A Lipidated Single-B-Chain Derivative of Relaxin Exhibits Improved In Vitro Serum Stability without Altering Activity
Abstract
1. Introduction
2. Results and Discussion
2.1. Different Lengths of Fatty Acids
2.2. Solubilizing Tag
2.3. Different Lengths of Spacer
2.4. In Vitro Serum Stability of AcK(PalmGlu)-PEG12-B7-33
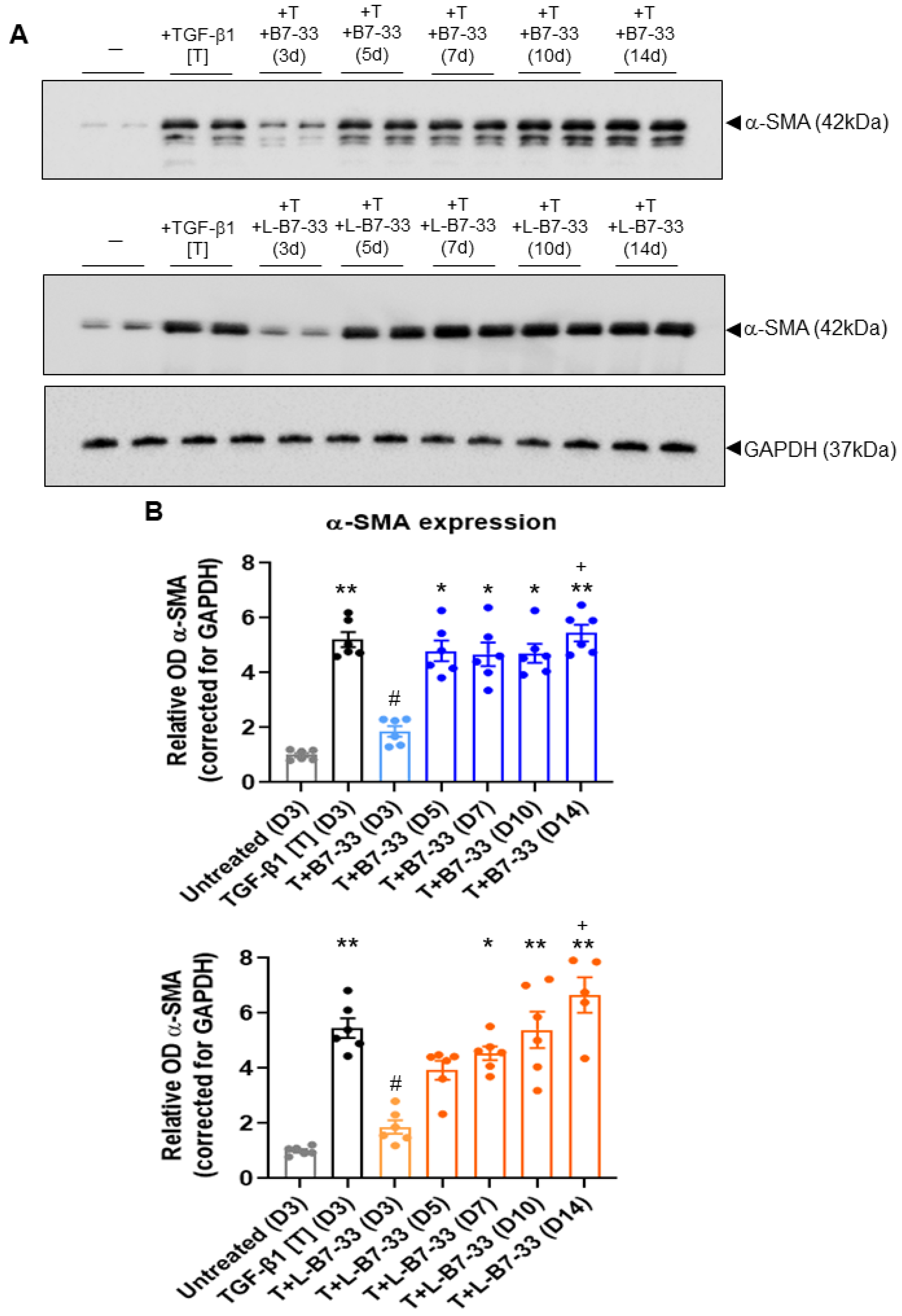
2.5. The Effects of Lipdated B7-33 (L-B7-33), AcK(PalmGlu)-PEG12-B7-33 on Myofibroblast Differentiation In Vitro
3. Materials and Methods
3.1. Materials
3.2. Solid-Phase Peptide Synthesis
3.3. Peptide Cleavage from Solid Support
3.4. Peptide Purification and Characterization
3.5. Amino Acid Analysis
3.6. HEK-7BP Binding Assays
3.7. In Vitro Serum Stability Assay
3.8. Determination of the Effects of Lipidated (L)-B7-33 on Myofibroblast Differentiation In Vitro
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Hisaw, F.L. Experimental relaxation of the pubic ligament of the guinea pig. Proc. Soc. Exp. Biol. Med. 1926, 23, 661–663. [Google Scholar] [CrossRef]
- Samuel, C.S.; Cendrawan, S.; Gao, X.M.; Ming, Z.; Zhao, C.; Kiriazis, H.; Xu, Q.; Tregear, G.W.; Bathgate, R.A.; Du, X.J. Relaxin remodels fibrotic healing following myocardial infarction. Lab. Investig. 2011, 91, 675–690. [Google Scholar] [CrossRef] [PubMed]
- Dschietzig, T.B. Relaxin-2 for heart failure with preserved ejection fraction (HFpEF): Rationale for future clinical trials. Mol. Cell Endocrinol. 2019, 487, 54–58. [Google Scholar] [CrossRef]
- Samuel, C.S.; Bennett, R.G. Relaxin as an anti-fibrotic treatment: Perspectives, challenges and future directions. Biochem. Pharmacol. 2022, 197, 114884. [Google Scholar] [CrossRef]
- Hsu, S.Y.; Nakabayashi, K.; Nishi, S.; Kumagai, J.; Kudo, M.; Sherwood, O.D.; Hsueh, A.J. Activation of orphan receptors by the hormone relaxin. Science 2002, 295, 671–674. [Google Scholar] [CrossRef] [PubMed]
- Kumagai, J.; Hsu, S.Y.; Matsumi, H.; Roh, J.S.; Fu, P.; Wade, J.D.; Bathgate, R.A.; Hsueh, A.J. INSL3/Leydig insulin-like peptide activates the LGR8 receptor important in testis descent. J. Biol. Chem. 2002, 277, 31283–31286. [Google Scholar] [CrossRef]
- Dschietzig, T.; Bartsch, C.; Stangl, V.; Baumann, G.; Stangl, K. Identification of the pregnancy hormone relaxin as glucocorticoid receptor agonist. Faseb J. 2004, 18, 1536–1538. [Google Scholar] [CrossRef] [PubMed]
- Sethi, A.; Bruell, S.; Patil, N.; Hossain, M.A.; Scott, D.J.; Petrie, E.J.; Bathgate, R.A.D.; Gooley, P.R. The complex binding mode of the peptide hormone H2 relaxin to its receptor RXFP1. Nat. Commun. 2016, 7, 11344. [Google Scholar] [CrossRef]
- Hossain, M.A.; Kocan, M.; Yao, S.T.; Royce, S.G.; Nair, V.B.; Siwek, C.; Patil, N.A.; Harrison, I.P.; Rosengren, K.J.; Selemidis, S.; et al. A single-chain derivative of the relaxin hormone is a functionally selective agonist of the G protein-coupled receptor, RXFP1. Chem. Sci. 2016, 7, 3805–3819. [Google Scholar] [CrossRef]
- Devarakonda, T.; Mauro, A.G.; Guzman, G.; Hovsepian, S.; Cain, C.; Das, A.; Praveen, P.; Hossain, M.A.; Salloum, F.N. B7-33, a functionally selective relaxin receptor 1 agonist, attenuates myocardial infarction-related adverse cardiac remodeling in mice. J. Am. Heart Assoc. 2020, 9, e015748. [Google Scholar] [CrossRef]
- Bhuiyan, S.; Shen, M.; Chelvaretnam, S.; Tan, A.Y.; Ho, G.; Hossain, M.A.; Widdop, R.E.; Samuel, C.S. Assessment of renal fibrosis and anti-fibrotic agents using a novel diagnostic and stain-free second-harmonic generation platform. Faseb J. 2021, 35, e21595. [Google Scholar] [CrossRef]
- Alam, F.; Gaspari, T.A.; Kemp-Harper, B.K.; Low, E.; Aw, A.; Ferens, D.; Spizzo, I.; Jefferis, A.M.; Praveen, P.; Widdop, R.E.; et al. The single-chain relaxin mimetic, B7-33, maintains the cardioprotective effects of relaxin and more rapidly reduces left ventricular fibrosis compared to perindopril in an experimental model of cardiomyopathy. Biomed. Pharmacother. 2023, 160, 114370. [Google Scholar] [CrossRef]
- Bathgate, R.A.D.; Kocan, M.; Scott, D.J.; Hossain, M.A.; Good, S.V.; Yegorov, S.; Bogerd, J.; Gooley, P.R. The relaxin receptor as a therapeutic target—Perspectives from evolution and drug targeting. Pharmacol. Ther. 2018, 187, 114–132. [Google Scholar] [CrossRef]
- Chow, B.S.; Kocan, M.; Bosnyak, S.; Sarwar, M.; Wigg, B.; Jones, E.S.; Widdop, R.E.; Summers, R.J.; Bathgate, R.A.; Hewitson, T.D.; et al. Relaxin requires the angiotensin II type 2 receptor to abrogate renal interstitial fibrosis. Kidney Int. 2014, 86, 75–85. [Google Scholar] [CrossRef] [PubMed]
- Werle, M.; Bernkop-Schnürch, A. Strategies to improve plasma half life time of peptide and protein drugs. Amino Acids 2006, 30, 351–367. [Google Scholar] [CrossRef]
- Bruno, B.J.; Miller, G.D.; Lim, C.S. Basics and recent advances in peptide and protein drug delivery. Ther. Deliv. 2013, 4, 1443–1467. [Google Scholar] [CrossRef]
- Knauf, M.J.; Bell, D.P.; Hirtzer, P.; Luo, Z.P.; Young, J.D.; Katre, N.V. Relationship of effective molecular size to systemic clearance in rats of recombinant interleukin-2 chemically modified with water-soluble polymers. J. Biol. Chem. 1988, 263, 15064–15070. [Google Scholar] [CrossRef] [PubMed]
- Chen, S.A.; Perlman, A.J.; Spanski, N.; Peterson, C.M.; Sanders, S.W.; Jaffe, R.; Martin, M.; Yalcinkaya, T.; Cefalo, R.C.; Chescheir, N.C.; et al. The pharmacokinetics of recombinant human relaxin in nonpregnant women after intravenous, intravaginal, and intracervical administration. Pharm. Res. 1993, 10, 834–838. [Google Scholar] [CrossRef]
- Chen, S.A.; Reed, B.; Nguyen, T.; Gaylord, N.; Fuller, G.B.; Mordenti, J. The pharmacokinetics and absorption of recombinant human relaxin in nonpregnant rabbits and rhesus monkeys after intravenous and intravaginal administration. Pharm. Res. 1993, 10, 223–227. [Google Scholar] [CrossRef] [PubMed]
- Teerlink, J.R.; Cotter, G.; Davison, B.A.; Felker, G.M.; Filippatos, G.; Greenberg, B.H.; Ponikowski, P.; Unemori, E.; Voors, A.A.; Adams, K.F., Jr.; et al. Serelaxin, recombinant human relaxin-2, for treatment of acute heart failure (RELAX-AHF): A randomised, placebo-controlled trial. Lancet 2013, 381, 29–39. [Google Scholar] [CrossRef]
- Mallart, S.; Ingenito, R.; Bianchi, E.; Bresciani, A.; Esposito, S.; Gallo, M.; Magotti, P.; Monteagudo, E.; Orsatti, L.; Roversi, D.; et al. Identification of potent and long-acting single-chain peptide mimetics of human relaxin-2 for cardiovascular diseases. J. Med. Chem. 2021, 64, 2139–2150. [Google Scholar] [CrossRef]
- Kurtzhals, P.; Havelund, S.; Jonassen, I.; Kiehr, B.; Larsen, U.D.; Ribel, U.; Markussen, J. Albumin binding of insulins acylated with fatty acids: Characterization of the ligand-protein interaction and correlation between binding affinity and timing of the insulin effect in vivo. Biochem. J. 1995, 312 Pt 3, 725–731. [Google Scholar] [CrossRef] [PubMed]
- Simard, J.R.; Zunszain, P.A.; Hamilton, J.A.; Curry, S. Location of high and low affinity fatty acid binding sites on human serum albumin revealed by NMR drug-competition analysis. J. Mol. Biol. 2006, 361, 336–351. [Google Scholar] [CrossRef]
- Hijazi, Y. Prediction of half-life extension of peptides via serum albumin binding: Current challenges. Eur. J. Drug Metab. Pharmacokinet. 2021, 46, 163–172. [Google Scholar] [CrossRef] [PubMed]
- Havelund, S.; Plum, A.; Ribel, U.; Jonassen, I.; Vølund, A.; Markussen, J.; Kurtzhals, P. The mechanism of protraction of insulin detemir, a long-acting, acylated analog of human insulin. Pharm. Res. 2004, 21, 1498–1504. [Google Scholar] [CrossRef]
- Jonassen, I.; Havelund, S.; Hoeg-Jensen, T.; Steensgaard, D.B.; Wahlund, P.O.; Ribel, U. Design of the novel protraction mechanism of insulin degludec, an ultra-long-acting basal insulin. Pharm. Res. 2012, 29, 2104–2114. [Google Scholar] [CrossRef]
- Guryanov, I.; Bondesan, A.; Visentini, D.; Orlandin, A.; Biondi, B.; Toniolo, C.; Formaggio, F.; Ricci, A.; Zanon, J.; Cabri, W. Innovative chemical synthesis and conformational hints on the lipopeptide liraglutide. J. Pept. Sci. 2016, 22, 471–479. [Google Scholar] [CrossRef]
- Marso, S.P.; Bain, S.C.; Consoli, A.; Eliaschewitz, F.G.; Jódar, E.; Leiter, L.A.; Lingvay, I.; Rosenstock, J.; Seufert, J.; Warren, M.L.; et al. Semaglutide and cardiovascular outcomes in patients with type 2 diabetes. N. Engl. J. Med. 2016, 375, 1834–1844. [Google Scholar] [CrossRef]
- Zorzi, A.; Linciano, S.; Angelini, A. Non-covalent albumin-binding ligands for extending the circulating half-life of small biotherapeutics. Medchemcomm 2019, 10, 1068–1081. [Google Scholar] [CrossRef]
- Scott, D.J.; Rosengren, K.J.; Bathgate, R.A. The different ligand-binding modes of relaxin family peptide receptors RXFP1 and RXFP2. Mol. Endocrinol. 2012, 26, 1896–1906. [Google Scholar] [CrossRef] [PubMed]
- Shabanpoor, F.; Bathgate, R.A.; Belgi, A.; Chan, L.J.; Nair, V.B.; Wade, J.D.; Hossain, M.A. Site-specific conjugation of a lanthanide chelator and its effects on the chemical synthesis and receptor binding affinity of human relaxin-2 hormone. Biochem. Biophys. Res. Commun. 2012, 420, 253–256. [Google Scholar] [CrossRef] [PubMed]
- Hahn, W.C.; Counter, C.M.; Lundberg, A.S.; Beijersbergen, R.L.; Brooks, M.W.; Weinberg, R.A. Creation of human tumour cells with defined genetic elements. Nature 1999, 400, 464–468. [Google Scholar] [CrossRef] [PubMed]
- Chow, B.S.; Chew, E.G.; Zhao, C.; Bathgate, R.A.; Hewitson, T.D.; Samuel, C.S. Relaxin signals through a RXFP1-pERK-nNOS-NO-cGMP-dependent pathway to up-regulate matrix metalloproteinases: The additional involvement of iNOS. PLoS ONE 2012, 7, e42714. [Google Scholar] [CrossRef] [PubMed]
- Du, X.J.; Bathgate, R.A.; Samuel, C.S.; Dart, A.M.; Summers, R.J. Cardiovascular effects of relaxin: From basic science to clinical therapy. Nat. Rev. Cardiol. 2010, 7, 48–58. [Google Scholar] [CrossRef]
- Pinar, A.A.; Yuferov, A.; Gaspari, T.A.; Samuel, C.S. Relaxin can mediate its anti-fibrotic effects by targeting the myofibroblast NLRP3 inflammasome at the level of caspase-1. Front. Pharmacol. 2020, 11, 1201. [Google Scholar] [CrossRef] [PubMed]

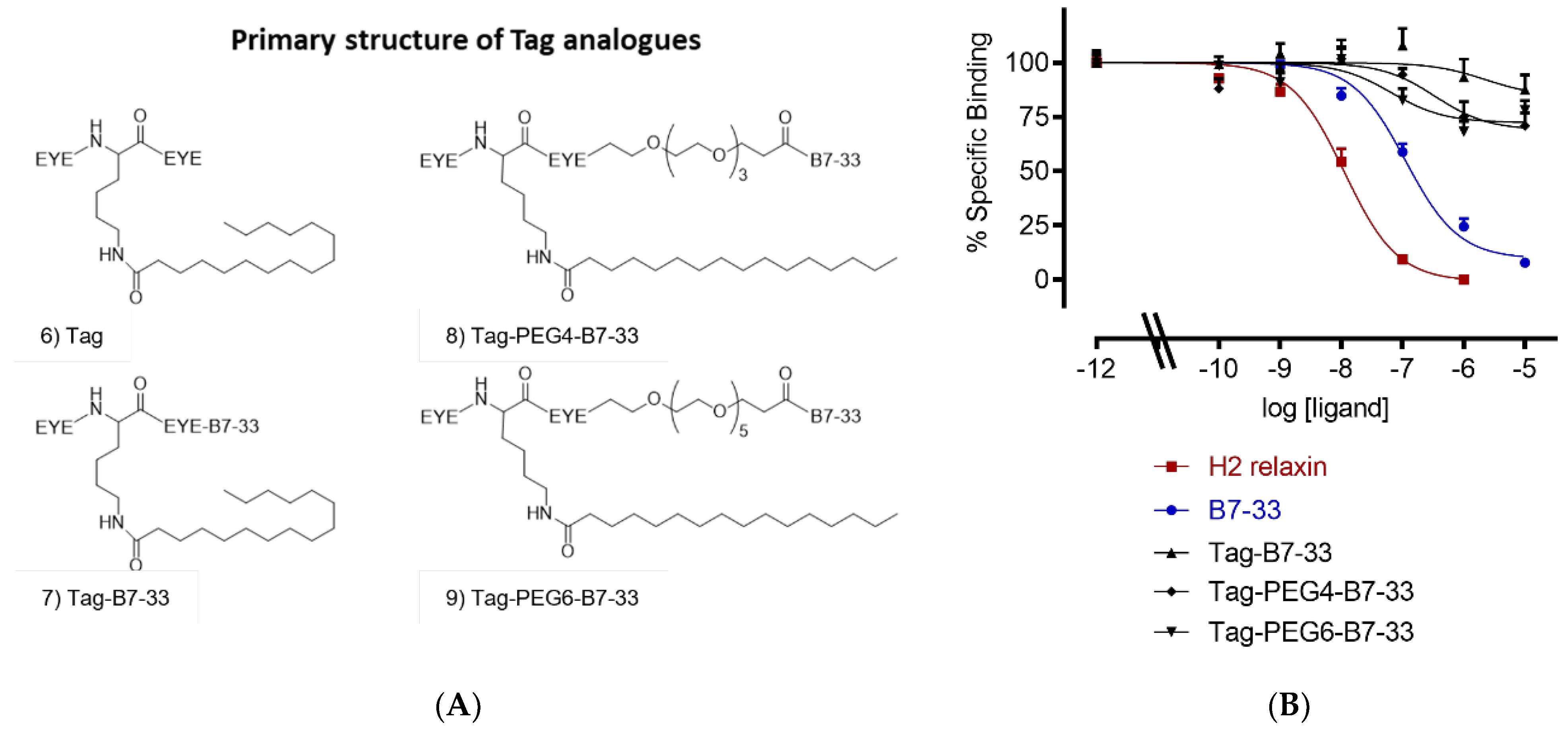
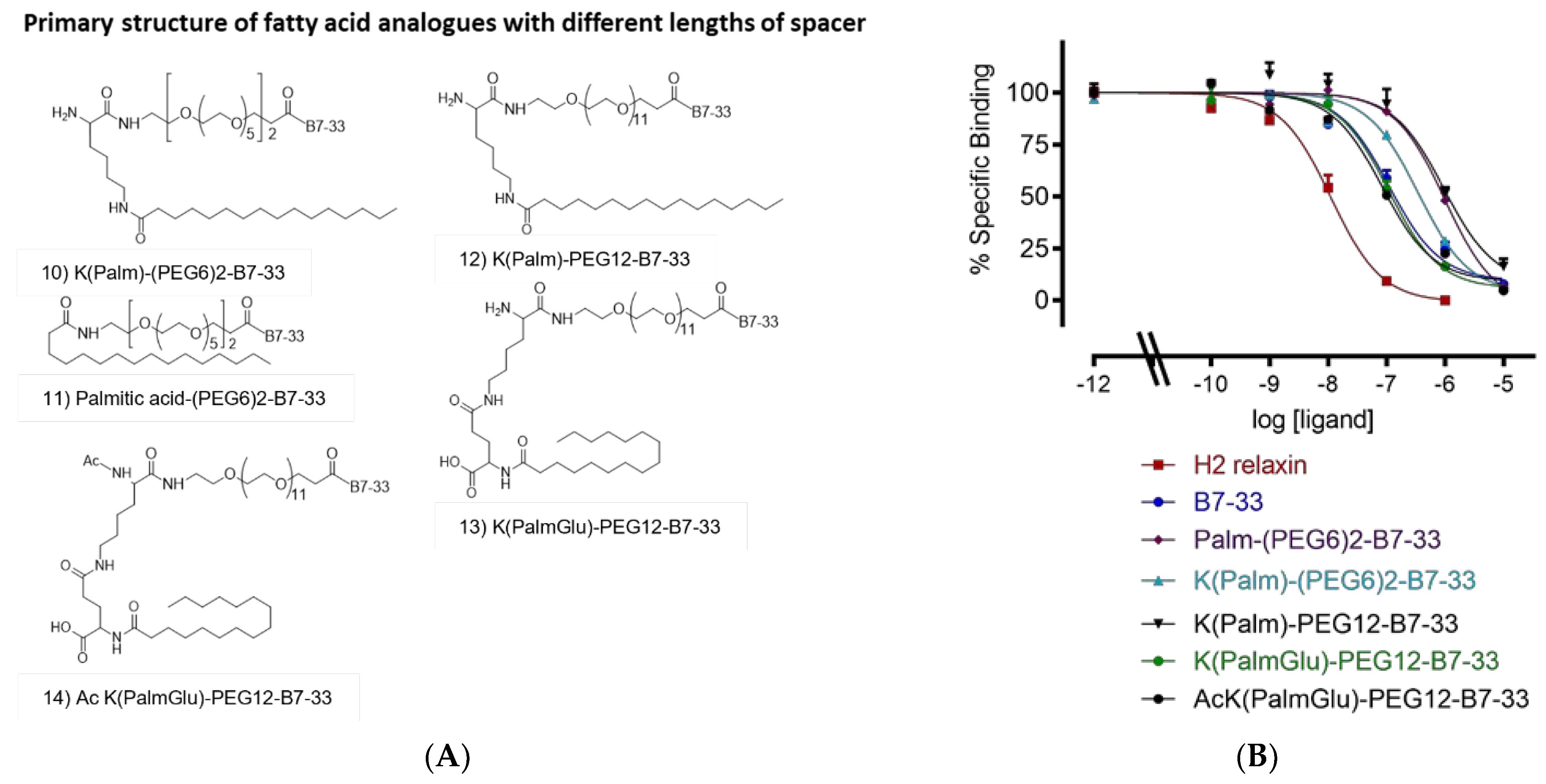

| Analogue | HEK-7BP Binding B.S.A. Free Eu-H2 pKi (n) |
|---|---|
| B7-33 | 7.28 ± 0.11 (7) |
| Decanoic acid-PEG6-B7-33 | 6.63 ± 0.14 (3) |
| Myristic acid-PEG6-B7-33 | 6.0 ± 0.11 (3) ** |
| Palmitic acid-PEG6-B7-33 | <5 (3) |
| K(Palm)-PEG6-B7-33 | 5.71 ± 0.14 (3) *** |
| B7-33_9K(Palm) | <5 (3) |
| Tag-B7-33 | <5 (3) |
| Tag-PEG4-B7-33 | <5 (3) |
| Tag-PEG6-B7-33 | <5 (3) |
| K(Palm)-(PEG6)2-B7-33 | 6.80 ± 0.16 (3) |
| PA-(PEG6)2-B7-33 | 6.40 ± 0.10 (3) ** |
| K(Palm)-PEG12-B7-33 | 6.56 ± 0.52 (3) ** |
| K(PalmGlu)-PEG12-B7-33 | 7.37 ± 0.10 (3) |
| Ac K(PalmGlu)-PEG12-B7-33 | 7.52 ± 0.13 (3) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Praveen, P.; Wang, C.; Handley, T.N.G.; Wu, H.; Samuel, C.S.; Bathgate, R.A.D.; Hossain, M.A. A Lipidated Single-B-Chain Derivative of Relaxin Exhibits Improved In Vitro Serum Stability without Altering Activity. Int. J. Mol. Sci. 2023, 24, 6616. https://doi.org/10.3390/ijms24076616
Praveen P, Wang C, Handley TNG, Wu H, Samuel CS, Bathgate RAD, Hossain MA. A Lipidated Single-B-Chain Derivative of Relaxin Exhibits Improved In Vitro Serum Stability without Altering Activity. International Journal of Molecular Sciences. 2023; 24(7):6616. https://doi.org/10.3390/ijms24076616
Chicago/Turabian StylePraveen, Praveen, Chao Wang, Thomas N. G. Handley, Hongkang Wu, Chrishan S. Samuel, Ross A. D. Bathgate, and Mohammed Akhter Hossain. 2023. "A Lipidated Single-B-Chain Derivative of Relaxin Exhibits Improved In Vitro Serum Stability without Altering Activity" International Journal of Molecular Sciences 24, no. 7: 6616. https://doi.org/10.3390/ijms24076616
APA StylePraveen, P., Wang, C., Handley, T. N. G., Wu, H., Samuel, C. S., Bathgate, R. A. D., & Hossain, M. A. (2023). A Lipidated Single-B-Chain Derivative of Relaxin Exhibits Improved In Vitro Serum Stability without Altering Activity. International Journal of Molecular Sciences, 24(7), 6616. https://doi.org/10.3390/ijms24076616







