Hydroxyapatite or Fluorapatite—Which Bioceramic Is Better as a Base for the Production of Bone Scaffold?—A Comprehensive Comparative Study
Abstract
1. Introduction
2. Results and Discussion
2.1. Characterization of Fabricated Biomaterials
2.2. Apatite-Forming Ability
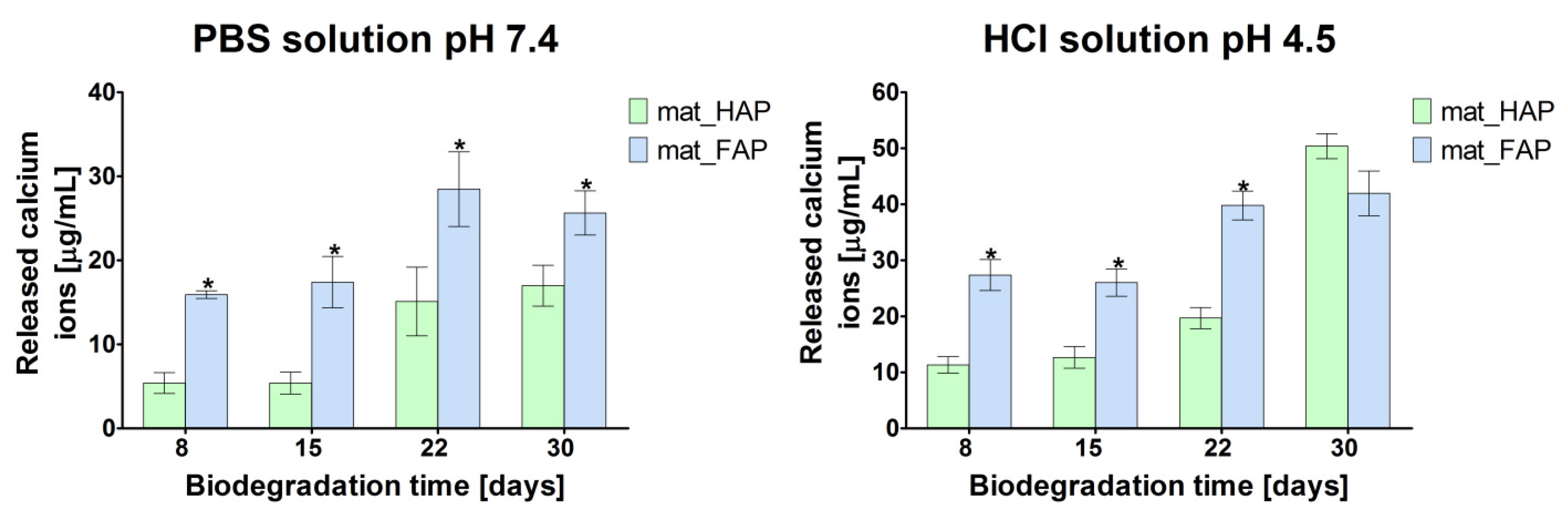
2.3. Biodegradation Assessment
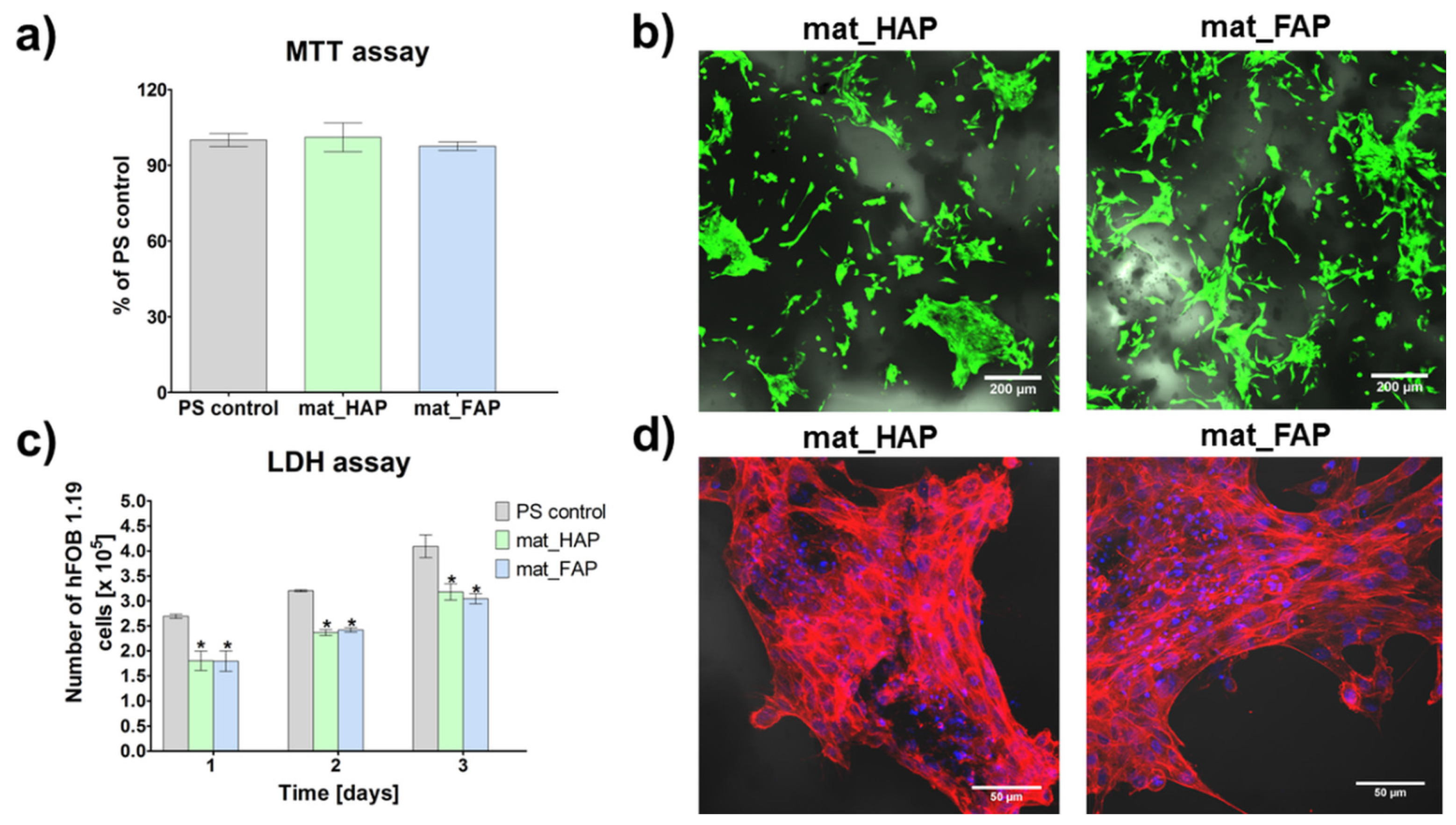
2.4. Evaluation of Biological Response to Fabricated Biomaterials
3. Materials and Methods
3.1. Fabrication of the Apatite-Based Biomaterials
3.2. Microstructure Characterization
3.3. Compression Test
3.4. Bioactivity Assessment
3.5. Biodegradation Assessment
3.6. Cell Culture Experiments
3.6.1. Cytotoxicity Assessment
3.6.2. Evaluation of Cell Proliferation
3.6.3. Osteogenic Differentiation Assessment
3.6.4. Evaluation of ROS/RNS Generation by Immune Cells
3.7. Statistical Analysis
4. Conclusions
5. Patents
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Rose, F.R.A.J.; Oreffo, R.O.C. Bone Tissue Engineering: Hope vs Hype. Biochem. Biophys. Res. Commun. 2002, 292, 1–7. [Google Scholar] [CrossRef] [PubMed]
- Orciani, M.; Fini, M.; Di Primio, R.; Mattioli-Belmonte, M. Biofabrication and Bone Tissue Regeneration: Cell Source, Approaches, and Challenges. Front. Bioeng. Biotechnol. 2017, 5, 17. [Google Scholar] [CrossRef] [PubMed]
- Yeatts, A.B.; Fisher, J.P. Bone Tissue Engineering Bioreactors: Dynamic Culture and the Influence of Shear Stress. Bone 2011, 48, 171–181. [Google Scholar] [CrossRef]
- Rauh, J.; Milan, F.; Günther, K.P.; Stiehler, M. Bioreactor Systems for Bone Tissue Engineering. Tissue Eng. Part B Rev. 2011, 17, 263–280. [Google Scholar] [CrossRef] [PubMed]
- Turnbull, G.; Clarke, J.; Picard, F.; Riches, P.; Jia, L.; Han, F.; Li, B.; Shu, W. 3D Bioactive Composite Scaffolds for Bone Tissue Engineering. Bioact. Mater. 2018, 3, 278–314. [Google Scholar] [CrossRef] [PubMed]
- Velasco, M.A.; Narváez-Tovar, C.A.; Garzón-Alvarado, D.A. Design, Materials, and Mechanobiology of Biodegradable Scaffolds for Bone Tissue Engineering. Biomed. Res. Int. 2015, 2015, 729076. [Google Scholar] [CrossRef]
- Mohd Pu’ad, N.A.S.; Abdul Haq, R.H.; Mohd Noh, H.; Abdullah, H.Z.; Idris, M.I.; Lee, T.C. Synthesis Method of Hydroxyapatite: A Review. Mater. Today Proc. 2020, 29, 233–239. [Google Scholar] [CrossRef]
- Choudhury, P.; Agrawal, D.C. Hydroxyapatite (HA) Coatings for Biomaterials. In Nanomedicine; Webstar, T., Ed.; Woodhead Publishing Ltd.: Cambridge, UK, 2012; pp. 84–127. [Google Scholar]
- Nayak, A.K. Hydroxyapatite Synthesis Methodologies: An Overview. Int. J. Chemtech. Res. 2010, 2, 903–907. [Google Scholar]
- Ginebra, M.P.; Espanol, M.; Maazouz, Y.; Bergez, V.; Pastorino, D. Bioceramics and Bone Healing. EFORT Open Rev. 2018, 3, 173–183. [Google Scholar] [CrossRef]
- Eliaz, N.; Metoki, N. Calcium Phosphate Bioceramics: A Review of Their History, Structure, Properties, Coating Technologies and Biomedical Applications. Materials 2017, 10, 334. [Google Scholar] [CrossRef]
- Przekora, A.; Klimek, K.; Wojcik, M.; Palka, K.; Ginalska, G. New Method for HA/Glucan Bone Scaffold Preparation Reduces Cytotoxic Effect of Highly Reactive Bioceramics. Mater. Lett. 2017, 190, 213–216. [Google Scholar] [CrossRef]
- Klimek, K.; Belcarz, A.; Pazik, R.; Sobierajska, P.; Han, T.; Wiglusz, R.J.; Ginalska, G. “False” Cytotoxicity of Ions-Adsorbing Hydroxyapatite—Corrected Method of Cytotoxicity Evaluation for Ceramics of High Specific Surface Area. Mater. Sci. Eng. C 2016, 65, 70–79. [Google Scholar] [CrossRef] [PubMed]
- Malafaya, P.B.; Reis, R.L. Bilayered Chitosan-Based Scaffolds for Osteochondral Tissue Engineering: Influence of Hydroxyapatite on in Vitro Cytotoxicity and Dynamic Bioactivity Studies in a Specific Double-Chamber Bioreactor. Acta Biomater. 2009, 5, 644–660. [Google Scholar] [CrossRef] [PubMed]
- Evis, Z.; Webster, T.J. Nanosize Hydroxyapatite: Doping with Various Ions. Adv. Appl. Ceram. 2011, 110, 311–321. [Google Scholar] [CrossRef]
- Mumith, A.; Cheong, V.S.; Fromme, P.; Coathup, M.J.; Blunn, G.W. The Effect of Strontium and Silicon Substituted Hydroxyapatite Electrochemical Coatings on Bone Ingrowth and Osseointegration of Selective Laser Sintered Porous Metal Implants. PLoS ONE 2020, 15, e0227232. [Google Scholar] [CrossRef]
- Meng, G.; Wu, X.; Yao, R.; He, J.; Yao, W.; Wu, F. Effect of Zinc Substitution in Hydroxyapatite Coating on Osteoblast and Osteoclast Differentiation under Osteoblast/Osteoclast Co-Culture. Regen. Biomater. 2019, 6, 349–359. [Google Scholar] [CrossRef]
- Chen, S.; Shi, Y.; Zhang, X.; Ma, J. Biomimetic Synthesis of Mg-Substituted Hydroxyapatite Nanocomposites and Three-Dimensional Printing of Composite Scaffolds for Bone Regeneration. J. Biomed. Mater. Res. A 2019, 107, 2512–2521. [Google Scholar] [CrossRef]
- Borkowski, L.; Przekora, A.; Belcarz, A.; Palka, K.; Jojczuk, M.; Lukasiewicz, P.; Nogalski, A.; Ginalska, G. Highly Porous Fluorapatite/Β-1,3-glucan Composite for Bone Tissue Regeneration: Characterization and in Vitro Assessment of Biomedical Potential. Int. J. Mol. Sci. 2021, 22, 10414. [Google Scholar] [CrossRef]
- Gentleman, E.; Stevens, M.M.; Hill, R.G.; Brauer, D.S. Surface Properties and Ion Release from Fluoride-Containing Bioactive Glasses Promote Osteoblast Differentiation and Mineralization in Vitro. Acta Biomater. 2013, 9, 5771–5779. [Google Scholar] [CrossRef]
- Li, Z.; Huang, B.; Mai, S.; Wu, X.; Zhang, H.; Qiao, W.; Luo, X.; Chen, Z. Effects of Fluoridation of Porcine Hydroxyapatite on Osteoblastic Activity of Human MG63 Cells. Sci. Technol. Adv. Mater. 2015, 16, 035006. [Google Scholar] [CrossRef]
- Yao, F.; LeGeros, R.Z. Carbonate and Fluoride Incorporation in Synthetic Apatites: Comparative Effect on Physico-Chemical Properties and in Vitro Bioactivity in Fetal Bovine Serum. Mater. Sci. Eng. C 2010, 30, 423–430. [Google Scholar] [CrossRef]
- Tredwin, C.J.; Young, A.M.; Abou Neel, E.A.; Georgiou, G.; Knowles, J.C. Hydroxyapatite, Fluor-Hydroxyapatite and Fluorapatite Produced via the Sol-Gel Method: Dissolution Behaviour and Biological Properties after Crystallisation. J. Mater. Sci. Mater. Med. 2014, 25, 47–53. [Google Scholar] [CrossRef]
- Kazimierczak, P.; Benko, A.; Palka, K.; Canal, C.; Kolodynska, D.; Przekora, A. Novel Synthesis Method Combining a Foaming Agent with Freeze-Drying to Obtain Hybrid Highly Macroporous Bone Scaffolds. J. Mater. Sci. Technol. 2020, 43, 52–63. [Google Scholar] [CrossRef]
- Sachot, N.; Engel, E.; Castano, O. Hybrid Organic-Inorganic Scaffolding Biomaterials for Regenerative Therapies. Curr. Org. Chem. 2014, 18, 2299–2314. [Google Scholar] [CrossRef]
- Wojnarowska-Nowak, R.; Rzeszutko, J.; Barylyak, A.; Nechyporenko, G.; Zinchenko, V.; Leszczyńska, D.; Bobitski, Y.; Kus-Liśkiewicz, M. Structural, Physical and Antibacterial Properties of Pristine and Ag+ Doped Fluoroapatite Nanomaterials. Adv. Appl. Ceram. 2017, 116, 108–117. [Google Scholar] [CrossRef]
- Liang, W.; Niu, Y.; Ge, S.; Song, S.; Su, J.; Luo, Z. Effects of Hydrothermal Treatment on the Properties of Nanoapatite Crystals. Int. J. Nanomed. 2012, 7, 5151–5158. [Google Scholar] [CrossRef]
- Okada, M.; Matsumoto, T. Synthesis and Modification of Apatite Nanoparticles for Use in Dental and Medical Applications. Jpn. Dent. Sci. Rev. 2015, 51, 85–95. [Google Scholar] [CrossRef]
- Wagoner Johnson, A.J.; Herschler, B.A. A Review of the Mechanical Behavior of CaP and CaP/Polymer Composites for Applications in Bone Replacement and Repair. Acta Biomater. 2011, 7, 16–30. [Google Scholar] [CrossRef]
- Bohner, M.; Lemaitre, J. Can Bioactivity Be Tested in Vitro with SBF Solution? Biomaterials 2009, 30, 2175–2179. [Google Scholar] [CrossRef]
- Kim, H.M.; Himeno, T.; Kawashita, M.; Kokubo, T.; Nakamura, T. The Mechanism of Biomineralization of Bone-like Apatite on Synthetic Hydroxyapatite: An in Vitro Assessment. J. R. Soc. Interface 2004, 1, 17–22. [Google Scholar] [CrossRef]
- Zhou, R.; Xu, W.; Chen, F.; Qi, C.; Lu, B.Q.; Zhang, H.; Wu, J.; Qian, Q.R.; Zhu, Y.J. Amorphous Calcium Phosphate Nanospheres/Polylactide Composite Coated Tantalum Scaffold: Facile Preparation, Fast Biomineralization and Subchondral Bone Defect Repair Application. Colloids Surf. B Biointerfaces 2014, 123, 236–245. [Google Scholar] [CrossRef]
- Xie, Y.; Liu, X.; Chu, P.K.; Ding, C. Nucleation and Growth of Calcium-Phosphate on Ca-Implanted Titanium Surface. Surf. Sci. 2006, 600, 651–656. [Google Scholar] [CrossRef]
- Borkowski, L.; Przekora, A.; Belcarz, A.; Palka, K.; Jozefaciuk, G.; Lübek, T.; Jojczuk, M.; Nogalski, A.; Ginalska, G. Fluorapatite Ceramics for Bone Tissue Regeneration: Synthesis, Characterization and Assessment of Biomedical Potential. Mater. Sci. Eng. C 2020, 116, 111211. [Google Scholar] [CrossRef] [PubMed]
- Florencio-Silva, R.; Sasso, G.R.; Sasso-Cerri, E.; Simões, M.J.; Cerri, P.S. Biology of Bone Tissue: Structure, Function, and Factors That Influence Bone Cells. Biomed. Res. Int. 2015, 2015, 421746. [Google Scholar] [CrossRef]
- Sumathi, S.; Gopal, B. In Vitro Degradation of Multisubstituted Hydroxyapatite and Fluorapatite in the Physiological Condition. J. Cryst. Growth 2015, 422, 36–43. [Google Scholar] [CrossRef]
- Morais, J.M.; Papadimitrakopoulos, F.; Burgess, D.J. Biomaterials/Tissue Interactions: Possible Solutions to Overcome Foreign Body Response. Am. Assoc. Pharm. Sci. 2010, 12, 188–196. [Google Scholar] [CrossRef] [PubMed]
- Przekora, A. The Summary of the Most Important Cell-Biomaterial Interactions That Need to Be Considered during in Vitro Biocompatibility Testing of Bone Scaffolds for Tissue Engineering Applications. Mater. Sci. Eng. C 2019, 97, 1036–1051. [Google Scholar] [CrossRef] [PubMed]
- Kazimierczak, P.; Benko, A.; Nocun, M.; Przekora, A. Novel Chitosan/Agarose/Hydroxyapatite Nanocomposite Scaffold for Bone Tissue Engineering Applications: Comprehensive Evaluation of Biocompatibility and Osteoinductivity with the Use of Osteoblasts and Mesenchymal Stem Cells. Int. J. Nanomed. 2019, 14, 6615–6630. [Google Scholar] [CrossRef]
- Tang, Z.; Li, X.; Tan, Y.; Fan, H.; Zhang, X. The Material and Biological Characteristics of Osteoinductive Calcium Phosphate Ceramics. Regen. Biomater. 2018, 5, 43–59. [Google Scholar] [CrossRef]
- Sun, J.; Wu, T.; Fan, Q.; Hu, Q.; Shi, B. Comparative Study of Hydroxyapatite, Fluor-Hydroxyapatite and Si-Substituted Hydroxyapatite Nanoparticles on Osteogenic, Osteoclastic and Antibacterial Ability. RSC Adv. 2019, 9, 16106–16118. [Google Scholar] [CrossRef]
- Kushwaha, M.; Pan, X.; Holloway, J.A.; Denry, I.L. Differentiation of Human Mesenchymal Stem Cells on Niobium-Doped Fluorapatite Glass-Ceramics. Dent. Mater. 2012, 28, 252–260. [Google Scholar] [CrossRef] [PubMed]
- Mansoorianfar, M.; Mansourianfar, M.; Fathi, M.; Bonakdar, S.; Ebrahimi, M.; Zahrani, E.M.; Hojjati-Najafabadi, A.; Li, D. Surface Modification of Orthopedic Implants by Optimized Fluorine-Substituted Hydroxyapatite Coating: Enhancing Corrosion Behavior and Cell Function. Ceram. Int. 2020, 46, 2139–2146. [Google Scholar] [CrossRef]
- Divieto, C.; Sassi, M.P. A First Approach to Evaluate the Cell Dose in Highly Porous Scaffolds by Using a Nondestructive Metabolic Method. Future Sci. OA 2015, 1, FOS58. [Google Scholar] [CrossRef] [PubMed]
- Kazimierczak, P.; Kolmas, J.; Przekora, A. Biological Response to Macroporous Chitosan-Agarose Bone Scaffolds Comprising Mg- and Zn-Doped Nano-Hydroxyapatite. Int. J. Mol. Sci. 2019, 20, 3835. [Google Scholar] [CrossRef] [PubMed]
- Sridharan, R.; Cameron, A.R.; Kelly, D.J.; Kearney, C.J.; O’Brien, F.J. Biomaterial Based Modulation of Macrophage Polarization: A Review and Suggested Design Principles. Mater. Today 2015, 18, 313–325. [Google Scholar] [CrossRef]
- Velard, F.; Laurent-Maquin, D.; Guillaume, C.; Bouthors, S.; Jallot, E.; Nedelec, J.M.; Belaaouaj, A.; Laquerriere, P. Polymorphonuclear Neutrophil Response to Hydroxyapatite Particles, Implication in Acute Inflammatory Reaction. Acta Biomater. 2009, 5, 1708–1715. [Google Scholar] [CrossRef] [PubMed]
- Velard, F.; Laurent-Maquin, D.; Braux, J.; Guillaume, C.; Bouthors, S.; Jallot, E.; Nedelec, J.M.; Belaaouaj, A.; Laquerriere, P. The Effect of Zinc on Hydroxyapatite-Mediated Activation of Human Polymorphonuclear Neutrophils and Bone Implant-Associated Acute Inflammation. Biomaterials 2010, 31, 2001–2009. [Google Scholar] [CrossRef]
- Nechyporenko, G.V.; Zinchenko, V.F. Novel Synthesis Method and Biomedical Applications of Doped and Undoped Hydroxyapatites and Fluoroapatites. In Proceedings of the International Symposium on Biomedical Engineering and Medical Physics, IFMBE Proceedings, Riga, Latvia, 10–12 October 2012; Dekhtyar, Y., Katashev, A., Lancere, L., Eds.; Springer: Berlin, Germany, 2013; Volume 38, pp. 170–173. [Google Scholar] [CrossRef]
- Wessely-Szponder, J.; Michalska, J.; Szponder, T.; Żylińska, B.; Tarczyńska, M.; Szubstarski, M. The Role of Antimicrobial Neutrophil Extract in Modification of the Inflammatory Response during Osteochondral Autograft and Allograft Transplantation in Rabbits. J. Comp. Pathol. 2020, 175, 49–63. [Google Scholar] [CrossRef]
- Zdziennicka, J.; Szponder, T.; Wessely-Szponder, J. Application of Natural Neutrophil Products for Stimulation of Monocyte-Derived Macrophages Obtained before and after Osteochondral or Bone Injury. Microorganisms 2021, 9, 124. [Google Scholar] [CrossRef]
- Przekora, A.; Czechowska, J.; Pijocha, D.; Ślósarczyk, A.; Ginalska, G. Do Novel Cement-Type Biomaterials Reveal Ion Reactivity That Affects Cell Viability in Vitro? Cent Eur. J. Biol. 2014, 9, 277–289. [Google Scholar] [CrossRef]
- Kolmas, J.; Pajor, K.; Pajchel, L.; Przekora, A.; Ginalska, G.; Oledzka, E.; Sobczak, M. Fabrication and Physicochemical Characterization of Porous Composite Microgranules with Selenium Oxyanions and Risedronate Sodium for Potential Applications in Bone Tumors. Int. J. Nanomed. 2017, 12, 5633–5642. [Google Scholar] [CrossRef] [PubMed]
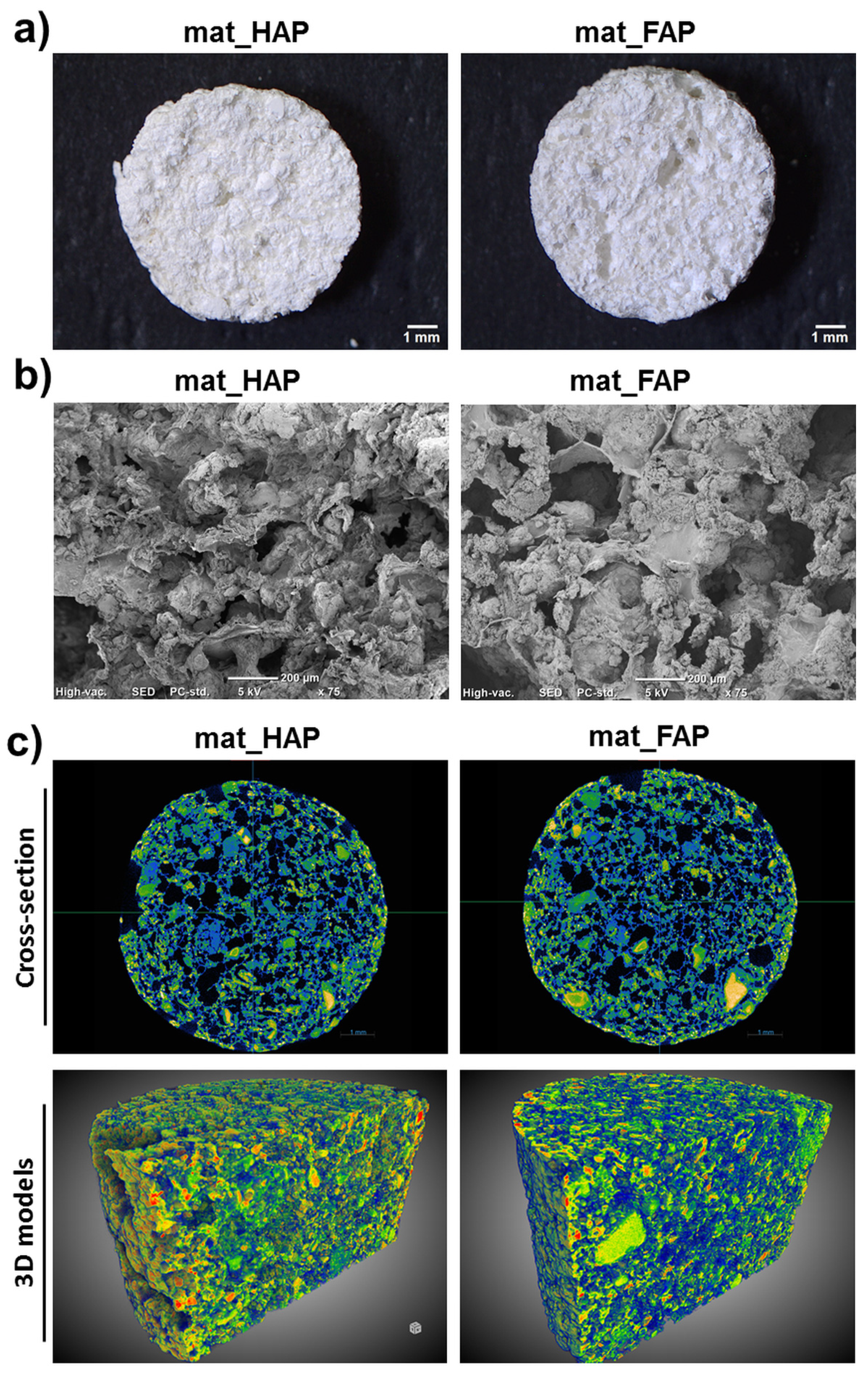




| Porosity [%] | mat_HAP | mat_FAP |
|---|---|---|
| Total | 37.52 ± 1.87 | 47.52 ± 2.95 * |
| Closed | 30.43 ± 2.59 | 36.06 ± 4.62 * |
| Open | 7.09 ± 2.34 | 11.46 ± 6.35 * |
| Mechanical Parameters [MPa] | mat_HAP | mat_FAP |
| Compressive strength | 3.01 ± 0.59 | 2.58 ± 0.75 |
| Young’s modulus | 19.14 ± 13.45 | 19.08 ± 10.38 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kazimierczak, P.; Wessely-Szponder, J.; Palka, K.; Barylyak, A.; Zinchenko, V.; Przekora, A. Hydroxyapatite or Fluorapatite—Which Bioceramic Is Better as a Base for the Production of Bone Scaffold?—A Comprehensive Comparative Study. Int. J. Mol. Sci. 2023, 24, 5576. https://doi.org/10.3390/ijms24065576
Kazimierczak P, Wessely-Szponder J, Palka K, Barylyak A, Zinchenko V, Przekora A. Hydroxyapatite or Fluorapatite—Which Bioceramic Is Better as a Base for the Production of Bone Scaffold?—A Comprehensive Comparative Study. International Journal of Molecular Sciences. 2023; 24(6):5576. https://doi.org/10.3390/ijms24065576
Chicago/Turabian StyleKazimierczak, Paulina, Joanna Wessely-Szponder, Krzysztof Palka, Adriana Barylyak, Viktor Zinchenko, and Agata Przekora. 2023. "Hydroxyapatite or Fluorapatite—Which Bioceramic Is Better as a Base for the Production of Bone Scaffold?—A Comprehensive Comparative Study" International Journal of Molecular Sciences 24, no. 6: 5576. https://doi.org/10.3390/ijms24065576
APA StyleKazimierczak, P., Wessely-Szponder, J., Palka, K., Barylyak, A., Zinchenko, V., & Przekora, A. (2023). Hydroxyapatite or Fluorapatite—Which Bioceramic Is Better as a Base for the Production of Bone Scaffold?—A Comprehensive Comparative Study. International Journal of Molecular Sciences, 24(6), 5576. https://doi.org/10.3390/ijms24065576








