Inhibition of BET Proteins Regulates Fcγ Receptor Function and Reduces Inflammation in Rheumatoid Arthritis
Abstract
1. Introduction
2. Results and Discussionγ
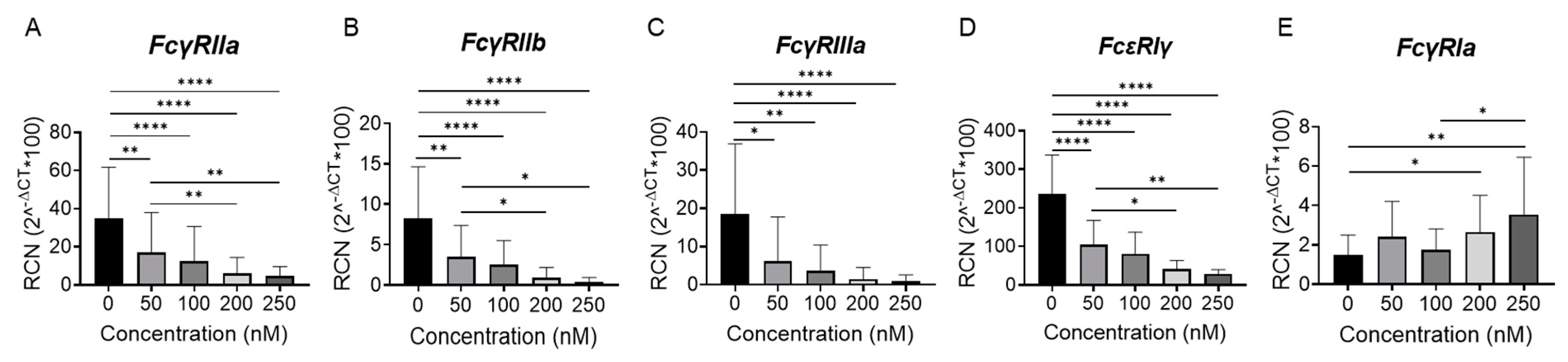
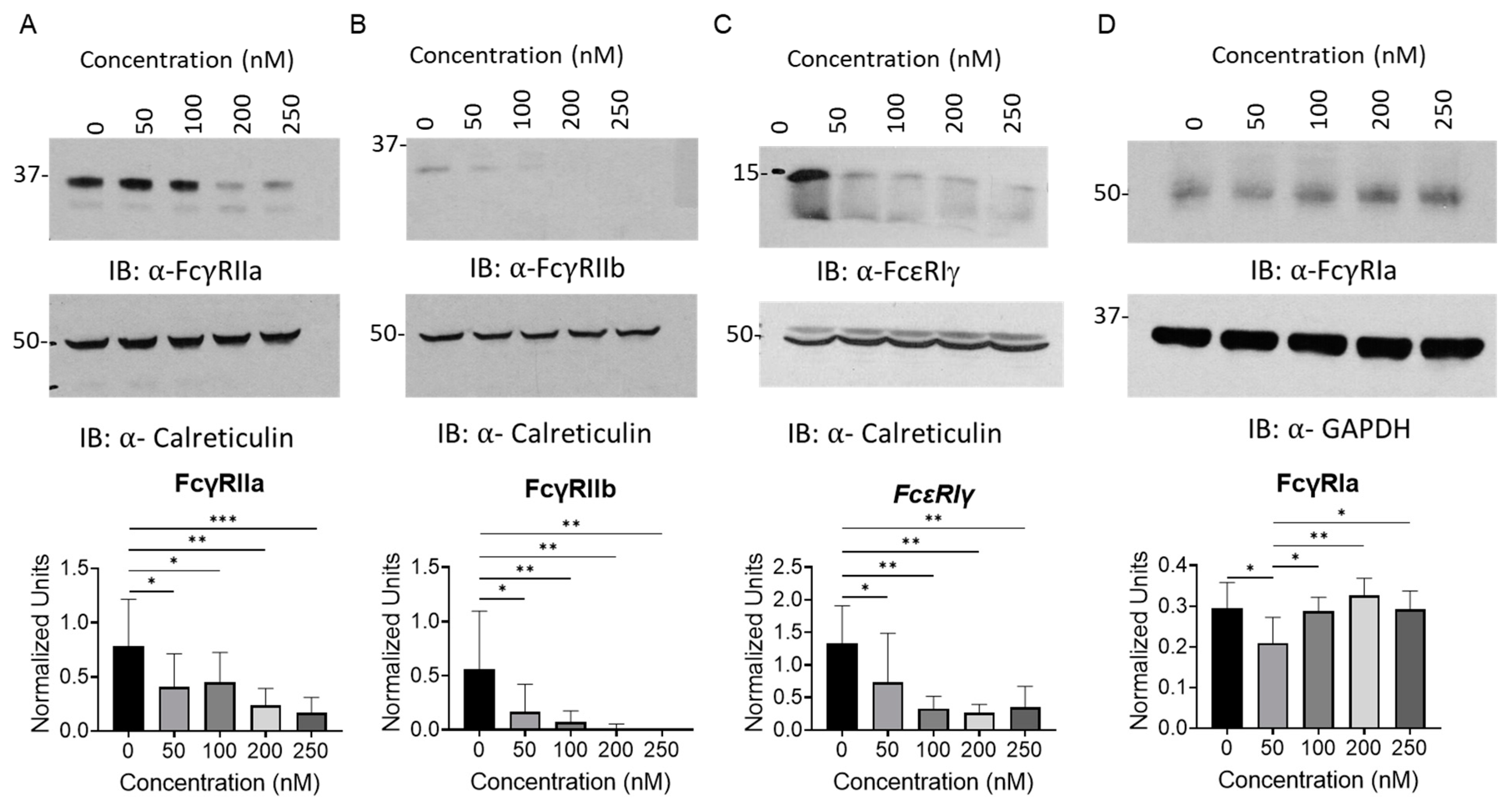
2.1. BET Inhibition Reduces Fcγ Receptor Expression in Peripheral Blood Monocytes
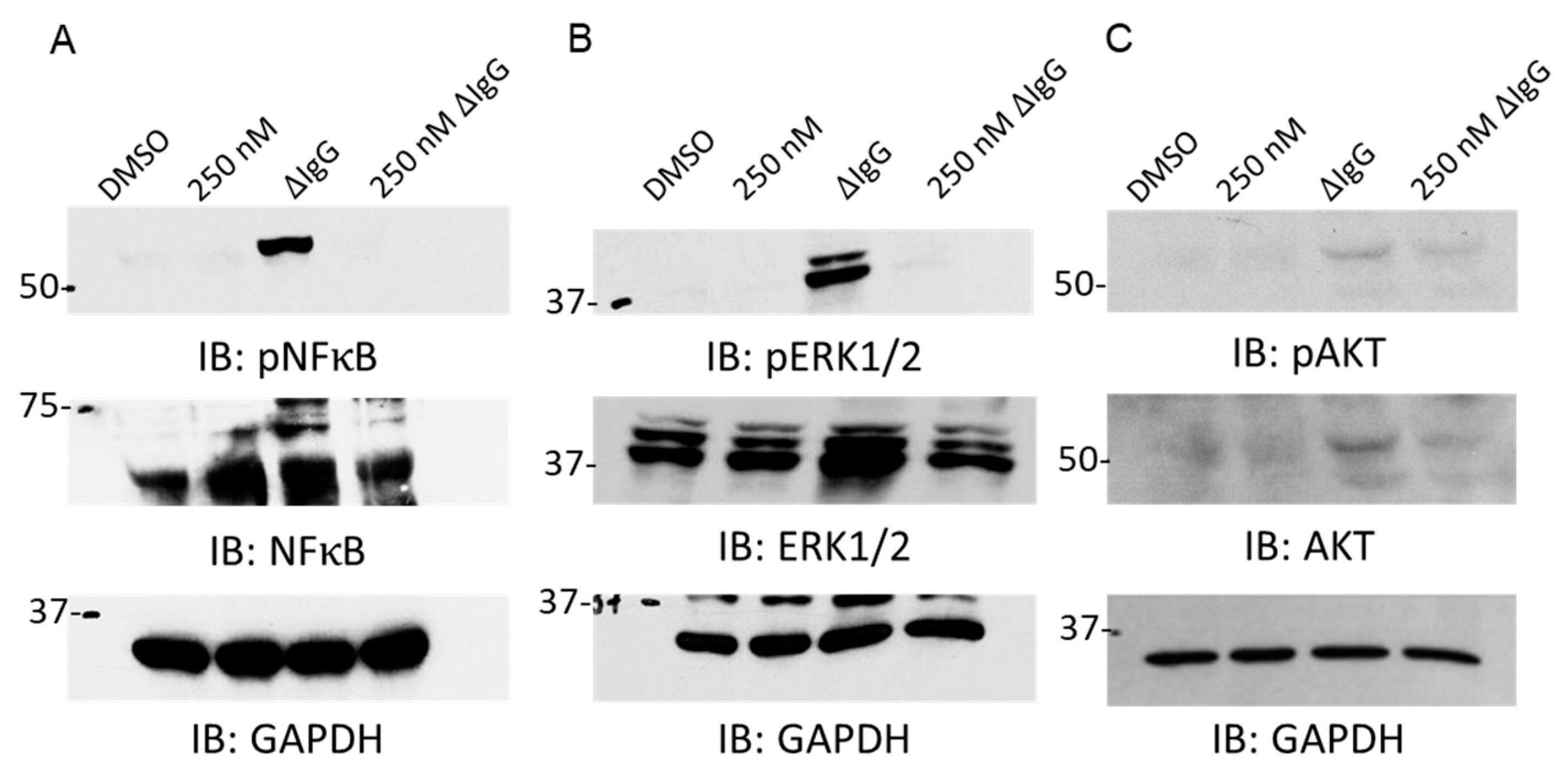
2.2. BET Inhibition Downregulates Fcγ Receptor-Mediated Signaling
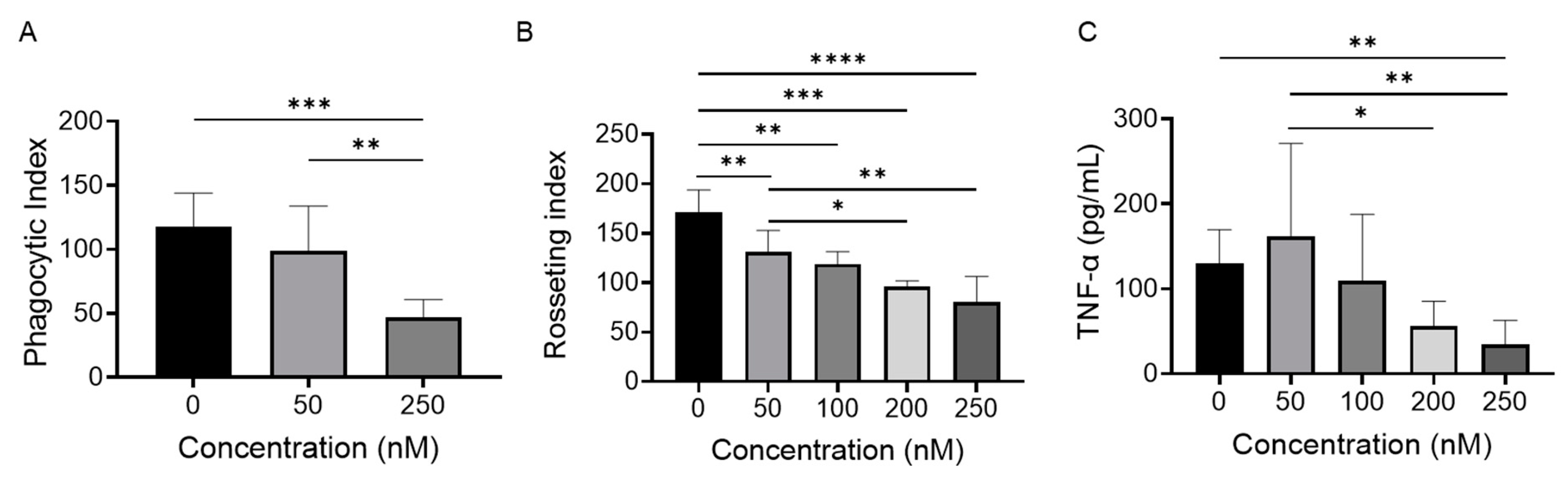
2.3. BET Inhibition Attenuates Monocyte FcγR Function
2.4. BET Inhibition Regulates Fc𝛾 Receptor Expression and Function in Monocytes from Rheumatoid Arthritis Patients
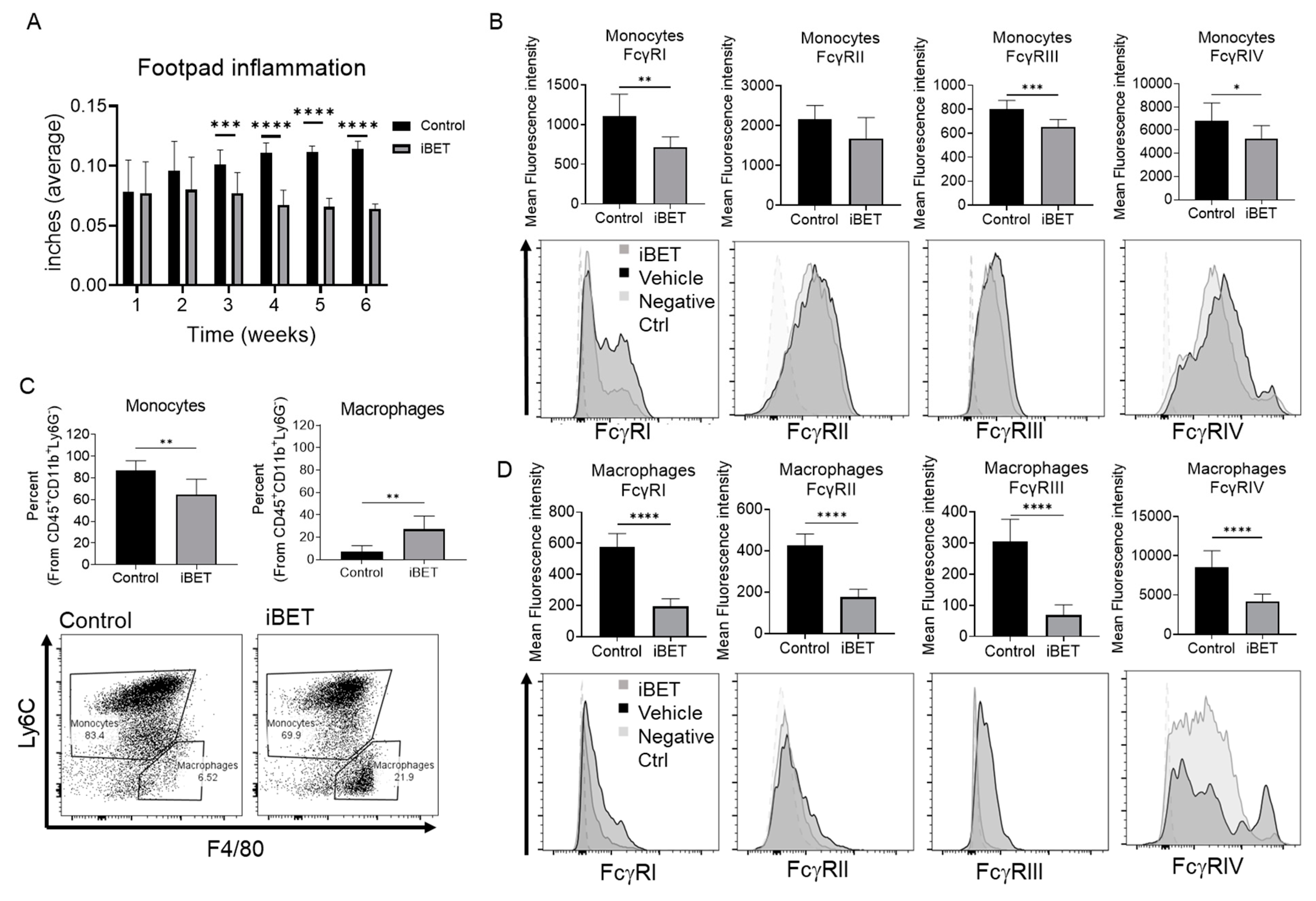
2.5. BET Inhibition Reduces Footpad Inflammation and FcγR Expression In Vivo
3. Materials and Methods
3.1. Peripheral Blood Monocyte Isolation
3.2. Cell Culture Treatments
3.3. Immunoblotting
3.4. Phagocytosis
3.5. Rosetting
3.6. Real-Time Polymerase Chain Reaction (qPCR)
3.7. Enzyme Linked Immunosorbence Assay (ELISA)
3.8. Fc𝛾 Receptor Flow Cytometry
3.9. Collagen-Induced Arthritis Mouse Model
3.10. Statistical Analysis
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Pisetsky, D.S. Advances in the Treatment of Rheumatoid Arthritis: Costs and Challenges. N. C. Med. J. 2017, 78, 337–340. [Google Scholar] [CrossRef] [PubMed]
- Gravallese, E.M.; Firestein, G.S. Rheumatoid Arthritis—Common Origins, Divergent Mechanisms. N. Engl. J. Med. 2023, 388, 529–542. [Google Scholar] [CrossRef] [PubMed]
- Ding, Q.; Hu, W.; Wang, R.; Yang, Q.; Zhu, M.; Li, M.; Cai, J.; Rose, P.; Mao, J.; Zhu, Y.Z. Signaling pathways in rheumatoid arthritis: Implications for targeted therapy. Signal Transduct. Target. Ther. 2023, 8, 68. [Google Scholar] [CrossRef] [PubMed]
- Song, Y.; Kang, E. Autoantibodies in rheumatoid arthritis: Rheumatoid factors and anticitrullinated protein antibodies. QJM Int. J. Med. 2009, 103, 139–146. [Google Scholar] [CrossRef]
- Magnusson, S.E.; Wennerberg, E.; Matt, P.; Lindqvist, U.; Kleinau, S. Dysregulated Fc receptor function in active rheumatoid arthritis. Immunol. Lett. 2014, 162 Pt A, 200–206. [Google Scholar] [CrossRef]
- Ben Mkaddem, S.; Benhamou, M.; Monteiro, R.C. Understanding Fc Receptor Involvement in Inflammatory Diseases: From Mechanisms to New Therapeutic Tools. Front. Immunol. 2019, 10, 811. [Google Scholar] [CrossRef]
- Guo, Q.; Wang, Y.; Xu, D.; Nossent, J.; Pavlos, N.J.; Xu, J. Rheumatoid arthritis: Pathological mechanisms and modern pharmacologic therapies. Bone Res. 2018, 6, 15. [Google Scholar] [CrossRef]
- Bullock, J.; Rizvi, S.A.; Saleh, A.M.; Ahmed, S.S.; Do, D.P.; Ansari, R.A.; Ahmed, J. Rheumatoid Arthritis: A Brief Overview of the Treatment. Med. Princ. Pract. 2018, 27, 501–507. [Google Scholar] [CrossRef]
- Mok, C.C. Rituximab for the treatment of rheumatoid arthritis: An update. Drug Des. Dev. Ther. 2013, 8, 87–100. [Google Scholar] [CrossRef]
- Watanabe, R.; Okano, T.; Gon, T.; Yoshida, N.; Fukumoto, K.; Yamada, S.; Hashimoto, M. Difficult-to-treat rheumatoid arthritis: Current concept and unsolved problems. Front. Med. 2022, 9, 1049875. [Google Scholar] [CrossRef]
- Taniguchi, Y. The Bromodomain and Extra-Terminal Domain (BET) Family: Functional Anatomy of BET Paralogous Proteins. Int. J. Mol. Sci. 2016, 17, 1849. [Google Scholar] [CrossRef] [PubMed]
- Nicodeme, E.; Jeffrey, K.L.; Schaefer, U.; Beinke, S.; Dewell, S.; Chung, C.-W.; Chandwani, R.; Marazzi, I.; Wilson, P.; Coste, H.; et al. Suppression of inflammation by a synthetic histone mimic. Nature 2010, 468, 1119–1123. [Google Scholar] [CrossRef] [PubMed]
- Ozer, H.G.; El-Gamal, D.; Powell, B.; Hing, Z.A.; Blachly, J.S.; Harrington, B.; Mitchell, S.; Grieselhuber, N.R.; Williams, K.; Lai, T.-H.; et al. BRD4 Profiling Identifies Critical Chronic Lymphocytic Leukemia Oncogenic Circuits and Reveals Sensitivity to PLX51107, a Novel Structurally Distinct BET Inhibitor. Cancer Discov. 2018, 8, 458–477. [Google Scholar] [CrossRef] [PubMed]
- Rocklin, R.E.; Winston, C.T.; David, J.R. Activation of human blood monocytes by products of sensitized lymphocytes. J. Clin. Investig. 1974, 53, 559–564. [Google Scholar] [CrossRef] [PubMed]
- Johnson, W.D., Jr.; Mei, B.; Cohn, Z.A. The separation, long-term cultivation, and maturation of the human monocyte. J. Exp. Med. 1977, 146, 1613–1626. [Google Scholar] [CrossRef] [PubMed]
- Guilliams, M.; Mildner, A.; Yona, S. Developmental and Functional Heterogeneity of Monocytes. Immunity 2018, 49, 595–613. [Google Scholar] [CrossRef]
- van Vugt, M.J.; Heijnen, I.A.; Capel, P.J.; Park, S.Y.; Ra, C.; Saito, T.; Verbeek, J.S.; van de Winkel, J.G. FcR gamma-chain is essential for both surface expression and function of human Fc gamma RI (CD64) in vivo. Blood 1996, 87, 3593–3599. [Google Scholar] [CrossRef]
- Makarov, S.S. NF-κB in rheumatoid arthritis: A pivotal regulator of inflammation, hyperplasia, and tissue destruction. Arthritis Res. Ther. 2001, 3, 200. [Google Scholar] [CrossRef]
- Malemud, C.J. Intracellular Signaling Pathways in Rheumatoid Arthritis. J. Clin. Cell. Immunol. 2013, 4, 160. [Google Scholar] [CrossRef]
- Pietrosimone, K.; Jin, M.; Poston, B.; Liu, P. Collagen-induced Arthritis: A Model for Murine Autoimmune Arthritis. Bio-Protocol 2015, 5, e1626. [Google Scholar] [CrossRef] [PubMed]
- Nimmerjahn, F.; Bruhns, P.; Horiuchi, K.; Ravetch, J.V. FcgammaRIV: A novel FcR with distinct IgG subclass specificity. Immunity 2005, 23, 41–51. [Google Scholar] [CrossRef]
- Nimmerjahn, F.; Ravetch, J.V. Fcgamma receptors: Old friends and new family members. Immunity 2006, 24, 19–28. [Google Scholar] [CrossRef] [PubMed]
- Ren, L.; Campbell, A.; Fang, H.; Gautam, S.; Elavazhagan, S.; Fatehchand, K.; Mehta, P.; Stiff, A.; Reader, B.F.; Mo, X.; et al. Analysis of the Effects of the Bruton’s tyrosine kinase (Btk) Inhibitor Ibrutinib on Monocyte Fcγ Receptor (FcγR) Function. J. Biol. Chem. 2016, 291, 3043–3052. [Google Scholar] [CrossRef] [PubMed]
- Merchand-Reyes, G.; Robledo-Avila, F.H.; Buteyn, N.J.; Gautam, S.; Santhanam, R.; Fatehchand, K.; Mo, X.; Partida-Sanchez, S.; Butchar, J.P.; Tridandapani, S. CD31 Acts as a Checkpoint Molecule and Is Modulated by FcγR-Mediated Signaling in Monocytes. J. Immunol. 2019, 203, 3216–3224. [Google Scholar] [CrossRef] [PubMed]
- Buteyn, N.J.; Santhanam, R.; Merchand-Reyes, G.; Murugesan, R.A.; Dettorre, G.M.; Byrd, J.C.; Sarkar, A.; Vasu, S.; Mundy-Bosse, B.L.; Butchar, J.P.; et al. Activation of the Intracellular Pattern Recognition Receptor NOD2 Promotes Acute Myeloid Leukemia (AML) Cell Apoptosis and Provides a Survival Advantage in an Animal Model of AML. J. Immunol. 2020, 204, 1988–1997. [Google Scholar] [CrossRef] [PubMed]
- Nemtsova, M.V.; Zaletaev, D.V.; Bure, I.V.; Mikhaylenko, D.S.; Kuznetsova, E.B.; Alekseeva, E.A.; Beloukhova, M.; Deviatkin, A.A.; Lukashev, A.N.; Zamyatnin, A.A. Epigenetic Changes in the Pathogenesis of Rheumatoid Arthritis. Front. Genet. 2019, 10, 570. [Google Scholar] [CrossRef] [PubMed]
- Shorstova, T.; Foulkes, W.D.; Witcher, M. Achieving clinical success with BET inhibitors as anti-cancer agents. Br. J. Cancer 2021, 124, 1478–1490. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Q.-G.; Qian, J.; Zhu, Y.-C. Targeting bromodomain-containing protein 4 (BRD4) benefits rheumatoid arthritis. Immunol. Lett. 2015, 166, 103–108. [Google Scholar] [CrossRef]
- Li, X.; Kimberly, R.P. Targeting the Fc receptor in autoimmune disease. Expert Opin. Ther. Targets 2014, 18, 335–350. [Google Scholar] [CrossRef]
- Ji, H.; Ohmura, K.; Mahmood, U.; Lee, D.M.; Hofhuis, F.M.; Boackle, S.A.; Takahashi, K.; Holers, V.M.; Walport, M.; Gerard, C.; et al. Arthritis Critically Dependent on Innate Immune System Players. Immunity 2002, 16, 157–168. [Google Scholar] [CrossRef]
- Clavel, C.; Nogueira, L.; Laurent, L.; Iobagiu, C.; Vincent, C.; Sebbag, M.; Serre, G. Induction of macrophage secretion of tumor necrosis factor alpha through Fcgamma receptor IIa engagement by rheumatoid arthritis-specific autoantibodies to citrullinated proteins complexed with fibrinogen. Arthritis Rheum. 2008, 58, 678–688. [Google Scholar] [CrossRef] [PubMed]
- Wijngaarden, S.; van de Winkel, J.G.; Jacobs, K.M.; Bijlsma, J.W.; Lafeber, F.P.; van Roon, J.A. A shift in the balance of inhibitory and activating Fcgamma receptors on monocytes toward the inhibitory Fcgamma receptor IIb is associated with prevention of monocyte activation in rheumatoid arthritis. Arthritis Rheum. 2004, 50, 3878–3887. [Google Scholar] [CrossRef] [PubMed]
- Malemud, C.J. The role of the JAK/STAT signal pathway in rheumatoid arthritis. Ther. Adv. Musculoskelet. Dis. 2018, 10, 117–127. [Google Scholar] [CrossRef] [PubMed]
- Hajmirza, A.; Emadali, A.; Gauthier, A.; Casasnovas, O.; Gressin, R.; Callanan, M.B. BET Family Protein BRD4: An Emerging Actor in NFκB Signaling in Inflammation and Cancer. Biomedicines 2018, 6, 16. [Google Scholar] [CrossRef] [PubMed]
- Handel, M.L.; Mcmorrow, L.B.; Gravallese, E.M. Nuclear factor–kB in rheumatoid synovium. Localization of P50 and P65. Arthritis Rheum. 1995, 38, 1762–1770. [Google Scholar] [CrossRef]
- Vasanthi, P.; Nalini, G.; Rajasekhar, G. Role of tumor necrosis factor-alpha in rheumatoid arthritis: A review. Int. J. Rheum. Dis. 2007, 10, 270–274. [Google Scholar] [CrossRef]
- Seeling, M.; Hillenhoff, U.; David, J.P.; Schett, G.; Tuckermann, J.; Lux, A.; Nimmerjahn, F. Inflammatory monocytes and Fcγ receptor IV on osteoclasts are critical for bone destruction during inflammatory arthritis in mice. Proc. Natl. Acad. Sci. USA 2013, 110, 10729–10734. [Google Scholar] [CrossRef]
- Yuasa, T.; Kubo, S.; Yoshino, T.; Ujike, A.; Matsumura, K.; Ono, M.; Ravetch, J.V.; Takai, T. Deletion of Fcγ Receptor IIB Renders H-2b Mice Susceptible to Collagen-induced Arthritis. J. Exp. Med. 1999, 189, 187–194. [Google Scholar] [CrossRef]
- Kratofil, R.M.; Kubes, P.; Deniset, J.F. Monocyte Conversion During Inflammation and Injury. Arter. Thromb. Vasc. Biol. 2017, 37, 35–42. [Google Scholar] [CrossRef]
- Puchner, A.; Saferding, V.; Bonelli, M.; Mikami, Y.; Hofmann, M.; Brunner, J.S.; Caldera, M.; Goncalves-Alves, E.; Binder, N.B.; Fischer, A.; et al. Non-classical monocytes as mediators of tissue destruction in arthritis. Ann. Rheum. Dis. 2018, 77, 1490–1497. [Google Scholar] [CrossRef]
- Watanabe, S.; Alexander, M.; Misharin, A.V.; Budinger, G.S. The role of macrophages in the resolution of inflammation. J. Clin. Investig. 2019, 129, 2619–2628. [Google Scholar] [CrossRef] [PubMed]


Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Shankar, D.; Merchand-Reyes, G.; Buteyn, N.J.; Santhanam, R.; Fang, H.; Kumar, K.; Mo, X.; Ganesan, L.P.; Jarjour, W.; Butchar, J.P.; et al. Inhibition of BET Proteins Regulates Fcγ Receptor Function and Reduces Inflammation in Rheumatoid Arthritis. Int. J. Mol. Sci. 2023, 24, 7623. https://doi.org/10.3390/ijms24087623
Shankar D, Merchand-Reyes G, Buteyn NJ, Santhanam R, Fang H, Kumar K, Mo X, Ganesan LP, Jarjour W, Butchar JP, et al. Inhibition of BET Proteins Regulates Fcγ Receptor Function and Reduces Inflammation in Rheumatoid Arthritis. International Journal of Molecular Sciences. 2023; 24(8):7623. https://doi.org/10.3390/ijms24087623
Chicago/Turabian StyleShankar, Divya, Giovanna Merchand-Reyes, Nathaniel J. Buteyn, Ramasamy Santhanam, Huiqing Fang, Krishan Kumar, Xiaokui Mo, Latha P. Ganesan, Wael Jarjour, Jonathan P. Butchar, and et al. 2023. "Inhibition of BET Proteins Regulates Fcγ Receptor Function and Reduces Inflammation in Rheumatoid Arthritis" International Journal of Molecular Sciences 24, no. 8: 7623. https://doi.org/10.3390/ijms24087623
APA StyleShankar, D., Merchand-Reyes, G., Buteyn, N. J., Santhanam, R., Fang, H., Kumar, K., Mo, X., Ganesan, L. P., Jarjour, W., Butchar, J. P., & Tridandapani, S. (2023). Inhibition of BET Proteins Regulates Fcγ Receptor Function and Reduces Inflammation in Rheumatoid Arthritis. International Journal of Molecular Sciences, 24(8), 7623. https://doi.org/10.3390/ijms24087623







