Kinetic Aspects of Benzene Degradation over TiO2-N and Composite Fe/Bi2WO6/TiO2-N Photocatalysts under Irradiation with Visible Light
Abstract
1. Introduction
2. Results and Discussion
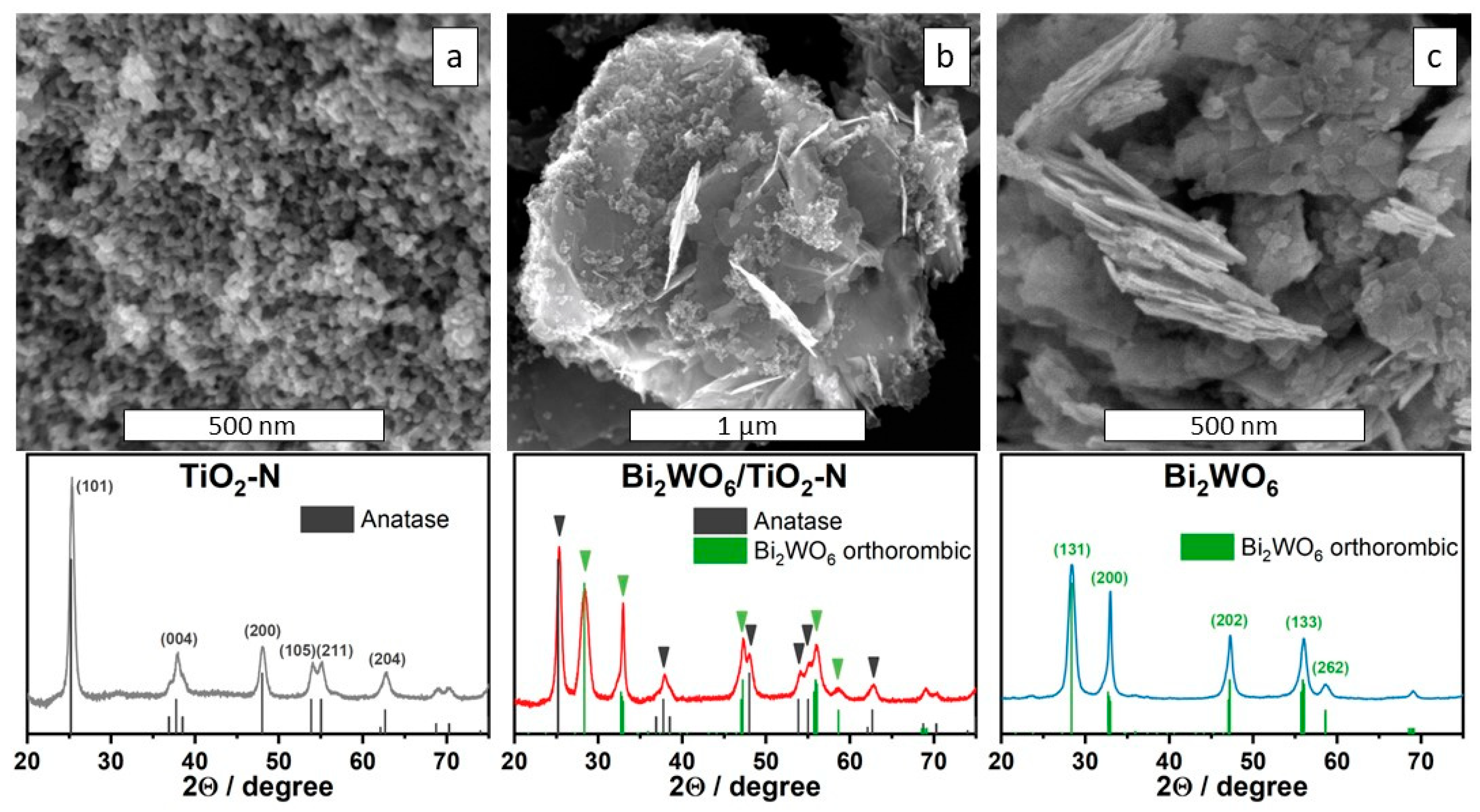
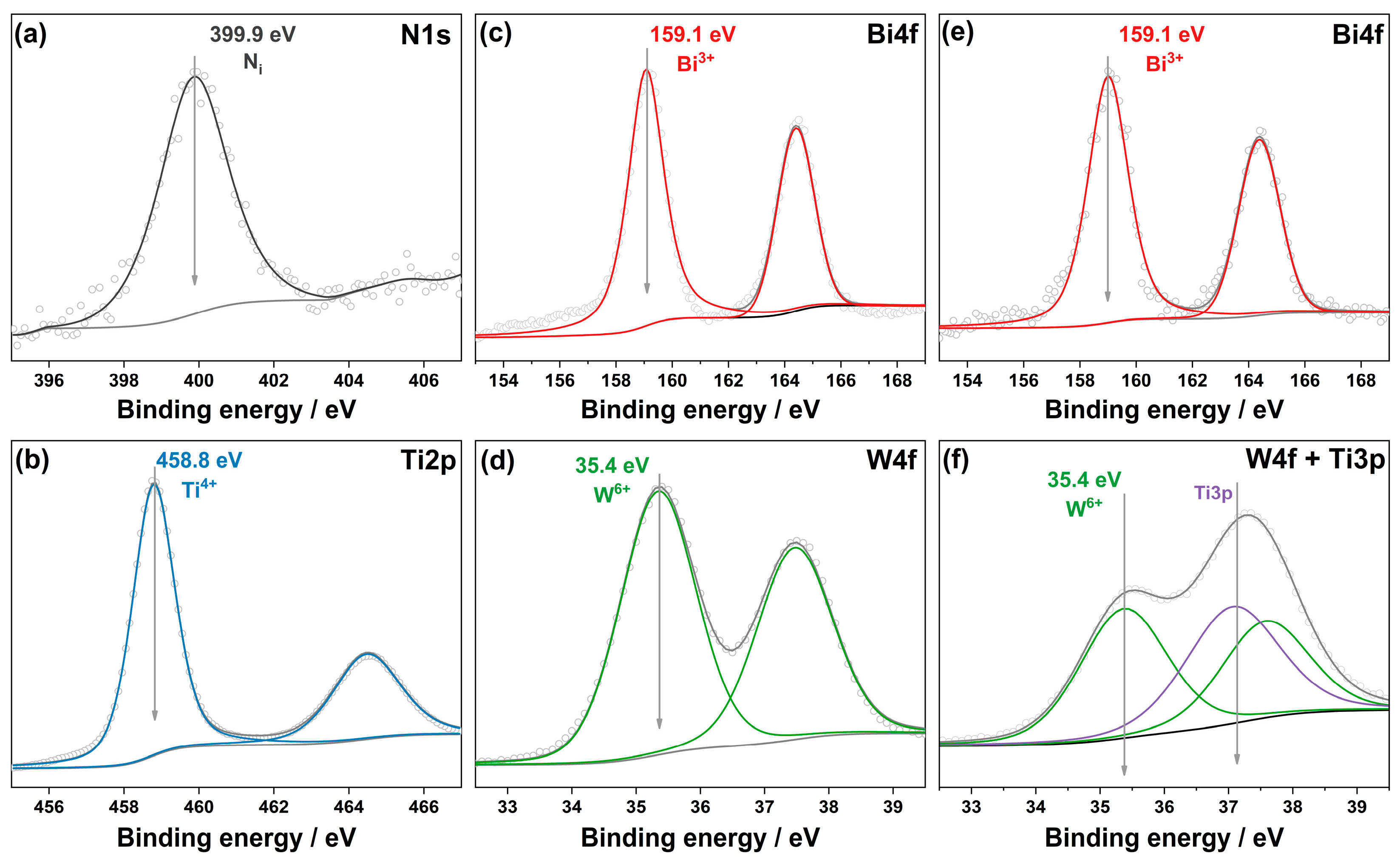
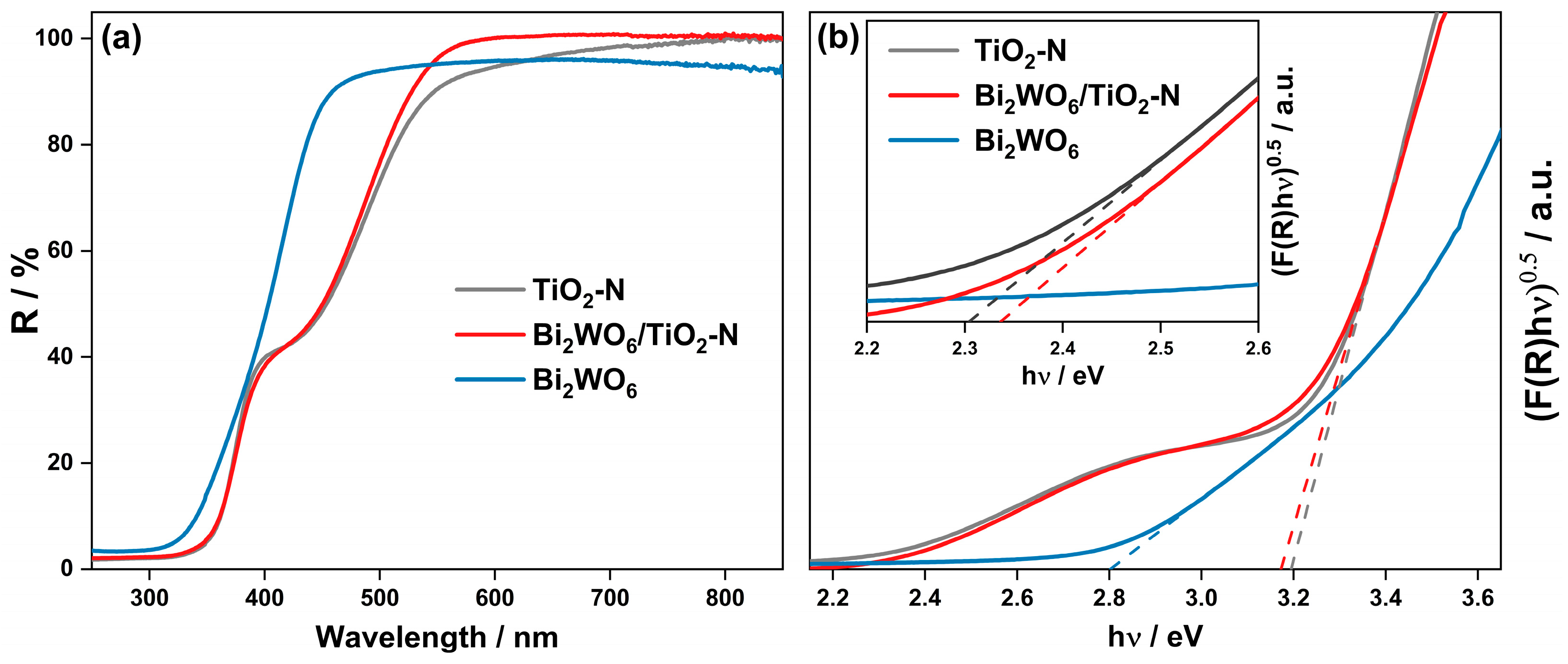
2.1. Characteristics of Synthesized Photocatalysts
2.2. Degradation of Volatile Organic Compounds
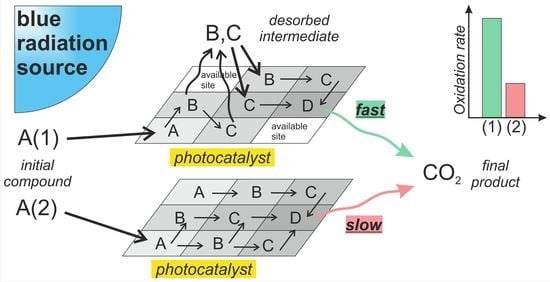
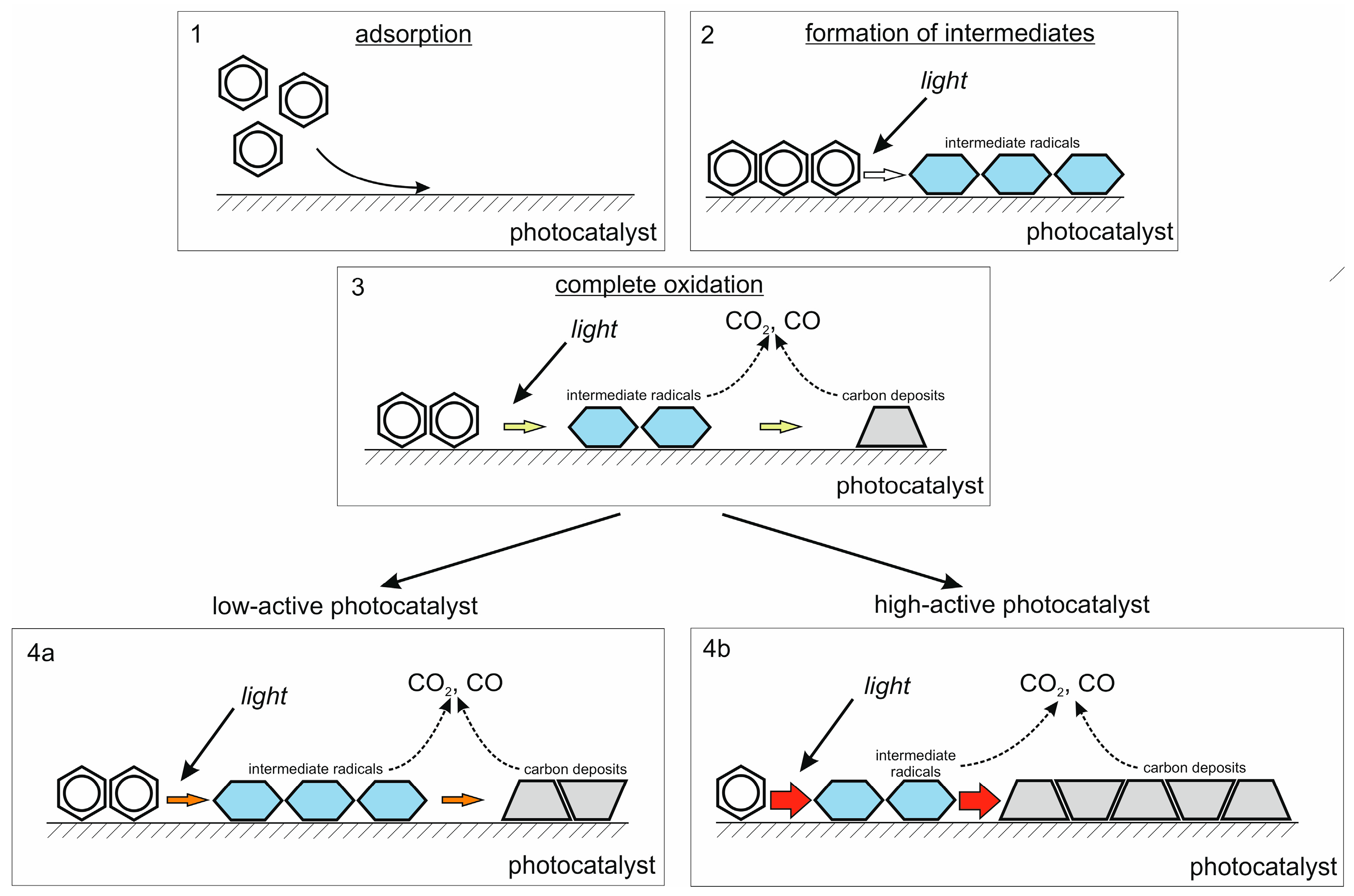
- The PCO of benzene occurs without the formation of gas-phase intermediates but leads to strongly adsorbed species formed on the surface of the photocatalyst due to the polymerization of intermediate radicals [44,45,46]. Furthermore, oxidation pathways with the opening of the benzene ring lead to an increase in the total number of species, thus resulting in the gradual deactivation of the photocatalyst due to competition for adsorption sites with the initial compound [47,48].
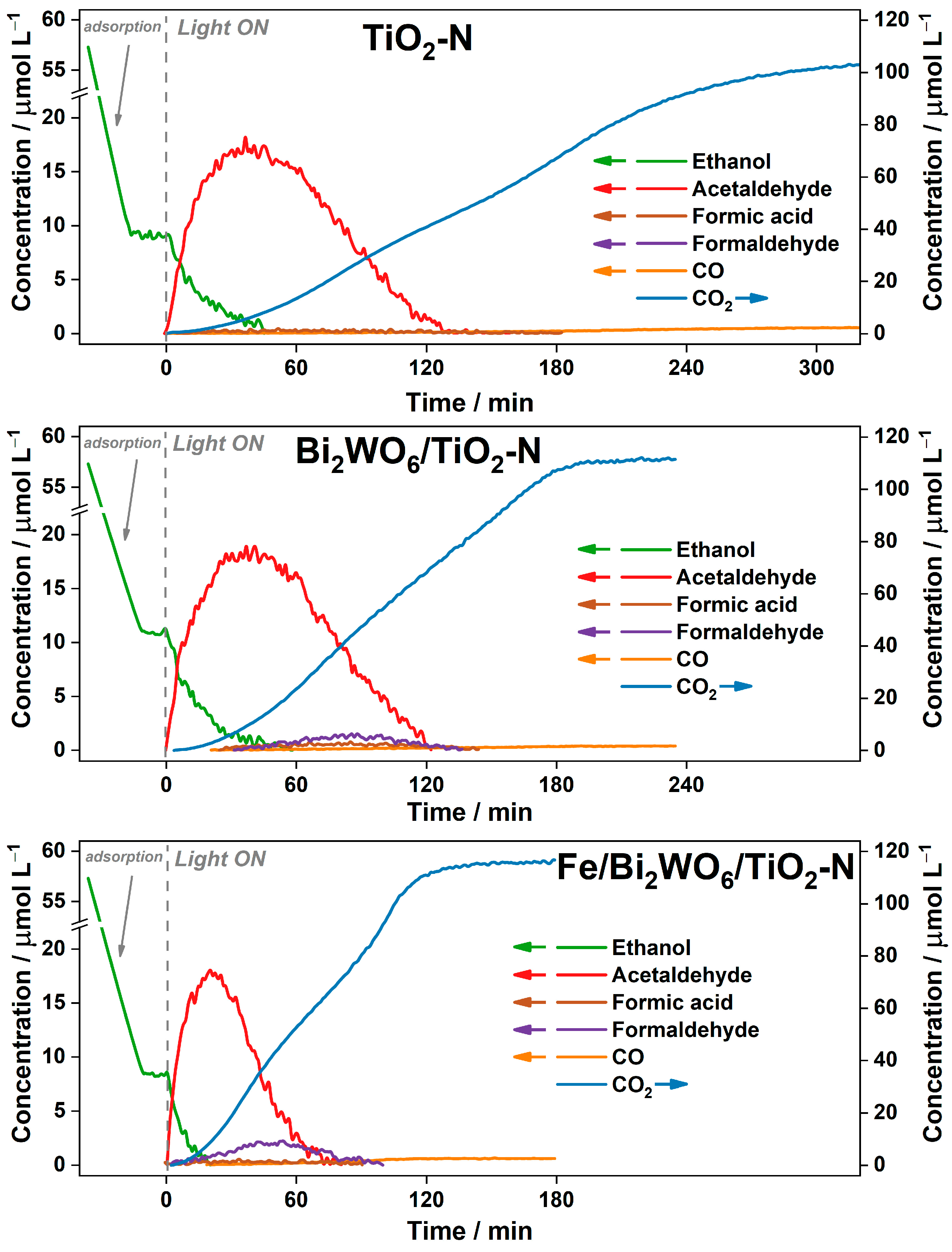
2.2.1. Degradation of Ethanol in Batch Reactor
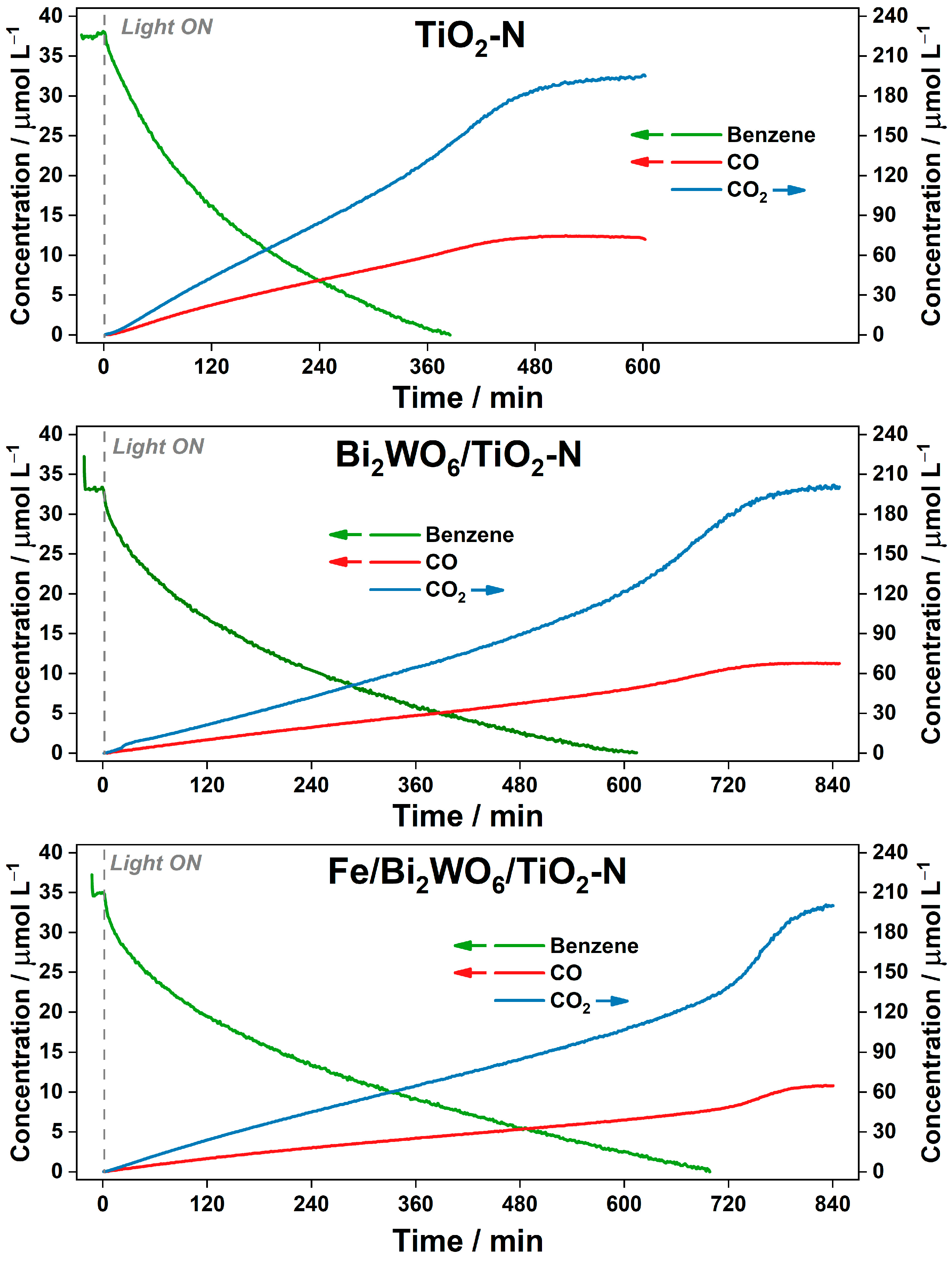
2.2.2. Degradation of Benzene in Batch Reactor
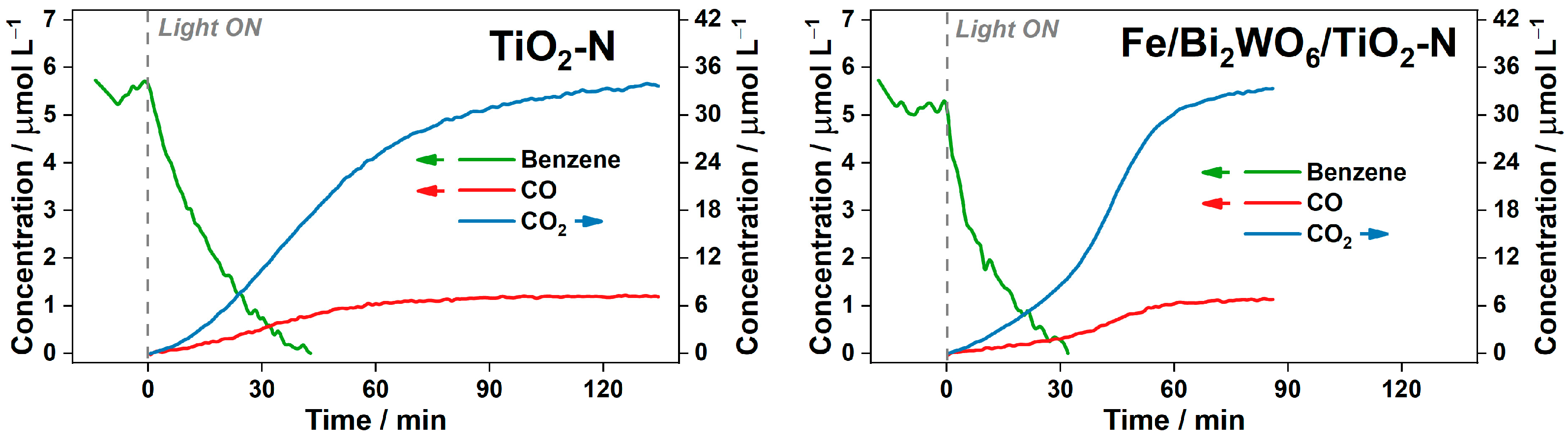
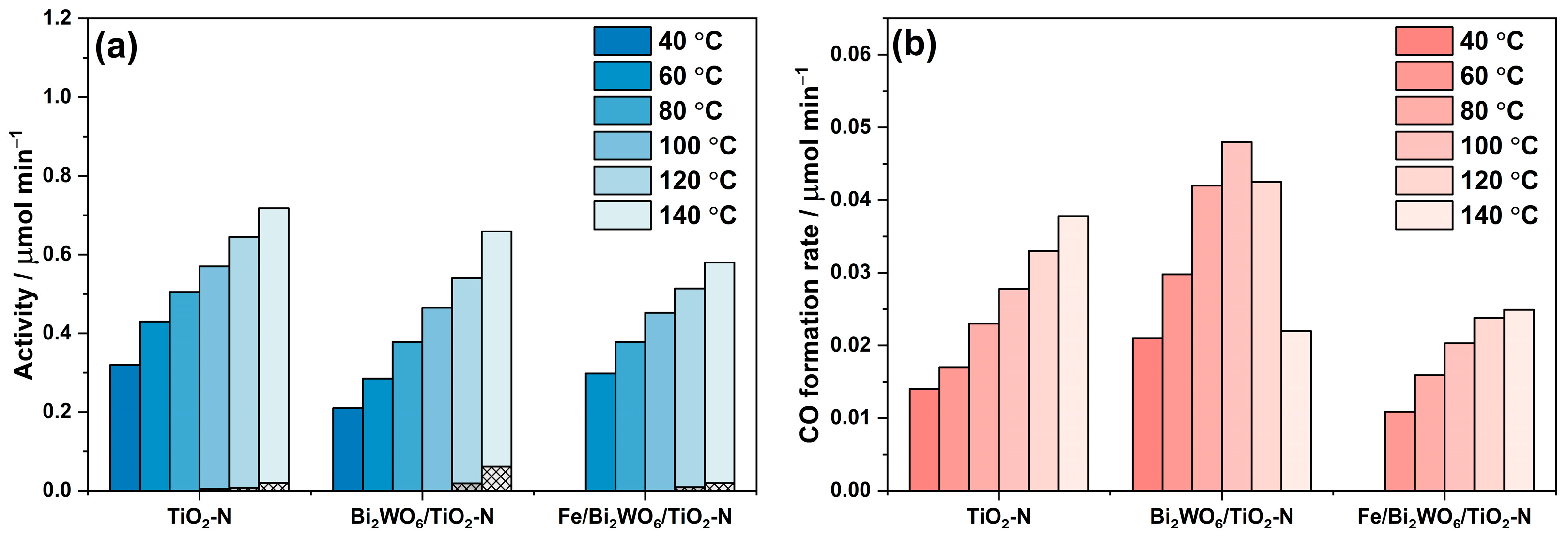
2.2.3. Degradation of Benzene in Continuous-Flow Reactor
- The activity of all samples in benzene degradation increases as the reaction temperature increases, even after overcoming a value of 80 °C, which is commonly regarded as the inflection point in the oxidation processes [55], because “the exothermic adsorption of reactant becomes disfavored and tends to become the rate limiting-step”. This means that there is no actual limitation in the thermoactivation of benzene oxidation because benzene is poorly adsorbed on the photocatalyst surface.
- Under the conditions of this experiment, TiO2-N is more active than the composite Bi2WO6/TiO2-N and Fe/Bi2WO6/TiO2-N photocatalysts over the whole temperature range (the reasons for that are discussed in the previous section). However, if we look at the formation rates of both final products: CO2 as the product of complete oxidation (Figure 10a) and CO as the product of incomplete oxidation (Figure 10b), the Fe/Bi2WO6/TiO2–N photocatalyst gives a lower amount of CO and provides more selective oxidation of benzene compared to other samples. This means that this photocatalyst would more effectively reduce the total hazard of air polluted with benzene vapor.
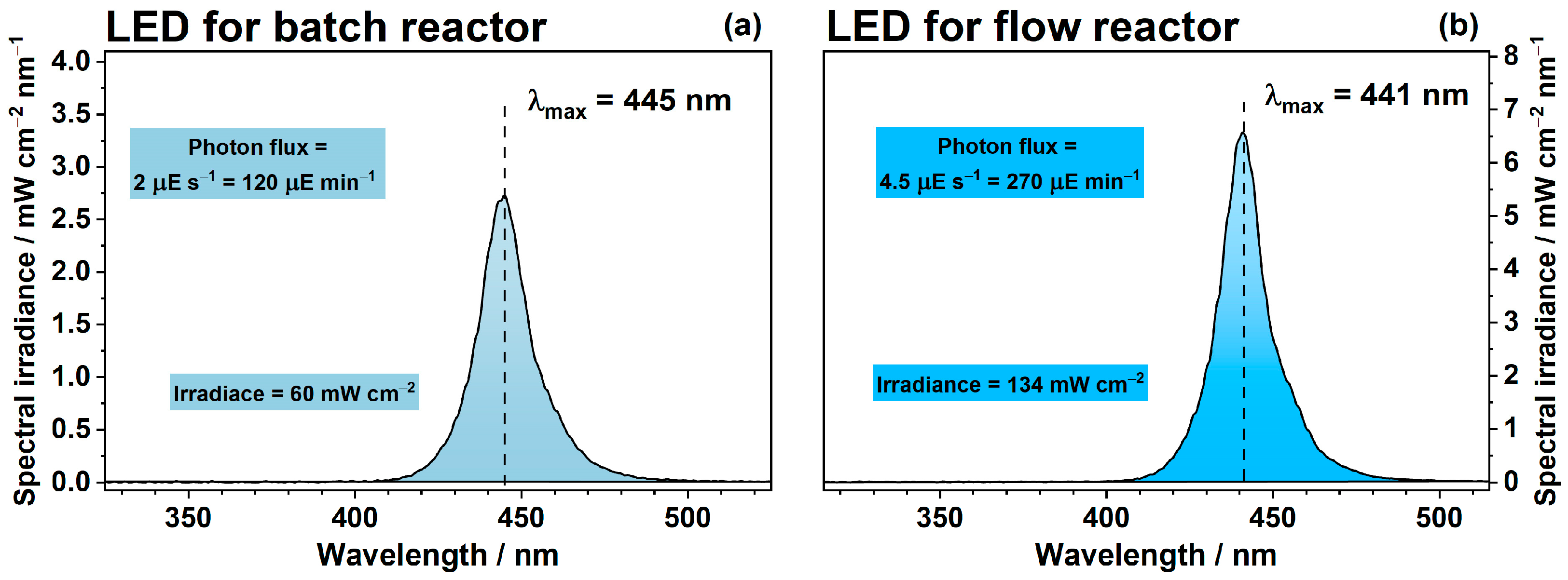
3. Materials and Methods
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Mata, T.M.; Martins, A.A.; Calheiros, C.S.C.; Villanueva, F.; Alonso-Cuevilla, N.P.; Gabriel, M.F.; Silva, G.V. Indoor Air Quality: A Review of Cleaning Technologies. Environments 2022, 9, 118. [Google Scholar] [CrossRef]
- Shayegan, Z.; Lee, C.-S.; Haghighat, F. TiO2 Photocatalyst for Removal of Volatile Organic Compounds in Gas Phase—A Review. Chem. Eng. J. 2018, 334, 2408–2439. [Google Scholar] [CrossRef]
- Salvadores, F.; Reli, M.; Alfano, O.M.; Kočí, K.; Ballari, M.D.L.M. Efficiencies Evaluation of Photocatalytic Paints Under Indoor and Outdoor Air Conditions. Front. Chem. 2020, 8, 551710. [Google Scholar] [CrossRef] [PubMed]
- Yang, X.; Wang, D. Photocatalysis: From Fundamental Principles to Materials and Applications. ACS Appl. Energy Mater. 2018, 1, 6657–6693. [Google Scholar] [CrossRef]
- Rashid, M.M.; Simončič, B.; Tomšič, B. Recent Advances in TiO2-Functionalized Textile Surfaces. Surf. Interfaces 2021, 22, 100890. [Google Scholar] [CrossRef]
- Chen, D.; Cheng, Y.; Zhou, N.; Chen, P.; Wang, Y.; Li, K.; Huo, S.; Cheng, P.; Peng, P.; Zhang, R.; et al. Photocatalytic Degradation of Organic Pollutants Using TiO2-Based Photocatalysts: A Review. J. Clean. Prod. 2020, 268, 121725. [Google Scholar] [CrossRef]
- Solovyeva, M.; Selishchev, D.; Cherepanova, S.; Stepanov, G.; Zhuravlev, E.; Richter, V.; Kozlov, D. Self-Cleaning Photoactive Cotton Fabric Modified with Nanocrystalline TiO2 for Efficient Degradation of Volatile Organic Compounds and DNA Contaminants. Chem. Eng. J. 2020, 388, 124167. [Google Scholar] [CrossRef]
- Daghrir, R.; Drogui, P.; Robert, D. Modified TiO2 For Environmental Photocatalytic Applications: A Review. Ind. Eng. Chem. Res. 2013, 52, 3581–3599. [Google Scholar] [CrossRef]
- Ong, W.-J.; Tan, L.-L.; Ng, Y.H.; Yong, S.-T.; Chai, S.-P. Graphitic Carbon Nitride (g-C3N4)-Based Photocatalysts for Artificial Photosynthesis and Environmental Remediation: Are We a Step Closer to Achieving Sustainability? Chem. Rev. 2016, 116, 7159–7329. [Google Scholar] [CrossRef]
- Amirulsyafiee, A.; Khan, M.M.; Harunsani, M.H. Ag3PO4 and Ag3PO4–Based Visible Light Active Photocatalysts: Recent Progress, Synthesis, and Photocatalytic Applications. Catal. Commun. 2022, 172, 106556. [Google Scholar] [CrossRef]
- Stelo, F.; Kublik, N.; Ullah, S.; Wender, H. Recent Advances in Bi2MoO6 Based Z-Scheme Heterojunctions for Photocatalytic Degradation of Pollutants. J. Alloys Compd. 2020, 829, 154591. [Google Scholar] [CrossRef]
- Gu, M.; Yang, Y.; Zhang, L.; Zhu, B.; Liang, G.; Yu, J. Efficient Sacrificial-Agent-Free Solar H2O2 Production over All-Inorganic S-Scheme Composites. Appl. Catal. B Environ. 2023, 324, 122227. [Google Scholar] [CrossRef]
- Qamar, M.A.; Shahid, S.; Javed, M.; Iqbal, S.; Sher, M.; Akbar, M.B. Highly Efficient g-C3N4/Cr-ZnO Nanocomposites with Superior Photocatalytic and Antibacterial Activity. J. Photochem. Photobiol. Chem. 2020, 401, 112776. [Google Scholar] [CrossRef]
- Weon, S.; He, F.; Choi, W. Status and Challenges in Photocatalytic Nanotechnology for Cleaning Air Polluted with Volatile Organic Compounds: Visible Light Utilization and Catalyst Deactivation. Environ. Sci. Nano 2019, 6, 3185–3214. [Google Scholar] [CrossRef]
- Wang, Z.; Liu, Y.; Huang, B.; Dai, Y.; Lou, Z.; Wang, G.; Zhang, X.; Qin, X. Progress on Extending the Light Absorption Spectra of Photocatalysts. Phys. Chem. Chem. Phys. 2014, 16, 2758. [Google Scholar] [CrossRef] [PubMed]
- Park, H.; Park, Y.; Kim, W.; Choi, W. Surface Modification of TiO2 Photocatalyst for Environmental Applications. J. Photochem. Photobiol. C Photochem. Rev. 2013, 15, 1–20. [Google Scholar] [CrossRef]
- Asahi, R.; Morikawa, T.; Irie, H.; Ohwaki, T. Nitrogen-Doped Titanium Dioxide as Visible-Light-Sensitive Photocatalyst: Designs, Developments, and Prospects. Chem. Rev. 2014, 114, 9824–9852. [Google Scholar] [CrossRef] [PubMed]
- Vaiano, V.; Sacco, O.; Sannino, D.; Ciambelli, P. Nanostructured N-Doped TiO2 Coated on Glass Spheres for the Photocatalytic Removal of Organic Dyes under UV or Visible Light Irradiation. Appl. Catal. B Environ. 2015, 170–171, 153–161. [Google Scholar] [CrossRef]
- Ansari, S.A.; Khan, M.M.; Ansari, M.O.; Cho, M.H. Nitrogen-Doped Titanium Dioxide (N-Doped TiO2) for Visible Light Photocatalysis. New J. Chem. 2016, 40, 3000–3009. [Google Scholar] [CrossRef]
- Bakar, S.A.; Ribeiro, C. Nitrogen-Doped Titanium Dioxide: An Overview of Material Design and Dimensionality Effect over Modern Applications. J. Photochem. Photobiol. C Photochem. Rev. 2016, 27, 1–29. [Google Scholar] [CrossRef]
- Ariza-Tarazona, M.C.; Villarreal-Chiu, J.F.; Barbieri, V.; Siligardi, C.; Cedillo-González, E.I. New Strategy for Microplastic Degradation: Green Photocatalysis Using a Protein-Based Porous N-TiO2 Semiconductor. Ceram. Int. 2019, 45, 9618–9624. [Google Scholar] [CrossRef]
- Sun, Z.; Fang, Y. Electrical Tuning Effect for Schottky Barrier and Hot-Electron Harvest in a Plasmonic Au/TiO2 Nanostructure. Sci. Rep. 2021, 11, 338. [Google Scholar] [CrossRef] [PubMed]
- Higashimoto, S.; Tanihata, W.; Nakagawa, Y.; Azuma, M.; Ohue, H.; Sakata, Y. Effective Photocatalytic Decomposition of VOC under Visible-Light Irradiation on N-Doped TiO2 Modified by Vanadium Species. Appl. Catal. Gen. 2008, 340, 98–104. [Google Scholar] [CrossRef]
- Kamat, P.V. Photoinduced Transformations in Semiconductormetal Nanocomposite Assemblies. Pure Appl. Chem. 2002, 74, 1693–1706. [Google Scholar] [CrossRef]
- Iwata, K.; Takaya, T.; Hamaguchi, H.; Yamakata, A.; Ishibashi, T.; Onishi, H.; Kuroda, H. Carrier Dynamics in TiO2 and Pt/TiO2 Powders Observed by Femtosecond Time-Resolved Near-Infrared Spectroscopy at a Spectral Region of 0.9–1.5 Μm with the Direct Absorption Method. J. Phys. Chem. B 2004, 108, 20233–20239. [Google Scholar] [CrossRef]
- Fan, X.; Hua, N.; Jia, H.; Zhu, Y.; Wang, Z.; Xu, J.; Wang, C. Synthesis and Evaluation of Visible-Light Photocatalyst: Nitrogen-Doped TiO2/Bi2O3 Heterojunction Structures. Sci. Adv. Mater. 2014, 6, 1892–1899. [Google Scholar] [CrossRef]
- Yao, Y.; Qin, J.; Chen, H.; Wei, F.; Liu, X.; Wang, J.; Wang, S. One-Pot Approach for Synthesis of N-Doped TiO2/ZnFe2O4 Hybrid as an Efficient Photocatalyst for Degradation of Aqueous Organic Pollutants. J. Hazard. Mater. 2015, 291, 28–37. [Google Scholar] [CrossRef]
- Shi, X.; Fujitsuka, M.; Lou, Z.; Zhang, P.; Majima, T. In Situ Nitrogen-Doped Hollow-TiO2/g-C3N4 Composite Photocatalysts with Efficient Charge Separation Boosting Water Reduction under Visible Light. J. Mater. Chem. A 2017, 5, 9671–9681. [Google Scholar] [CrossRef]
- Zhao, Z.; Zhang, X.; Fan, J.; Xue, D.; Zhang, B.; Yin, S. N-TiO2/g-C3N4/Up-Conversion Phosphor Composites for the Full-Spectrum Light-Responsive DeNOxphotocatalysis. J. Mater. Sci. 2018, 53, 7266–7278. [Google Scholar] [CrossRef]
- Tang, X.; Wang, Z.; Huang, W.; Jing, Q.; Liu, N. Construction of N-Doped TiO2/MoS2 Heterojunction with Synergistic Effect for Enhanced Visible Photodegradation Activity. Mater. Res. Bull. 2018, 105, 126–132. [Google Scholar] [CrossRef]
- Kovalevskiy, N.; Cherepanova, S.; Gerasimov, E.; Lyulyukin, M.; Solovyeva, M.; Prosvirin, I.; Kozlov, D.; Selishchev, D. Enhanced Photocatalytic Activity and Stability of Bi2WO6—TiO2-N Nanocomposites in the Oxidation of Volatile Pollutants. Nanomaterials 2022, 12, 359. [Google Scholar] [CrossRef]
- Kovalevskiy, N.; Svintsitskiy, D.; Cherepanova, S.; Yakushkin, S.; Martyanov, O.; Selishcheva, S.; Gribov, E.; Kozlov, D.; Selishchev, D. Visible-Light-Active N-Doped TiO2 Photocatalysts: Synthesis from TiOSO4, Characterization, and Enhancement of Stability Via Surface Modification. Nanomaterials 2022, 12, 4146. [Google Scholar] [CrossRef] [PubMed]
- Bellardita, M.; Addamo, M.; Paola, A.D.; Palmisano, L.; Venezia, A.M. Preparation of N-Doped TiO2: Characterization and Photocatalytic Performance under UV and Visible Light. Phys. Chem. Chem. Phys. 2009, 11, 4084–4093. [Google Scholar] [CrossRef]
- Japa, M.; Tantraviwat, D.; Phasayavan, W.; Nattestad, A.; Chen, J.; Inceesungvorn, B. Simple Preparation of Nitrogen-Doped TiO2 and Its Performance in Selective Oxidation of Benzyl Alcohol and Benzylamine under Visible Light. Colloids Surf. Physicochem. Eng. Asp. 2021, 610, 125743. [Google Scholar] [CrossRef]
- Liu, S.J.; Ma, Q.; Gao, F.; Song, S.H.; Gao, S. Relationship between N-Doping Induced Point Defects by Annealing in Ammonia and Enhanced Thermal Stability for Anodized Titania Nanotube Arrays. J. Alloys Compd. 2012, 543, 71–78. [Google Scholar] [CrossRef]
- Lee, S.; Cho, I.-S.; Lee, D.K.; Kim, D.W.; Noh, T.H.; Kwak, C.H.; Park, S.; Hong, K.S.; Lee, J.-K.; Jung, H.S. Influence of Nitrogen Chemical States on Photocatalytic Activities of Nitrogen-Doped TiO2 Nanoparticles under Visible Light. J. Photochem. Photobiol. Chem. 2010, 213, 129–135. [Google Scholar] [CrossRef]
- Zhu, Z.; Wan, S.; Zhao, Y.; Qin, Y.; Ge, X.; Zhong, Q.; Bu, Y. Recent Progress in Bi2WO6-Based Photocatalysts for Clean Energy and Environmental Remediation: Competitiveness, Challenges, and Future Perspectives. Nano Sel. 2021, 2, 187–215. [Google Scholar] [CrossRef]
- Yang, C.; Huang, Y.; Li, F.; Li, T. One-Step Synthesis of Bi2WO6/TiO2 Heterojunctions with Enhanced Photocatalytic and Superhydrophobic Property via Hydrothermal Method. J. Mater. Sci. 2016, 51, 1032–1042. [Google Scholar] [CrossRef]
- Wang, R.; Xu, M.; Xie, J.; Ye, S.; Song, X. A Spherical TiO2-Bi2WO6 Composite Photocatalyst for Visible-Light Photocatalytic Degradation of Ethylene. Colloids Surf. Physicochem. Eng. Asp. 2020, 602, 125048. [Google Scholar] [CrossRef]
- He, F.; Muliane, U.; Weon, S.; Choi, W. Substrate-Specific Mineralization and Deactivation Behaviors of TiO2 as an Air-Cleaning Photocatalyst. Appl. Catal. B Environ. 2020, 275, 119145. [Google Scholar] [CrossRef]
- Salvadores, F.; Alfano, O.M.; Ballari, M.M. Kinetic Study of Air Treatment by Photocatalytic Paints under Indoor Radiation Source: Influence of Ambient Conditions and Photocatalyst Content. Appl. Catal. B Environ. 2020, 268, 118694. [Google Scholar] [CrossRef]
- Sauer, M.L.; Ollis, D.F. Photocatalyzed Oxidation of Ethanol and Acetaldehyde in Humidified Air. J. Catal. 1996, 158, 570–582. [Google Scholar] [CrossRef]
- Muggli, D.S.; McCue, J.T.; Falconer, J.L. Mechanism of the Photocatalytic Oxidation of Ethanol on TiO2. J. Catal. 1998, 173, 470–483. [Google Scholar] [CrossRef]
- d’Hennezel, O.; Pichat, P.; Ollis, D.F. Benzene and Toluene Gas-Phase Photocatalytic Degradation over H2O and HCL Pretreated TiO2: By-Products and Mechanisms. J. Photochem. Photobiol. Chem. 1998, 118, 197–204. [Google Scholar] [CrossRef]
- Einaga, H.; Futamura, S.; Ibusuki, T. Photocatalytic Decomposition of Benzene over TiO2 in a Humidified Airstream. Phys. Chem. Chem. Phys. 1999, 1, 4903–4908. [Google Scholar] [CrossRef]
- Einaga, H. Heterogeneous Photocatalytic Oxidation of Benzene, Toluene, Cyclohexene and Cyclohexane in Humidified Air: Comparison of Decomposition Behavior on Photoirradiated TiO2 Catalyst. Appl. Catal. B Environ. 2002, 38, 215–225. [Google Scholar] [CrossRef]
- Kozlov, D.V. Titanium Dioxide in Gas-Phase Photocatalytic Oxidation of Aromatic and Heteroatom Organic Substances: Deactivation and Reactivation of Photocatalyst. Theor. Exp. Chem. 2014, 50, 133–154. [Google Scholar] [CrossRef]
- Bui, T.D.; Kimura, A.; Higashida, S.; Ikeda, S.; Matsumura, M. Two Routes for Mineralizing Benzene by TiO2-Photocatalyzed Reaction. Appl. Catal. B Environ. 2011, 107, 119–127. [Google Scholar] [CrossRef]
- Zhuang, H.; Gu, Q.; Long, J.; Lin, H.; Lin, H.; Wang, X. Visible Light-Driven Decomposition of Gaseous Benzene on Robust Sn2+ -Doped Anatase TiO2 Nanoparticles. RSC Adv 2014, 4, 34315–34324. [Google Scholar] [CrossRef]
- Vikrant, K.; Park, C.M.; Kim, K.-H.; Kumar, S.; Jeon, E.-C. Recent Advancements in Photocatalyst-Based Platforms for the Destruction of Gaseous Benzene: Performance Evaluation of Different Modes of Photocatalytic Operations and against Adsorption Techniques. J. Photochem. Photobiol. C Photochem. Rev. 2019, 41, 100316. [Google Scholar] [CrossRef]
- Bathla, A.; Vikrant, K.; Kukkar, D.; Kim, K.-H. Photocatalytic Degradation of Gaseous Benzene Using Metal Oxide Nanocomposites. Adv. Colloid Interface Sci. 2022, 305, 102696. [Google Scholar] [CrossRef] [PubMed]
- He, F.; Ma, F.; Li, T.; Li, G. Solvothermal Synthesis of N-Doped TiO2 Nanoparticles Using Different Nitrogen Sources, and Their Photocatalytic Activity for Degradation of Benzene. Chin. J. Catal. 2013, 34, 2263–2270. [Google Scholar] [CrossRef]
- Selishchev, D.S.; Kolinko, P.A.P.; Kozlov, D.V. Adsorbent as an Essential Participant in Photocatalytic Processes of Water and Air Purification: Computer Simulation Study. Appl. Catal. Gen. 2010, 377, 140–149. [Google Scholar] [CrossRef]
- Lyulyukin, M.; Kovalevskiy, N.; Prosvirin, I.; Selishchev, D.; Kozlov, D. Thermo-Photoactivity of Pristine and Modified Titania Photocatalysts under UV and Blue Light. J. Photochem. Photobiol. Chem. 2022, 425, 113675. [Google Scholar] [CrossRef]
- Herrmann, J.-M. Heterogeneous Photocatalysis: Fundamentals and Applications to the Removal of Various Types of Aqueous Pollutants. Catal. Today 1999, 53, 115–129. [Google Scholar] [CrossRef]
- Selishchev, D.; Svintsitskiy, D.; Kovtunova, L.; Gerasimov, E.; Gladky, A.; Kozlov, D. Surface Modification of TiO2 with Pd Nanoparticles for Enhanced Photocatalytic Oxidation of Benzene Micropollutants. Colloids Surf. Physicochem. Eng. Asp. 2021, 612, 125959. [Google Scholar] [CrossRef]


| O/Ti | N/Ti | Bi/Ti | W/Ti | |
|---|---|---|---|---|
| Before reaction | 3.99 | 0.10 | 0.60 | 0.42 |
| After reaction | 3.81 | 0.10 | 0.61 | 0.41 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lyulyukin, M.; Kovalevskiy, N.; Bukhtiyarov, A.; Kozlov, D.; Selishchev, D. Kinetic Aspects of Benzene Degradation over TiO2-N and Composite Fe/Bi2WO6/TiO2-N Photocatalysts under Irradiation with Visible Light. Int. J. Mol. Sci. 2023, 24, 5693. https://doi.org/10.3390/ijms24065693
Lyulyukin M, Kovalevskiy N, Bukhtiyarov A, Kozlov D, Selishchev D. Kinetic Aspects of Benzene Degradation over TiO2-N and Composite Fe/Bi2WO6/TiO2-N Photocatalysts under Irradiation with Visible Light. International Journal of Molecular Sciences. 2023; 24(6):5693. https://doi.org/10.3390/ijms24065693
Chicago/Turabian StyleLyulyukin, Mikhail, Nikita Kovalevskiy, Andrey Bukhtiyarov, Denis Kozlov, and Dmitry Selishchev. 2023. "Kinetic Aspects of Benzene Degradation over TiO2-N and Composite Fe/Bi2WO6/TiO2-N Photocatalysts under Irradiation with Visible Light" International Journal of Molecular Sciences 24, no. 6: 5693. https://doi.org/10.3390/ijms24065693
APA StyleLyulyukin, M., Kovalevskiy, N., Bukhtiyarov, A., Kozlov, D., & Selishchev, D. (2023). Kinetic Aspects of Benzene Degradation over TiO2-N and Composite Fe/Bi2WO6/TiO2-N Photocatalysts under Irradiation with Visible Light. International Journal of Molecular Sciences, 24(6), 5693. https://doi.org/10.3390/ijms24065693