IL-1 Mediates Chronic Stress-Induced Hyperalgesia Accompanied by Microglia and Astroglia Morphological Changes in Pain-Related Brain Regions in Mice
Abstract
1. Introduction
2. Results
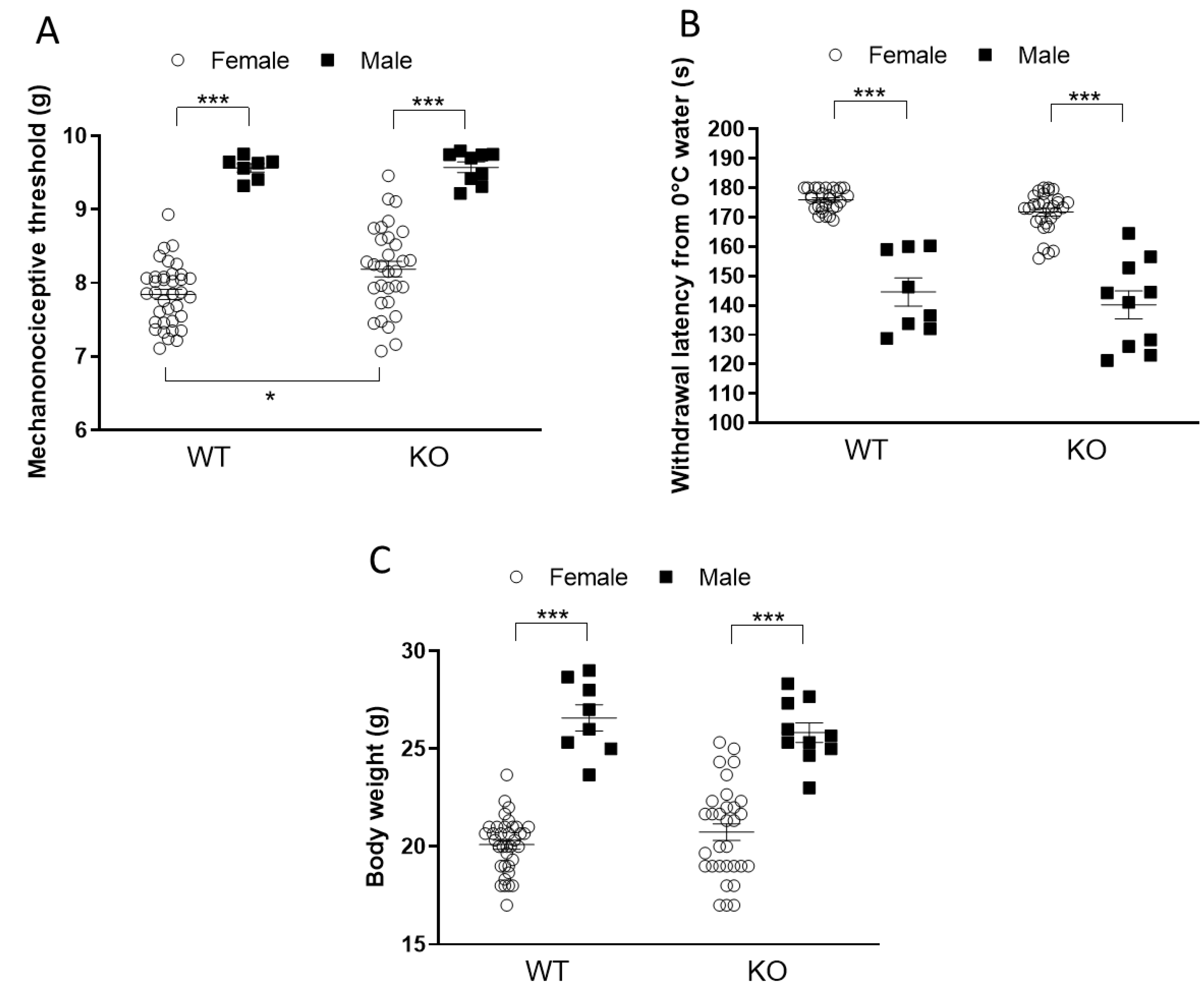
2.1. Female Mice Are More Sensitive to Mechanical Stimuli but Less Sensitive to Cold Stimuli than Males
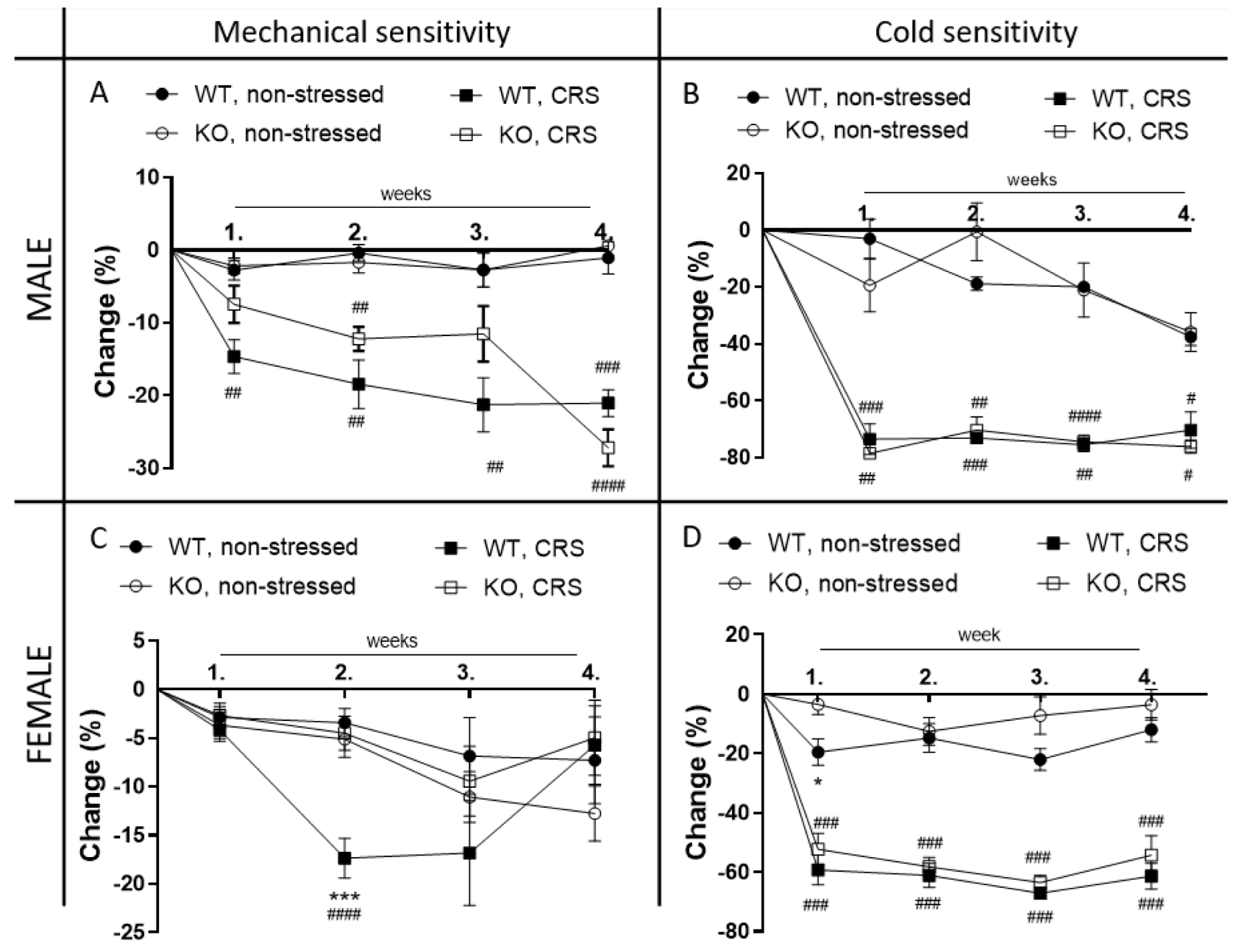
2.2. Chronic Restraint Stress (CRS)-Induced Mechanical Hyperalgesia, but Not Cold Hyperalgesia, Is Significantly Reduced in Female IL-1 KO Mice
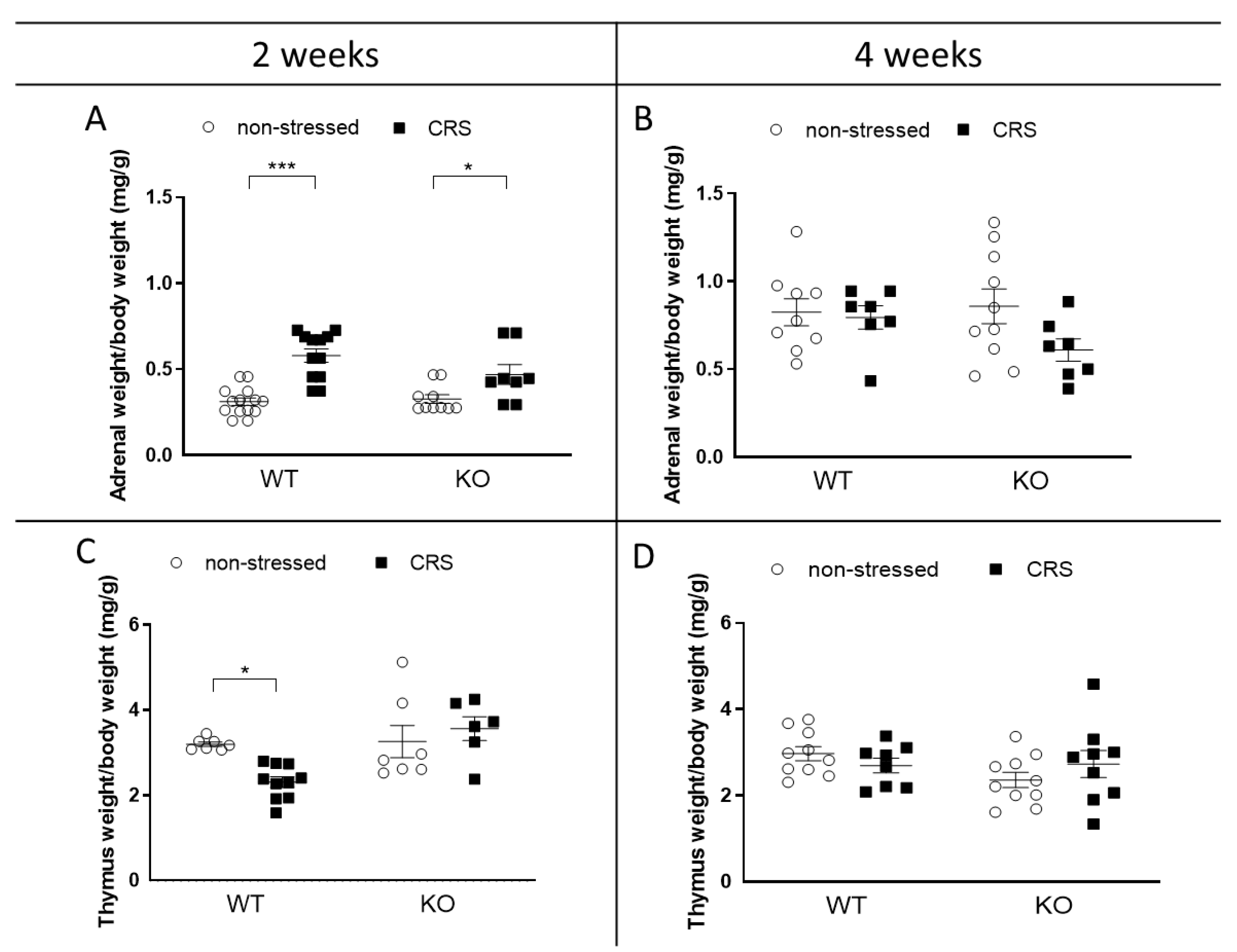
2.3. CRS-Induced Relative Thymus and Adrenal Weight Changes at 2 Weeks Vanished after 4 Weeks of CRS
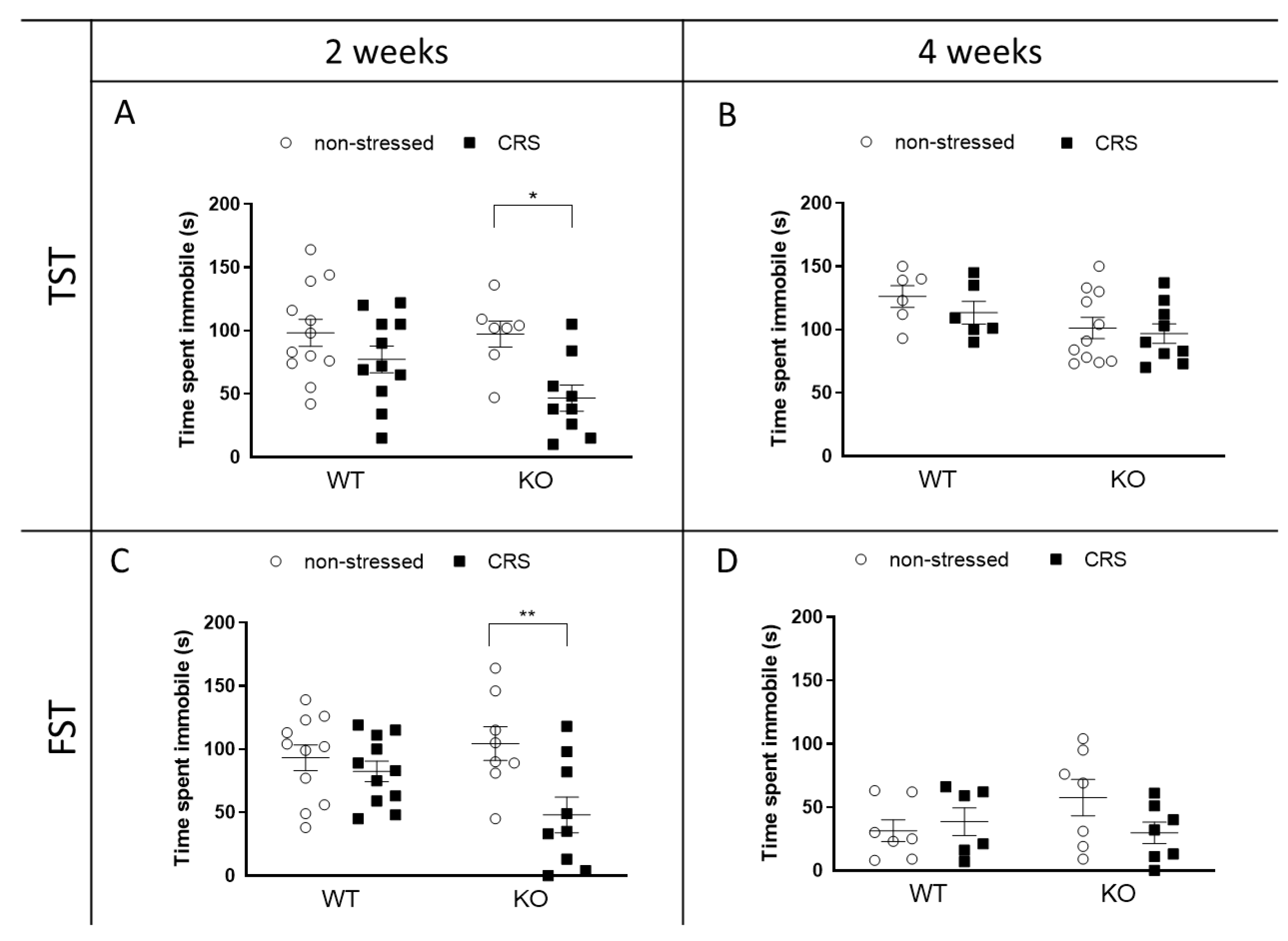
2.4. CRS-Induced Immobility Decreases in KO but Not in WT Mice after 2 Weeks of CRS
2.4.1. Depression-like Behavior Changes following CRS in Female Animals
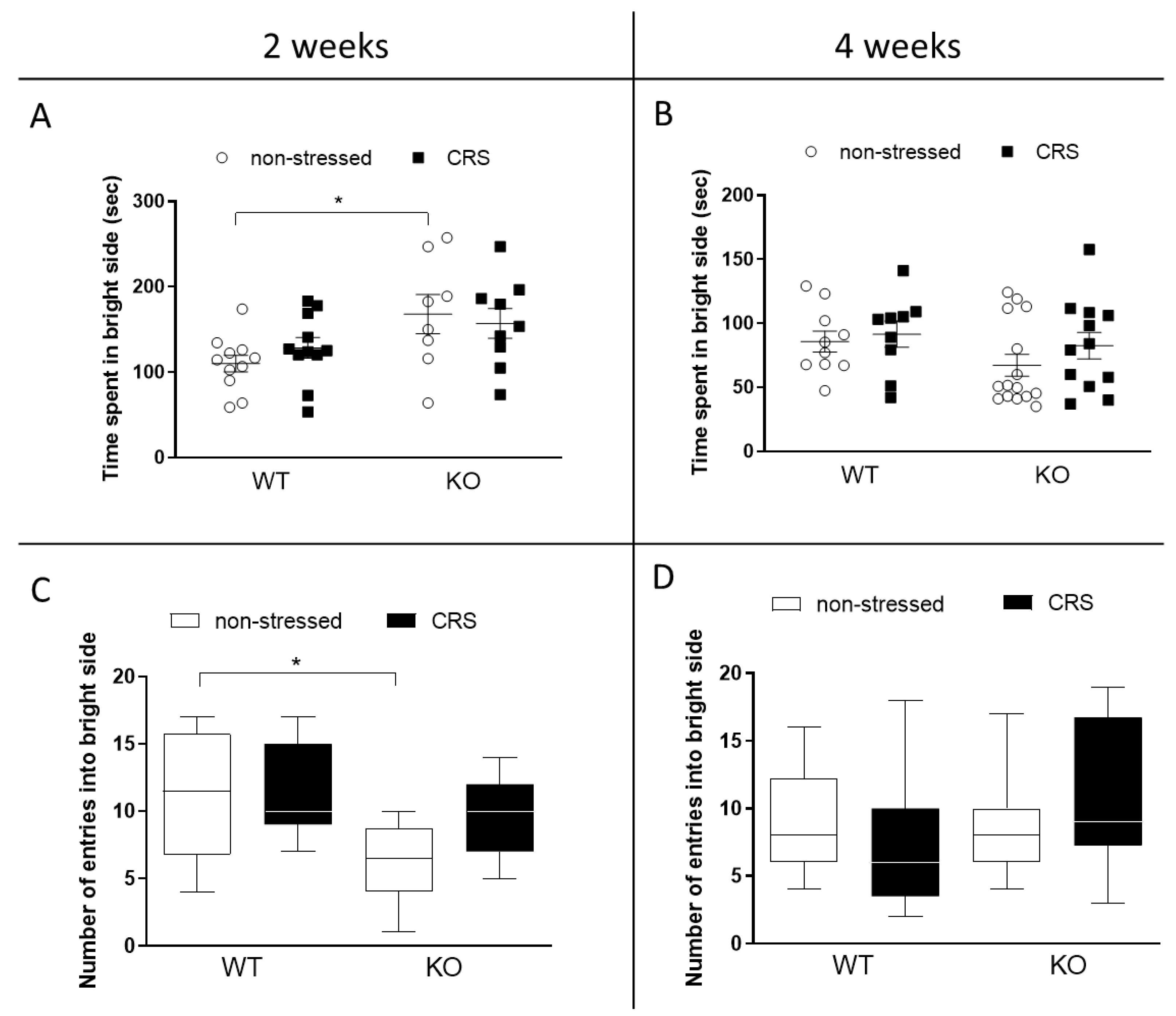
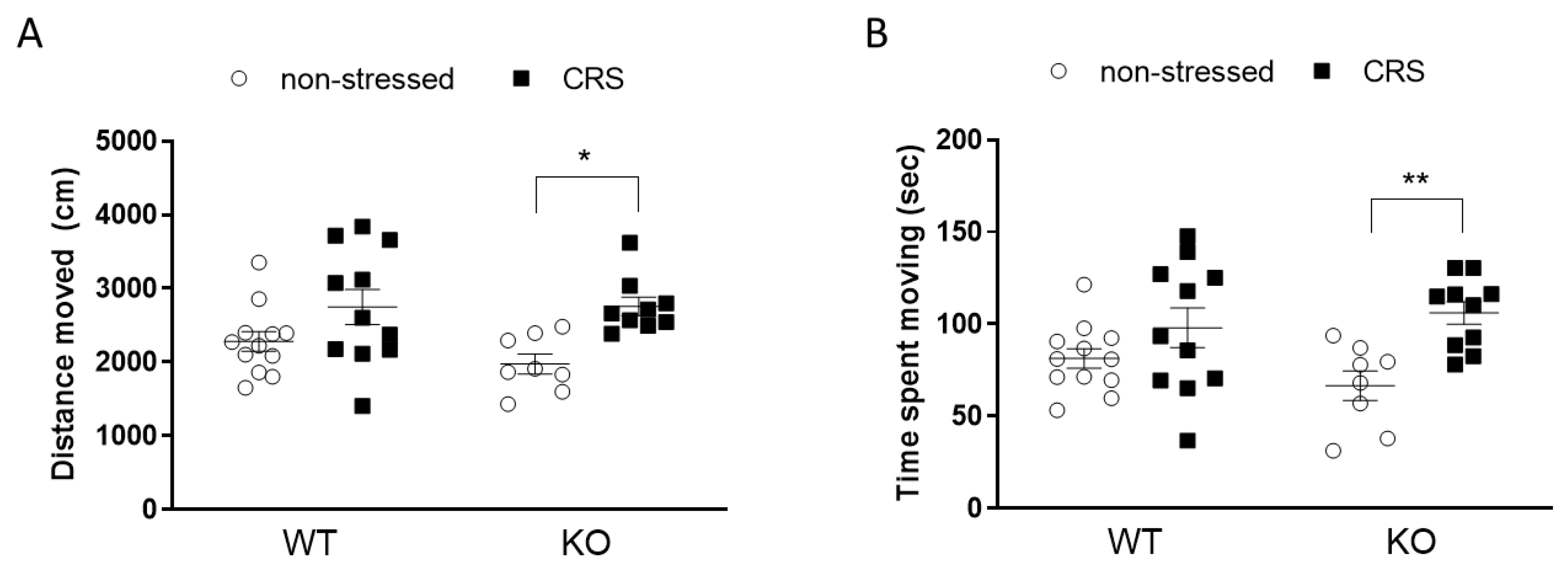
2.4.2. CRS Induced No Alteration in Anxiety-like Behaviors in Female Mice
Light–Dark Box (LDB) Test
Open Field Test (OFT)
2.5. CRS-Induced Microglia and Astrocyte Changes in Pain-Related Brain Regions of WT and IL-1 KO Female Mice
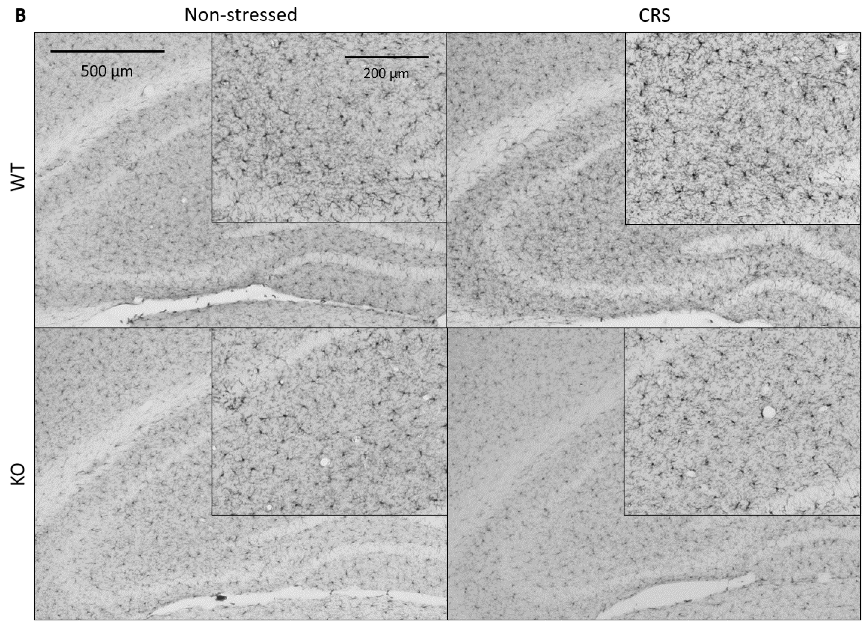
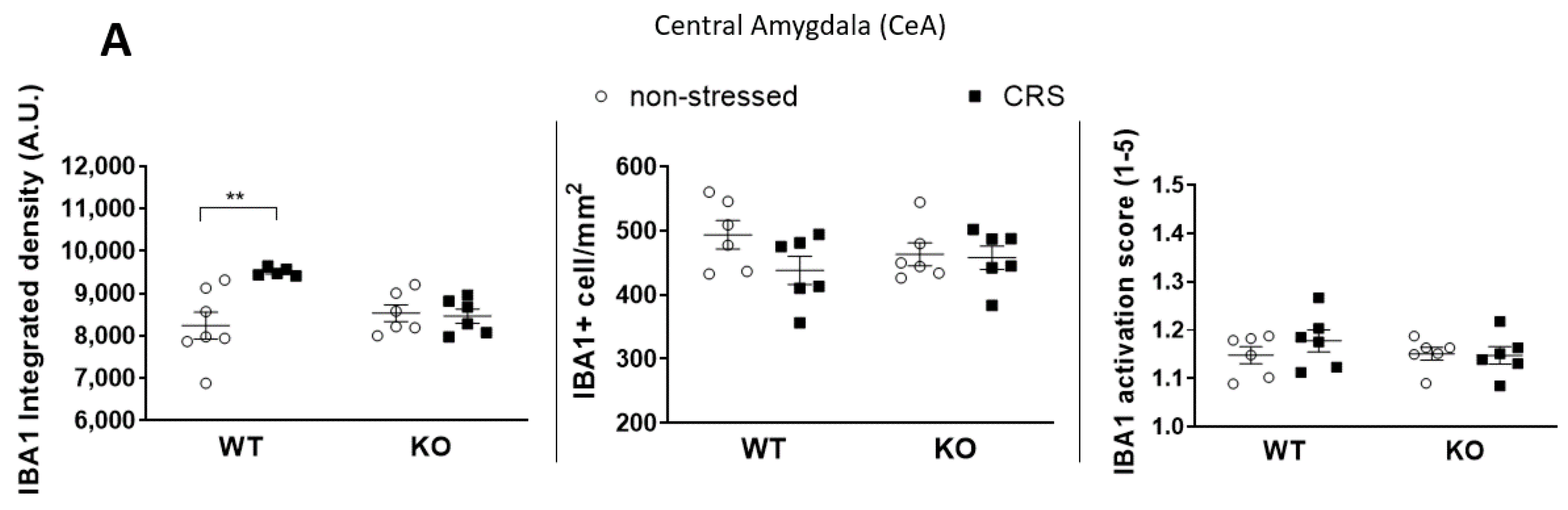
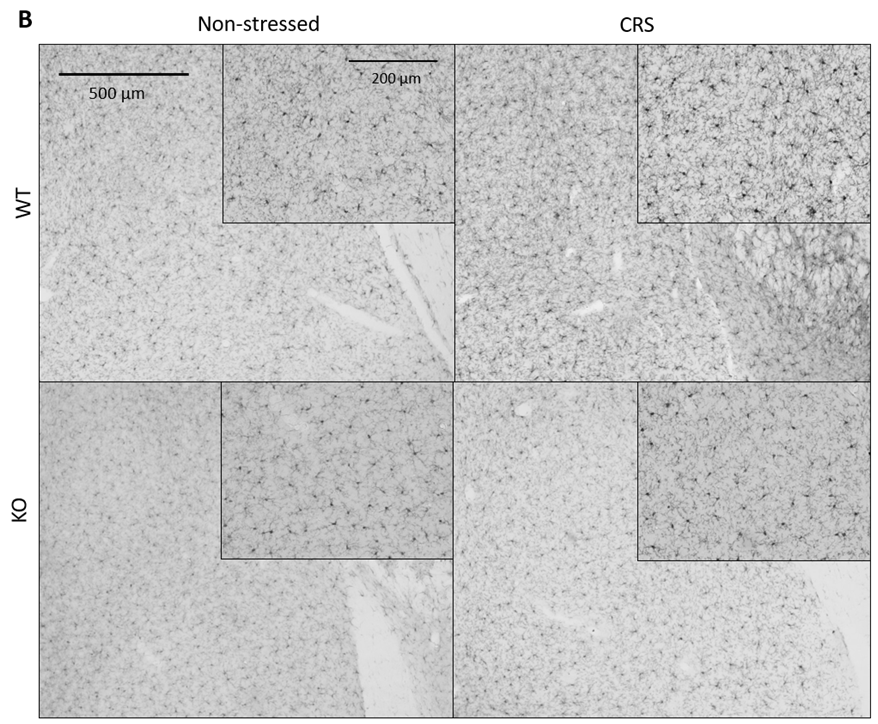
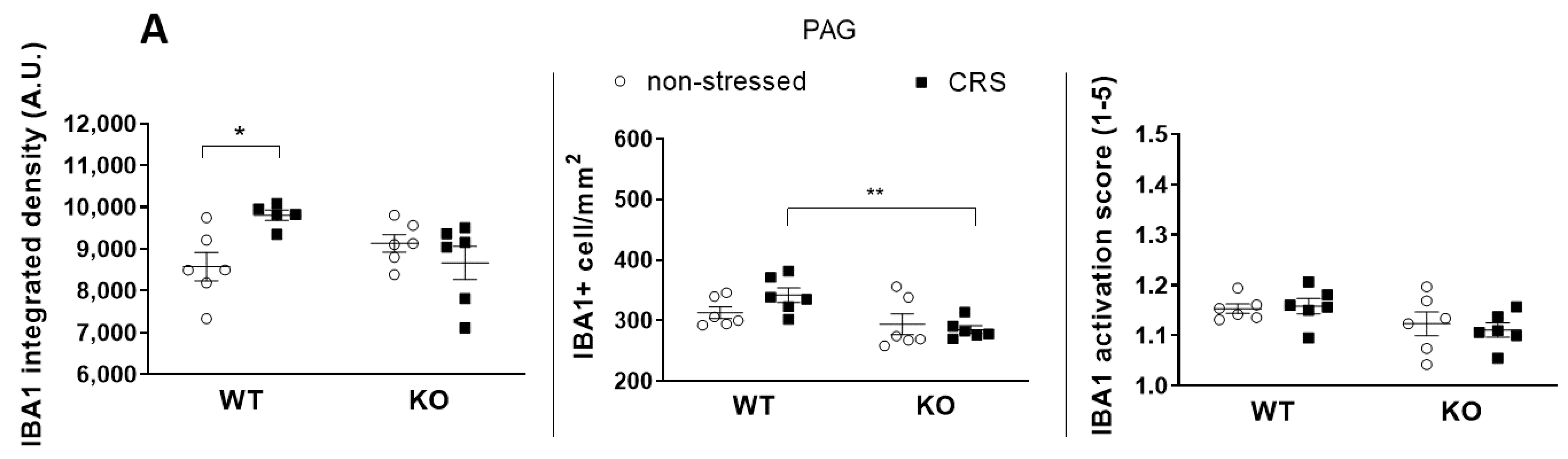
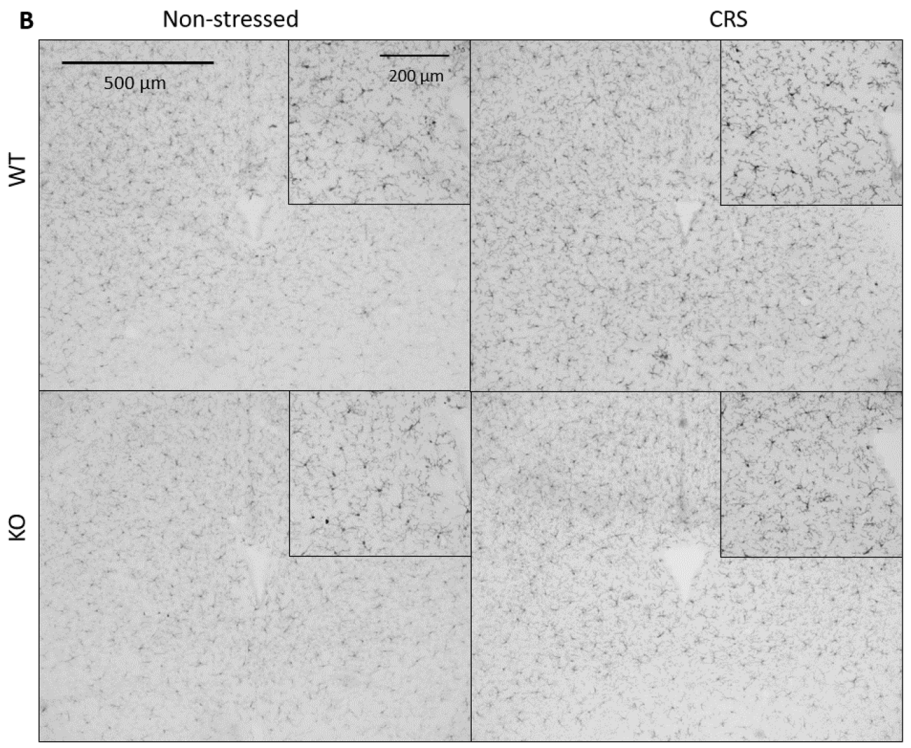
2.5.1. CRS-Induced Higher Integrated Density of Ionized Calcium-Binding Adaptor Molecule 1 (IBA1)-Positive Microglia in Pain-Related Brain Areas with a Greater IBA1+ Microglia Cell Count in the Periaqueductal Gray (PAG)
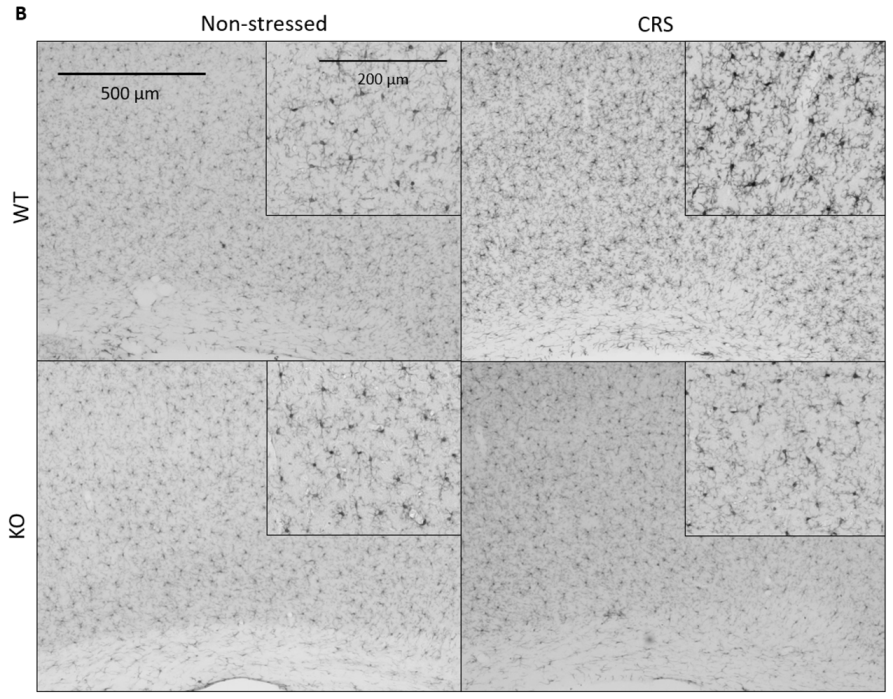
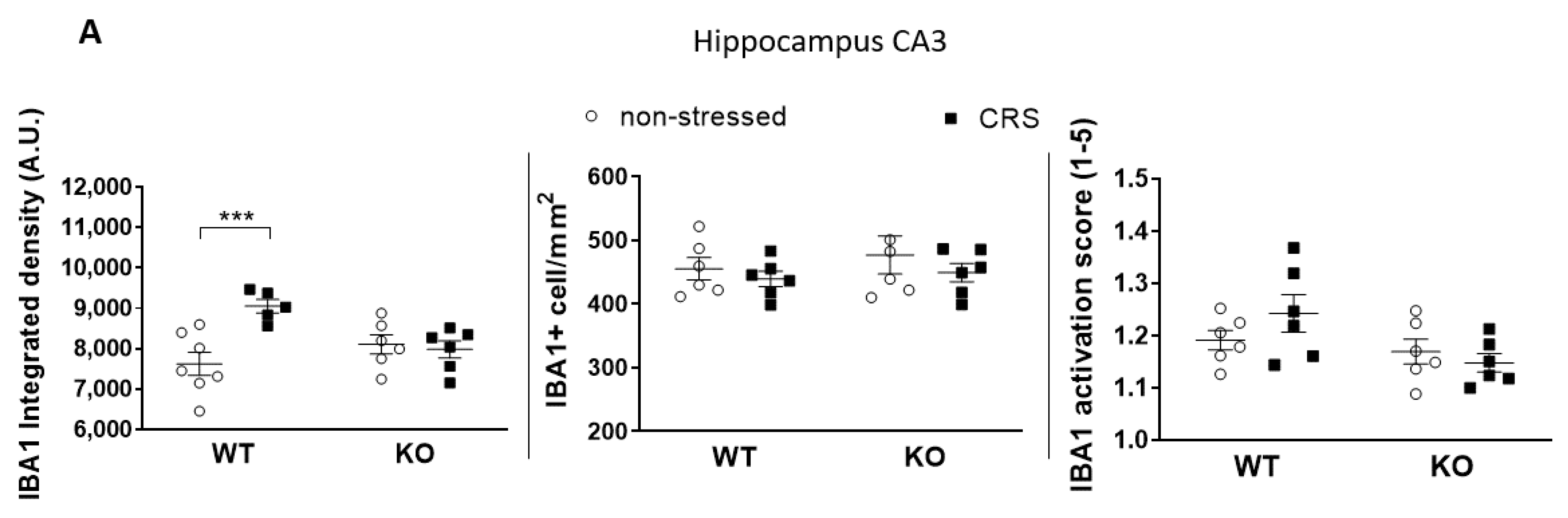
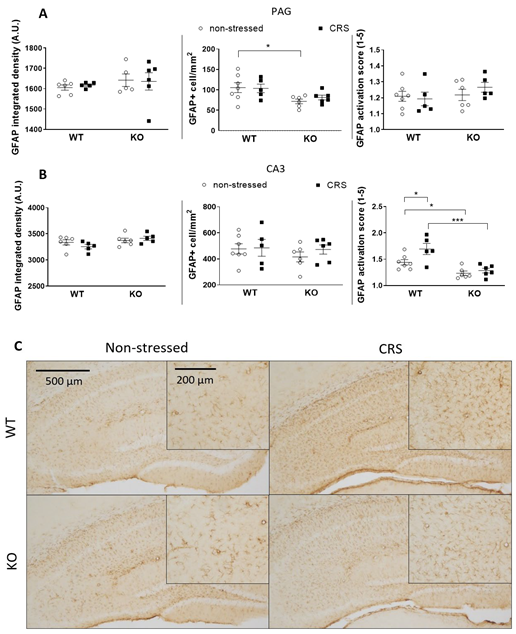
2.5.2. CRS-Induced Glial Fibrillary Acidic Protein (GFAP)-Positive Astroglia Activation in the Hippocampal CA3
3. Discussion
4. Materials and Methods
4.1. Animals
4.2. The CRS Paradigm
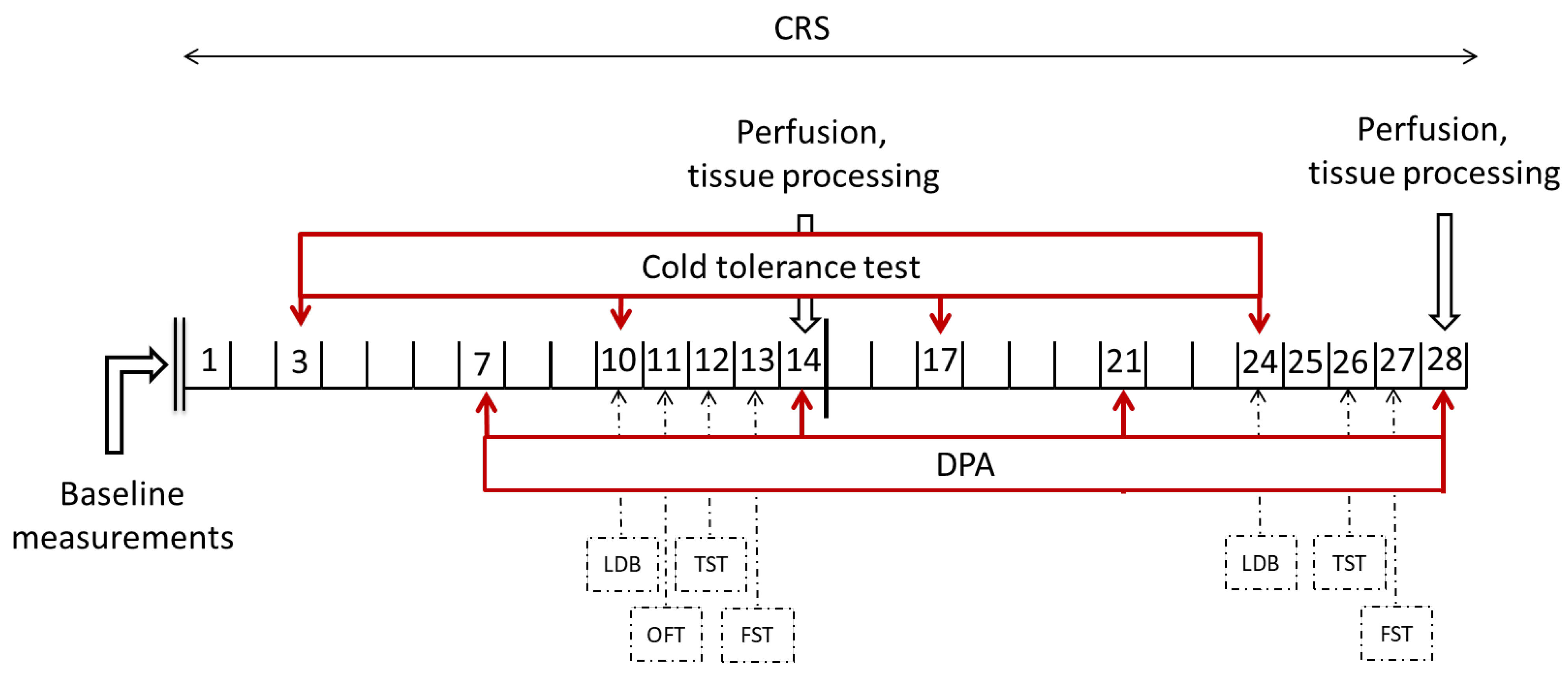
4.3. Experimental Design
4.4. Nociceptive Measurements
4.4.1. Dynamic Plantar Aesthesiometry (DPA)
4.4.2. Cold Tolerance Test
4.5. Behavioral Tests
4.5.1. Open Field Test (OFT)
4.5.2. Light–Dark Box Test (LDB)
4.5.3. Tail Suspension Test (TST)
4.5.4. Forced Swim Test (FST)
4.6. Perfusion and Tissue Processing
4.7. Immunohistochemistry: IBA1
4.8. Immunohistochemistry: GFAP
4.9. Immunohistochemistry Data Analysis
4.10. Statistical Analysis
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| ANOVA | analysis of variance |
| CA3 | hippocampus cornu ammonis area 3 |
| CeA | central nucleus of amygdala |
| CRPS | complex regional pain syndrome |
| CRS | chronic restraint stress |
| DAB | diaminobenzidine |
| DPA | dynamic plantar esthesiometry |
| FST | forced swim test |
| GFAP | glial fibrillary acidic protein |
| IBA1 | ionized calcium-binding adapter protein 1 |
| ICD | International Classification of Disease |
| IL-1 | interleukine-1 |
| IL-1R1 | interleukine-1 receptor 1 |
| IL-6 | interleukine-6 |
| IL-8 | interlekuine-8 |
| KO | knock-out |
| LDB | light–dark box test |
| MAP | mitogen-activated protein kinase |
| OFT | open field test |
| PAG | periaqueductal gray matter |
| PBS | phosphate-buffered saline |
| PFA | paraformaldehyde |
| PKC | protein kinase C |
| S1HL | primary somatosensory cortex—representation of the hind limb |
| TST | tail suspension test |
| WHO | World Health Organization |
| WT | wild-type |
References
- National Institute for Health and Care Excellence. NICE Chronic Pain (Primary and Secondary) in over 16s: Assessment of All Chronic Pain and Management of Chronic Primary Pain; National Institute for Health and Care Excellence (NICE): London, UK, 2021. [Google Scholar]
- Scholz, J.; Finnerup, N.B.; Attal, N.; Aziz, Q.; Baron, R.; Bennett, M.I.; Benoliel, R.; Cohen, M.; Cruccu, G.; Davis, K.D.; et al. The IASP Classification of Chronic Pain for ICD-11: Chronic Neuropathic Pain. Pain 2019, 160, 53. [Google Scholar] [CrossRef]
- Nicholas, M.; Vlaeyen, J.W.S.; Rief, W.; Barke, A.; Aziz, Q.; Benoliel, R.; Cohen, M.; Evers, S.; Giamberardino, M.A.; Goebel, A.; et al. The IASP Classification of Chronic Pain for ICD-11: Chronic Primary Pain. Pain 2019, 160, 28–37. [Google Scholar] [CrossRef] [PubMed]
- Gatzounis, R.; den Hollander, M.; Meulders, A. Optimizing Long-Term Outcomes of Exposure for Chronic Primary Pain from the Lens of Learning Theory. J. Pain 2021, 22, 1315–1327. [Google Scholar] [CrossRef] [PubMed]
- Vincent, A.; Lahr, B.D.; Wolfe, F.; Clauw, D.J.; Whipple, M.O.; Oh, T.H.; Barton, D.L.; St. Sauver, J. Prevalence of Fibromyalgia: A Population-Based Study in Olmsted County, Minnesota, Utilizing the Rochester Epidemiology Project. Arthritis Care Res. 2013, 65, 786–792. [Google Scholar] [CrossRef] [PubMed]
- Clinical Manifestations and Diagnosis of Fibromyalgia in Adults—UpToDate. Available online: https://www.uptodate.com/contents/clinical-manifestations-and-diagnosis-of-fibromyalgia-in-adults?search=fibromyalgia&source=search_result&selectedTitle=1~150&usage_type=default&display_rank=1 (accessed on 7 June 2022).
- Treatment of Irritable Bowel Syndrome in Adults—UpToDate. Available online: https://www.uptodate.com/contents/treatment-of-irritable-bowel-syndrome-in-adults?search=ibs&source=search_result&selectedTitle=1~150&usage_type=default&display_rank=1 (accessed on 7 June 2022).
- Bellato, E.; Marini, E.; Castoldi, F.; Barbasetti, N.; Mattei, L.; Bonasia, D.E.; Blonna, D. Fibromyalgia Syndrome: Etiology, Pathogenesis, Diagnosis, and Treatment. Pain Res. Treat. 2012, 2012, 17. [Google Scholar] [CrossRef]
- Marques, A.P.; de Sousa do Espírito Santo, A.; Berssaneti, A.A.; Matsutani, L.A.; Yuan, S.L.K. Prevalence of Fibromyalgia: Literature Review Update. Rev. Bras. Reumatol. 2017, 57, 356–363. [Google Scholar] [CrossRef]
- Sluka, K.A.; Clauw, D.J. Neurobiology of Fibromyalgia and Chronic Widespread Pain. Neuroscience 2016, 338, 114–129. [Google Scholar] [CrossRef]
- Bennett, R.M.; Jones, K.D.; Aebischer, J.H.; St. John, A.W.; Friend, R. Which Symptoms Best Distinguish Fibromyalgia Patients from Those with Other Chronic Pain Disorders? J. Eval. Clin. Pract. 2022, 28, 225–234. [Google Scholar] [CrossRef]
- Giorgi, V.; Sirotti, S.; Romano, M.E.; Marotto, D.; Ablin, J.N.; Salaffi, F.; Sarzi-Puttini, P. Fibromyalgia: One Year in Review 2022. Clin. Exp. Rheumatol. 2022, 40, 1065–1072. [Google Scholar] [CrossRef]
- Scheich, B.; Vincze, P.; Szőke, É.; Borbély, É.; Hunyady, Á.; Szolcsányi, J.; Dénes, Á.; Környei, Z.; Gaszner, B.; Helyes, Z. Chronic Stress-Induced Mechanical Hyperalgesia Is Controlled by Capsaicin-Sensitive Neurones in the Mouse. Eur. J. Pain 2017, 21, 1417–1431. [Google Scholar] [CrossRef]
- Bardin, L.; Malfetes, N.; Newman-Tancredi, A.; Depoortère, R. Chronic Restraint Stress Induces Mechanical and Cold Allodynia, and Enhances Inflammatory Pain in Rat: Relevance to Human Stress-Associated Painful Pathologies. Behav. Brain Res. 2009, 205, 360–366. [Google Scholar] [CrossRef]
- Balasingam, V.; Tejada-Berges, T.; Wright, E.; Bouckova, R.; Yong, V.W. Reactive Astrogliosis in the Neonatal Mouse Brain and Its Modulation by Cytokines. J. Neurosci. 1994, 14, 846–856. [Google Scholar] [CrossRef]
- Tynan, R.J.; Naicker, S.; Hinwood, M.; Nalivaiko, E.; Buller, K.M.; Pow, D.V.; Day, T.A.; Walker, F.R. Chronic Stress Alters the Density and Morphology of Microglia in a Subset of Stress-Responsive Brain Regions. Brain Behav. Immun. 2010, 24, 1058–1068. [Google Scholar] [CrossRef]
- Sugama, S.; Takenouchi, T.; Hashimoto, M.; Ohata, H.; Takenaka, Y.; Kakinuma, Y. Stress-Induced Microglial Activation Occurs through β-Adrenergic Receptor: Noradrenaline as a Key Neurotransmitter in Microglial Activation. J. Neuroinflamm. 2019, 16, 1–16. [Google Scholar] [CrossRef]
- Kreisel, T.; Frank, M.G.; Licht, T.; Reshef, R.; Ben-Menachem-Zidon, O.; Baratta, M.V.; Maier, S.F.; Yirmiya, R. Dynamic Microglial Alterations Underlie Stress-Induced Depressive-like Behavior and Suppressed Neurogenesis. Mol. Psychiatry 2014, 19, 699–709. [Google Scholar] [CrossRef]
- Becher, B.; Spath, S.; Goverman, J. Cytokine Networks in Neuroinflammation. Nat. Rev. Immunol. 2017, 17, 49–59. [Google Scholar] [CrossRef] [PubMed]
- Fukuoka, H.; Kawatani, M.; Hisamitsu, T.; Takeshige, C. Cutaneous Hyperalgesia Induced by Peripheral Injection of Interleukin-1 Beta in the Rat. Brain Res. 1994, 657, 133–140. [Google Scholar] [CrossRef] [PubMed]
- Albrecht, D.S.; Forsberg, A.; Sandström, A.; Bergan, C.; Kadetoff, D.; Protsenko, E.; Lampa, J.; Lee, Y.C.; Höglund, C.O.; Catana, C.; et al. Brain Glial Activation in Fibromyalgia—A Multi-Site Positron Emission Tomography Investigation. Brain Behav. Immun. 2019, 75, 72–83. [Google Scholar] [CrossRef]
- Abd-Ellatief, R.B.; Mohamed, H.K.; Kotb, H.I. Reactive Astrogliosis in an Experimental Model of Fibromyalgia: Effect of Dexmedetomidine. Cells Tissues Organs 2018, 205, 105–119. [Google Scholar] [CrossRef]
- Menkin, V. Chemical Basis of Fever. Science 1944, 100, 337–338. [Google Scholar] [CrossRef] [PubMed]
- Mizel, S.B.; Farrar, J.J. Revised Nomenclature for Antigen-Nonspecific T-Cell Proliferation and Helper Factors. Cell. Immunol. 1979, 48, 433–436. [Google Scholar] [CrossRef]
- Moynagh, P.N.; Williams, D.C.; O’Neill, L.A. Activation of NF-Kappa B and Induction of Vascular Cell Adhesion Molecule-1 and Intracellular Adhesion Molecule-1 Expression in Human Glial Cells by IL-1. Modulation by Antioxidants. J. Immunol. 1994, 153, 2681–2690. [Google Scholar] [CrossRef]
- Moynagh, P.N. The Interleukin-1 Signalling Pathway in Astrocytes: A Key Contributor to Inflammation in the Brain. J. Anat. 2005, 207, 265. [Google Scholar] [CrossRef]
- Goshen, I.; Yirmiya, R. Interleukin-1 (IL-1): A Central Regulator of Stress Responses. Front. Neuroendocrinol. 2009, 30, 30–45. [Google Scholar] [CrossRef]
- Jenei-Lanzl, Z.; Meurer, A.; Zaucke, F. Interleukin-1β Signaling in Osteoarthritis—Chondrocytes in Focus. Cell. Signal. 2019, 53, 212–223. [Google Scholar] [CrossRef]
- Cohen, S.B.; Moreland, L.W.; Cush, J.J.; Greenwald, M.W.; Block, S.; Shergy, W.J.; Hanrahan, P.S.; Khraishi, M.M.; Patel, A.; Sun, G.; et al. A Multicentre, Double Blind, Randomised, Placebo Controlled Trial of Anakinra (Kineret), a Recombinant Interleukin 1 Receptor Antagonist, in Patients with Rheumatoid Arthritis Treated with Background Methotrexate. Ann. Rheum. Dis. 2004, 63, 1062–1068. [Google Scholar] [CrossRef]
- Farooq, R.K.; Asghar, K.; Kanwal, S.; Zulqernain, A. Role of Inflammatory Cytokines in Depression: Focus on Interleukin-1β (Review). Biomed. Rep. 2017, 6, 15–20. [Google Scholar] [CrossRef]
- Barrett, A.C.; Smith, E.S.; Picker, M.J. Sex-Related Differences in Mechanical Nociception and Antinociception Produced by μ- and κ-Opioid Receptor Agonists in Rats. Eur. J. Pharmacol. 2002, 452, 163–173. [Google Scholar] [CrossRef] [PubMed]
- Bourgeois, J.R.; Feustel, P.J.; Kopec, A.M. Sex Differences in Choice-Based Thermal Nociceptive Tests in Adult Rats. Behav. Brain Res. 2022, 429, 113919. [Google Scholar] [CrossRef] [PubMed]
- Body Weight Information for C57BL/6J|The Jackson Laboratory. Available online: https://www.jax.org/jax-mice-and-services/strain-data-sheet-pages/body-weight-chart-000664 (accessed on 12 November 2022).
- Shornick, L.P.; De Togni, P.; Mariathasan, S.; Goellner, J.; Strauss-Schoenberger, J.; Karr, R.W.; Ferguson, T.A.; Chaplin, D.D. Mice Deficient in IL-1β Manifest Impaired Contact Hypersensitivity to Trinitrochlorobenzene. J. Exp. Med. 1996, 183, 1427–1436. [Google Scholar] [CrossRef] [PubMed]
- Wolf, G.; Yirmiya, R.; Goshen, I.; Iverfeldt, K.; Holmlund, L.; Takeda, K.; Shavit, Y. Impairment of Interleukin-1 (IL-1) Signaling Reduces Basal Pain Sensitivity in Mice: Genetic, Pharmacological and Developmental Aspects. Pain 2003, 104, 471–480. [Google Scholar] [CrossRef]
- Torres, R.; Macdonald, L.; Croll, S.D.; Reinhardt, J.; Dore, A.; Stevens, S.; Hylton, D.M.; Rudge, J.S.; Liu-Bryan, R.; Terkeltaub, R.A.; et al. Hyperalgesia, Synovitis and Multiple Biomarkers of Inflammation Are Suppressed by Interleukin 1 Inhibition in a Novel Animal Model of Gouty Arthritis. Ann. Rheum. Dis. 2009, 68, 1602–1608. [Google Scholar] [CrossRef]
- Honore, P.; Wade, C.L.; Zhong, C.; Harris, R.R.; Wu, C.; Ghayur, T.; Iwakura, Y.; Decker, M.W.; Faltynek, C.; Sullivan, J.; et al. Interleukin-1alphabeta Gene-Deficient Mice Show Reduced Nociceptive Sensitivity in Models of Inflammatory and Neuropathic Pain but Not Post-Operative Pain. Behav. Brain Res. 2006, 167, 355–364. [Google Scholar] [CrossRef]
- Binshtok, A.M.; Wang, H.; Zimmermann, K.; Amaya, F.; Vardeh, D.; Shi, L.; Brenner, G.J.; Ji, R.R.; Bean, B.P.; Woolf, C.J.; et al. Nociceptors Are Interleukin-1β Sensors. J. Neurosci. 2008, 28, 14062. [Google Scholar] [CrossRef] [PubMed]
- Ebbinghaus, M.; Uhlig, B.; Richter, F.; Von Banchet, G.S.; Gajda, M.; Bräuer, R.; Schaible, H.G. The Role of Interleukin-1β in Arthritic Pain: Main Involvement in Thermal, but Not Mechanical, Hyperalgesia in Rat Antigen-Induced Arthritis. Arthritis Rheum. 2012, 64, 3897–3907. [Google Scholar] [CrossRef] [PubMed]
- Schaible, H.G. Nociceptive Neurons Detect Cytokines in Arthritis. Arthritis Res. Ther. 2014, 16, 470. [Google Scholar] [CrossRef]
- Mailhot, B.; Christin, M.; Tessandier, N.; Sotoudeh, C.; Bretheau, F.; Turmel, R.; Pellerin, E.; Wang, F.; Bories, C.; Joly-Beauparlant, C.; et al. Neuronal Interleukin-1 Receptors Mediate Pain in Chronic Inflammatory Diseases. J. Exp. Med. 2020, 217, e20191430. [Google Scholar] [CrossRef]
- Guesdon, F.; Saklatvala, J. Identification of a Cytoplasmic Protein Kinase Regulated by IL-1 That Phosphorylates the Small Heat Shock Protein, Hsp27. J. Immunol. 1991, 147, 3402–3407. [Google Scholar] [CrossRef] [PubMed]
- Ahlers, A.; Belka, C.; Gaestel, M.; Lamping, N.; Sott, C.; Herrmann, F.; Brach, M.A. Interleukin-1-Induced Intracellular Signaling Pathways Converge in the Activation of Mitogen-Activated Protein Kinase and Mitogen-Activated Protein Kinase-Activated Protein Kinase 2 and the Subsequent Phosphorylation of the 27-Kilodalton Heat Shock Protein in Monocytic Cells. Mol. Pharmacol. 1994, 46, 1077–1083. [Google Scholar] [PubMed]
- Weber, A.; Wasiliew, P.; Kracht, M. Interleukin-1 (IL-1) Pathway. Sci. Signal. 2010, 3, 1. [Google Scholar] [CrossRef]
- Denes, A.; Wilkinson, F.; Bigger, B.; Chu, M.; Rothwell, N.J.; Allan, S.M. Central and Haematopoietic Interleukin-1 Both Contribute to Ischaemic Brain Injury in Mice. Dis. Model. Mech. 2013, 6, 1043–1048. [Google Scholar] [CrossRef]
- Denes, A.; Drake, C.; Stordy, J.; Chamberlain, J.; McColl, B.W.; Gram, H.; Crossman, D.; Francis, S.; Allan, S.M.; Rothwell, N.J. Interleukin-1 Mediates Neuroinflammatory Changes Associated with Diet-Induced Atherosclerosis. J. Am. Heart Assoc. 2012, 1, e002006. [Google Scholar] [CrossRef]
- Pugh, C.R.; Nguyen, K.T.; Gonyea, J.L.; Fleshner, M.; Wakins, L.R.; Maier, S.F.; Rudy, J.W. Role of Interleukin-1 Beta in Impairment of Contextual Fear Conditioning Caused by Social Isolation. Behav. Brain Res. 1999, 106, 109–118. [Google Scholar] [CrossRef] [PubMed]
- Minami, M.; Kuraishi, Y.; Yamaguchi, T.; Nakai, S.; Hirai, Y.; Satoh, M. Immobilization Stress Induces Interleukin-1β MRNA in the Rat Hypothalamus. Neurosci. Lett. 1991, 123, 254–256. [Google Scholar] [CrossRef]
- Swiergiel, A.H.; Dunn, A.J. Effects of Interleukin-1β and Lipopolysaccharide on Behavior of Mice in the Elevated Plus-Maze and Open Field Tests. Pharmacol. Biochem. Behav. 2007, 86, 651. [Google Scholar] [CrossRef]
- Söderlund, J.; Olsson, S.K.; Samuelsson, M.; Walther-Jallow, L.; Johansson, C.; Erhardt, S.; Landén, M.; Engberg, G. Elevation of Cerebrospinal Fluid Interleukin-1β in Bipolar Disorder. J. Psychiatry Neurosci. 2011, 36, 114. [Google Scholar] [CrossRef] [PubMed]
- Deinzer, R.; Förster, P.; Fuck, L.; Herforth, A.; Stiller-Winkler, R.; Idel, H. Increase of Crevicular Interleukin 1beta under Academic Stress at Experimental Gingivitis Sites and at Sites of Perfect Oral Hygiene. J. Clin. Periodontol. 1999, 26, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Reeve, A.J.; Patel, S.; Fox, A.; Walker, K.; Urban, L. Intrathecally Administered Endotoxin or Cytokines Produce Allodynia, Hyperalgesia and Changes in Spinal Cord Neuronal Responses to Nociceptive Stimuli in the Rat. Eur. J. Pain 2000, 4, 247–257. [Google Scholar] [CrossRef]
- Gui, W.S.; Wei, X.; Mai, C.L.; Murugan, M.; Wu, L.J.; Xin, W.J.; Zhou, L.J.; Liu, X.G. Interleukin-1β Overproduction Is a Common Cause for Neuropathic Pain, Memory Deficit, and Depression Following Peripheral Nerve Injury in Rodents. Mol. Pain 2016, 12, 1744806916646784. [Google Scholar] [CrossRef]
- Teodorczyk-Injeyan, J.A.; Triano, J.J.; Injeyan, H.S. Nonspecific Low Back Pain: Inflammatory Profiles of Patients With Acute and Chronic Pain. Clin. J. Pain 2019, 35, 818. [Google Scholar] [CrossRef]
- Lindenlaub, T.; Sommer, C. Cytokines in Sural Nerve Biopsies from Inflammatory and Non-Inflammatory Neuropathies. Acta Neuropathol. 2003, 105, 593–602. [Google Scholar] [CrossRef] [PubMed]
- O’Mahony, L.F.; Srivastava, A.; Mehta, P.; Ciurtin, C. Is Fibromyalgia Associated with a Unique Cytokine Profile? A Systematic Review and Meta-Analysis. Rheumatology 2021, 60, 2602. [Google Scholar] [CrossRef] [PubMed]
- Fillingim, R.B.; King, C.D.; Ribeiro-Dasilva, M.C.; Rahim-Williams, B.; Riley, J.L. Sex, Gender, and Pain: A Review of Recent Clinical and Experimental Findings. J. Pain 2009, 10, 447–485. [Google Scholar] [CrossRef] [PubMed]
- Tang, Y.R.; Yang, W.W.; Wang, Y.L.; Lin, L. Sex Differences in the Symptoms and Psychological Factors That Influence Quality of Life in Patients with Irritable Bowel Syndrome. Eur. J. Gastroenterol. Hepatol. 2012, 24, 702–707. [Google Scholar] [CrossRef]
- Derbyshire, S.W.G.; Nichols, T.E.; Firestone, L.; Townsend, D.W.; Jones, A.K.P. Gender Differences in Patterns of Cerebral Activation during Equal Experience of Painful Laser Stimulation. J. Pain 2002, 3, 401–411. [Google Scholar] [CrossRef]
- Berman, S.; Munakata, J.; Naliboff, B.D.; Chang, L.; Mandelkern, M.; Silverman, D.; Kovalik, E.; Mayer, E.A. Gender Differences in Regional Brain Response to Visceral Pressure in IBS Patients. Eur. J. Pain 2000, 4, 157–172. [Google Scholar] [CrossRef]
- Craft, R.M.; Mogil, J.S.; Maria Aloisi, A. Sex Differences in Pain and Analgesia: The Role of Gonadal Hormones. Eur. J. Pain 2004, 8, 397–411. [Google Scholar] [CrossRef]
- Fillingim, R.B.; Ness, T.J. Sex-Related Hormonal Influences on Pain and Analgesic Responses. Neurosci. Biobehav. Rev. 2000, 24, 485–501. [Google Scholar] [CrossRef]
- Prendergast, B.J.; Onishi, K.G.; Zucker, I. Female Mice Liberated for Inclusion in Neuroscience and Biomedical Research. Neurosci. Biobehav. Rev. 2014, 40, 1–5. [Google Scholar] [CrossRef]
- Mogil, J.S.; Chanda, M.L. The Case for the Inclusion of Female Subjects in Basic Science Studies of Pain. Pain 2005, 117, 1–5. [Google Scholar] [CrossRef]
- Sorge, R.E.; Mapplebeck, J.C.S.; Rosen, S.; Beggs, S.; Taves, S.; Alexander, J.K.; Martin, L.J.; Austin, J.S.; Sotocinal, S.G.; Chen, D.; et al. Different Immune Cells Mediate Mechanical Pain Hypersensitivity in Male and Female Mice. Nat. Neurosci. 2015, 18, 1081. [Google Scholar] [CrossRef]
- Sorge, R.E.; LaCroix-Fralish, M.L.; Tuttle, A.H.; Sotocinal, S.G.; Austin, J.S.; Ritchie, J.; Chanda, M.L.; Graham, A.C.; Topham, L.; Beggs, S.; et al. Spinal Cord Toll-Like Receptor 4 Mediates Inflammatory and Neuropathic Hypersensitivity in Male But Not Female Mice. J. Neurosci. 2011, 31, 15450. [Google Scholar] [CrossRef] [PubMed]
- Ruh, M.F.; Bi, Y.; D’Alonzo, R.; Bellone, C.J. Effect of Estrogens on IL-1beta Promoter Activity. J. Steroid Biochem. Mol. Biol. 1998, 66, 203–210. [Google Scholar] [CrossRef]
- Arakawa, K.; Arakawa, H.; Hueston, C.M.; Deak, T. Effects of the Estrous Cycle and Ovarian Hormones on Central Expression of Interleukin-1 Evoked by Stress in Female Rats. Neuroendocrinology 2014, 100, 162–177. [Google Scholar] [CrossRef]
- Pacifici, R. Estrogen, Cytokines, and Pathogenesis of Postmenopausal Osteoporosis. J. Bone Miner. Res. 1996, 11, 1043–1051. [Google Scholar] [CrossRef] [PubMed]
- Meyer, R.A.; Ringkamp, M.; Campbell, J.N.; Raja, S.N. Peripheral Mechanisms of Cutaneous Nociception. Wall Melzack’s Textb. Pain 2006, 6, 3–34. [Google Scholar] [CrossRef]
- Kreutzberg, G.W. Microglia: A Sensor for Pathological Events in the CNS. Trends Neurosci. 1996, 19, 312–318. [Google Scholar] [CrossRef]
- Ossipov, M.H.; Morimura, K.; Porreca, F. Descending Pain Modulation and Chronification of Pain. Curr. Opin. Support. Palliat. Care 2014, 8, 143. [Google Scholar] [CrossRef]
- del Fernández-Arjona, M.M.; Grondona, J.M.; Fernández-Llebrez, P.; López-Ávalos, M.D. Microglial Morphometric Parameters Correlate With the Expression Level of IL-1β, and Allow Identifying Different Activated Morphotypes. Front. Cell. Neurosci. 2019, 13, 472. [Google Scholar] [CrossRef]
- Zhang, D.; Hu, X.; Qian, L.; O’Callaghan, J.P.; Hong, J.S. Astrogliosis in CNS Pathologies: Is There A Role for Microglia? Mol. Neurobiol. 2010, 41, 232. [Google Scholar] [CrossRef]
- Sofroniew, M.V. Astrogliosis. Cold Spring Harb. Perspect. Biol. 2015, 7, a020420. [Google Scholar] [CrossRef]
- Sofroniew, M.V. Multiple Roles for Astrocytes as Effectors of Cytokines and Inflammatory Mediators. Neuroscientist 2014, 20, 160–172. [Google Scholar] [CrossRef] [PubMed]
- Sofroniew, M.V. Molecular Dissection of Reactive Astrogliosis and Glial Scar Formation. Trends Neurosci. 2009, 32, 638–647. [Google Scholar] [CrossRef] [PubMed]
- Czéh, B.; Simon, M.; Schmelting, B.; Hiemke, C.; Fuchs, E. Astroglial Plasticity in the Hippocampus Is Affected by Chronic Psychosocial Stress and Concomitant Fluoxetine Treatment. Neuropsychopharmacology 2006, 31, 1616–1626. [Google Scholar] [CrossRef] [PubMed]
- Lambert, K.G.; Gerecke, K.M.; Quadros, P.S.; Doudera, E.; Jasnow, A.M.; Kinsley, C.H. Activity-Stress Increases Density of GFAP-Immunoreactive Astrocytes in the Rat Hippocampus. Stress 2000, 3, 275–284. [Google Scholar] [CrossRef]
- Kwon, M.S.; Seo, Y.J.; Lee, J.K.; Lee, H.K.; Jung, J.S.; Jang, J.E.; Park, S.H.; Suh, H.W. The Repeated Immobilization Stress Increases IL-1beta Immunoreactivities in Only Neuron, but Not Astrocyte or Microglia in Hippocampal CA1 Region, Striatum and Paraventricular Nucleus. Neurosci. Lett. 2008, 430, 258–263. [Google Scholar] [CrossRef]
- Naskar, S.; Chattarji, S. Stress Elicits Contrasting Effects on the Structure and Number of Astrocytes in the Amygdala versus Hippocampus. eNeuro 2019, 6, 1–14. [Google Scholar] [CrossRef]
- Marchette, R.C.N.; Bicca, M.A.; da Santos, E.C.S.; de Lima, T.C.M. Distinctive Stress Sensitivity and Anxiety-like Behavior in Female Mice: Strain Differences Matter. Neurobiol. Stress 2018, 9, 55–63. [Google Scholar] [CrossRef]
- Shilpa, B.M.; Bhagya, V.; Harish, G.; Srinivas Bharath, M.M.; Shankaranarayana Rao, B.S. Environmental Enrichment Ameliorates Chronic Immobilisation Stress-Induced Spatial Learning Deficits and Restores the Expression of BDNF, VEGF, GFAP and Glucocorticoid Receptors. Prog. Neuro-Psychopharmacol. Biol. Psychiatry 2017, 76, 88–100. [Google Scholar] [CrossRef]
- Selye, H. Stress and the General Adaptation Syndrome. Br. Med. J. 1950, 1, 1383–1392. [Google Scholar] [CrossRef]
- Lee, J.H.; Kim, T.J.; Kim, J.W.; Yoon, J.S.; Kim, H.S.; Lee, K.M. The Anti-Apoptotic Effect of Ghrelin on Restraint Stress-Induced Thymus Atrophy in Mice. Immune Netw. 2016, 16, 242–248. [Google Scholar] [CrossRef]
- Marin, M.T.; Cruz, F.C.; Planeta, C.S. Chronic Restraint or Variable Stresses Differently Affect the Behavior, Corticosterone Secretion and Body Weight in Rats. Physiol. Behav. 2007, 90, 29–35. [Google Scholar] [CrossRef] [PubMed]
- Magariños, A.M.; McEwen, B.S. Stress-Induced Atrophy of Apical Dendrites of Hippocampal CA3c Neurons: Comparison of Stressors. Neuroscience 1995, 69, 83–88. [Google Scholar] [CrossRef] [PubMed]
- Steru, L.; Chermat, R.; Thierry, B.; Simon, P. The Tail Suspension Test: A New Method for Screening Antidepressants in Mice. Psychopharmacology 1985, 85, 367–370. [Google Scholar] [CrossRef]
- Porsolt, R.D.; Anton, G.; Blavet, N.; Jalfre, M. Behavioural Despair in Rats: A New Model Sensitive to Antidepressant Treatments. Eur. J. Pharmacol. 1978, 47, 379–391. [Google Scholar] [CrossRef] [PubMed]
- Gould, T.D.; Dao, D.T.; Kovacsics, C.E. The Open Field Test. In Neuromethods; Humana Press: Totowa NJ, USA, 2009; Volume 42, pp. 1–20. [Google Scholar]
- Sadler, A.M.; Bailey, S.J. Repeated Daily Restraint Stress Induces Adaptive Behavioural Changes in Both Adult and Juvenile Mice. Physiol. Behav. 2016, 167, 313–323. [Google Scholar] [CrossRef]
- Dubovicky, M.; Jezova, D. Effect of Chronic Emotional Stress on Habituation Processes in Open Field in Adult Rats. Ann. N. Y. Acad. Sci. 2004, 1018, 199–206. [Google Scholar] [CrossRef]
- Horai, R.; Asano, M.; Sudo, K.; Kanuka, H.; Suzuki, M.; Nishihara, M.; Takahashi, M.; Iwakura, Y. Production of Mice Deficient in Genes for Interleukin (IL)-1α, IL-1β, IL-1α/β, and IL-1 Receptor Antagonist Shows That IL-1β Is Crucial in Turpentine-Induced Fever Development and Glucocorticoid Secretion. J. Exp. Med. 1998, 187, 1463. [Google Scholar] [CrossRef]
- Dinarello, C.A. Immunological and Inflammatory Functions of the Interleukin-1 Family. Annu. Rev. Immunol. 2009, 27, 519–550. [Google Scholar] [CrossRef]
- Uri-Belapolsky, S.; Shaish, A.; Eliyahu, E.; Grossman, H.; Levi, M.; Chuderland, D.; Ninio-Many, L.; Hasky, N.; Shashar, D.; Almog, T.; et al. Interleukin-1 Deficiency Prolongs Ovarian Lifespan in Mice. Proc. Natl. Acad. Sci. USA 2014, 111, 12492–12497. [Google Scholar] [CrossRef]
- Ihne, J.L.; Fitzgerald, P.J.; Hefner, K.R.; Holmes, A. Pharmacological Modulation of Stress-Induced Behavioral Changes in the Light/Dark Exploration Test in Male C57BL/6J Mice. Neuropharmacology 2012, 62, 464–473. [Google Scholar] [CrossRef] [PubMed]
- Borbély, É.; Botz, B.; Bölcskei, K.; Kenyér, T.; Kereskai, L.; Kiss, T.; Szolcsányi, J.; Pintér, E.; Csepregi, J.Z.; Mócsai, A.; et al. Capsaicin-Sensitive Sensory Nerves Exert Complex Regulatory Functions in the Serum-Transfer Mouse Model of Autoimmune Arthritis. Brain Behav. Immun. 2015, 45, 50–59. [Google Scholar] [CrossRef] [PubMed]
- Tékus, V.; Hajna, Z.; Borbély, É.; Markovics, A.; Bagoly, T.; Szolcsányi, J.; Thompson, V.; Kemény, Á.; Helyes, Z.; Goebel, A. A CRPS-IgG-Transfer-Trauma Model Reproducing Inflammatory and Positive Sensory Signs Associated with Complex Regional Pain Syndrome. Pain 2014, 155, 299–308. [Google Scholar] [CrossRef]
- Holland, H.C.; Weldon, E. A Note on a New Technique of Recording Ambulation in the Open Field Test and Its Validation. Acta Psychol. 1968, 28, 293–300. [Google Scholar] [CrossRef]
- Gaszner, B.; Kormos, V.; Kozicz, T.; Hashimoto, H.; Reglodi, D.; Helyes, Z. The Behavioral Phenotype of Pituitary Adenylate-Cyclase Activating Polypeptide-Deficient Mice in Anxiety and Depression Tests Is Accompanied by Blunted c-Fos Expression in the Bed Nucleus of the Stria Terminalis, Central Projecting Edinger-Westphal Nucleus, Ventral Lateral Septum, and Dorsal Raphe Nucleus. Neuroscience 2012, 202, 283–299. [Google Scholar] [CrossRef]
- Nielsen, D.M.; Carey, G.J.; Gold, L.H. Antidepressant-like Activity of Corticotropin-Releasing Factor Type-1 Receptor Antagonists in Mice. Eur. J. Pharmacol. 2004, 499, 135–146. [Google Scholar] [CrossRef] [PubMed]
- Ghasemi, M.; Montaser-Kouhsari, L.; Shafaroodi, H.; Nezami, B.G.; Ebrahimi, F.; Dehpour, A.R. NMDA Receptor/Nitrergic System Blockage Augments Antidepressant-like Effects of Paroxetine in the Mouse Forced Swimming Test. Psychopharmacology 2009, 206, 325–333. [Google Scholar] [CrossRef] [PubMed]
- Gibson-Corley, K.N.; Olivier, A.K.; Meyerholz, D.K. Principles for Valid Histopathologic Scoring in Research. Vet. Pathol. 2013, 50, 1007–1015. [Google Scholar] [CrossRef]
- Harrison, L.; Pfuhlmann, K.; Schriever, S.C.; Pfluger, P.T. Profound Weight Loss Induces Reactive Astrogliosis in the Arcuate Nucleus of Obese Mice. Mol. Metab. 2019, 24, 149–155. [Google Scholar] [CrossRef]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Fülöp, B.; Hunyady, Á.; Bencze, N.; Kormos, V.; Szentes, N.; Dénes, Á.; Lénárt, N.; Borbély, É.; Helyes, Z. IL-1 Mediates Chronic Stress-Induced Hyperalgesia Accompanied by Microglia and Astroglia Morphological Changes in Pain-Related Brain Regions in Mice. Int. J. Mol. Sci. 2023, 24, 5479. https://doi.org/10.3390/ijms24065479
Fülöp B, Hunyady Á, Bencze N, Kormos V, Szentes N, Dénes Á, Lénárt N, Borbély É, Helyes Z. IL-1 Mediates Chronic Stress-Induced Hyperalgesia Accompanied by Microglia and Astroglia Morphological Changes in Pain-Related Brain Regions in Mice. International Journal of Molecular Sciences. 2023; 24(6):5479. https://doi.org/10.3390/ijms24065479
Chicago/Turabian StyleFülöp, Barbara, Ágnes Hunyady, Noémi Bencze, Viktória Kormos, Nikolett Szentes, Ádám Dénes, Nikolett Lénárt, Éva Borbély, and Zsuzsanna Helyes. 2023. "IL-1 Mediates Chronic Stress-Induced Hyperalgesia Accompanied by Microglia and Astroglia Morphological Changes in Pain-Related Brain Regions in Mice" International Journal of Molecular Sciences 24, no. 6: 5479. https://doi.org/10.3390/ijms24065479
APA StyleFülöp, B., Hunyady, Á., Bencze, N., Kormos, V., Szentes, N., Dénes, Á., Lénárt, N., Borbély, É., & Helyes, Z. (2023). IL-1 Mediates Chronic Stress-Induced Hyperalgesia Accompanied by Microglia and Astroglia Morphological Changes in Pain-Related Brain Regions in Mice. International Journal of Molecular Sciences, 24(6), 5479. https://doi.org/10.3390/ijms24065479






