PD-1/PD-L1 Control of Antigen-Specifically Activated CD4 T-Cells of Neonates
Abstract
1. Introduction
2. Results
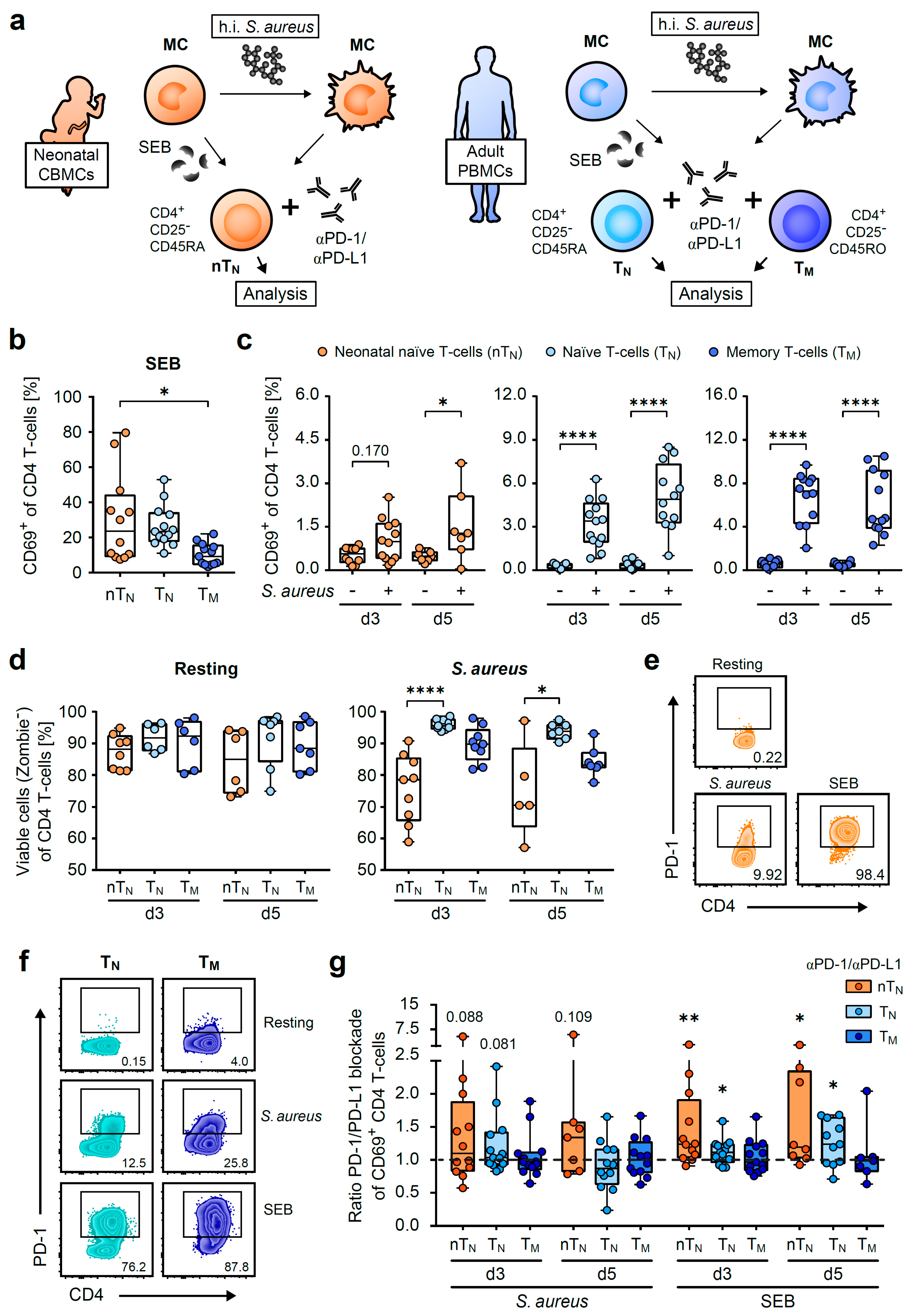
2.1. Neonatal CD4 T-Cells Show Widespread Activation by S. aureus and SEB
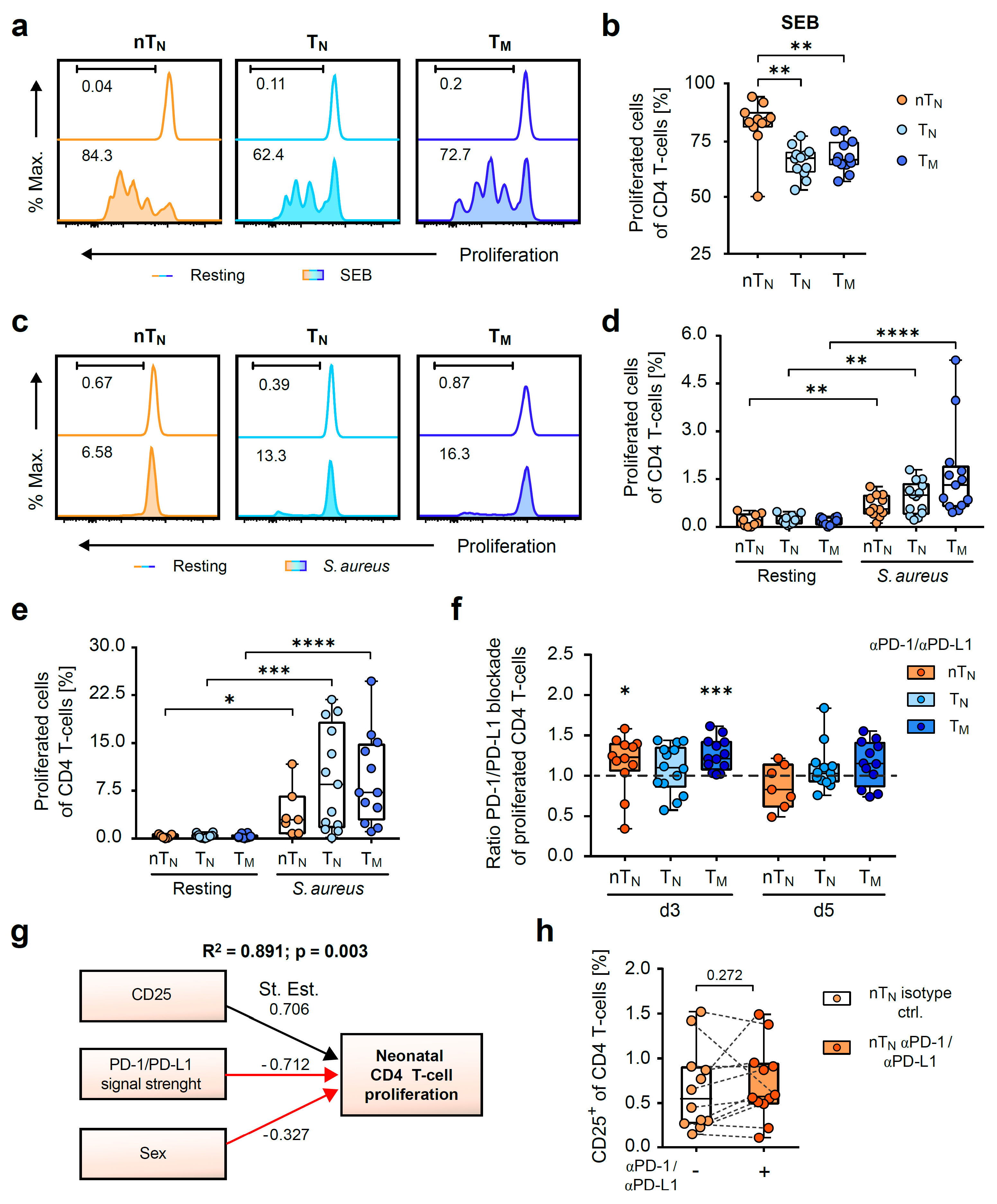
2.2. PD-1 Controls the Capacity of S. aureus-Specific Proliferative Responses of Neonatal CD4 T-Cells
2.3. PD-1/PD-L1 Blockade Increases the Magnitude of S. aureus-Antigen-Specific T-Helper Cells in Neonates
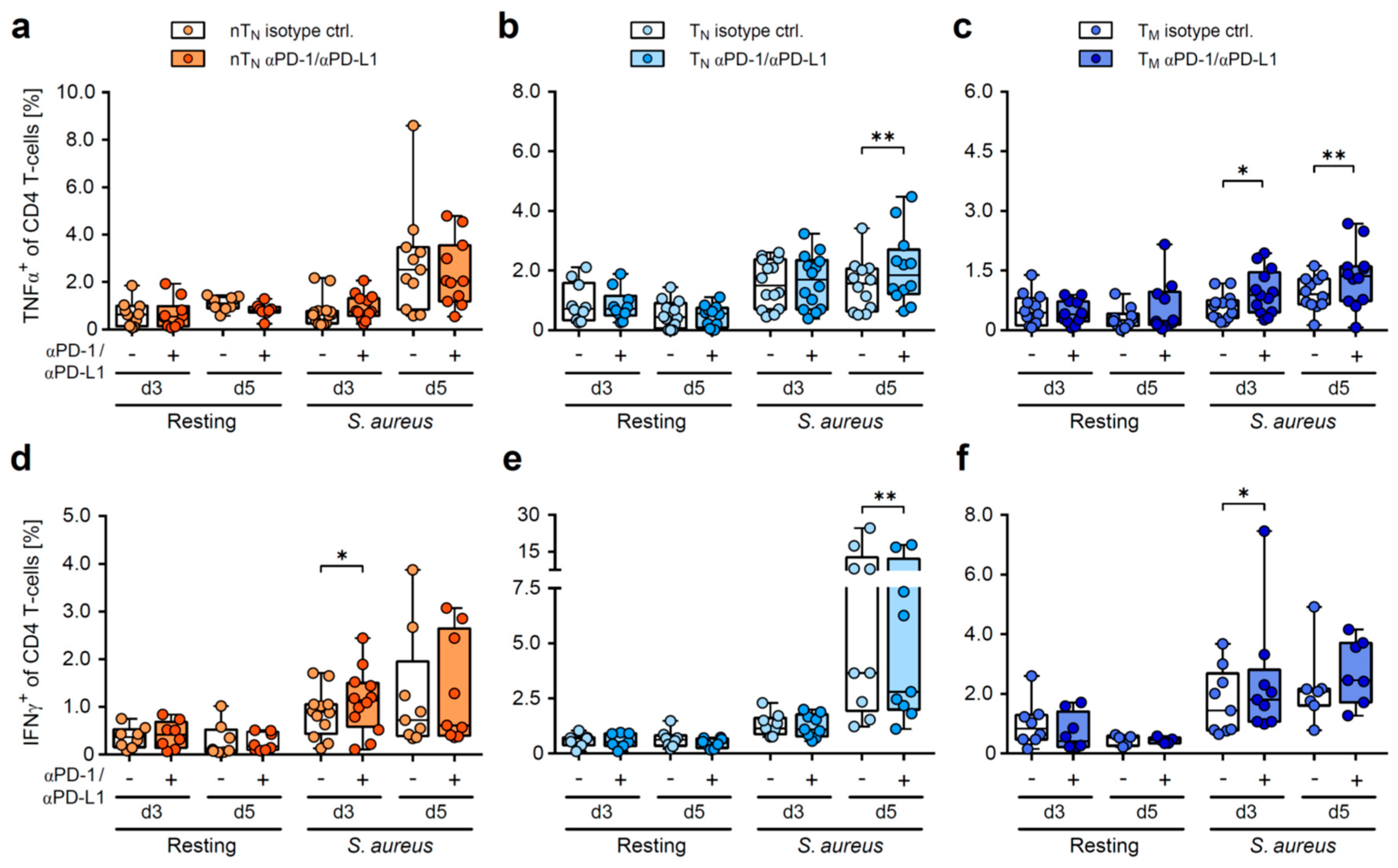
2.4. PD-1/PD-L1 Blockade Increases the Frequency of S. aureus-Induced Inflammatory Cytokine Producers in Neonatal T-Helper Cells
3. Discussion
4. Materials and Methods
4.1. Samples
4.2. Cell Isolation
4.3. Cell Culture and Cell Activation
4.4. Flow Cytometric Analysis
4.5. Statistical Analysis
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- World Health Organization. Newborn Infections. Available online: https://www.who.int/teams/maternal-newborn-child-adolescent-health-and-ageing/newborn-health/newborn-infections (accessed on 14 February 2023).
- Filleron, A.; Beauregard-Birba, S.; Mura, T.; Aujoulat, F.; Michon, A.L.; Rodière, M.; Tran, T.A.; Jeziorski, E.; Marchandin, H. Survey of Staphylococcus aureus in a general pediatric population and focus on isolates with three clinically relevant toxin-encoding genes. World J. Pediatr. 2018, 14, 35–43. [Google Scholar] [CrossRef] [PubMed]
- Ondusko, D.S.; Nolt, D. Staphylococcus aureus. Pediatr. Rev. 2018, 39, 287–298. [Google Scholar] [CrossRef] [PubMed]
- Kaplan, S.L. Staphylococcus aureus Infections in Children: The Implications of Changing Trends. Pediatrics 2016, 137, e20160101. [Google Scholar] [CrossRef] [PubMed]
- Antimicrobial Resistance Collaborators. Global burden of bacterial antimicrobial resistance in 2019: A systematic analysis. Lancet 2022, 399, 629–655. [Google Scholar] [CrossRef]
- Pierau, M.; Arra, A.; Brunner-Weinzierl, M.C. Preventing Atopic Diseases During Childhood—Early Exposure Matters. Front. Immunol. 2021, 12, 617731. [Google Scholar] [CrossRef]
- Fujimura, K.E.; Sitarik, A.R.; Havstad, S.; Lin, D.L.; LeVan, S.; Fadrosh, D.; Panzer, A.R.; LaMere, B.; Rackaityte, E.; Lukacs, N.W.; et al. Neonatal gut microbiota associates with childhood multisensitized atopy and T cell differentiation. Nat. Med. 2016, 22, 1187–1191. [Google Scholar] [CrossRef]
- Risnes, K.R.; Belanger, K.; Murk, W.; Bracken, M.B. Antibiotic Exposure by 6 Months and Asthma and Allergy at 6 Years: Findings in a Cohort of 1,401 US Children. Am. J. Epidemiol. 2010, 173, 310–318. [Google Scholar] [CrossRef]
- Shaw, S.Y.; Blanchard, J.F.; Bernstein, C.N. Association Between the Use of Antibiotics in the First Year of Life and Pediatric Inflammatory Bowel Disease. Am. J. Gastroenterol. 2010, 105, 2687–2692. [Google Scholar] [CrossRef]
- Kronman, M.P.; Zaoutis, T.E.; Haynes, K.; Feng, R.; Coffin, S.E. Antibiotic Exposure and IBD Development Among Children: A Population-Based Cohort Study. Pediatrics 2012, 130, e794–e803. [Google Scholar] [CrossRef]
- Rudd, B.D. Neonatal T Cells: A Reinterpretation. Annu. Rev. Immunol. 2020, 38, 229–247. [Google Scholar] [CrossRef]
- Mold, J.E.; Venkatasubrahmanyam, S.; Burt, T.D.; Michaëlsson, J.; Rivera, J.M.; Galkina, S.A.; Weinberg, K.; Stoddart, C.A.; McCune, J.M. Fetal and Adult Hematopoietic Stem Cells Give Rise to Distinct T Cell Lineages in Humans. Science 2010, 330, 1695–1699. [Google Scholar] [CrossRef] [PubMed]
- Schaub, B.; Liu, J.; Höppler, S.; Schleich, I.; Huehn, J.; Olek, S.; Wieczorek, G.; Illi, S.; von Mutius, E. Maternal farm exposure modulates neonatal immune mechanisms through regulatory T cells. J. Allergy Clin. Immunol. 2009, 123, 774–782.e5. [Google Scholar] [CrossRef] [PubMed]
- Schmiedeberg, K.; Krause, H.; Röhl, F.-W.; Hartig, R.; Jorch, G.; Brunner-Weinzierl, M.C. T Cells of Infants Are Mature, but Hyporeactive Due to Limited Ca2+ Influx. PLoS ONE 2016, 11, e0166633. [Google Scholar] [CrossRef] [PubMed]
- Li, G.; Yu, M.; Lee, W.-W.; Tsang, M.; Krishnan, E.; Weyand, C.M.; Goronzy, J.J. Decline in miR-181a expression with age impairs T cell receptor sensitivity by increasing DUSP6 activity. Nat. Med. 2012, 18, 1518–1524. [Google Scholar] [CrossRef] [PubMed]
- Hebel, K.; Weinert, S.; Kuropka, B.; Knolle, J.; Kosak, B.; Jorch, G.; Arens, C.; Krause, E.; Braun-Dullaeus, R.C.; Brunner-Weinzierl, M.C. CD4+ T Cells from Human Neonates and Infants Are Poised Spontaneously to Run a Nonclassical IL-4 Program. J. Immunol. 2014, 192, 5160–5170. [Google Scholar] [CrossRef]
- Halonen, M.; Lohman, I.C.; Stern, D.A.; Spangenberg, A.; Anderson, D.; Mobley, S.; Ciano, K.; Peck, M.; Wright, A.L. Th1/Th2 Patterns and Balance in Cytokine Production in the Parents and Infants of a Large Birth Cohort. J. Immunol. 2009, 182, 3285–3293. [Google Scholar] [CrossRef]
- Gibbons, D.; Fleming, P.; Virasami, A.; Michel, M.-L.; Sebire, N.; Costeloe, K.; Carr, R.; Klein, N.; Hayday, A. Interleukin-8 (CXCL8) production is a signatory T cell effector function of human newborn infants. Nat. Med. 2014, 20, 1206–1210. [Google Scholar] [CrossRef]
- Hebel, K.; Rudolph, M.; Kosak, B.; Chang, H.-D.; Butzmann, J.; Brunner-Weinzierl, M.C. IL-1β and TGF-β Act Antagonistically in Induction and Differentially in Propagation of Human Proinflammatory Precursor CD4+ T Cells. J. Immunol. 2011, 187, 5627–5635. [Google Scholar] [CrossRef]
- Razzaghian, H.R.; Sharafian, Z.; Sharma, A.A.; Boyce, G.K.; Lee, K.; Da Silva, R.; Orban, P.C.; Sekaly, R.-P.; Ross, C.J.; Lavoie, P.M. Neonatal T Helper 17 Responses Are Skewed Towards an Immunoregulatory Interleukin-22 Phenotype. Front. Immunol. 2021, 12, 655027. [Google Scholar] [CrossRef]
- Vogel, K.; Pierau, M.; Arra, A.; Lampe, K.; Schlueter, D.; Arens, C.; Brunner-Weinzierl, M.C. Developmental induction of human T-cell responses against Candida albicans and Aspergillus fumigatus. Sci. Rep. 2018, 8, 16904. [Google Scholar] [CrossRef]
- Jubel, J.M.; Barbati, Z.R.; Burger, C.; Wirtz, D.C.; Schildberg, F.A. The Role of PD-1 in Acute and Chronic Infection. Front. Immunol. 2020, 11, 487. [Google Scholar] [CrossRef] [PubMed]
- Dietz, S.; Molnar, K.; Riedel, H.; Haag, L.; Spring, B.; Orlikowsky, T.W.; Poets, C.F.; Gille, C.; Köstlin-Gille, N. Expression of immune checkpoint molecules on adult and neonatal T-cells. Immunol. Res. 2022, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Titov, A.; Kaminskiy, Y.; Ganeeva, I.; Zmievskaya, E.; Valiullina, A.; Rakhmatullina, A.; Petukhov, A.; Miftakhova, R.; Rizvanov, A.; Bulatov, E. Knowns and Unknowns about CAR-T Cell Dysfunction. Cancers 2022, 14, 1078. [Google Scholar] [CrossRef]
- Arra, A.; Pech, M.; Fu, H.; Lingel, H.; Braun, F.; Beyer, C.; Spiliopoulou, M.; Bröker, B.M.; Lampe, K.; Arens, C.; et al. Immune-checkpoint blockade of CTLA-4 (CD152) in antigen-specific human T-cell responses differs profoundly between neonates, children, and adults. Oncoimmunology 2021, 10, 1938475. [Google Scholar] [CrossRef]
- Sharma, A.A.; Jen, R.; Butler, A.; Lavoie, P.M. The developing human preterm neonatal immune system: A case for more research in this area. Clin. Immunol. 2012, 145, 61–68. [Google Scholar] [CrossRef] [PubMed]
- Bae, J.S.; Da, F.; Liu, R.; He, L.; Lv, H.; Fisher, E.L.; Rajagopalan, G.; Li, M.; Cheung, G.Y.C.; Otto, M. Contribution of Staphylococcal Enterotoxin B to Staphylococcus aureus Systemic Infection. J. Infect. Dis. 2021, 223, 1766–1775. [Google Scholar] [CrossRef]
- Splawski, J.B.; Nishioka, J.; E Lipsky, P. CD40 ligand is expressed and functional on activated neonatal T cells. J. Immunol. 1996, 156, 119–127. [Google Scholar] [CrossRef] [PubMed]
- Darrah, P.A.; Patel, D.T.; De Luca, P.M.; Lindsay, R.W.B.; Davey, D.F.; Flynn, B.J.; Hoff, S.T.; Andersen, P.; Reed, S.G.; Morris, S.L.; et al. Multifunctional TH1 cells define a correlate of vaccine-mediated protection against Leishmania major. Nat. Med. 2007, 13, 843–850. [Google Scholar] [CrossRef]
- Rabe, H.; Nordström, I.; Andersson, K.; Lundell, A.; Rudin, A. Staphylococcus aureus convert neonatal conventional CD4(+) T cells into FOXP3(+) CD25(+) CD127(low) T cells via the PD-1/PD-L1 axis. Immunology 2014, 141, 467–481. [Google Scholar] [CrossRef] [PubMed]
- Siefker, D.T.; Adkins, B. Rapid CD8+ Function Is Critical for Protection of Neonatal Mice from an Extracellular Bacterial Enteropathogen. Front. Pediatr. 2016, 4, 141. [Google Scholar] [CrossRef]
- Klein, S.L.; Flanagan, K.L. Sex differences in immune responses. Nat. Rev. Immunol. 2016, 16, 626–638. [Google Scholar] [CrossRef] [PubMed]
- Takahashi, T.; Ellingson, M.K.; Wong, P.; Israelow, B.; Lucas, C.; Klein, J.; Silva, J.; Mao, T.; Oh, J.E.; Tokuyama, M.; et al. Sex differences in immune responses that underlie COVID-19 disease outcomes. Nature 2020, 588, 315–320. [Google Scholar] [CrossRef] [PubMed]
- Vázquez-Martínez, E.R.; García-Gómez, E.; Camacho-Arroyo, I.; González-Pedrajo, B. Sexual dimorphism in bacterial infections. Biol. Sex Differ. 2018, 9, 27. [Google Scholar] [CrossRef]
- Drevenstedt, G.L.; Crimmins, E.M.; Vasunilashorn, S.; Finch, C.E. The rise and fall of excess male infant mortality. Proc. Natl. Acad. Sci. USA 2008, 105, 5016–5021. [Google Scholar] [CrossRef]
- Federal Institute for Population Research. Sex Dependent Mortality of Infants in Germany (1990–2020). Available online: https://www.bib.bund.de/Permalink.html?cms_permaid=1217856 (accessed on 13 February 2023).
- Luna, R.M.L.; Körmendy, D.; Brunner-Weinzierl, M.C. Female-biased incidence of experimental autoimmune encephalomyelitis reflects sexually dimorphic expression of surface CTLA-4 (CD152) on T lymphocytes. Gend. Med. 2010, 7, 296–308. [Google Scholar] [CrossRef]
- Körmendy, D.; Hoff, H.; Hoff, P.; Bröker, B.M.; Burmester, G.-R.; Brunner-Weinzierl, M.C. Impact of the CTLA-4/CD28 axis on the processes of joint inflammation in rheumatoid arthritis. Arthritis Rheum. 2013, 65, 81–87. [Google Scholar] [CrossRef] [PubMed]
- Komai-Koma, M.; Jones, L.; Ogg, G.S.; Xu, D.; Liew, F.Y. TLR2 is expressed on activated T cells as a costimulatory receptor. Proc. Natl. Acad. Sci. USA 2004, 101, 3029–3034. [Google Scholar] [CrossRef] [PubMed]
- Sinnott, B.D.; Park, B.; Boer, M.C.; Lewinsohn, D.A.; Lancioni, C.L. Direct TLR-2 Costimulation Unmasks the Proinflammatory Potential of Neonatal CD4+ T Cells. J. Immunol. 2016, 197, 68–77. [Google Scholar] [CrossRef]


Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Majer, C.; Lingel, H.; Arra, A.; Heuft, H.-G.; Bretschneider, D.; Balk, S.; Vogel, K.; Brunner-Weinzierl, M.C. PD-1/PD-L1 Control of Antigen-Specifically Activated CD4 T-Cells of Neonates. Int. J. Mol. Sci. 2023, 24, 5662. https://doi.org/10.3390/ijms24065662
Majer C, Lingel H, Arra A, Heuft H-G, Bretschneider D, Balk S, Vogel K, Brunner-Weinzierl MC. PD-1/PD-L1 Control of Antigen-Specifically Activated CD4 T-Cells of Neonates. International Journal of Molecular Sciences. 2023; 24(6):5662. https://doi.org/10.3390/ijms24065662
Chicago/Turabian StyleMajer, Christiane, Holger Lingel, Aditya Arra, Hans-Gert Heuft, Dirk Bretschneider, Silke Balk, Katrin Vogel, and Monika C. Brunner-Weinzierl. 2023. "PD-1/PD-L1 Control of Antigen-Specifically Activated CD4 T-Cells of Neonates" International Journal of Molecular Sciences 24, no. 6: 5662. https://doi.org/10.3390/ijms24065662
APA StyleMajer, C., Lingel, H., Arra, A., Heuft, H.-G., Bretschneider, D., Balk, S., Vogel, K., & Brunner-Weinzierl, M. C. (2023). PD-1/PD-L1 Control of Antigen-Specifically Activated CD4 T-Cells of Neonates. International Journal of Molecular Sciences, 24(6), 5662. https://doi.org/10.3390/ijms24065662




