Aconitum carmichaelii Debx. Attenuates Heart Failure through Inhibiting Inflammation and Abnormal Vascular Remodeling
Abstract
1. Introduction
2. Result
2.1. Analysis of the Chemical Composition of WETA
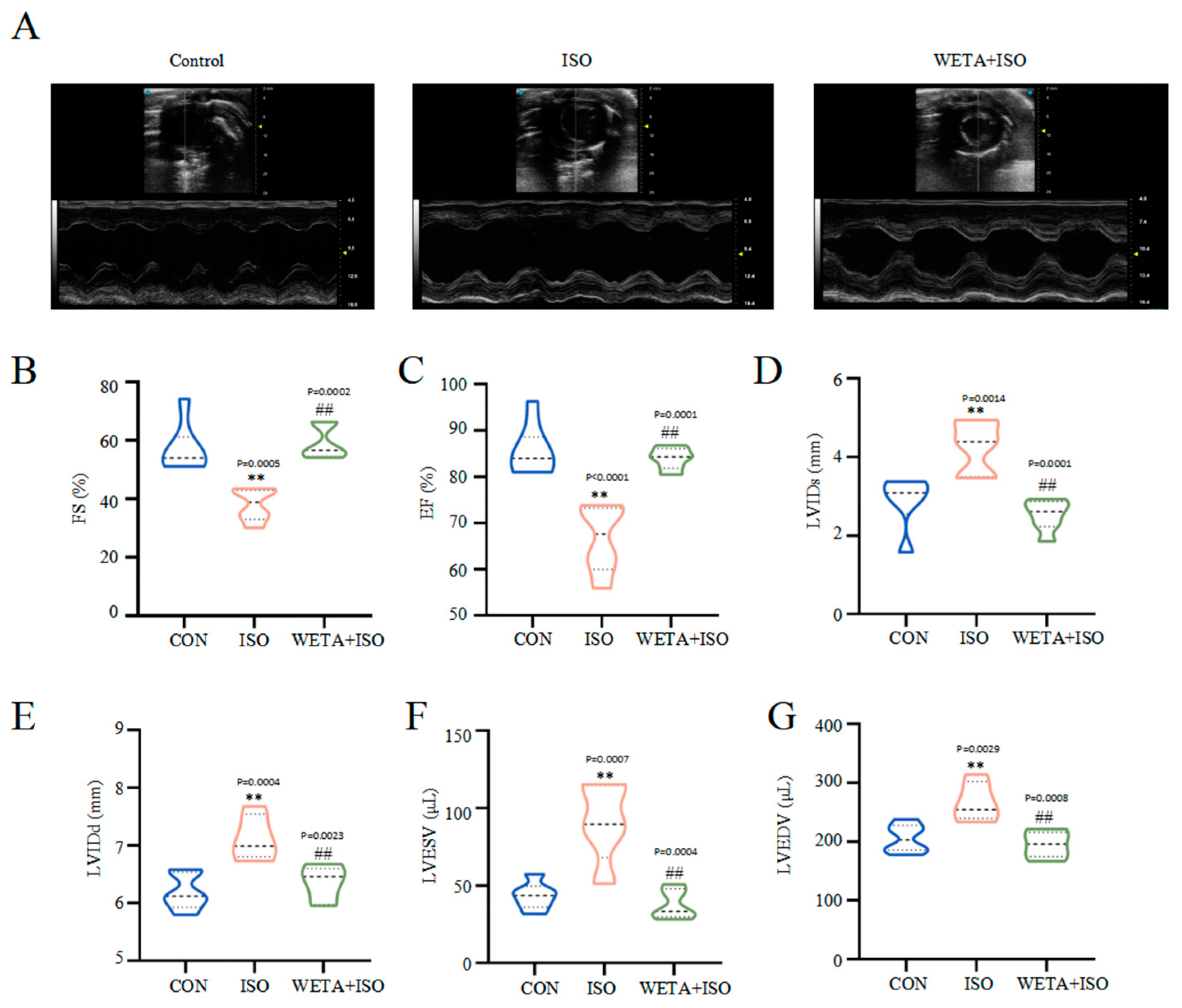
2.2. Effects of WETA on LV Hypertrophy and Systolic Function
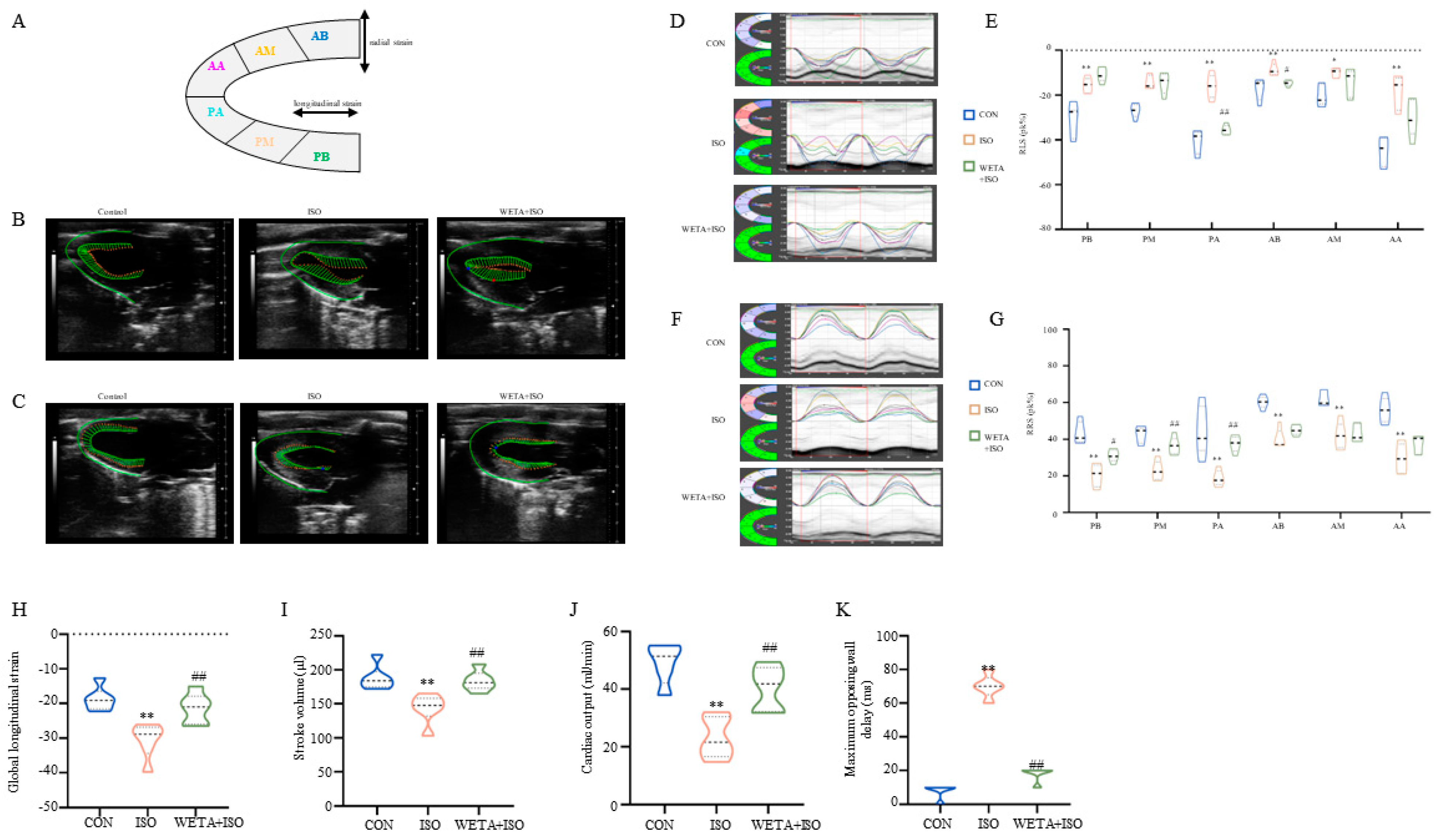
2.3. Effects of WETA on Segmental Myocardial Strain Caused by ISO
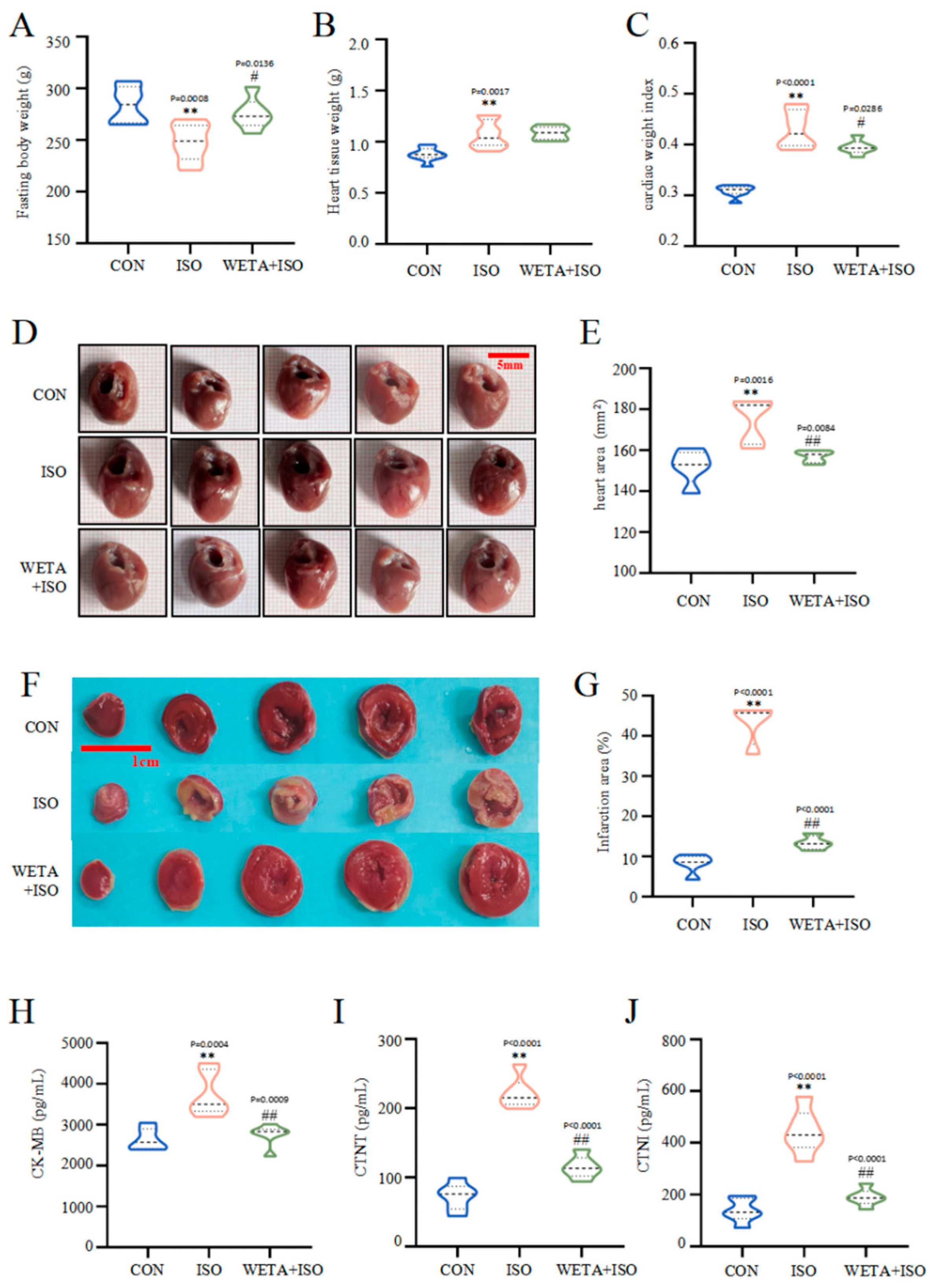
2.4. Effects of WETA on Cardiac Morphology and Serum Heart Function Indicators
2.5. Effects of WETA on Attenuating Histopathological Changes
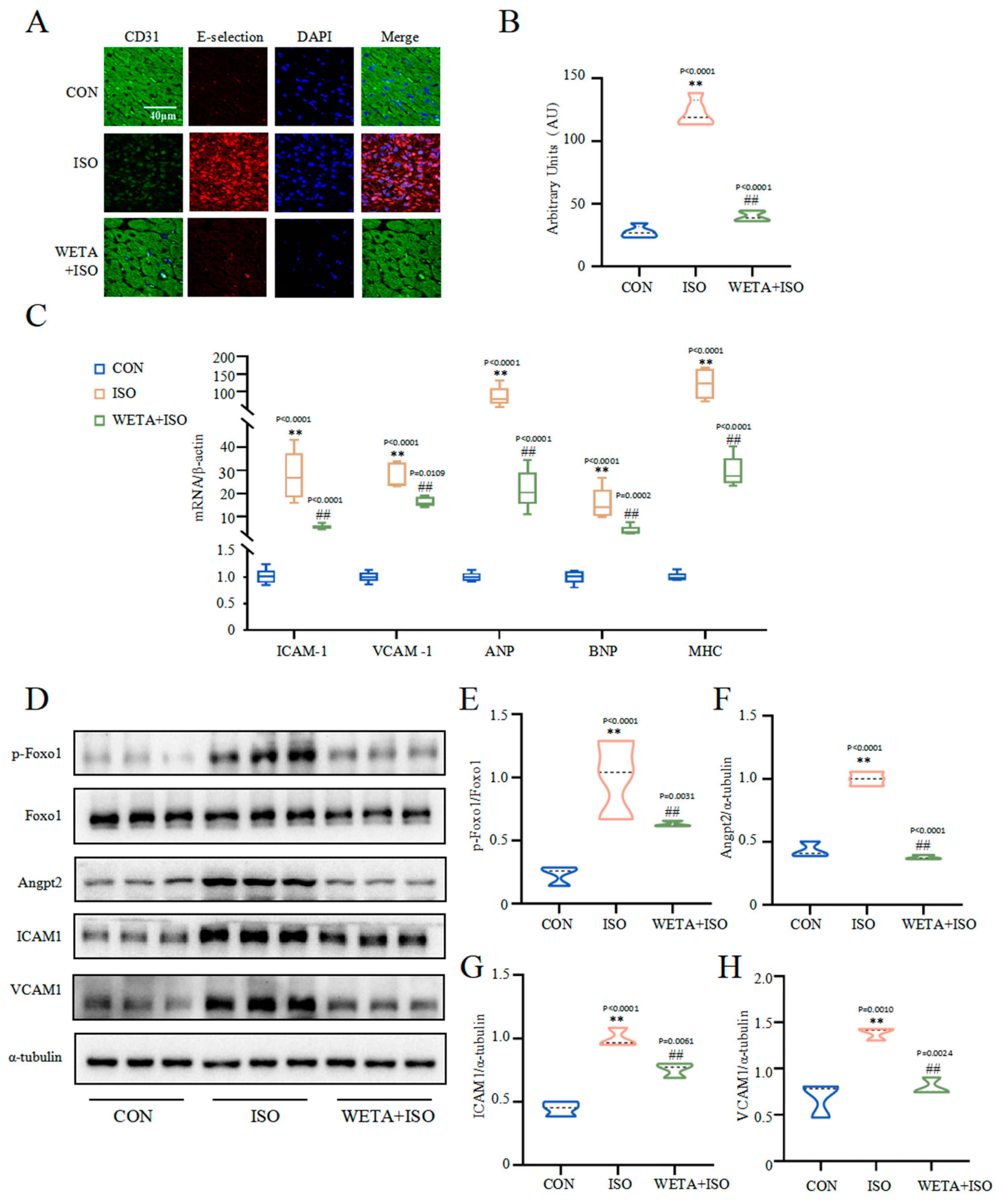
2.6. Effects of WETA on Inhibiting Inflammatory Response
2.7. Effects of WETA on Improving Myocardial Damage
3. Discussion
4. Methods
4.1. The Extraction Process of Aconitum carmichaelii Debx.
4.2. Qualitative Analysis of WETA
4.3. Experimental Animals and Treatment
4.4. Echocardiographic Analysis
4.5. Speckle-Tracking Echocardiographic
4.6. Collection and Detection of Cardiac Tissue
4.7. TTC Staining and Measurement of the Infarction Area
4.8. Determination of Serum Biomarkers
4.9. H&E Staining and Masson’s Trichrome Staining
4.10. qRT-PCR Analysis
4.11. Immunofluorescence Analysis
4.12. Western Blot Analysis
4.13. Statistical Analysis
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Conflicts of Interest
References
- Baman, J.R.; Ahmad, F.S. Heart Failure. JAMA 2020, 324, 1015. [Google Scholar] [CrossRef]
- Heidenreich, P.A.; Bozkurt, B.; Aguilar, D.; Allen, L.A.; Byun, J.J.; Colvin, M.M.; Deswal, A.; Drazner, M.H.; Dunlay, S.M.; Evers, L.R.; et al. 2022 AHA/ACC/HFSA Guideline for the Management of Heart Failure: Executive Summary: A Report of the American College of Cardiology/American Heart Association Joint Committee on Clinical Practice Guidelines. Circulation 2022, 145, e876–e894. [Google Scholar] [CrossRef] [PubMed]
- Ezekowitz, J.A.; O’Meara, E.; McDonald, M.A.; Abrams, H.; Chan, M.; Ducharme, A.; Giannetti, N.; Grzeslo, A.; Hamilton, P.G.; Heckman, G.A.; et al. 2017 Comprehensive Update of the Canadian Cardiovascular Society Guidelines for the Management of Heart Failure. Can. J. Cardiol. 2017, 33, 1342–1433. [Google Scholar] [CrossRef] [PubMed]
- Anderson, T.; Hummel, S.L.; Konerman, M.C. Epidemiology, Diagnosis, Pathophysiology, and Initial Approach to Heart Failure with Preserved Ejection Fraction. Cardiol. Clin. 2022, 40, 397–413. [Google Scholar] [CrossRef]
- Gonzalez, A.; Schelbert, E.B.; Diez, J.; Butler, J. Myocardial Interstitial Fibrosis in Heart Failure: Biological and Translational Perspectives. J. Am. Coll. Cardiol. 2018, 71, 1696–1706. [Google Scholar] [CrossRef] [PubMed]
- Burchfield, J.S.; Xie, M.; Hill, J.A. Pathological ventricular remodeling: Mechanisms: Part 1 of 2. Circulation 2013, 128, 388–400. [Google Scholar] [CrossRef]
- Anzai, A.; Ko, S.; Fukuda, K. Immune and Inflammatory Networks in Myocardial Infarction: Current Research and Its Potential Implications for the Clinic. Int. J. Mol. Sci. 2022, 23, 5214. [Google Scholar] [CrossRef]
- Laskarin, G.; Zaputovic, L.; Persic, V.; Ruzic, A.; Sotosek Tokmadzic, V. Harmful immune reactions during acute myocardial infarction. Med. Hypotheses 2012, 78, 703–706. [Google Scholar] [CrossRef]
- Hwang, S.J.; Ballantyne, C.M.; Sharrett, A.R.; Smith, L.C.; Davis, C.E.; Gotto, A.M., Jr.; Boerwinkle, E. Circulating adhesion molecules VCAM-1, ICAM-1, and E-selectin in carotid atherosclerosis and incident coronary heart disease cases: The Atherosclerosis Risk In Communities (ARIC) study. Circulation 1997, 96, 4219–4225. [Google Scholar] [CrossRef]
- Satoh, M.; Minami, Y.; Takahashi, Y.; Nakamura, M. Immune modulation: Role of the inflammatory cytokine cascade in the failing human heart. Curr. Heart Fail. Rep. 2008, 5, 69–74. [Google Scholar] [CrossRef]
- Peisker, F.; Halder, M.; Nagai, J.; Ziegler, S.; Kaesler, N.; Hoeft, K.; Li, R.; Bindels, E.M.J.; Kuppe, C.; Moellmann, J.; et al. Mapping the cardiac vascular niche in heart failure. Nat. Commun. 2022, 13, 3027. [Google Scholar] [CrossRef]
- Shiojima, I.; Sato, K.; Izumiya, Y.; Schiekofer, S.; Ito, M.; Liao, R.; Colucci, W.S.; Walsh, K. Disruption of coordinated cardiac hypertrophy and angiogenesis contributes to the transition to heart failure. J. Clin. Investig. 2005, 115, 2108–2118. [Google Scholar] [CrossRef]
- Dor, Y.; Djonov, V.; Abramovitch, R.; Itin, A.; Fishman, G.I.; Carmeliet, P.; Goelman, G.; Keshet, E. Conditional switching of VEGF provides new insights into adult neovascularization and pro-angiogenic therapy. EMBO J. 2002, 21, 1939–1947. [Google Scholar] [CrossRef]
- Peplinski, B.S.; Houston, B.A.; Bluemke, D.A.; Kawut, S.M.; Kolb, T.M.; Kronmal, R.A.; Lima, J.A.C.; Ralph, D.D.; Rayner, S.G.; Steinberg, Z.L.; et al. Associations of Angiopoietins With Heart Failure Incidence and Severity. J. Card. Fail. 2021, 27, 786–795. [Google Scholar] [CrossRef] [PubMed]
- Lee, S.J.; Lee, C.K.; Kang, S.; Park, I.; Kim, Y.H.; Kim, S.K.; Hong, S.P.; Bae, H.; He, Y.; Kubota, Y.; et al. Angiopoietin-2 exacerbates cardiac hypoxia and inflammation after myocardial infarction. J. Clin. Investig. 2018, 128, 5018–5033. [Google Scholar] [CrossRef] [PubMed]
- Rubio, B.; Mora, C.; Pintado, C.; Mazuecos, L.; Fernandez, A.; Lopez, V.; Andres, A.; Gallardo, N. The nutrient sensing pathways FoxO1/3 and mTOR in the heart are coordinately regulated by central leptin through PPARbeta/delta. Implications in cardiac remodeling. Metabolism 2021, 115, 154453. [Google Scholar] [CrossRef]
- Zhang, L.; Lu, X.; Wang, J.; Li, P.; Li, H.; Wei, S.; Zhou, X.; Li, K.; Wang, L.; Wang, R.; et al. Zingiberis rhizoma mediated enhancement of the pharmacological effect of aconiti lateralis radix praeparata against acute heart failure and the underlying biological mechanisms. Biomed. Pharmacother. 2017, 96, 246–255. [Google Scholar] [CrossRef]
- Zhou, G.; Tang, L.; Zhou, X.; Wang, T.; Kou, Z.; Wang, Z. A review on phytochemistry and pharmacological activities of the processed lateral root of Aconitum carmichaelii Debeaux. J. Ethnopharmacol. 2015, 160, 173–193. [Google Scholar] [CrossRef] [PubMed]
- Xu, X.; Xie, X.; Zhang, H.; Wang, P.; Li, G.; Chen, J.; Chen, G.; Cao, X.; Xiong, L.; Peng, F.; et al. Water-soluble alkaloids extracted from Aconiti Radix lateralis praeparata protect against chronic heart failure in rats via a calcium signaling pathway. Biomed. Pharmacother. 2021, 135, 111184. [Google Scholar] [CrossRef]
- Wen, J.X.; Zou, W.J.; Wang, R.L.; Liu, H.H.; Yang, Y.X.; Li, H.T.; Wei, S.Z.; Li, R.S.; Cai, H.D.; Wang, J.; et al. Cardioprotective effects of Aconiti Lateralis Radix Praeparata combined with Zingiberis Rhizoma on doxorubicin-induced chronic heart failure in rats and potential mechanisms. J. Ethnopharmacol. 2019, 238, 111880. [Google Scholar] [CrossRef]
- Wu, J.J.; Guo, Z.Z.; Zhu, Y.F.; Huang, Z.J.; Gong, X.; Li, Y.H.; Son, W.J.; Li, X.Y.; Lou, Y.M.; Zhu, L.J.; et al. A systematic review of pharmacokinetic studies on herbal drug Fuzi: Implications for Fuzi as personalized medicine. Phytomedicine 2018, 44, 187–203. [Google Scholar] [CrossRef] [PubMed]
- Lei, H.; Zhang, Y.; Ye, J.; Cheng, T.; Liang, Y.; Zu, X.; Zhang, W. A comprehensive quality evaluation of Fuzi and its processed product through integration of UPLC-QTOF/MS combined MS/MS-based mass spectral molecular networking with multivariate statistical analysis and HPLC-MS/MS. J. Ethnopharmacol. 2021, 266, 113455. [Google Scholar] [CrossRef]
- Wang, Y.; Wang, Q.; Li, C.; Lu, L.; Zhang, Q.; Zhu, R.; Wang, W. A Review of Chinese Herbal Medicine for the Treatment of Chronic Heart Failure. Curr. Pharm. Des. 2017, 23, 5115–5124. [Google Scholar] [CrossRef]
- Willerson, J.T. The Medical and Device-Related Treatment of Heart Failure. Circ. Res. 2019, 124, 1519. [Google Scholar] [CrossRef]
- Luis, S.A.; Chan, J.; Pellikka, P.A. Echocardiographic Assessment of Left Ventricular Systolic Function: An Overview of Contemporary Techniques, Including Speckle-Tracking Echocardiography. Mayo Clin. Proc. 2019, 94, 125–138. [Google Scholar] [CrossRef]
- Writing Committee, M.; Douglas, P.S.; Carabello, B.A.; Lang, R.M.; Lopez, L.; Pellikka, P.A.; Picard, M.H.; Thomas, J.D.; Varghese, P.; Wang, T.Y.; et al. 2019 ACC/AHA/ASE Key Data Elements and Definitions for Transthoracic Echocardiography: A Report of the American College of Cardiology/American Heart Association Task Force on Clinical Data Standards (Writing Committee to Develop Clinical Data Standards for Transthoracic Echocardiography) and the American Society of Echocardiography. J. Am. Soc. Echocardiogr. 2019, 32, 1161–1248. [Google Scholar]
- van Bommel, R.J.; Bax, J.J.; Abraham, W.T.; Chung, E.S.; Pires, L.A.; Tavazzi, L.; Zimetbaum, P.J.; Gerritse, B.; Kristiansen, N.; Ghio, S. Characteristics of heart failure patients associated with good and poor response to cardiac resynchronization therapy: A PROSPECT (Predictors of Response to CRT) sub-analysis. Eur. Heart J. 2009, 30, 2470–2477. [Google Scholar] [CrossRef] [PubMed]
- Ahmad, F.; Tomar, D.; Aryal, A.C.S.; Elmoselhi, A.B.; Thomas, M.; Elrod, J.W.; Tilley, D.G.; Force, T. Nicotinamide riboside kinase-2 alleviates ischemia-induced heart failure through P38 signaling. Biochim. Biophys. Acta Mol. Basis Dis. 2020, 1866, 165609. [Google Scholar] [CrossRef]
- Walter, J.; du Fay de Lavallaz, J.; Koechlin, L.; Zimmermann, T.; Boeddinghaus, J.; Honegger, U.; Strebel, I.; Twerenbold, R.; Amrein, M.; Nestelberger, T.; et al. Using High-Sensitivity Cardiac Troponin for the Exclusion of Inducible Myocardial Ischemia in Symptomatic Patients: A Cohort Study. Ann. Intern. Med. 2020, 172, 175–185. [Google Scholar] [CrossRef]
- Liu, P.; Li, J.; Liu, M.; Zhang, M.; Xue, Y.; Zhang, Y.; Han, X.; Jing, X.; Chu, L. Hesperetin modulates the Sirt1/Nrf2 signaling pathway in counteracting myocardial ischemia through suppression of oxidative stress, inflammation, and apoptosis. Biomed. Pharmacother. 2021, 139, 111552. [Google Scholar] [CrossRef]
- Keihanian, F.; Moohebati, M.; Saeidinia, A.; Mohajeri, S.A.; Madaeni, S. Therapeutic effects of medicinal plants on isoproterenol-induced heart failure in rats. Biomed. Pharmacother. 2021, 134, 111101. [Google Scholar] [CrossRef]
- Frantz, S.; Hundertmark, M.J.; Schulz-Menger, J.; Bengel, F.M.; Bauersachs, J. Left ventricular remodelling post-myocardial infarction: Pathophysiology, imaging, and novel therapies. Eur. Heart J. 2022, 43, 2549–2561. [Google Scholar] [CrossRef] [PubMed]
- Tian, C.X.; Li, M.Y.; Shuai, X.X.; Jiang, F.; Dong, Y.L.; Gui, Y.; Zhang, Z.L.; Qin, R.J.; Kang, Z.Y.; Lin, L.; et al. Berberine plays a cardioprotective role by inhibiting macrophage Wnt5a/beta-catenin pathway in the myocardium of mice after myocardial infarction. Phytother. Res. 2022, 37, 50–61. [Google Scholar] [CrossRef]
- Paulus, W.J.; Tschope, C. A Novel Paradigm for Heart Failure With Preserved Ejection Fraction Comorbidities Drive Myocardial Dysfunction and Remodeling Through Coronary Microvascular Endothelial Inflammation. J. Am. Coll. Cardiol. 2013, 62, 263–271. [Google Scholar] [CrossRef]
- Frangogiannis, N.G. The immune system and the remodeling infarcted heart: Cell biological insights and therapeutic opportunities. J. Cardiovasc. Pharmacol. 2014, 63, 185–195. [Google Scholar] [CrossRef] [PubMed]
- Hulsmans, M.; Sam, F.; Nahrendorf, M. Monocyte and macrophage contributions to cardiac remodeling. J. Mol. Cell. Cardiol. 2016, 93, 149–155. [Google Scholar] [CrossRef]
- Heusch, G.; Libby, P.; Gersh, B.; Yellon, D.; Bohm, M.; Lopaschuk, G.; Opie, L. Cardiovascular remodelling in coronary artery disease and heart failure. Lancet 2014, 383, 1933–1943. [Google Scholar] [CrossRef]
- Wong, S.C.; Fukuchi, M.; Melnyk, P.; Rodger, I.; Giaid, A. Induction of cyclooxygenase-2 and activation of nuclear factor-kappaB in myocardium of patients with congestive heart failure. Circulation 1998, 98, 100–103. [Google Scholar] [CrossRef]
- Hamid, T.; Gu, Y.; Ortines, R.V.; Bhattacharya, C.; Wang, G.; Xuan, Y.T.; Prabhu, S.D. Divergent tumor necrosis factor receptor-related remodeling responses in heart failure: Role of nuclear factor-kappaB and inflammatory activation. Circulation 2009, 119, 1386–1397. [Google Scholar] [CrossRef]
- Gordon, J.W.; Shaw, J.A.; Kirshenbaum, L.A. Multiple facets of NF-kappaB in the heart: To be or not to NF-kappaB. Circ. Res. 2011, 108, 1122–1132. [Google Scholar] [CrossRef] [PubMed]
- McKinsey, T.A.; Kass, D.A. Small-molecule therapies for cardiac hypertrophy: Moving beneath the cell surface. Nat. Rev. Drug Discov. 2007, 6, 617–635. [Google Scholar] [CrossRef] [PubMed]
- Oka, T.; Akazawa, H.; Naito, A.T.; Komuro, I. Angiogenesis and cardiac hypertrophy: Maintenance of cardiac function and causative roles in heart failure. Circ. Res. 2014, 114, 565–571. [Google Scholar] [CrossRef] [PubMed]
- Daly, C.; Pasnikowski, E.; Burova, E.; Wong, V.; Aldrich, T.H.; Griffiths, J.; Ioffe, E.; Daly, T.J.; Fandl, J.P.; Papadopoulos, N.; et al. Angiopoietin-2 functions as an autocrine protective factor in stressed endothelial cells. Proc. Natl. Acad. Sci. USA 2006, 103, 15491–15496. [Google Scholar] [CrossRef] [PubMed]
- Fiedler, U.; Reiss, Y.; Scharpfenecker, M.; Grunow, V.; Koidl, S.; Thurston, G.; Gale, N.W.; Witzenrath, M.; Rosseau, S.; Suttorp, N.; et al. Angiopoietin-2 sensitizes endothelial cells to TNF-alpha and has a crucial role in the induction of inflammation. Nat. Med. 2006, 12, 235–239. [Google Scholar] [CrossRef]
- Kim, I.; Moon, S.O.; Kim, S.H.; Kim, H.J.; Koh, Y.S.; Koh, G.Y. Vascular endothelial growth factor expression of intercellular adhesion molecule 1 (ICAM-1), vascular cell adhesion molecule 1 (VCAM-1), and E-selectin through nuclear factor-kappa B activation in endothelial cells. J. Biol. Chem. 2001, 276, 7614–7620. [Google Scholar] [CrossRef]
- Wahid, M.; Saqib, F.; Chicea, L.; Ahmedah, H.T.; Sajer, B.H.; Marc Vlaic, R.A.; Pop, O.L.; Moga, M.; Gavris, C. Metabolomics analysis delineates the therapeutic effects of hydroethanolic extract of Cucumis sativus L. seeds on hypertension and isoproterenol-induced myocardial infarction. Biomed. Pharmacother. 2022, 148, 112704. [Google Scholar] [CrossRef]
- Li, D.; Yang, C.; Zhu, J.Z.; Lopez, E.; Zhang, T.; Tong, Q.; Peng, C.; Lin, L.G. Berberine remodels adipose tissue to attenuate metabolic disorders by activating sirtuin 3. Acta Pharmacol. Sin. 2022, 43, 1285–1298. [Google Scholar] [CrossRef]
- Li, D.; Xing, Z.; Yu, T.; Dong, W.; Wang, Z.; Peng, C.; Yang, C. Pogostone attenuates adipose tissue inflammation by regulating the adipocyte-macrophage crosstalk via activating SIRT1. Food Funct. 2022, 13, 11853–11864. [Google Scholar] [CrossRef]
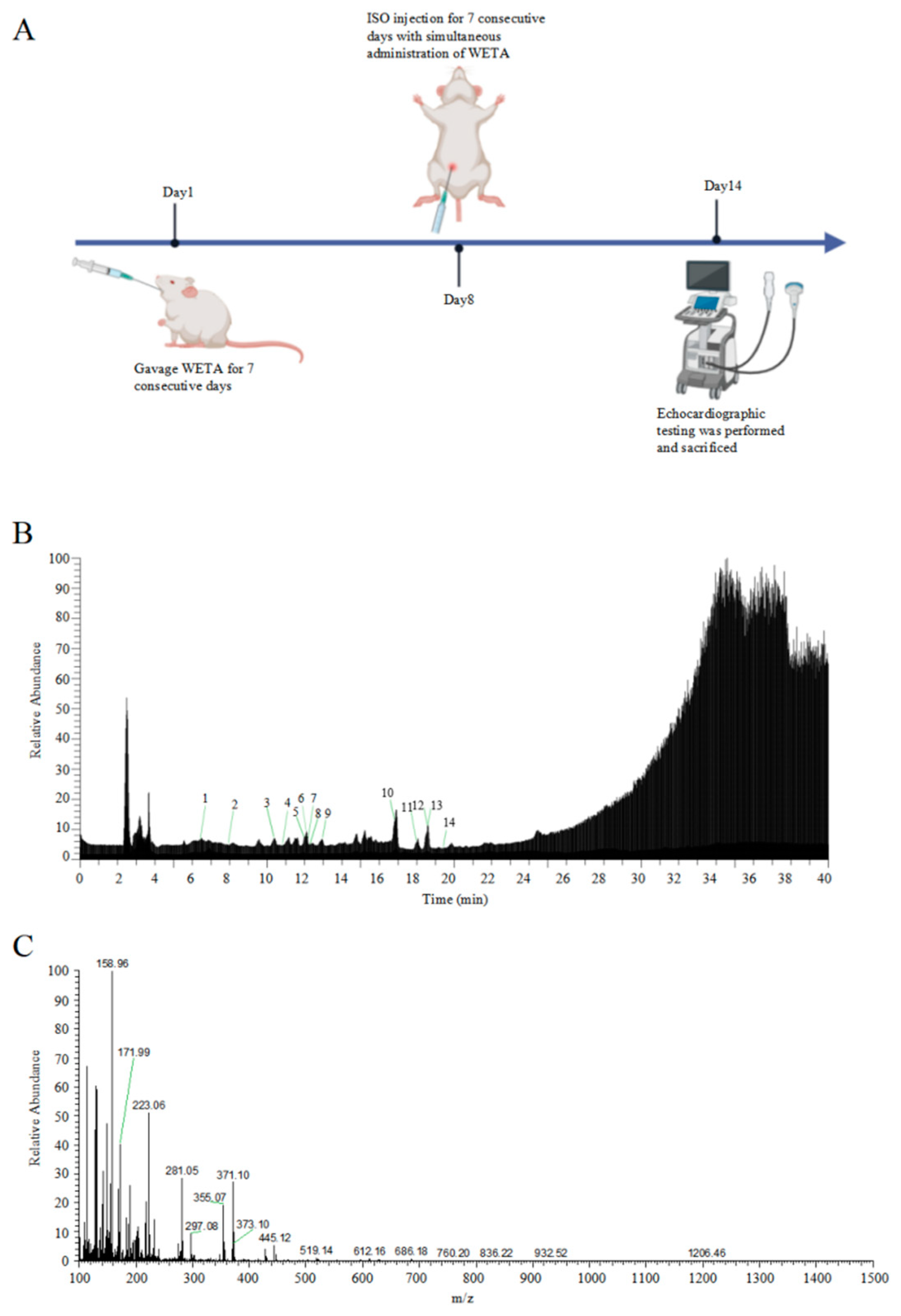


| Peak No. | tR (min) | Compounds | Molecular Formula | Calculated MS (m/z) | Observed (m/z) | Error (ppm) |
|---|---|---|---|---|---|---|
| 1 | 6.418 | Salsolinol | C10H13NO2 | 179.09463 | 179.09502 | 2.18 |
| 2 | 7.937 | Karakoline | C22H35NO4 | 378.2635 | 377.25716 | 1.46 |
| 3 | 10.377 | Isotalatizidine | C23H37NO5 | 408.2742 | 407.26772 | 1.34 |
| 4 | 10.812 | Napelline | C22H33NO3 | 360.2525 | 359.24672 | 1.89 |
| 5 | 11.98 | Neoline/Bullatine B | C24H39NO6 | 438.2862 | 437.27834 | 1.37 |
| 6 | 12.103 | Neoline | C24H39NO6 | 437.27774 | 437.27834 | 1.37 |
| 7 | 12.177 | Lycoctonine | C25H41NO7 | 468.2955 | 467.28935 | 2.25 |
| 8 | 12.289 | Fuziline (15-α-Hydroxyneoline) | C24H39NO7 | 453.27265 | 454.2793 | 1.5 |
| 9 | 12.969 | Talatisamine | C24H39NO5 | 421.28282 | 421.28365 | 1.96 |
| 10 | 16.770 | Benzoylmesaconine | C31H43NO10 | 589.47383 | 589.28905 | 0.6 |
| 11 | 17.980 | Benzoylaconine | C32H45NO10 | 603.30435 | 603.30528 | 1.55 |
| 12 | 18.566 | Hypaconitine | C33H45NO10 | 615.30435 | 615.30461 | 0.43 |
| 13 | 18.590 | Benzoylhypaconine | C31H43NO9 | 573.29378 | 573.29418 | 0.69 |
| 14 | 19.410 | Aconitine | C34 H47 NO11 | 645.31491 | 645.31664 | 2.67 |
| Gene | Primer Sequence (5′ to 3′) |
|---|---|
| TNF-α | F: TACTCCCAGGTTCTCTTCAAGG |
| R: GGAGGCTGACTTTCTCCTGGTA | |
| IL-6 | F: GAGTTGTGCAATGGCAATTC |
| R: ACTCCAGAAGACCAGAGCAG | |
| IL-1β | F: CACCTCTCAAGCAGAGCACAG |
| R: GGGTTCCATGGTGAAGTCAAC | |
| ANP | F: CTGCTAGACCACCTGGAGGA |
| R: AAGCTGTTGCAGCCTAGTCC | |
| BNP | F: GATCCAGGAGAGACTTCGAAA |
| R: CGGTCTATCTTCTGCCCAA | |
| MHC | F: GAGGAGAGGGCGGACATT |
| R: ACTCTTCATTCAGGCCCTTG | |
| ICAM-1 | F: AGATCATACGGGTTTGGGCTTC |
| R: TATGACTCGTGAAAGAAATCAGCTC | |
| VCAM-1 | F: TTTGCAAGAAAAGCCAACATGAAAG |
| R: TCTCCAACAGTTCAGACGTTAGC | |
| β-actin | F: GAAGTGTGACGTTGACATCCG |
| R: TGCTGATCCACATCTGCTGGA |
| Antibodies | Source | Production Company | Catalog Numbers |
|---|---|---|---|
| anti-phospho-IKKα/β (Ser176/180) | Rabbit | Cell Signaling Technology | #2697 |
| anti-IKKα | Mouse | Cell Signaling Technology | #11930 |
| anti-IKKβ | Rabbit | Cell Signaling Technology | #8943 |
| anti-phospho-IκBα (Ser32) | Rabbit | Cell Signaling Technology | #2859 |
| anti-IκBα | Mouse | Cell Signaling Technology | #4814 |
| anti-phospho-p65 (Ser536) | Rabbit | Cell Signaling Technology | #3033 |
| anti-p65 | Rabbit | Cell Signaling Technology | #8242 |
| anti-p-Foxo1 | Rabbit | Cell Signaling Technology | #2599 |
| anti-Foxo1 | Rabbit | Cell Signaling Technology | #2880 |
| anti-VCAM1 | Rabbit | ABclonal | A11236 |
| anti-ICAM1 | Rabbit | ABclonal | A5597 |
| anti-CD68 | Rabbit | ABclonal | A6554 |
| anti-E-selectin | Rabbit | proteintech | 20894-1-AP |
| anti-CD31 | Mouse | proteintech | 66065-2-Ig |
| anti-Angpt2 | Rabbit | affinity | #DF6137 |
| α-Tubulin | Mouse | ABclonal | AC012 |
| Anti-rabbit IgG, HRP-linked Antibody | Goat | Cell Signaling Technology | #7074 |
| Anti-mouse IgG, HRP-linked Antibody | Horse | Cell Signaling Technology | #7076 |
| Alexa Flour 488 goat anti-mouse IgG | Goat | Thermo Fisher Scientific | A11029 |
| Alexa Flour 594 goat anti-Rabbit IgG | Goat | Thermo Fisher Scientific | A11037 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Xing, Z.; Chen, J.; Yu, T.; Li, X.; Dong, W.; Peng, C.; Li, D. Aconitum carmichaelii Debx. Attenuates Heart Failure through Inhibiting Inflammation and Abnormal Vascular Remodeling. Int. J. Mol. Sci. 2023, 24, 5838. https://doi.org/10.3390/ijms24065838
Xing Z, Chen J, Yu T, Li X, Dong W, Peng C, Li D. Aconitum carmichaelii Debx. Attenuates Heart Failure through Inhibiting Inflammation and Abnormal Vascular Remodeling. International Journal of Molecular Sciences. 2023; 24(6):5838. https://doi.org/10.3390/ijms24065838
Chicago/Turabian StyleXing, Ziwei, Junren Chen, Tingting Yu, Xu Li, Wei Dong, Cheng Peng, and Dan Li. 2023. "Aconitum carmichaelii Debx. Attenuates Heart Failure through Inhibiting Inflammation and Abnormal Vascular Remodeling" International Journal of Molecular Sciences 24, no. 6: 5838. https://doi.org/10.3390/ijms24065838
APA StyleXing, Z., Chen, J., Yu, T., Li, X., Dong, W., Peng, C., & Li, D. (2023). Aconitum carmichaelii Debx. Attenuates Heart Failure through Inhibiting Inflammation and Abnormal Vascular Remodeling. International Journal of Molecular Sciences, 24(6), 5838. https://doi.org/10.3390/ijms24065838






