Targeting ER Stress with Saikosaponin A to Overcome Resistance under Radiation in Gastric Cancer Cells
Abstract
1. Introduction
2. Results
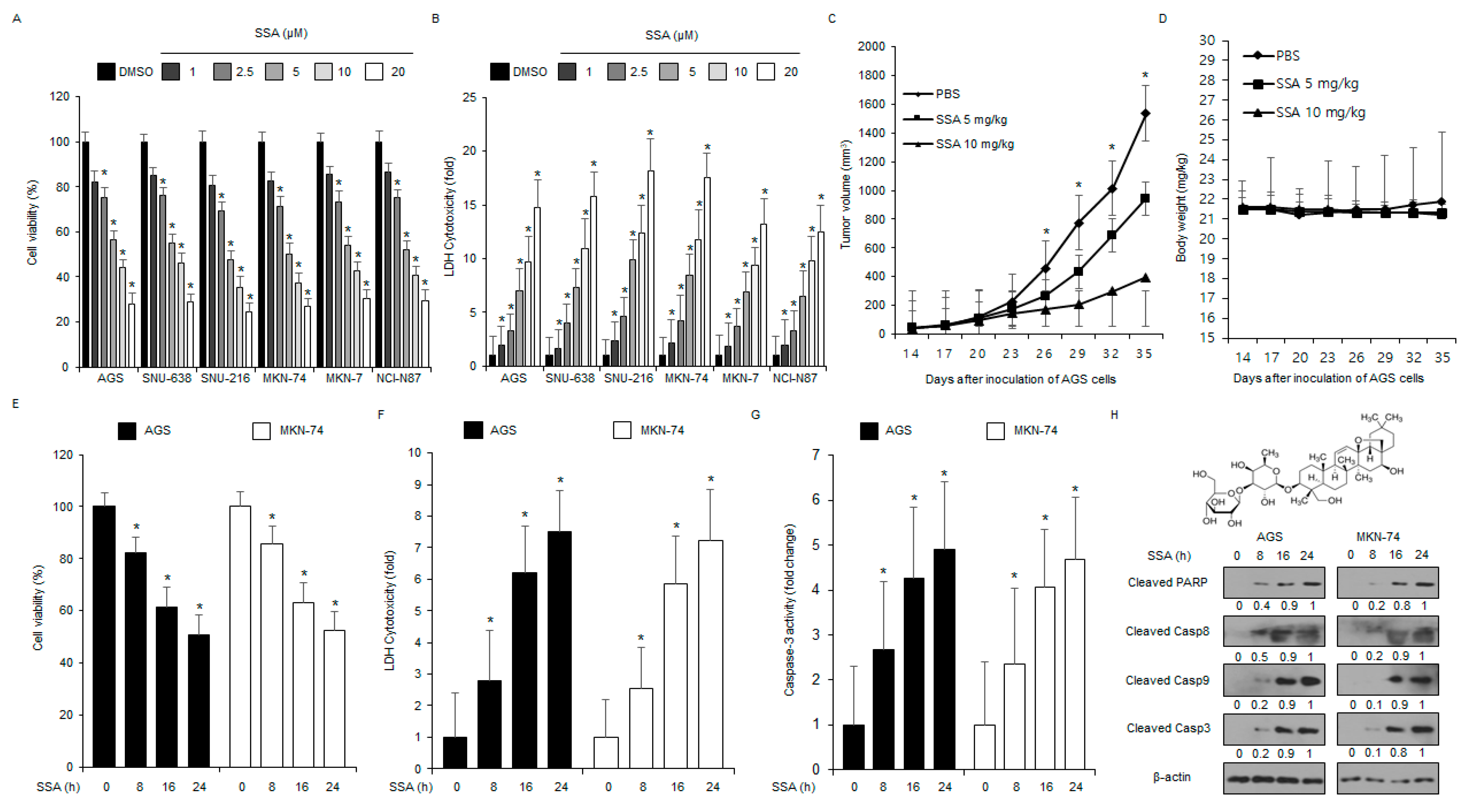
2.1. SSA Treatment Induces Apoptosis in GC Cells
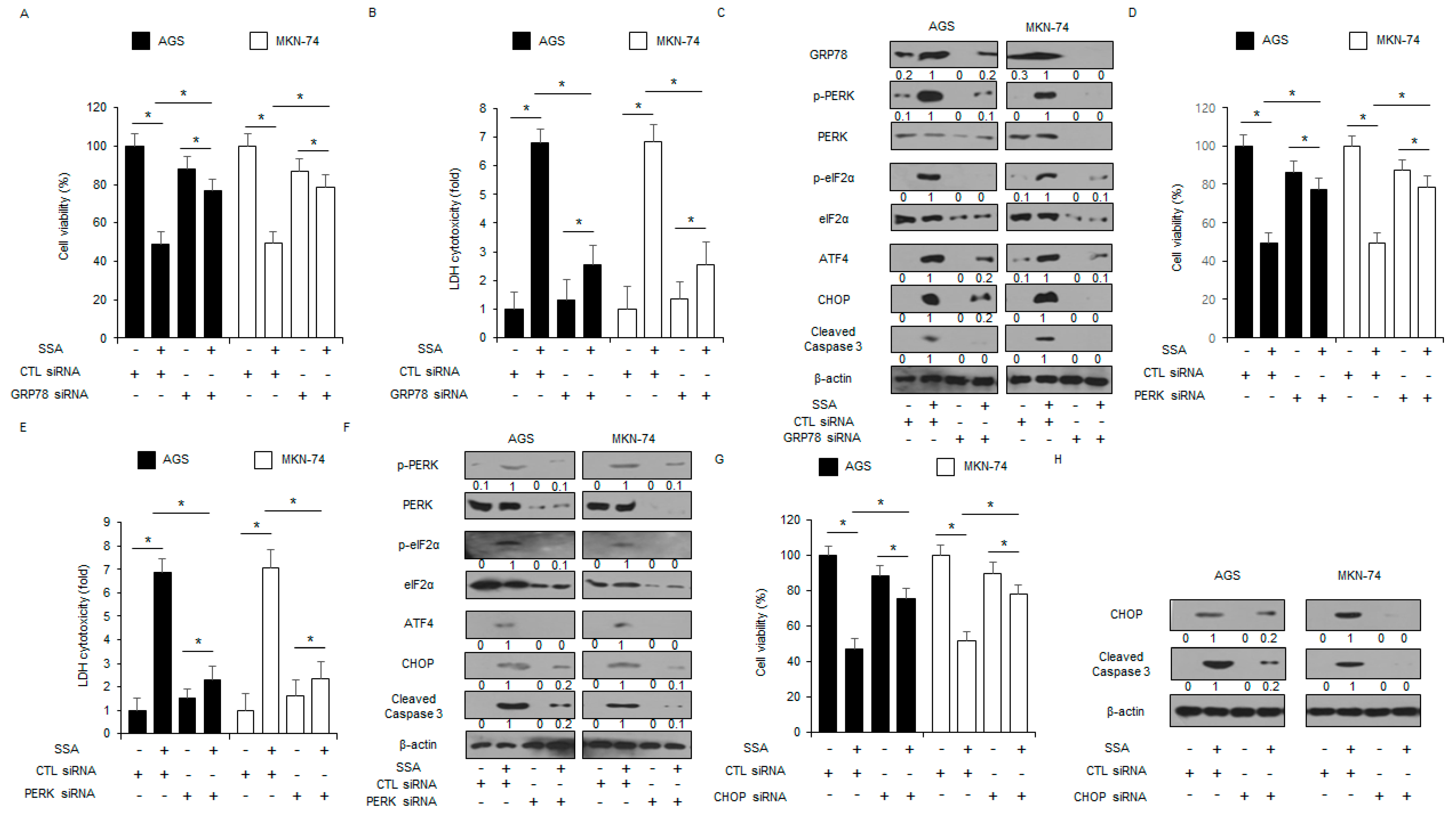
2.2. SSA Mediates Apoptotic Cell Death through the ER Stress Pathway in GC Cells
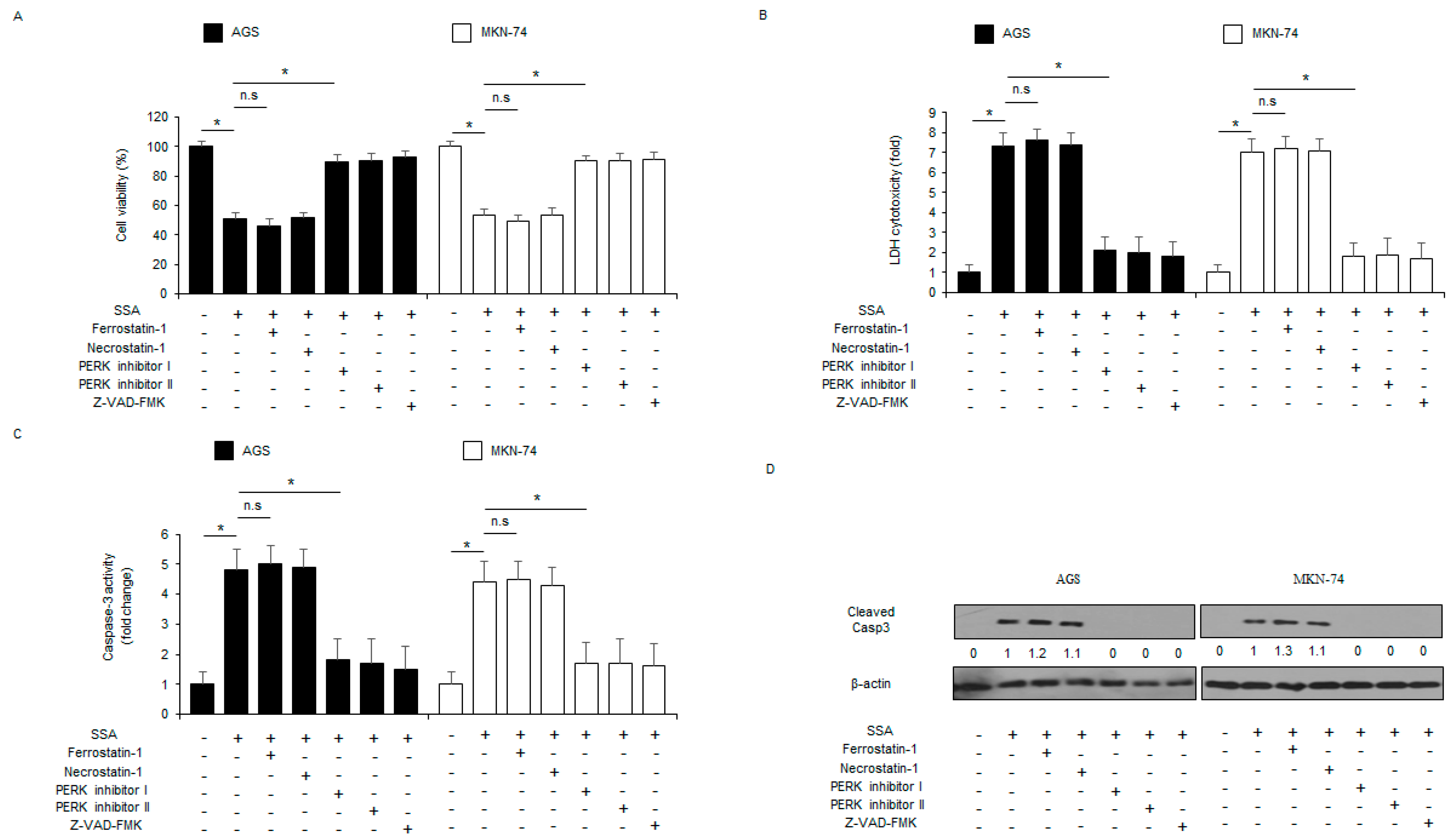
2.3. The Inhibition of ER Stress Signaling Blocks SSA-Induced Apoptotic Cell Death
2.4. SSA Induces ER Stress and Cell Death via ROS and Ca2+ Generation in GC Cells
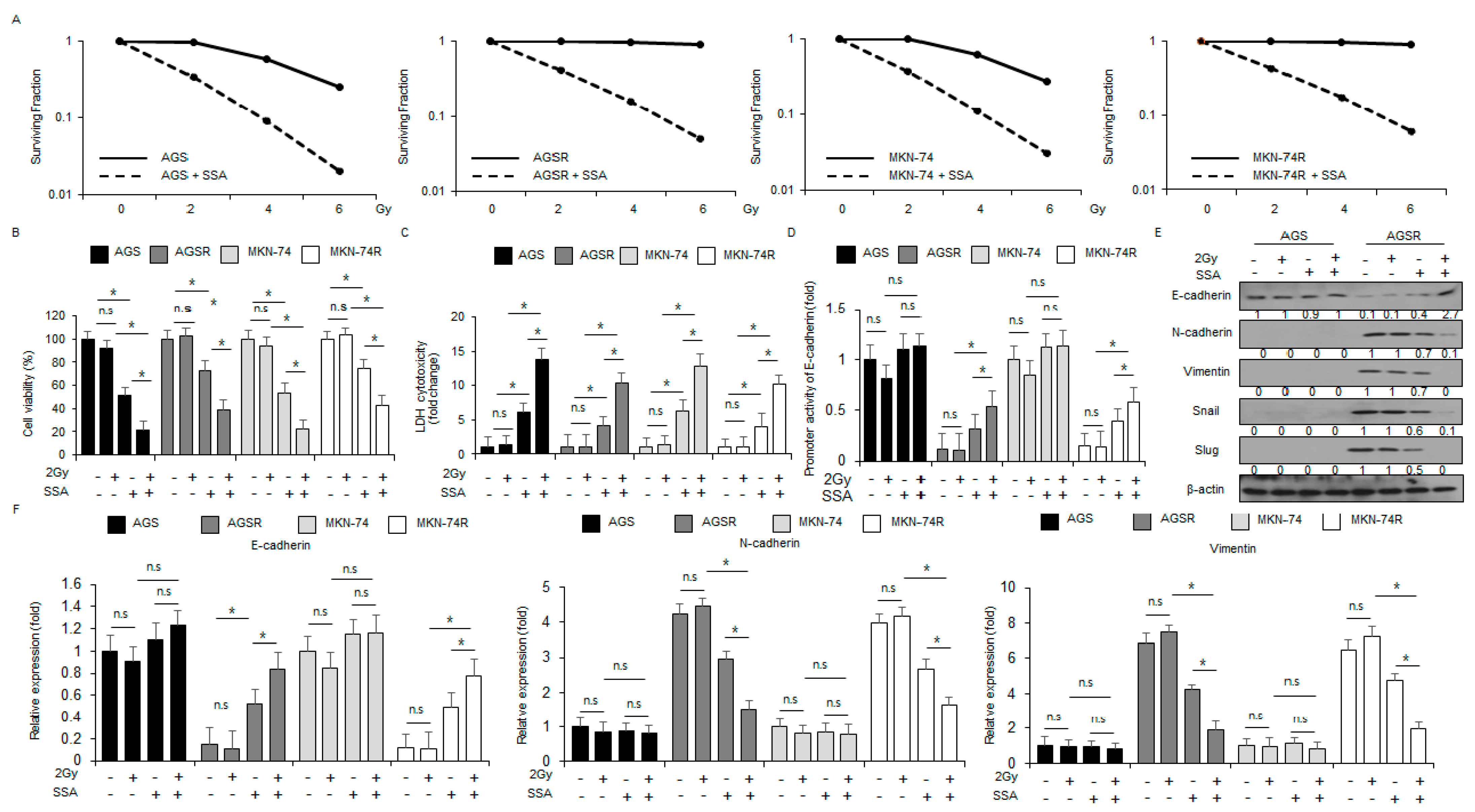
2.5. SSA Treatment Overcomes Radio-Resistance in GC Cells
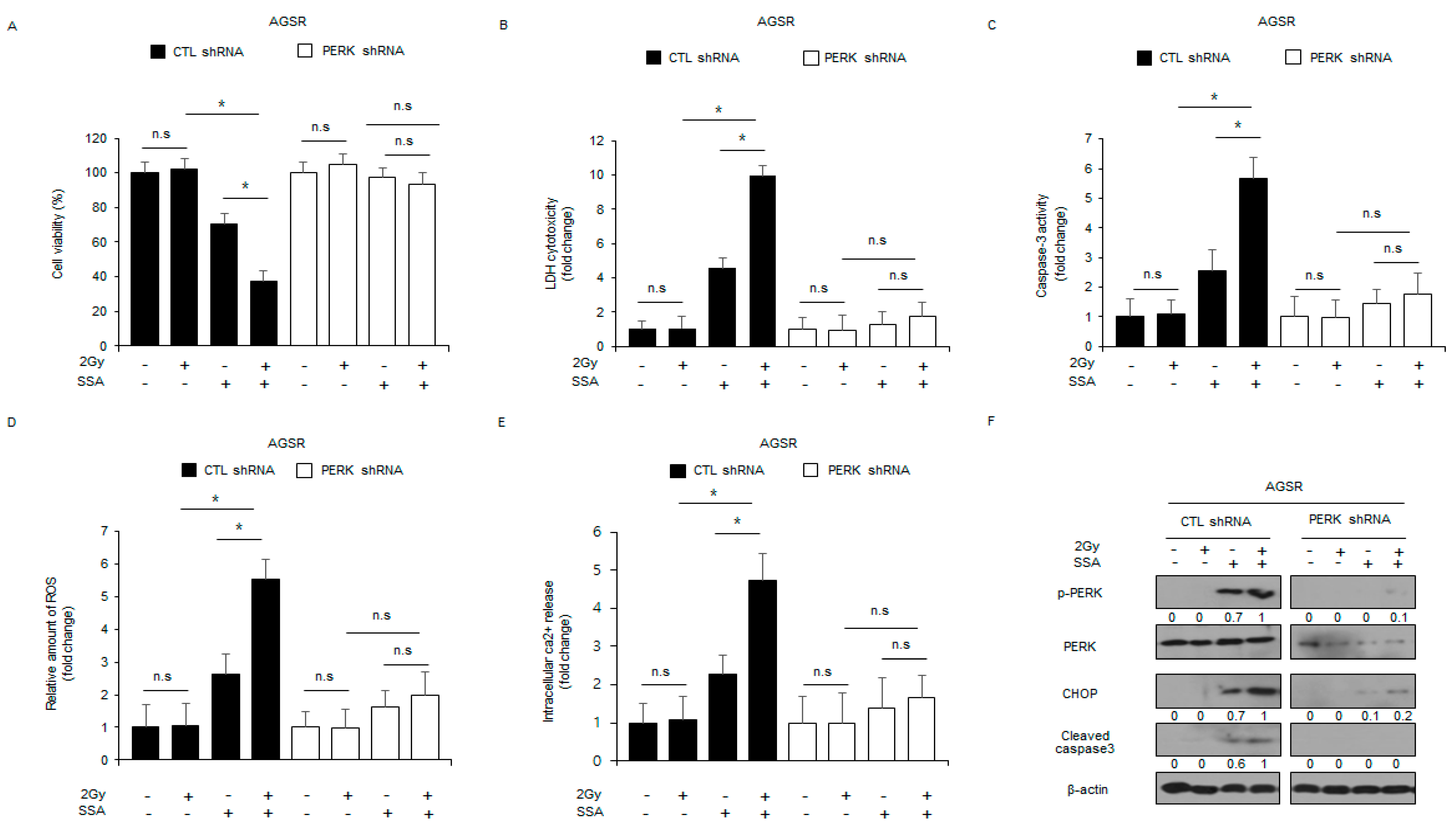
2.6. Targeting PERK Blocks SSA-Induced Cell Death under Radiation Exposure in GC Cells
3. Discussion
4. Materials and Methods
4.1. Reagents
4.2. Cell Culture
4.3. Cell Viability
4.4. LDH Assay
4.5. Caspase Activity Assay
4.6. Ionizing Radiation (IR)
4.7. Development of Acquired Radio-Resistant GC Cell Lines
4.8. Colony Formation Assay
4.9. Transfection
4.10. Isolation of Total RNA
4.11. Real-Time RT-PCR
4.12. Luciferase Reporter Assay
4.13. Isolation of Protein
4.14. Western Blotting Analyses
4.15. Measuring of Reactive Oxygen Species (ROS)
4.16. Exosomes Isolation
4.17. Intracellular Ca2+ Assays
4.18. Animals
4.19. Tumor Xenograft Mouse Models
4.20. Statistical Analysis
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Rawla, P.; Barsouk, A. Epidemiology of gastric cancer: Global trends, risk factors and prevention. Prz. Gastroenterol. 2019, 14, 26–38. [Google Scholar] [CrossRef]
- Brace, C. Thermal tumor ablation in clinical use. IEEE Pulse 2011, 2, 28–38. [Google Scholar] [CrossRef]
- Gutierrez, J.A.; Gish, R.G. Efficacy of combination treatment modalities for intermediate and advanced hepatocellular carcinoma: Intra-arterial therapies, sorafenib and novel small molecules. Transl. Cancer Res. 2013, 2, 460–471. [Google Scholar]
- Li, M.; Zhang, W.Y.; Wang, B.; Gao, Y.; Song, Z.; Zheng, Q.C. Ligand-based targeted therapy: A novel strategy for hepatocellular carcinoma. Int. J. Nanomed. 2016, 11, 5645–5669. [Google Scholar] [CrossRef] [PubMed]
- Hashiguchi, N.; Schenker, N.; Rottner, L.; Reißmann, B.; Rillig, A.; Maurer, T.; Lemes, C.; Kuck, K.H.; Ouyang, F.; Mathew, S. Absence of detectable effect of radiotherapy and chemotherapy for breast cancer on the presence of low voltage areas in patients receiving left atrial catheter ablation. Acta Cardiol. 2021, 76, 1061–1068. [Google Scholar] [CrossRef]
- Li, Y.; Li, X.; Cuiping, C.; Pu, R.; Weihua, Y. Study on the Anticancer Effect of an Astragaloside- and Chlorogenic Acid-Containing Herbal Medicine (RLT-03) in Breast Cancer. Evid. -Based Complement. Altern. Med. 2020, 2020, 1515081. [Google Scholar] [CrossRef]
- Yang, Y.; Chen, Q.; Yu, W.Y.; Zhang, H.H.; Zhong, Y.S.; Zhang, S.Z.; Wang, J.F.; Yu, C.H. Herbal Active Ingredients: An Emerging Potential for the Prevention and Treatment of Papillary Thyroid Carcinoma. BioMed Res. Int. 2020, 2020, 1340153. [Google Scholar] [CrossRef] [PubMed]
- Yin, S.Y.; Wei, W.C.; Jian, F.Y.; Yang, N.S. Therapeutic applications of herbal medicines for cancer patients. Evid. -Based Complement. Altern. Med. 2013, 2013, 302426. [Google Scholar] [CrossRef]
- Kim, B.M. The Role of Saikosaponins in Therapeutic Strategies for Age-Related Diseases. Oxid. Med. Cell. Longev. 2018, 2018, 8275256. [Google Scholar] [CrossRef] [PubMed]
- Kim, B.M.; Hong, S.H. Sequential caspase-2 and caspase-8 activation is essential for saikosaponin a-induced apoptosis of human colon carcinoma cell lines. Apoptosis 2011, 16, 184–197. [Google Scholar] [CrossRef] [PubMed]
- Wong, V.K.; Zhang, M.M.; Zhou, H.; Lam, K.Y.; Chan, P.L.; Law, C.K.; Yue, P.Y.; Liu, L. Saikosaponin-d Enhances the Anticancer Potency of TNF-α via Overcoming Its Undesirable Response of Activating NF-Kappa B Signalling in Cancer Cells. Evid.-Based Complement. Altern. Med. 2013, 2013, 745295. [Google Scholar] [CrossRef]
- Chen, J.C.; Chang, N.W.; Chung, J.G.; Chen, K.C. Saikosaponin-A induces apoptotic mechanism in human breast MDA-MB-231 and MCF-7 cancer cells. Am. J. Chin. Med. 2003, 31, 363–377. [Google Scholar] [CrossRef] [PubMed]
- Wang, Q.; Zheng, X.L.; Yang, L.; Shi, F.; Gao, L.B.; Zhong, Y.J.; Sun, H.; He, F.; Lin, Y.; Wang, X. Reactive oxygen species-mediated apoptosis contributes to chemosensitization effect of saikosaponins on cisplatin-induced cytotoxicity in cancer cells. J. Exp. Clin. Cancer Res. 2010, 29, 159. [Google Scholar] [CrossRef] [PubMed]
- Tang, J.C.; Long, F.; Zhao, J.; Hang, J.; Ren, Y.G.; Chen, J.Y.; Mu, B. The Effects and Mechanisms by which Saikosaponin-D Enhances the Sensitivity of Human Non-small Cell Lung Cancer Cells to Gefitinib. J. Cancer. 2019, 10, 6666–6672. [Google Scholar] [CrossRef] [PubMed]
- Tundis, R.; Bonesi, M.; Deguin, B.; Loizzo, M.R.; Menichini, F.; Conforti, F.; Tillequin, F.; Menichini, F. Cytotoxic activity and inhibitory effect on nitric oxide production of triterpene saponins from the roots of Physospermum verticillatum (Waldst & Kit) (Apiaceae). Bioorg. Med. Chem. 2009, 17, 4542–4547. [Google Scholar]
- Tsuyoshi, H.; Wong, V.K.W.; Han, Y.; Orisaka, M.; Yoshida, Y.; Tsang, B.K. Saikosaponin-d, a calcium mobilizing agent, sensitizes chemoresistant ovarian cancer cells to cisplatin-induced apoptosis by facilitating mitochondrial fission and G2/M arrest. Oncotarget 2017, 8, 99825–99840. [Google Scholar] [CrossRef]
- Lemmer, I.L.; Willemsen, N.; Hilal, N.; Bartelt, A. A guide to understanding endoplasmic reticulum stress in metabolic disorders. Mol. Metab. 2021, 47, 101169. [Google Scholar] [CrossRef] [PubMed]
- Gardner, B.M.; Walter, P. Unfolded proteins are Ire1-activating ligands that directly induce the unfolded protein response. Science 2011, 333, 1891–1894. [Google Scholar] [CrossRef] [PubMed]
- Oyadomari, S.; Mori, M. Roles of CHOP/GADD153 in endoplasmic reticulum stress. Cell Death Differ. 2004, 11, 381–389. [Google Scholar] [CrossRef]
- Dadey, D.Y.A.; Kapoor, V.; Khudanyan, A.; Thotala, D.; Hallahan, D.E. PERK Regulates Glioblastoma Sensitivity to ER Stress Although Promoting Radiation Resistance. Mol. Cancer Res. 2018, 16, 1447–1453. [Google Scholar] [CrossRef]
- Hsieh, C.L.; Huang, H.S.; Chen, K.C.; Saka, T.; Chiang, C.Y.; Chung, L.W.K.; Sung, S.Y. A Novel Salicylanilide Derivative Induces Autophagy Cell Death in Castration-Resistant Prostate Cancer via ER Stress-Activated PERK Signaling Pathway. Mol. Cancer Ther. 2020, 19, 101–111. [Google Scholar] [CrossRef] [PubMed]
- He, C.; Hua, W.; Liu, J.; Fan, L.; Wang, H.; Sun, G. Exosomes derived from endoplasmic reticulum-stressed liver cancer cells enhance the expression of cytokines in macrophages via the STAT3 signaling pathway. Oncol. Lett. 2020, 20, 589–600. [Google Scholar] [CrossRef] [PubMed]
- Bahar, E.; Kim, J.Y.; Yoon, H.N. Chemotherapy resistance explained through endoplasmic reticulum stress-dependent signaling. Cancers 2019, 11, 338. [Google Scholar] [CrossRef] [PubMed]
- Banerjee, A.; Banerjee, V.; Czinn, S.; Blanchard, T. Increased reactive oxygen species levels cause ER stress and cytotoxicity in andrographolide treated colon cancer cells. Oncotarget 2017, 8, 26142–26153. [Google Scholar] [CrossRef] [PubMed]
- Weyemi, U.; Lagente-Chevallier, O.; Boufraqech, M.; Prenois, F.; Courtin, F.; Caillou, B.; Talbot, M.; Dardalon, M.; Ghuzlan, A.A.; Bidart, J.M.; et al. ROS-generating NADPH oxidase NOX4 is a critical mediator in oncogenic H-Ras-induced DNA damage and subsequent senescence. Oncogene 2012, 31, 1117–1129. [Google Scholar] [CrossRef] [PubMed]
- Spencer, B.G.; Finnie, J.W. The role of endoplasmic reticulum stress in cell survival and death. J. Comp. Pathol. 2020, 181, 86–91. [Google Scholar] [CrossRef]
- Sano, R.; Reed, J.C. ER stress-induced cell death mechanisms. Biochim. Biophys. Acta 2013, 1833, 3460–3470. [Google Scholar] [CrossRef]
- Paschen, W.; Frandsen, A. Endoplasmic reticulum dysfunction—A common denominator for cell injury in acute and degenerative diseases of the brain? J. Neurochem. 2001, 79, 719–725. [Google Scholar] [CrossRef]
- Rao, R.V.; Ellerby, H.M.; Bredesen, D.E. Coupling endoplasmic reticulum stress to the cell death program. Cell Death Differ. 2004, 11, 372–380. [Google Scholar] [CrossRef]
- Szegezdi, E.; Loque, S.E.; Gorman, A.H.; Samali, A. Mediators of endoplasmicreticulum stress-induced apoptosis. EMBO Rep. 2006, 7, 880–885. [Google Scholar] [CrossRef]
- Faitova, J.; Krekac, D.; Hrstka, R.; Vojtesek, B. Endoplasmic reticulum stress and apoptosis. Cell. Mol. Biol. Lett. 2006, 11, 489–505. [Google Scholar] [CrossRef]
- Bahar, E.; Kim, H.S.; Yoon, H.O. ER stress-mediated signaling: Action potential and Ca2+ as key players. Int. J. Mol. Sci. 2016, 17, 1558. [Google Scholar] [CrossRef]
- Braakman, I.; Bulleid, N.J. Protein folding and modification in the mammalian endoplasmic reticulum. Annu. Rev. Biochem. 2011, 80, 71–99. [Google Scholar] [CrossRef] [PubMed]
- Shibasaki, F.; McKeon, F. Calcineurin functions in Ca2+-activated cell death in mammalian cells. J. Cell Biol. 1995, 131, 735–743. [Google Scholar] [CrossRef] [PubMed]
- Bergeron, J.J.; Brenner, M.B.; Thomas, D.Y.; Williams, D.B. Calnexin: A membrane-bound chaperone of the endoplasmic reticulum. Trends Biochem. Sci. 1994, 19, 124–128. [Google Scholar] [CrossRef] [PubMed]
- Barrero, A.F.; Haïdour, A.; Sedqui, A.; Mansour, A.I.; Rodríguez-García, I.; López, A.; Muñoz-Dorado, M. Saikosaponins from roots of Bupleurum gibraltaricum and Bupleurum spinosum. Phytochemistry 2000, 54, 741–745. [Google Scholar] [CrossRef]
- Hsu, Y.L.; Kuo, P.L.; Lin, C.C. The proliferative inhibition and apoptotic mechanism of Saikosaponin D in human non-small cell lung cancer A549 cells. Life Sci. 2004, 75, 1231–1242. [Google Scholar] [CrossRef]
- Hsu, Y.L.; Kuo, P.L.; Chiang, L.C.; Lin, C.C. Involvement of p53, nuclear factor kappaB and Fas/Fas ligand in induction of apoptosis and cell cycle arrest by saikosaponin d in human hepatoma cell lines. Cancer Lett. 2004, 213, 213–221. [Google Scholar] [CrossRef]
- Wu, S.; Chen, W.; Liu, K.; Ren, F.; Zheng, D.; Xu, F.; Wu, H. Saikosaponin D inhibits proliferation and induces apoptosis of non-small cell lung cancer cells by inhibiting the STAT3 pathway. J. Int. Med. Res. 2020, 48, 300060520937163. [Google Scholar] [CrossRef] [PubMed]
- Liu, R.Y.; Li, J.P. Saikosaponin-d inhibits proliferation of human undifferentiated thyroid carcinoma cells through induction of apoptosis and cell cycle arrest. Eur. Rev. Med. Pharmacol. Sci. 2014, 18, 2435–2443. [Google Scholar]
- Yao, M.; Yang, J.; Cao, L.; Zhang, L.; Qu, S.; Gao, H. Saikosaponin-d inhibits proliferation of DU145 human prostate cancer cells by inducing apoptosis and arresting the cell cycle at G0/G1 phase. Mol. Med. Rep. 2014, 10, 365–372. [Google Scholar] [CrossRef]
- Cai, C.; Zhang, H.; Ou, Y.; Jiang, Y.; Zhong, D.; Qi, H.; Dang, Q. Saikosaponin-d suppresses cell growth in renal cell carcinoma through EGFR/p38 signaling pathway. Neoplasma 2017, 64, 518–525. [Google Scholar] [CrossRef]
- Wang, B.F.; Dai, Z.J.; Wang, X.J.; Bai, M.H.; Lin, S.; Ma, H.B.; Wang, Y.L.; Song, L.Q.; Ma, X.L.; Zan, Y.; et al. Saikosaponin-d increases the radiosensitivity of smmc-7721 hepatocellular carcinoma cells by adjusting the g0/g1 and g2/m checkpoints of the cell cycle. BMC Complement. Altern. Med. 2013, 13, 263. [Google Scholar] [CrossRef] [PubMed]
- Wong, V.K.; Li, T.; Law, B.Y.; Ma, E.D.; Yip, N.C.; Michelangeli, F.; Law, C.K.; Zhang, M.M.; Lam, K.Y.; Chan, P.L.; et al. Saikosaponin-d, a novel SERCA inhibitor, induces autophagic cell death in apoptosis-defective cells. Cell Death Dis. 2013, 4, e720. [Google Scholar] [CrossRef] [PubMed]
- Chen, C.H.; Chen, M.F.; Huang, S.J.; Huang, C.Y.; Wang, H.K.; Hsieh, W.C.; Huang, C.H.; Liu, L.F.; Shiu, L.Y. Saikosaponin a Induces Apoptosis through Mitochondria-Dependent Pathway in Hepatic Stellate Cells. Am. J. Chin. Med. 2017, 45, 351–368. [Google Scholar] [CrossRef] [PubMed]
- Ye, R.P.; Chen, Z.D. Saikosaponin A, an active glycoside from Radix bupleuri, reverses P-glycoprotein-mediated multidrug resistance in MCF-7/ADR cells and HepG2/ADM cells. Xenobiotica 2017, 47, 176–184. [Google Scholar] [CrossRef]
- Denmeade, S.R.; Isaacs, J.T. The SERCA pump as a therapeutic target: Making a “smart bomb” for prostate cancer. Cancer Biol. Ther. 2005, 4, 14–22. [Google Scholar] [CrossRef]


Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kim, T.W. Targeting ER Stress with Saikosaponin A to Overcome Resistance under Radiation in Gastric Cancer Cells. Int. J. Mol. Sci. 2023, 24, 5661. https://doi.org/10.3390/ijms24065661
Kim TW. Targeting ER Stress with Saikosaponin A to Overcome Resistance under Radiation in Gastric Cancer Cells. International Journal of Molecular Sciences. 2023; 24(6):5661. https://doi.org/10.3390/ijms24065661
Chicago/Turabian StyleKim, Tae Woo. 2023. "Targeting ER Stress with Saikosaponin A to Overcome Resistance under Radiation in Gastric Cancer Cells" International Journal of Molecular Sciences 24, no. 6: 5661. https://doi.org/10.3390/ijms24065661
APA StyleKim, T. W. (2023). Targeting ER Stress with Saikosaponin A to Overcome Resistance under Radiation in Gastric Cancer Cells. International Journal of Molecular Sciences, 24(6), 5661. https://doi.org/10.3390/ijms24065661





