Nuclear Epidermal Growth Factor Receptor Overexpression as a Survival Predictor in Oral Squamous Cell Carcinoma
Abstract
:1. Introduction
2. Results
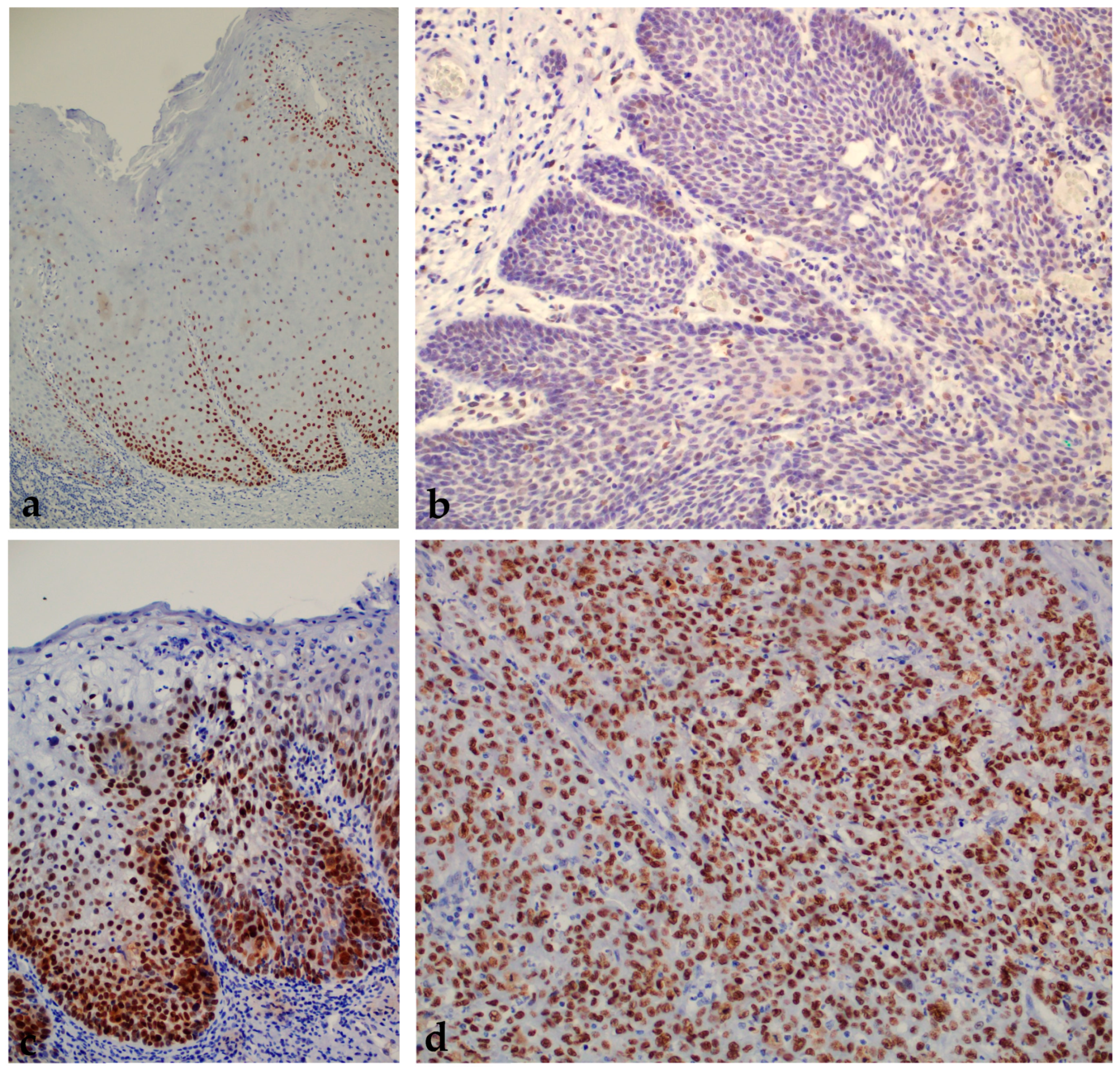
2.1. Results of Immunohistochemical Staining
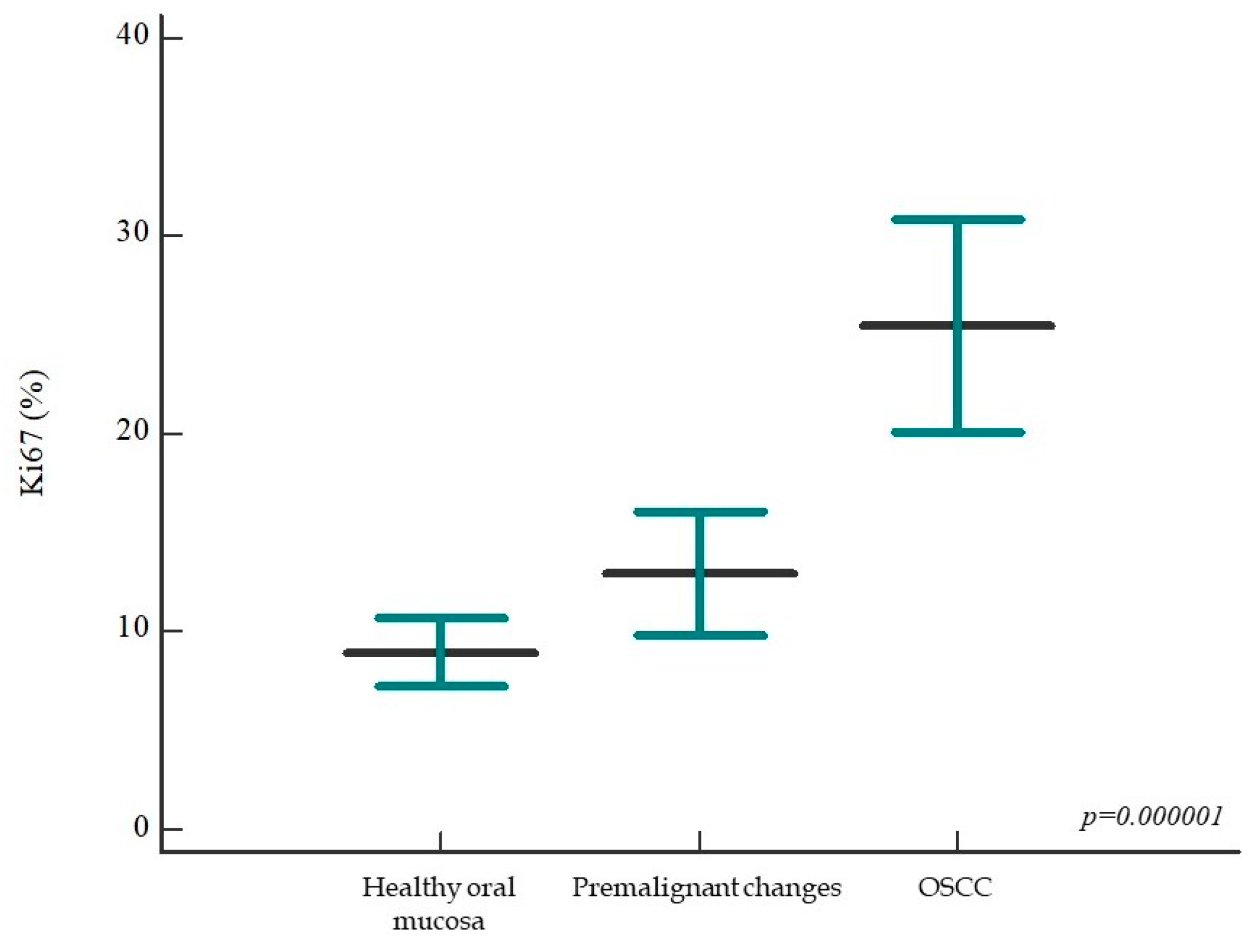
2.1.1. Expression of Ki-67 in Healthy Oral Mucosa, Premalignant Changes, and Invasive Oral Squamous Cell Carcinoma
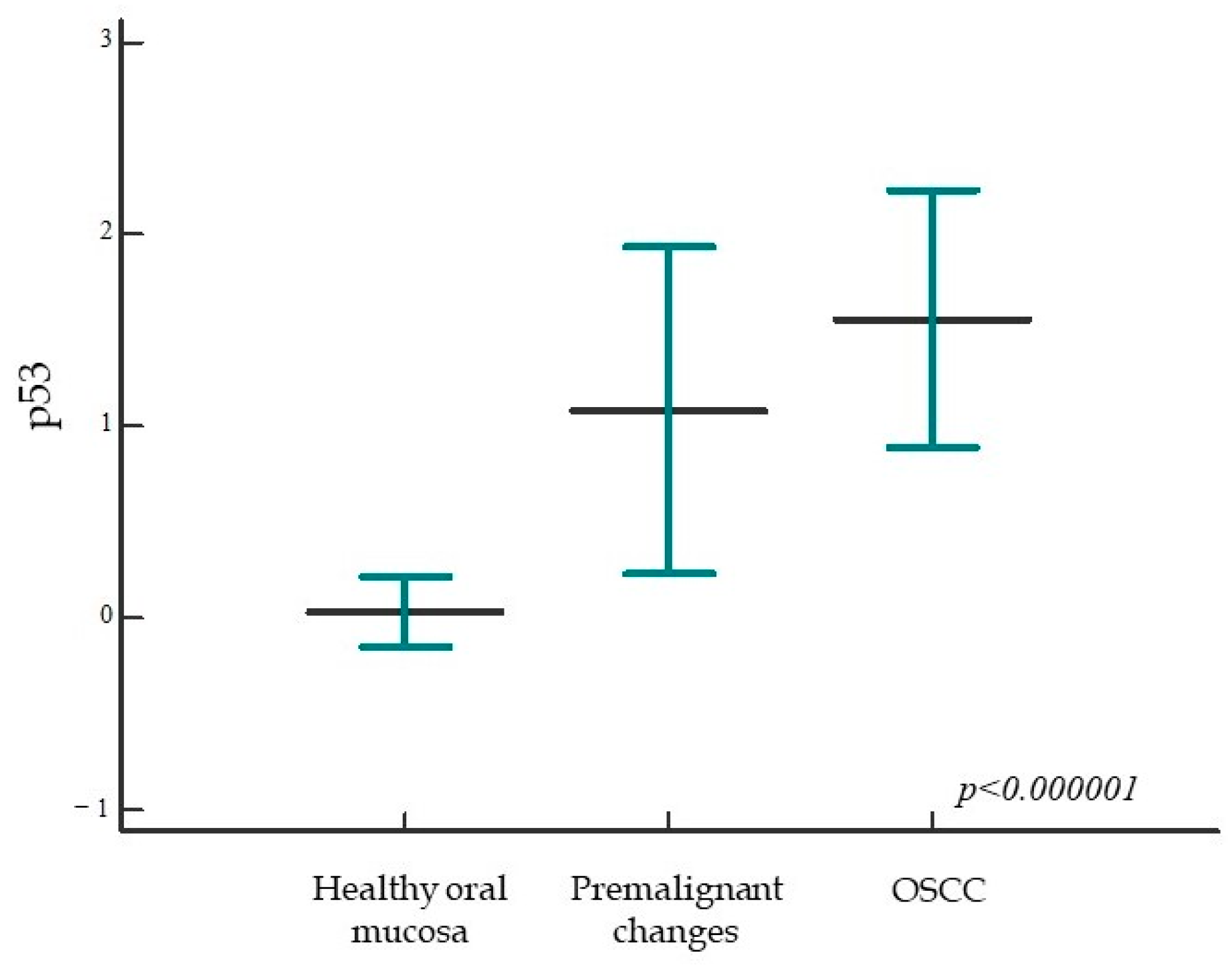
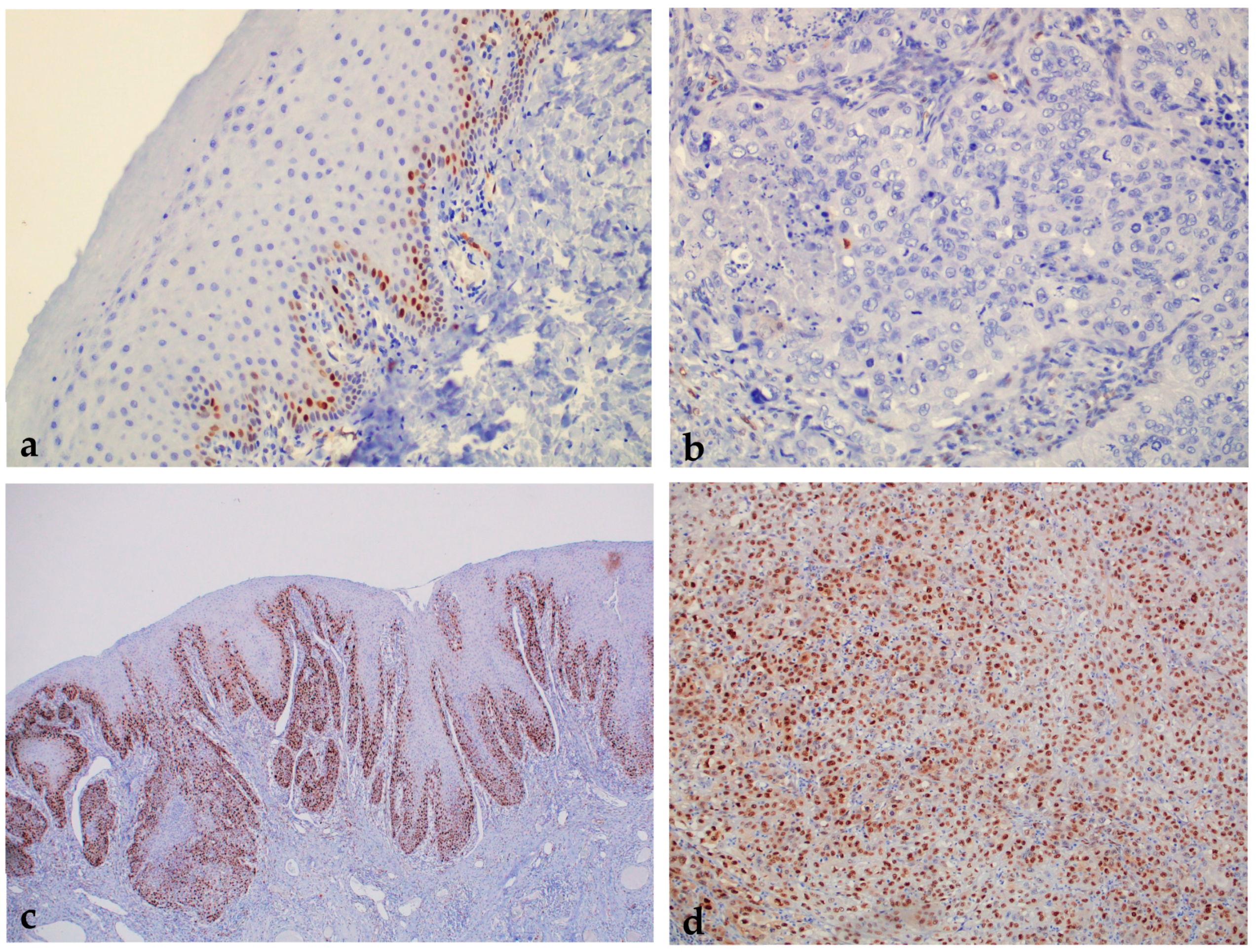
2.1.2. Expression of p53 in Healthy Oral Mucosa, Premalignant Changes, and Invasive Oral Squamous Cell Carcinoma
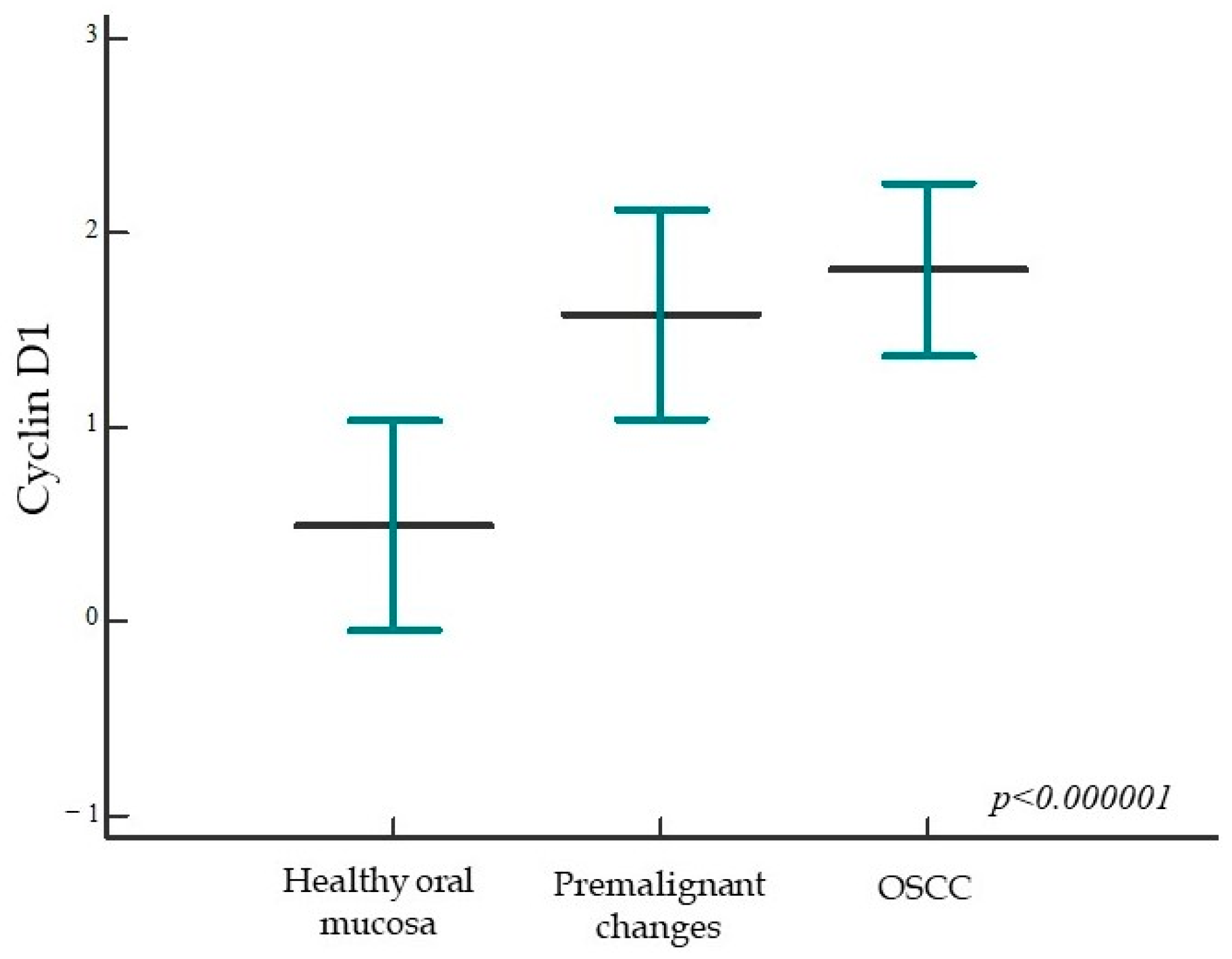
2.1.3. Expression of Cyclin D1 in Healthy Oral Mucosa, Premalignant Changes, and Invasive Oral Squamous Cell Carcinoma
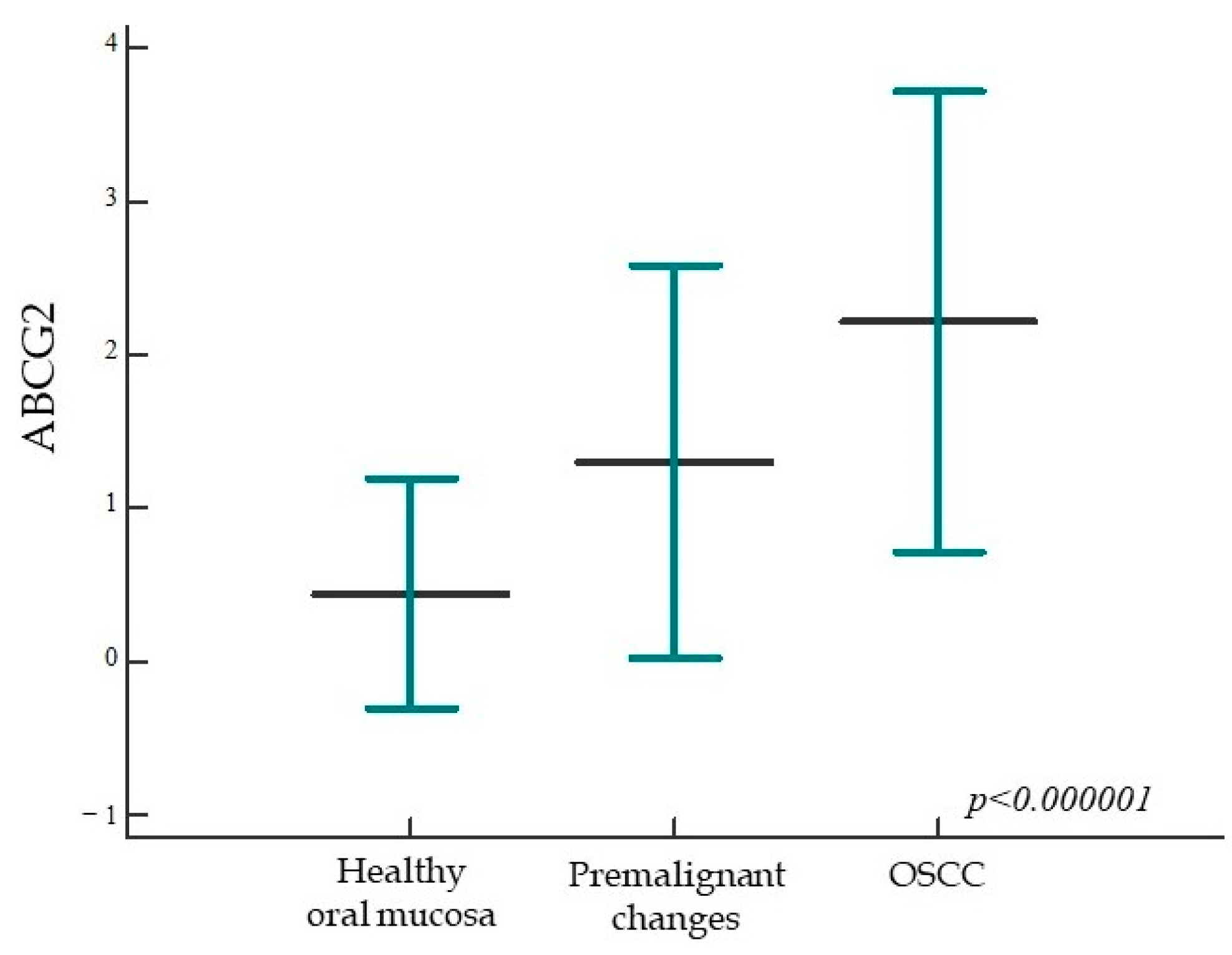
2.1.4. ABCG2 Expression in Healthy Oral Mucosa, Premalignant Changes, and Invasive Oral Squamous Cell Carcinoma
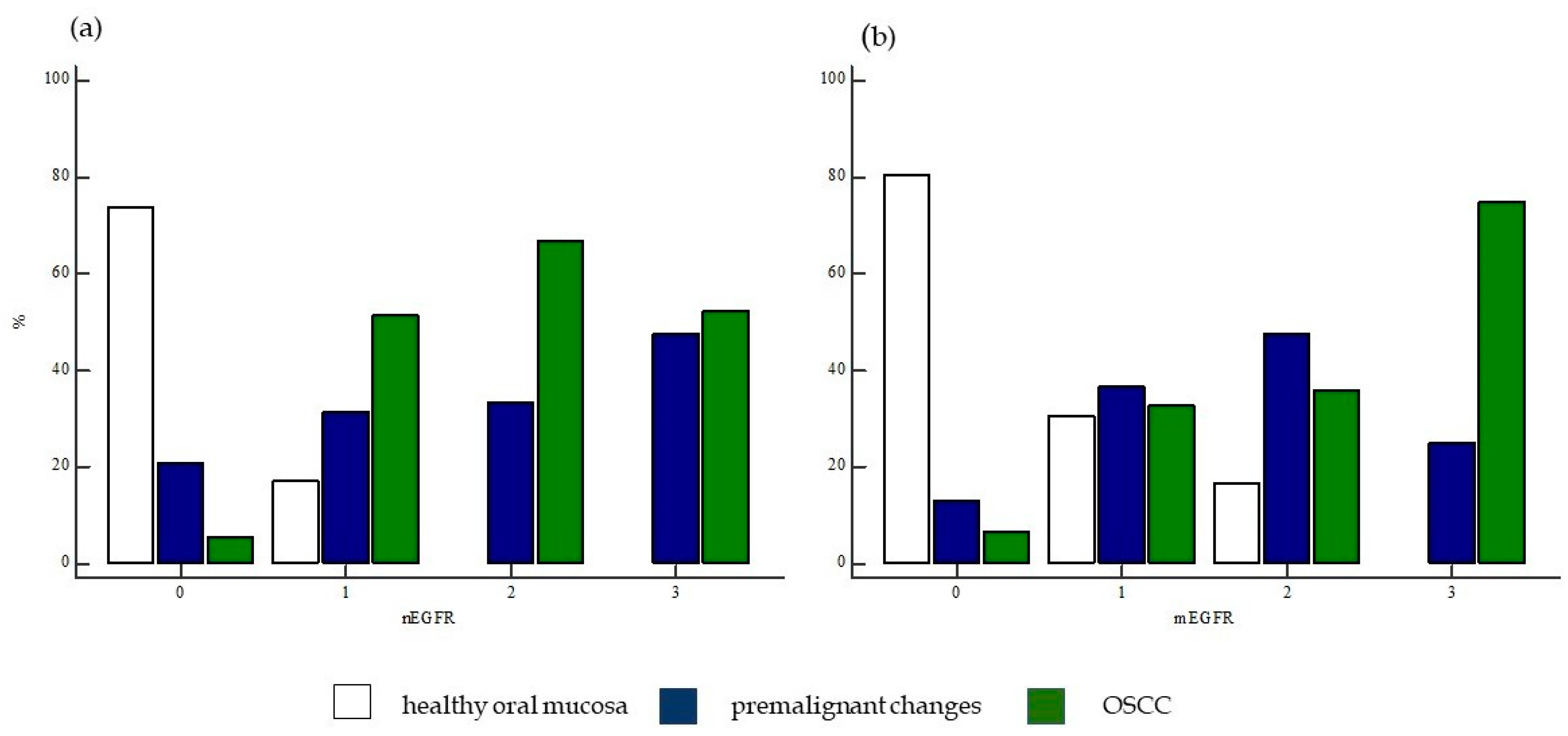
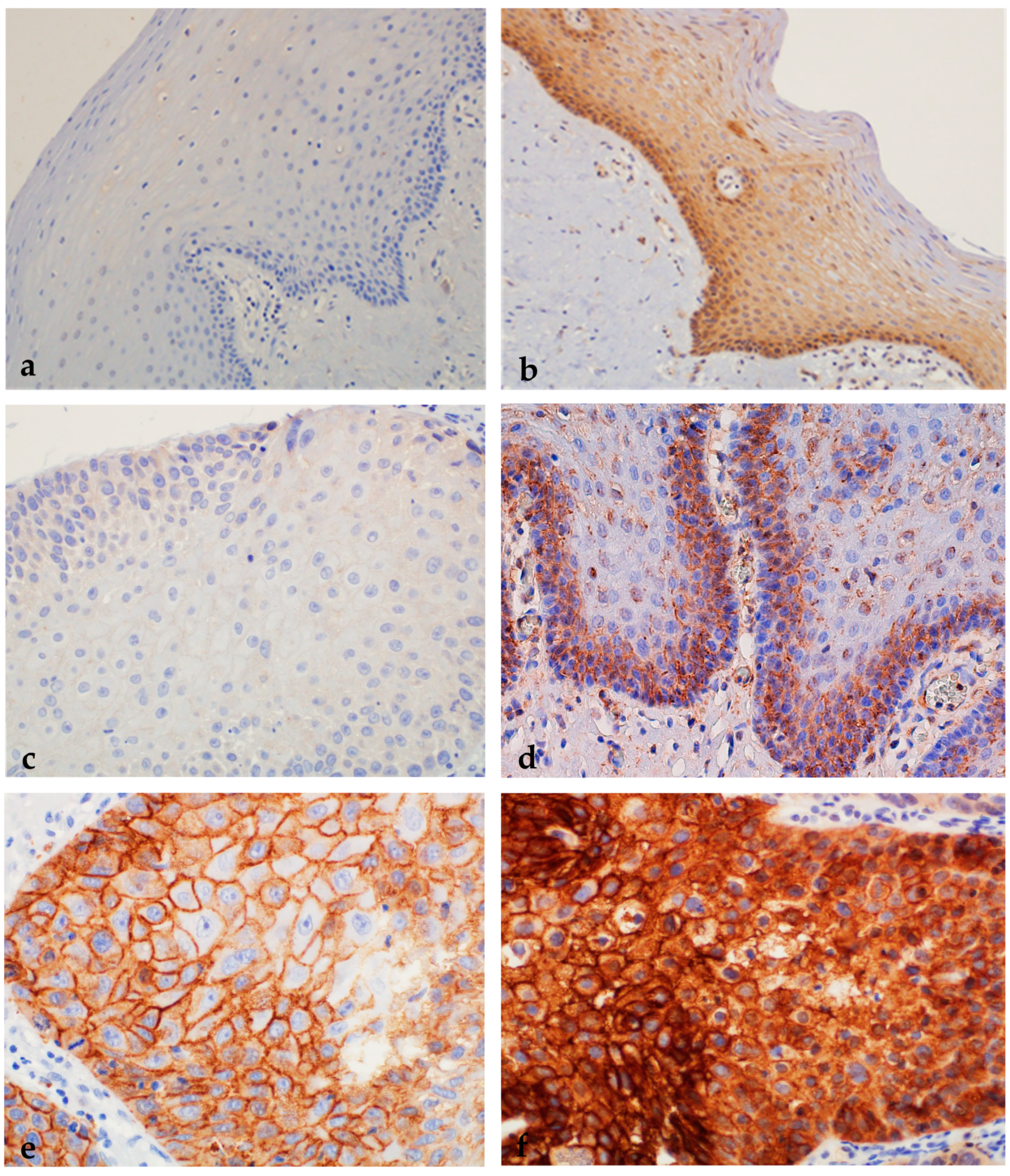
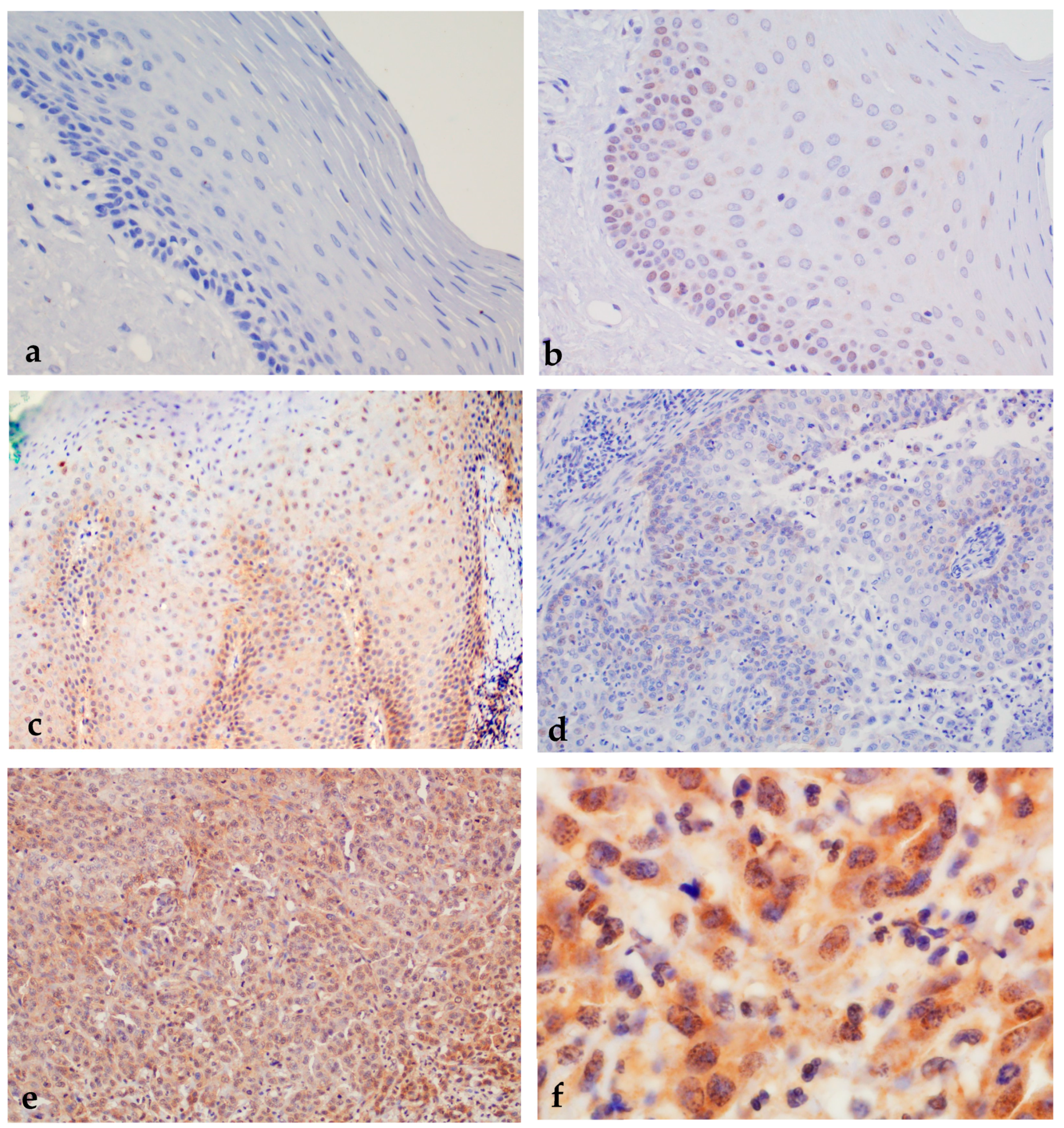
2.1.5. Expression of nEGFR and mEGFR in Healthy Oral Mucosa, Premalignant Changes, and Invasive Oral Squamous Cell Carcinoma
2.1.6. Correlation of nEGFR Expression with mEGFR and Markers of Cell Cycle, Cell Proliferation, and Tumor Stem Cells in Healthy Oral Mucosa, Premalignant Changes, and Invasive Oral Squamous Cell Carcinoma
2.1.7. Association of Protein Expression of nEGFR and mEGFR and of Ki-67, p53, Cyclin D1, and ABCG2 with Clinicopathologic Parameters in Invasive Oral Squamous Cell Carcinoma
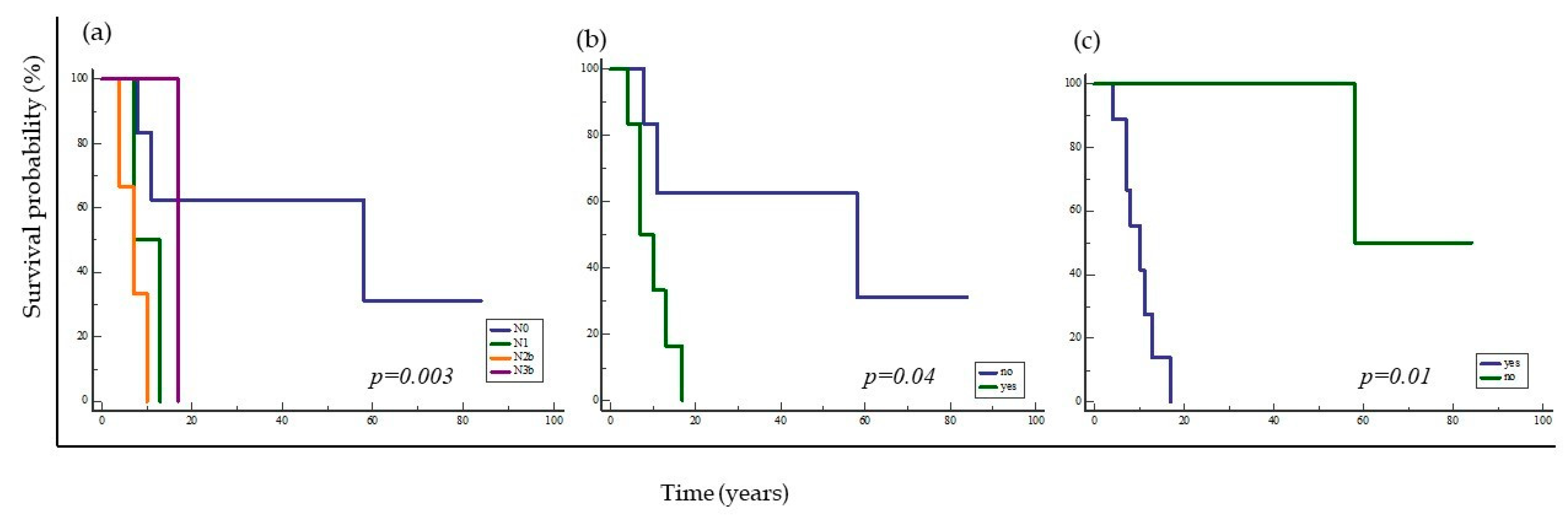
2.2. Survival Analysis
3. Discussion
4. Materials and Methods
4.1. Patients
4.2. Pathohistological Samples
4.3. Immunohistochemical Staining
4.4. Evaluation of Immunohistochemical Staining
4.5. Statistical Analysis
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Shah, J.; Patel, S.; Singh, B. Jatin Shah’s Head and Neck Surgery and Oncology, 4th ed.; Mosby: Maryland Heights, MI, USA, 2019. [Google Scholar]
- Sung, H.; Ferlay, J.; Siegel, R.L.; Laversanne, M.; Soerjomataram, I.; Jemal, A.; Bray, F. Global Cancer Statistics 2020: GLOBOCAN Estimates of Incidence and Mortality Worldwide for 36 Cancers in 185 Countries. CA Cancer J. Clin. 2021, 71, 209–249. [Google Scholar] [CrossRef] [PubMed]
- Patel, S.C.; Carpenter, W.R.; Tyree, S.; Couch, M.E.; Weissler, M.; Hackman, T.; Hayes, D.N.; Shores, C.; Chera, B.S. Increasing incidence of oral tongue squamous cell carcinoma in young white women, age 18 to 44 years. J. Clin. Oncol. 2011, 29, 1488–1494. [Google Scholar] [CrossRef] [PubMed]
- Neville, B.W.; Day, T.A. Oral cancer and precancerous lesions. CA Cancer J. Clin. 2002, 52, 195–215. [Google Scholar] [CrossRef] [Green Version]
- Budach, V.; Tinhofer, I. Novel prognostic clinical factors and biomarkers for outcome prediction in head and neck cancer: A systematic review. Lancet Oncol. 2019, 20, e313–e326. [Google Scholar] [CrossRef] [PubMed]
- Hsieh, J.C.; Wang, H.-M.; Wu, M.-H.; Chang, K.-P.; Chang, P.-H.; Liao, C.-T.; Liau, C.-T. Review of emerging biomarkers in head and neck squamous cell carcinoma in the era of immunotherapy and targeted therapy. Head Neck 2019, 41 (Suppl. 1), 19–45. [Google Scholar] [CrossRef] [Green Version]
- Lin, S.-Y.; Makino, K.; Xia, W.; Matin, A.; Wen, Y.; Kwong, K.Y.; Bourguignon, L.; Hung, M.-C. Nuclear localization of EGF receptor and its potential new role as a transcription factor. Nature 2001, 3, 802–808. [Google Scholar] [CrossRef] [PubMed]
- Lo, H.-W.; Xia, W.; Wei, Y.; Ali-Seyed, M.; Huang, S.-F.; Hung, M.-C. Novel prognostic value of nuclear epidermal growth factor receptor in breast cancer. Cancer Res 2005, 65, 338–348. [Google Scholar] [CrossRef]
- Hanada, N.; Lo, H.-W.; Day, C.-P.; Pan, Y.; Nakajima, Y.; Hung, M.-C. Co-regulation of B-Myb expression by E2F1 and EGF receptor. Mol. Carcinog. 2006, 45, 10–17. [Google Scholar] [CrossRef]
- Hung, L.-Y.; Tseng, J.T.-C.; Lee, Y.-C.; Xia, W.; Wang, Y.-N.; Wu, M.-L.; Chuang, Y.-H.; Lai, C.-H.; Chang, W.-C. Nuclear epidermal growth factor receptor (EGFR) interacts with signal transducer and activator of transcription 5 (STAT5) in activating Aurora-A gene expression. Nucleic Acids Res. 2008, 36, 4337–4351. [Google Scholar] [CrossRef]
- Hadžisejdić, I.; Mustać, E.; Jonjić, N.; Petković, M.; Grahovac, B. Nuclear EGFR in ductal invasive breast cancer: Correlation with cyclin-D1 and prognosis. Mod. Pathol. 2010, 23, 392–403. [Google Scholar] [CrossRef] [Green Version]
- Kamio, T.; Shigematsu, K.; Sou, H.; Kawai, K.; Tsuchiyama, H. Immunohistochemical expression of epidermal growth factor receptors in human adrenocortical carcinoma. Hum. Pathol. 1990, 21, 277–282. [Google Scholar] [CrossRef]
- Lipponen, P.; Eskelinen, M. Expression of epidermal growth factor receptor in bladder cancer as related to established prognostic factors, oncoprotein (c-erbB-2, p53) expression and long-term prognosis. Br. J. Cancer 1994, 69, 1120–1125. [Google Scholar] [CrossRef] [Green Version]
- Psyrri, A.; Yu, Z.; Weinberger, P.M.; Sasaki, C.; Haffty, B.; Camp, R.; Rimm, D.; Burtness, B.A. Quantitative determination of nuclear and cytoplasmic epidermal growth factor receptor expression in oropharyngeal squamous cell cancer by using automated quantitative analysis. Clin. Cancer Res. 2005, 11, 5856–5862. [Google Scholar] [CrossRef] [Green Version]
- Xia, W.; Wei, Y.; Du, Y.; Liu, J.; Chang, B.; Yu, Y.-L.; Huo, L.-F.; Miller, S.; Hung, M.-C. Nuclear expression of epidermal growth factor receptor is a novel prognostic value in patients with ovarian cancer. Mol. Carcinog. 2009, 48, 610–617. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Marijić, B.; Braut, T.M.; Babarović, E.M.; Krstulja, M.M.; Maržić, D.; Avirović, M.M.; Kujundžić, M.M.; Hadžisejdić, I.M. Nuclear EGFR Expression Is Associated With Poor Survival in Laryngeal Carcinoma. Appl. Immunohistochem. Mol. Morphol. 2021, 29, 576–584. [Google Scholar] [CrossRef]
- Cho, E.Y.; Choi, Y.-L.; Han, J.J.; Kim, K.-M.; Oh, Y.L. Expression and amplification of Her2, EGFR and cyclin D1 in breast cancer: Immunohistochemistry and chromogenic in situ hybridization. Pathol. Int. 2008, 58, 17–25. [Google Scholar] [CrossRef]
- Parakh, M.K.; Ulaganambi, S.; Ashifa, N.; Premkumar, R.; Jain, A.L. Oral potentially malignant disorders: Clinical diagnosis and current screening aids: A narrative review. Eur. J. Cancer Prev. 2020, 29, 65–72. [Google Scholar] [CrossRef] [PubMed]
- Elbehi, A.M.; Anu, R.; Ekine-Afolabi, B.; Cash, E. Emerging role of immune checkpoint inhibitors and predictive biomarkers in head and neck cancers. Oral Oncol. 2020, 109, 104977. [Google Scholar] [CrossRef] [PubMed]
- Farah, C.S. Molecular, genomic and mutational landscape of oral leukoplakia. Oral Dis. 2021, 27, 803–812. [Google Scholar] [CrossRef] [PubMed]
- Ranganathan, K.; Kavitha, L. Oral epithelial dysplasia: Classifications and clinical relevance in risk assessment of oral potentially malignant disorders. J. Oral Maxillofac. Pathol. 2019, 23, 19–27. [Google Scholar] [CrossRef] [PubMed]
- Economopoulou, P.; De Bree, R.; Kotsantis, I.; Psyrri, A. Diagnostic Tumor Markers in Head and Neck Squamous Cell Carcinoma (HNSCC) in the Clinical Setting. Front. Oncol. 2019, 9, 827. [Google Scholar] [CrossRef] [PubMed]
- Li, R.; Koch, W.M.; Fakhry, C.; Gourin, C.G. Distinct Epidemiologic Characteristics of Oral Tongue Cancer Patients. Otolaryngol. Neck Surg. 2013, 148, 792–796. [Google Scholar] [CrossRef] [PubMed]
- Sarode, G.; Maniyar, N.; Sarode, S.C.; Jafer, M.; Patil, S.; Awan, K.H. Epidemiologic aspects of oral cancer. Dis. Mon. 2020, 66, 100988. [Google Scholar] [CrossRef] [PubMed]
- Sorbye, S.W.; Kilvaer, T.K.; Valkov, A.; Donnem, T.; Smeland, E.; Al-Shibli, K.; Bremnes, R.M.; Busund, L.-T. Prognostic Impact of Jab1, p16, p21, p62, Ki67 and Skp2 in Soft Tissue Sarcomas. PLoS ONE 2012, 7, e47068. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Josefsson, A.; Wikström, P.; Egevad, L.; Granfors, T.; Karlberg, L.; Stattin, P.; Bergh, A. Low endoglin vascular density and Ki67 index in Gleason score 6 tumours may identify prostate cancer patients suitable for surveillance. Scand. J. Urol. Nephrol. 2012, 46, 247–257. [Google Scholar] [CrossRef]
- Ishihara, M.; Mukai, H.; Nagai, S.; Onozawa, M.; Nihei, K.; Shimada, T.; Wada, N. Retrospective analysis of risk factors for central nervous system metastases in operable breast cancer: Effects of biologic subtype and Ki67 overexpression on survival. Oncology 2013, 84, 135–140. [Google Scholar] [CrossRef]
- Jing, Y.; Zhou, Q.; Zhu, H.; Zhang, Y.; Song, Y.; Zhang, X.; Huang, X.; Yang, Y.; Ni, Y.; Hu, Q. Ki-67 is an independent prognostic marker for the recurrence and relapse of oral squamous cell carcinoma. Oncol. Lett. 2019, 17, 974–980. [Google Scholar] [CrossRef] [Green Version]
- Takkem, A.; Barakat, C.; Zakarea, S.; Zaid, K.; Najmeh, J.; Ayoub, M.; Seirawan, M.Y. Ki-67 Prognostic Value in Different Histological Grades of Oral Epithelial Dysplasia and Oral Squamous Cell Carcinoma. Asian Pac. J. Cancer Prev. 2018, 19, 3279–3286. [Google Scholar] [CrossRef] [Green Version]
- Lopes, V.K.M.; de Jesus, A.S.; de Souza, L.L.; Miyahara, L.A.N.; Guimarães, D.M.; Pontes, H.A.R.; Pontes, F.S.C.; de Carvalho, P.L. Ki-67 protein predicts survival in oral squamous carcinoma cells: An immunohistochemical study. Braz. Oral Res. 2017, 31, e66. [Google Scholar] [CrossRef] [Green Version]
- Xie, S.; Liu, Y.; Qiao, X.; Hua, R.-X.; Wang, K.; Shan, X.-F.; Cai, Z.-G. What is the Prognostic Significance of Ki-67 Positivity in Oral Squamous Cell Carcinoma? J. Cancer 2016, 7, 758–767. [Google Scholar] [CrossRef] [Green Version]
- Sharma, S.; Maheshwari, V.; Narula, V.; Verma, S.; Jain, A.; Alam, K. Prognostic and Predictive Impact of Ki-67 in Premalignant and Malignant Squamous Cell Lesions of Oral Cavity. Int. J. Head Neck Surg. 2013, 4, 61–65. [Google Scholar] [CrossRef]
- Mondal, K.; Mandal, R.; Sarkar, B.C. Importance of Ki-67 Labeling in Oral Leukoplakia with Features of Dysplasia and Carcinomatous Transformation: An Observational Study over 4 Years. South Asian J. Cancer 2020, 9, 099–104. [Google Scholar] [CrossRef]
- Dwivedi, N.; Chandra, S.; Kashyap, B.; Raj, V.; Agarwal, A. Suprabasal expression of Ki-67 as a marker for the severity of oral epithelial dysplasia and oral squamous cell carcinoma. Contemp. Clin. Dent. 2013, 4, 7–12. [Google Scholar] [CrossRef]
- Birajdar, S.S.; Radhika, M.; Paremala, K.; Gadivan, M.; Sudhakara, M.; Soumya, M. Expression of Ki-67 in normal oral epithelium, leukoplakic oral epithelium and oral squamous cell carcinoma. J. Oral Maxillofac. Pathol. 2014, 18, 169–176. [Google Scholar] [CrossRef] [Green Version]
- Ozaki, T.; Nakagawara, A. Role of p53 in Cell Death and Human Cancers. Cancers 2011, 3, 994–1013. [Google Scholar] [CrossRef] [Green Version]
- Renzi, A.; De Bonis, P.; Morandi, L.; Lenzi, J.; Tinto, D.; Rigillo, A.; Bettini, G.; Bellei, E.; Sabattini, S. Prevalence of p53 dysregulations in feline oral squamous cell carcinoma and non-neoplastic oral mucosa. PLoS ONE 2019, 14, e0215621. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tandon, S.; Tudur-Smith, C.; Riley, R.D.; Boyd, M.T.; Jones, T.M. A systematic review of p53 as a prognostic factor of survival in squamous cell carcinoma of the four main anatomical subsites of the head and neck. Cancer Epidemiol. Biomark. Prev. 2010, 19, 574–587. [Google Scholar] [CrossRef] [Green Version]
- Banerjee, R.; Van Tubergen, E.A.; Scanlon, C.S.; Broek, R.V.; Lints, J.P.; Liu, M.; Russo, N.; Inglehart, R.C.; Wang, Y.; Polverini, P.J.; et al. The G protein–coupled receptor GALR2 promotes angiogenesis in head and neck cancer. Mol. Cancer Ther. 2014, 13, 1323–1333. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kanazawa, T.; Misawa, K.; Misawa, Y.; Maruta, M.; Uehara, T.; Kawada, K.; Nagatomo, T.; Ichimura, K. Galanin receptor 2 utilizes distinct signaling pathways to suppress cell proliferation and induce apoptosis in HNSCC. Mol. Med. Rep. 2014, 10, 1289–1294. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Khan, Z.; Tiwari, R.P.; Mulherkar, R.; Sah, N.K.; Prasad, G.B.K.S.; Shrivastava, B.R.; Bisen, P.S. Detection of survivin and p53 in human oral cancer: Correlation with clinicopathologic findings. Head Neck 2009, 31, 1039–1048. [Google Scholar] [CrossRef]
- Ögmundsdóttir, H.M.; Björnsson, J.; Holbrook, W.P. Role of TP53 in the progression of pre-malignant and malignant oral mucosal lesions. A follow-up study of 144 patients. J. Oral Pathol. Med. 2009, 38, 565–571. [Google Scholar] [CrossRef] [PubMed]
- Baldin, V.; Lukas, J.; Marcote, M.J.; Pagano, M.; Draetta, G. Cyclin D1 is a nuclear protein required for cell cycle progression in G1. Genes Dev. 1993, 7, 812–821. [Google Scholar] [CrossRef] [Green Version]
- Bartkova, J.; Lukas, J.; Strauss, M.; Bartek, J. Cyclin D1 oncoprotein aberrantly accumulates in malignancies of diverse histogenesis. Oncogene 1995, 10, 775–778. [Google Scholar] [PubMed]
- Fu, M.; Wang, C.; Li, Z.; Sakamaki, T.; Pestell, R.G. Minireview: Cyclin D1: Normal and Abnormal Functions. Endocrinology 2004, 145, 5439–5447. [Google Scholar] [CrossRef] [Green Version]
- Binabaj, M.M.; Bahrami, A.; Khazaei, M.; Ryzhikov, M.; Ferns, G.A.; Avan, A.; Hassanian, S.M. The prognostic value of cyclin D1 expression in the survival of cancer patients: A meta-analysis. Gene 2020, 728, 144283. [Google Scholar] [CrossRef]
- Moharil, R.B.; Khandekar, S.; Dive, A.; Bodhade, A. Cyclin D1 in oral premalignant lesions and oral squamous cell carcinoma: An immunohistochemical study. J. Oral Maxillofac. Pathol. 2020, 24, 397. [Google Scholar] [CrossRef] [PubMed]
- de Vicente, J.C.; Herrero-Zapatero, A.; Fresno, M.F.; López-Arranz, J.S. Expression of cyclin D1 and Ki-67 in squamous cell carcinoma of the oral cavity: Clinicopathological and prognostic significance. Oral Oncol. 2002, 38, 301–308. [Google Scholar] [CrossRef] [PubMed]
- Gupta, S.; Khan, H.; Husain, N.; Misra, S.; Singh, N.; Negi, M. Prognostics of Cyclin-D1 expression with chemoradiation response in patients of locally advanced oral squamous cell carcinoma. J. Cancer Res. Ther. 2014, 10, 258–264. [Google Scholar] [CrossRef]
- Guimaraes, E.; De Carli, M.; Sperandio, F.; Hanemann, J.; Pereira, A. Cyclin D1 and Ki-67 expression correlates to tumor staging in tongue squamous cell carcinoma. Med. Oral Patol. Oral Cir. Bucal. 2015, 20, e657–e663. [Google Scholar] [CrossRef]
- Zhao, Y.; Yu, D.; Li, H.; Nie, P.; Zhu, Y.; Liu, S.; Zhu, M.; Fang, B. Cyclin D1 overexpression is associated with poor clinicopathological outcome and survival in oral squamous cell carcinoma in asian populations: Insights from a meta-analysis. PLoS ONE 2014, 9, e93210. [Google Scholar] [CrossRef]
- Wang, L.; Liu, T.; Nishioka, M.; Aguirre, R.L.; Win, S.S.; Okada, N. Activation of ERK1/2 and cyclin D1 expression in oral tongue squamous cell carcinomas: Relationship between clinicopathological appearances and cell proliferation. Oral Oncol. 2006, 42, 625–631. [Google Scholar] [CrossRef]
- Noorlag, R.; Van Kempen, P.M.W.; Stegeman, I.; Koole, R.; Van Es, R.J.J.; Willems, S.M. The diagnostic value of 11q13 amplification and protein expression in the detection of nodal metastasis from oral squamous cell carcinoma: A systematic review and meta-analysis. Virchows Arch. 2015, 466, 363–373. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Huang, S.-F.; Cheng, S.-D.; Chuang, W.-Y.; Chen, I.-H.; Liao, C.-T.; Wang, H.-M.; Hsieh, L.-L. Cyclin D1 overexpression and poor clinical outcomes in Taiwanese oral cavity squamous cell carcinoma. World J. Surg. Oncol. 2012, 10, 40. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lam, K.Y.; Ng, I.O.L.; Yuen, A.P.W.; Kwong, R.L.W.; Wei, W. Cyclin D1 expression in oral squamous cell carcinomas: Clinicopathological relevance and correlation with p53 expression. J. Oral Pathol. Med. 2000, 29, 167–172. [Google Scholar] [CrossRef]
- Saawarn, S.; Astekar, M.; Saawarn, N.; Dhakar, N.; Sagari, S.G. Cyclin D1 Expression and Its Correlation with Histopathological Differentiation in Oral Squamous Cell Carcinoma. Sci. World J. 2012, 2012, 978327. [Google Scholar] [CrossRef] [Green Version]
- Angadi, P.V.; Krishnapillai, R. Cyclin D1 expression in oral squamous cell carcinoma and verrucous carcinoma: Correlation with histological differentiation. Oral Surg. Oral Med. Oral Pathol. Oral Radiol. Endodontol. 2007, 103, e30–e35. [Google Scholar] [CrossRef]
- Mishra, R.; Das, B.R. Cyclin D1 expression and its possible regulation in chewing tobacco mediated oral squamous cell carcinoma progression. Arch. Oral Biol. 2009, 54, 917–923. [Google Scholar] [CrossRef] [PubMed]
- Mallya, S.M.; Woods, M.; Pant, R. Cyclin D1 and cyclin D-dependent kinases enhance oral keratinocyte proliferation but do not block keratinocyte differentiation. Int. J. Oncol. 2010, 37, 1471–1475. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ohnishi, Y.; Watanabe, M.; Wato, M.; Tanaka, A.; Kakudo, K.; Nozaki, M. Cyclin D1 expression is correlated with cell differentiation and cell proliferation in oral squamous cell carcinomas. Oncol. Lett. 2014, 7, 1123–1127. [Google Scholar] [CrossRef] [Green Version]
- Tamatani, T.; Takamaru, N.; Ohe, G.; Akita, K.; Nakagawa, T.; Miyamoto, Y. Expression of CD44, CD44v9, ABCG2, CD24, Bmi-1 and ALDH1 in stage I and II oral squamous cell carcinoma and their association with clinicopathological factors. Oncol. Lett. 2018, 16, 1133–1140. [Google Scholar] [CrossRef] [Green Version]
- Shi, P.; Liu, W.; Zhou, Z.-T.; He, Q.-B.; Jiang, W.-W. Podoplanin and ABCG2: Malignant Transformation Risk Markers for Oral Lichen Planus. Cancer Epidemiol. Biomark. Prev. 2010, 19, 844–849. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Feng, J.-Q.; Mi, J.-G.; Wu, L.; Ma, L.-W.; Shi, L.-J.; Yang, X.; Liu, W.; Zhang, C.-P.; Zhou, Z.-T. Expression of podoplanin and ABCG2 in oral erythroplakia correlate with oral cancer development. Oral Oncol. 2012, 48, 848–852. [Google Scholar] [CrossRef]
- Diestra, J.E.; Scheffer, G.L.; Català, I.; Maliepaard, M.; Schellens, J.H.M.; Scheper, R.J.; Germà-Lluch, J.R.; Izquierdo, M.A. Frequent expression of the multi-drug resistance-associated protein BCRP/MXR/ABCP/ABCG2 in human tumours detected by the BXP-21 monoclonal antibody in paraffin-embedded material. J. Pathol. 2002, 198, 213–219. [Google Scholar] [CrossRef]
- Mo, W.; Zhang, J.T. Human ABCG2: Structure, function, and its role in multidrug resistance. Int. J. Biochem. Mol. Biol. 2012, 3, 1–27. [Google Scholar]
- Zhou, S.; Schuetz, J.D.; Bunting, K.D.; Colapietro, A.M.; Sampath, J.; Morris, J.J.; Lagutina, I.; Grosveld, G.C.; Osawa, M.; Nakauchi, H.; et al. The ABC transporter Bcrp1/ABCG2 is expressed in a wide variety of stem cells and is a molecular determinant of the side-population phenotype. Nat. Med. 2001, 7, 1028–1034. [Google Scholar] [CrossRef] [PubMed]
- Bhatia, P.; Bernier, M.; Sanghvi, M.; Moaddel, R.; Schwarting, R.; Ramamoorthy, A.; Wainer, I.W. Breast cancer resistance protein (BCRP/ABCG2) localises to the nucleus in glioblastoma multiforme cells. Xenobiotica 2012, 42, 748–755. [Google Scholar] [CrossRef] [Green Version]
- Liang, S.-C.; Yang, C.-Y.; Tseng, J.-Y.; Wang, H.-L.; Tung, C.-Y.; Liu, H.-W.; Chen, C.-Y.; Yeh, Y.-C.; Chou, T.-Y.; Yang, M.-H.; et al. ABCG2 Localizes to the Nucleus and Modulates CDH1 Expression in Lung Cancer Cells. Neoplasia 2015, 17, 265–278. [Google Scholar] [CrossRef] [Green Version]
- Triaca, V.; Fico, E.; Rosso, P.; Ralli, M.; Corsi, A.; Severini, C.; Crevenna, A.; Agostinelli, E.; Rullo, E.; Riminucci, M.; et al. Pilot Investigation on p75ICD Expression in Laryngeal Squamous Cell Carcinoma. Cancers 2022, 14, 2622. [Google Scholar] [CrossRef] [PubMed]
- Yanamoto, S.; Yamada, S.-I.; Takahashi, H.; Naruse, T.; Matsushita, Y.; Ikeda, H.; Shiraishi, T.; Seki, S.; Fujita, S.; Ikeda, T.; et al. Expression of the cancer stem cell markers CD44v6 and ABCG2 in tongue cancer: Effect of neoadjuvant chemotherapy on local recurrence. Int. J. Oncol. 2014, 44, 1153–1162. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cognetti, D.M.; Weber, R.S.; Lai, S.Y. Head and neck cancer: An evolving treatment paradigm. Cancer 2008, 113, 1911–1932. [Google Scholar] [CrossRef]
- Kalyankrishna, S.; Grandis, J.R. Epidermal growth factor receptor biology in head and neck cancer. J. Clin. Oncol. 2006, 24, 2666–2672. [Google Scholar] [CrossRef]
- Bauml, J.M.; Vinnakota, R.; Park, Y.A.; Bates, S.E.; Fojo, T.; Aggarwal, C.; Di Stefano, J.; Knepley, C.; Limaye, S.; Mamtani, R.; et al. Cisplatin versus cetuximab with definitive concurrent radiotherapy for head and neck squamous cell carcinoma: An analysis of Veterans Health Affairs data. Cancer 2019, 125, 406–415. [Google Scholar] [CrossRef] [Green Version]
- Gillison, M.L.; Trotti, A.M.; Harris, J.; Eisbruch, A.; Harari, P.M.; Adelstein, D.J.; Jordan, R.C.K.; Zhao, W.; Sturgis, E.M.; Burtness, B.; et al. Radiotherapy plus cetuximab or cisplatin in human papillomavirus-positive oropharyngeal cancer (NRG Oncology RTOG 1016): A randomised, multicentre, non-inferiority trial. Lancet 2019, 393, 40–50. [Google Scholar] [CrossRef]
- Burdick, J.S.; Chung, E.; Tanner, G.; Sun, M.; Paciga, J.E.; Cheng, J.Q.; Washington, K.; Goldenring, J.R.; Coffey, R.J. Treatment of Ménétrier’s Disease with a Monoclonal Antibody against the Epidermal Growth Factor Receptor. N. Engl. J. Med. 2000, 343, 1697–1701. [Google Scholar] [CrossRef] [PubMed]
- Sigismund, S.; Avanzato, D.; Lanzetti, L. Emerging functions of theEGFRin cancer. Mol. Oncol. 2018, 12, 3–20. [Google Scholar] [CrossRef] [Green Version]
- Mirza, Y.; Ali, S.M.A.; Awan, M.S.; Idress, R.; Naeem, S.; Zahid, N.; Qadeer, U. Overexpression of EGFR in Oral Premalignant Lesions and OSCC and Its Impact on Survival and Recurrence. Oncomedicine 2018, 3, 28–36. [Google Scholar] [CrossRef] [Green Version]
- Singla, S.; Singla, G.; Zaheer, S.; Rawat, D.S.; Mandal, A.K. Expression of p53, epidermal growth factor receptor, c-erbB2 in oral leukoplakias and oral squamous cell carcinomas. J. Cancer Res. Ther. 2018, 14, 388–393. [Google Scholar] [CrossRef]
- Ries, J.; Vairaktaris, E.; Agaimy, A.; Bechtold, M.; Gorecki, P.; Neukam, F.W.; Nkenke, E. The relevance of EGFR overexpression for the prediction of the malignant transformation of oral leukoplakia. Oncol. Rep. 2013, 30, 1149–1156. [Google Scholar] [CrossRef] [Green Version]
- Swaminathan, U.; Joshua, E.; Rao, U.K.; Ranganathan, K. Expression of p53 and Cyclin D1 in oral squamous cell carcinoma and normal mucosa: An Immunohistochemical study. J. Oral Maxillofac. Pathol. 2012, 16, 172–177. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kaur, J.; Srivastava, A.; Ralhan, R. Overexpression of p53 protein in betel- and tobacco-related human oral dysplasia and squamous-cell carcinoma in India. Int. J. Cancer 1994, 58, 340–345. [Google Scholar] [CrossRef] [PubMed]
- Kerdpon, D.; Rich, A.M.; Reade, P.C. Expression of p53 in oral mucosal hyperplasia, dysplasia and squamous cell carcinoma. Oral Dis. 1997, 3, 86–92. [Google Scholar] [CrossRef]
- Liu, K.; Jiang, T.; Ouyang, Y.; Shi, Y.; Zang, Y.; Li, N.; Lu, S.; Chen, D. Nuclear EGFR impairs ASPP2-p53 complex-induced apoptosis by inducing SOS1 expression in hepatocellular carcinoma. Oncotarget 2015, 6, 16507–16516. [Google Scholar] [CrossRef] [Green Version]
- Ghorbanpour, M.; Shahsavari, F.; Miri, R. Expression of epidermal growth factor receptor in oral and esophageal squamous-cell carcinoma. Dent. Res. J. 2020, 17, 85. [Google Scholar] [CrossRef]
- Costa, V.; Kowalski, L.; Coutinho-Camillo, C.; Begnami, M.; Calsavara, V.; Neves, J.; Kaminagakura, E. EGFR amplification and expression in oral squamous cell carcinoma in young adults. Int. J. Oral Maxillofac. Surg. 2018, 47, 817–823. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Abbas, E.A.; Abdel-Aal, W.E.; Samy, A.A. Evaluation Of Transforming Growth Factor (Tgf-) And Epidermal Growth Factor Receptor (Egfr) Expression In Oral Squamous Cell Carcinoma. Egypt. J. Hosp. Med. 2002, 7, 168–176. [Google Scholar] [CrossRef]
- Brands, M.T.; Smeekens, E.A.J.; Takes, R.P.; Kaanders, J.H.A.M.; Verbeek, A.L.M.; Merkx, M.A.W.; Geurts, S.M.E. Time patterns of recurrence and second primary tumors in a large cohort of patients treated for oral cavity cancer. Cancer Med. 2019, 8, 5810–5819. [Google Scholar] [CrossRef] [Green Version]
- Schmidt-Ullrich, R.K.; Valerie, K.; Fogleman, P.B.; Walters, J. Radiation-induced autophosphorylation of epidermal growth factor receptor in human malignant mammary and squamous epithelial cells. Radiat. Res. 1996, 145, 81–85. [Google Scholar] [CrossRef]
- Dittmann, K.; Mayer, C.; Fehrenbacher, B.; Schaller, M.; Raju, U.; Milas, L.; Chen, D.J.; Kehlbach, R.; Rodemann, H.P. Radiation-induced epidermal growth factor receptor nuclear import is linked to activation of DNA-dependent protein kinase. J. Biol. Chem. 2005, 280, 31182–31189. [Google Scholar] [CrossRef] [Green Version]
- da Silva, L.A.B.; Lopes, M.L.D.D.S.; Sá, M.C.; Freitas, R.D.A.; Della Coletta, R.; da Silveira, E.J.D.; Miguel, M.C.D.C. Histopathologic grading and its relationship with outcome in oral tongue squamous cell carcinoma. J. Oral Pathol. Med. 2021, 50, 183–190. [Google Scholar] [CrossRef]
- Elsheikh, S.; Green, A.R.; Aleskandarany, M.A.; Grainge, M.; Paish, C.E.; Lambros, M.B.K.; Reis-Filho, J.S.; Ellis, I.O. CCND1 amplification and cyclin D1 expression in breast cancer and their relation with proteomic subgroups and patient outcome. Breast Cancer Res. Treat. 2008, 109, 325–335. [Google Scholar] [CrossRef]
- Zhang, Y.-Y.; Xu, Z.-N.; Wang, J.-X.; Wei, D.-M.; Pan, X.-L. G1/S-specific cyclin-D1 might be a prognostic biomarker for patients with laryngeal squamous cell carcinoma. Asian Pac. J. Cancer Prev. 2012, 13, 2133–2137. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- AbdulMajeed, A.A.; Dalley, A.J.; Farah, C.S. Putative cancer stem cell marker expression in oral epithelial dysplasia and squamous cell carcinoma. J. Oral Pathol. Med. 2013, 42, 755–760. [Google Scholar] [CrossRef] [PubMed]




| Group of Subjects | Age (Years) | Gender | Number of Subjects |
|---|---|---|---|
| Control group with healthy oral mucosa | 56.56 ± 11.97 54.28 ± 12.22 52.7 ± 11.84 | ♂ 32 ♀ 27 | 59 |
| Patients with premalignant changes | 64.22 ± 14.35 64.6 ± 9.92 63.9 ± 17.46 | ♂ 23 ♀ 27 | 50 |
| Patients with Oral squamous cell carcinoma | 55 ± 10.91 56 ± 10.87 54 ± 11.22 | ♂ 35 ♀ 17 | 52 |
| Molecular Biomarker | Ki-67 | p53 | Cyclin D1 | ABCG2 | nEGFR | mEGFR | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| IHC scoring | ≤30% | > 30% | 0/+ | ++ | 0/+ | ++ | 0/+ | ++/+++ | 0/+ | ++/+++ | 0/+ | ++/+++ | ||||||
| Subject number | n = 30 | n = 22 | n = 9 | n = 43 | n = 9 | n = 43 | n = 9 | n = 43 | n = 22 | n = 30 | n = 19 | n = 33 | ||||||
| harmful habits | ||||||||||||||||||
| none | 0 | 8 | χ2 = 0.359, p = 0.55 | 3 | 9 | χ2 = 1.46, p = 0.48 | 3 | 10 | χ2 = 2.20, p = 0.33 | 4 | 9 | χ2 = 0.28, p = 0.59 | 7 | 8 | χ2 = 9.71, p = 0.002 | 6 | 7 | χ2 = 1.85, p = 0.60 |
| alcohol abuse | 18 | 15 | 9 | 25 | 3 | 16 | 4 | 15 | 8 | 11 | 6 | 13 | ||||||
| smoking | 8 | 17 | 8 | 22 | 6 | 33 | 5 | 34 | 17 | 22 | 13 | 26 | ||||||
| both factors | 8 | 15 | 8 | 22 | 2 | 15 | 3 | 14 | 8 | 11 | 6 | 13 | ||||||
| TNM disease stage | ||||||||||||||||||
| I | 9 | 5 | χ2 = 2.02, p = 0.73 | 4 | 10 | χ2 = 9.76, p = 0.28 | 2 | 12 | χ2 = 5.48, p = 0.70 | 4 | 10 | χ2 = 4.67, p = 0.32 | 2 | 12 | χ2 = 14.58, p = 0.26 | 4 | 10 | χ2 = 6.66, p = 0.88 |
| II | 7 | 5 | 4 | 8 | 2 | 10 | 3 | 9 | 8 | 4 | 5 | 7 | ||||||
| III | 6 | 4 | 1 | 9 | 1 | 9 | 0 | 10 | 4 | 6 | 3 | 7 | ||||||
| IV A | 5 | 7 | 7 | 5 | 4 | 8 | 2 | 10 | 5 | 7 | 5 | 7 | ||||||
| IV B | 3 | 1 | 2 | 2 | 0 | 4 | 0 | 4 | 3 | 1 | 2 | 2 | ||||||
| IV C | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | ||||||
| histological tumor grade | ||||||||||||||||||
| 1 | 18 | 10 | χ2 = 1.08, p = 0.58 | 9 | 19 | χ2 = 3.16, p = 0.53 | 6 | 22 | χ2 = 3.47, p = 0.48 | 6 | 22 | χ2 = 1.24, p = 0.53 | 14 | 14 | χ2 = 6.2, p = 0.40 | 10 | 16 | χ2 = 5.93, p = 0.43 |
| 2 | 10 | 10 | 7 | 13 | 3 | 17 | 3 | 17 | 6 | 14 | 6 | 14 | ||||||
| 3 | 2 | 2 | 2 | 2 | 0 | 4 | 0 | 4 | 2 | 2 | 3 | 1 | ||||||
| lymphovascular invasion | ||||||||||||||||||
| absent | 24 | 16 | p = 0.55 * | 14 | 26 | p = 0.53 * | 7 | 33 | p = 0.85 * | 9 | 31 | p = 0.07 * | 18 | 22 | p = 0.88 * | 15 | 25 | p = 0.78 * |
| present | 6 | 6 | 4 | 8 | 2 | 10 | 0 | 12 | 4 | 8 | 4 | 8 | ||||||
| perineural invasion | ||||||||||||||||||
| absent | 16 | 12 | p = 0.93 * | 9 | 19 | p = 0.02 * | 6 | 22 | p = 0.54 * | 8 | 20 | p = 0.02 * | 12 | 16 | p = 0.25 * | 10 | 18 | p = 0.80 * |
| present | 14 | 10 | 9 | 15 | 3 | 21 | 1 | 26 | 10 | 14 | 9 | 15 | ||||||
| extranodal extension | ||||||||||||||||||
| absent | 26 | 20 | p = 0.64 * | 15 | 31 | p = 0.67 | 9 | 37 | p = 0.49 * | 9 | 37 | p = 0.23 | 18 | 28 | p = 0.5 * | 18 | 19 | p = 0.25 * |
| present | 4 | 2 | 3 | 3 | 0 | 6 | 0 | 6 | 4 | 2 | 2 | 4 | ||||||
| disease progression | ||||||||||||||||||
| no | 24 | 16 | p = 0.54 * | 12 | 28 | χ2 = 1.59, p = 0.66 | 8 | 32 | p = 0.62 * | 9 | 31 | χ2 = 1.59, p = 0.07 | 16 | 21 | p = 0.51 * | 14 | 26 | p = 0.66 * |
| yes | 6 | 6 | 6 | 6 | 1 | 11 | 0 | 12 | 6 | 6 | 5 | 7 | ||||||
| HPV status | ||||||||||||||||||
| negative | 28 | 22 | p = 0.22 * | 16 | 34 | p = 0.11 * | 8 | 42 | p = 0.38 * | 9 | 41 | p = 0.51 * | 21 | 26 | p = 0.88 * | 17 | 33 | p = 0.19 * |
| positive | 2 | 0 | 2 | 0 | 1 | 1 | 0 | 2 | 1 | 1 | 2 | 0 | ||||||
| second primary tumor | ||||||||||||||||||
| no | 27 | 16 | p = 0.11 * | 17 | 26 | p = 0.24 * | 7 | 36 | p = 0.75 * | 8 | 35 | p = 0.58 * | 18 | 23 | p = 0.35 * | 16 | 27 | p = 0.87 * |
| yes | 3 | 6 | 1 | 8 | 2 | 7 | 9 | 0 | 4 | 3 | 3 | 6 | ||||||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tarle, M.; Raguž, M.; Muller, D.; Lukšić, I. Nuclear Epidermal Growth Factor Receptor Overexpression as a Survival Predictor in Oral Squamous Cell Carcinoma. Int. J. Mol. Sci. 2023, 24, 5816. https://doi.org/10.3390/ijms24065816
Tarle M, Raguž M, Muller D, Lukšić I. Nuclear Epidermal Growth Factor Receptor Overexpression as a Survival Predictor in Oral Squamous Cell Carcinoma. International Journal of Molecular Sciences. 2023; 24(6):5816. https://doi.org/10.3390/ijms24065816
Chicago/Turabian StyleTarle, Marko, Marina Raguž, Danko Muller, and Ivica Lukšić. 2023. "Nuclear Epidermal Growth Factor Receptor Overexpression as a Survival Predictor in Oral Squamous Cell Carcinoma" International Journal of Molecular Sciences 24, no. 6: 5816. https://doi.org/10.3390/ijms24065816
APA StyleTarle, M., Raguž, M., Muller, D., & Lukšić, I. (2023). Nuclear Epidermal Growth Factor Receptor Overexpression as a Survival Predictor in Oral Squamous Cell Carcinoma. International Journal of Molecular Sciences, 24(6), 5816. https://doi.org/10.3390/ijms24065816








