Myopia Is Suppressed by Digested Lactoferrin or Holo-Lactoferrin Administration
Abstract
1. Introduction
2. Results
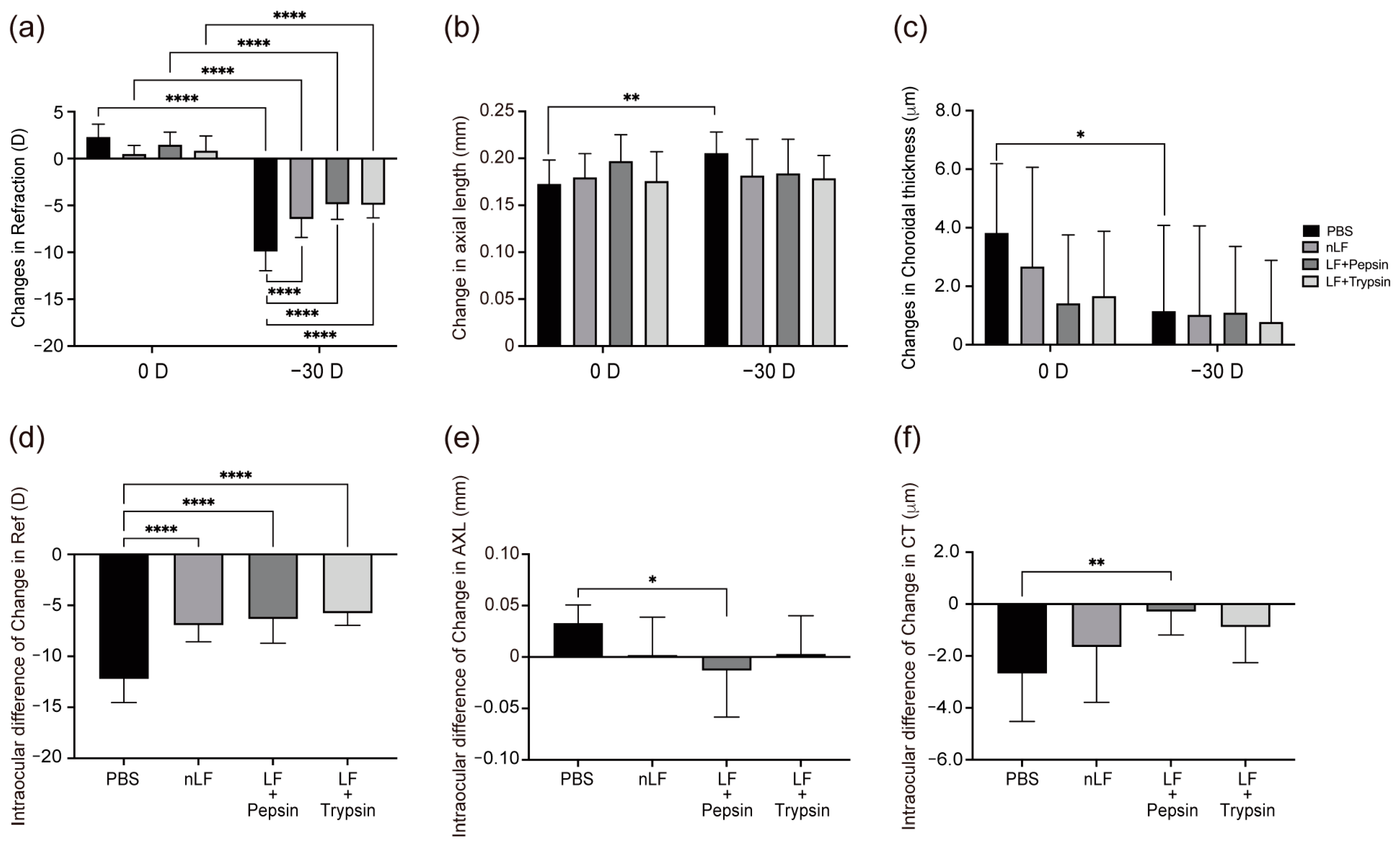
2.1. Changes in Refraction, AL, and CT of Digested-LF-Administered LIM Mice
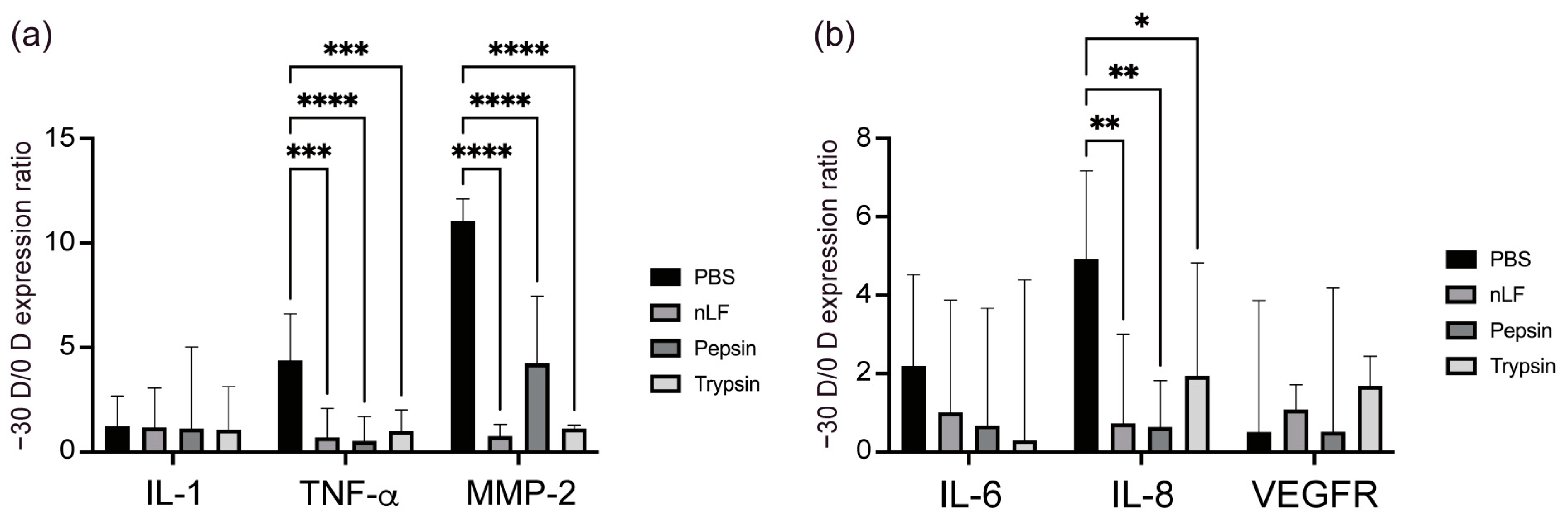
2.2. Changes in the Inflammatory Status of the Choroid and Sclera owing to Oral Administration of LF and Its Derivatives
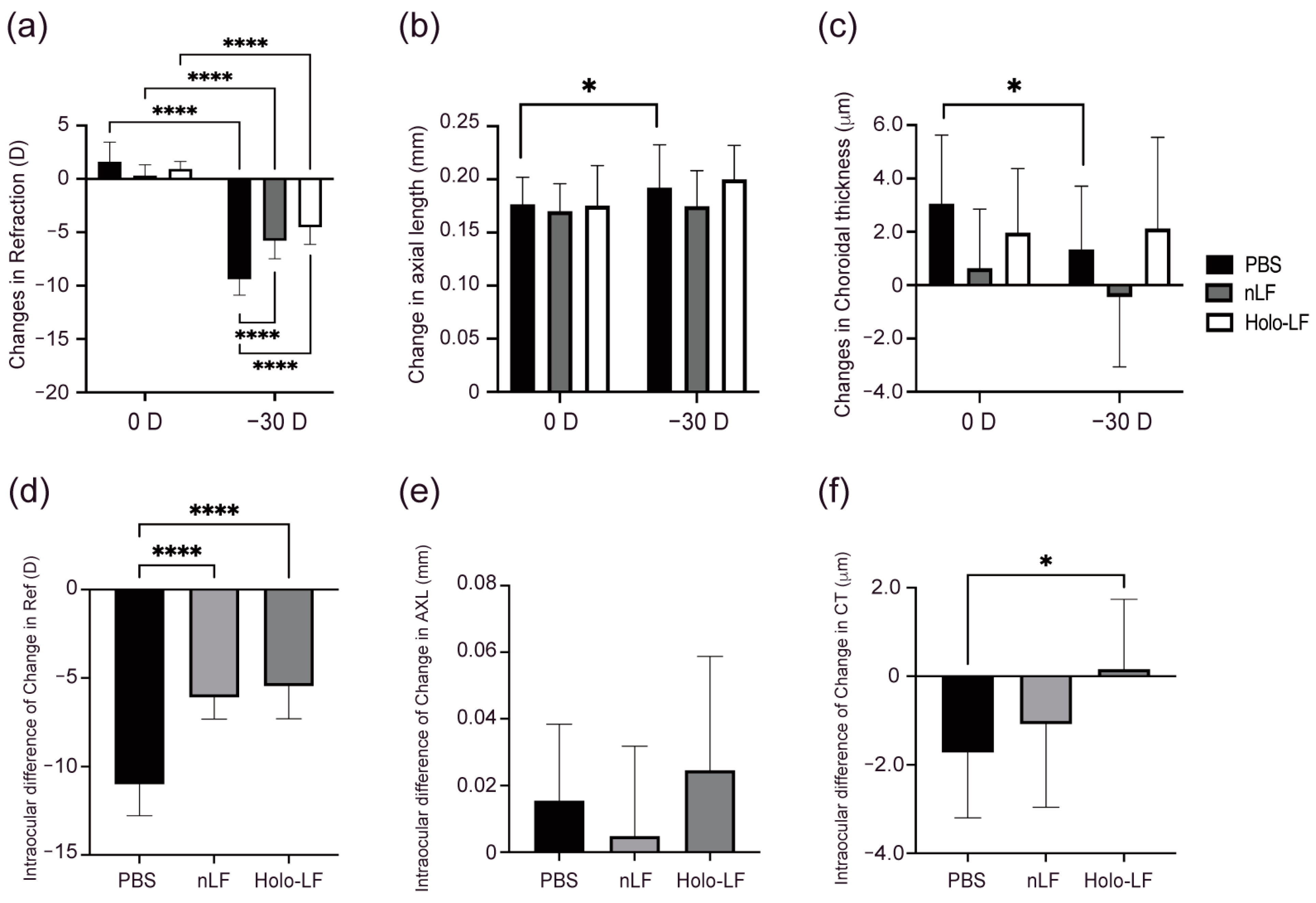
2.3. Changes in Refraction, AL, and CT of Holo-LF-Administered LIM Mice
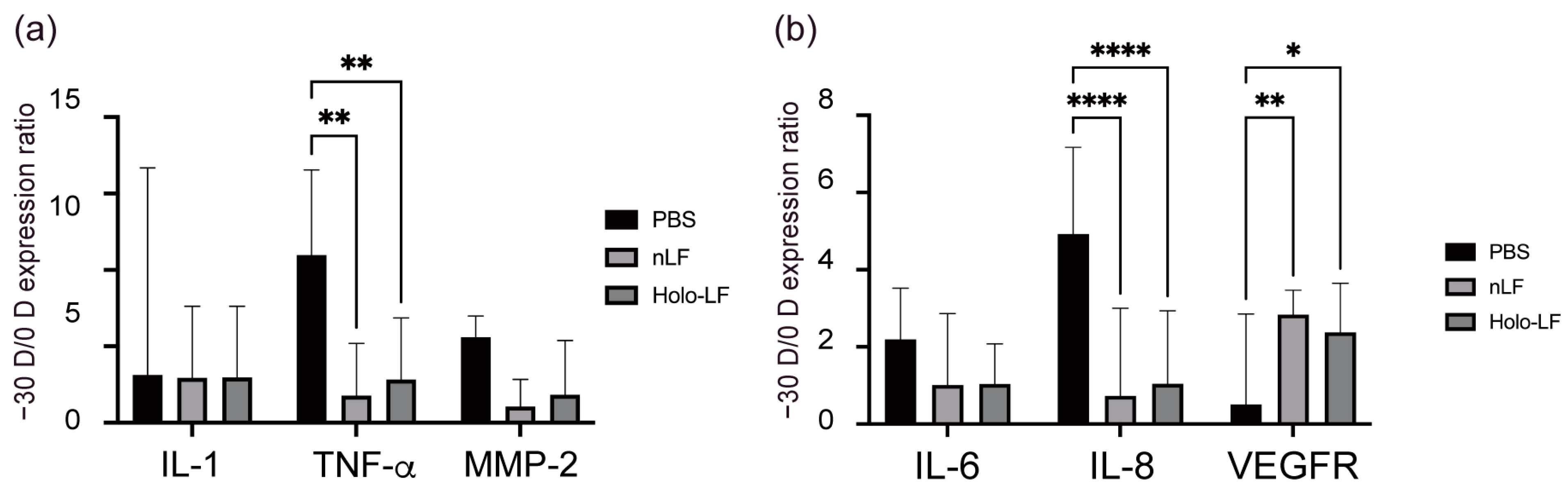
2.4. Changes in the Inflammatory Status of the Choroid and Sclera following Oral Administration of LF and Holo-LF
3. Discussion
4. Materials and Methods
4.1. Preparation of Native-LF, LF Hydrolysates, and Holo-LF
4.2. Animals
4.2.1. LF Administration
4.2.2. LIM in Mice
4.2.3. Refraction, Axial Length (AL), and Choroid Thickness (CT) Measurements
4.3. RNA Expression Test
4.3.1. Sampling
4.3.2. RNA Extraction
4.3.3. Reverse Transcription-Polymerase Chain Reaction (RT-PCR)
4.4. Statistical Analyses
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
References
- Dolgin, E. The myopia boom. Nature 2015, 519, 276–278. [Google Scholar] [CrossRef] [PubMed]
- Holden, B.A.; Fricke, T.R.; Wilson, D.A.; Jong, M.; Naidoo, K.S.; Sankaridurg, P.; Wong, T.Y.; Naduvilath, T.J.; Resnikoff, S. Global Prevalence of Myopia and High Myopia and Temporal Trends from 2000 through 2050. Ophthalmology 2016, 123, 1036–1042. [Google Scholar] [CrossRef] [PubMed]
- Tideman, J.W.L.; Snabel, M.C.C.; Tedja, M.S.; van Rijn, G.A.; Wong, K.T.; Kuijpers, R.W.A.M.; Vingerling, J.R.; Hofman, A.; Buitendijk, G.H.S.; Keunen, J.E.E.; et al. Association of Axial Length With Risk of Uncorrectable Visual Impairment for Europeans With Myopia. JAMA Ophthalmol. 2016, 134, 1355–1363. [Google Scholar] [CrossRef] [PubMed]
- Flitcroft, D.I.; He, M.; Jonas, J.B.; Jong, M.; Naidoo, K.; Ohno-Matsui, K.; Rahi, J.; Resnikoff, S.; Vitale, S.; Yannuzzi, L. IMI—Defining and Classifying Myopia: A Proposed Set of Standards for Clinical and Epidemiologic Studies. Investig. Ophthalmol. Vis. Sci. 2019, 60, M20–M30. [Google Scholar] [CrossRef]
- Yotsukura, E.; Torii, H.; Ozawa, H.; Hida, R.Y.; Shiraishi, T.; Corso Teixeira, I.; Rautha YV, B.L.; Moraes Do Nascimento, C.F.; Mori, K.; Uchino, M.; et al. Axial Length and Prevalence of Myopia among Schoolchildren in the Equatorial Region of Brazil. J. Clin. Med. 2020, 10, 115. [Google Scholar] [CrossRef]
- Yotsukura, E.; Torii, H.; Inokuchi, M.; Tokumura, M.; Uchino, M.; Nakamura, K.; Hyodo, M.; Mori, K.; Jiang, X.; Ikeda, S.-I.; et al. Current Prevalence of Myopia and Association of Myopia With Environmental Factors Among Schoolchildren in Japan. JAMA Ophthalmol. 2019, 137, 1233. [Google Scholar] [CrossRef]
- Meng, W.; Butterworth, J.; Malecaze, F.; Calvas, P. Axial Length of Myopia: A Review of Current Research. Ophthalmologica 2011, 225, 127–134. [Google Scholar] [CrossRef]
- Chen, M.J.; Liu, Y.T.; Tsai, C.C.; Chen, Y.C.; Chou, C.K.; Lee, S.M. Relationship between central corneal thickness, refractive error, corneal curvature, anterior chamber depth and axial length. J. Chin. Med. Assoc. 2009, 72, 133–137. [Google Scholar] [CrossRef]
- Mallen, E.A.; Gammoh, Y.; Al-Bdour, M.; Sayegh, F.N. Refractive error and ocular biometry in Jordanian adults. Ophthalmic. Physiol. Opt. 2005, 25, 302–309. [Google Scholar] [CrossRef] [PubMed]
- Baird, P.N.; Saw, S.M.; Lanca, C.; Guggenheim, J.A.; Smith Iii, E.L.; Zhou, X.; Matsui, K.O.; Wu, P.C.; Sankaridurg, P.; Chia, A.; et al. Myopia. Nat. Rev. Dis. Primers 2020, 6, 99. [Google Scholar] [CrossRef] [PubMed]
- Haarman, A.E.G.; Enthoven, C.A.; Tideman, J.W.L.; Tedja, M.S.; Verhoeven, V.J.M.; Klaver, C.C.W. The Complications of Myopia: A Review and Meta-Analysis. Investig. Ophthalmol. Vis. Sci. 2020, 61, 49. [Google Scholar] [CrossRef] [PubMed]
- Zieliński, G.; Wójcicki, M.; Rapa, M.; Matysik-Woźniak, A.; Baszczowski, M.; Ginszt, M.; Litko-Rola, M.; Szkutnik, J.; Różyło-Kalinowska, I.; Rejdak, R.; et al. Masticatory Muscle Thickness and Activity Correlates to Eyeball Length, Intraocular Pressure, Retinal and Choroidal Thickness in Healthy Women versus Women with Myopia. J. Pers. Med. 2022, 12, 626. [Google Scholar] [CrossRef] [PubMed]
- Zieliński, G.; Baszczowski, M.; Rapa, M.; Matysik-Woźniak, A.; Zawadka, M.; Szkutnik, J.; Gawda, P.; Rejdak, R.; Majcher, P.; Ginszt, M. The Axial Length of the Eyeball and Bioelectrical Activity of Masticatory and Neck Muscles: A Preliminary Report. Pain Res. Manag. 2022, 2022, 6115782. [Google Scholar] [CrossRef]
- Zheng, Y.-F.; Pan, C.-W.; Chay, J.; Wong, T.Y.; Finkelstein, E.; Saw, S.-M. The Economic Cost of Myopia in Adults Aged Over 40 Years in Singapore. Investig. Ophthalmol. Vis. Sci. 2013, 54, 7532–7537. [Google Scholar] [CrossRef] [PubMed]
- Backhouse, S.; Gentle, A. Scleral remodelling in myopia and its manipulation: A review of recent advances in scleral strengthening and myopia control. Ann. Eye Sci. 2018, 3, 5. [Google Scholar] [CrossRef]
- Herbort, C.P.; Papadia, M.; Neri, P. Myopia and inflammation. J. Ophthalmic. Vis. Res. 2011, 6, 270–283. [Google Scholar]
- Lin, H.J.; Wei, C.C.; Chang, C.Y.; Chen, T.H.; Hsu, Y.A.; Hsieh, Y.C.; Chen, H.J.; Wan, L. Role of Chronic Inflammation in Myopia Progression: Clinical Evidence and Experimental Validation. EBioMedicine 2016, 10, 269–281. [Google Scholar] [CrossRef] [PubMed]
- Baker, E.N. Lactoferrin. Cell. Mol. Life Sci. 2005, 62, 2529–2530. [Google Scholar] [CrossRef]
- Legrand, D. Lactoferrin, a key molecule in immune and inflammatory processes. Biochem. Cell Biol. 2012, 90, 252–268. [Google Scholar] [CrossRef]
- Masson, P.L.; Heremans, J.F.; Dive, C.H. An iron-binding protein common to many external secretions. Clin. Chim. Acta 1966, 14, 735–739. [Google Scholar] [CrossRef]
- Saito, S.; Takayama, Y.; Mizumachi, K.; Suzuki, C. Lactoferrin promotes hyaluronan synthesis in human dermal fibroblasts. Biotechnol. Lett. 2011, 33, 33–39. [Google Scholar] [CrossRef]
- Ono, T.; Morishita, S.; Fujisaki, C.; Ohdera, M.; Murakoshi, M.; Iida, N.; Kato, H.; Miyashita, K.; Iigo, M.; Yoshida, T.; et al. Effects of pepsin and trypsin on the anti-adipogenic action of lactoferrin against pre-adipocytes derived from rat mesenteric fat. Br. J. Nutr. 2011, 105, 200–211. [Google Scholar] [CrossRef]
- Gifford, J.L.; Hunter, H.N.; Vogel, H.J. Lactoferricin. Cell. Mol. Life Sci. 2005, 62, 2588. [Google Scholar] [CrossRef] [PubMed]
- Jones, E.M.; Smart, A.; Bloomberg, G.; Burgess, L.; Millar, M.R. Lactoferricin, a new antimicrobial peptide. J. Appl. Bacteriol. 1994, 77, 208–214. [Google Scholar] [CrossRef] [PubMed]
- Crouch, S.; Slater, K.; Fletcher, J. Regulation of cytokine release from mononuclear cells by the iron- binding protein lactoferrin. Blood 1992, 80, 235–240. [Google Scholar] [CrossRef]
- Ikeda, S.-I.; Kurihara, T.; Toda, M.; Jiang, X.; Torii, H.; Tsubota, K. Oral Bovine Milk Lactoferrin Administration Suppressed Myopia Development through Matrix Metalloproteinase 2 in a Mouse Model. Nutrients 2020, 12, 3744. [Google Scholar] [CrossRef] [PubMed]
- Zhu, X.; Zhang, K.; He, W.; Yang, J.; Sun, X.; Jiang, C.; Dai, J.; Lu, Y. Proinflammatory status in the aqueous humor of high myopic cataract eyes. Exp. Eye Res. 2016, 142, 13–18. [Google Scholar] [CrossRef]
- Kossakowska, A.E.; Edwards, D.R.; Prusinkiewicz, C.; Zhang, M.C.; Guo, D.; Urbanski, S.J.; Grogan, T.; Marquez, L.A.; Janowska-Wieczorek, A. Interleukin-6 Regulation of Matrix Metalloproteinase (MMP-2 and MMP-9) and Tissue Inhibitor of Metalloproteinase (TIMP-1) Expression in Malignant Non-Hodgkin’s Lymphomas. Blood 1999, 94, 2080–2089. [Google Scholar] [CrossRef]
- Wei, C.C.; Kung, Y.J.; Chen, C.S.; Chang, C.Y.; Lin, C.J.; Tien, P.T.; Chang, H.Y.; Chen, H.J.; Huang, Y.S.; Lin, H.J.; et al. Allergic Conjunctivitis-induced Retinal Inflammation Promotes Myopia Progression. EBioMedicine 2018, 28, 274–286. [Google Scholar] [CrossRef] [PubMed]
- Saint-Geniez, M.; Kurihara, T.; Sekiyama, E.; Maldonado, A.E.; D’Amore, P.A. An essential role for RPE-derived soluble VEGF in the maintenance of the choriocapillaris. Proc. Natl. Acad. Sci. USA 2009, 106, 18751–18756. [Google Scholar] [CrossRef]
- Wong, C.W.; Yanagi, Y.; Tsai, A.S.H.; Shihabuddeen, W.A.; Cheung, N.; Lee, S.Y.; Jonas, J.B.; Cheung, C.M.G. Correlation of axial length and myopic macular degeneration to levels of molecular factors in the aqueous. Sci. Rep. 2019, 9, 15708. [Google Scholar] [CrossRef]
- Zhang, Y.; Jeong, H.; Mori, K.; Ikeda, S.-I.; Shoda, C.; Miwa, Y.; Nakai, A.; Chen, J.; Ma, Z.; Jiang, X.; et al. Vascular endothelial growth factor from retinal pigment epithelium is essential in choriocapillaris and axial length maintenance. PNAS Nexus 2022, 1, pgac166. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Liu, F.; An, Q.; Wang, W.; Cheng, Z.; Dai, Y.; Meng, Q.; Zhang, Y. Lactoferrin Deficiency Impairs Proliferation of Satellite Cells via Downregulating the ERK1/2 Signaling Pathway. Int. J. Mol. Sci. 2022, 23, 7478. [Google Scholar] [CrossRef]
- Kitakaze, T.; Oshimo, M.; Kobayashi, Y.; Ryu, M.; Suzuki, Y.; Inui, H.; Harada, N.; Yamaji, R. Lactoferrin promotes murine C2C12 myoblast proliferation and differentiation and myotube hypertrophy. Mol. Med. Rep. 2018, 17, 5912–5920. [Google Scholar] [CrossRef]
- Stern, R. Devising a pathway for hyaluronan catabolism: Are we there yet? Glycobiology 2003, 13, 105R–115R. [Google Scholar] [CrossRef]
- Engelmayer, J.; Blezinger, P.; Varadhachary, A. Talactoferrin Stimulates Wound Healing With Modulation of Inflammation. J. Surg. Res. 2008, 149, 278–286. [Google Scholar] [CrossRef] [PubMed]
- Takayama, Y.; Takahashi, H.; Mizumachi, K.; Takezawa, T. Low Density Lipoprotein Receptor-related Protein (LRP) Is Required for Lactoferrin-enhanced Collagen Gel Contractile Activity of Human Fibroblasts. J. Biol. Chem. 2003, 278, 22112–22118. [Google Scholar] [CrossRef]
- Tang, L.; Cui, T.; Wu, J.J.; Liu-Mares, W.; Huang, N.; Li, J. A rice-derived recombinant human lactoferrin stimulates fibroblast proliferation, migration, and sustains cell survival. Wound Repair Regen. 2010, 18, 123–131. [Google Scholar] [CrossRef] [PubMed]
- Brock, J.H.; Arzabe, F.; Lampreave, F.; Piñeiro, A. The effect of trypsin on bovine transferrin and lactoferrin. Biochim. Biophys. Acta (BBA) Protein Struct. 1976, 446, 214–225. [Google Scholar] [CrossRef]
- Wakabayashi, H.; Takase, M.; Tomita, M. Lactoferricin derived from milk protein lactoferrin. Curr. Pharm. Des. 2003, 9, 1277–1287. [Google Scholar] [CrossRef]
- Yang, N.; Rekdal, Ø.; Stensen, W.; Svendsen, J.S. Enhanced antitumor activity and selectivity of lactoferrin-derived peptides. J. Peptide Res. 2002, 60, 187–197. [Google Scholar] [CrossRef]
- Kawashima, M.; Kawakita, T.; Inaba, T.; Okada, N.; Ito, M.; Shimmura, S.; Watanabe, M.; Shinmura, K.; Tsubota, K. Dietary Lactoferrin Alleviates Age-Related Lacrimal Gland Dysfunction in Mice. PLoS ONE 2012, 7, e33148. [Google Scholar] [CrossRef] [PubMed]
- Kawashima, M.; Nakamura, S.; Izuta, Y.; Inoue, S.; Tsubota, K. Dietary Supplementation with a Combination of Lactoferrin, Fish Oil, and Enterococcus faecium WB2000 for Treating Dry Eye: A Rat Model and Human Clinical Study. Ocul. Surf. 2016, 14, 255–263. [Google Scholar] [CrossRef]
- Connell, S.; Kawashima, M.; Nakamura, S.; Imada, T.; Yamamoto, H.; Tsubota, K.; Fukuda, S. Lactoferrin Ameliorates Dry Eye Disease Potentially through Enhancement of Short-Chain Fatty Acid Production by Gut Microbiota in Mice. Int. J. Mol. Sci. 2021, 22, 12384. [Google Scholar] [CrossRef]
- Hazra, D.; Yotsukura, E.; Torii, H.; Mori, K.; Maruyama, T.; Ogawa, M.; Hanyuda, A.; Tsubota, K.; Kurihara, T.; Negishi, K. Relation between dry eye and myopia based on tear film breakup time, higher order aberration, choroidal thickness, and axial length. Sci. Rep. 2022, 12, 10891. [Google Scholar] [CrossRef] [PubMed]
- Cai, Y. Determination of Select Trace Elements in Hair of College Students in Jinzhou, China. Biol. Trace Elem. Res. 2011, 144, 469–474. [Google Scholar] [CrossRef]
- Zhang, Z.; Lu, M.; Chen, C.; Tong, X.; Li, Y.; Yang, K.; Lv, H.; Xu, J.; Qin, L. Holo-lactoferrin: The link between ferroptosis and radiotherapy in triple-negative breast cancer. Theranostics 2021, 11, 3167–3182. [Google Scholar] [CrossRef]
- Jiang, X.; Kurihara, T.; Kunimi, H.; Miyauchi, M.; Ikeda, S.-I.; Mori, K.; Tsubota, K.; Torii, H.; Tsubota, K. A highly efficient murine model of experimental myopia. Sci. Rep. 2018, 8, 2026. [Google Scholar] [CrossRef] [PubMed]
- Jiang, X.; Kurihara, T.; Ikeda, S.-I.; Kunimi, H.; Mori, K.; Torii, H.; Tsubota, K. Inducement and Evaluation of a Murine Model of Experimental Myopia. J. Vis. Exp. 2019, e58822. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Liang, Y.; Ikeda, S.-i.; Chen, J.; Zhang, Y.; Negishi, K.; Tsubota, K.; Kurihara, T. Myopia Is Suppressed by Digested Lactoferrin or Holo-Lactoferrin Administration. Int. J. Mol. Sci. 2023, 24, 5815. https://doi.org/10.3390/ijms24065815
Liang Y, Ikeda S-i, Chen J, Zhang Y, Negishi K, Tsubota K, Kurihara T. Myopia Is Suppressed by Digested Lactoferrin or Holo-Lactoferrin Administration. International Journal of Molecular Sciences. 2023; 24(6):5815. https://doi.org/10.3390/ijms24065815
Chicago/Turabian StyleLiang, Yifan, Shin-ichi Ikeda, Junhan Chen, Yan Zhang, Kazuno Negishi, Kazuo Tsubota, and Toshihide Kurihara. 2023. "Myopia Is Suppressed by Digested Lactoferrin or Holo-Lactoferrin Administration" International Journal of Molecular Sciences 24, no. 6: 5815. https://doi.org/10.3390/ijms24065815
APA StyleLiang, Y., Ikeda, S.-i., Chen, J., Zhang, Y., Negishi, K., Tsubota, K., & Kurihara, T. (2023). Myopia Is Suppressed by Digested Lactoferrin or Holo-Lactoferrin Administration. International Journal of Molecular Sciences, 24(6), 5815. https://doi.org/10.3390/ijms24065815









