Endocrine Disruptor Compounds in Environment: Focus on Women’s Reproductive Health and Endometriosis
Abstract
1. Introduction
2. Endocrine Disruptors
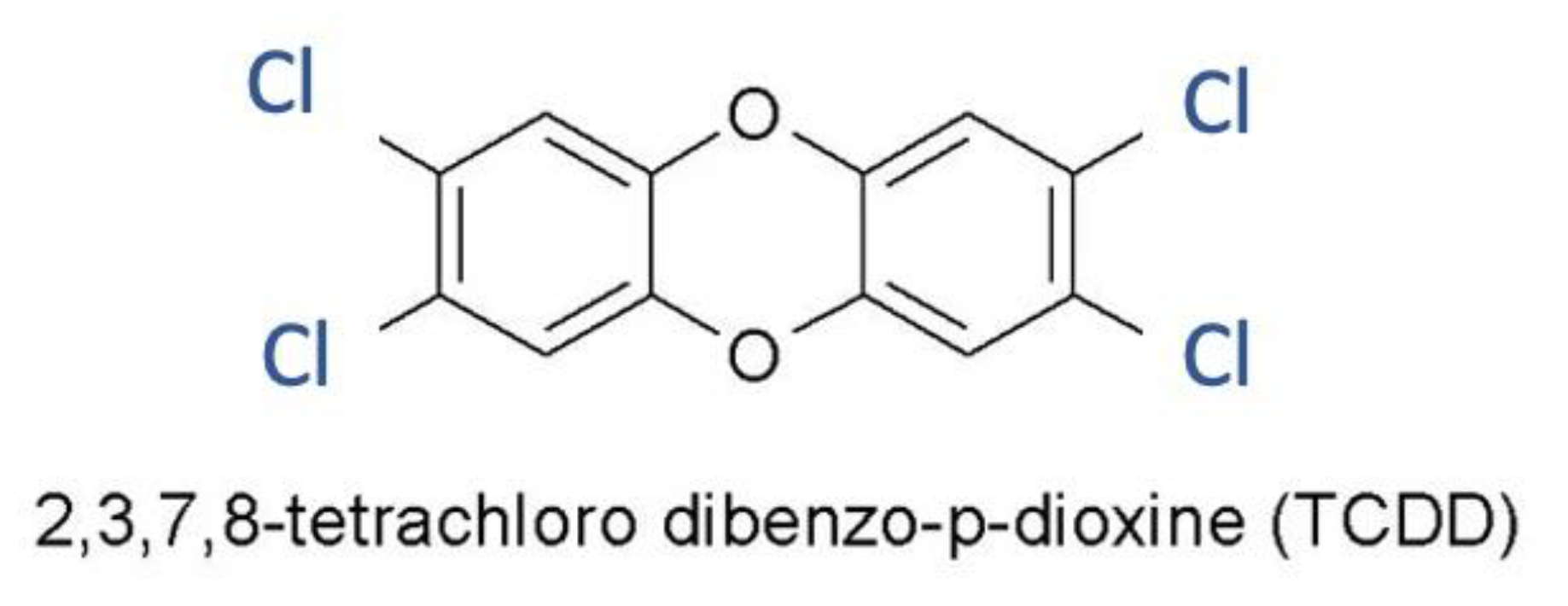
2.1. Dioxin
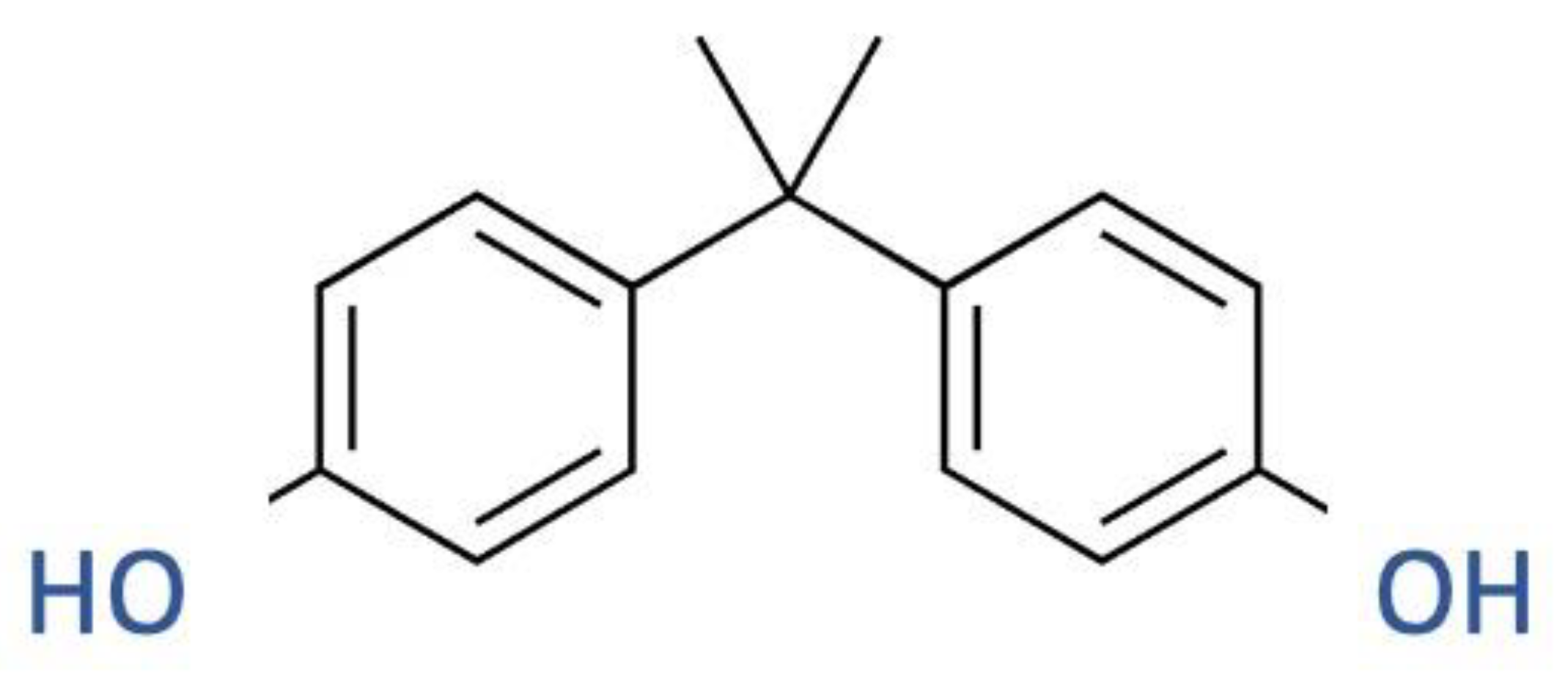
2.2. BPA
2.3. Phthalates
| Compounds | Sources | Exposition Effects |
|---|---|---|
| di-(2-ethylhexyl) phthalate (DEHP) | Perfumes, PVC plastics used in household products (e.g., toys, floor tiles and furniture upholstery, cables, garden hoses, wall coverings, and gloves), food packaging, blood storage bags, and medical devices. | [30] |
| diethyl phthalate (DEP) | Used as solvents and fixatives in fragrances, additives in cosmetics, medical devices, and household and personal care products. | [31] |
| di-n-butyl phthalate (DBP) | Cellulose acetate plastics, solvent for oil-soluble dyes, pesticides, personal care products (e.g., nail polish and cosmetics), lacquers, varnishes, and coatings (e.g., pharmaceuticals). | [32] |
2.4. Pesticides
3. Epidemiological Studies
4. Endocrine Disruptors and Women’s Reproductive System
5. Endocrine Disruptors and Endometriosis
6. Endometriosis and Treatment
7. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Hiller-Sturmhöfel, S.; Bartke, A. The endocrine system: An overview. Alcohol Health Res. World 1998, 22, 153. [Google Scholar]
- Chahal, H.; Drake, W. The endocrine system and ageing. J. Pathol. A J. Pathol. Soc. Great Br. Irel. 2007, 211, 173–180. [Google Scholar] [CrossRef] [PubMed]
- Sbraccia, P.; Guglielmi, V. Endocrine disruptors ediabetetipo2. Diabetes 2012, 24, 155–165. [Google Scholar]
- Gültekin, I.; Ince, N.H. Synthetic endocrine disruptors in the environment and water remediation by advanced oxidation processes. J. Environ. Manag. 2007, 85, 816–832. [Google Scholar] [CrossRef] [PubMed]
- Preda, C.; Ungureanu, M.C.; Vulpoi, C. Endocrine disruptors in the environment and their impact on human health. Environ. Eng. Manag. J. 2012, 11, 1697–1706. [Google Scholar] [CrossRef]
- Hoshi, N. Adverse effects of pesticides on regional biodiversity and their mechanisms. In Risks and Regulation of New Technologies; Springer: Berlin/Heidelberg, Germany, 2021; pp. 235–247. [Google Scholar]
- Mnif, W.; Hassine, A.I.H.; Bouaziz, A.; Bartegi, A.; Thomas, O.; Roig, B. Effect of endocrine disruptor pesticides: A review. Int. J. Environ. Res. Public Health 2011, 8, 2265–2303. [Google Scholar] [CrossRef] [PubMed]
- Maqbool, F.; Mostafalou, S.; Bahadar, H.; Abdollahi, M. Review of endocrine disorders associated with environmental toxicants and possible involved mechanisms. Life Sci. 2016, 145, 265–273. [Google Scholar] [CrossRef] [PubMed]
- Alonso-Magdalena, P.; Quesada, I.; Nadal, A. Endocrine disruptors in the etiology of type 2 diabetes mellitus. Nat. Rev. Endocrinol. 2011, 7, 346–353. [Google Scholar] [CrossRef]
- Masuo, Y.; Ishido, M. Neurotoxicity of endocrine disruptors: Possible involvement in brain development and neurodegeneration. J. Toxicol. Environ. Health Part B 2011, 14, 346–369. [Google Scholar] [CrossRef]
- Lintelmann, J.; Katayama, A.; Kurihara, N.; Shore, L.; Wenzel, A. Endocrine disruptors in the environment (IUPAC Technical Report). Pure Appl. Chem. 2003, 75, 631–681. [Google Scholar] [CrossRef]
- Baker, M.E.; Vidal-Dorsch, D.E.; Ribecco, C.; Sprague, L.J.; Angert, M.; Lekmine, N.; Ludka, C.; Martella, A.; Ricciardelli, E.; Bay, S.M. Molecular analysis of endocrine disruption in hornyhead turbot at wastewater outfalls in southern california using a second generation multi-species microarray. PLoS ONE 2013, 8, e75553. [Google Scholar] [CrossRef] [PubMed]
- Swedenborg, E.; Pongratz, I.; Gustafsson, J.Å. Endocrine disruptors targeting ERβ function. Int. J. Androl. 2010, 33, 288–297. [Google Scholar] [CrossRef] [PubMed]
- Rosenfeld, C.S.; Cooke, P.S. Endocrine disruption through membrane estrogen receptors and novel pathways leading to rapid toxicological and epigenetic effects. J. Steroid Biochem. Mol. Biol. 2019, 187, 106–117. [Google Scholar] [CrossRef]
- Boutin, J.A.; Norbeck, K.; Moldeus, P.; Genton, A.; Paraire, M.; Bizzari, J.-P.; Lavielle, G.; Cudennec, C.A. Effects of the new nitrosourea derivative, fotemustine, on the glutathione reductase activity in rat tissues in vivo and in isolated rat hepatocytes. Eur. J. Cancer Clin. Oncol. 1989, 25, 1311–1316. [Google Scholar] [CrossRef] [PubMed]
- Mulero-Navarro, S.; Fernandez-Salguero, P.M. New Trends in Aryl Hydrocarbon Receptor Biology. Front. Cell Dev. Biol. 2016, 4, 45. [Google Scholar] [CrossRef]
- Bruner-Tran, K.L.; Osteen, K.G. Developmental exposure to TCDD reduces fertility and negatively affects pregnancy outcomes across multiple generations. Reprod. Toxicol. 2011, 31, 344–350. [Google Scholar] [CrossRef]
- Kahn, L.G.; Philippat, C.; Nakayama, S.F.; Slama, R.; Trasande, L. Endocrine-disrupting chemicals: Implications for human health. Lancet Diabetes Endocrinol 2020, 8, 703–718. [Google Scholar] [CrossRef] [PubMed]
- Nicolopoulou-Stamati, P.; Pitsos, M. The impact of endocrine disrupters on the female reproductive system. Hum. Reprod. Update 2001, 7, 323–330. [Google Scholar] [CrossRef]
- Bruner-Tran, K.L.; Gnecco, J.; Ding, T.; Glore, D.R.; Pensabene, V.; Osteen, K.G. Exposure to the environmental endocrine disruptor TCDD and human reproductive dysfunction: Translating lessons from murine models. Reprod Toxicol 2017, 68, 59–71. [Google Scholar] [CrossRef]
- Piazza, M.J.; Urbanetz, A.A. Environmental toxins and the impact of other endocrine disrupting chemicals in women’s reproductive health. JBRA Assist. Reprod. 2019, 23, 154. [Google Scholar] [CrossRef]
- Iughetti, L.; Lucaccioni, L.; Bernasconi, S.; Predieri, B. Effetti Degli Interferenti Endocrini su Crescita e Sviluppo Puberale. 2020. Available online: https://hdl.handle.net/11380/1202122 (accessed on 1 January 2020).
- Lang, I.A.; Galloway, T.S.; Scarlett, A.; Henley, W.E.; Depledge, M.; Wallace, R.B.; Melzer, D. Association of urinary bisphenol A concentration with medical disorders and laboratory abnormalities in adults. Jama 2008, 300, 1303–1310. [Google Scholar] [CrossRef] [PubMed]
- Grün, F.; Blumberg, B. Environmental obesogens: Organotins and endocrine disruption via nuclear receptor signaling. Endocrinology 2006, 147, S50–S55. [Google Scholar] [CrossRef] [PubMed]
- Le, H.H. Definition of Rapid 17β-Estradiol Signaling Networks in Developing Cerebellar Granule Cells; University of Cincinnati: Cincinnati, OH, USA, 2008. [Google Scholar]
- Heudorf, U.; Mersch-Sundermann, V.; Angerer, J. Phthalates: Toxicology and exposure. Int. J. Hyg. Environ. Health 2007, 210, 623–634. [Google Scholar] [CrossRef]
- Davis, B.; Maronpot, R.; Heindel, J. Di-(2-ethylhexyl) phthalate suppresses estradiol and ovulation in cycling rats. Toxicol. Appl. Pharmacol. 1994, 128, 216–223. [Google Scholar] [CrossRef] [PubMed]
- Kumar, S.; Sharma, A.; Kshetrimayum, C. Environmental & occupational exposure & female reproductive dysfunction. Indian J. Med. Res. 2019, 150, 532. [Google Scholar] [CrossRef]
- Thomsen, A.M.L.; Riis, A.H.; Olsen, J.; Jönsson, B.A.; Lindh, C.H.; Hjollund, N.H.; Jensen, T.K.; Bonde, J.P.; Toft, G. Female exposure to phthalates and time to pregnancy: A first pregnancy planner study. Hum. Reprod. 2017, 32, 232–238. [Google Scholar] [CrossRef]
- Sathyanarayana, S.; Barrett, E.; Butts, S.; Wang, C.; Swan, S.H. Phthalate exposure and reproductive hormone concentrations in pregnancy. Reproduction 2014, 147, 401. [Google Scholar] [CrossRef]
- Taheri, E.; Amin, M.M.; Daniali, S.S.; Abdollahpour, I.; Fatehizadeh, A.; Kelishadi, R. Health risk assessment of exposure to chlorpyrifos in pregnant women using deterministic and probabilistic approaches. PLoS ONE 2022, 17, e0262127. [Google Scholar] [CrossRef]
- Heidari, T.; Batavani, R.A.; Malekinejad, H.; Hobbenaghi, R. Evaluation of di-n-butyl phthalate reproductive toxicity in pregnant rats and their offspring and assessment of vitamin E administration in reducing toxicity. In Veterinary Research Forum; Faculty of Veterinary Medicine, Urmia University: Urmia, Iran, 2022; p. 201. [Google Scholar]
- Ji, C.; Song, Q.; Chen, Y.; Zhou, Z.; Wang, P.; Liu, J.; Sun, Z.; Zhao, M. The potential endocrine disruption of pesticide transformation products (TPs): The blind spot of pesticide risk assessment. Environ. Int. 2020, 137, 105490. [Google Scholar] [CrossRef]
- al-Saleh, I.A. Pesticides: A review article. J. Environ. Pathol. Toxicol. Oncol. 1994, 13, 151–161. [Google Scholar]
- Negatu, B.; Dugassa, S.; Mekonnen, Y. Environmental and Health Risks of Pesticide Use in Ethiopia. J. Health Pollut. 2021, 11, 210601. [Google Scholar] [CrossRef] [PubMed]
- He, Y.; Guo, C.; Lv, J.; Deng, Y.; Xu, J. Occurrence, sources, and ecological risks of three classes of insecticides in sediments of the Liaohe River basin, China. Environ. Sci. Pollut. Res. 2021, 28, 62726–62735. [Google Scholar] [CrossRef] [PubMed]
- Kaushal, J.; Khatri, M.; Arya, S.K. A treatise on Organophosphate pesticide pollution: Current strategies and advancements in their environmental degradation and elimination. Ecotoxicol. Environ. Saf. 2021, 207, 111483. [Google Scholar] [CrossRef] [PubMed]
- Mahmood, I.; Imadi, S.R.; Shazadi, K.; Gul, A.; Hakeem, K.R. Effects of pesticides on environment. In Plant, Soil and Microbes; Springer: Berlin/Heidelberg, Germany, 2016; pp. 253–269. [Google Scholar] [CrossRef]
- Anand, N.; Chakraborty, P.; Ray, S. Human exposure to organochlorine, pyrethroid and neonicotinoid pesticides: Comparison between urban and semi-urban regions of India. Environ. Pollut. 2021, 270, 116156. [Google Scholar] [CrossRef] [PubMed]
- Achour, A.; Derouiche, A.; Barhoumi, B.; Kort, B.; Cherif, D.; Bouabdallah, S.; Sakly, M.; Rhouma, K.B.; Touil, S.; Driss, M.R.; et al. Organochlorine pesticides and polychlorinated biphenyls in human adipose tissue from northern Tunisia: Current extent of contamination and contributions of socio-demographic characteristics and dietary habits. Environ. Res. 2017, 156, 635–643. [Google Scholar] [CrossRef] [PubMed]
- Jugan, J.; Lind, P.M.; Salihovic, S.; Stubleski, J.; Karrman, A.; Lind, L.; La Merrill, M.A. The associations between p,p′-DDE levels and plasma levels of lipoproteins and their subclasses in an elderly population determined by analysis of lipoprotein content. Lipids Health Dis. 2020, 19, 249. [Google Scholar] [CrossRef]
- Yaglova, N.; Tsomartova, D.; Yaglov, V. Differences in production of adrenal steroid hormones in pubertal rats exposed to low doses of the endocrine disruptor DDT during prenatal and postnatal development. Biochem. (Mosc.) Suppl. Ser. B Biomed. Chem. 2018, 12, 80–86. [Google Scholar] [CrossRef]
- Wang, L.; Qie, Y.; Yang, Y.; Zhao, Q. Binding and Activation of Estrogen-Related Receptor γ: A Novel Molecular Mechanism for the Estrogenic Disruption Effects of DDT and Its Metabolites. Environ. Sci. Technol. 2022, 56, 12358–12367. [Google Scholar] [CrossRef]
- Yaglova, N.V.; Tsomartova, D.A.; Obernikhin, S.S.; Yaglov, V.V.; Nazimova, S.V.; Tsomartova, E.S.; Chereshneva, E.V.; Ivanova, M.Y.; Lomanovskaya, T.A. Differential disrupting effects of prolonged low-dose exposure to dichlorodiphenyltrichloroethane on androgen and estrogen production in males. Int. J. Mol. Sci. 2021, 22, 3155. [Google Scholar] [CrossRef]
- Kassotis, C.D.; Vandenberg, L.N.; Demeneix, B.A.; Porta, M.; Slama, R.; Trasande, L. Endocrine-disrupting chemicals: Economic, regulatory, and policy implications. Lancet Diabetes Endocrinol. 2020, 8, 719–730. [Google Scholar] [CrossRef]
- Pistollato, F.; Madia, F.; Corvi, R.; Munn, S.; Grignard, E.; Paini, A.; Worth, A.; Bal-Price, A.; Prieto, P.; Casati, S. Current EU regulatory requirements for the assessment of chemicals and cosmetic products: Challenges and opportunities for introducing new approach methodologies. Arch. Toxicol. 2021, 95, 1867–1897. [Google Scholar] [CrossRef] [PubMed]
- Haug, L.S.; Sakhi, A.K.; Cequier, E.; Casas, M.; Maitre, L.; Basagana, X.; Andrusaityte, S.; Chalkiadaki, G.; Chatzi, L.; Coen, M. In-utero and childhood chemical exposome in six European mother-child cohorts. Environ. Int. 2018, 121, 751–763. [Google Scholar] [CrossRef] [PubMed]
- Haggerty, D.K.; Upson, K.; Pacyga, D.C.; Franko, J.E.; Braun, J.M.; Strakovsky, R.S. REPRODUCTIVE TOXICOLOGY: Pregnancy exposure to endocrine disrupting chemicals: Implications for women’s health. Reproduction 2021, 162, F169–F180. [Google Scholar] [CrossRef] [PubMed]
- Rolfo, A.; Nuzzo, A.M.; De Amicis, R.; Moretti, L.; Bertoli, S.; Leone, A. Fetal–maternal exposure to endocrine disruptors: Correlation with diet intake and pregnancy outcomes. Nutrients 2020, 12, 1744. [Google Scholar] [CrossRef]
- Bliznashka, L.; Diao, N.; Christiani, D.; Calafat, A.; Ospina, M.; Mazumdar, M.; Hasan, M.O.S.I.; Wright, R.; Quamruzzaman, Q.; Jaacks, L. Prenatal Pesticide Exposure Is Associated with Lower Cognitive, Language, and Motor Development Scores in Children 20–40 Months of Age Rural Bangladesh. Curr. Dev. Nutr. 2022, 6, 550. [Google Scholar] [CrossRef]
- Fouyet, S.; Olivier, E.; Leproux, P.; Dutot, M.; Rat, P. Pregnant Women and Endocrine Disruptors: Role of P2X7 Receptor and Mitochondrial Alterations in Placental Cell Disorders. Cells 2022, 11, 495. [Google Scholar] [CrossRef]
- Keith, L.H. Environmental endocrine disruptors. Pure Appl. Chem. 1998, 70, 2319–2326. [Google Scholar] [CrossRef]
- Karzi, V.; Tzatzarakis, M.; Katsikantami, I.; Stavroulaki, A.; Alegakis, A.; Vakonaki, E.; Xezonaki, P.; Sifakis, S.; Rizos, A.; Tsatsakis, A. Investigating exposure to endocrine disruptors via hair analysis of pregnant women. Environ. Res. 2019, 178, 108692. [Google Scholar] [CrossRef]
- Hauser, R.; Skakkebaek, N.E.; Hass, U.; Toppari, J.; Juul, A.; Andersson, A.M.; Kortenkamp, A.; Heindel, J.J.; Trasande, L. Male reproductive disorders, diseases, and costs of exposure to endocrine-disrupting chemicals in the European Union. J. Clin. Endocrinol. Metab. 2015, 100, 1267–1277. [Google Scholar] [CrossRef]
- Jeng, H.A. Exposure to endocrine disrupting chemicals and male reproductive health. Front. Public Health 2014, 2, 55. [Google Scholar] [CrossRef]
- Wu, Y.; Wang, J.; Wei, Y.; Chen, J.; Kang, L.; Long, C.; Wu, S.; Shen, L.; Wei, G. Contribution of prenatal endocrine-disrupting chemical exposure to genital anomalies in males: The pooled results from current evidence. Chemosphere 2022, 286, 131844. [Google Scholar] [CrossRef]
- Harley, K.G.; Kogut, K.; Madrigal, D.S.; Cardenas, M.; Vera, I.A.; Meza-Alfaro, G.; She, J.; Gavin, Q.; Zahedi, R.; Bradman, A. Reducing phthalate, paraben, and phenol exposure from personal care products in adolescent girls: Findings from the HERMOSA intervention study. Environ. Health Perspect. 2016, 124, 1600–1607. [Google Scholar] [CrossRef] [PubMed]
- Jagne, J.; White, D.; Jefferson, F. Endocrine-disrupting chemicals: Adverse effects of bisphenol A and parabens to women’s health. Water Air Soil Pollut. 2016, 227, 182. [Google Scholar] [CrossRef]
- Mantovani, A.; Baldi, F. Valutazione del rischio in sicurezza alimentare: L’esempio degli interferenti endocrini. Annali di Igiene 2013. [Google Scholar]
- Rattan, S.; Flaws, J.A. The epigenetic impacts of endocrine disruptors on female reproduction across generations. Biol. Reprod. 2019, 101, 635–644. [Google Scholar] [CrossRef]
- Waring, R.; Harris, R. Endocrine disrupters—A threat to women’s health? Maturitas 2011, 68, 111–115. [Google Scholar] [CrossRef]
- Lite, C.; Raja, G.L.; Juliet, M.; Sridhar, V.V.; Subhashree, K.D.; Kumar, P.; Chakraborty, P.; Arockiaraj, J. In utero exposure to endocrine-disrupting chemicals, maternal factors and alterations in the epigenetic landscape underlying later-life health effects. Environ. Toxicol. Pharmacol. 2022, 89, 103779. [Google Scholar] [CrossRef]
- Chiang, C.; Mahalingam, S.; Flaws, J.A. Environmental Contaminants Affecting Fertility and Somatic Health. Semin. Reprod. Med. 2017, 35, 241–249. [Google Scholar] [CrossRef]
- Lovekamp-Swan, T.; Davis, B.J. Mechanisms of phthalate ester toxicity in the female reproductive system. Environ. Health Perspect. 2003, 111, 139–145. [Google Scholar] [CrossRef]
- Rier, S.E.; Martin, D.C.; Bowman, R.E.; Dmowski, W.P.; Becker, J.L. Endometriosis in rhesus monkeys (Macaca mulatta) following chronic exposure to 2,3,7,8-tetrachlorodibenzo-p-dioxin. Fundam. Appl. Toxicol. 1993, 21, 433–441. [Google Scholar] [CrossRef]
- Treinen, K.A.; Dodson, W.C.; Heindel, J.J. Inhibition of FSH-stimulated cAMP accumulation and progesterone production by mono (2-ethylhexyl) phthalate in rat granulosa cell cultures. Toxicol. Appl. Pharmacol. 1990, 106, 334–340. [Google Scholar] [CrossRef] [PubMed]
- Spaczynski, R.Z.; Duleba, A.J. Diagnosis of endometriosis. Semin. Reprod. Med. 2003, 21, 193–208. [Google Scholar] [PubMed]
- García-Peñarrubia, P.; Ruiz-Alcaraz, A.J.; Martínez-Esparza, M.; Marín, P.; Machado-Linde, F. Hypothetical roadmap towards endometriosis: Prenatal endocrine-disrupting chemical pollutant exposure, anogenital distance, gut-genital microbiota and subclinical infections. Hum. Reprod. Update 2020, 26, 214–246. [Google Scholar] [CrossRef] [PubMed]
- Cramer, D.W.; Missmer, S.A. The epidemiology of endometriosis. Ann. N. Y. Acad. Sci. 2002, 955, 11–22. [Google Scholar] [CrossRef] [PubMed]
- Agarwal, N.; Subramanian, A. Endometriosis-morphology, clinical presentations and molecular pathology. J. Lab. Physicians 2010, 2, 001–009. [Google Scholar] [CrossRef] [PubMed]
- Cordaro, M.; Trovato Salinaro, A.; Siracusa, R.; D’Amico, R.; Impellizzeri, D.; Scuto, M.; Ontario, M.L.; Interdonato, L.; Crea, R.; Fusco, R. Hidrox® and endometriosis: Biochemical evaluation of oxidative stress and pain. Antioxidants 2021, 10, 720. [Google Scholar] [CrossRef]
- Fusco, R.; D’amico, R.; Cordaro, M.; Gugliandolo, E.; Siracusa, R.; Peritore, A.F.; Crupi, R.; Impellizzeri, D.; Cuzzocrea, S.; Di Paola, R. Absence of formyl peptide receptor 1 causes endometriotic lesion regression in a mouse model of surgically-induced endometriosis. Oncotarget 2018, 9, 31355. [Google Scholar] [CrossRef]
- Signorile, P.G.; Spugnini, E.P.; Mita, L.; Mellone, P.; D’Avino, A.; Bianco, M.; Diano, N.; Caputo, L.; Rea, F.; Viceconte, R.; et al. Pre-natal exposure of mice to bisphenol A elicits an endometriosis-like phenotype in female offspring. Gen. Comp. Endocrinol. 2010, 168, 318–325. [Google Scholar] [CrossRef]
- Signorile, P.G.; Spugnini, E.P.; Citro, G.; Viceconte, R.; Vincenzi, B.; Baldi, F.; Baldi, A. Endocrine disruptors in utero cause ovarian damages linked to endometriosis. Front. Biosci. -Elite 2012, 4, 1724–1730. [Google Scholar] [CrossRef]
- WHO. State of the Science of Endocrine Disrupting Chemicals 2012: Summary for Decision-Makers; WHO: Geneva, Switzerland, 2012. [Google Scholar]
- Moreira Fernandez, M.A.; Cardeal, Z.L.; Carneiro, M.M.; André, L.C. Study of possible association between endometriosis and phthalate and bisphenol A by biomarkers analysis. J. Pharm. Biomed. Anal. 2019, 172, 238–242. [Google Scholar] [CrossRef]
- Xue, W.; Yao, X.; Ting, G.; Ling, J.; Huimin, L.; Yuan, Q.; Chun, Z.; Ming, Z.; Yuanzhen, Z. BPA modulates the WDR5/TET2 complex to regulate ERβ expression in eutopic endometrium and drives the development of endometriosis. Environ. Pollut. 2021, 268, 115748. [Google Scholar] [CrossRef] [PubMed]
- Liu, R.; Zacharewski, T.R.; Conolly, R.B.; Zhang, Q. A Physiologically Based Pharmacokinetic (PBPK) Modeling Framework for Mixtures of Dioxin-like Compounds. Toxics 2022, 10, 700. [Google Scholar] [CrossRef] [PubMed]
- Aoudeh, E.; Oz, E.; Khan, M.R.; Oz, F. Dioxins and dioxin-like compounds in meat and meat products. Теoрия И Практика Перерабoтки Мяса 2022, 7, 4–15. [Google Scholar] [CrossRef]
- Kettel, B. Women, health and the environment. Soc. Sci. Med. 1996, 42, 1367–1379. [Google Scholar] [CrossRef] [PubMed]
- Rumph, J.T.; Stephens, V.R.; Archibong, A.E.; Osteen, K.G.; Bruner-Tran, K.L. Environmental Endocrine Disruptors and Endometriosis. Adv. Anat. Embryol. Cell. Biol. 2020, 232, 57–78. [Google Scholar] [CrossRef]
- Bredhult, C.; Bäcklin, B.-M.; Olovsson, M. Effects of some endocrine disruptors on the proliferation and viability of human endometrial endothelial cells in vitro. Reprod. Toxicol. 2007, 23, 550–559. [Google Scholar] [CrossRef]
- Krikun, G.; Schatz, F.; Taylor, R.; Critchley, H.O.D.; Rogers, P.A.W.; Huang, J.; Lockwood, C.J. Endometrial Endothelial Cell Steroid Receptor Expression and Steroid Effects on Gene Expression. J. Clin. Endocrinol. Metab. 2005, 90, 1812–1818. [Google Scholar] [CrossRef]
- González-Ramos, R.; Donnez, J.; Defrère, S.; Leclercq, I.; Squifflet, J.; Lousse, J.-C.; Van Langendonckt, A. Nuclear factor-kappa B is constitutively activated in peritoneal endometriosis. Mol. Hum. Reprod. 2007, 13, 503–509. [Google Scholar] [CrossRef]
- Olive, D.L.; Pritts, E.A. Treatment of endometriosis. N. Engl. J. Med. 2001, 345, 266–275. [Google Scholar] [CrossRef]
- Valle, R.F.; Sciarra, J.J. Endometriosis: Treatment strategies. Ann. N. Y. Acad. Sci. 2003, 997, 229–239. [Google Scholar] [CrossRef]
- Bruner-Tran, K.L.; Osteen, K.G.; Taylor, H.S.; Sokalska, A.; Haines, K.; Duleba, A.J. Resveratrol inhibits development of experimental endometriosis in vivo and reduces endometrial stromal cell invasiveness in vitro. Biol. Reprod. 2011, 84, 106–112. [Google Scholar] [CrossRef] [PubMed]
- Guan, Q.; Velho, R.V.; Sehouli, J.; Mechsner, S. Endometriosis and Opioid Receptors: Are Opioids A Possible/Promising Treatment for Endometriosis? Int. J. Mol. Sci. 2023, 24, 1633. [Google Scholar] [CrossRef] [PubMed]
- Restaino, S.; Mereu, L.; Finelli, A.; Spina, M.R.; Marini, G.; Catena, U.; Turco, L.C.; Moroni, R.; Milani, M.; Cela, V. Robotic surgery vs laparoscopic surgery in patients with diagnosis of endometriosis: A systematic review and meta-analysis. J. Robot. Surg. 2020, 14, 687–694. [Google Scholar] [CrossRef] [PubMed]
| Example of Research Question | Example of Study Objective | Study Examples |
|---|---|---|
| What environmental contaminants are pregnant women exposed to during pregnancy? | To characterize concentrations of a large number of environmental contaminants in pregnant European women. | [47,48,49,50,51,52,53] |
| What proportion of male reproductive disorders and diseases would be prevented by a ban on EDCs in the European Union? | To estimate the incidence and prevalence of selected male reproductive disorders and diseases attributed to EDC exposure in the European Union. | [54,55,56] |
| Can using “low-chemical” personal care products significantly lower levels of EDCs in the body? | To evaluate whether changing personal care products to those labeled as “low chemical” reduces urinary concentrations of metabolites of phthalates, parabens, and phenols in adolescent girls. | [57,58] |
| Example of Research Question | Example of Study Objective | Study Examples |
|---|---|---|
| Are Opioids a Possible/Promising Treatment for Endometriosis? | The mechanisms of pain development in endometriosis disease, the endogenous opioid system and pain, as well as the opioid receptors and endometriosis-associated pain. | [88] |
| Does the surgical treatment of endometriosis in conjunction with assisted reproduction improve pregnancy rates? | The year following the assisted reproductive technology (ART) cycle, a significantly higher spontaneous pregnancy rate was seen in those undergoing surgery. | [85] |
| Robotic surgery or laparoscopic surgery? | The study aimed to evaluate the safety and efficacy of robotic-assisted laparoscopic surgery (RAS) versus conventional laparoscopic surgery (LPS) in the treatment of endometriosis. This meta-analysis demonstrated that robotic surgery is safe and practical in endometriosis patients. We may argue that RAS is a viable option that could be regarded as an alternative to LPS, particularly in advanced situations. | [89] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Interdonato, L.; Siracusa, R.; Fusco, R.; Cuzzocrea, S.; Di Paola, R. Endocrine Disruptor Compounds in Environment: Focus on Women’s Reproductive Health and Endometriosis. Int. J. Mol. Sci. 2023, 24, 5682. https://doi.org/10.3390/ijms24065682
Interdonato L, Siracusa R, Fusco R, Cuzzocrea S, Di Paola R. Endocrine Disruptor Compounds in Environment: Focus on Women’s Reproductive Health and Endometriosis. International Journal of Molecular Sciences. 2023; 24(6):5682. https://doi.org/10.3390/ijms24065682
Chicago/Turabian StyleInterdonato, Livia, Rosalba Siracusa, Roberta Fusco, Salvatore Cuzzocrea, and Rosanna Di Paola. 2023. "Endocrine Disruptor Compounds in Environment: Focus on Women’s Reproductive Health and Endometriosis" International Journal of Molecular Sciences 24, no. 6: 5682. https://doi.org/10.3390/ijms24065682
APA StyleInterdonato, L., Siracusa, R., Fusco, R., Cuzzocrea, S., & Di Paola, R. (2023). Endocrine Disruptor Compounds in Environment: Focus on Women’s Reproductive Health and Endometriosis. International Journal of Molecular Sciences, 24(6), 5682. https://doi.org/10.3390/ijms24065682









