Co-Catalyzed Asymmetric Hydrogenation. The Same Enantioselection Pattern for Different Mechanisms
Abstract
1. Introduction
2. Results and Discussion
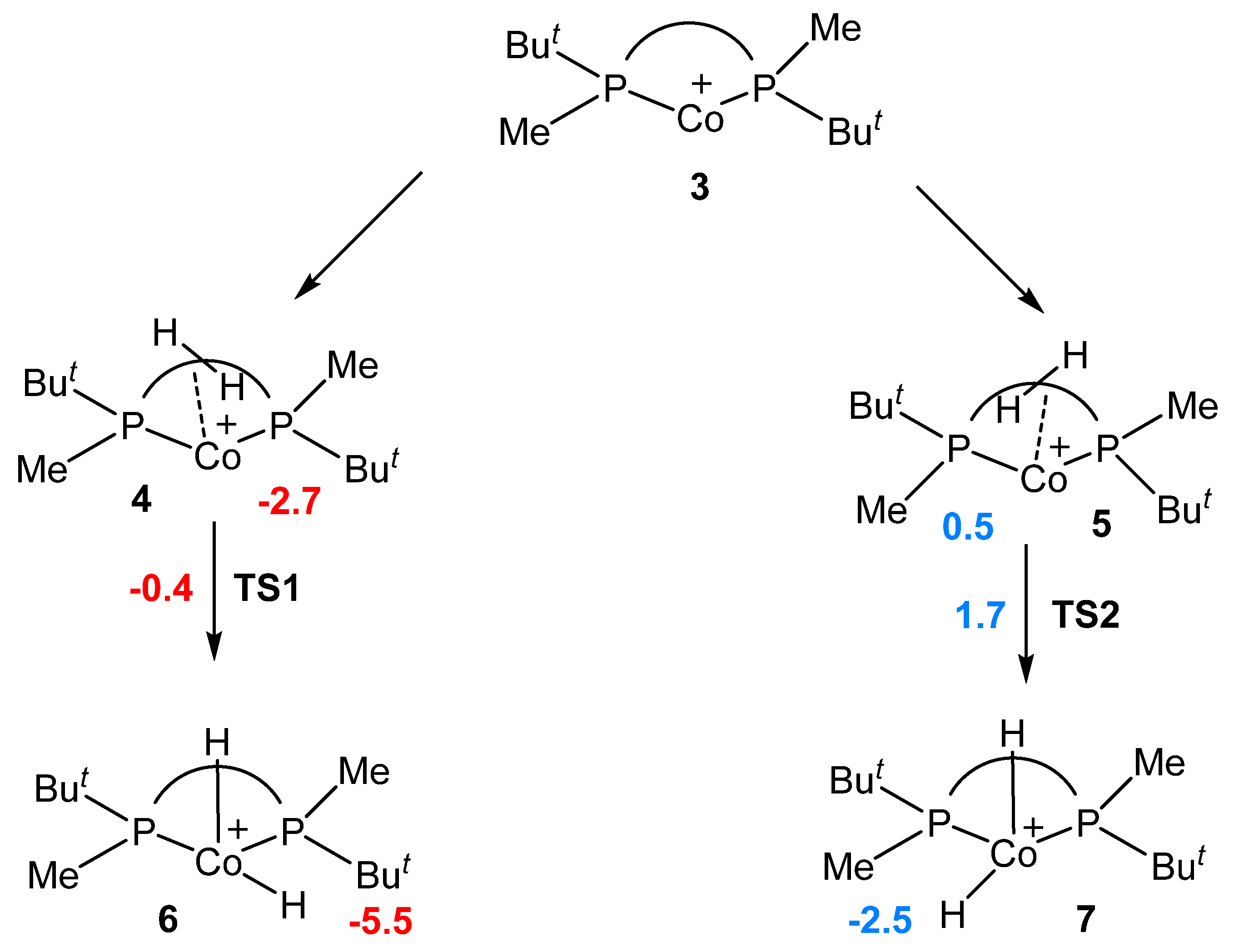
2.1. Formation of Solvate Dihydrides
2.2. Oxidative Addition to Catalyst–Substrate Complexes
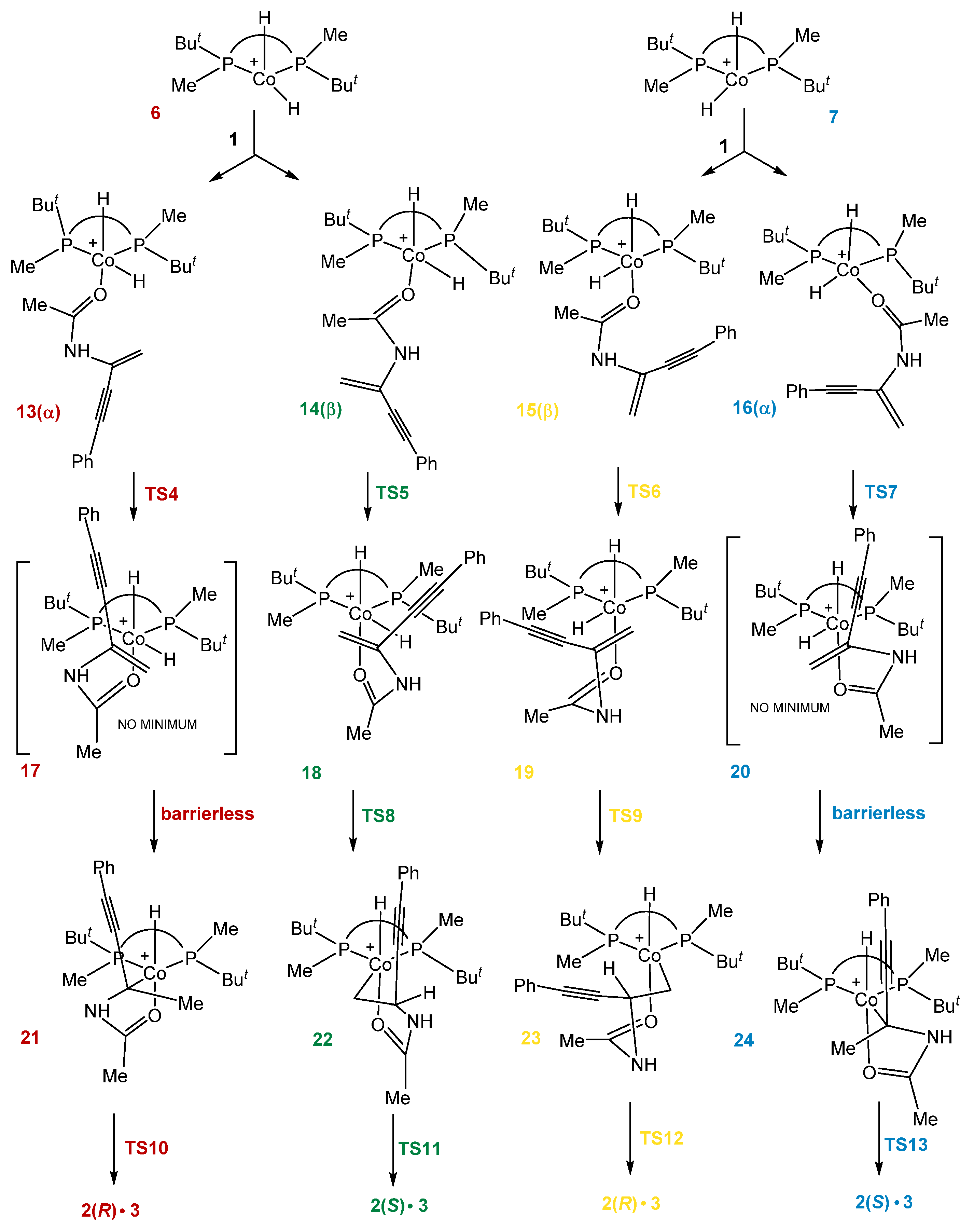
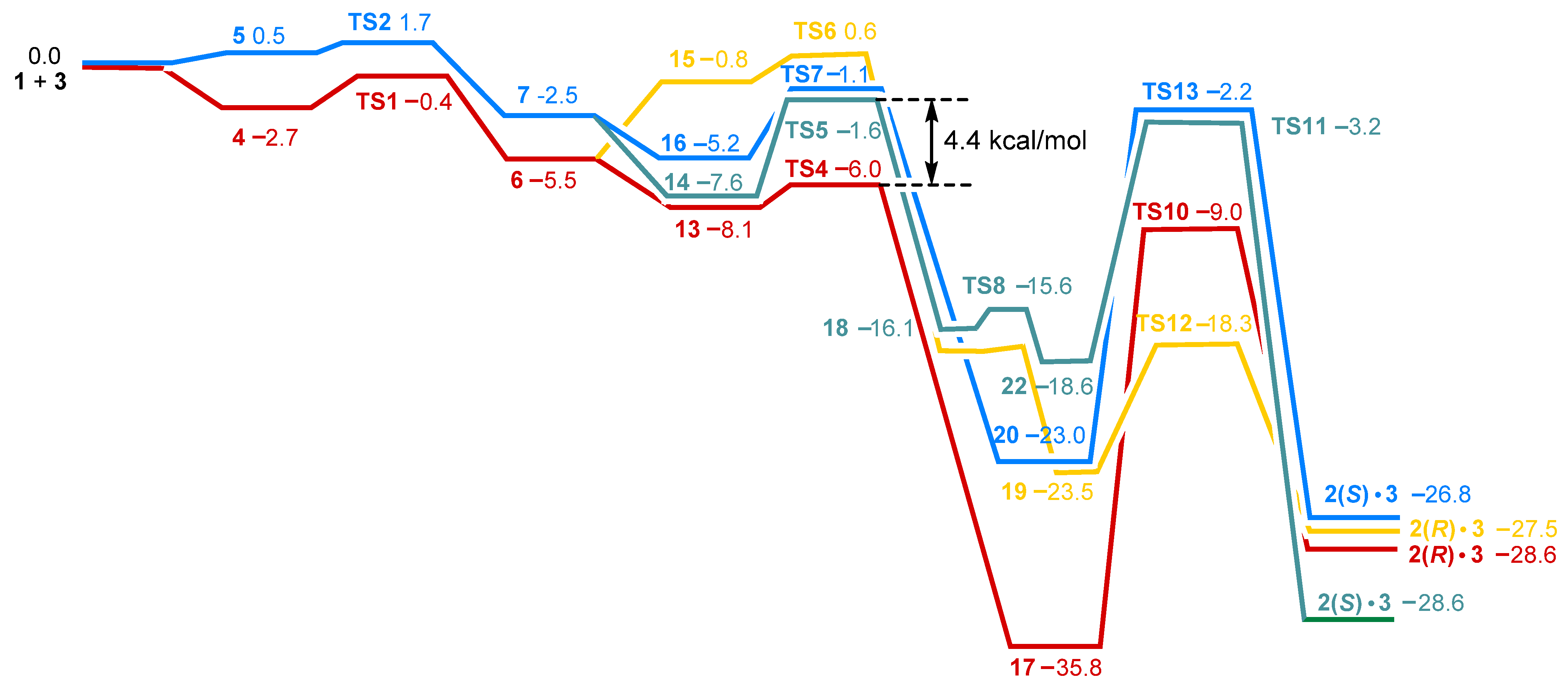
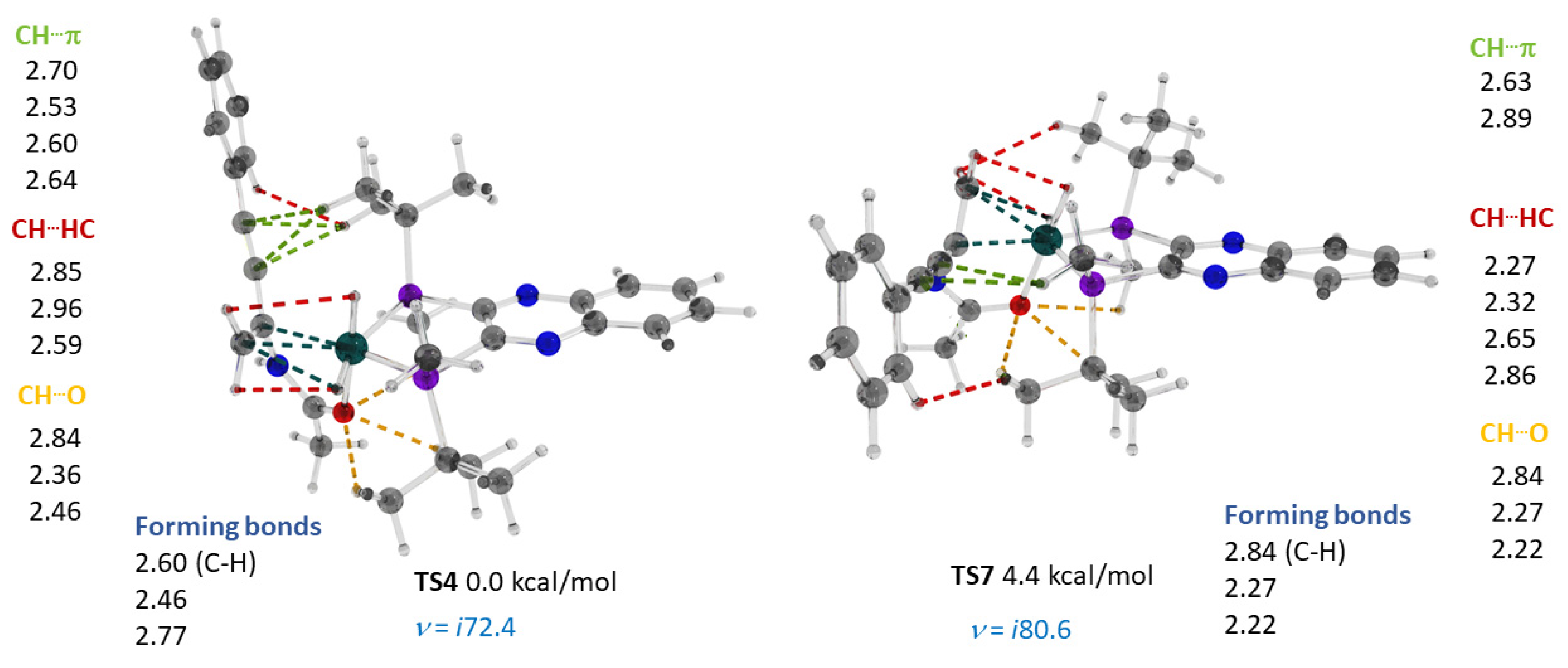
2.3. Stereoselective Formation of Chelating Dihydrides—Four Competing Catalytic Cycles
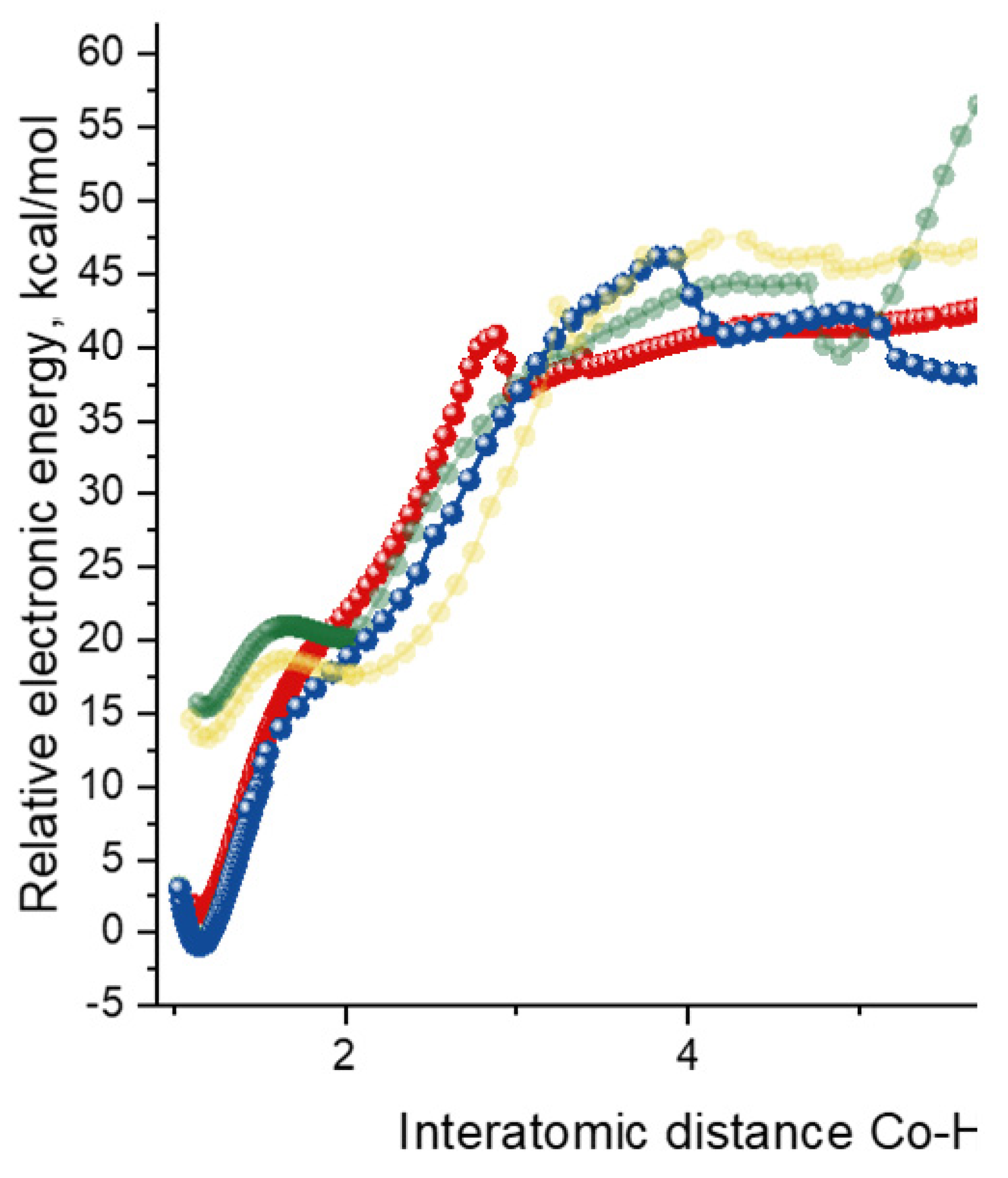
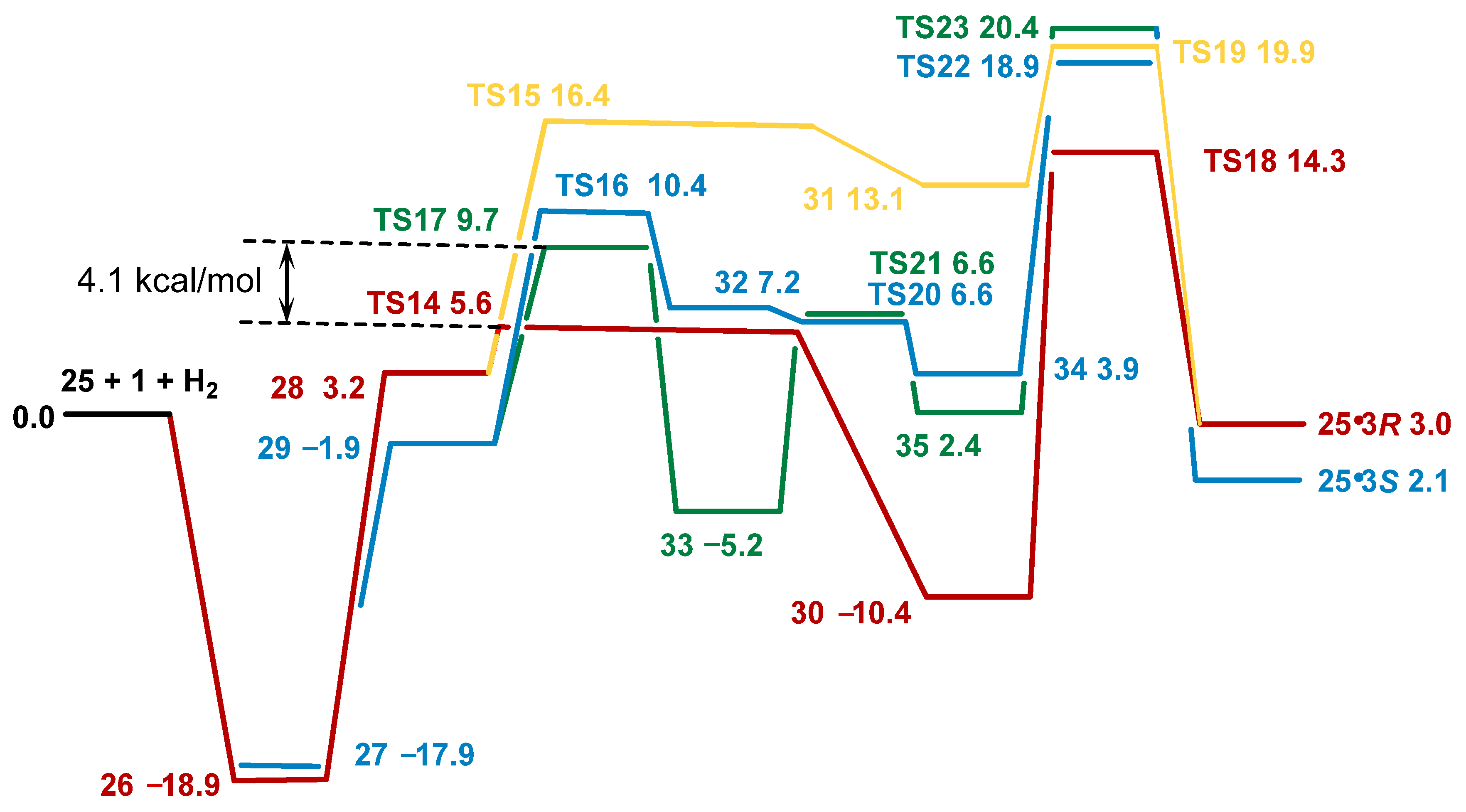
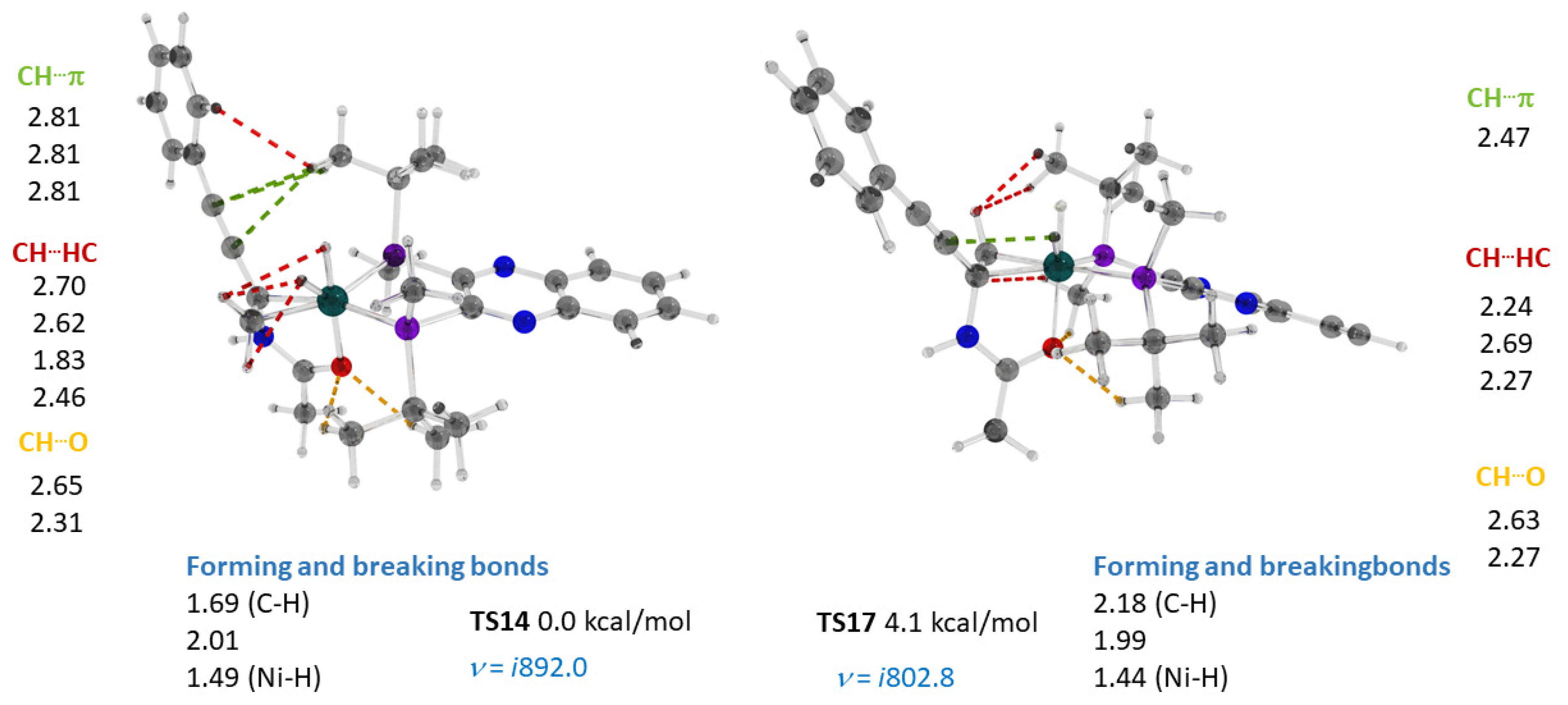
2.4. Co(0)-Co(II) Mechanism
3. Conclusions
4. Materials and Methods
Computational Details
Supplementary Materials
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Hartwig, J. Organotransition Metal Chemistry: From Bonding to Catalysis, 1st ed.; University Science Books: Sausalito, CA, USA, 2009. [Google Scholar]
- Barton, A. Organometallic Transition Metal Catalysis: A Hostic Approach to Understanding and Predicting Their Mechanisms; Lulu: Kerala, India, 2022. [Google Scholar]
- Brown, J.M. Hydrogenation of Functionalized Carbon-Carbon Double Bonds; Jacobsen, E.N., Pfalz, A., Yamamoto, H., Eds.; Springer: Berlin/Heidelberg, Germany, 1999; Volume 1, pp. 119–182. [Google Scholar]
- Halpern, J. Mechanism of Stereoselectivity of Asymmetric Hydrogenation. Science 1982, 217, 401–407. [Google Scholar] [CrossRef] [PubMed]
- Landis, C.R.; Halpern, J. Asymmetric Hydrogenation of Methyl-(Z)-α-acetamidocinnamate Catalyzed by {1,2-Bis((phenyl-o-anisoyl)phosphino)ethane}rhodium(I): Kinetics, Mechanism, and Origin of Enantioselection. J. Am. Chem. Soc. 1987, 109, 1746–1754. [Google Scholar] [CrossRef]
- Giernoth, R.; Heinrich, H.; Adams, N.J.; Deeth, R.J.; Bargon, J.; Brown, J.M. PHIP Detection of a Transient Rhodium Dihydride Intermediate in the Homogeneous Hydrogenation of Dehydroamino Acids. J. Am. Chem. Soc. 2000, 122, 12381–12382. [Google Scholar] [CrossRef]
- Gridnev, I.D.; Imamoto, T. On the Mechanism of Stereoselection in Rh-Catalyzed Asymmetric Hydrogenation: A General Approach for Predicting the Sense of Enantioselectivity. Acc. Chem. Res. 2004, 37, 633–644. [Google Scholar] [CrossRef] [PubMed]
- Gridnev, I.D.; Imamoto, T. Mechanism of Enantioselection in Rh-Catalyzed Asymmetric Hydrogenation. The Origin of Utmost Catalytic Performance. Chem. Commun. 2009, 7447–7464. [Google Scholar] [CrossRef]
- Verdolino, V.; Forbes, A.; Helquist, P.; Norrby, P.-O.; Wiest, O. On the mechanism of the rhodium catalyzed acrylamide hydrogenation. J. Mol. Catal. A Chem. 2010, 324, 9–14. [Google Scholar] [CrossRef]
- Imamoto, T.; Tamura, K.; Zhang, Z.; Horiuchi, Y.; Sugiya, M.; Yoshida, K.; Yanagisawa, A.; Gridnev, I.D. Rigid P-chiral phosphine ligands with tert-butylmethylphosphino groups for rhodium-catalyzed asymmetric hydrogenation of functionalized alkenes. J. Am. Chem. Soc. 2012, 134, 1754–1769. [Google Scholar] [CrossRef]
- Gridnev, I.D.; Imamoto, T. Challenging the major/minor concept in Rh-catalyzed asymmetric hydrogenation. ACS Catal. 2015, 5, 2911–2915. [Google Scholar] [CrossRef]
- Gridnev, I.D.; Dub, P.A. Enantioselection in Asymmetric Catalysis; CRC Press: Boca Raton, FL, USA, 2016; p. 234. [Google Scholar]
- Kitamura, M.; Tsukamoto, M.; Bessho, Y.; Yoshimura, M.; Kobs, U.; Widhalm, M.; Noyori, R. Mechanism of Asymmetric Hydrogenation of α-(Acylamino)acrylic Esters Catalyzed by BINAP-Ruthenium(II) Diacetate. J. Am. Chem. Soc. 2002, 124, 6649–6667. [Google Scholar] [CrossRef]
- Dub, P.A.; Gordon, J.C. The role of the metal-bound N–H functionality in Noyori-type molecular catalysts. Nat. Rev. Chem. 2018, 2, 396–408. [Google Scholar] [CrossRef]
- Dub, P.A.; Ikariya, T. Quantum Chemical Calculations with the Inclusion of Nonspecific and Specific Solvation: Asymmetric Transfer Hydrogenation with Bifunctional Ruthenium Catalysts. J. Am. Chem. Soc. 2013, 135, 2604–2619. [Google Scholar] [CrossRef] [PubMed]
- Dub, P.A.; Gordon, J.C. The mechanism of enantioselective ketone reduction with Noyori and Noyori–Ikariya bifunctional catalysts. Dalton Trans 2016, 45, 6756–6781. [Google Scholar] [CrossRef]
- Roseblade, J.; Pfaltz, A. Iridium-catalyzed asymmetric hydrogenation of olefins. Acc. Chem. Res. 2007, 40, 1402–1411. [Google Scholar] [CrossRef] [PubMed]
- Hopmann, K.H.; Bayer, A. On the Mechanism of Iridium-Catalyzed Asymmetric Hydrogenation of Imines and Alkenes: A Theoretical Study. Organometallics 2011, 30, 2483–2497. [Google Scholar] [CrossRef]
- Liu, Y.; Gridnev, I.D.; Zhang, W. Mechanism of Asymmetric Hydrogenation of Exocyclic alpha, beta-Unsaturated Carbonyl Compounds with an Iridium/BiphPhopx Catalyst: NMR and DFT Studies. Angew. Chem. Intl. Ed. 2014, 53, 1901–1905. [Google Scholar] [CrossRef]
- Sparta, M.; Riplinger, C.; Neese, F. Mechanism of Olefin Asymmetric Hydrogenation Catalyzed by Iridium Phosphino-Oxazoline: A Pair Natural Orbital Coupled Cluster Study. J. Chem. Theory Comput. 2014, 10, 1099–1108. [Google Scholar] [CrossRef] [PubMed]
- Tutkowski, B.; Kerdphon, S.; Limé, E.; Helquist, P.; Andersson, P.G.; Wiest, O.; Norrby, P.-O. Revisiting the Stereodetermining Step in Enantioselective Iridium-Catalyzed Imine Hydrogenation. ACS Catal. 2018, 8, 615–623. [Google Scholar] [CrossRef]
- Cui, C.X.; Chen, H.H.; Li, S.J.; Zhang, T.; Qu, L.B.; Lan, Y. Mechanism of Ir-catalyzed hydrogenation: A theoretical view. Coord. Chem. Rev. 2020, 412, 213251. [Google Scholar] [CrossRef]
- Mas-Roselló, J.; Smejkal, T.; Cramer, N. Iridium-catalyzed acid-assisted asymmetric hydrogenation of oximes to hydroxylamines. Science 2020, 368, 1098–1102. [Google Scholar] [CrossRef]
- Vogiatzis, K.D.; Polynski, M.V.; Kirkland, J.K.; Townsend, J.; Hashemi, A.; Liu, C.; Pidko, E.A. Computational Approach to Molecular Catalysis by 3d Transition Metals: Challenges and Opportunities. Chem. Rev. 2019, 119, 2453–2523. [Google Scholar] [CrossRef]
- Zhang, Z.; Butt, N.A.; Zhou, M.; Liu, D.; Zhang, W. Asymmetric Transfer and Pressure Hydrogenation with Earth-Abundant Transition Metal Catalysts. Chin. J. Chem. 2018, 36, 443–454. [Google Scholar] [CrossRef]
- Seo, C.S.G.; Morris, R.H. Catalytic Homogeneous Asymmetric Hydrogenation: Successes and Opportunities. Organometallics 2019, 38, 47–65. [Google Scholar] [CrossRef]
- Li, B.; Chen, J.; Zhang, Z.; Gridnev, I.D.; Zhang, W. Nickel-catalyzed asymmetric hydrogenation of N-sulfonyl imines. Angew. Chem. Int. Ed. 2019, 58, 7329–7334. [Google Scholar] [CrossRef]
- Liu, Y.; Dong, X.-Q.; Zhang, X. Recent advances of nickel-catalyzed homogeneous asymmetric hydrogenation. Chin. J. Org. Chem. 2020, 40, 1096–1104. [Google Scholar] [CrossRef]
- Hu, Y.; Chen, J.; Li, B.; Zhang, Z.; Gridnev, I.D.; Zhang, W. Nickel-catalyzed asymmetric hydrogenation of 2-amidoacrylates. Angew. Chem. Int. Ed. 2020, 59, 5371–5375. [Google Scholar] [CrossRef] [PubMed]
- Liu, D.; Li, B.; Chen, J.; Gridnev, I.D.; Yan, D.; Zhang, W. Ni-catalyzed asymmetric hydrogenation of N-aryl imino esters for the efficient synthesis of chiral α-aryl glycines. Nat. Commun. 2020, 11, 5935. [Google Scholar] [CrossRef]
- Li, B.; Chen, J.; Liu, D.; Gridnev, I.D.; Zhang, W. Nickel-catalysed asymmetric hydrogenation of oximes. Nat. Chem. 2022, 14, 920–927. [Google Scholar] [CrossRef]
- Monfette, S.; Turner, Z.R.; Semproni, S.P.; Chirik, P.J. Enantiopure C1-Symmetric Bis(imino)pyridine Cobalt Complexes for Asymmetric Alkene Hydrogenation. J. Am. Chem. Soc. 2012, 134, 4561–4564. [Google Scholar] [CrossRef]
- Zhang, G.; Vasudevan, K.V.; Scott, B.L.; Hanson, S.K. Understanding the Mechanisms of Cobalt-Catalyzed Hydrogenation and Dehydrogenation Reactions. J. Am. Chem. Soc. 2013, 135, 8668–8681. [Google Scholar] [CrossRef]
- Zhong, H.; Friedfeld, M.R.; Chirik, P.J. Syntheses and Catalytic Hydrogenation Performance of Cationic Bis(phosphine) Cobalt(I) Diene and Arene Compounds. Angew. Chem. Int. Ed. 2019, 58, 9194–9198. [Google Scholar] [CrossRef] [PubMed]
- Hopmann, K.H. Cobalt–Bis(imino)pyridine-Catalyzed Asymmetric Hydrogenation: Electronic Structure, Mechanism, and Stereoselectivity. Organometallics 2013, 32, 6388–6399. [Google Scholar] [CrossRef]
- Zhong, H.; Friedfeld, M.R.; Camacho-Bunquin, J.; Sohn, H.; Yang, C.; Delferro, M.; Chirik, P.J. Exploring the Alcohol Stability of Bis(phosphine) Cobalt Dialkyl Precatalysts in Asymmetric Alkene Hydrogenation. Organometallics 2019, 38, 149–156. [Google Scholar] [CrossRef]
- Friedfeld, M.R.; Zhong, H.; Ruck, R.T.; Shevlin, M.; Chirik, P.J. Cobalt-catalyzed asymmetric hydrogenation of enamides enabled by single-electron reduction. Science 2018, 360, 888–893. [Google Scholar] [CrossRef]
- Chen, J.; Chen, C.; Ji, C.; Lu, Z. Cobalt-Catalyzed Asymmetric Hydrogenation of 1,1-Diarylethenes. Org. Lett. 2016, 18, 1594–1597. [Google Scholar] [CrossRef] [PubMed]
- Friedfeld, M.R.; Shevlin, M.; Margulieux, G.W.; Campeau, L.-C.; Chirik, P.J. Cobalt-Catalyzed Enantioselective Hydrogenation of Minimally Functionalized Alkenes: Isotopic Labeling Provides Insight into the Origin of Stereoselectivity and Alkene Insertion Preferences. J. Am. Chem. Soc. 2016, 138, 3314–3324. [Google Scholar] [CrossRef] [PubMed]
- Ma, X.; Lei, M. Mechanistic Insights into the Directed Hydrogenation of Hydroxylated Alkene Catalyzed by Bis(phosphine)Cobalt Dialkyl Complexes. J. Org. Chem. 2017, 82, 2703–2712. [Google Scholar] [CrossRef]
- Hu, Y.; Zhang, Z.; Zhang, J.; Liu, Y.; Gridnev, I.D.; Zhang, W. Cobalt-Catalyzed Asymmetric Hydrogenation of C=N Bonds Enabled by Assisted Coordination and Nonbonding Interactions. Angew. Chem. Int. Ed. 2019, 58, 15767–15771. [Google Scholar] [CrossRef]
- Zhong, H.; Shevlin, M.; Chirik, P.J. Cobalt-Catalyzed Asymmetric Hydrogenation of α,β-Unsaturated Carboxylic Acids by Homolytic H2 Cleavage. J. Am. Chem. Soc. 2020, 142, 5272–5281. [Google Scholar] [CrossRef] [PubMed]
- Du, X.; Xiao, Y.; Yang, Y.; Duan, Y.-N.; Li, F.; Hu, Q.; Chung, L.W.; Chen, G.-Q.; Zhang, X. Enantioselective Hydrogenation of Tetrasubstituted α,β-Unsaturated Carboxylic Acids Enabled by Cobalt(II) Catalysis: Scope and Mechanistic Insights. Angew. Chem. Int. Ed. 2021, 60, 11384–11390. [Google Scholar] [CrossRef]
- Du, X.; Xiao, Y.; Huang, J.-M.; Zhang, Y.; Duan, Y.-N.; Wang, H.; Shi, C.; Chen, G.-Q.; Zhang, X. Cobalt-catalyzed highly enantioselective hydrogenation of α,β-unsaturated carboxylic acids. Nat. Commun. 2020, 11, 3239. [Google Scholar] [CrossRef] [PubMed]
- Hu, Y.; Zhang, Z.; Liu, Y.; Zhang, W. Cobalt-Catalyzed Chemo- and Enantioselective Hydrogenation of Conjugated Enynes. Angew. Chem. Int. Ed. 2021, 60, 16989–16993. [Google Scholar] [CrossRef] [PubMed]
- Mendelsohn, L.N.; Pavlovic, L.; Zhong, H.; Friedfeld, M.R.; Shevlin, M.; Hopmann, K.H.; Chirik, P.J. Mechanistic Investigations of the Asymmetric Hydrogenation of Enamides with Neutral Bis(phosphine) Cobalt Precatalysts. J. Am. Chem. Soc. 2022, 144, 15764–15778. [Google Scholar] [CrossRef]
- Pavlovich, L.; Mendelsohn, L.N.; Zhong, H.Y.; Chirik, P.J.; Hopmann, K.H. Cobalt-Catalyzed Asymmetric Hydrogenation of Enamides: Insights into Mechanisms and Solvent Effects. Organometallics 2022, 41, 1872–1882. [Google Scholar] [CrossRef]
- Zhou, S.; Fleischer, S.; Junge, K.; Das, S.; Addis, D.; Beller, M. Enantioselective Synthesis of Amines: General, Efficient Iron-Catalyzed Asymmetric Transfer Hydrogenation of Imines. Angew. Chem. Int. Ed. 2010, 49, 8121–8125. [Google Scholar] [CrossRef] [PubMed]
- Lagaditis, P.O.; Sues, P.E.; Sonnenberg, J.F.; Wan, K.Y.; Lough, A.J.; Morris, R.H. Iron(II) Complexes Containing Unsymmetrical P–N–P′ Pincer Ligands for the Catalytic Asymmetric Hydrogenation of Ketones and Imines. J. Am. Chem. Soc. 2014, 136, 1367–1380. [Google Scholar] [CrossRef]
- Smith, S.A.M.; Lagaditis, P.O.; Lüpke, A.; Lough, A.J.; Morris, R.H. Unsymmetrical Iron P-NH-P′ Catalysts for the Asymmetric Pressure Hydrogenation of Aryl Ketones. Chem.-Eur. J. 2017, 23, 7212–7216. [Google Scholar] [CrossRef]
- Sonnenberg, J.F.; Wan, K.Y.; Sues, P.E.; Morris, R.H. Ketone Asymmetric Hydrogenation Catalyzed by P-NH-P′ Pincer Iron Catalysts: An Experimental and Computational Study. ACS Catal. 2017, 7, 316–326. [Google Scholar] [CrossRef]
- Seo, C.S.G.; Tannoux, T.; Smith, S.A.M.; Lough, A.J.; Morris, R.H. Enantioselective hydrogenation of activated aryl imines catalyzed by an iron(II) P-NH-P′ complex. J. Org. Chem. 2019, 84, 12040–12049. [Google Scholar] [CrossRef]
- Zhang, L.; Tang, Y.; Han, Z.; Ding, K. Lutidine-based chiral pincer manganese catalysts for enantioselective hydrogenation of ketones. Angew. Chem. Int. Ed. 2019, 58, 4973–4977. [Google Scholar] [CrossRef]
- Zhang, L.; Wang, Z.; Han, Z.; Ding, K. Manganese-catalyzed anti-selective asymmetric hydrogenation of α-substituted β-ketoamides. Angew. Chem. Int. Ed. 2020, 59, 15565–15569. [Google Scholar] [CrossRef]
- Knowles, W.S. Asymmetric Hydrogenation. Acc. Chem. Res. 1983, 16, 106–112. [Google Scholar] [CrossRef]
- Gridnev, I.D. Attraction versus Repulsion in Rhodium-Catalyzed Asymmetric Hydrogenation. ChemCatChem 2016, 8, 3463–3468. [Google Scholar] [CrossRef]
- Burk, M.J.; Casy, G.; Johnson, N.B. A Three-Step Procedure for Asymmetric Catalytic Reductive Amidation of Ketones. J. Org. Chem. 1998, 63, 6084–6085. [Google Scholar] [CrossRef]
- Gridnev, I.D.; Higashi, N.; Imamoto, T. On the Origin of opposite Stereoselection in the Asymmetric Hydrogenation of Phenyl- and tert-Butyl-Substituted Enamides. J. Am. Chem. Soc. 2000, 122, 10486–10487. [Google Scholar] [CrossRef]
- Gridnev, I.D.; Yasutake, M.; Higashi, N.; Imamoto, T. Asymmetric Hydrogenation of Enamides with Rh-BisP* and Rh-MiniPHOS Catalysts. Scope, Limitations, and Mechanism. J. Am. Chem. Soc. 2001, 123, 5268–5276. [Google Scholar] [CrossRef]
- Chai, J.-D.; Head-Gordon, M. Long-Range Corrected Hybrid Density Functionals with Damped Atom–Atom Dispersion Corrections. Phys. Chem. Chem. Phys. 2008, 10, 6615–6620. [Google Scholar] [CrossRef]
- Frisch, M.J.; Trucks, G.W.; Schlegel, H.B.; Scuseria, G.E.; Robb, M.A.; Cheeseman, J.R.; Scalmani, G.; Barone, V.; Mennucci, B.; Petersson, G.A.; et al. Gaussian 09, rev. D.01; Gaussian, Inc.: Wallingford, CT, USA, 2013. [Google Scholar]
- Becke, A.D. Density-functional exchange-energy approximation with correct asymptotic behavior. Phys. Rev. A 1988, 38, 3098–3100. [Google Scholar] [CrossRef]
- Ditchfield, R.; Hehre, W.J.; Pople, J.A. Self-Consistent Molecular-Orbital Methods. IX. An Extended Gaussian-Type Basis for Molecular-Orbital Studies of Organic Molecules. J. Chem. Phys. 1971, 54, 724–728. [Google Scholar] [CrossRef]
- Hehre, W.J.; Ditchfield, R.; Pople, J.A. Self-Consistent Molecular Orbital Methods. XII. Further Extensions of Gaussian-Type Basis Sets for Use in Molecular Orbital Studies of Organic Moleculesl. J. Chem. Phys. 1972, 56, 2257–2261. [Google Scholar] [CrossRef]
- Hariharan, P.C.; Pople, J.A. The influence of polarization functions on molecular orbital hydrogenation energies. Theor. Chim. Acta 1973, 28, 213–222. [Google Scholar] [CrossRef]
- Francl, M.M.; Pietro, W.J.; Hehre, W.J.; Binkley, J.S.; Gordon, M.S.; DeFrees, D.J.; Pople, J.A. Self-consistent molecular orbital methods. XXIII. A polarization-type basis set for second-row elements. J. Chem. Phys. 1982, 77, 3654–3665. [Google Scholar]
- Gordon, M.S.; Binkley, J.S.; Pople, J.A.; Pietro, W.J.; Hehre, W.J. Self-consistent molecular-orbital methods. 22. Small split-valence basis sets for second-row elements. J. Am. Chem. Soc. 1982, 104, 2797–2803. [Google Scholar] [CrossRef]
- Rassolov, V.A.; Pople, J.A.; Ratner, M.A.; Windus, T.L. 6-31G* basis set for atoms K through Zn. J. Chem. Phys. 1998, 109, 1223–1229. [Google Scholar] [CrossRef]
- Krishnan, R.; Binkley, J.S.; Seeger, R.; Pople, J.A. Self-consistent molecular orbital methods. XX. A basis set for correlated wave functions. J. Chem. Phys. 1980, 72, 650–654. [Google Scholar] [CrossRef]
- McLean, A.D.; Chandler, G.S. Contracted Gaussian basis sets for molecular calculations. I. Second row atoms, Z=11–18. J. Chem. Phys. 1980, 72, 5639–5648. [Google Scholar] [CrossRef]
- Clark, T.; Chandrasekhar, J.; Spitznagel, G.W.; Schleyer, P.V.R. Efficient diffuse function-augmented basis sets for anion calculations. III. The 3-21+G basis set for first-row elements, Li–F. J. Comput. Chem. 1983, 4, 294–301. [Google Scholar] [CrossRef]
- Spitznagel, G.W.; Clark, T.; Schleyer, P.V.R.; Hehre, W.J. An evaluation of the performance of diffuse function-augmented basis sets for second row elements, Na-Cl. J. Comput. Chem. 1987, 8, 1109–1116. [Google Scholar] [CrossRef]
- Raghavachari, K.; Trucks, G.W. Highly correlated systems. Excitation energies of first row transition metals Sc–Cu. J. Chem. Phys. 1989, 91, 1062–1065. [Google Scholar] [CrossRef]
- Marenich, A.V.; Cramer, C.J.; Truhlar, D.G. Universal Solvation Model Based on Solute Electron Density and on a Continuum Model of the Solvent Defined by the Bulk Dielectric Constant and Atomic Surface Tensions. J. Phys. Chem. B 2009, 113, 6378–6396. [Google Scholar] [CrossRef]
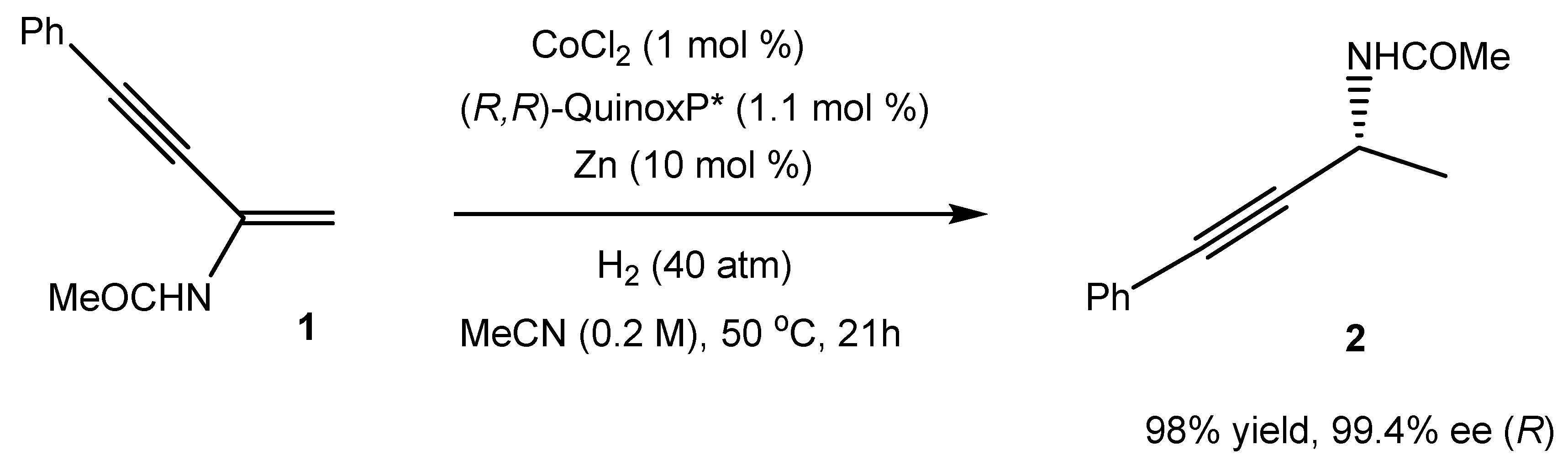



Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gridnev, I.D. Co-Catalyzed Asymmetric Hydrogenation. The Same Enantioselection Pattern for Different Mechanisms. Int. J. Mol. Sci. 2023, 24, 5568. https://doi.org/10.3390/ijms24065568
Gridnev ID. Co-Catalyzed Asymmetric Hydrogenation. The Same Enantioselection Pattern for Different Mechanisms. International Journal of Molecular Sciences. 2023; 24(6):5568. https://doi.org/10.3390/ijms24065568
Chicago/Turabian StyleGridnev, Ilya D. 2023. "Co-Catalyzed Asymmetric Hydrogenation. The Same Enantioselection Pattern for Different Mechanisms" International Journal of Molecular Sciences 24, no. 6: 5568. https://doi.org/10.3390/ijms24065568
APA StyleGridnev, I. D. (2023). Co-Catalyzed Asymmetric Hydrogenation. The Same Enantioselection Pattern for Different Mechanisms. International Journal of Molecular Sciences, 24(6), 5568. https://doi.org/10.3390/ijms24065568





