Enhanced Exciton Effect and Singlet Oxygen Generation Triggered by Tunable Oxygen Vacancies on Bi2MoO6 for Efficient Photocatalytic Degradation of Sodium Pentachlorophenol
Abstract
1. Introduction
2. Results and Discussion
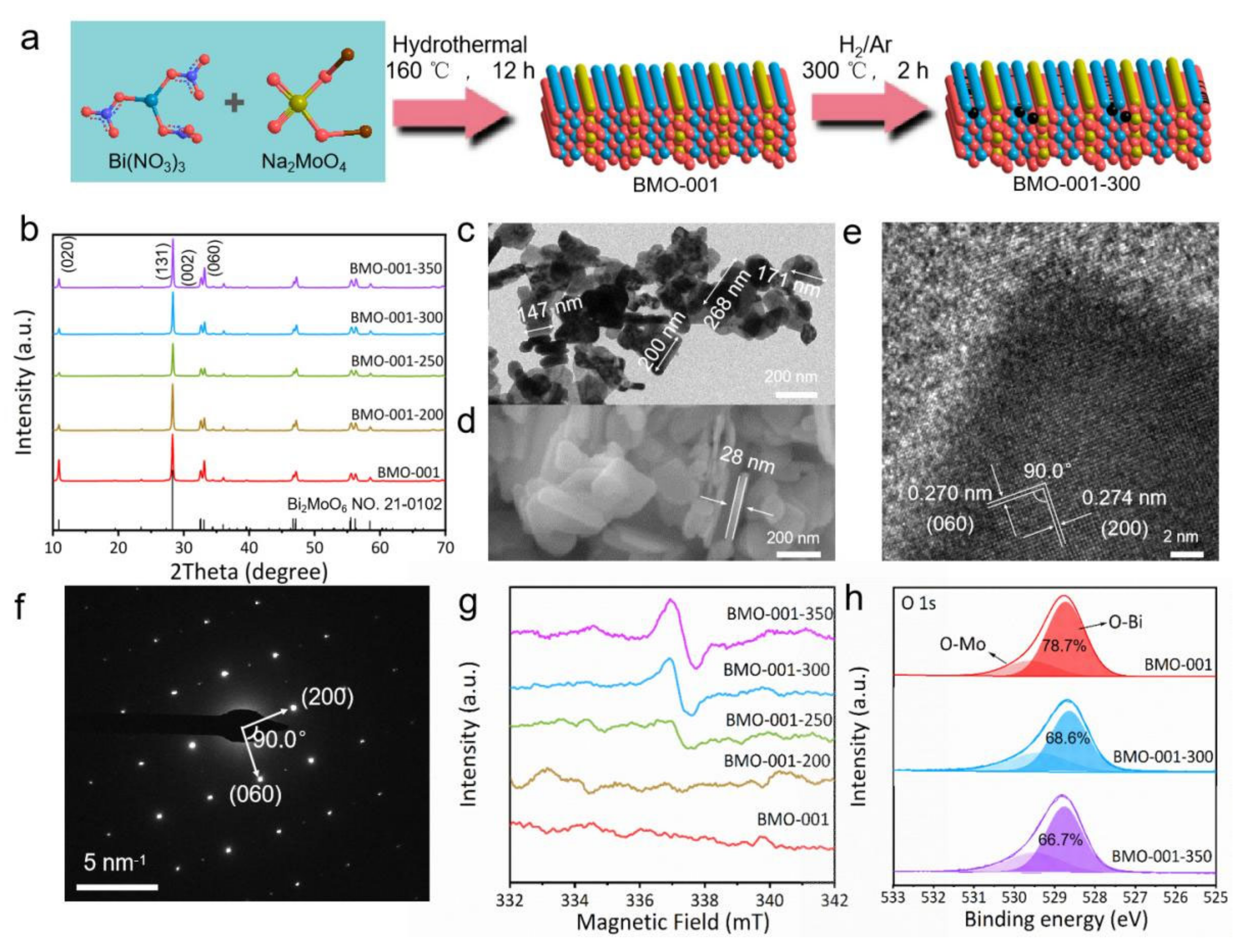
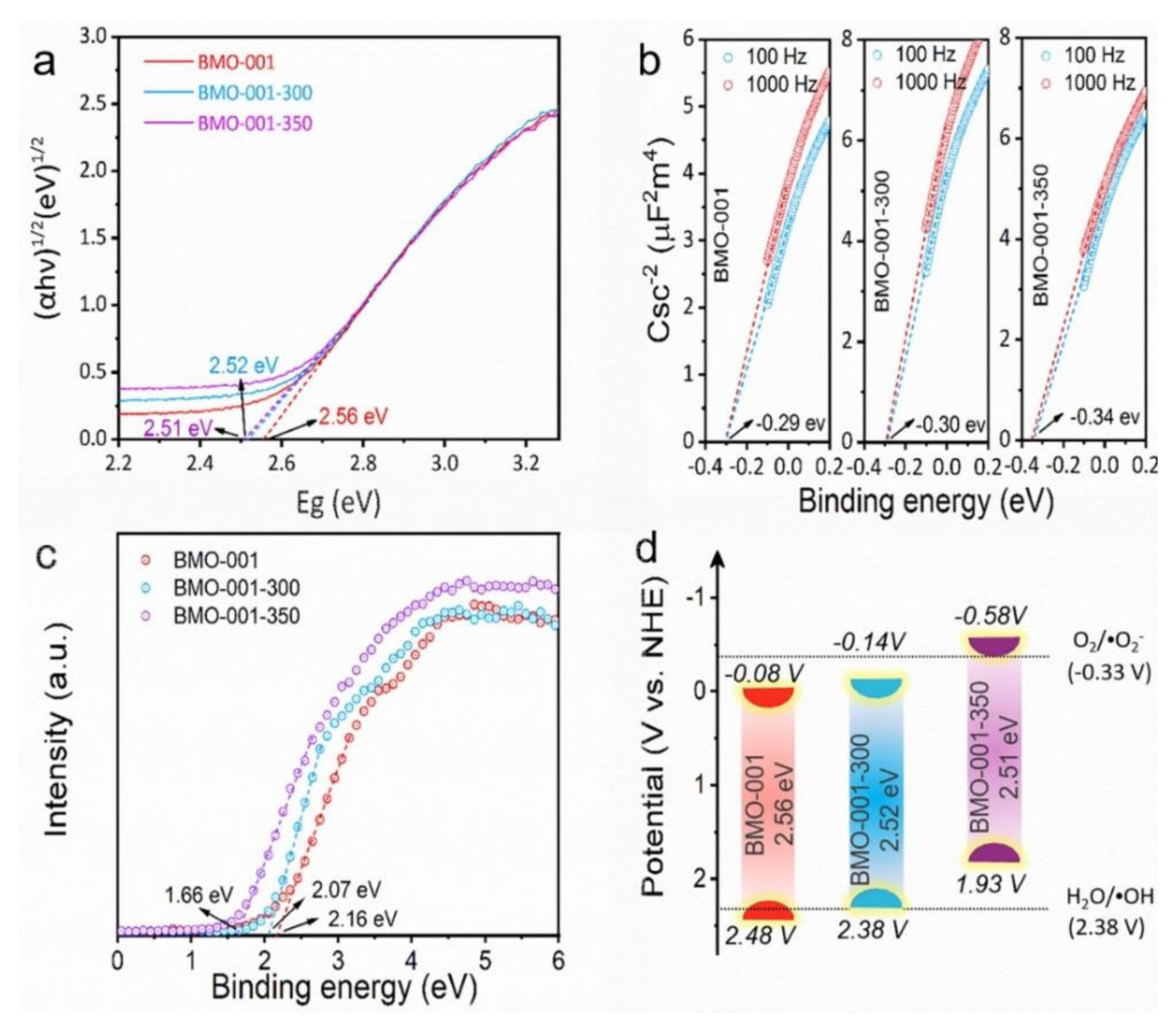
2.1. Characterization of the Samples
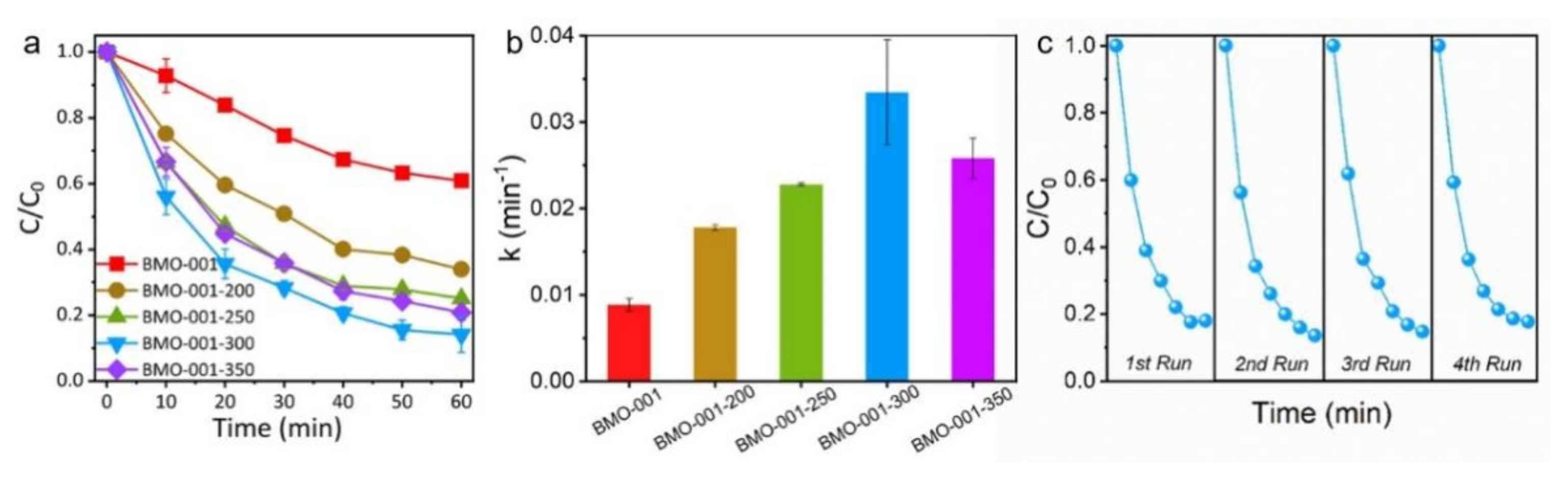
2.2. Photocatalytic Activity
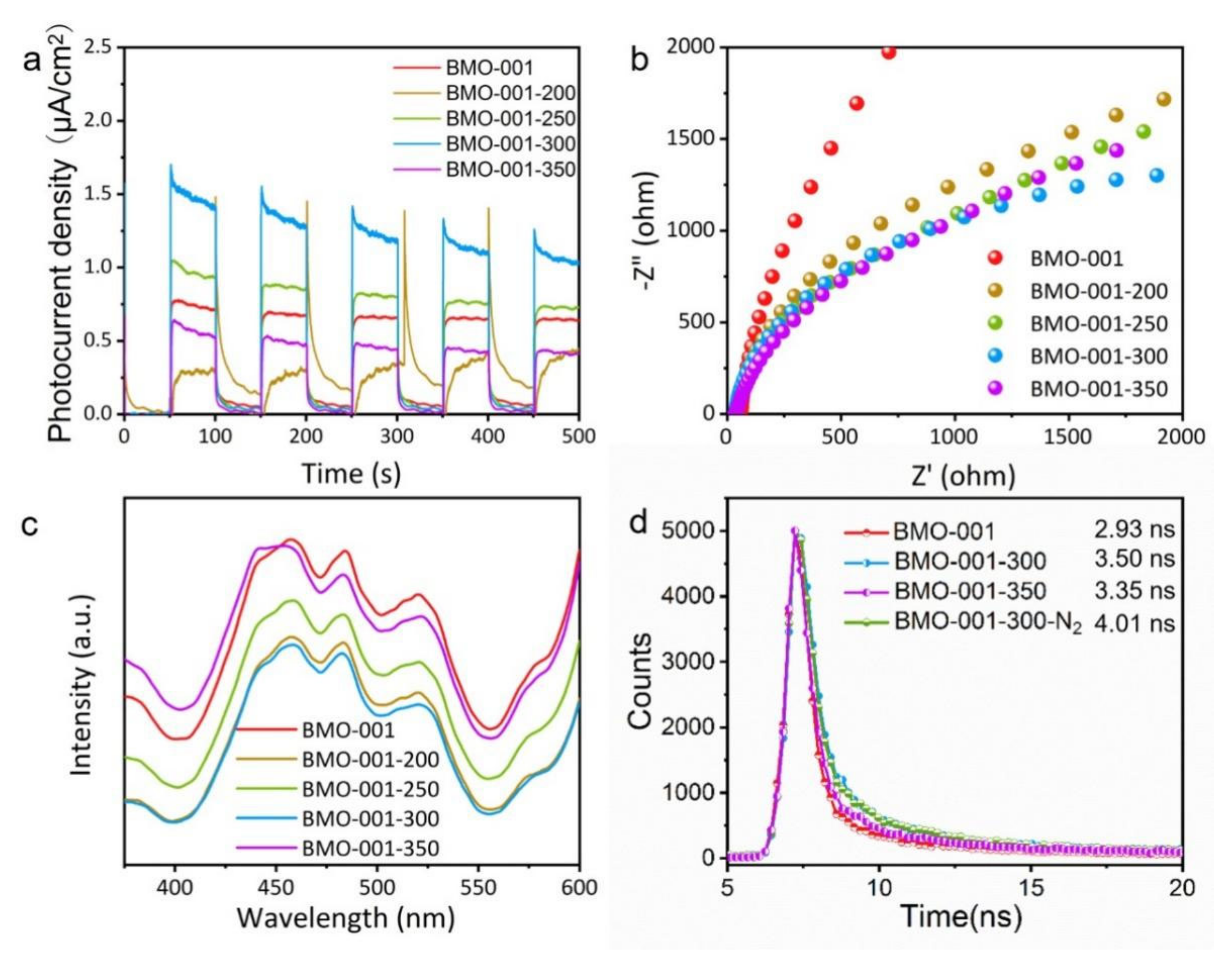
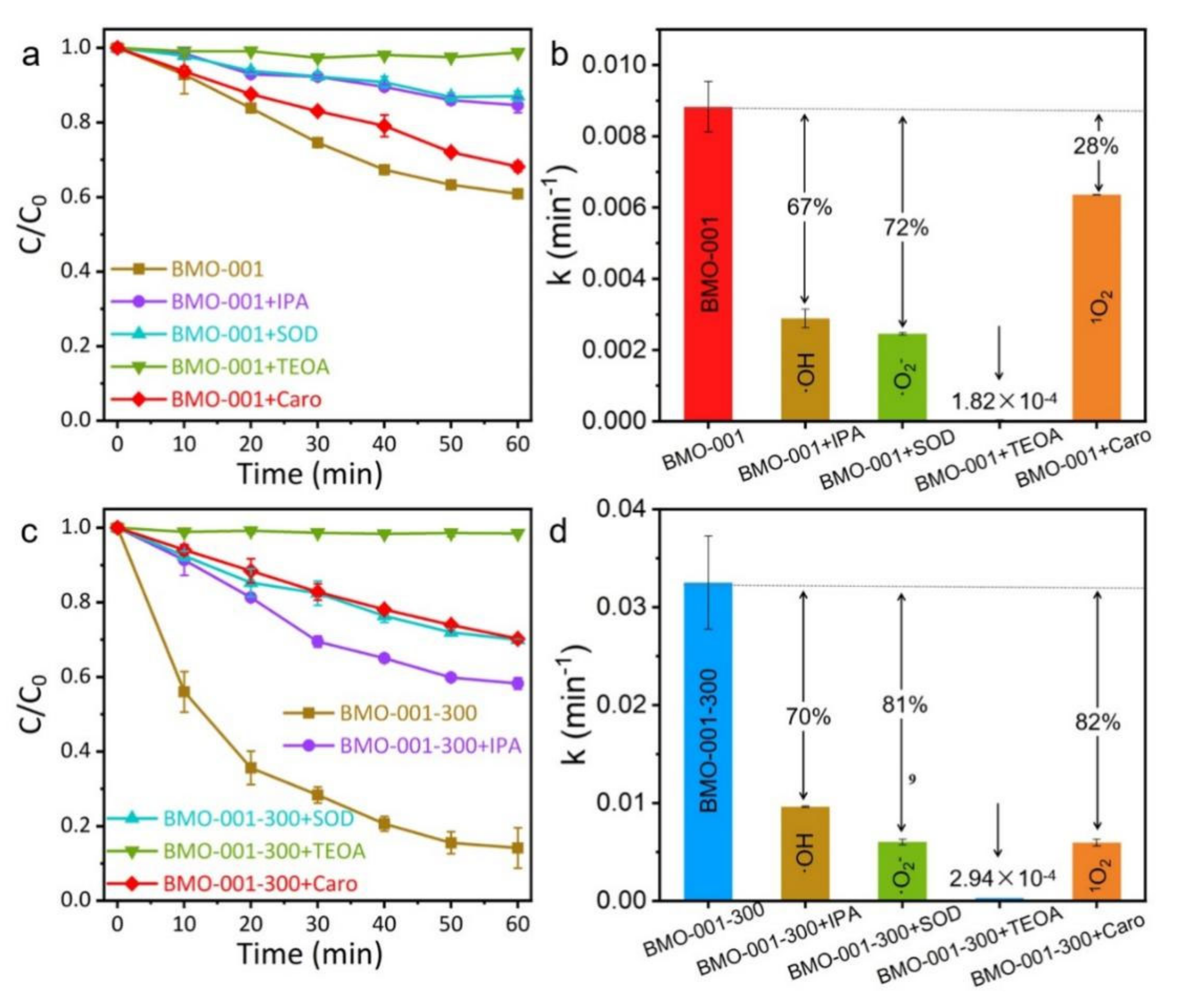
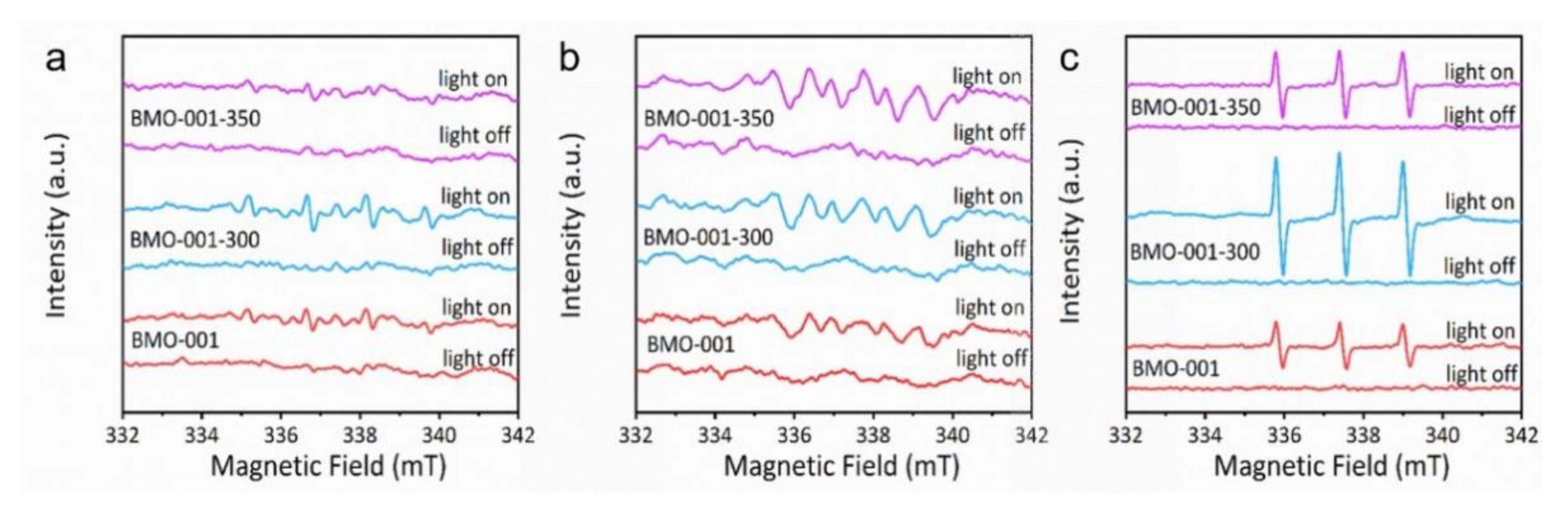
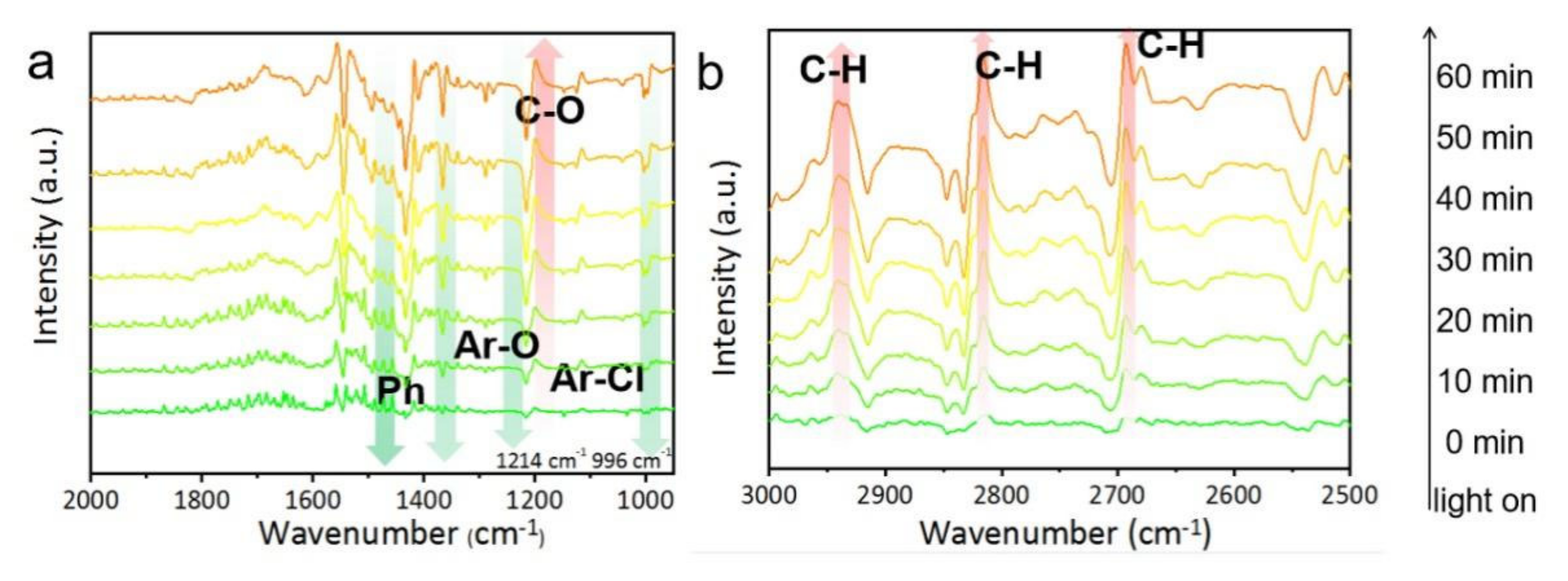
2.3. Mechanisms of Photocatalytic NaPCP Degradation on BMO-001-300
2.4. Conclusions
3. Materials and Methods
3.1. Sample Preparation
3.2. Sample Characterization
3.3. Photocatalytic Activity Test
3.4. In-Situ DRIFTS Experiments
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Ding, X.; Zhao, K.; Zhang, L. Enhanced photocatalytic removal of sodium pentachlorophenate with self-doped Bi2WO6 under visible light by generating more superoxide ions. Environ. Sci. Technol. 2014, 48, 5823–5831. [Google Scholar] [CrossRef] [PubMed]
- Weon, S.; He, F.; Choi, W. Status and challenges in photocatalytic nanotechnology for cleaning air polluted with volatile organic compounds: Visible light utilization and catalyst deactivation. Environ. Sci-Nano 2019, 6, 3185–3214. [Google Scholar] [CrossRef]
- Hunge, Y.M.; Uchida, A.; Tominaga, Y.; Fujii, Y.; Yadav, A.A.; Kang, S.-W.; Suzuki, N.; Shitanda, I.; Kondo, T.; Itagaki, M.; et al. Visible Light-Assisted Photocatalysis Using Spherical-Shaped BiVO4 Photocatalyst. Catalysts 2021, 11, 460. [Google Scholar] [CrossRef]
- Ding, X.; Yang, X.; Xiong, Z.; Chen, H.; Zhang, L. Environment Pollutants Removal with Bi-Based Photocatalysts. Prog. Chem. 2017, 29, 1115–1126. [Google Scholar]
- Hodges, B.C.; Cates, E.L.; Kim, J.H. Challenges and prospects of advanced oxidation water treatment processes using catalytic nanomaterials. Nat. Nanotechnol. 2018, 13, 642–650. [Google Scholar] [CrossRef]
- Loeb, S.K.; Alvarez, P.J.J.; Brame, J.A.; Cates, E.L.; Choi, W.; Crittenden, J.; Dionysiou, D.D.; Li, Q.; Li-Puma, G.; Quan, X.; et al. The Technology Horizon for Photocatalytic Water Treatment: Sunrise or Sunset? Environ. Sci. Technol. 2019, 53, 2937–2947. [Google Scholar] [CrossRef]
- Hunge, Y.M.; Yadav, A.A.; Kang, S.-W.; Jun Lim, S.; Kim, H. Visible light activated MoS2/ZnO composites for photocatalytic degradation of ciprofloxacin antibiotic and hydrogen production. J. Photochem. Photobiol. A 2023, 434, 114250. [Google Scholar] [CrossRef]
- Hunge, Y.M.; Yadav, A.A.; Khan, S.; Takagi, K.; Suzuki, N.; Teshima, K.; Terashima, C.; Fujishima, A. Photocatalytic degradation of bisphenol A using titanium dioxide@nanodiamond composites under UV light illumination. J. Colloid Interface Sci. 2021, 582, 1058–1066. [Google Scholar] [CrossRef] [PubMed]
- Xu, M.; Chen, Y.; Qin, J.; Feng, Y.; Li, W.; Chen, W.; Zhu, J.; Li, H.; Bian, Z. Unveiling the Role of Defects on Oxygen Activation and Photodegradation of Organic Pollutants. Environ. Sci. Technol. 2018, 52, 13879–13886. [Google Scholar] [CrossRef]
- Li, Z.; Meng, X. New Insight into Reactive Oxidation Species (ROS) for Bismuth-based Photocatalysis in Phenol Removal. J. Hazard. Mater. 2020, 399, 122939. [Google Scholar] [CrossRef] [PubMed]
- Yang, X.; Xiang, Y.; Qu, Y.; Ding, X.; Chen, H. Novel in situ fabrication of conjugated microporous poly(benzothiadiazole)–Bi2MoO6 Z-scheme heterojunction with enhanced visible light photocatalytic activity. J. Catal. 2017, 345, 319–328. [Google Scholar] [CrossRef]
- Li, S.; Huang, T.; Du, P.; Liu, W.; Hu, J. Photocatalytic transformation fate and toxicity of ciprofloxacin related to dissociation species: Experimental and theoretical evidences. Water Res. 2020, 185, 116286. [Google Scholar] [CrossRef]
- Zeng, L.; Guo, X.; He, C.; Duan, C. Metal–Organic Frameworks: Versatile Materials for Heterogeneous Photocatalysis. ACS Catal. 2016, 6, 7935–7947. [Google Scholar] [CrossRef]
- Qian, Y.; Li, D.; Han, Y.; Jiang, H.-L. Photocatalytic Molecular Oxygen Activation by Regulating Excitonic Effects in Covalent Organic Frameworks. J. Am. Chem. Soc. 2020, 142, 20763–20771. [Google Scholar] [CrossRef]
- Liu, Y.; Liu, L.; Wang, Y. A Critical Review on Removal of Gaseous Pollutants Using Sulfate Radical-based Advanced Oxidation Technologies. Environ. Sci. Technol. 2021, 55, 9691–9710. [Google Scholar] [CrossRef]
- Xu, X.; Wang, J.; Chen, T.; Yang, N.; Wang, S.; Ding, X.; Chen, H. Deep insight into ROS mediated direct and hydroxylated dichlorination process for efficient photocatalytic sodium pentachlorophenate mineralization. Appl. Catal. B Environ. 2021, 296, 120352. [Google Scholar] [CrossRef]
- Xu, Q.; Zhang, L.; Cheng, B.; Fan, J.; Yu, J. S-Scheme Heterojunction Photocatalyst. Chem 2020, 6, 1543–1559. [Google Scholar] [CrossRef]
- Qiu, B.; Du, M.; Ma, Y.; Zhu, Q.; Xing, M.; Zhang, J. Integration of redox cocatalysts for artificial photosynthesis. Energy Environ. Sci. 2021, 14, 5260–5288. [Google Scholar] [CrossRef]
- Yang, X.; Wang, S.; Yang, N.; Zhou, W.; Wang, P.; Jiang, K.; Li, S.; Song, H.; Ding, X.; Chen, H.; et al. Oxygen vacancies induced special CO2 adsorption modes on Bi2MoO6 for highly selective conversion to CH4. Appl. Catal. B-Environ. 2019, 259, 118088–118095. [Google Scholar] [CrossRef]
- Xu, X.; Liu, H.; Wang, J.; Chen, T.; Ding, X.; Chen, H. Insight into surface hydroxyl groups for environmental purification: Characterizations, applications and advances. Surf. Interfaces 2021, 25, 101272. [Google Scholar] [CrossRef]
- Huang, Y.; Yu, Y.; Yu, Y.; Zhang, B. Oxygen Vacancy Engineering in Photocatalysis. Sol. RRL 2020, 4, 2000037. [Google Scholar] [CrossRef]
- Wu, X.; Li, J.; Xie, S.; Duan, P.; Zhang, H.; Feng, J.; Zhang, Q.; Cheng, J.; Wang, Y. Selectivity Control in Photocatalytic Valorization of Biomass-Derived Platform Compounds by Surface Engineering of Titanium Oxide. Chem 2020, 6, 3038–3053. [Google Scholar] [CrossRef]
- Wang, S.; He, T.; Chen, P.; Du, A.; Ostrikov, K.; Huang, W.; Wang, L. In Situ Formation of Oxygen Vacancies Achieving Near-Complete Charge Separation in Planar BiVO4 Photoanodes. Adv. Mater. 2020, 32, 2001385. [Google Scholar] [CrossRef] [PubMed]
- Li, P.; Zhou, Z.; Wang, Q.; Guo, M.; Chen, S.; Low, J.; Long, R.; Liu, W.; Ding, P.; Wu, Y.; et al. Visible-Light-Driven Nitrogen Fixation Catalyzed by Bi5O7Br Nanostructures: Enhanced Performance by Oxygen Vacancies. J. Am. Chem. Soc. 2020, 142, 12430–12439. [Google Scholar] [CrossRef] [PubMed]
- Wei, Z.; Wang, W.; Li, W.; Bai, X.; Zhao, J.; Tse, E.C.M.; Phillips, D.L.; Zhu, Y. Steering Electron–Hole Migration Pathways Using Oxygen Vacancies in Tungsten Oxides to Enhance Their Photocatalytic Oxygen Evolution Performance. Angew. Chem. Int. Ed. 2021, 60, 8236–8242. [Google Scholar] [CrossRef] [PubMed]
- Nam, Y.; Li, L.; Lee, J.Y.; Prezhdo, O.V. Strong Influence of Oxygen Vacancy Location on Charge Carrier Losses in Reduced TiO2 Nanoparticles. J. Phys. Chem. Lett. 2019, 10, 2676–2683. [Google Scholar] [CrossRef] [PubMed]
- Li, H.; Shi, J.; Zhao, K.; Zhang, L. Sustainable molecular oxygen activation with oxygen vacancies on the {001} facets of BiOCl nanosheets under solar light. Nanoscale 2014, 6, 14168–14173. [Google Scholar] [CrossRef]
- Li, H.; Shang, J.; Zhu, H.; Yang, Z.; Ai, Z.; Zhang, L. Oxygen Vacancy Structure Associated Photocatalytic Water Oxidation of BiOCl. ACS Catal. 2016, 6, 8276–8285. [Google Scholar] [CrossRef]
- Chen, F.; Liu, L.-L.; Zhang, Y.-J.; Wu, J.-H.; Huang, G.-X.; Yang, Q.; Chen, J.-J.; Yu, H.-Q. Enhanced full solar spectrum photocatalysis by nitrogen-doped graphene quantum dots decorated BiO2-x nanosheets: Ultrafast charge transfer and molecular oxygen activation. Appl. Catal. B Environ. 2020, 227, 119218. [Google Scholar] [CrossRef]
- Wang, S.; Ding, X.; Yang, N.; Zhan, G.; Zhang, X.; Dong, G.; Zhang, L.; Chen, H. Insight into the effect of bromine on facet-dependent surface oxygen vacancies construction and stabilization of Bi2MoO6 for efficient photocatalytic NO removal. Appl. Catal. B Environ. 2020, 265, 118585. [Google Scholar] [CrossRef]
- Ma, Z.; Li, P.; Ye, L.; Zhou, Y.; Su, F.; Ding, C.; Xie, H.; Bai, Y.; Wong, P.K. Oxygen vacancies induced exciton dissociation of flexible BiOCl nanosheets for effective photocatalytic CO2 conversion. J. Mater. Chem. A 2017, 5, 24995–25004. [Google Scholar] [CrossRef]
- Wang, H.; Chen, S.; Yong, D.; Zhang, X.; Li, S.; Shao, W.; Sun, X.; Pan, B.; Xie, Y. Giant Electron-Hole Interactions in Confined Layered Structures for Molecular Oxygen Activation. J. Am. Chem. Soc. 2017, 139, 4737–4742. [Google Scholar] [CrossRef]
- Rousseau, R.; Glezakou, V.-A.; Selloni, A. Theoretical insights into the surface physics and chemistry of redox-active oxides. Nat. Rev. Mater. 2020, 5, 460–475. [Google Scholar] [CrossRef]
- Shi, Y.; Li, H.; Mao, C.; Zhan, G.; Yang, Z.; Ling, C.; Wei, K.; Liu, X.; Ai, Z.; Zhang, L. Manipulating Excitonic Effects in Layered Bismuth Oxyhalides for Photocatalysis. ACS ES&T Engineer. 2022, 2, 957–974. [Google Scholar]
- Shi, Y.; Zhan, G.; Li, H.; Wang, X.; Liu, X.; Shi, L.; Wei, K.; Ling, C.; Li, Z.; Wang, H.; et al. Simultaneous Manipulation of Bulk Excitons and Surface Defects for Ultrastable and Highly Selective CO2 Photoreduction. Adv. Mater. 2021, 33, 2100143. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.; Liu, W.; He, X.; Zhang, P.; Zhang, X.; Xie, Y. An Excitonic Perspective on Low-Dimensional Semiconductors for Photocatalysis. J. Am. Chem. Soc. 2020, 142, 14007–14022. [Google Scholar] [CrossRef] [PubMed]
- Xu, X.; Yang, N.; Wang, P.; Wang, S.; Xiang, Y.; Zhang, X.; Ding, X.; Chen, H. Highly Intensified Molecular Oxygen Activation on Bi@Bi2MoO6 via a Metallic Bi-Coordinated Facet-Dependent Effect. ACS Appl. Mater. Interfaces 2020, 12, 1867–1876. [Google Scholar] [CrossRef]
- Xu, X.; Ding, X.; Yang, X.; Wang, P.; Li, S.; Lu, Z.; Chen, H. Oxygen vacancy boosted photocatalytic decomposition of ciprofloxacin over Bi2MoO6: Oxygen vacancy engineering, biotoxicity evaluation and mechanism study. J. Hazard. Mater. 2019, 364, 691–699. [Google Scholar] [CrossRef] [PubMed]
- Wang, S.; Xiong, Z.; Yang, N.; Ding, X.; Chen, H. Iodine-doping-assisted tunable introduction of oxygen vacancies on bismuth tungstate photocatalysts for highly efficient molecular oxygen activation and pentachlorophenol mineralization. Chin. J. Catal. 2020, 41, 1544–1553. [Google Scholar] [CrossRef]
- Li, K.; Fang, X.; Fu, Z.; Yang, Y.; Nabi, I.; Feng, Y.; Bacha, A.-U.-R.; Zhang, L. Boosting photocatalytic chlorophenols remediation with addition of sulfite and mechanism investigation by in-situ DRIFTs. J. Hazard. Mater. 2020, 398, 123007. [Google Scholar] [CrossRef]
- Chang, Y.; Liu, Z.; Shen, X.; Zhu, B.; Macharia, D.K.; Chen, Z.; Zhang, L. Synthesis of Au nanoparticle-decorated carbon nitride nanorods with plasmon-enhanced photoabsorption and photocatalytic activity for removing various pollutants from water. J. Hazard. Mater. 2018, 344, 1188–1197. [Google Scholar] [CrossRef]
- Wang, Q.; Wang, W.; Zhong, L.; Liu, D.; Cao, X.; Cui, F. Oxygen vacancy-rich 2D/2D BiOCl-g-C3N4 ultrathin heterostructure nanosheets for enhanced visible-light-driven photo-catalytic activity in environmental remediation. Appl. Catal. B- Environ. 2018, 220, 290–302. [Google Scholar] [CrossRef]
- Huang, S.; Tian, F.; Dai, J.; Tian, X.; Li, G.; Liu, Y.; Chen, Z.; Chen, R. Highly efficient degradation of chlorophenol over bismuth oxides upon near-infrared irradiation: Unraveling the effect of Bi-O-Bi-O defects cluster and 1O2 involved process. Appl. Catal. B-Environ. 2021, 298, 120576. [Google Scholar] [CrossRef]
- Ma, H.-Y.; Zhao, L.; Guo, L.-H.; Zhang, H.; Chen, F.-J.; Yu, W.-C. Roles of reactive oxygen species (ROS) in the photocatalytic degradation of pentachlorophenol and its main toxic intermediates by TiO2/UV. J. Hazard. Mater. 2019, 369, 719–726. [Google Scholar] [CrossRef]
- Li, H.; Zhang, L. Oxygen vacancy induced selective silver deposition on the {001} facets of BiOCl single-crystalline nanosheets for enhanced Cr(VI) and sodium pentachlorophenate removal under visible light. Nanoscale 2014, 6, 7805–7810. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Xu, X.; Yang, X.; Tao, Y.; Zhu, W.; Ding, X.; Zhu, J.; Chen, H. Enhanced Exciton Effect and Singlet Oxygen Generation Triggered by Tunable Oxygen Vacancies on Bi2MoO6 for Efficient Photocatalytic Degradation of Sodium Pentachlorophenol. Int. J. Mol. Sci. 2022, 23, 15221. https://doi.org/10.3390/ijms232315221
Xu X, Yang X, Tao Y, Zhu W, Ding X, Zhu J, Chen H. Enhanced Exciton Effect and Singlet Oxygen Generation Triggered by Tunable Oxygen Vacancies on Bi2MoO6 for Efficient Photocatalytic Degradation of Sodium Pentachlorophenol. International Journal of Molecular Sciences. 2022; 23(23):15221. https://doi.org/10.3390/ijms232315221
Chicago/Turabian StyleXu, Xiao, Xianglong Yang, Yunlong Tao, Wen Zhu, Xing Ding, Junjiang Zhu, and Hao Chen. 2022. "Enhanced Exciton Effect and Singlet Oxygen Generation Triggered by Tunable Oxygen Vacancies on Bi2MoO6 for Efficient Photocatalytic Degradation of Sodium Pentachlorophenol" International Journal of Molecular Sciences 23, no. 23: 15221. https://doi.org/10.3390/ijms232315221
APA StyleXu, X., Yang, X., Tao, Y., Zhu, W., Ding, X., Zhu, J., & Chen, H. (2022). Enhanced Exciton Effect and Singlet Oxygen Generation Triggered by Tunable Oxygen Vacancies on Bi2MoO6 for Efficient Photocatalytic Degradation of Sodium Pentachlorophenol. International Journal of Molecular Sciences, 23(23), 15221. https://doi.org/10.3390/ijms232315221






