Circulating miRNA-451a and miRNA-328-3p as Potential Markers of Coronary Artery Aneurysmal Disease
Abstract
1. Introduction
2. Results
2.1. Clinical Characteristics of the Study Population
2.2. Expression Profile of miRNAs in the Plasma of Group 1 Versus Group 2 and Group 3
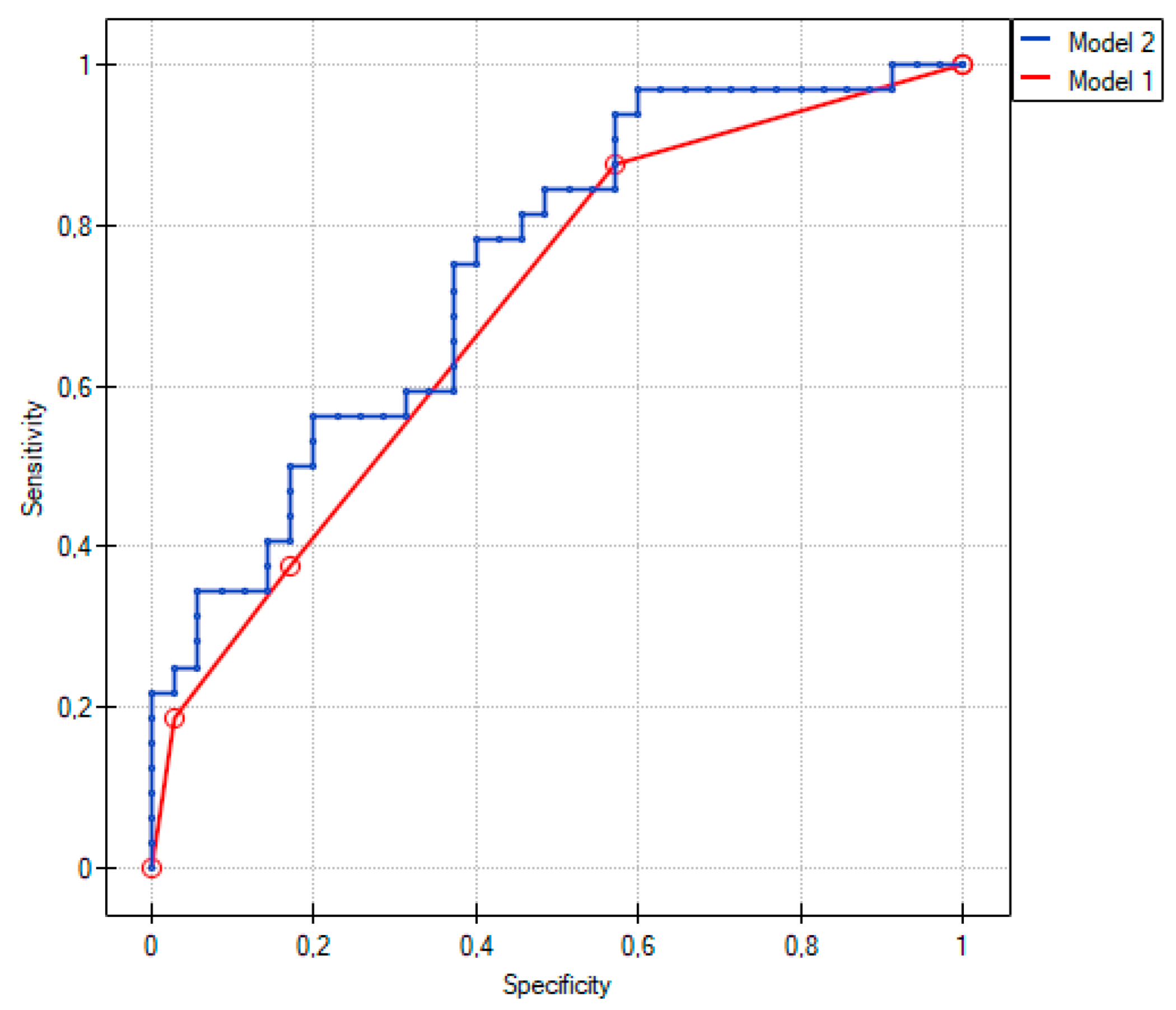
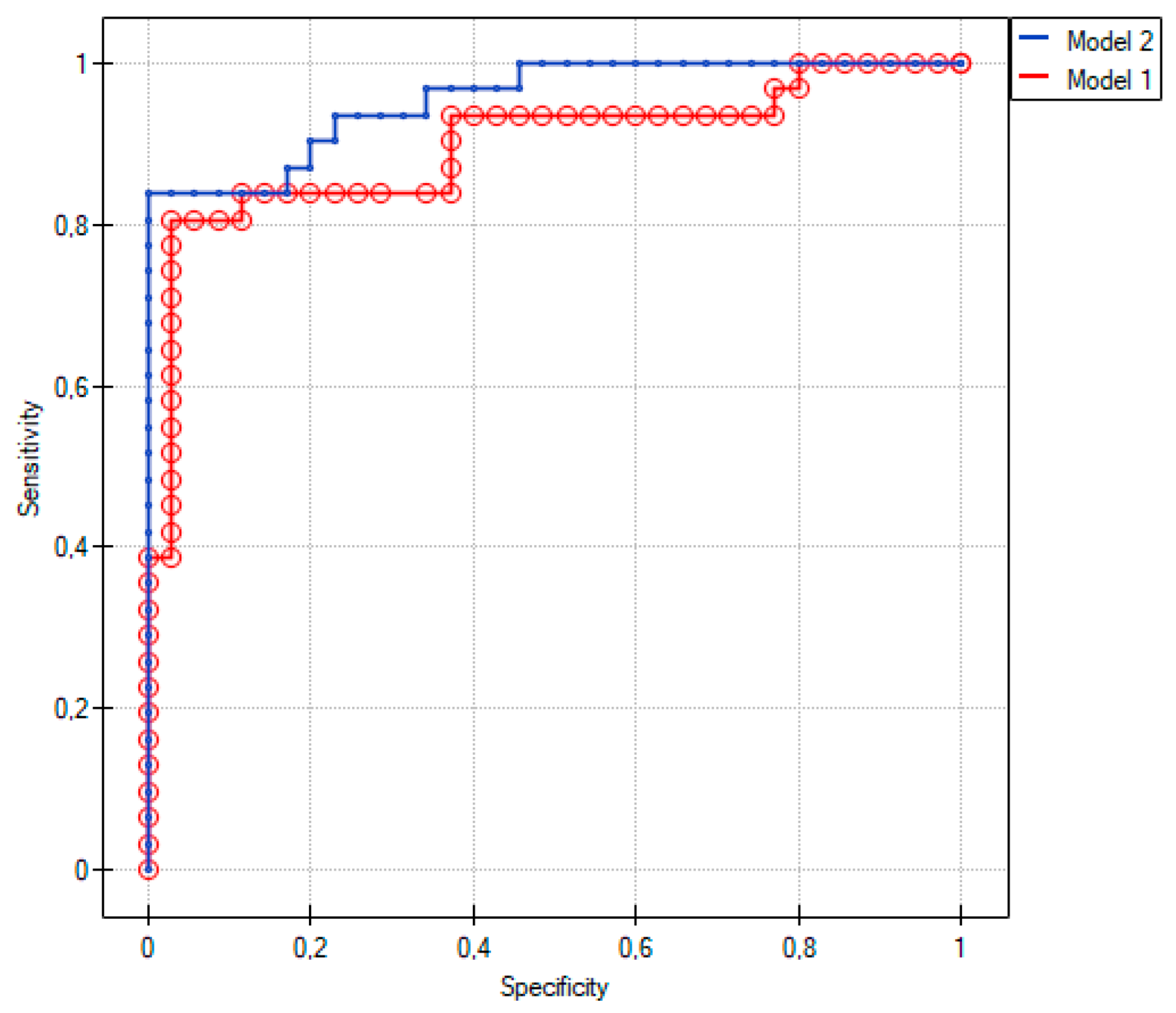
2.3. Comparison of Group 1 with Group 2
2.4. Comparison between Group 1 and Group 3
2.5. Functional Enrichment Analysis
3. Discussion
4. Materials and Methods
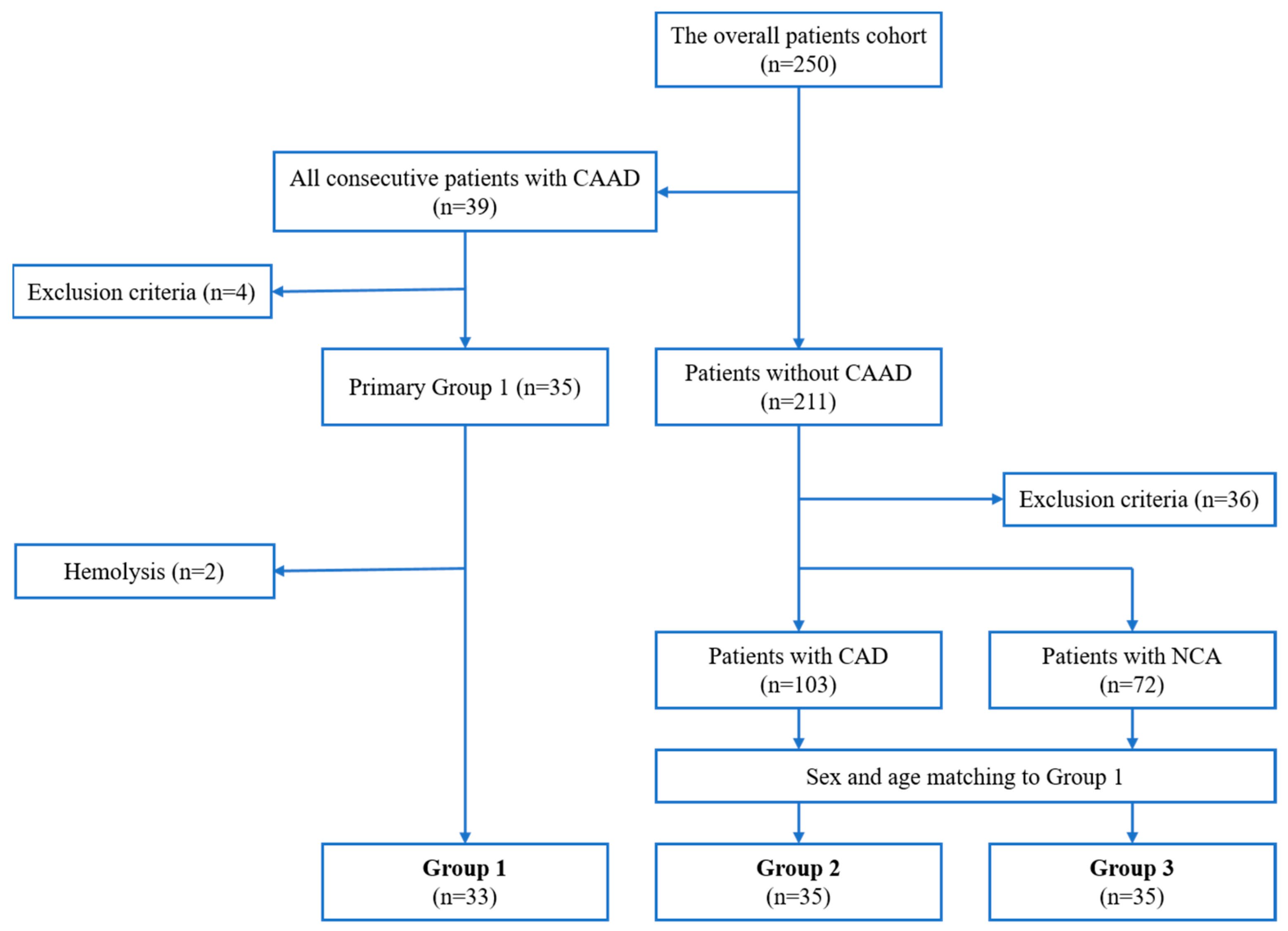
4.1. Study Design and Patient Selection
4.2. Blood Samples Collection and Evaluation of miRNA Profiles
4.3. Statistical Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Appendix A
References
- Fichtlscherer, S.; Zeiher, A.M.; Dimmeler, S. Circulating MicroRNAs: Biomarkers or Mediators of Cardiovascular Diseases? Arterioscler. Thromb. Vasc. Biol. 2011, 31, 2383–2390. [Google Scholar] [CrossRef] [PubMed]
- Huntzinger, E.; Izaurralde, E. Gene Silencing by MicroRNAs: Contributions of Translational Repression and MRNA Decay. Nat. Rev. Genet. 2011, 12, 99–110. [Google Scholar] [CrossRef]
- Rocchi, A.; Chiti, E.; Maiese, A.; Turillazzi, E.; Spinetti, I. MicroRNAs: An Update of Applications in Forensic Science. Diagnostics 2020, 11, 32. [Google Scholar] [CrossRef]
- Swaye, P.S.; Fisher, L.D.; Litwin, P.; Vignola, P.A.; Judkins, M.P.; Kemp, H.G.; Mudd, J.G.; Gosselin, A.J. Aneurysmal Coronary Artery Disease. Circulation 1983, 67, 134–138. [Google Scholar] [CrossRef]
- Baman, T.S.; Cole, J.H.; Devireddy, C.M.; Sperling, L.S. Risk Factors and Outcomes in Patients with Coronary Artery Aneurysms. Am. J. Cardiol. 2004, 93, 1549–1551. [Google Scholar] [CrossRef] [PubMed]
- Warisawa, T.; Naganuma, T.; Tomizawa, N.; Fujino, Y.; Ishiguro, H.; Tahara, S.; Kurita, N.; Nojo, T.; Nakamura, S.; Nakamura, S. High Prevalence of Coronary Artery Events and Non-Coronary Events in Patients with Coronary Artery Aneurysm in the Observational Group. Int. J. Cardiol. Heart Vasc. 2016, 10, 29–31. [Google Scholar] [CrossRef] [PubMed]
- Markis, J.E.; Joffe, C.D.; Cohn, P.F.; Feen, D.J.; Herman, M.V.; Gorlin, R. Clinical Significance of Coronary Arterial Ectasia. Am. J. Cardiol. 1976, 37, 217–222. [Google Scholar] [CrossRef]
- Kühl, M.; Varma, C. A Case of Acute Coronary Thrombosis in Diffuse Coronary Artery Ectasia. J. Invasive Cardiol. 2008, 20, E23–E25. [Google Scholar]
- Doi, T.; Kataoka, Y.; Noguchi, T.; Shibata, T.; Nakashima, T.; Kawakami, S.; Nakao, K.; Fujino, M.; Nagai, T.; Kanaya, T.; et al. Coronary Artery Ectasia Predicts Future Cardiac Events in Patients With Acute Myocardial Infarction. Arterioscler. Thromb. Vasc. Biol. 2017, 37, 2350–2355. [Google Scholar] [CrossRef]
- Janus, M.; Iwańczyk, S.; Stanisławska, K.; Mularek-Kubzdela, T.; Lesiak, M. Endovascular Closure of Coronary Artery to Pulmonary Artery Fistula with Giant Aneurysm. Kardiol. Pol. 2022, 80, 621–622. [Google Scholar] [CrossRef]
- Iwańczyk, S.; Borger, M.; Kamiński, M.; Chmara, E.; Cieślewicz, A.; Tykarski, A.; Radziemski, A.; Krasiński, Z.; Lesiak, M.; Araszkiewicz, A. Inflammatory response in patients with coronary artery ectasia and coronary artery disease. Kardiol. Pol. 2019, 77, 713–715. [Google Scholar] [CrossRef] [PubMed]
- Iwańczyk, S.; Lehmann, T.; Cieślewicz, A.; Radziemski, A.; Malesza, K.; Wrotyński, M.; Jagodziński, P.P.; Grygier, M.; Lesiak, M.; Araszkiewicz, A. Involvement of Angiogenesis in the Pathogenesis of Coronary Aneurysms. Biomedicines 2021, 9, 1269. [Google Scholar] [CrossRef] [PubMed]
- Wojciechowska, A.; Braniewska, A.; Kozar-Kamińska, K. MicroRNA in Cardiovascular Biology and Disease. Adv. Clin. Exp. Med. 2017, 26, 865–874. [Google Scholar] [CrossRef]
- Iwańczyk, S.; Lehmann, T.; Cieślewicz, A.; Radziemski, A.; Malesza, K.; Wrotyński, M.; Jagodziński, P.P.; Grygier, M.; Lesiak, M.; Araszkiewicz, A. Circulating MicroRNAs in Patients with Aneurysmal Dilatation of Coronary Arteries. Exp. Ther. Med. 2022, 23, 404. [Google Scholar] [CrossRef]
- Wang, L.; Liu, J.; Xu, B.; Liu, Y.-L.; Liu, Z. Reduced Exosome MiR-425 and MiR-744 in the Plasma Represents the Progression of Fibrosis and Heart Failure. Kaohsiung J. Med. Sci. 2018, 34, 626–633. [Google Scholar] [CrossRef] [PubMed]
- Subramanian, A.; Tamayo, P.; Mootha, V.K.; Mukherjee, S.; Ebert, B.L.; Gillette, M.A.; Paulovich, A.; Pomeroy, S.L.; Golub, T.R.; Lander, E.S.; et al. Gene Set Enrichment Analysis: A Knowledge-Based Approach for Interpreting Genome-Wide Expression Profiles. Proc. Natl. Acad. Sci. USA 2005, 102, 15545–15550. [Google Scholar] [CrossRef]
- Zaha, V.G.; Young, L.H. AMP-Activated Protein Kinase Regulation and Biological Actions in the Heart. Circ. Res. 2012, 111, 800–814. [Google Scholar] [CrossRef] [PubMed]
- Lei, L.; Zhou, Y.; Wang, T.; Zheng, Z.; Chen, L.; Pan, Y. Activation of AMP-Activated Protein Kinase Ablated the Formation of Aortic Dissection by Suppressing Vascular Inflammation and Phenotypic Switching of Vascular Smooth Muscle Cells. Int. Immunopharmacol. 2022, 112, 109177. [Google Scholar] [CrossRef]
- Zhao, F.; Satyanarayana, G.; Zhang, Z.; Zhao, J.; Ma, X.-L.; Wang, Y. Endothelial Autophagy in Coronary Microvascular Dysfunction and Cardiovascular Disease. Cells 2022, 11, 2081. [Google Scholar] [CrossRef] [PubMed]
- Ni, D.; Mo, Z.; Yi, G. Recent Insights into Atherosclerotic Plaque Cell Autophagy. Exp. Biol. Med. 2021, 246, 2553–2558. [Google Scholar] [CrossRef] [PubMed]
- Henderson, J.M.; Weber, C.; Santovito, D. Beyond Self-Recycling: Cell-Specific Role of Autophagy in Atherosclerosis. Cells 2021, 10, 625. [Google Scholar] [CrossRef]
- Naglova, H.; Bucova, M. HMGB1 and Its Physiological and Pathological Roles. Bratisl. Lekárske Listy 2012, 113, 163–171. [Google Scholar] [CrossRef] [PubMed]
- Vavassori, C.; Cipriani, E.; Colombo, G.I. Circulating MicroRNAs as Novel Biomarkers in Risk Assessment and Prognosis of Coronary Artery Disease. Eur. Cardiol. Rev. 2022, 17, e06. [Google Scholar] [CrossRef]
- Samra, M.; Srivastava, K. Non-Coding RNA and Their Potential Role in Cardiovascular Diseases. Gene 2023, 851, 147011. [Google Scholar] [CrossRef] [PubMed]
- Kabłak-Ziembicka, A.; Badacz, R.; Przewłocki, T. Clinical Application of Serum MicroRNAs in Atherosclerotic Coronary Artery Disease. J. Clin. Med. 2022, 11, 6849. [Google Scholar] [CrossRef]
- Bergami, M.; Fabin, N.; Cenko, E.; Bugiardini, R.; Manfrini, O. MicroRNAs as Potential Biomarkers in Coronary Artery Disease. Curr. Top. Med. Chem. 2023, 23. [Google Scholar] [CrossRef]
- Shen, N.-N.; Wang, J.-L.; Fu, Y.-P. The MicroRNA Expression Profiling in Heart Failure: A Systematic Review and Meta-Analysis. Front. Cardiovasc. Med. 2022, 9, 856358. [Google Scholar] [CrossRef]
- Sigutova, R.; Evin, L.; Stejskal, D.; Ploticova, V.; Svagera, Z. Specific MicroRNAs and Heart Failure: Time for the next Step toward Application? Biomed. Pap. Med. Fac. Univ. Palacky Olomouc Czechoslov. 2022, 166, 359–368. [Google Scholar] [CrossRef]
- Wang, Y.-F.; Lian, X.-L.; Zhong, J.-Y.; Su, S.-X.; Xu, Y.-F.; Xie, X.-F.; Wang, Z.-P.; Li, W.; Zhang, L.; Che, D.; et al. Serum Exosomal MicroRNA Let-7i-3p as Candidate Diagnostic Biomarker for Kawasaki Disease Patients with Coronary Artery Aneurysm. IUBMB Life 2019, 71, 891–900. [Google Scholar] [CrossRef]
- Xu, L.; Tian, L.; Yan, Z.; Wang, J.; Xue, T.; Sun, Q. Diagnostic and Prognostic Value of MiR-486-5p, MiR-451a, MiR-21-5p and Monocyte to High-Density Lipoprotein Cholesterol Ratio in Patients with Acute Myocardial Infarction. Heart Vessel. 2023, 38, 318–331. [Google Scholar] [CrossRef] [PubMed]
- Deng, H.-Y.; He, Z.-Y.; Dong, Z.-C.; Zhang, Y.-L.; Han, X.; Li, H.-H. MicroRNA-451a Attenuates Angiotensin II-Induced Cardiac Fibrosis and Inflammation by Directly Targeting T-Box1. J. Physiol. Biochem. 2022, 78, 257–269. [Google Scholar] [CrossRef]
- Scrimgeour, N.R.; Wrobel, A.; Pinho, M.J.; Høydal, M.A. MicroRNA-451a Prevents Activation of Matrix Metalloproteinases 2 and 9 in Human Cardiomyocytes during Pathological Stress Stimulation. Am. J. Physiol. Cell Physiol. 2020, 318, C94–C102. [Google Scholar] [CrossRef] [PubMed]
- He, F.; Lv, P.; Zhao, X.; Wang, X.; Ma, X.; Meng, W.; Meng, X.; Dong, S. Predictive Value of Circulating MiR-328 and MiR-134 for Acute Myocardial Infarction. Mol. Cell. Biochem. 2014, 394, 137–144. [Google Scholar] [CrossRef] [PubMed]
- Zhao, D.; Li, C.; Yan, H.; Li, T.; Qian, M.; Zheng, N.; Jiang, H.; Liu, L.; Xu, B.; Wu, Q.; et al. Cardiomyocyte Derived MiR-328 Promotes Cardiac Fibrosis by Paracrinely Regulating Adjacent Fibroblasts. Cell. Physiol. Biochem. 2018, 46, 1555–1565. [Google Scholar] [CrossRef]
- Li, C.; Li, X.; Gao, X.; Zhang, R.; Zhang, Y.; Liang, H.; Xu, C.; Du, W.; Zhang, Y.; Liu, X.; et al. MicroRNA-328 as a Regulator of Cardiac Hypertrophy. Int. J. Cardiol. 2014, 173, 268–276. [Google Scholar] [CrossRef] [PubMed]
- Lu, Y.; Zhang, Y.; Wang, N.; Pan, Z.; Gao, X.; Zhang, F.; Zhang, Y.; Shan, H.; Luo, X.; Bai, Y.; et al. MicroRNA-328 Contributes to Adverse Electrical Remodeling in Atrial Fibrillation. Circulation 2010, 122, 2378–2387. [Google Scholar] [CrossRef] [PubMed]
- Du, W.; Liang, H.; Gao, X.; Li, X.; Zhang, Y.; Pan, Z.; Li, C.; Wang, Y.; Liu, Y.; Yuan, W.; et al. MicroRNA-328, a Potential Anti-Fibrotic Target in Cardiac Interstitial Fibrosis. Cell. Physiol. Biochem. 2016, 39, 827–836. [Google Scholar] [CrossRef]
- Ghafouri-Fard, S.; Gholipour, M.; Taheri, M. Role of MicroRNAs in the Pathogenesis of Coronary Artery Disease. Front. Cardiovasc. Med. 2021, 8, 632392. [Google Scholar] [CrossRef]
- Wang, S.; He, W.; Wang, C. MiR-23a Regulates the Vasculogenesis of Coronary Artery Disease by Targeting Epidermal Growth Factor Receptor. Cardiovasc. Ther. 2016, 34, 199–208. [Google Scholar] [CrossRef]
- Guan, Y.; Song, X.; Sun, W.; Wang, Y.; Liu, B. Effect of Hypoxia-Induced MicroRNA-210 Expression on Cardiovascular Disease and the Underlying Mechanism. Oxidative Med. Cell. Longev. 2019, 2019, 4727283. [Google Scholar] [CrossRef]
- Karakas, M.; Schulte, C.; Appelbaum, S.; Ojeda, F.; Lackner, K.J.; Münzel, T.; Schnabel, R.B.; Blankenberg, S.; Zeller, T. Circulating MicroRNAs Strongly Predict Cardiovascular Death in Patients with Coronary Artery Disease-Results from the Large AtheroGene Study. Eur. Heart J. 2017, 38, 516–523. [Google Scholar] [CrossRef] [PubMed]
- Li, T.; Cao, H.; Zhuang, J.; Wan, J.; Guan, M.; Yu, B.; Li, X.; Zhang, W. Identification of MiR-130a, MiR-27b and MiR-210 as Serum Biomarkers for Atherosclerosis Obliterans. Clin. Chim. Acta 2011, 412, 66–70. [Google Scholar] [CrossRef] [PubMed]
- Landgraf, P.; Rusu, M.; Sheridan, R.; Sewer, A.; Iovino, N.; Aravin, A.; Pfeffer, S.; Rice, A.; Kamphorst, A.O.; Landthaler, M.; et al. A Mammalian MicroRNA Expression Atlas Based on Small RNA Library Sequencing. Cell 2007, 129, 1401–1414. [Google Scholar] [CrossRef]
- Wei, F.; Ren, W.; Zhang, X.; Wu, P.; Fan, J. MiR-425-5p Is Negatively Associated with Atrial Fibrosis and Promotes Atrial Remodeling by Targeting CREB1 in Atrial Fibrillation. J. Cardiol. 2022, 79, 202–210. [Google Scholar] [CrossRef] [PubMed]
- Neumann, F.J.; Sousa-Uva, M.; Ahlsson, A.; Alfonso, F.; Banning, A.P.; Benedetto, U.; Byrne, R.A.; Collet, J.P.; Falk, V.; Head, S.J.; et al. 2018 ESC/EACTS Guidelines on myocardial revascularization. Eur. Heart J. 2019, 40, 87–165. [Google Scholar] [CrossRef]
| Variable | Group 1 (n = 33) | Group 2 (n = 35) | Group 3 (n = 35) | p-Value | ||
|---|---|---|---|---|---|---|
| Group 1 vs. Group 2 | Group 1 vs. Group 3 | Group 2 vs. Group 3 | ||||
| Sex, male | 28 (84.8) | 28 (80.0) | 27 (77.1) | 0.41 | 0.69 | 0.18 |
| Age, years | 65.9 ± 9.4 | 67.1 ± 8.3 | 64.6 ± 12.3 | 0.88 | 0.85 | 0.55 |
| BMI, kg/m2 | 29.7 (26.5–32.9) | 28.9 (25.3–31.9) | 30.1 (27.2–33.7) | 0.72 | 0.72 | 0.63 |
| Hypertension | 29 (87.9) | 30 (85.7) | 31 (88.6) | 0.81 | 0.90 | 0.72 |
| Hyperlipidemia | 20 (60.6) | 29 (82.9) | 27 (77.1) | 0.09 | 0.26 | 0.55 |
| Diabetes mellitus | 10 (30.3) | 10 (28.6) | 11 (31.4) | 0.97 | 0.77 | 0.79 |
| HF, NYHA ≥ 2 | 6 (18.2) | 9 (25.7) | 4 (11.4) | 0.12 | 0.40 | 0.12 |
| LVEF, % | 57.5 (55–60) | 60 (50–60) | 60 (55–60) | 0.87 | 0.87 | 0.87 |
| Stroke | 4 (12.1) | 3 (8.6) | 1 (2.9) | 0.90 | 0.30 | 0.60 |
| Smoking | 11 (33.3) | 11 (31.4) | 8 (22.8) | 0.80 | 0.30 | 0.42 |
| CKD, eGFR ≤ 60 mL/min | 2 (6.1) | 7 (20) | 9 (25.7) | 0.45 | 0.88 | 0.20 |
| Previous MI | 10 (33.3) | 13 (37.1) | 0 | 0.61 | 0.01 | <0.01 |
| Previous PCI | 10 (33.3) | 20 (57.1) | 0 | 0.03 | <0.01 | <0.01 |
| Previous CABG | 1 (3.0) | 3 (8.6) | 0 | 0.67 | 0.46 | 0.24 |
| Drugs administration | ||||||
| Aspirin | 25 (75.8) | 26 (74.3) | 22 (62.9) | 0.60 | 0.01 | 0.05 |
| P2Y12 inhibitor | 14 (42.4) | 20 (57.1) | 1 (2.9) | 0.24 | <0.01 | <0.01 |
| Beta-blocker | 23 (69.7) | 25 (71.4) | 22 (62.9) | 0.84 | 0.15 | 0.04 |
| Statin | 26 (78.8) | 28 (80.0) | 30 (85.7) | 0.95 | 0.12 | 0.02 |
| ACEI/ARB | 25 (75.8) | 26 (74.3) | 31 (88.6) | 0.78 | 0.82 | 1.00 |
| OHA | 7 (21.2) | 6 (17.1) | 12 (34.3) | 0.96 | 0.33 | 0.29 |
| Insulin | 2 (6.1) | 1 (2.9) | 3 (8.6) | 0.90 | 0.74 | 1.00 |
| Laboratory test | ||||||
| WBC, 109/L | 7.2 (5.9–9.1) | 7.5 (6.1–9.6) | 6.5 (5.5–7.7) | 0.58 | 0.15 | 0.05 |
| HGB, mmol/L | 9.2 (8.3–9.9) | 9 (8.1–9.5) | 9 (8.4–9.8) | 0.45 | 0.85 | 0.45 |
| PLT, 109/L | 223.5 (188.5–257) | 204 (180–239) | 201 (176.5–249.5) | 0.91 | 0.91 | 0.91 |
| LDL-C, mmol/L | 2.1 (1.6–2.6) | 1.9 (1.6–3.2) | 2.8 (2.0–3.3) | 0.78 | 0.33 | 0.44 |
| Creatinine, mmol/L | 85.7 (77.6–99.5) | 93 (80–99.5) | 80.9 (69.4–86.0) | 0.45 | 0.04 | <0.01 |
| Glucose, mmol/L | 5.6 (5.1–6.3) | 5.8 (5.2–6.6) | 5.8 (5.3–6.4) | 0.86 | 0.86 | 0.86 |
| Symbol | Up-Down Regulation | |||||
|---|---|---|---|---|---|---|
| Group 2 Compared to Group 1 | Group 3 Compared to Group 1 | Group 3 Compared to Group 2 | ||||
| Fold Regulation | p-Value | Fold Regulation | p-Value | Fold Regulation | p-Value | |
| hsa-miR-451a | −2.18 | 0.03 | −2.64 | 0.01 | −1.51 | 0.39 |
| hsa-miR-23a-3p | 1.54 | 0.01 | 2.11 | <0.01 | 1.10 | 0.59 |
| hsa-miR-125b-5p | −1.51 | 0.28 | −1.78 | 0.07 | −1.51 | 0.14 |
| hsa-miR-210-3p | −2.07 | 0.22 | −4.71 | <0.01 | −1.50 | 0.03 |
| hsa-miR-328-3p | −1.08 | 0.53 | −2.25 | 0.03 | −1.88 | 0.03 |
| hsa-miR-425-3p | −1.96 | 0.17 | −3.30 | 0.01 | −1.63 | 0.14 |
| hsa-miR-483-5p | −1.28 | 0.45 | −1.66 | 0.30 | −1.47 | 0.02 |
| hsa-miR-16-5p | −1.34 | 0.07 | −1.23 | 0.15 | −1.15 | 0.49 |
| hsa-miR-30d-5p | −1.18 | 0.96 | −1.49 | 0.15 | −1.59 | 0.18 |
| hsa-miR-320d | −1.38 | 0.27 | −1.46 | 0.04 | −1.38 | 0.10 |
| Variable | Univariate Analysis | Multivariate Analysis | ||
|---|---|---|---|---|
| OR [95% CI] | p-Value | OR [95% CI] | p-Value | |
| Previous PCI | 0.3 [0.1; 0.9] | 0.03 | 0.2 [0.1; 0.7] | 0.01 |
| Dyslipidemia | 0.2 [0.1; 1.1] | 0.06 | 0.2 [0.1; 0.8] | 0.02 |
| hsa-miR-451a | 0.7 [0.5; 0.9] | 0.03 | 0.7 [0.5; 0.99] | 0.049 |
| Variable | Univariate Analysis | |
|---|---|---|
| OR [95% CI] | p-Value | |
| Previous MI | 7.5 [1.5; 37.6] | 0.01 |
| P2Y12 inhibitor | 36.3 [4.4; 229.0] | <0.01 |
| Aspirin | 5.5 [1.4; 21.8] | 0.01 |
| WBC, 109/L | 1.2 [0.9; 1.6] | 0.06 |
| RBC, 109/L | 3.2 [1.1; 10.0] | 0.04 |
| Creatinine, mmol/L | 1.02 [1.0; 1.1] | 0.03 |
| hsa-miR-328-3p | 0.7 [0.5; 0.9] | <0.01 |
| hsa-miR-451a | 0.6 [0.4; 0.9] | <0.01 |
| hsa-miR-23a-3p | 2.6 [1.5; 4.7] | <0.01 |
| hsa-miR-210-3p | 0.7 [0.5; 0.8] | <0.01 |
| hsa-miR-425-3p | 0.8 [0.6; 0.9] | 0.01 |
| Variable | Multivariate Analysis | |
|---|---|---|
| OR [95% CI] | p-Value | |
| Previous MI | 7.0 [0.9; 51.7] | 0.06 |
| P2Y12 inhibitor | 80.3 [5.8; 1108.4] | <0.01 |
| Aspirin | 3.7 [0.4; 29.8] | 0.22 |
| WBC, 109/L | 1.3 [0.9; 1.9] | 0.08 |
| RBC, 109/L | 4.5 [0.8; 24.6] | 0.11 |
| Creatinine, mmol/L | 1.0 [0.97; 1.1] | 0.54 |
| hsa-miR-328-3p | 0.2 [0.1; 0.7] | <0.01 |
| Pathways | Genes | p-Value, Adjusted | p-Value (BH) |
|---|---|---|---|
| Non-alcoholic fatty liver disease (NAFLD) | IL6R NDUFV3 PIK3R1 SDHD UQCRFS RAC1 | <0.01 | 0.01 |
| AMPK signaling pathway | TSC1, PIK3R1 PPARGC1A FOXO3 SCD | <0.01 | 0.01 |
| Autophagy | TSC1 ATG7 PIK3R1 HMGB1 VMP1 | <0.01 | <0.01 |
| Regulation of actin cytoskeleton | VAV3 EZR PIK3R1 MYH10 RAC1 | 0.02 | 0.06 |
| Thermogenesis | TSC1 NDUFV3 SDHD PPARGC1A UQCRFS1 | 0.03 | 0.06 |
| Huntington disease | NDUFV SDHD PPARGC1A ACTR1A UQCRFS1 | 0.05 | 0.08 |
| PI3K-Akt signaling pathway | TSC1 IL6R PIK3R1 FOXO3 RAC1 | 0.12 | 0.18 |
| Pathways in cancer | MITF IL6R TPM3 RUNX1T1 PIK3R1 RAC1 | 0.20 | 0.25 |
| Herpes simplex virus 1 infection | TSC1 ZNF275 ZNF268 ZNF701 PIK3R1 | 0.29 | 0.32 |
| Metabolic pathways | NDUFV3 HMGCS1 GLS SDHD STT3A MGAT5 FUT4 SCD UQCRFS1 STT3B | 0.62 | 0.62 |
| 1 | 2 | |
|---|---|---|
| A | UniSp2 | hsa-miR-125b-5p |
| B | UniSp4 | hsa-miR-210-3p |
| C | UniSp5 | hsa-miR-328-3p |
| D | UniSp6 | hsa-miR-425-3p |
| E | cel-miR-39-3p | hsa-miR-483-5p |
| F | UniSp3 | hsa-miR-16-5p |
| G | hsa-miR-451a | hsa-miR-30d-5p |
| H | hsa-miR-23a-3p | hsa-miR-320d |
| Position | Mature ID | Meaning of the Gene |
|---|---|---|
| 1 | UniSp2 | Control |
| 2 | UniSp4 | Control |
| 3 | UniSp5 | Control |
| 4 | UniSp6 | Control |
| 5 | cel-miR-39-3p | Control |
| 6 | UniSp3 | Control |
| 7 | hsa-miR-451a | Hemolysis marker |
| 8 | hsa-miR-23a-3p | Hemolysis marker |
| 9 | hsa-miR-125b-5p | Marker |
| 10 | hsa-miR-210-3p | Marker |
| 11 | hsa-miR-328-3p | Marker |
| 12 | hsa-miR-425-3p | Marker |
| 13 | hsa-miR-483-5p | Marker |
| 14 | hsa-miR-16-5p | Reference marker |
| 15 | hsa-miR-30d-5p | Reference marker |
| 16 | hsa-miR-320d | Reference marker |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Iwańczyk, S.; Lehmann, T.; Cieślewicz, A.; Malesza, K.; Woźniak, P.; Hertel, A.; Krupka, G.; Jagodziński, P.P.; Grygier, M.; Lesiak, M.; et al. Circulating miRNA-451a and miRNA-328-3p as Potential Markers of Coronary Artery Aneurysmal Disease. Int. J. Mol. Sci. 2023, 24, 5817. https://doi.org/10.3390/ijms24065817
Iwańczyk S, Lehmann T, Cieślewicz A, Malesza K, Woźniak P, Hertel A, Krupka G, Jagodziński PP, Grygier M, Lesiak M, et al. Circulating miRNA-451a and miRNA-328-3p as Potential Markers of Coronary Artery Aneurysmal Disease. International Journal of Molecular Sciences. 2023; 24(6):5817. https://doi.org/10.3390/ijms24065817
Chicago/Turabian StyleIwańczyk, Sylwia, Tomasz Lehmann, Artur Cieślewicz, Katarzyna Malesza, Patrycja Woźniak, Agnieszka Hertel, Grzegorz Krupka, Paweł P. Jagodziński, Marek Grygier, Maciej Lesiak, and et al. 2023. "Circulating miRNA-451a and miRNA-328-3p as Potential Markers of Coronary Artery Aneurysmal Disease" International Journal of Molecular Sciences 24, no. 6: 5817. https://doi.org/10.3390/ijms24065817
APA StyleIwańczyk, S., Lehmann, T., Cieślewicz, A., Malesza, K., Woźniak, P., Hertel, A., Krupka, G., Jagodziński, P. P., Grygier, M., Lesiak, M., & Araszkiewicz, A. (2023). Circulating miRNA-451a and miRNA-328-3p as Potential Markers of Coronary Artery Aneurysmal Disease. International Journal of Molecular Sciences, 24(6), 5817. https://doi.org/10.3390/ijms24065817








