Physiological and Pathophysiological Roles of IgM Fc Receptor (FcµR) Isoforms
Abstract
1. Introduction
2. Disputes on the Cellular Distribution of FcµR
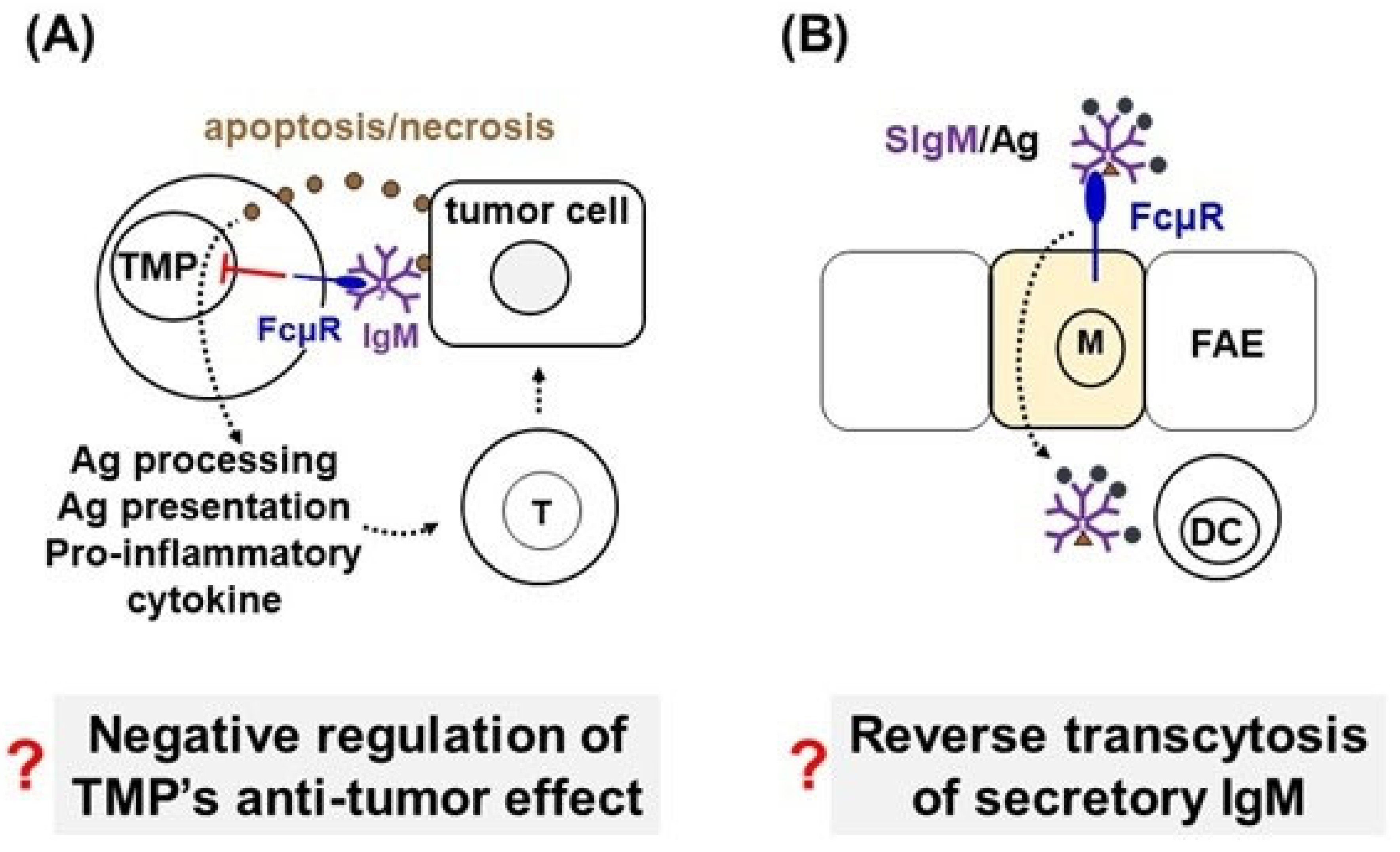
2.1. Questioning Whether FcµR Is Expressed by Mouse Innate Immune Cells
2.2. Reverse Transcytosis of Secretory IgM via FcµR Expressed on M Cells in Peyer’s Patches
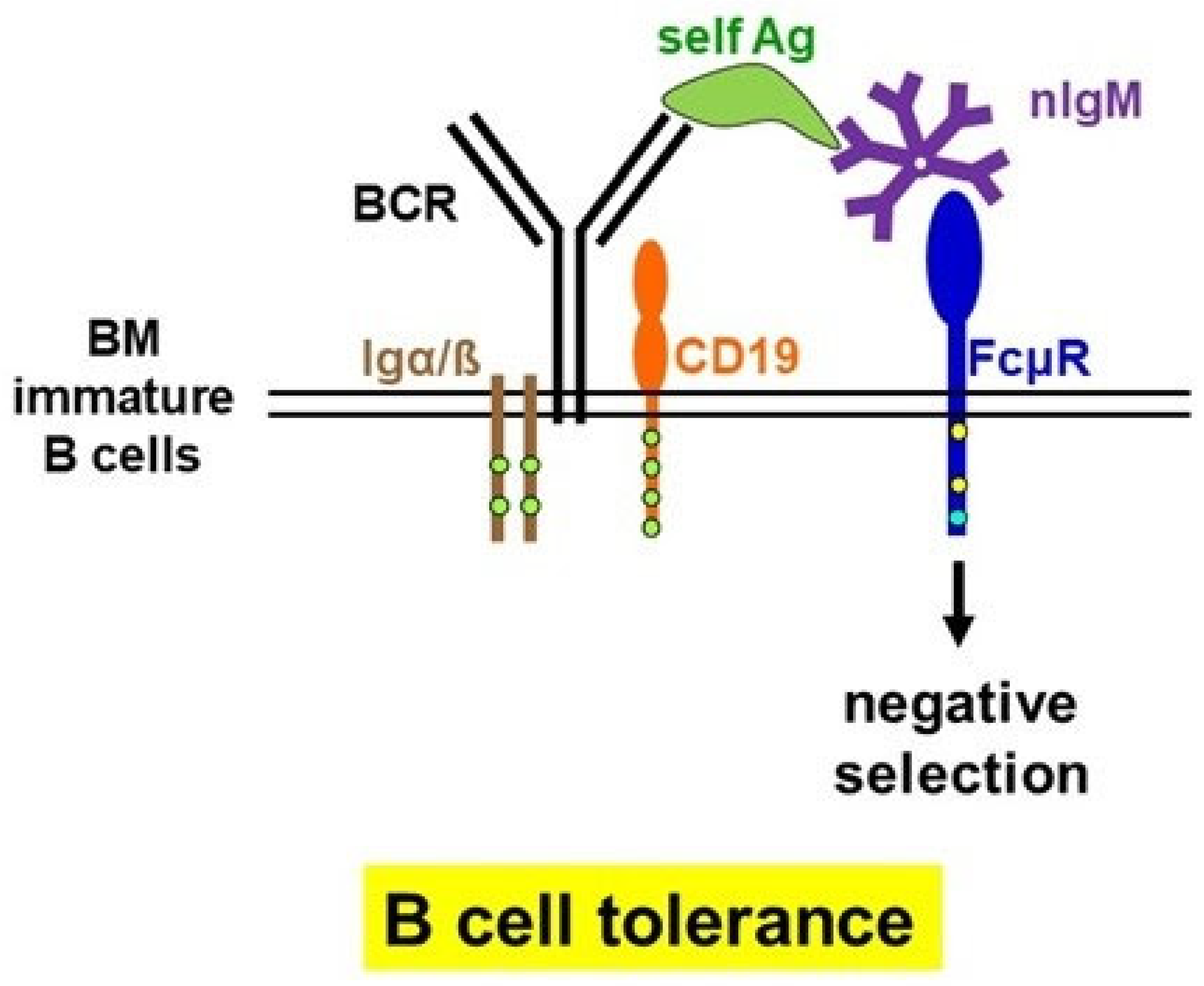
3. Common and Conflicting Findings in Fcmr-Deficient Mice
4. Structural and Signaling Aspects of FcµR
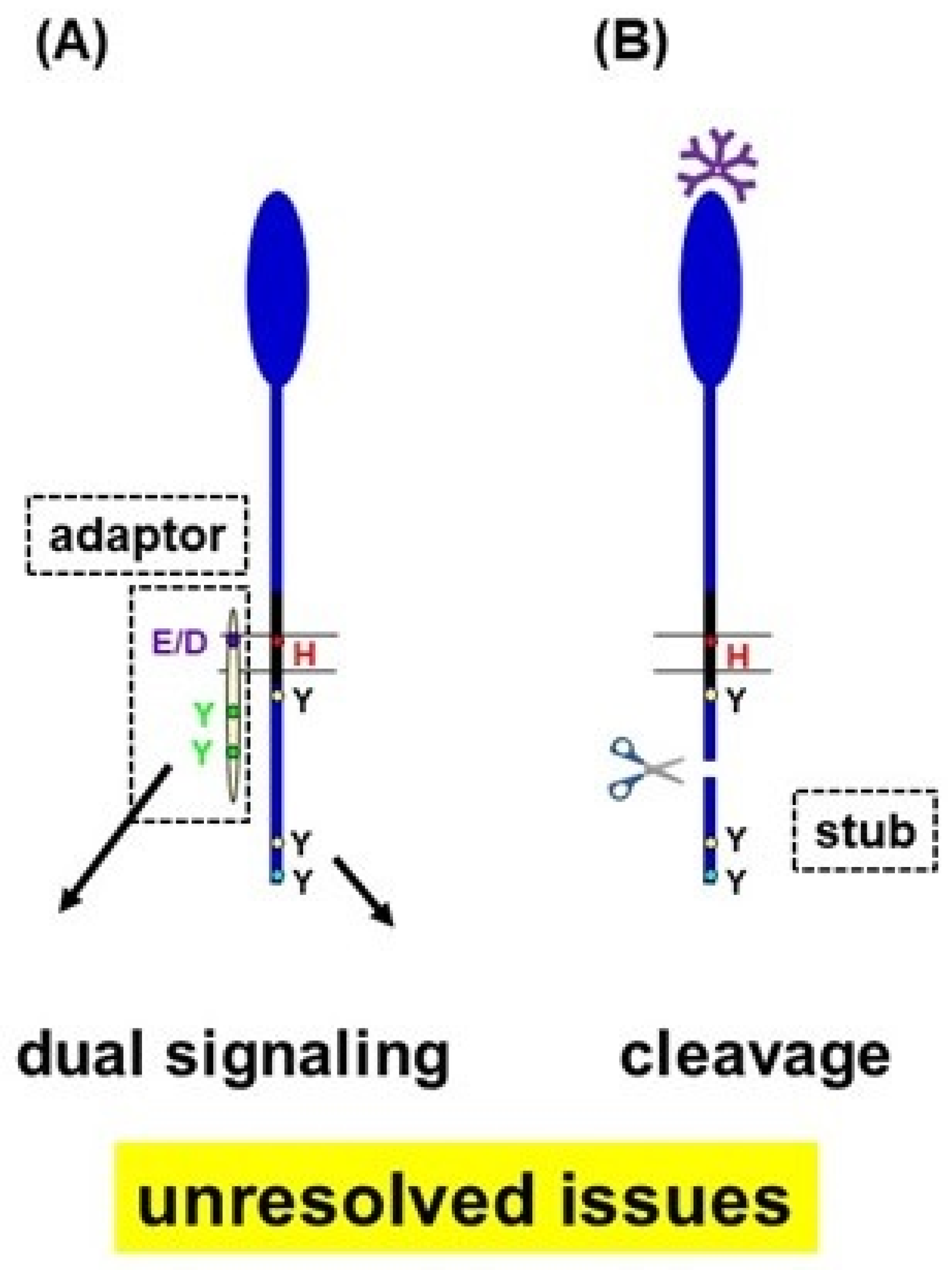
4.1. Dual-Signaling Potential of FcµR
4.2. Signaling Function of the Ig-Tail Tyrosine (ITT)-like Motif of FcµR
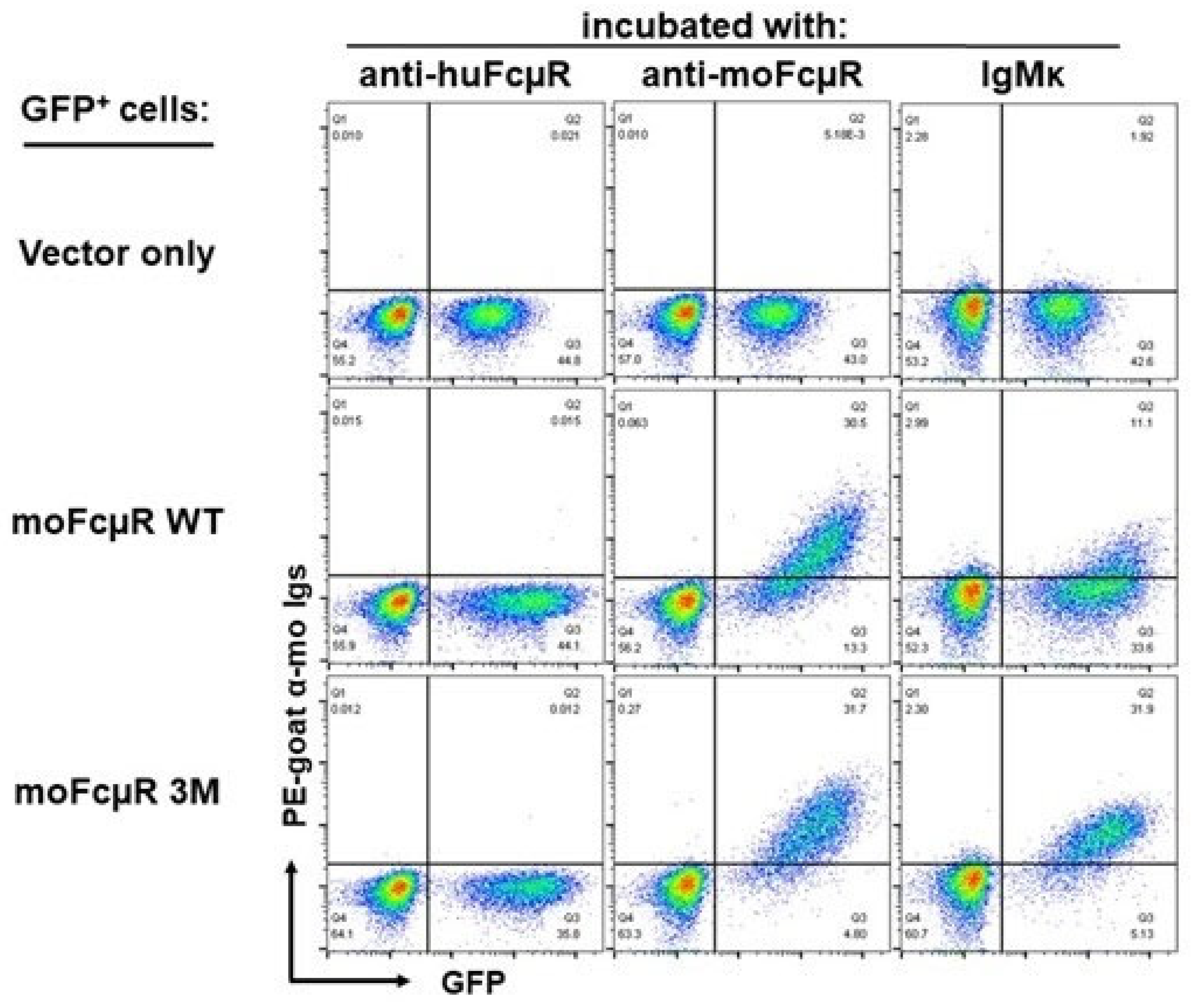
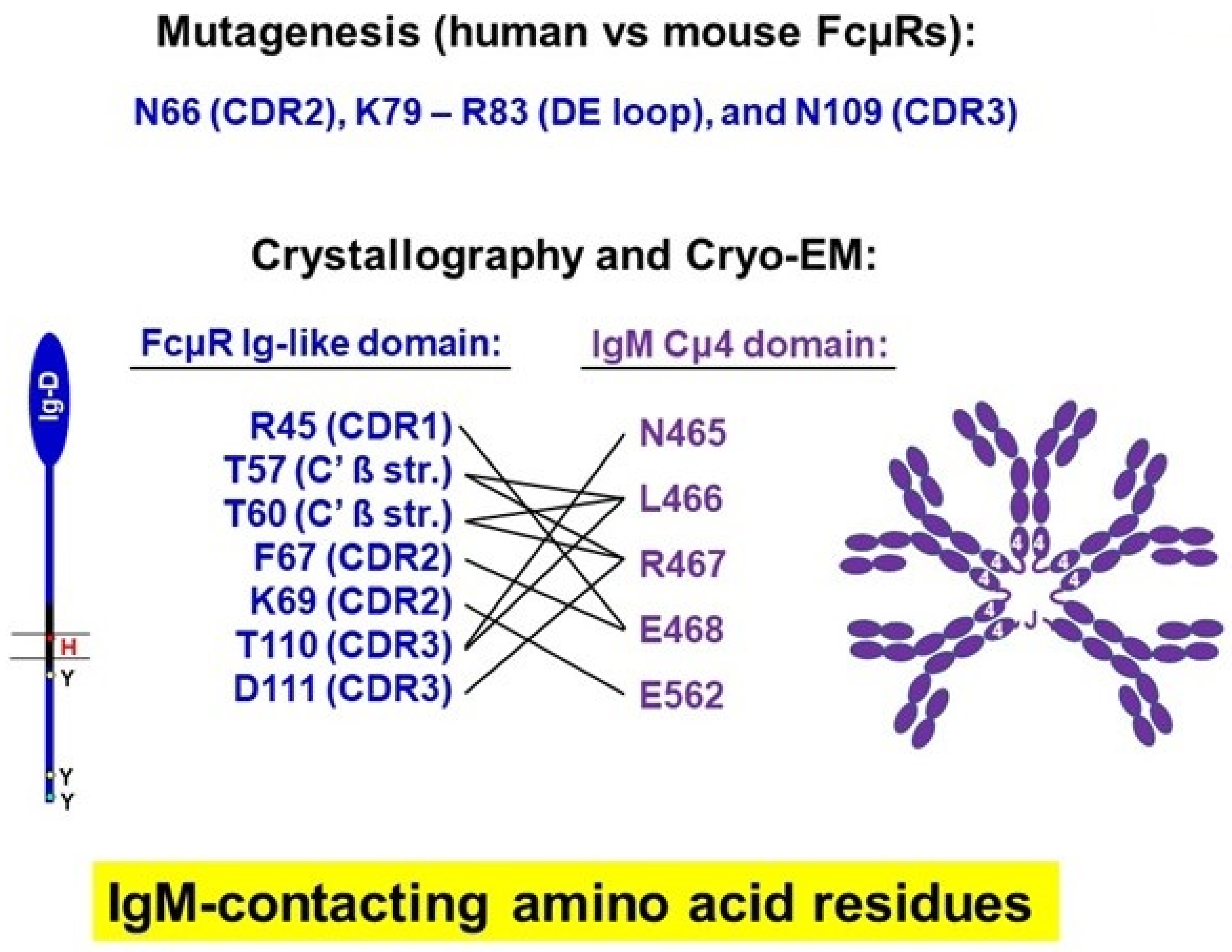
4.3. Identification of Amino Acid Residues of FcµR Critical for IgM Binding
4.4. Crystallographic Analysis of Human FcµR
5. Ex Vivo Functions of Human FcµR and Its Association with Diseases
5.1. Ex Vivo Functions
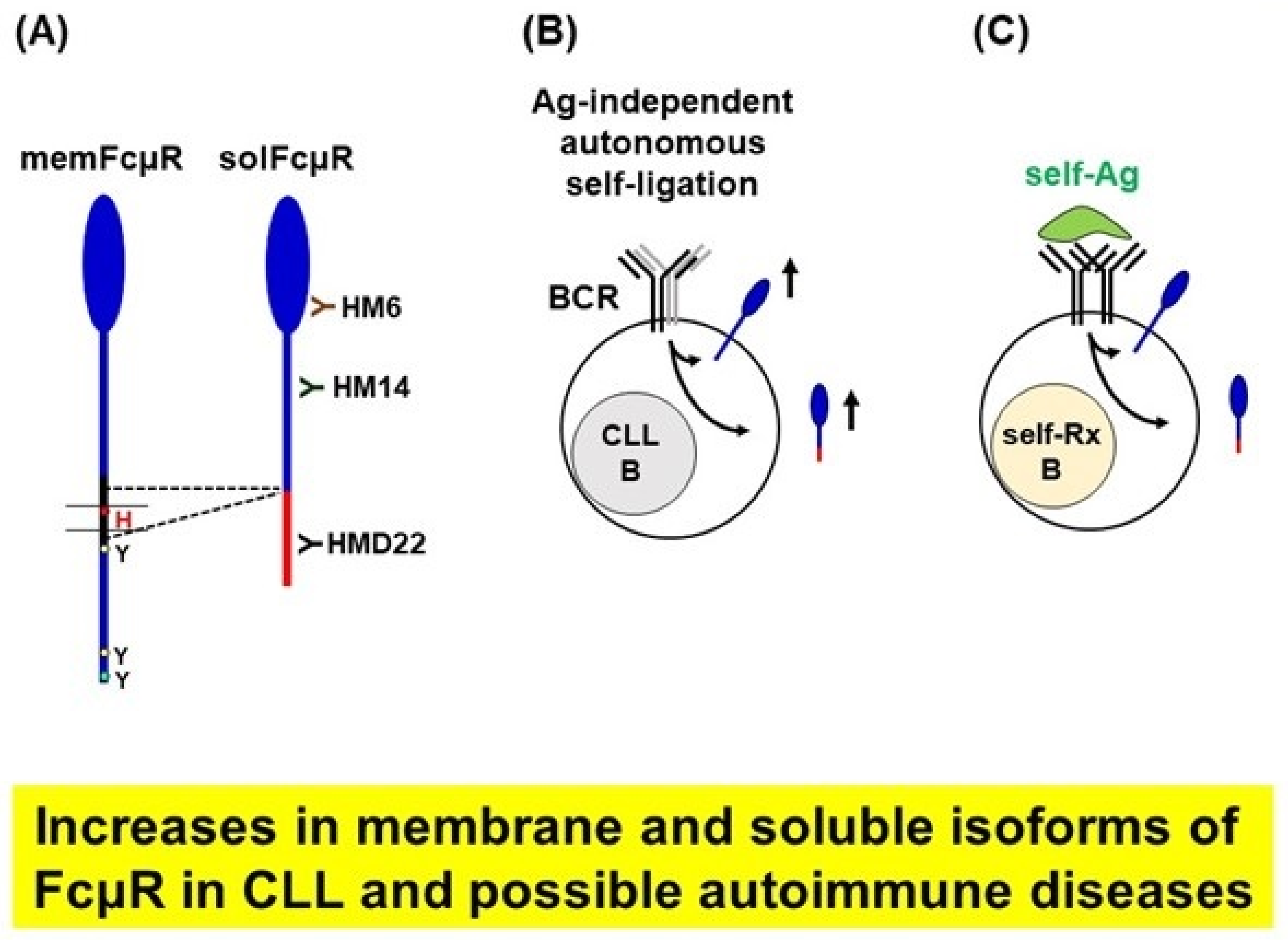
5.2. Enhanced Levels of the Soluble Form of FcµR in Patients with Chronic Lymphocytic Leukemia
5.3. Lessons from Studies on FcµR in CLL
5.4. Generation of mAbs Specific for solFcµR
6. Epilogue
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Cooper, M.D.; Miller, J. Discovery of 2 Distinctive Lineages of Lymphocytes, T Cells and B Cells, as the Basis of the Adaptive Immune System and Immunologic Function: 2019 Albert Lasker Basic Medical Research Award. JAMA 2019, 322, 1247–1248. [Google Scholar] [CrossRef]
- Gomes de Castro, M.A.; Wildhagen, H.; Sograte-Idrissi, S.; Hitzing, C.; Binder, M.; Trepel, M.; Engels, N.; Opazo, F. Differential organization of tonic and chronic B cell antigen receptors in the plasma membrane. Nat. Commun. 2019, 10, 820. [Google Scholar] [CrossRef]
- Ehrenstein, M.R.; Notley, C.A. The importance of natural IgM: Scavenger, protector and regulator. Nat. Rev. Immunol. 2010, 10, 778–786. [Google Scholar] [CrossRef]
- Blandino, R.; Baumgarth, N. Secreted IgM: New tricks for an old molecule. J. Leukoc. Biol. 2019, 106, 1021–1034. [Google Scholar] [CrossRef] [PubMed]
- Heyman, B. Regulation of antibody responses via antibodies, complement, and Fc receptors. Annu. Rev. Immunol. 2000, 18, 709–737. [Google Scholar] [CrossRef]
- Ding, Z.; Bergman, A.; Rutemark, C.; Ouchida, R.; Ohno, H.; Wang, J.Y.; Heyman, B. Complement-activating IgM enhances the humoral but not the T cell immune response in mice. PLoS ONE 2013, 8, e81299. [Google Scholar] [CrossRef]
- Kubagawa, H.; Oka, S.; Kubagawa, Y.; Torii, I.; Takayama, E.; Kang, D.W.; Gartland, G.L.; Bertoli, L.F.; Mori, H.; Takatsu, H.; et al. Identity of the elusive IgM Fc receptor (FcμR) in humans. J. Exp. Med. 2009, 206, 2779–2793. [Google Scholar] [CrossRef]
- Klimovich, V.B. IgM and its receptors: Structural and functional aspects. Biochemistry 2011, 76, 534–549. [Google Scholar] [CrossRef] [PubMed]
- Kubagawa, H.; Oka, S.; Kubagawa, Y.; Torii, I.; Takayama, E.; Kang, D.W.; Jones, D.; Nishida, N.; Miyawaki, T.; Bertoli, L.F.; et al. The long elusive IgM Fc receptor, FcmR. J. Clin. Immunol. 2014, 34 (Suppl. S1), S35–S45. [Google Scholar] [CrossRef] [PubMed]
- Kubagawa, H.; Kubagawa, Y.; Jones, D.; Nasti, T.H.; Walter, M.R.; Honjo, K. The old but new IgM Fc receptor (FcmR). Curr. Top. Microbiol. Immunol. 2014, 382, 3–28. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.; Coligan, J.E.; Morse, H.C., 3rd. Emerging functions of natural IgM and its Fc receptor FCMR in immune homeostasis. Front. Immunol. 2016, 7, 99. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.; Wang, Y.; Xiong, E.; Hong, R.; Lu, Q.; Ohno, H.; Wang, J.Y. Role of the IgM Fc Receptor in Immunity and Tolerance. Front. Immunol. 2019, 10, 529. [Google Scholar] [CrossRef] [PubMed]
- Kubagawa, H.; Honjo, K.; Ohkura, N.; Sakaguchi, S.; Radbruch, A.; Melchers, F.; Jani, P.K. Functional roles of the IgM Fc receptor in the immune system. Front. Immunol. 2019, 10, 945. [Google Scholar] [CrossRef]
- Ravetch, J.V.; Nimmerjahn, F. Fundamental Immunology, 6th ed.; Paul, W.E., Ed.; Lippincott Williams & Wilkins: Philadelphia, PA, USA, 2008; pp. 684–705. [Google Scholar]
- Shima, H.; Takatsu, H.; Fukuda, S.; Ohmae, M.; Hase, K.; Kubagawa, H.; Wang, J.Y.; Ohno, H. Identification of TOSO/FAIM3 as an Fc receptor for IgM. Int. Immunol. 2010, 22, 149–156. [Google Scholar] [CrossRef]
- Lang, K.S.; Lang, P.A.; Meryk, A.; Pandyra, A.A.; Boucher, L.M.; Pozdeev, V.I.; Tusche, M.W.; Gothert, J.R.; Haight, J.; Wakeham, A.; et al. Involvement of Toso in activation of monocytes, macrophages, and granulocytes. Proc. Natl. Acad. Sci. USA 2013, 110, 2593–2598. [Google Scholar] [CrossRef]
- Brenner, D.; Brustle, A.; Lin, G.H.; Lang, P.A.; Duncan, G.S.; Knobbe-Thomsen, C.B.; St, P.M.; Reardon, C.; Tusche, M.W.; Snow, B.; et al. Toso controls encephalitogenic immune responses by dendritic cells and regulatory T cells. Proc. Natl. Acad. Sci. USA 2014, 111, 1060–1065. [Google Scholar] [CrossRef] [PubMed]
- Gaublomme, J.T.; Yosef, N.; Lee, Y.; Gertner, R.S.; Yang, L.V.; Wu, C.; Pandolfi, P.P.; Mak, T.; Satija, R.; Shalek, A.K.; et al. Single-cell genomics unveils critical regulators of Th17 cell pathogenicity. Cell 2015, 163, 1400–1412. [Google Scholar] [CrossRef]
- Kubli, S.P.; Vornholz, L.; Duncan, G.; Zhou, W.; Ramachandran, P.; Fortin, J.; Cox, M.; Han, S.; Nechanitzky, R.; Nechanitzky, D.; et al. Fcmr regulates mononuclear phagocyte control of anti-tumor immunity. Nat. Commun. 2019, 10, 2678. [Google Scholar] [CrossRef]
- Skopnik, C.M.; Riedel, R.; Addo, R.K.; Heinz, G.A.; Heinrich, F.; Honjo, K.; Durek, P.; Enghard, P.; Mashreghi, M.F.; Radbruch, A.; et al. Questioning whether IgM Fc receptor (FcmicroR) is expressed by innate immune cells. Nat. Commun. 2022, 13, 3951. [Google Scholar] [CrossRef] [PubMed]
- Summers, K.M.; Bush, S.J.; Hume, D.A. Network analysis of transcriptomic diversity amongst resident tissue macrophages and dendritic cells in the mouse mononuclear phagocyte system. PLoS Biol. 2020, 18, e3000859. [Google Scholar] [CrossRef] [PubMed]
- Millard, S.M.; Heng, O.; Opperman, K.S.; Sehgal, A.; Irvine, K.M.; Kaur, S.; Sandrock, C.J.; Wu, A.C.; Magor, G.W.; Batoon, L.; et al. Fragmentation of tissue-resident macrophages during isolation confounds analysis of single-cell preparations from mouse hematopoietic tissues. Cell Rep. 2021, 37, 110058. [Google Scholar] [CrossRef]
- Kubli, S.P.; Ramachandran, P.; Duncan, G.; Brokx, R.; Mak, T.W. Reply to: Questioning whether the IgM Fc receptor (FcmuR) is expressed by innate immune cells. Nat. Commun. 2022, 13, 3950. [Google Scholar] [CrossRef] [PubMed]
- Owen, R.L. Uptake and transport of intestinal macromolecules and microorganisms by M cells in Peyer’s patches—A personal and historical perspective. Semin. Immunol. 1999, 11, 157–163. [Google Scholar] [CrossRef] [PubMed]
- Dillon, A.; Lo, D.D. M Cells: Intelligent Engineering of Mucosal Immune Surveillance. Front. Immunol. 2019, 10, 1499. [Google Scholar] [CrossRef] [PubMed]
- Rochereau, N.; Drocourt, D.; Perouzel, E.; Pavot, V.; Redelinghuys, P.; Brown, G.D.; Tiraby, G.; Roblin, X.; Verrier, B.; Genin, C.; et al. Dectin-1 is essential for reverse transcytosis of glycosylated SIgA-antigen complexes by intestinal M cells. PLoS Biol. 2013, 11, e1001658. [Google Scholar] [CrossRef]
- Rochereau, N.; Michaud, E.; Waeckel, L.; Killian, M.; Gayet, R.; Goguyer-Deschaumes, R.; Roblin, X.; Biolley, G.; Corthesy, B.; Paul, S. Essential role of TOSO/FAIM3 in intestinal IgM reverse transcytosis. Cell Rep. 2021, 37, 110006. [Google Scholar] [CrossRef]
- Kitagawa, Y.; Ohkura, N.; Kidani, Y.; Vandenbon, A.; Hirota, K.; Kawakami, R.; Yasuda, K.; Motooka, D.; Nakamura, S.; Kondo, M.; et al. Guidance of regulatory T cell development by Satb1-dependent super-enhancer establishment. Nat. Immunol. 2017, 18, 173–183. [Google Scholar] [CrossRef] [PubMed]
- Ouchida, R.; Mori, H.; Hase, K.; Takatsu, H.; Kurosaki, T.; Tokuhisa, T.; Ohno, H.; Wang, J.Y. Critical role of the IgM Fc receptor in IgM homeostasis, B-cell survival, and humoral immune responses. Proc. Natl. Acad. Sci. USA 2012, 109, E2699–E2706. [Google Scholar] [CrossRef] [PubMed]
- Jani, P.K.; Kubagawa, H.; Melchers, F. A rheostat sets B-cell receptor repertoire selection to distinguish self from non-self. Curr. Opin. Immunol. 2020, 67, 42–49. [Google Scholar] [CrossRef]
- Engels, N.; Konig, L.M.; Heemann, C.; Lutz, J.; Tsubata, T.; Griep, S.; Schrader, V.; Wienands, J. Recruitment of the cytoplasmic adaptor Grb2 to surface IgG and IgE provides antigen receptor-intrinsic costimulation to class-switched B cells. Nat. Immunol. 2009, 10, 1018–1025. [Google Scholar] [CrossRef]
- Engels, N.; Wienands, J. The signaling tool box for tyrosine-based costimulation of lymphocytes. Curr. Opin. Immunol. 2011, 23, 324–329. [Google Scholar] [CrossRef] [PubMed]
- Vire, B.; David, A.; Wiestner, A. TOSO, the Fcμ receptor, is highly expressed on chronic lymphocytic leukemia B cells, internalizes upon IgM binding, shuttles to the lysosome, and is downregulated in response to TLR activation. J. Immunol. 2011, 187, 4040–4050. [Google Scholar] [CrossRef] [PubMed]
- Honjo, K.; Kubagawa, Y.; Kearney, J.F.; Kubagawa, H. Unique ligand-binding property of the human IgM Fc receptor. J. Immunol. 2015, 194, 1975–1982. [Google Scholar] [CrossRef] [PubMed]
- Kubagawa, H.; Chen, C.C.; Ho, L.H.; Shimada, T.S.; Gartland, L.; Mashburn, C.; Uehara, T.; Ravetch, J.V.; Cooper, M.D. Biochemical nature and cellular distribution of the paired immunoglobulin-like receptors, PIR-A and PIR-B. J. Exp. Med. 1999, 189, 309–318. [Google Scholar] [CrossRef] [PubMed]
- Gardiner, E.E.; Karunakaran, D.; Arthur, J.F.; Mu, F.T.; Powell, M.S.; Baker, R.I.; Hogarth, P.M.; Kahn, M.L.; Andrews, R.K.; Berndt, M.C. Dual ITAM-mediated proteolytic pathways for irreversible inactivation of platelet receptors: De-ITAM-izing FcgRIIa. Blood 2008, 111, 165–174. [Google Scholar] [CrossRef]
- Hamburger, A.E.; West, A.P., Jr.; Bjorkman, P.J. Crystal structure of a polymeric immunoglobulin binding fragment of the human polymeric immunoglobulin receptor. Structure 2004, 12, 1925–1935. [Google Scholar] [CrossRef]
- Stadtmueller, B.M.; Huey-Tubman, K.E.; Lopez, C.J.; Yang, Z.; Hubbell, W.L.; Bjorkman, P.J. The structure and dynamics of secretory component and its interactions with polymeric immunoglobulins. eLife 2016, 5, e10640. [Google Scholar] [CrossRef]
- Skopnik, C.M.; Al-Qaisi, K.; Calvert, R.A.; Enghard, P.; Radbruch, A.; Sutton, B.J.; Kubagawa, H. Identification of amino acid residues in human IgM Fc receptor (FcmicroR) critical for IgM binding. Front. Immunol. 2021, 11, 618327. [Google Scholar] [CrossRef]
- Kubagawa, H.; Skopnik, C.M.; Al-Qaisi, K.; Calvert, R.A.; Honjo, K.; Kubagawa, Y.; Teuber, R.; Aliabadi, P.M.; Enghard, P.; Radbruch, A.; et al. Differences between human and mouse IgM Fc receptor (FcmicroR). Int. J. Mol. Sci. 2021, 22, 7024. [Google Scholar] [CrossRef]
- Hitoshi, Y.; Lorens, J.; Kitada, S.I.; Fisher, J.; LaBarge, M.; Ring, H.Z.; Francke, U.; Reed, J.C.; Kinoshita, S.; Nolan, G.P. Toso, a cell surface, specific regulator of Fas-induced apoptosis in T cells. Immunity 1998, 8, 461–471. [Google Scholar] [CrossRef]
- Murakami, Y.; Narayanan, S.; Su, S.; Childs, R.; Krzewski, K.; Borrego, F.; Weck, J.; Coligan, J.E. Toso, a functional IgM receptor, is regulated by IL-2 in T and NK cells. J. Immunol. 2012, 189, 587–597. [Google Scholar] [CrossRef]
- Pallasch, C.P.; Schulz, A.; Kutsch, N.; Schwamb, J.; Hagist, S.; Kashkar, H.; Ultsch, A.; Wickenhauser, C.; Hallek, M.; Wendtner, C.M. Overexpression of TOSO in CLL is triggered by B-cell receptor signaling and associated with progressive disease. Blood 2008, 112, 4213–4219. [Google Scholar] [CrossRef] [PubMed]
- Proto-Siqueira, R.; Panepucci, R.A.; Careta, F.P.; Lee, A.; Clear, A.; Morris, K.; Owen, C.; Rizzatti, E.G.; Silva, W.A., Jr.; Falcao, R.P.; et al. SAGE analysis demonstrates increased expression of TOSO contributing to Fas-mediated resistance in CLL. Blood 2008, 112, 394–397. [Google Scholar] [CrossRef] [PubMed]
- Li, F.J.; Kubagawa, Y.; McCollum, M.K.; Wilson, L.; Motohashi, T.; Bertoli, L.F.; Barton, J.C.; Barnes, S.; Davis, R.S.; Kubagawa, H. Enhanced levels of both membrane-bound and soluble forms of IgM Fc receptor (FcmR) in patients with chronic lymphocytic leukemia. Blood 2011, 118, 4902–4909. [Google Scholar] [CrossRef]
- Meryk, A.; Pangrazzi, L.; Hagen, M.; Hatzmann, F.; Jenewein, B.; Jakic, B.; Hermann-Kleiter, N.; Baier, G.; Jylhava, J.; Hurme, M.; et al. Fcmu receptor as a Costimulatory Molecule for T Cells. Cell Rep. 2019, 26, 2681–2691.e2685. [Google Scholar] [CrossRef] [PubMed]
- Pichler, W.J.; Knapp, W. Receptors for IgM-coated erythrocytes on chronic lymphatic leukemia cells. J. Immunol. 1977, 118, 1010–1015. [Google Scholar] [CrossRef] [PubMed]
- Ferrarini, M.; Hoffman, T.; Fu, S.M.; Winchester, R.; Kunkel, H.G. Receptors for IgM on certain human B lymphocytes. J. Immunol. 1977, 119, 1525–1529. [Google Scholar] [CrossRef]
- Sanders, S.K.; Kubagawa, H.; Suzuki, T.; Butler, J.L.; Cooper, M.D. IgM binding protein expressed by activated B cells. J. Immunol. 1987, 139, 188–193. [Google Scholar] [CrossRef]
- Ohno, T.; Kubagawa, H.; Sanders, S.K.; Cooper, M.D. Biochemical nature of an Fcm receptor on human B-lineage cells. J. Exp. Med. 1990, 172, 1165–1175. [Google Scholar] [CrossRef]
- Rosenwald, A.; Alizadeh, A.A.; Widhopf, G.; Simon, R.; Davis, R.E.; Yu, X.; Yang, L.; Pickeral, O.K.; Rassenti, L.Z.; Powell, J.; et al. Relation of gene expression phenotype to immunoglobulin mutation genotype in B cell chronic lymphocytic leukemia. J. Exp. Med. 2001, 194, 1639–1647. [Google Scholar] [CrossRef]
- Duhren-von, M.M.; Ubelhart, R.; Schneider, D.; Wossning, T.; Bach, M.P.; Buchner, M.; Hofmann, D.; Surova, E.; Follo, M.; Kohler, F.; et al. Chronic lymphocytic leukaemia is driven by antigen-independent cell-autonomous signalling. Nature 2012, 489, 309–312. [Google Scholar]
- Minici, C.; Gounari, M.; Ubelhart, R.; Scarfo, L.; Duhren-von Minden, M.; Schneider, D.; Tasdogan, A.; Alkhatib, A.; Agathangelidis, A.; Ntoufa, S.; et al. Distinct homotypic B-cell receptor interactions shape the outcome of chronic lymphocytic leukaemia. Nat. Commun. 2017, 8, 15746. [Google Scholar] [CrossRef] [PubMed]
- Landeira-Vinuela, A.; Arias-Hidalgo, C.; Juanes-Velasco, P.; Alcoceba, M.; Navarro-Bailon, A.; Pedreira, C.E.; Lecrevisse, Q.; Diaz-Munoz, L.; Sanchez-Santos, J.M.; Hernandez, A.P.; et al. Unravelling soluble immune checkpoints in chronic lymphocytic leukemia: Physiological immunomodulators or immune dysfunction. Front. Immunol. 2022, 13, 965905. [Google Scholar] [CrossRef] [PubMed]
- Mahmoudi Aliabadi, P.; Teuber, R.; Jani, P.K.; Wilson, L.; Enghard, P.; Barnes, S.; Chiorazzi, N.; Radbruch, A.; Melchers, F.; Kubagawa, H. Soluble Fc Receptor for IgM in Sera From Subsets of Patients With Chronic Lymphocytic Leukemia as Determined by a New Mouse Monoclonal Antibody. Front. Immunol. 2022, 13, 863895. [Google Scholar] [CrossRef] [PubMed]
- Akula, S.; Mohammadamin, S.; Hellman, L. Fc receptors for immunoglobulins and their appearance during vertebrate evolution. PLoS ONE 2014, 9, e96903. [Google Scholar] [CrossRef] [PubMed]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kubagawa, H.; Clark, C.; Skopnik, C.M.; Mahmoudi Aliabadi, P.; Al-Qaisi, K.; Teuber, R.; Jani, P.K.; Radbruch, A.; Melchers, F.; Engels, N.; et al. Physiological and Pathophysiological Roles of IgM Fc Receptor (FcµR) Isoforms. Int. J. Mol. Sci. 2023, 24, 5728. https://doi.org/10.3390/ijms24065728
Kubagawa H, Clark C, Skopnik CM, Mahmoudi Aliabadi P, Al-Qaisi K, Teuber R, Jani PK, Radbruch A, Melchers F, Engels N, et al. Physiological and Pathophysiological Roles of IgM Fc Receptor (FcµR) Isoforms. International Journal of Molecular Sciences. 2023; 24(6):5728. https://doi.org/10.3390/ijms24065728
Chicago/Turabian StyleKubagawa, Hiromi, Caren Clark, Christopher M. Skopnik, Pedram Mahmoudi Aliabadi, Khlowd Al-Qaisi, Ruth Teuber, Peter K. Jani, Andreas Radbruch, Fritz Melchers, Niklas Engels, and et al. 2023. "Physiological and Pathophysiological Roles of IgM Fc Receptor (FcµR) Isoforms" International Journal of Molecular Sciences 24, no. 6: 5728. https://doi.org/10.3390/ijms24065728
APA StyleKubagawa, H., Clark, C., Skopnik, C. M., Mahmoudi Aliabadi, P., Al-Qaisi, K., Teuber, R., Jani, P. K., Radbruch, A., Melchers, F., Engels, N., & Wienands, J. (2023). Physiological and Pathophysiological Roles of IgM Fc Receptor (FcµR) Isoforms. International Journal of Molecular Sciences, 24(6), 5728. https://doi.org/10.3390/ijms24065728






