Synthesis of 3-(Pyridin-2-yl)quinazolin-2,4(1H,3H)-diones via Annulation of Anthranilic Esters with N-pyridyl Ureas †
Abstract
1. Introduction
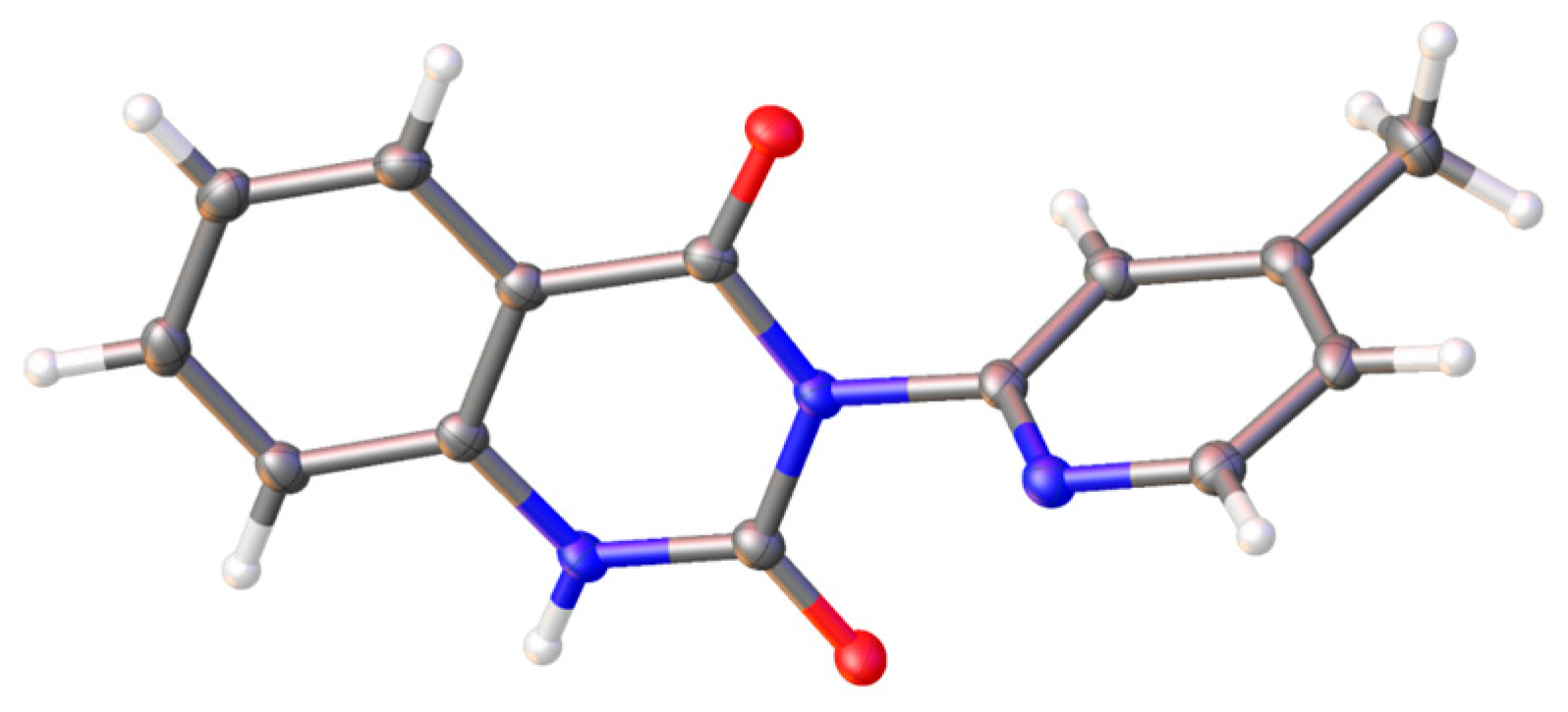
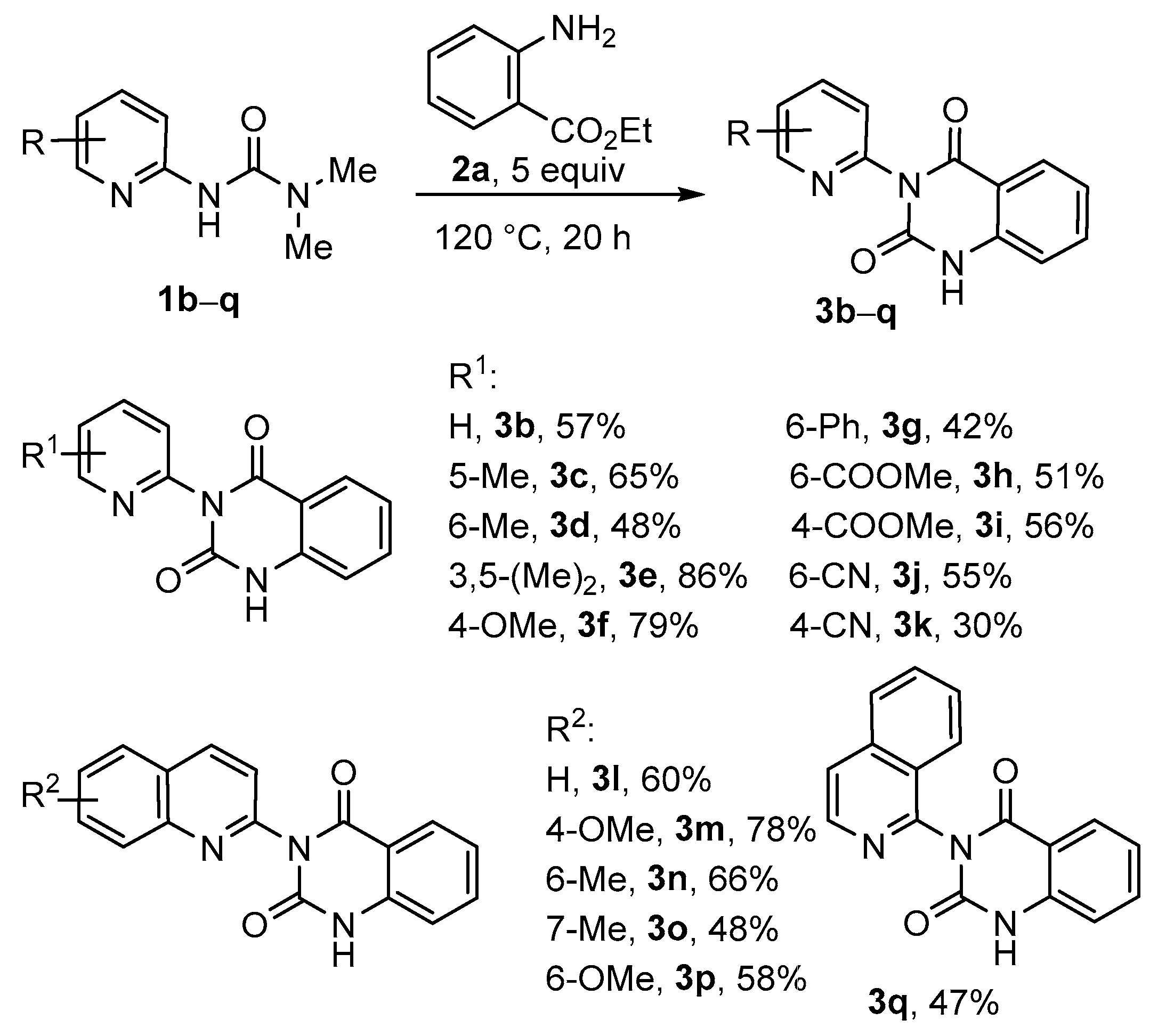
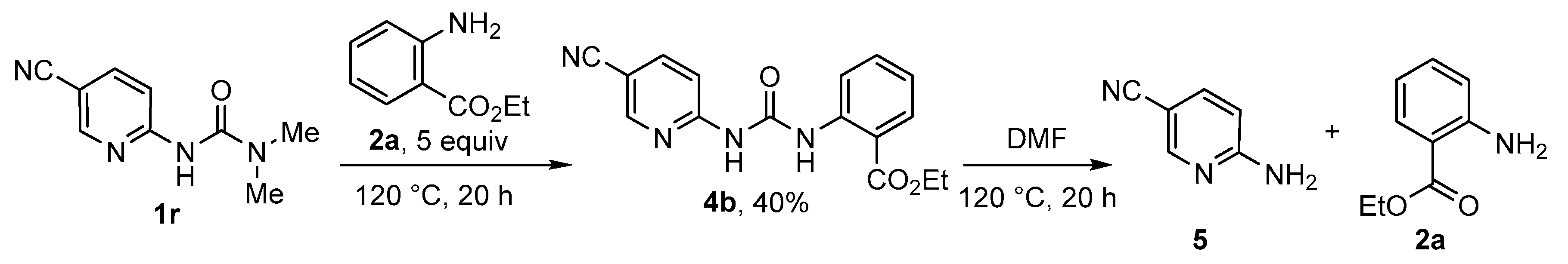
2. Results and Discussion
3. Material and Methods
3.1. General
3.2. Preparation of Starting Ureas 1a–r
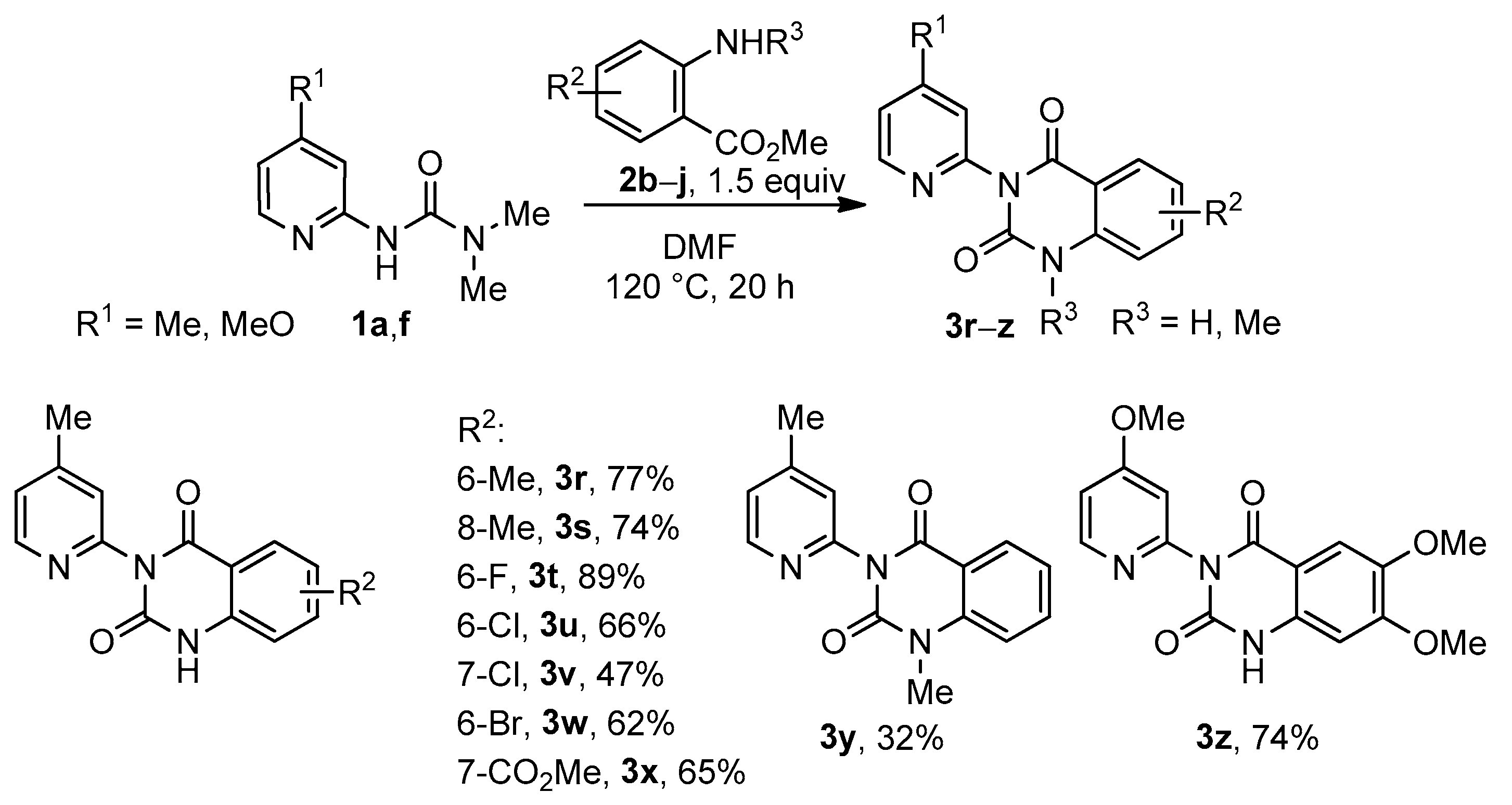
3.3. Synthesis of Quinazoline-2,4-Diones 3
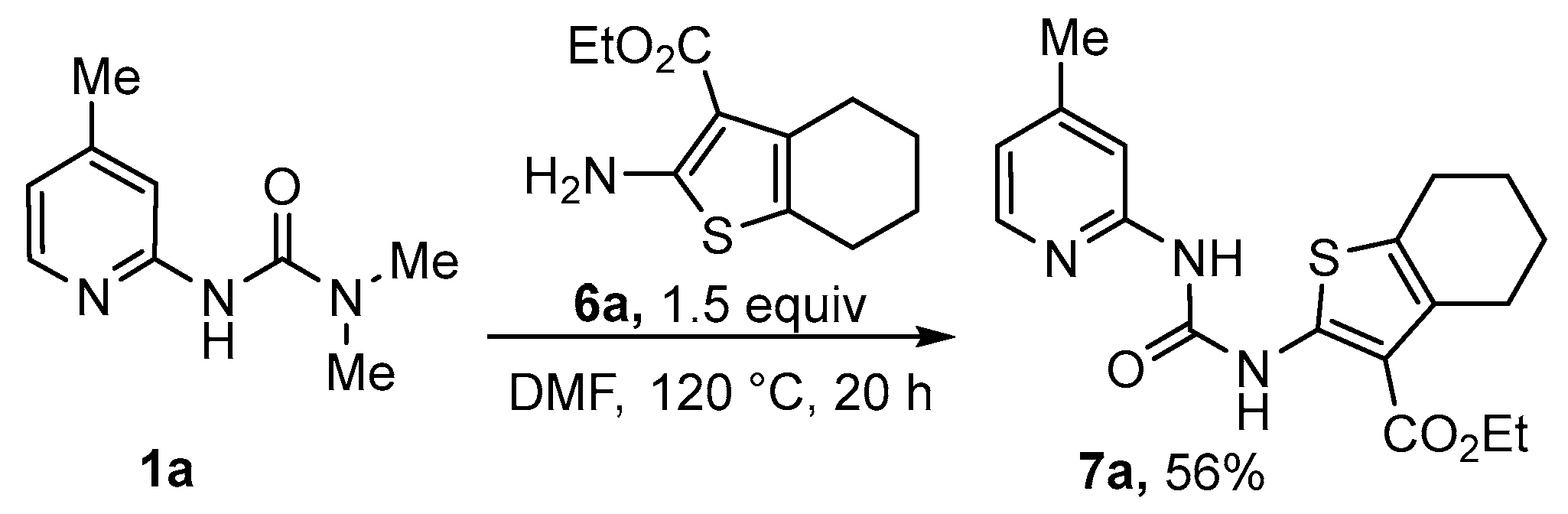
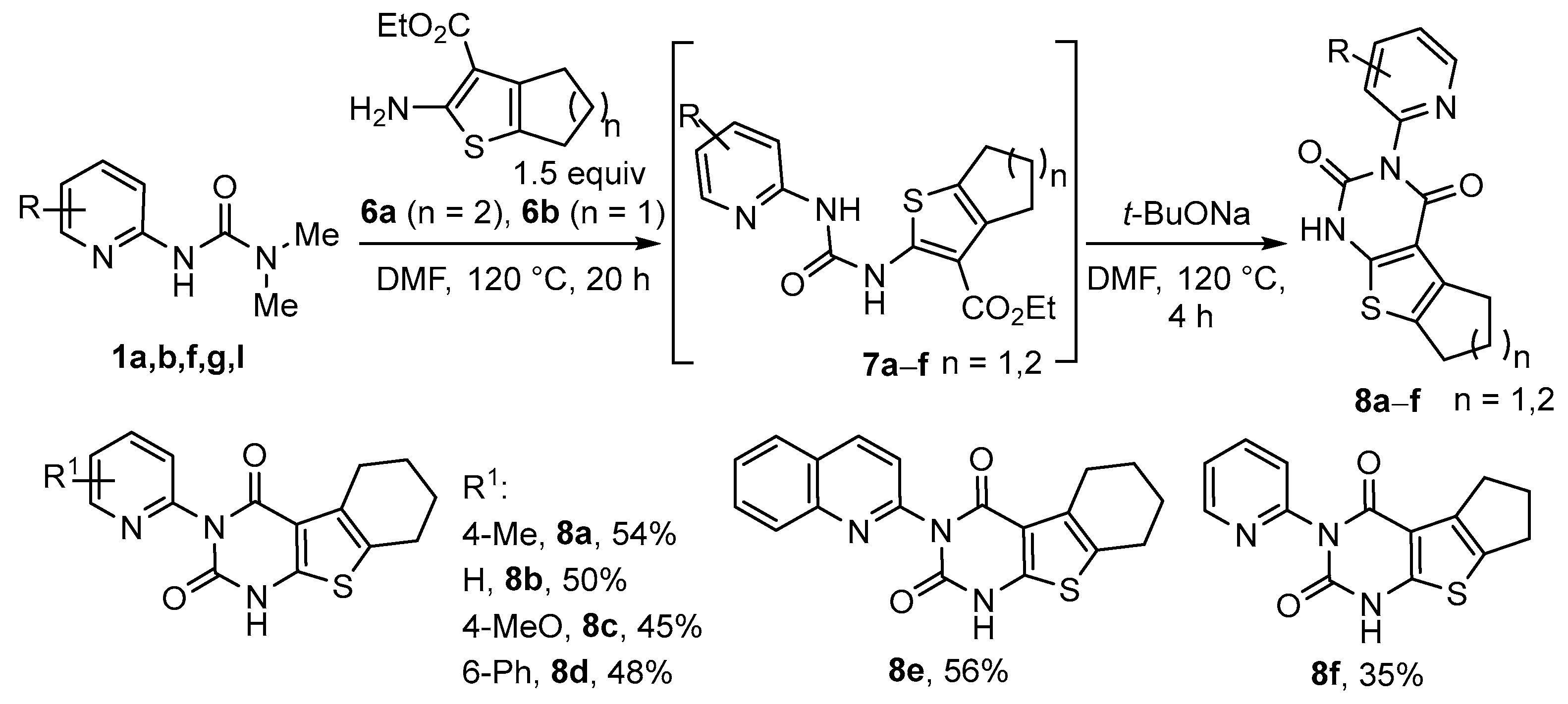
3.4. Synthesis of Thienopyrimidine-2,4-Diones 8
3.5. Gram-Scale Synthesis of 3a and 8a
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Faught, E. BGG492 (Selurampanel), an AMPA/Kainate Receptor Antagonist Drug for Epilepsy. Expert Opin. Investig. Drugs 2014, 23, 107–113. [Google Scholar] [CrossRef] [PubMed]
- Orain, D.; Tasdelen, E.; Haessig, S.; Koller, M.; Picard, A.; Dubois, C.; Lingenhoehl, K.; Desrayaud, S.; Floersheim, P.; Carcache, D.; et al. Design and Synthesis of Selurampanel, a Novel Orally Active and Competitive AMPA Receptor Antagonist. ChemMedChem 2017, 12, 197–201. [Google Scholar] [CrossRef]
- Ueno, M.; Rao, S.V.; Angiolillo, D.J. Elinogrel: Pharmacological Principles, Preclinical and Early Phase Clinical Testing. Future Cardiol. 2010, 6, 445–453. [Google Scholar] [CrossRef]
- Welsh, R.C.; Rao, S.V.; Zeymer, U.; Thompson, V.P.; Huber, K.; Kochman, J.; McClure, M.W.; Gretler, D.D.; Bhatt, D.L.; Gibson, C.M.; et al. A Randomized, Double-Blind, Active-Controlled Phase 2 Trial to Evaluate a Novel Selective and Reversible Intravenous and Oral P2Y 12 Inhibitor Elinogrel Versus Clopidogrel in Patients Undergoing Nonurgent Percutaneous Coronary Intervention. Circ. Cardiovasc. Interv. 2012, 5, 336–346. [Google Scholar] [CrossRef]
- Siller-Matula, J.M.; Krumphuber, J.; Jilma, B. Pharmacokinetic, Pharmacodynamic and Clinical Profile of Novel Antiplatelet Drugs Targeting Vascular Diseases. Br. J. Pharmacol. 2010, 159, 502–517. [Google Scholar] [CrossRef] [PubMed]
- Brown, M.J.; Bird, S.J.; Watling, S.; Kaleta, H.; Hayes, L.; Eckert, S.; Foyt, H.L. Natural Progression of Diabetic Peripheral Neuropathy in the Zenarestat Study Population. Diabetes Care 2004, 27, 1153–1159. [Google Scholar] [CrossRef]
- Shimoshige, Y.; Minoura, K.; Matsuoka, N.; Takakura, S.; Mutoh, S.; Kamijo, M. Thirteen-Month Inhibition of Aldose Reductase by Zenarestat Prevents Morphological Abnormalities in the Dorsal Root Ganglia of Streptozotocin-Induced Diabetic Rats. Brain Res. 2009, 1247, 182–187. [Google Scholar] [CrossRef]
- Vanhoutte, P.; Amery, A.; Birkenhäger, W.; Breckenridge, A.; Bühler, F.; Distler, A.; Dormandy, J.; Doyle, A.; Frohlich, E.; Hansson, L. Serotoninergic Mechanisms in Hypertension. Focus on the Effects of Ketanserin. Hypertension 1988, 11, 111–133. [Google Scholar] [CrossRef]
- Liechti, M. Psychological and Physiological Effects of MDMA (“Ecstasy”) after Pretreatment with the 5-HT2 Antagonist Ketanserin in Healthy Humans. Neuropsychopharmacology 2000, 23, 396–404. [Google Scholar] [CrossRef] [PubMed]
- Dhillon, S. Carotegrast Methyl: First Approval. Drugs 2022, 82, 1011–1016. [Google Scholar] [CrossRef]
- Sugiura, T.; Kageyama, S.; Andou, A.; Miyazawa, T.; Ejima, C.; Nakayama, A.; Dohi, T.; Eda, H. Oral Treatment with a Novel Small Molecule Alpha 4 Integrin Antagonist, AJM300, Prevents the Development of Experimental Colitis in Mice. J. Crohn’s Colitis 2013, 7, e533–e542. [Google Scholar] [CrossRef]
- Hu, X.; Zhang, J.; Zhang, Y.; Jiao, F.; Wang, J.; Chen, H.; Ouyang, L.; Wang, Y. Dual-Target Inhibitors of Poly (ADP-Ribose) Polymerase-1 for Cancer Therapy: Advances, Challenges, and Opportunities. Eur. J. Med. Chem. 2022, 230, 114094. [Google Scholar] [CrossRef]
- Peng, X.; Pan, W.; Jiang, F.; Chen, W.; Qi, Z.; Peng, W.; Chen, J. Selective PARP1 Inhibitors, PARP1-Based Dual-Target Inhibitors, PROTAC PARP1 Degraders, and Prodrugs of PARP1 Inhibitors for Cancer Therapy. Pharmacol. Res. 2022, 186, 106529. [Google Scholar] [CrossRef]
- Gao, B.; Voskoboynik, M.; Cooper, A.; Wilkinson, K.; Hoon, S.; Hsieh, C.; Cai, S.; Tian, Y.E.; Bao, J.; Ma, N.; et al. A Phase 1 Dose-escalation Study of the Poly(ADP-ribose) Polymerase Inhibitor Senaparib in Australian Patients with Advanced Solid Tumors. Cancer 2023, 129, 1041–1050. [Google Scholar] [CrossRef] [PubMed]
- Gillooly, K.M.; Pulicicchio, C.; Pattoli, M.A.; Cheng, L.; Skala, S.; Heimrich, E.M.; McIntyre, K.W.; Taylor, T.L.; Kukral, D.W.; Dudhgaonkar, S.; et al. Bruton’s Tyrosine Kinase Inhibitor BMS-986142 in Experimental Models of Rheumatoid Arthritis Enhances Efficacy of Agents Representing Clinical Standard-of-Care. PLoS ONE 2017, 12, e0181782. [Google Scholar] [CrossRef] [PubMed]
- Watterson, S.H.; De Lucca, G.V.; Shi, Q.; Langevine, C.M.; Liu, Q.; Batt, D.G.; Beaudoin Bertrand, M.; Gong, H.; Dai, J.; Yip, S.; et al. Discovery of 6-Fluoro-5-(R)-(3-S)-(8-fluoro-1-methyl-2,4-dioxo-1,2-dihydroquinazolin-3(4H)-yl)-2-methylphenyl)-2-(S)-(2-hydroxypropan-2-yl)-2,3,4,9-tetrahydro-1H-carbazole-8-carboxamide (BMS-986142): A Reversible Inhibitor of Bruton’s Tyrosine. J. Med. Chem. 2016, 59, 9173–9200. [Google Scholar] [CrossRef]
- Lee, S.K.; Xing, J.; Catlett, I.M.; Adamczyk, R.; Griffies, A.; Liu, A.; Murthy, B.; Nowak, M. Safety, Pharmacokinetics, and Pharmacodynamics of BMS-986142, a Novel Reversible BTK Inhibitor, in Healthy Participants. Eur. J. Clin. Pharmacol. 2017, 73, 689–698. [Google Scholar] [CrossRef] [PubMed]
- Nencini, A.; Pratelli, C.; Quinn, J.M.; Salerno, M.; Tunici, P.; De Robertis, A.; Valensin, S.; Mennillo, F.; Rossi, M.; Bakker, A.; et al. Structure–Activity Relationship and Properties Optimization of a Series of Quinazoline-2,4-diones as Inhibitors of the Canonical Wnt Pathway. Eur. J. Med. Chem. 2015, 95, 526–545. [Google Scholar] [CrossRef]
- Falsini, M.; Squarcialupi, L.; Catarzi, D.; Varano, F.; Betti, M.; Di Cesare Mannelli, L.; Tenci, B.; Ghelardini, C.; Tanc, M.; Angeli, A.; et al. 3-Hydroxy-1H-quinazoline-2,4-dione as a New Scaffold To Develop Potent and Selective Inhibitors of the Tumor-Associated Carbonic Anhydrases IX and XII. J. Med. Chem. 2017, 60, 6428–6439. [Google Scholar] [CrossRef]
- Yu, C.-W.; Hung, P.-Y.; Yang, H.-T.; Ho, Y.-H.; Lai, H.-Y.; Cheng, Y.-S.; Chern, J.-W. Quinazolin-2,4-dione-based Hydroxamic Acids as Selective Histone Deacetylase-6 Inhibitors for Treatment of Non-Small Cell Lung Cancer. J. Med. Chem. 2019, 62, 857–874. [Google Scholar] [CrossRef] [PubMed]
- El-Adl, K.; El-Helby, A.A.; Sakr, H.; El-Hddad, S.S.A. Design, Synthesis, Molecular Docking, and Anticancer Evaluations of 1-benzylquinazoline-2,4(1H,3H)-dione Bearing Different Moieties as VEGFR-2 Inhibitors. Arch. Pharm. 2020, 353, 2000068. [Google Scholar] [CrossRef] [PubMed]
- Qin, D.; Lin, X.; Liu, Z.; Chen, Y.; Zhang, Z.; Wu, C.; Liu, L.; Pan, Y.; Laquerre, S.; Emery, J.; et al. Discovery of Orally Bioavailable Ligand Efficient Quinazolindiones as Potent and Selective Tankyrases Inhibitors. ACS Med. Chem. Lett. 2021, 12, 1005–1010. [Google Scholar] [CrossRef] [PubMed]
- Kakuta, H.; Tanatani, A.; Nagasawa, K.; Hashimoto, Y. Specific Nonpeptide Inhibitors of Puromycin-Sensitive Aminopeptidase with a 2,4(1H,3H)-Quinazolinedione Skeleton. Chem. Pharm. Bull. 2003, 51, 1273–1282. [Google Scholar] [CrossRef] [PubMed]
- Luo, Z.; Liu, H.; Yu, Y.; Gropler, R.J.; Klein, R.S.; Tu, Z. Synthesis and Evaluation of Highly Selective Quinazoline-2,4-dione Ligands for Sphingosine-1-phosphate Receptor 2. RSC Med. Chem. 2022, 13, 202–207. [Google Scholar] [CrossRef]
- Gazzillo, E.; Terracciano, S.; Ruggiero, D.; Potenza, M.; Chini, M.G.; Lauro, G.; Fischer, K.; Hofstetter, R.K.; Giordano, A.; Werz, O.; et al. Repositioning of Quinazolinedione-Based Compounds on Soluble Epoxide Hydrolase (SEH) through 3D Structure-Based Pharmacophore Model-Driven Investigation. Molecules 2022, 27, 3866. [Google Scholar] [CrossRef]
- Kirincich, S.J.; Xiang, J.; Green, N.; Tam, S.; Yang, H.Y.; Shim, J.; Shen, M.W.H.; Clark, J.D.; McKew, J.C. Benzhydrylquinazolinediones: Novel Cytosolic Phospholipase A2α Inhibitors with Improved Physicochemical Properties. Bioorg. Med. Chem. 2009, 17, 4383–4405. [Google Scholar] [CrossRef]
- Elansary, A.K.; Kadry, H.H.; Ahmed, E.M.; Sonousi, A.S.M. Design, Synthesis, and Biological Activity of Certain Quinazolinedione Derivatives as Potent Phosphodiestrase4 Inhibitors. Med. Chem. Res. 2012, 21, 3557–3567. [Google Scholar] [CrossRef]
- Głowacka, I.E.; Gawron, K.; Piotrowska, D.G.; Graus, M.; Andrei, G.; Schols, D.; Snoeck, R.; Camps, A.; Vanhulle, E.; Vermeire, K. Design and Synthesis of a New Series of Hybrids of Functionalised N1-[(1H-1,2,3-Triazol-4-yl)methyl]quinazoline-2,4-dione with Antiviral Activity against Respiratory Syncytial Virus. Antivir. Res. 2023, 209, 105518. [Google Scholar] [CrossRef]
- Tatarinov, D.A.; Garifullin, B.F.; Belenok, M.G.; Andreeva, O.V.; Strobykina, I.Y.; Shepelina, A.V.; Zarubaev, V.V.; Slita, A.V.; Volobueva, A.S.; Saifina, L.F.; et al. The First 5′-Phosphorylated 1,2,3-Triazolyl Nucleoside Analogues with Uracil and Quinazoline-2,4-Dione Moieties: A Synthesis and Antiviral Evaluation. Molecules 2022, 27, 6214. [Google Scholar] [CrossRef]
- Boshta, N.M.; El-Essawy, F.A.; Alshammari, M.B.; Noreldein, S.G.; Darwesh, O.M. Discovery of Quinazoline-2,4(1H,3H)-Dione Derivatives as Potential Antibacterial Agent: Design, Synthesis, and Their Antibacterial Activity. Molecules 2022, 27, 3853. [Google Scholar] [CrossRef]
- Lansdon, E.B.; Liu, Q.; Leavitt, S.A.; Balakrishnan, M.; Perry, J.K.; Lancaster-Moyer, C.; Kutty, N.; Liu, X.; Squires, N.H.; Watkins, W.J.; et al. Structural and Binding Analysis of Pyrimidinol Carboxylic Acid and N-Hydroxy Quinazolinedione HIV-1 RNase H Inhibitors. Antimicrob. Agents Chemother. 2011, 55, 2905–2915. [Google Scholar] [CrossRef] [PubMed]
- Malik, M.; Marks, K.R.; Mustaev, A.; Zhao, X.; Chavda, K.; Kerns, R.J.; Drlica, K. Fluoroquinolone and Quinazolinedione Activities against Wild-Type and Gyrase Mutant Strains of Mycobacterium Smegmatis. Antimicrob. Agents Chemother. 2011, 55, 2335–2343. [Google Scholar] [CrossRef] [PubMed]
- Enciso, E.; Sarmiento-Sánchez, J.I.; López-Moreno, H.S.; Ochoa-Terán, A.; Osuna-Martínez, U.; Beltrán-López, E. Synthesis of New Quinazolin-2,4-diones as Anti-Leishmania Mexicana Agents. Mol. Divers. 2016, 20, 821–828. [Google Scholar] [CrossRef] [PubMed]
- Mourot, L.; Schmitt, M.; Mouray, E.; Spichty, M.; Florent, I.; Albrecht, S. Structure-Activity Relationship and Molecular Modelling Studies of Quinazolinedione Derivatives MMV665916 as Potential Antimalarial Agent. Bioorg. Med. Chem. 2021, 51, 116513. [Google Scholar] [CrossRef]
- Swann, J.; Corey, V.; Scherer, C.A.; Kato, N.; Comer, E.; Maetani, M.; Antonova-Koch, Y.; Reimer, C.; Gagaring, K.; Ibanez, M.; et al. High-Throughput Luciferase-Based Assay for the Discovery of Therapeutics That Prevent Malaria. ACS Infect. Dis. 2016, 2, 281–293. [Google Scholar] [CrossRef]
- Ji, Q.; Yang, D.; Wang, X.; Chen, C.; Deng, Q.; Ge, Z.; Yuan, L.; Yang, X.; Liao, F. Design, Synthesis and Evaluation of Novel Quinazoline-2,4-dione Derivatives as Chitin Synthase Inhibitors and Antifungal Agents. Bioorg. Med. Chem. 2014, 22, 3405–3413. [Google Scholar] [CrossRef]
- Ali, E.M.H.; Abdel-Maksoud, M.S.; Oh, C.-H. Thieno [2,3-d]pyrimidine as a Promising Scaffold in Medicinal Chemistry: Recent Advances. Bioorg. Med. Chem. 2019, 27, 1159–1194. [Google Scholar] [CrossRef]
- Markham, A. Relugolix: First Global Approval. Drugs 2019, 79, 675–679. [Google Scholar] [CrossRef]
- Nakata, D.; Masaki, T.; Tanaka, A.; Yoshimatsu, M.; Akinaga, Y.; Asada, M.; Sasada, R.; Takeyama, M.; Miwa, K.; Watanabe, T.; et al. Suppression of the Hypothalamic–Pituitary–Gonadal Axis by TAK-385 (Relugolix), a Novel, Investigational, Orally Active, Small Molecule Gonadotropin-Releasing Hormone (GnRH) Antagonist: Studies in Human GnRH Receptor Knock-in Mice. Eur. J. Pharmacol. 2014, 723, 167–174. [Google Scholar] [CrossRef]
- Miwa, K.; Hitaka, T.; Imada, T.; Sasaki, S.; Yoshimatsu, M.; Kusaka, M.; Tanaka, A.; Nakata, D.; Furuya, S.; Endo, S.; et al. Discovery of 1-{4-[1-(2,6-Difluorobenzyl)-5-[(dimethylamino)methyl]-3-(6-methoxypyridazin-3-yl)-2,4-dioxo-1,2,3,4-tetrahydrothieno [2,3-d]pyrimidin-6-yl]phenyl}-3-methoxyurea (TAK-385) as a Potent, Orally Active, Non-Peptide Antagonist of the Human Gonad. J. Med. Chem. 2011, 54, 4998–5012. [Google Scholar] [CrossRef]
- Zou, F.; Wang, Y.; Yu, D.; Liu, C.; Lu, J.; Zhao, M.; Ma, M.; Wang, W.; Jiang, W.; Gao, Y.; et al. Discovery of the Thieno [2,3-d]pyrimidine-2,4-dione Derivative 21a: A Potent and Orally Bioavailable Gonadotropin-Releasing Hormone Receptor Antagonist. Eur. J. Med. Chem. 2022, 242, 114679. [Google Scholar] [CrossRef] [PubMed]
- Guo, Z.; Chen, Y.; Wu, D.; Zhu, Y.-F.; Struthers, R.S.; Saunders, J.; Xie, Q.; Chen, C. Synthesis and Structure–Activity Relationships of Thieno [2,3-d]pyrimidine-2,4-dione Derivatives as Potent GnRH Receptor Antagonists. Bioorg. Med. Chem. Lett. 2003, 13, 3617–3622. [Google Scholar] [CrossRef] [PubMed]
- Sasaki, S.; Cho, N.; Nara, Y.; Harada, M.; Endo, S.; Suzuki, N.; Furuya, S.; Fujino, M. Discovery of a Thieno [2,3-d]pyrimidine-2,4-dione Bearing a p-Methoxyureidophenyl Moiety at the 6-Position: A Highly Potent and Orally Bioavailable Non-Peptide Antagonist for the Human Luteinizing Hormone-Releasing Hormone Receptor. J. Med. Chem. 2003, 46, 113–124. [Google Scholar] [CrossRef] [PubMed]
- Alkhouri, N.; Lawitz, E.; Noureddin, M.; DeFronzo, R.; Shulman, G.I. GS-0976 (Firsocostat): An Investigational Liver-Directed Acetyl-CoA Carboxylase (ACC) Inhibitor for the Treatment of Non-Alcoholic Steatohepatitis (NASH). Expert Opin. Investig. Drugs 2020, 29, 135–141. [Google Scholar] [CrossRef]
- Loomba, R.; Noureddin, M.; Kowdley, K.V.; Kohli, A.; Sheikh, A.; Neff, G.; Bhandari, B.R.; Gunn, N.; Caldwell, S.H.; Goodman, Z.; et al. Combination Therapies Including Cilofexor and Firsocostat for Bridging Fibrosis and Cirrhosis Attributable to NASH. Hepatology 2021, 73, 625–643. [Google Scholar] [CrossRef]
- Alkhouri, N.; Herring, R.; Kabler, H.; Kayali, Z.; Hassanein, T.; Kohli, A.; Huss, R.S.; Zhu, Y.; Billin, A.N.; Damgaard, L.H.; et al. Safety and Efficacy of Combination Therapy with Semaglutide, Cilofexor and Firsocostat in Patients with Non-Alcoholic Steatohepatitis: A Randomised, Open-Label Phase II Trial. J. Hepatol. 2022, 77, 607–618. [Google Scholar] [CrossRef]
- Wang, D.-W.; Lin, H.-Y.; Cao, R.-J.; Yang, S.-G.; Chen, Q.; Hao, G.-F.; Yang, W.-C.; Yang, G.-F. Synthesis and Herbicidal Evaluation of Triketone-Containing Quinazoline-2,4-diones. J. Agric. Food Chem. 2014, 62, 11786–11796. [Google Scholar] [CrossRef]
- Wang, D.-W.; Zhang, H.; Yu, S.-Y.; Zhang, R.-B.; Liang, L.; Wang, X.; Yang, H.-Z.; Xi, Z. Discovery of a Potent Thieno [2,3-d]Pyrimidine-2,4-dione-Based Protoporphyrinogen IX Oxidase Inhibitor through an In Silico Structure-Guided Optimization Approach. J. Agric. Food Chem. 2021, 69, 14115–14125. [Google Scholar] [CrossRef]
- Gheidari, D.; Mehrdad, M.; Maleki, S. The Quinazoline-2,4(1H,3H)-diones Skeleton: A Key Intermediate in Drug Synthesis. Sustain. Chem. Pharm. 2022, 27, 100696. [Google Scholar] [CrossRef]
- Gheidari, D.; Mehrdad, M.; Maleki, S. Recent Advances in Synthesis of Quinazoline-2,4(1H,3H)-diones: Versatile Building Blocks in N-Heterocyclic Compounds. Appl. Organomet. Chem. 2022, 36, e6631. [Google Scholar] [CrossRef]
- Govindan, K.; Jayaram, A.; Duraisamy, T.; Chen, N.-Q.; Lin, W.-Y. Metal-Free N–H/C–H Carbonylation by Phenyl Isocyanate: Divergent Synthesis of Six-Membered N-Heterocycles. J. Org. Chem. 2022, 87, 8719–8729. [Google Scholar] [CrossRef] [PubMed]
- Dong, X.; Ma, P.; Zhang, T.; Jalani, H.B.; Li, G.; Lu, H. Iridium-Catalyzed C–H Amination of Weinreb Amides: A Facile Pathway toward Anilines and Quinazolin-2,4-diones. J. Org. Chem. 2020, 85, 13096–13107. [Google Scholar] [CrossRef] [PubMed]
- Ghosh, A.; Shee, S.; Biju, A.T. A Benzannulation Strategy for Rapid Access to Quinazoline-2,4-diones via Oxidative N-Heterocyclic Carbene Catalysis. Org. Lett. 2022, 24, 2772–2777. [Google Scholar] [CrossRef] [PubMed]
- Abu-Hashem, A.A.; Abu-Zied, K.M.; El-Shehry, M.F. Synthetic Utility of Bifunctional Thiophene Derivatives and Antimicrobial Evaluation of the Newly Synthesized Agents. Monatsh. Chem. 2011, 142, 539–545. [Google Scholar] [CrossRef]
- Dewal, M.B.; Wani, A.S.; Vidaillac, C.; Oupický, D.; Rybak, M.J.; Firestine, S.M. Thieno [2,3-d]pyrimidinedione Derivatives as Antibacterial Agents. Eur. J. Med. Chem. 2012, 51, 145–153. [Google Scholar] [CrossRef] [PubMed]
- Lagardère, P.; Fersing, C.; Masurier, N.; Lisowski, V. Thienopyrimidine: A Promising Scaffold to Access Anti-Infective Agents. Pharmaceuticals 2021, 15, 35. [Google Scholar] [CrossRef]
- El-Meligie, S.E.M.; Khalil, N.A.; El-Nassan, H.B.; Ibraheem, A.A.M. New Synthetic Approaches to Thieno [3,2-d]pyrimidine and Thieno [3,4-b]pyridine Derivatives. Chem. Pap. 2020, 74, 2501–2514. [Google Scholar] [CrossRef]
- Ravi, O.; Ramaraju, A.; Sridhar, B.; Bathula, S.R. Copper-Catalyzed Domino C−C Bond Cleavage of 2,3-Unsubstituted Indoles/Indolines and Oxindoles via Oxidation and Directed Insertion of 2-Aminopyridines: Direct Access to Quinazolinediones. Adv. Synth. Catal. 2018, 360, 3009–3014. [Google Scholar] [CrossRef]
- Kasatkina, S.O.; Geyl, K.K.; Baykov, S.V.; Novikov, M.S.; Boyarskiy, V.P. “Urea to Urea” Approach: Access to Unsymmetrical Ureas Bearing Pyridyl Substituents. Adv. Synth. Catal. 2022, 364, 1295–1304. [Google Scholar] [CrossRef]
- Kasatkina, S.O.; Geyl, K.K.; Baykov, S.V.; Boyarskaya, I.A.; Boyarskiy, V.P. Catalyst-Free Synthesis of Substituted Pyridin-2-yl, Quinolin-2-yl, and Isoquinolin-1-yl Carbamates from the Corresponding Hetaryl Ureas and Alcohols. Org. Biomol. Chem. 2021, 19, 6059–6065. [Google Scholar] [CrossRef]
- Dobrynin, M.V.; Kasatkina, S.O.; Baykov, S.V.; Savko, P.Y.; Antonov, N.S.; Mikherdov, A.S.; Boyarskiy, V.P.; Islamova, R.M. Deprotonated Diaminocarbene Platinum Complexes for Thermoresponsive Luminescent Silicone Materials: Both Catalysts and Luminophores. Dalt. Trans. 2021, 50, 14994–14999. [Google Scholar] [CrossRef] [PubMed]
- Geyl, K.K.; Baykov, S.V.; Kasatkina, S.O.; Savko, P.Y.; Boyarskiy, V.P. Reaction of Coordinated Isocyanides with Substituted N-(2-Pyridyl) Ureas as a Route to New Cyclometallated Pd(II) Complexes. J. Organomet. Chem. 2022, 980–981, 122518. [Google Scholar] [CrossRef]
- Geyl, K.K.; Baykov, S.V.; Kalinin, S.A.; Bunev, A.S.; Troshina, M.A.; Sharonova, T.V.; Skripkin, M.Y.; Kasatkina, S.O.; Presnukhina, S.I.; Shetnev, A.A.; et al. Synthesis, Structure, and Antiproliferative Action of 2-Pyridyl Urea-Based Cu(II) Complexes. Biomedicines 2022, 10, 461. [Google Scholar] [CrossRef]
- Geyl, K.K.; Baykova, S.O.; Andoskin, P.A.; Sharoyko, V.V.; Eliseeva, A.A.; Baykov, S.V.; Semenov, K.N.; Boyarskiy, V.P. Palladium(II) and Platinum(II) Deprotonated Diaminocarbene Complexes Based on N-(2-Pyridyl)Ureas with Oxadiazole Periphery. Inorganics 2022, 10, 247. [Google Scholar] [CrossRef]
- Rassadin, V.A.; Zimin, D.P.; Raskil’dina, G.Z.; Ivanov, A.Y.; Boyarskiy, V.P.; Zlotskii, S.S.; Kukushkin, V.Y. Solvent- and Halide-Free Synthesis of Pyridine-2-yl Substituted Ureas through Facile C–H Functionalization of Pyridine N-Oxides. Green Chem. 2016, 18, 6630–6636. [Google Scholar] [CrossRef]
- Geyl, K.; Baykov, S.; Tarasenko, M.; Zelenkov, L.E.; Matveevskaya, V.; Boyarskiy, V.P. Convenient Entry to N-Pyridinylureas with Pharmaceutically Privileged Oxadiazole Substituents via the Acid-Catalyzed C–H Activation of N-Oxides. Tetrahedron Lett. 2019, 60, 151108. [Google Scholar] [CrossRef]
- Baykov, S.; Mikherdov, A.; Novikov, A.; Geyl, K.; Tarasenko, M.; Gureev, M.; Boyarskiy, V. π–π Noncovalent Interaction Involving 1,2,4- and 1,3,4-Oxadiazole Systems: The Combined Experimental, Theoretical, and Database Study. Molecules 2021, 26, 5672. [Google Scholar] [CrossRef]
- Baykov, S.V.; Boyarskiy, V.P. Metal-Free Functionalization of Azine N-Oxides with Electrophilic Reagents. Chem. Heterocycl. Compd. 2020, 56, 814–823. [Google Scholar] [CrossRef]
- Tian, X.-C.; Huang, X.; Wang, D.; Gao, F. Eco-Efficient One-Pot Synthesis of Quinazoline-2,4(1H,3H)-Diones at Room Temperature in Water. Chem. Pharm. Bull. 2014, 62, 824–829. [Google Scholar] [CrossRef]
- Inamoto, K.; Araki, Y.; Kikkawa, S.; Yonemoto, M.; Tanaka, Y.; Kondo, Y. Organocatalytic Functionalization of Heteroaromatic N-Oxides with C-Nucleophiles Using in Situ Generated Onium Amide Bases. Org. Biomol. Chem. 2013, 11, 4438. [Google Scholar] [CrossRef]
- Wengryniuk, S.E.; Weickgenannt, A.; Reiher, C.; Strotman, N.A.; Chen, K.; Eastgate, M.D.; Baran, P.S. Regioselective Bromination of Fused Heterocyclic N-Oxides. Org. Lett. 2013, 15, 792–795. [Google Scholar] [CrossRef] [PubMed]
- Hu, W.; Zhang, F.; Chen, C.; Qi, T.; Shen, Y.; Qian, G.; Rong, Z. Metal-Free C8–H Functionalization of Quinoline N-Oxides with Ynamides. Chem. Commun. 2021, 57, 6995–6998. [Google Scholar] [CrossRef] [PubMed]
- Sheldrick, G.M. SHELXT—Integrated Space-Group and Crystal-Structure Determination. Acta Crystallogr. Sect. A Found. Adv. 2015, 71, 3–8. [Google Scholar] [CrossRef] [PubMed]
- Sheldrick, G.M. Crystal Structure Refinement with SHELXL. Acta Crystallogr. Sect. C Struct. Chem. 2015, 71, 3–8. [Google Scholar] [CrossRef] [PubMed]
- Dolomanov, O.V.; Bourhis, L.J.; Gildea, R.J.; Howard, J.A.K.; Puschmann, H. OLEX2: A Complete Structure Solution, Refinement and Analysis Program. J. Appl. Crystallogr. 2009, 42, 339–341. [Google Scholar] [CrossRef]
- CrysAlis Pro. Data Collection and Processing Software for Agilent X-ray Diffractometers; Aglient Technologies: Yarnton, UK, 2013. [Google Scholar]

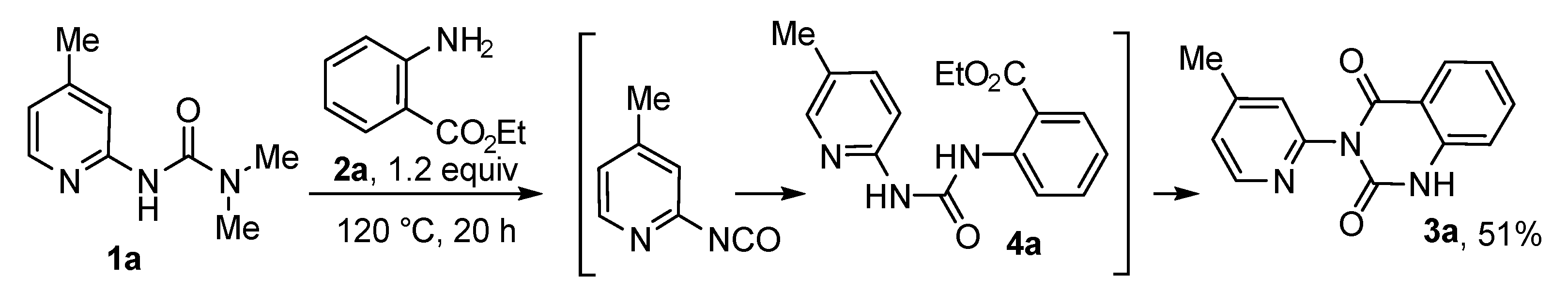

| Entry | Equiv. of 2a | Solvent | Temperature, °C | Time, h | Yield, % |
|---|---|---|---|---|---|
| 1 | 1.2 | DMF | 120 | 24 | 52 |
| 2 | 1.2 | DMF | 140 | 24 | 50 |
| 3 | 1.5 | DMF | 120 | 20 | 58 |
| 4 | 2.0 | DMF | 120 | 20 | 62 |
| 5 | 5.0 | DMF | 120 | 20 | 63 |
| 6 | 5.0 | neat | 120 | 20 | 70 |
| 7 | 10.0 | neat | 120 | 20 | 59 |
| 8 * | 1.5 | DMF | 120 | 20 | 46 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Baykova, S.O.; Geyl, K.K.; Baykov, S.V.; Boyarskiy, V.P. Synthesis of 3-(Pyridin-2-yl)quinazolin-2,4(1H,3H)-diones via Annulation of Anthranilic Esters with N-pyridyl Ureas. Int. J. Mol. Sci. 2023, 24, 7633. https://doi.org/10.3390/ijms24087633
Baykova SO, Geyl KK, Baykov SV, Boyarskiy VP. Synthesis of 3-(Pyridin-2-yl)quinazolin-2,4(1H,3H)-diones via Annulation of Anthranilic Esters with N-pyridyl Ureas. International Journal of Molecular Sciences. 2023; 24(8):7633. https://doi.org/10.3390/ijms24087633
Chicago/Turabian StyleBaykova, Svetlana O., Kirill K. Geyl, Sergey V. Baykov, and Vadim P. Boyarskiy. 2023. "Synthesis of 3-(Pyridin-2-yl)quinazolin-2,4(1H,3H)-diones via Annulation of Anthranilic Esters with N-pyridyl Ureas" International Journal of Molecular Sciences 24, no. 8: 7633. https://doi.org/10.3390/ijms24087633
APA StyleBaykova, S. O., Geyl, K. K., Baykov, S. V., & Boyarskiy, V. P. (2023). Synthesis of 3-(Pyridin-2-yl)quinazolin-2,4(1H,3H)-diones via Annulation of Anthranilic Esters with N-pyridyl Ureas. International Journal of Molecular Sciences, 24(8), 7633. https://doi.org/10.3390/ijms24087633







