Microbe-Derived Antioxidants Alleviate Liver and Adipose Tissue Lipid Disorders and Metabolic Inflammation Induced by High Fat Diet in Mice
Abstract
1. Introduction
2. Results
2.1. Body Weight, Energy Intake, Body Fat Rate and Organ Index Changes in Mice
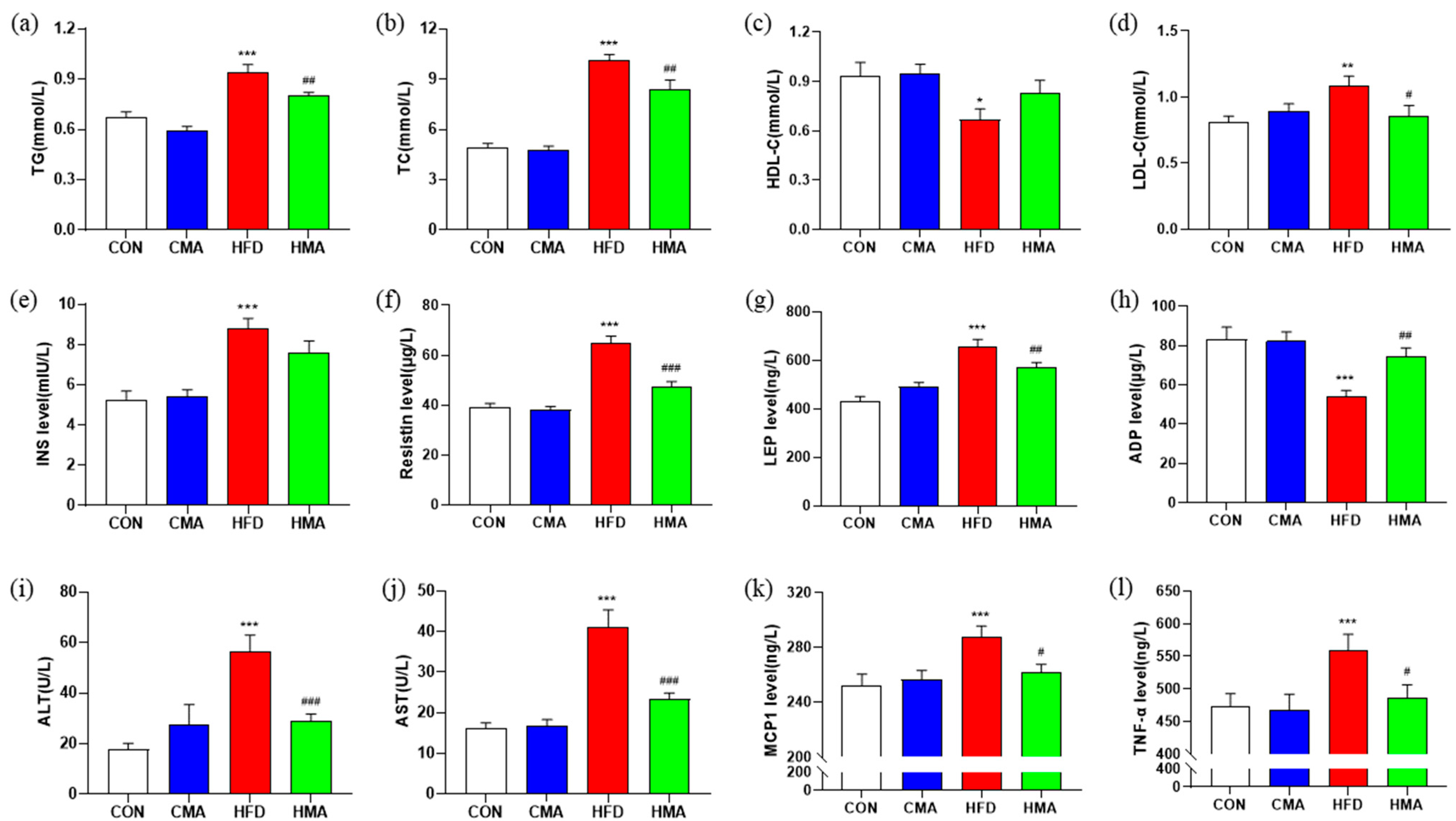
2.2. Lipid Metabolism and Inflammatory Response in Serum of Mice
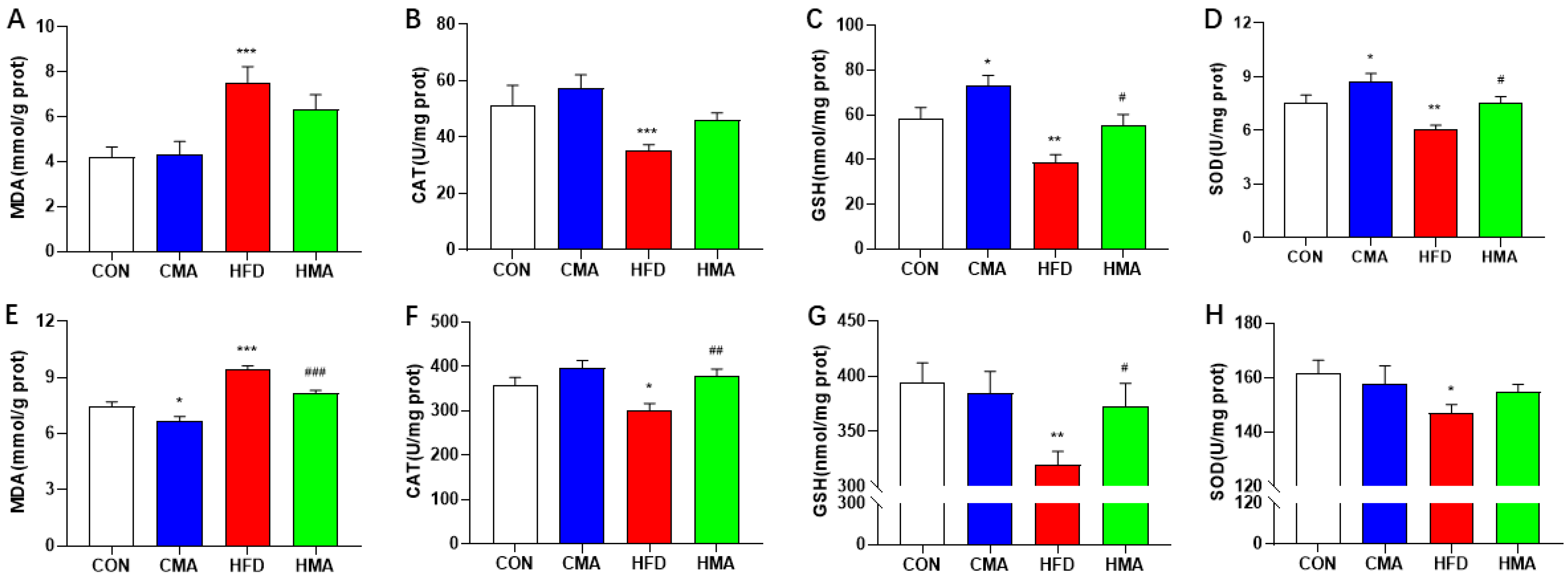
2.3. Oxidative Stress in Liver and EAT of Mice
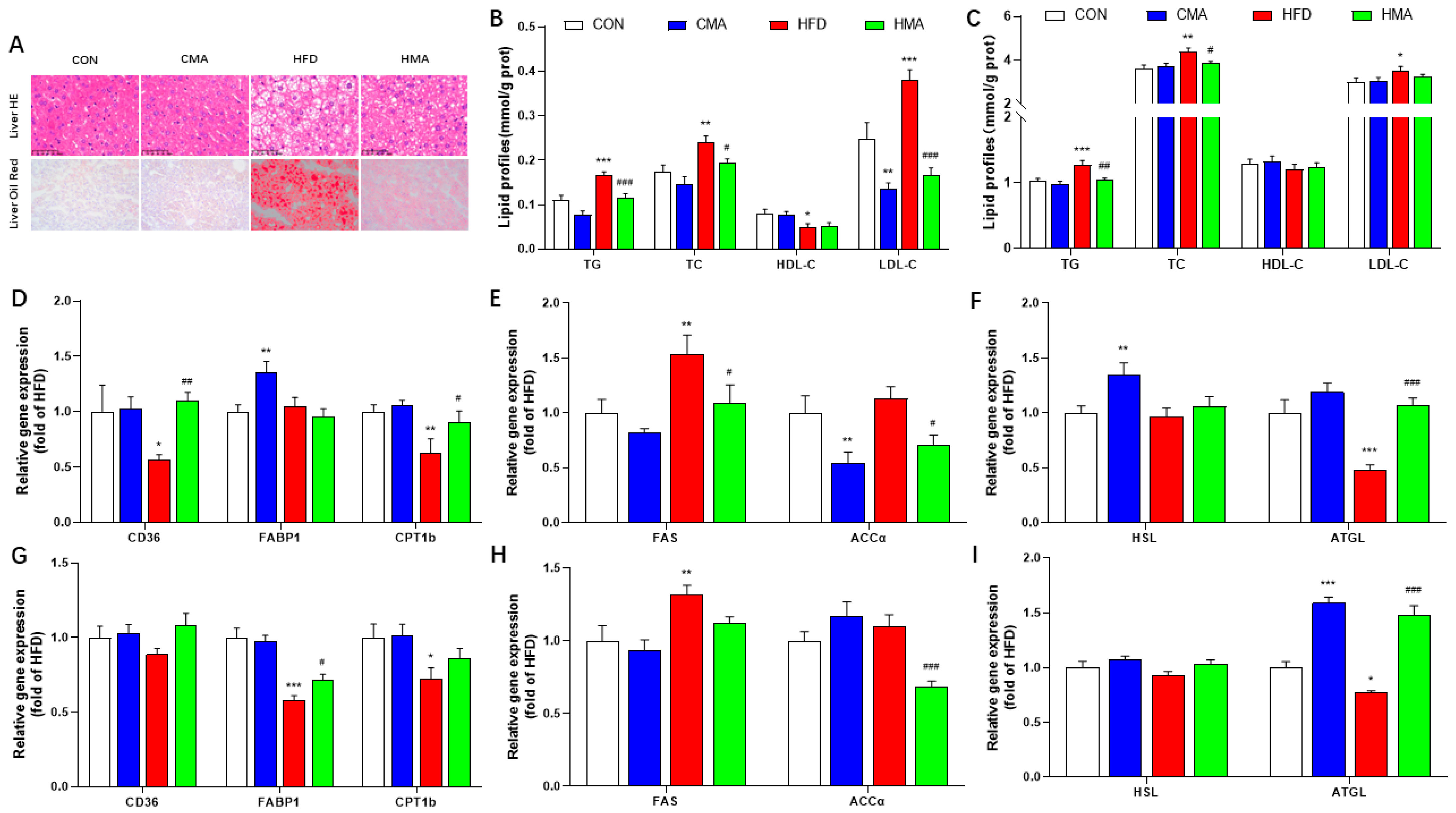
2.4. Lipid Metabolism in Liver and EAT of Mice
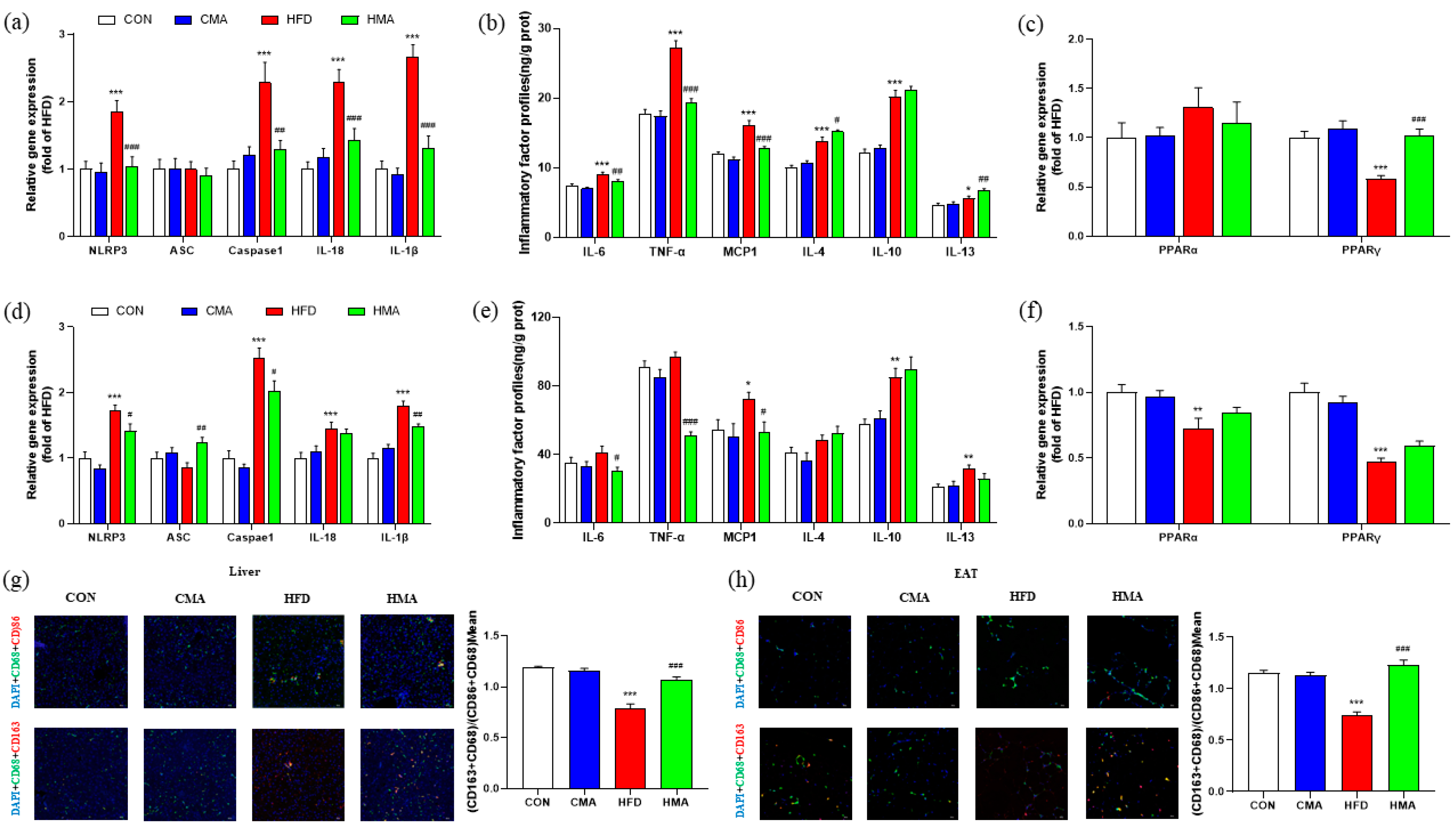
2.5. Inflammatory Reaction in Liver and EAT of Mice
3. Materials and Methods
3.1. Animal Grouping and Feeding Management
3.2. Sample Collection and Preparation
3.3. H&E Staining and Oil Red Staining Were Performed
3.4. Determination of TG, TC, HDL-C, LDL-C and Transaminase Activity
3.5. Determination of Oxidative Stress Index
3.6. An ELISA Assay Was Also Utilized
3.7. Immunofluorescence
3.8. Reverese Transcription–Quantitative Real-Time Polymerase Chain Reaction (RT-qPCR)
3.9. Data Analysis
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Spinelli, A.; Buoncristiano, M.; Nardone, P.; Starc, G.; Hejgaard, T.; Júlíusson, P.B.; Fismen, A.S.; Weghuber, D.; Musić Milanović, S.; García-Solano, M.; et al. Thinness, overweight, and obesity in 6- to 9-year-old children from 36 countries: The World Health Organization European Childhood Obesity Surveillance Initiative-COSI 2015–2017. Obes. Rev. 2021, 22 (Suppl. S6), e13214. [Google Scholar] [CrossRef] [PubMed]
- Chinese Nutrition Society. Chinese Food Guide; People’s Health Publishing House: Beijing, China, 2022; pp. 5–25. [Google Scholar]
- Hong, X.; Ye, Q.; He, J.; Wang, Z.; Yang, H.; Qi, S.; Chen, X.; Wang, C.; Zhou, H.; Li, C.; et al. Prevalence and clustering of cardiovascular risk factors: A cross-sectional survey among Nanjing adults in China. BMJ Open 2018, 8, e020530. [Google Scholar] [CrossRef] [PubMed]
- Purdy, J.C.; Shatzel, J.J. The hematologic consequences of obesity. Eur. J. Haematol. 2021, 106, 306–319. [Google Scholar] [CrossRef] [PubMed]
- Lau, L.H.S.; Wong, S.H. Microbiota, Obesity and NAFLD. Adv. Exp. Med. Biol. 2018, 1061, 111–125. [Google Scholar] [CrossRef]
- Artemniak-Wojtowicz, D.; Kucharska, A.M.; Pyrżak, B. Obesity and chronic inflammation crosslinking. Cent. Eur. J. Immunol. 2020, 45, 461–468. [Google Scholar] [CrossRef] [PubMed]
- Bayliak, M.M.; Abrat, O.B.; Storey, J.M.; Storey, K.B.; Lushchak, V.I. Interplay between diet-induced obesity and oxidative stress: Comparison between Drosophila and mammals. Comp. Biochem. Physiol. A Mol. Integr. Physiol. 2019, 228, 18–28. [Google Scholar] [CrossRef] [PubMed]
- Nagarajan, S.R.; Cross, E.; Sanna, F.; Hodson, L. Dysregulation of hepatic metabolism with obesity: Factors influencing glucose and lipid metabolism. Proc. Nutr. Soc. 2022, 81, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Lee, Y.S.; Wollam, J.; Olefsky, J.M. An Integrated View of Immunometabolism. Cell 2018, 172, 22–40. [Google Scholar] [CrossRef] [PubMed]
- Scherer, P.E. Adipose Tissue: From Lipid Storage Compartment to Endocrine Organ. Diabetes 2006, 55, 1537–1545. [Google Scholar] [CrossRef]
- Rui, L. Energy metabolism in the liver. Compr. Physiol. 2014, 4, 177–197. [Google Scholar] [CrossRef] [PubMed]
- Hartstra, A.V.; de Groot, P.F.; Mendes Bastos, D.; Levin, E.; Serlie, M.J.; Soeters, M.R.; Pekmez, C.T.; Dragsted, L.O.; Ackermans, M.T.; Groen, A.K.; et al. Correlation of plasma metabolites with glucose and lipid fluxes in human insulin resistance. Obes. Sci. Pract. 2020, 6, 340–349. [Google Scholar] [CrossRef] [PubMed]
- Ghaben, A.L.; Scherer, P.E. Adipogenesis and metabolic health. Nat. Rev. Mol. Cell Biol. 2019, 20, 242–258. [Google Scholar] [CrossRef]
- Cusi, K. Role of Obesity and Lipotoxicity in the Development of Nonalcoholic Steatohepatitis: Pathophysiology and Clinical Implications. Gastroenterology 2012, 142, 711–725.e716. [Google Scholar] [CrossRef]
- Aaseth, J.; Ellefsen, S.; Alehagen, U.; Sundfør, T.M.; Alexander, J. Diets and drugs for weight loss and health in obesity–An update. Biomed. Pharmacother. 2021, 140, 111789. [Google Scholar] [CrossRef]
- Li, S.; Tan, H.-Y.; Wang, N.; Zhang, Z.-J.; Lao, L.; Wong, C.-W.; Feng, Y. The Role of Oxidative Stress and Antioxidants in Liver Diseases. Int. J. Mol. Sci. 2015, 16, 26087–26124. [Google Scholar] [CrossRef] [PubMed]
- Pérez-Torres, I.; Castrejón-Téllez, V.; Soto, M.E.; Rubio-Ruiz, M.E.; Manzano-Pech, L.; Guarner-Lans, V. Oxidative Stress, Plant Natural Antioxidants, and Obesity. Int. J. Mol. Sci. 2021, 22, 1786. [Google Scholar] [CrossRef] [PubMed]
- Albillos, A.; de Gottardi, A.; Rescigno, M. The gut-liver axis in liver disease: Pathophysiological basis for therapy. J. Hepatol. 2020, 72, 558–577. [Google Scholar] [CrossRef]
- Anhê, F.F.; Schertzer, J.D.; Marette, A. Bacteria to alleviate metabolic syndrome. Nat. Med. 2019, 25, 1031–1033. [Google Scholar] [CrossRef]
- Xu, X.; Gao, Z.; Yang, F.; Yang, Y.; Chen, L.; Han, L.; Zhao, N.; Xu, J.; Wang, X.; Ma, Y.; et al. Antidiabetic Effects of Gegen Qinlian Decoction via the Gut Microbiota Are Attributable to Its Key Ingredient Berberine. Genom. Proteom. Bioinform. 2020, 18, 721–736. [Google Scholar] [CrossRef]
- Luo, Z.; Gao, Q.; Zhang, H.; Zhang, Y.; Zhou, S.; Zhang, J.; Xu, W.; Xu, J. Microbe-derived antioxidants attenuate cobalt chloride-induced mitochondrial function, autophagy and BNIP3-dependent mitophagy pathways in BRL3A cells. Ecotoxicol. Environ. Saf. 2022, 232, 113219. [Google Scholar] [CrossRef]
- Zhu, L.H.; Zhao, K.L.; Chen, X.L.; Xu, J.X. Impact of weaning and an antioxidant blend on intestinal barrier function and antioxidant status in pigs. J. Anim. Sci. 2012, 90, 2581–2589. [Google Scholar] [CrossRef] [PubMed]
- Luo, Z.; Xu, X.; Zhao, S.; Sho, T.; Luo, W.; Zhang, J.; Xu, W.; Hon, K.; Xu, J. Inclusion of microbe-derived antioxidant during pregnancy and lactation attenuates high-fat diet-induced hepatic oxidative stress, lipid disorders, and NLRP3 inflammasome in mother rats and offspring. Food Nutr. Res. 2019, 63, 3504. [Google Scholar] [CrossRef] [PubMed]
- Xu, X.; Zhang, J.; Xu, W.; Xu, J. Role of microbial-derived antioxidants on diquat-induced oxidative stress, endoplasmic reticulum stress and function in mice. Chin. J. Hepatol. 2020, 28, 441–445. [Google Scholar]
- Shi, H.; Li, Y.; Gu, Y. Effects of a micro-derived antioxidant on semen parameters of males with asthenozoospermia. New Biotechnol. 2018, 44, S31. [Google Scholar] [CrossRef]
- Shen, C.; Luo, Z.; Ma, S.; Yu, C.; Gao, Q.; Zhang, M.; Zhang, H.; Zhang, J.; Xu, W.; Yao, J.; et al. Microbe-Derived Antioxidants Reduce Lipopolysaccharide-Induced Inflammatory Responses by Activating the Nrf2 Pathway to Inhibit the ROS/NLRP3/IL-1β Signaling Pathway. Int. J. Mol. Sci. 2022, 23, 12477. [Google Scholar]
- Luo, Z.; Zhu, W.; Guo, Q.; Luo, W.; Zhang, J.; Xu, W.; Xu, J. Weaning Induced Hepatic Oxidative Stress, Apoptosis, and Aminotransferases through MAPK Signaling Pathways in Piglets. Oxidative Med. Cell. Longev. 2016, 2016, 4768541. [Google Scholar] [CrossRef]
- Sellayah, D.; Cagampang, F.R.; Cox, R.D. On the evolutionary origins of obesity: A new hypothesis. Endocrinology 2014, 155, 1573–1588. [Google Scholar] [CrossRef]
- Vekic, J.; Zeljkovic, A.; Stefanovic, A.; Jelic-Ivanovic, Z.; Spasojevic-Kalimanovska, V. Obesity and dyslipidemia. Metabolism 2019, 92, 71–81. [Google Scholar] [CrossRef]
- Bolsoni-Lopes, A.; Alonso-Vale, M.I. Lipolysis and lipases in white adipose tissue—An update. Arch. Endocrinol. Metab. 2015, 59, 335–342. [Google Scholar] [CrossRef]
- Jarc, E.; Petan, T. Lipid Droplets and the Management of Cellular Stress. Yale J. Biol. Med. 2019, 92, 435–452. [Google Scholar]
- Alves-Bezerra, M.; Cohen, D.E. Triglyceride Metabolism in the Liver. Compr. Physiol. 2017, 8, 1–8. [Google Scholar] [CrossRef]
- Fuchs, C.D.; Claudel, T.; Trauner, M. Role of metabolic lipases and lipolytic metabolites in the pathogenesis of NAFLD. Trends Endocrinol. Metab. 2014, 25, 576–585. [Google Scholar] [CrossRef] [PubMed]
- Liu, M.; Yu, X.; Lin, L.; Deng, J.; Wang, K.; Xia, Y.; Tang, X.; Hong, H. ATGL promotes the proliferation of hepatocellular carcinoma cells via the p-AKT signaling pathway. J. Biochem. Mol. Toxicol. 2019, 33, e22391. [Google Scholar] [CrossRef] [PubMed]
- Jocken, J.W.; Langin, D.; Smit, E.; Saris, W.H.; Valle, C.; Hul, G.B.; Holm, C.; Arner, P.; Blaak, E.E. Adipose triglyceride lipase and hormone-sensitive lipase protein expression is decreased in the obese insulin-resistant state. J. Clin. Endocrinol. Metab. 2007, 92, 2292–2299. [Google Scholar] [CrossRef]
- Prashar, Y.; Patel, N.J. Methanolic fruit extract of Myrica nagi protects the hypothalamus and attenuates inflammation associated with gold thioglucose- and high-fat diet-induced obesity via various adipokines. J. Ayurveda Integr. Med. 2022, 13, 100582. [Google Scholar] [CrossRef] [PubMed]
- Zhao, L.; Shen, Y.; Wang, Y.; Wang, L.; Zhang, L.; Zhao, Z.; Li, S. Lactobacillus plantarum S9 alleviates lipid profile, insulin resistance, and inflammation in high-fat diet-induced metabolic syndrome rats. Sci. Rep. 2022, 12, 15490. [Google Scholar] [CrossRef] [PubMed]
- Li, L.; Ma, S.; Zhang, J.; Xu, W.N.; Xu, J.X. Microbe-derived antioxidants relieve DSS-induced intestinal and liver damage. Chin. J. Anim. Nutr. 2021, 33, 4123–4132. [Google Scholar]
- Stienstra, R.; van Diepen, J.A.; Tack, C.J.; Zaki, M.H.; van de Veerdonk, F.L.; Perera, D.; Neale, G.A.; Hooiveld, G.J.; Hijmans, A.; Vroegrijk, I.; et al. Inflammasome is a central player in the induction of obesity and insulin resistance. Proc. Natl. Acad. Sci. USA 2011, 108, 15324–15329. [Google Scholar] [CrossRef]
- Vandanmagsar, B.; Youm, Y.H.; Ravussin, A.; Galgani, J.E.; Stadler, K.; Mynatt, R.L.; Ravussin, E.; Stephens, J.M.; Dixit, V.D. The NLRP3 inflammasome instigates obesity-induced inflammation and insulin resistance. Nat. Med. 2011, 17, 179–188. [Google Scholar] [CrossRef]
- Donath, M.Y.; Böni-Schnetzler, M.; Ellingsgaard, H.; Halban, P.A.; Ehses, J.A. Cytokine production by islets in health and diabetes: Cellular origin, regulation and function. Trends Endocrinol. Metab. 2010, 21, 261–267. [Google Scholar] [CrossRef]
- Kursawe, R.; Dixit, V.D.; Scherer, P.E.; Santoro, N.; Narayan, D.; Gordillo, R.; Giannini, C.; Lopez, X.; Pierpont, B.; Nouws, J.; et al. A Role of the Inflammasome in the Low Storage Capacity of the Abdominal Subcutaneous Adipose Tissue in Obese Adolescents. Diabetes 2016, 65, 610–618. [Google Scholar] [CrossRef] [PubMed]
- Sun, Q.W.; Lian, C.F.; Chen, Y.M.; Ye, J.; Chen, W.; Gao, Y.; Wang, H.L.; Gao, L.L.; Liu, Y.L.; Yang, Y.F. Ramulus Mori (Sangzhi) Alkaloids Ameliorate Obesity-Linked Adipose Tissue Metabolism and Inflammation in Mice. Nutrients 2022, 14, 5050. [Google Scholar] [CrossRef] [PubMed]
- Kong, X.; Gao, J. Macrophage polarization: A key event in the secondary phase of acute spinal cord injury. J. Cell. Mol. Med. 2017, 21, 941–954. [Google Scholar] [CrossRef] [PubMed]
- Fukui, S.; Iwamoto, N.; Takatani, A.; Igawa, T.; Shimizu, T.; Umeda, M.; Nishino, A.; Horai, Y.; Hirai, Y.; Koga, T.; et al. M1 and M2 Monocytes in Rheumatoid Arthritis: A Contribution of Imbalance of M1/M2 Monocytes to Osteoclastogenesis. Front. Immunol. 2017, 8, 1958. [Google Scholar] [CrossRef] [PubMed]
- Byrne, A.J.; Mathie, S.A.; Gregory, L.G.; Lloyd, C.M. Pulmonary macrophages: Key players in the innate defence of the airways. Thorax 2015, 70, 1189–1196. [Google Scholar] [CrossRef]
- Yuan, R.; Li, S.; Geng, H.; Wang, X.; Guan, Q.; Li, X.; Ren, C.; Yuan, X. Reversing the polarization of tumor-associated macrophages inhibits tumor metastasis. Int. Immunopharmacol. 2017, 49, 30–37. [Google Scholar] [CrossRef]
- Ferrante, C.J.; Leibovich, S.J. Regulation of Macrophage Polarization and Wound Healing. Adv. Wound Care 2012, 1, 10–16. [Google Scholar] [CrossRef]
- Schneider, C.; Nobs, S.P.; Kurrer, M.; Rehrauer, H.; Thiele, C.; Kopf, M. Induction of the nuclear receptor PPAR-γ by the cytokine GM-CSF is critical for the differentiation of fetal monocytes into alveolar macrophages. Nat. Immunol. 2014, 15, 1026–1037. [Google Scholar] [CrossRef]
- Kratz, M.; Coats, B.R.; Hisert, K.B.; Hagman, D.; Mutskov, V.; Peris, E.; Schoenfelt, K.Q.; Kuzma, J.N.; Larson, I.; Billing, P.S.; et al. Metabolic dysfunction drives a mechanistically distinct proinflammatory phenotype in adipose tissue macrophages. Cell Metab. 2014, 20, 614–625. [Google Scholar] [CrossRef]
- Odegaard, J.I.; Ricardo-Gonzalez, R.R.; Goforth, M.H.; Morel, C.R.; Subramanian, V.; Mukundan, L.; Red Eagle, A.; Vats, D.; Brombacher, F.; Ferrante, A.W.; et al. Macrophage-specific PPARgamma controls alternative activation and improves insulin resistance. Nature 2007, 28, 1116–1120. [Google Scholar] [CrossRef]
- Zhang, J.; Liu, X.; Wan, C.; Liu, Y.; Wang, Y.; Meng, C.; Zhang, Y.; Jiang, C. NLRP3 inflammasome mediates M1 macrophage polarization and IL-1β production in inflammatory root resorption. J. Clin. Periodontol. 2020, 47, 451–460. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.N.; Zhao, N.; Guo, F.F.; Wang, Y.R.; Liu, S.X.; Zeng, T. Diallyl disulfide suppresses the lipopolysaccharide-driven inflammatory response of macrophages by activating the Nrf2 pathway. Food Chem. Toxicol. 2022, 159, 112760. [Google Scholar] [CrossRef] [PubMed]
- Ciesarová, Z.; Murkovic, M.; Cejpek, K.; Kreps, F.; Tobolková, B.; Koplík, R.; Belajová, E.; Kukurová, K.; Daško, Ľ.; Panovská, Z.; et al. Why is sea buckthorn (Hippophae rhamnoides L.) so exceptional? A review. Food Res. Int. 2020, 133, 109170. [Google Scholar] [CrossRef] [PubMed]
- Bäck, M.; Yurdagul, A., Jr.; Tabas, I.; Öörni, K.; Kovanen, P.T. Inflammation and its resolution in atherosclerosis: Mediators and therapeutic opportunities. Nat. Rev. Cardiol. 2019, 16, 389–406. [Google Scholar] [CrossRef]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gao, Q.; Luo, Z.; Ma, S.; Yu, C.; Shen, C.; Xu, W.; Zhang, J.; Zhang, H.; Xu, J. Microbe-Derived Antioxidants Alleviate Liver and Adipose Tissue Lipid Disorders and Metabolic Inflammation Induced by High Fat Diet in Mice. Int. J. Mol. Sci. 2023, 24, 3269. https://doi.org/10.3390/ijms24043269
Gao Q, Luo Z, Ma S, Yu C, Shen C, Xu W, Zhang J, Zhang H, Xu J. Microbe-Derived Antioxidants Alleviate Liver and Adipose Tissue Lipid Disorders and Metabolic Inflammation Induced by High Fat Diet in Mice. International Journal of Molecular Sciences. 2023; 24(4):3269. https://doi.org/10.3390/ijms24043269
Chicago/Turabian StyleGao, Qingying, Zhen Luo, Sheng Ma, Chengbing Yu, Cheng Shen, Weina Xu, Jing Zhang, Hongcai Zhang, and Jianxiong Xu. 2023. "Microbe-Derived Antioxidants Alleviate Liver and Adipose Tissue Lipid Disorders and Metabolic Inflammation Induced by High Fat Diet in Mice" International Journal of Molecular Sciences 24, no. 4: 3269. https://doi.org/10.3390/ijms24043269
APA StyleGao, Q., Luo, Z., Ma, S., Yu, C., Shen, C., Xu, W., Zhang, J., Zhang, H., & Xu, J. (2023). Microbe-Derived Antioxidants Alleviate Liver and Adipose Tissue Lipid Disorders and Metabolic Inflammation Induced by High Fat Diet in Mice. International Journal of Molecular Sciences, 24(4), 3269. https://doi.org/10.3390/ijms24043269







