google Scholar] [CrossRef]
- Lu, X.; Chen, Z.; Mylarapu, N.; Watsky, M.A. Effects of 1,25 and 24,25 Vitamin D on Corneal Epithelial Proliferation, Migration and Vitamin D Metabolizing and Catabolizing Enzymes. Sci. Rep. 2017, 7, 16951. [Google Scholar] [CrossRef]
- Helwa, I.; Patel, R.; Karempelis, P.; Kaddour-Djebbar, I.; Choudhary, V.; Bollag, W.B. The antipsoriatic agent monomethylfumarate has antiproliferative, prodifferentiative, and anti-inflammatory effects on keratinocytes. J. Pharmacol. Exp. Ther. 2015, 352, 90–97. [Google Scholar] [CrossRef]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Fowler, T.E.; Choudhary, V.; Melnyk, S.; Farsi, M.; Chang, L.Y.; Fortingo, N.; Chen, X.; Watsky, M.A.; Bollag, W.B. Dioleoylphosphatidylglycerol Inhibits Heat Shock Protein B4 (HSPB4)-Induced Inflammatory Pathways In Vitro. Int. J. Mol. Sci. 2023, 24, 5839. https://doi.org/10.3390/ijms24065839
Fowler TE, Choudhary V, Melnyk S, Farsi M, Chang LY, Fortingo N, Chen X, Watsky MA, Bollag WB. Dioleoylphosphatidylglycerol Inhibits Heat Shock Protein B4 (HSPB4)-Induced Inflammatory Pathways In Vitro. International Journal of Molecular Sciences. 2023; 24(6):5839. https://doi.org/10.3390/ijms24065839
Chicago/Turabian StyleFowler, Teresa E., Vivek Choudhary, Samuel Melnyk, Mishma Farsi, Luke Y. Chang, Nyemkuna Fortingo, Xunsheng Chen, Mitchell A. Watsky, and Wendy B. Bollag. 2023. "Dioleoylphosphatidylglycerol Inhibits Heat Shock Protein B4 (HSPB4)-Induced Inflammatory Pathways In Vitro" International Journal of Molecular Sciences 24, no. 6: 5839. https://doi.org/10.3390/ijms24065839
APA StyleFowler, T. E., Choudhary, V., Melnyk, S., Farsi, M., Chang, L. Y., Fortingo, N., Chen, X., Watsky, M. A., & Bollag, W. B. (2023). Dioleoylphosphatidylglycerol Inhibits Heat Shock Protein B4 (HSPB4)-Induced Inflammatory Pathways In Vitro. International Journal of Molecular Sciences, 24(6), 5839. https://doi.org/10.3390/ijms24065839






