Unique, Specific CART Receptor-Independent Regulatory Mechanism of CART(55-102) Peptide in Spinal Nociceptive Transmission and Its Relation to Dipeptidyl-Peptidase 4 (DDP4)
Abstract
1. Introduction
2. Results
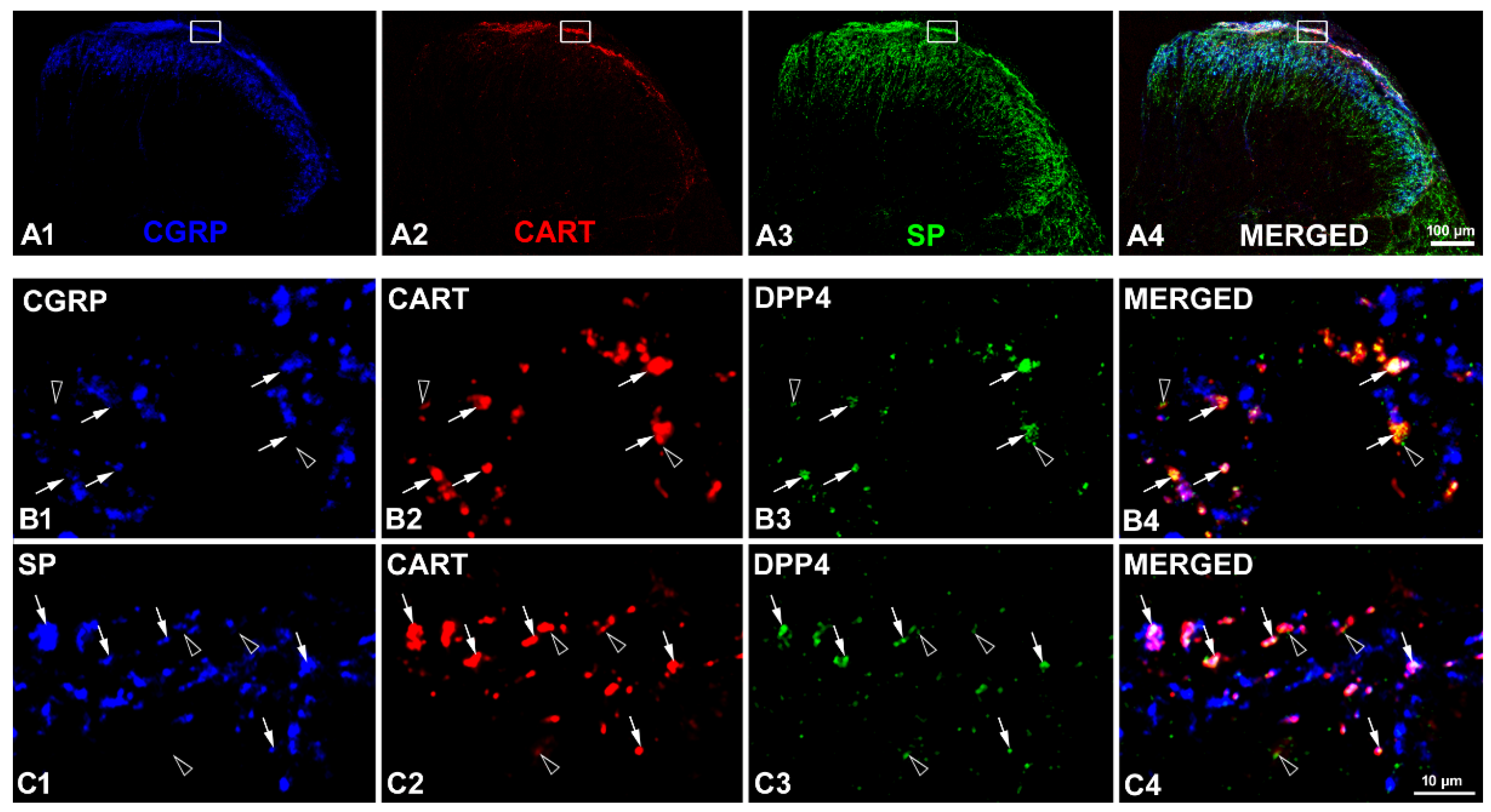
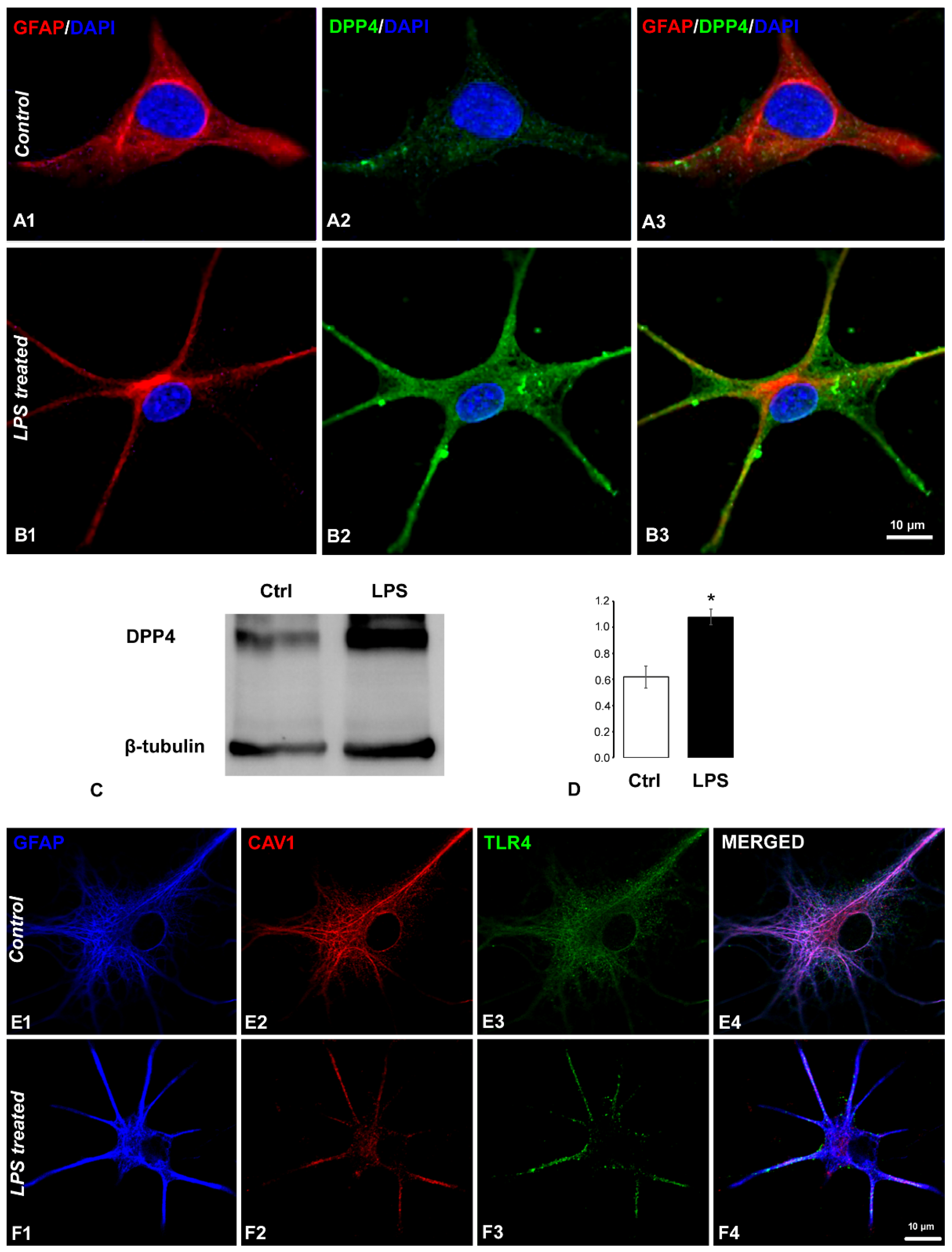
2.1. Coexistence of CART and DPP4 in the Spinal Nociceptive Pathways
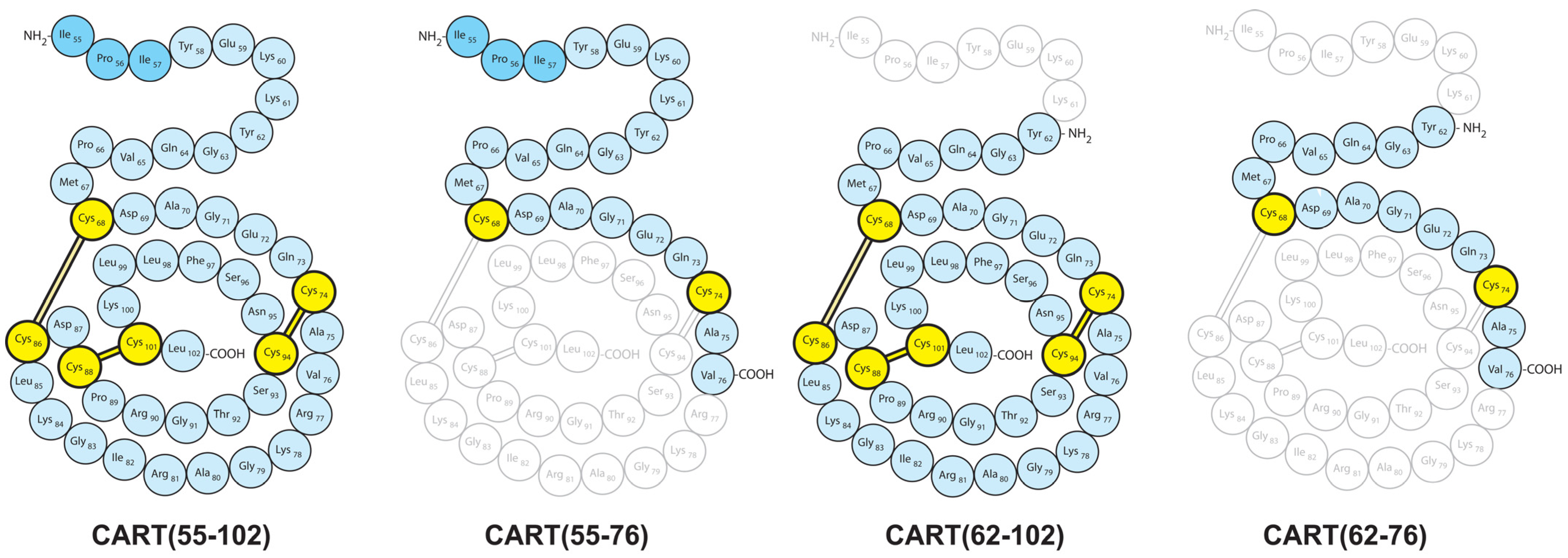
2.2. Presence of Both CART(55-102) and CART(62-102) in the Spinal Cord
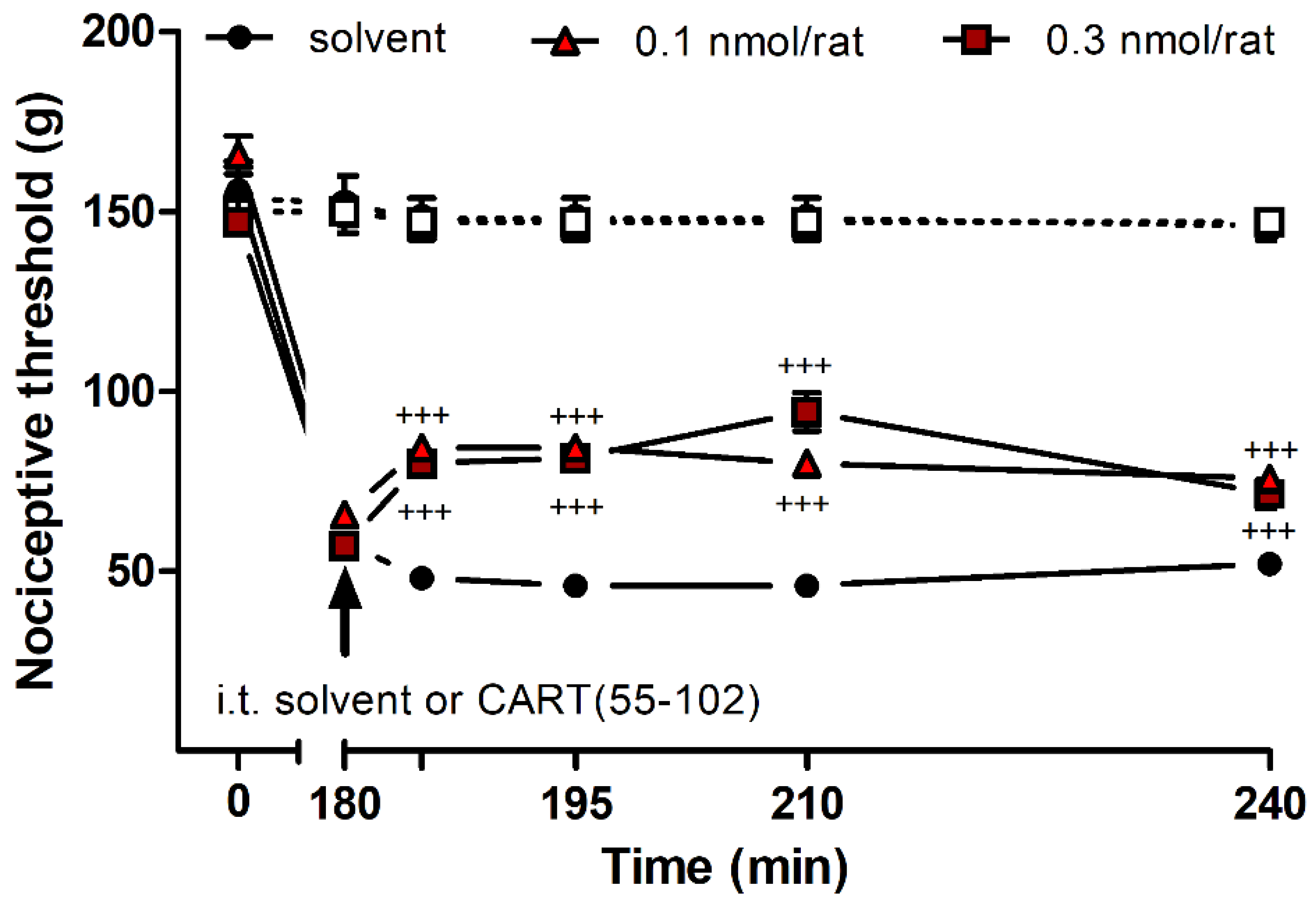
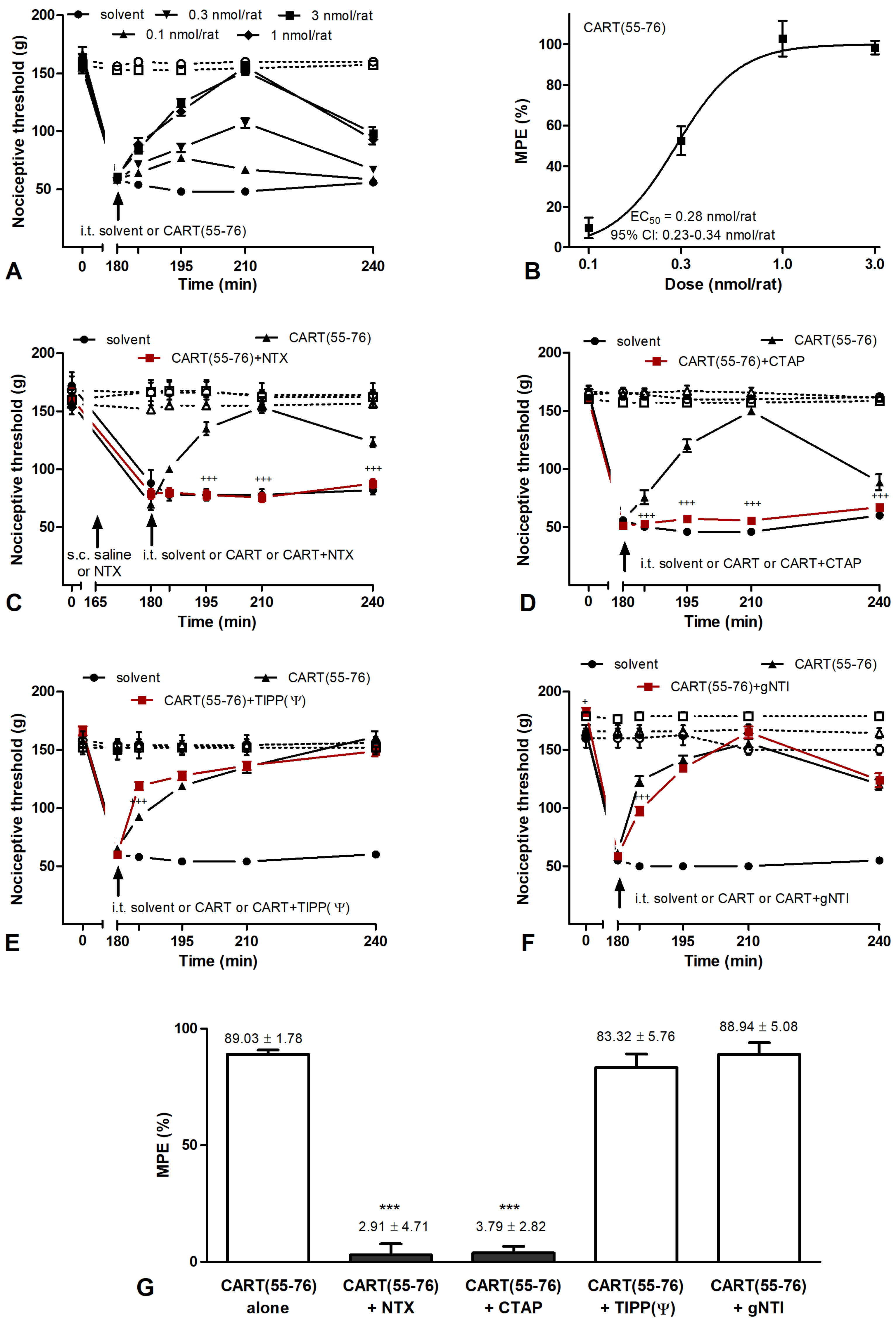
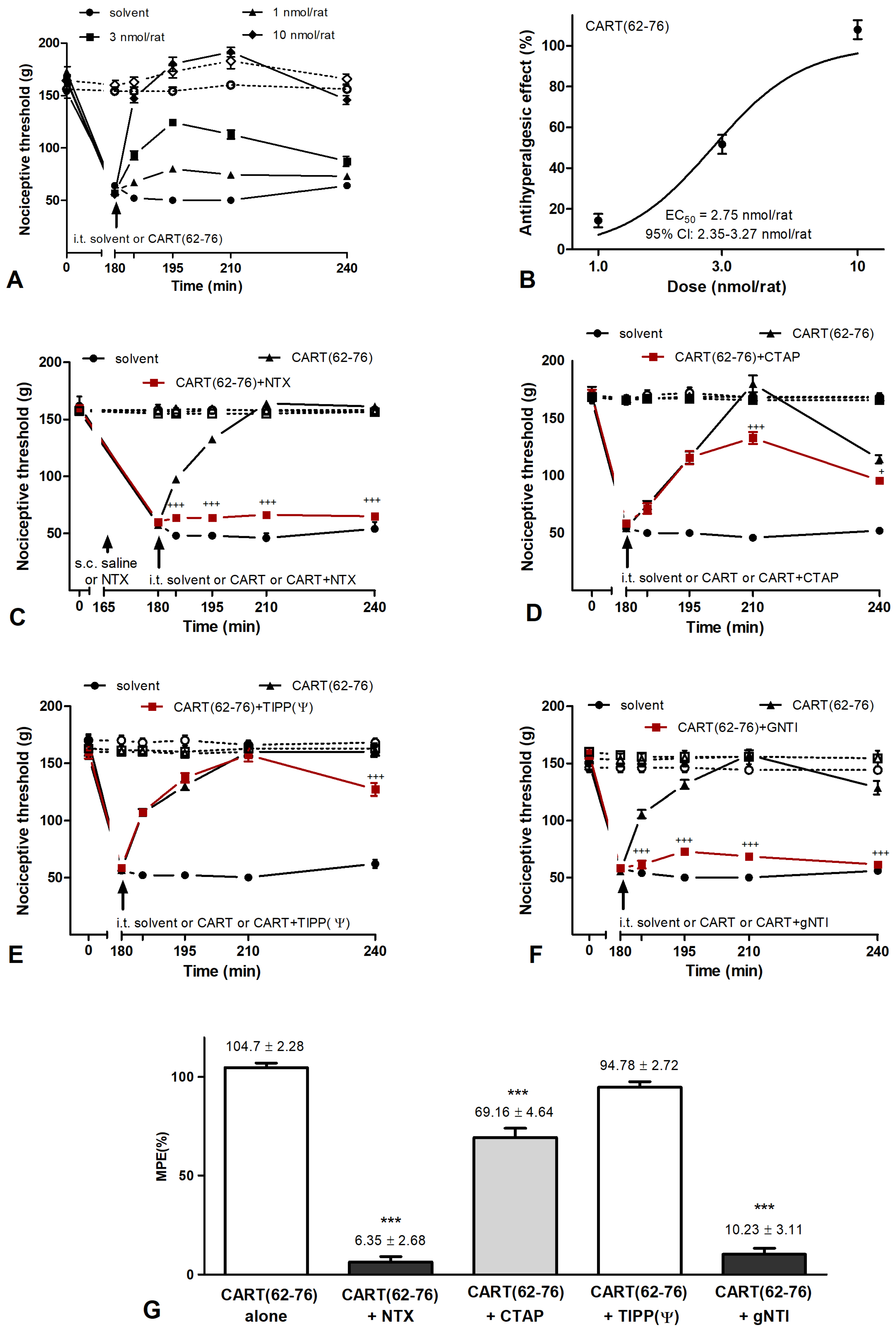
2.3. Effects of CART Peptides in Acute Nociceptive, Inflammatory, and Neuropathic Pain States
2.4. CART Peptides and Opioid Receptor Binding
2.5. Effects of CART(55-76) on the Activity of the DPP4 Enzyme
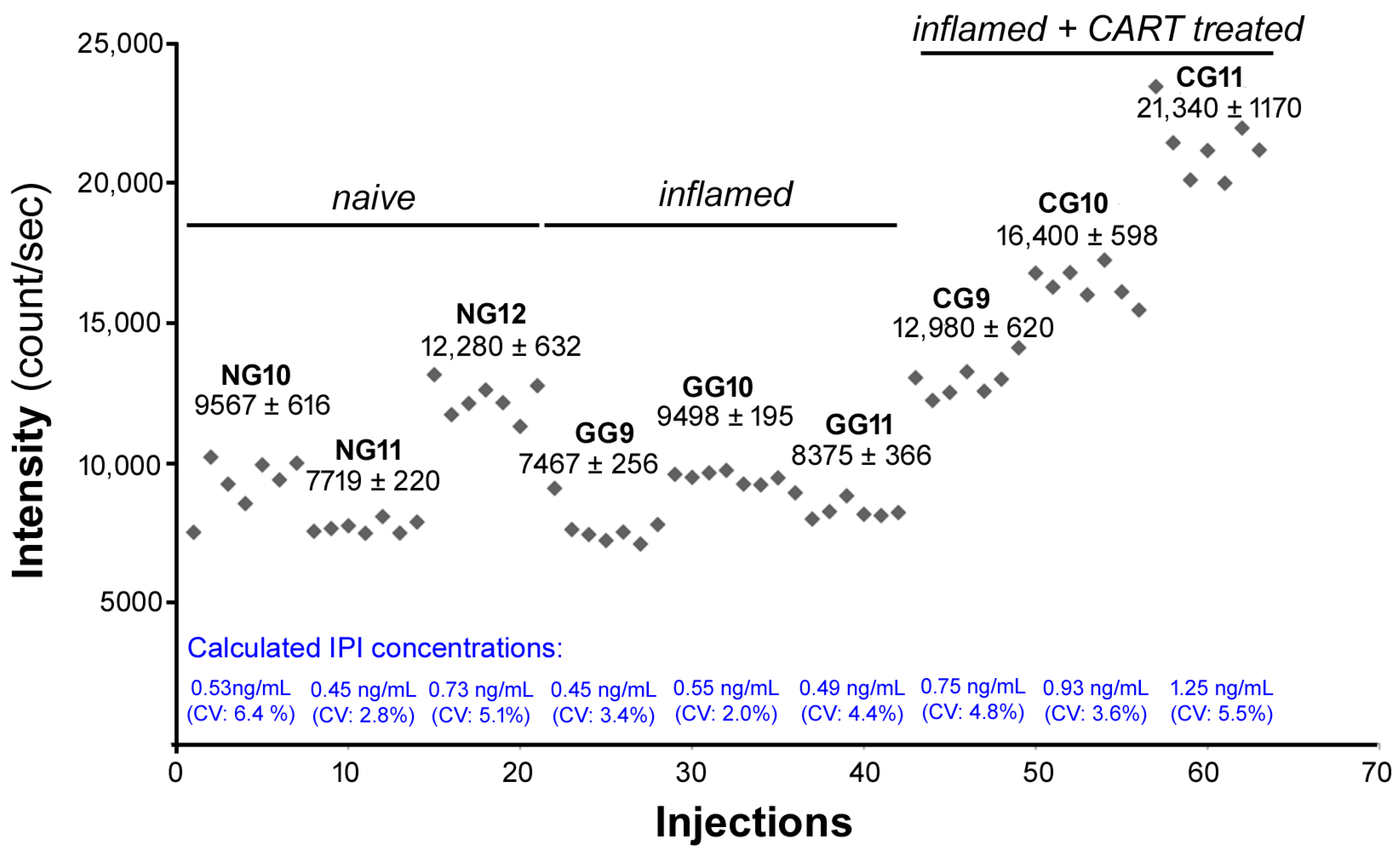
2.6. IPI Is Cleaved from CART(55-76) in the Rat Spinal Cord in Carrageenan-Induced Hind Paw Inflamation
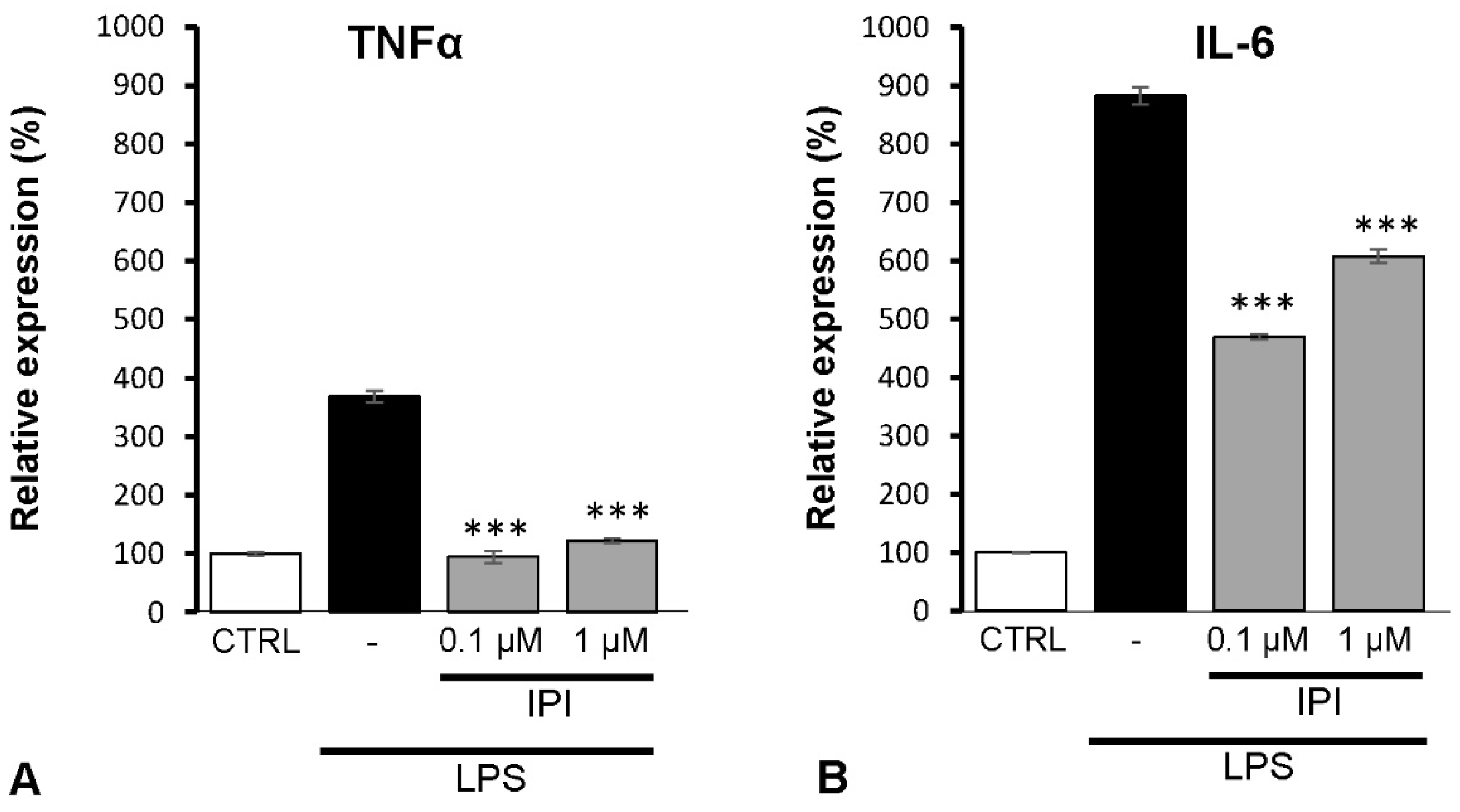
2.7. Effects of DPP4 Inhibitor IPI on IL-6 and TNFα Expression of Spinal Astrocytes
3. Discussion
3.1. CART Peptide in the Spinal Nociceptive System
3.2. CART Peptide in the Spinal Motor System
3.3. Contribution of DPP4 to Hyperalgesia Development
3.4. CART(55-76) and CART(62-76) Fragments
4. Materials and Methods
4.1. Animals
4.2. Drugs
4.3. Immunofluorescent Labelling
4.4. Western Blot
4.5. Behavioural Experiments
4.5.1. Measuring Acute Thermal Nociception
4.5.2. Determining Mechanical Hyperalgesia in Carrageenan-Induced Inflammation
4.5.3. Assessing Chronic Neuropathic Pain Parameters in the Partial Sciatic Nerve Ligation Model of Seltzer
4.6. Opioid Receptor Binding Assay
4.6.1. Preparation of Brain Samples for Binding Assays
4.6.2. Functional [35S]GTPγS Binding Experiments
4.6.3. Competitive Binding Experiments
4.7. DPP4 Inhibitor Screening Assay
4.8. Mass Spectrometry
4.8.1. Cerebrospinal Fluid Collection and Peptide Extraction
4.8.2. Peptide Extraction from Spinal Cord Segments
4.8.3. Measuring the Concentration of IPI
4.9. Cell Culture Experiments
4.9.1. Primary Astrocyte Cultures
4.9.2. Immunocytochemistry
4.9.3. Cell Treatments
4.9.4. Western Blot Analysis of Astrocytic DPP4 Expression
4.9.5. Enzyme Linked Immunosorbent Assay (ELISA)
4.10. Statistical Analysis
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Douglass, J.; McKinzie, A.A.; Couceyro, P. PCR differential display identifies a rat brain mRNA that is transcriptionally regulated by cocaine and amphetamine. J. Neurosci. 1995, 15, 2471–2481. [Google Scholar] [CrossRef] [PubMed]
- Douglass, J.; Daoud, S. Characterization of the human cDNA and genomic DNA encoding CART: A cocaine- and amphetamine-regulated transcript. Gene 1996, 169, 241–245. [Google Scholar] [CrossRef] [PubMed]
- Stanley, S.A.; Small, C.J.; Murphy, K.G.; Rayes, E.; Abbott, C.R.; Seal, L.J.; Morgan, D.G.; Sunter, D.; Dakin, C.L.; Kim, M.S.; et al. Actions of cocaine- and amphetamine-regulated transcript (CART) peptide on regulation of appetite and hypothalamo-pituitary axes in vitro and in vivo in male rats. Brain Res. 2001, 893, 186–194. [Google Scholar] [CrossRef] [PubMed]
- Fekete, C.; Lechan, R.M. Neuroendocrine implications for the association between cocaine- and amphetamine regulated transcript (CART) and hypophysiotropic thyrotropin-releasing hormone (TRH). Peptides 2006, 27, 2012–2018. [Google Scholar] [CrossRef]
- Subhedar, N.K.; Nakhate, K.T.; Upadhya, M.A.; Kokare, D.M. CART in the brain of vertebrates: Circuits, functions and evolution. Peptides 2014, 54, 108–130. [Google Scholar] [CrossRef] [PubMed]
- Meng, Q.; Kim, H.C.; Oh, S.; Lee, Y.M.; Hu, Z.; Oh, K.W. Cocaine- and Amphetamine-Regulated Transcript (CART) Peptide Plays Critical Role in Psychostimulant-Induced Depression. Biomol. Ther. 2018, 26, 425–431. [Google Scholar] [CrossRef]
- Thim, L.; Nielsen, P.F.; Judge, M.E.; Andersen, A.S.; Diers, I.; Egel-Mitani, M.; Hastrup, S. Purification and characterisation of a new hypothalamic satiety peptide, cocaine and amphetamine regulated transcript (CART), produced in yeast. FEBS Lett. 1998, 428, 263–268. [Google Scholar] [CrossRef]
- Lakatos, A.; Prinster, S.; Vicentic, A.; Hall, R.A.; Kuhar, M.J. Cocaine- and amphetamine-regulated transcript (CART) peptide activates the extracellular signal-regulated kinase (ERK) pathway in AtT20 cells via putative G-protein coupled receptors. Neurosci. Lett. 2005, 384, 198–202. [Google Scholar] [CrossRef]
- Vicentic, A.; Lakatos, A.; Kuhar, M.J. CART (cocaine- and amphetamine-regulated transcript) peptide receptors: Specific binding in AtT20 cells. Eur. J. Pharmacol. 2005, 528, 188–189. [Google Scholar] [CrossRef]
- Maletinska, L.; Maixnerova, J.; Matyskova, R.; Haugvicova, R.; Sloncova, E.; Elbert, T.; Slaninova, J.; Zelezna, B. Cocaine- and amphetamine-regulated transcript (CART) peptide specific binding in pheochromocytoma cells PC12. Eur. J. Pharmacol. 2007, 559, 109–114. [Google Scholar] [CrossRef]
- Nagelova, V.; Pirnik, Z.; Zelezna, B.; Maletinska, L. CART (cocaine- and amphetamine-regulated transcript) peptide specific binding sites in PC12 cells have characteristics of CART peptide receptors. Brain Res. 2014, 1547, 16–24. [Google Scholar] [CrossRef]
- Maixnerova, J.; Hlavacek, J.; Blokesova, D.; Kowalczyk, W.; Elbert, T.; Sanda, M.; Blechova, M.; Zelezna, B.; Slaninova, J.; Maletinska, L. Structure-activity relationship of CART (cocaine- and amphetamine-regulated transcript) peptide fragments. Peptides 2007, 28, 1945–1953. [Google Scholar] [CrossRef] [PubMed]
- Blechova, M.; Nagelova, V.; Zakova, L.; Demianova, Z.; Zelezna, B.; Maletinska, L. New analogs of the CART peptide with anorexigenic potency: The importance of individual disulfide bridges. Peptides 2013, 39, 138–144. [Google Scholar] [CrossRef] [PubMed]
- Kristensen, P.; Judge, M.E.; Thim, L.; Ribel, U.; Christjansen, K.N.; Wulff, B.S.; Clausen, J.T.; Jensen, P.B.; Madsen, O.D.; Vrang, N.; et al. Hypothalamic CART is a new anorectic peptide regulated by leptin. Nature 1998, 393, 72–76. [Google Scholar] [CrossRef] [PubMed]
- Koylu, E.O.; Couceyro, P.R.; Lambert, P.D.; Kuhar, M.J. Cocaine- and amphetamine-regulated transcript peptide immunohistochemical localization in the rat brain. J. Comp. Neurol. 1998, 391, 115–132. [Google Scholar] [CrossRef]
- Dun, S.L.; Chianca, D.A., Jr.; Dun, N.J.; Yang, J.; Chang, J.K. Differential expression of cocaine- and amphetamine-regulated transcript-immunoreactivity in the rat spinal preganglionic nuclei. Neurosci. Lett. 2000, 294, 143–146. [Google Scholar] [CrossRef] [PubMed]
- Ohsawa, M.; Dun, S.L.; Tseng, L.F.; Chang, J.; Dun, N.J. Decrease of hindpaw withdrawal latency by cocaine- and amphetamine-regulated transcript peptide to the mouse spinal cord. Eur. J. Pharmacol. 2000, 399, 165–169. [Google Scholar] [CrossRef]
- Kozsurek, M.; Lukacsi, E.; Fekete, C.; Puskar, Z. Nonselective innervation of lamina I projection neurons by cocaine- and amphetamine-regulated transcript peptide (CART)-immunoreactive fibres in the rat spinal dorsal horn. Eur. J. Neurosci. 2009, 29, 2375–2387. [Google Scholar] [CrossRef]
- Kozsurek, M.; Lukacsi, E.; Fekete, C.; Wittmann, G.; Rethelyi, M.; Puskar, Z. Cocaine- and amphetamine-regulated transcript peptide (CART) is present in peptidergic C primary afferents and axons of excitatory interneurons with a possible role in nociception in the superficial laminae of the rat spinal cord. Eur. J. Neurosci. 2007, 26, 1624–1631. [Google Scholar] [CrossRef]
- Chiu, H.Y.; Lin, H.H.; Lai, C.C. Potentiation of spinal NMDA-mediated nociception by cocaine- and amphetamine-regulated transcript peptide via PKA and PKC signaling pathways in rats. Regul. Pept. 2009, 158, 77–85. [Google Scholar] [CrossRef]
- Damaj, M.I.; Hunter, R.G.; Martin, B.R.; Kuhar, M.J. Intrathecal CART(55-102) enhances the spinal analgesic actions of morphine in mice. Brain Res. 2004, 1024, 146–149. [Google Scholar] [CrossRef] [PubMed]
- Damaj, M.I.; Zheng, J.; Martin, B.R.; Kuhar, M.J. Intrathecal CART(55-102) attenuates hyperlagesia and allodynia in a mouse model of neuropathic but not inflammatory pain. Peptides 2006, 27, 2019–2023. [Google Scholar] [CrossRef]
- Umezawa, H.; Aoyagi, T.; Ogawa, K.; Naganawa, H.; Hamada, M.; Takeuchi, T. Diprotins A and B, inhibitors of dipeptidyl aminopeptidase IV, produced by bacteria. J. Antibiot. 1984, 37, 422–425. [Google Scholar] [CrossRef] [PubMed]
- Rahfeld, J.; Schierhorn, M.; Hartrodt, B.; Neubert, K.; Heins, J. Are diprotin A (Ile-Pro-Ile) and diprotin B (Val-Pro-Leu) inhibitors or substrates of dipeptidyl peptidase IV? Biochim. Biophys. Acta 1991, 1076, 314–316. [Google Scholar] [CrossRef] [PubMed]
- Zhong, J.; Goud, A.; Rajagopalan, S. Glycemia Lowering and Risk for Heart Failure: Recent Evidence from Studies of Dipeptidyl Peptidase Inhibition. Circ. Heart Fail. 2015, 8, 819–825. [Google Scholar] [CrossRef]
- Rohrborn, D.; Wronkowitz, N.; Eckel, J. DPP4 in Diabetes. Front. Immunol. 2015, 6, 386. [Google Scholar] [CrossRef]
- Zhong, J.; Maiseyeu, A.; Davis, S.N.; Rajagopalan, S. DPP4 in cardiometabolic disease: Recent insights from the laboratory and clinical trials of DPP4 inhibition. Circ. Res. 2015, 116, 1491–1504. [Google Scholar] [CrossRef]
- Mulvihill, E.E.; Drucker, D.J. Pharmacology, physiology, and mechanisms of action of dipeptidyl peptidase-4 inhibitors. Endocr. Rev. 2014, 35, 992–1019. [Google Scholar] [CrossRef]
- Klemann, C.; Wagner, L.; Stephan, M.; von Horsten, S. Cut to the chase: A review of CD26/dipeptidyl peptidase-4′s (DPP4) entanglement in the immune system. Clin. Exp. Immunol. 2016, 185, 1–21. [Google Scholar] [CrossRef]
- Iacobellis, G. COVID-19 and diabetes: Can DPP4 inhibition play a role? Diabetes Res. Clin. Pract. 2020, 162, 108125. [Google Scholar] [CrossRef]
- Strollo, R.; Pozzilli, P. DPP4 inhibition: Preventing SARS-CoV-2 infection and/or progression of COVID-19? Diabetes Metab. Res. Rev. 2020, 36, e3330. [Google Scholar] [CrossRef] [PubMed]
- Barchetta, I.; Cavallo, M.G.; Baroni, M.G. COVID-19 and diabetes: Is this association driven by the DPP4 receptor? Potential clinical and therapeutic implications. Diabetes Res. Clin. Pract. 2020, 163, 108165. [Google Scholar] [CrossRef] [PubMed]
- Kiraly, K.; Kozsurek, M.; Lukacsi, E.; Barta, B.; Alpar, A.; Balazsa, T.; Fekete, C.; Szabon, J.; Helyes, Z.; Bolcskei, K.; et al. Glial cell type-specific changes in spinal dipeptidyl peptidase 4 expression and effects of its inhibitors in inflammatory and neuropatic pain. Sci. Rep. 2018, 8, 3490. [Google Scholar] [CrossRef] [PubMed]
- Sandkuhler, J.; Gruber-Schoffnegger, D. Hyperalgesia by synaptic long-term potentiation (LTP): An update. Curr. Opin. Pharmacol. 2012, 12, 18–27. [Google Scholar] [CrossRef] [PubMed]
- Kronschlager, M.T.; Drdla-Schutting, R.; Gassner, M.; Honsek, S.D.; Teuchmann, H.L.; Sandkuhler, J. Gliogenic LTP spreads widely in nociceptive pathways. Science 2016, 354, 1144–1148. [Google Scholar] [CrossRef]
- Ikeda, T.; Kumagai, E.; Iwata, S.; Yamakawa, A. Soluble CD26/Dipeptidyl Peptidase IV Enhances the Transcription of IL-6 and TNF-alpha in THP-1 Cells and Monocytes. PLoS ONE 2013, 8, e66520. [Google Scholar] [CrossRef]
- Dylag, T.; Kotlinska, J.; Rafalski, P.; Pachuta, A.; Silberring, J. The activity of CART peptide fragments. Peptides 2006, 27, 1926–1933. [Google Scholar] [CrossRef]
- Kim, J.; Lee, H.J.; Park, S.K.; Park, J.H.; Jeong, H.R.; Lee, S.; Lee, H.; Seol, E.; Hoe, H.S. Donepezil Regulates LPS and Abeta-Stimulated Neuroinflammation through MAPK/NLRP3 Inflammasome/STAT3 Signaling. Int. J. Mol. Sci. 2021, 22, 10637. [Google Scholar] [CrossRef]
- Ohnuma, K.; Uchiyama, M.; Yamochi, T.; Nishibashi, K.; Hosono, O.; Takahashi, N.; Kina, S.; Tanaka, H.; Lin, X.; Dang, N.H.; et al. Caveolin-1 triggers T-cell activation via CD26 in association with CARMA1. J. Biol. Chem. 2007, 282, 10117–10131. [Google Scholar] [CrossRef]
- Ciesielska, A.; Matyjek, M.; Kwiatkowska, K. TLR4 and CD14 trafficking and its influence on LPS-induced pro-inflammatory signaling. Cell. Mol. Life Sci. 2021, 78, 1233–1261. [Google Scholar] [CrossRef]
- Mousa, S.A.; Ayoub, B.M. Repositioning of dipeptidyl peptidase-4 inhibitors and glucagon like peptide-1 agonists as potential neuroprotective agents. Neural. Regen. Res. 2019, 14, 745–748. [Google Scholar] [CrossRef] [PubMed]
- Gayathiri, K.; Prabhavathi, A.; Tamilarasi, R.; Vimalavathini, R.; Kavimani, S. Role of Neprilysin in Various Diseases. Int. J. Pharmacol. Res. 2014, 4, 4. [Google Scholar]
- Iliff, J.J.; Alkayed, N.J.; Gloshani, K.J.; Traystman, R.J.; West, G.A. Cocaine- and amphetamine-regulated transcript (CART) peptide: A vasoactive role in the cerebral circulation. J. Cereb. Blood Flow. Metab. 2005, 25, 1376–1385. [Google Scholar] [CrossRef] [PubMed]
- Kasacka, I.; Janiuk, I.; Lewandowska, A.; Bekisz, A.; Lebkowski, W. Distribution pattern of CART-containing neurons and cells in the human pancreas. Acta Histochem. 2012, 114, 695–699. [Google Scholar] [CrossRef] [PubMed]
- Abels, M.; Riva, M.; Bennet, H.; Ahlqvist, E.; Dyachok, O.; Nagaraj, V.; Shcherbina, L.; Fred, R.G.; Poon, W.; Sorhede-Winzell, M.; et al. CART is overexpressed in human type 2 diabetic islets and inhibits glucagon secretion and increases insulin secretion. Diabetologia 2016, 59, 1928–1937. [Google Scholar] [CrossRef]
- Akoumianakis, I.; Antoniades, C. Dipeptidyl peptidase IV inhibitors as novel regulators of vascular disease. Vascul. Pharmacol. 2017, 96–98, 1–4. [Google Scholar] [CrossRef] [PubMed]
- Wierup, N.; Abels, M.; Shcherbina, L.; Lindqvist, A. The role of CART in islet biology. Peptides 2022, 149, 170708. [Google Scholar] [CrossRef]
- Deacon, C.F. Corrigendum: Physiology and Pharmacology of DPP-4 in Glucose Homeostasis and the Treatment of Type 2 Diabetes. Front. Endocrinol. 2019, 10, 275. [Google Scholar] [CrossRef]
- Guertin, P.A. Central pattern generator for locomotion: Anatomical, physiological, and pathophysiological considerations. Front. Neurol. 2012, 3, 183. [Google Scholar] [CrossRef]
- Keating, G.L.; Kuhar, M.J.; Rye, D.B. High dose CART peptide induces abnormal EEG activity and behavioral seizures. Neuropeptides 2008, 42, 199–204. [Google Scholar] [CrossRef]
- Volkoff, H.; Peter, R.E. Effects of CART peptides on food consumption, feeding and associated behaviors in the goldfish, Carassius auratus: Actions on neuropeptide Y- and orexin A-induced feeding. Brain Res. 2000, 887, 125–133. [Google Scholar] [CrossRef] [PubMed]
- Ruscheweyh, R.; Wilder-Smith, O.; Drdla, R.; Liu, X.G.; Sandkuhler, J. Long-term potentiation in spinal nociceptive pathways as a novel target for pain therapy. Mol. Pain 2011, 7, 20. [Google Scholar] [CrossRef] [PubMed]
- Ji, R.R.; Chamessian, A.; Zhang, Y.Q. Pain regulation by non-neuronal cells and inflammation. Science 2016, 354, 572–577. [Google Scholar] [CrossRef]
- Rothaug, M.; Becker-Pauly, C.; Rose-John, S. The role of interleukin-6 signaling in nervous tissue. Biochim. Biophys. Acta 2016, 1863, 1218–1227. [Google Scholar] [CrossRef] [PubMed]
- Zhang, R.X.; Li, A.; Liu, B.; Wang, L.; Ren, K.; Zhang, H.; Berman, B.M.; Lao, L. IL-1ra alleviates inflammatory hyperalgesia through preventing phosphorylation of NMDA receptor NR-1 subunit in rats. Pain 2008, 135, 232–239. [Google Scholar] [CrossRef] [PubMed]
- Liu, T.; Zhang, L.; Joo, D.; Sun, S.C. NF-kappaB signaling in inflammation. Signal Transduct. Target. Ther. 2017, 2, 17023. [Google Scholar] [CrossRef]
- Ta, N.N.; Schuyler, C.A.; Li, Y.; Lopes-Virella, M.F.; Huang, Y. DPP-4 (CD26) inhibitor alogliptin inhibits atherosclerosis in diabetic apolipoprotein E-deficient mice. J. Cardiovasc. Pharmacol. 2011, 58, 157–166. [Google Scholar] [CrossRef]
- Lewandowski, A.; Deaver, C.M.; Myers, M.J. Lambda-Carrageenan initiates inflammation via activation of heterodimers TLR2/6 and TLR4/6. J. Immunol. 2018, 200, 42–112. [Google Scholar] [CrossRef]
- Ruiz-Miyazawa, K.W.; Zarpelon, A.C.; Pinho-Ribeiro, F.A.; Pavao-de-Souza, G.F.; Casagrande, R.; Verri, W.A., Jr. Vinpocetine reduces carrageenan-induced inflammatory hyperalgesia in mice by inhibiting oxidative stress, cytokine production and NF-kappaB activation in the paw and spinal cord. PLoS ONE 2015, 10, e0118942. [Google Scholar] [CrossRef]
- Han, X.; Shao, J.; Ren, X.; Li, Y.; Yu, W.; Lin, C.; Li, L.; Sun, Y.; Xu, B.; Luo, H.; et al. The different mechanisms of peripheral and central TLR4 on chronic postsurgical pain in rats. J. Anat. 2021, 239, 111–124. [Google Scholar] [CrossRef]
- Lacagnina, M.J.; Watkins, L.R.; Grace, P.M. Toll-like receptors and their role in persistent pain. Pharmacol. Ther. 2018, 184, 145–158. [Google Scholar] [CrossRef]
- Ohnuma, K.; Yamochi, T.; Uchiyama, M.; Nishibashi, K.; Iwata, S.; Hosono, O.; Kawasaki, H.; Tanaka, H.; Dang, N.H.; Morimoto, C. CD26 mediates dissociation of Tollip and IRAK-1 from caveolin-1 and induces upregulation of CD86 on antigen-presenting cells. Mol. Cell. Biol. 2005, 25, 7743–7757. [Google Scholar] [CrossRef] [PubMed]
- Hiromura, M.; Nohtomi, K.; Mori, Y.; Kataoka, H.; Sugano, M.; Ohnuma, K.; Kuwata, H.; Hirano, T. Caveolin-1, a binding protein of CD26, is essential for the anti-inflammatory effects of dipeptidyl peptidase-4 inhibitors on human and mouse macrophages. Biochem. Biophys. Res. Commun. 2018, 495, 223–229. [Google Scholar] [CrossRef] [PubMed]
- El-Hage, N.; Podhaizer, E.M.; Sturgill, J.; Hauser, K.F. Toll-like receptor expression and activation in astroglia: Differential regulation by HIV-1 Tat, gp120, and morphine. Immunol. Investig. 2011, 40, 498–522. [Google Scholar] [CrossRef] [PubMed]
- Holm, T.H.; Draeby, D.; Owens, T. Microglia are required for astroglial Toll-like receptor 4 response and for optimal TLR2 and TLR3 response. Glia 2012, 60, 630–638. [Google Scholar] [CrossRef]
- Yoon, H.; Walters, G.; Paulsen, A.R.; Scarisbrick, I.A. Astrocyte heterogeneity across the brain and spinal cord occurs developmentally, in adulthood and in response to demyelination. PLoS ONE 2017, 12, e0180697. [Google Scholar] [CrossRef]
- Kiraly, K.; Lambeir, A.M.; Szalai, J.; Szentirmay, A.; Luyten, W.; Barna, I.; Puskar, Z.; Kozsurek, M.; Ronai, A.Z. The dipeptidyl peptidase IV (CD26, EC 3.4.14.5) inhibitor vildagliptin is a potent antihyperalgesic in rats by promoting endomorphin-2 generation in the spinal cord. Eur. J. Pharmacol. 2011, 650, 195–199. [Google Scholar] [CrossRef]
- Hutchinson, M.R.; Zhang, Y.; Shridhar, M.; Evans, J.H.; Buchanan, M.M.; Zhao, T.X.; Slivka, P.F.; Coats, B.D.; Rezvani, N.; Wieseler, J.; et al. Evidence that opioids may have toll-like receptor 4 and MD-2 effects. Brain Behav. Immun. 2010, 24, 83–95. [Google Scholar] [CrossRef]
- Hutchinson, M.R.; Lewis, S.S.; Coats, B.D.; Rezvani, N.; Zhang, Y.; Wieseler, J.L.; Somogyi, A.A.; Yin, H.; Maier, S.F.; Rice, K.C.; et al. Possible involvement of toll-like receptor 4/myeloid differentiation factor-2 activity of opioid inactive isomers causes spinal proinflammation and related behavioral consequences. Neuroscience 2010, 167, 880–893. [Google Scholar] [CrossRef]
- Wang, X.; Zhang, Y.; Peng, Y.; Hutchinson, M.R.; Rice, K.C.; Yin, H.; Watkins, L.R. Pharmacological characterization of the opioid inactive isomers (+)-naltrexone and (+)-naloxone as antagonists of toll-like receptor 4. Br. J. Pharmacol. 2016, 173, 856–869. [Google Scholar] [CrossRef]
- Grace, P.M.; Ramos, K.M.; Rodgers, K.M.; Wang, X.; Hutchinson, M.R.; Lewis, M.T.; Morgan, K.N.; Kroll, J.L.; Taylor, F.R.; Strand, K.A.; et al. Activation of adult rat CNS endothelial cells by opioid-induced toll-like receptor 4 (TLR4) signaling induces proinflammatory, biochemical, morphological, and behavioral sequelae. Neuroscience 2014, 280, 299–317. [Google Scholar] [CrossRef] [PubMed]
- Cant, R.; Dalgleish, A.G.; Allen, R.L. Naltrexone Inhibits IL-6 and TNFalpha Production in Human Immune Cell Subsets following Stimulation with Ligands for Intracellular Toll-Like Receptors. Front. Immunol. 2017, 8, 809. [Google Scholar] [CrossRef] [PubMed]
- Conway, S.M.; Mikati, M.O.; Al-Hasani, R. Challenges and new opportunities for detecting endogenous opioid peptides in reward. Addict. Neurosci. 2022, 2, 100016. [Google Scholar] [CrossRef] [PubMed]
- Zhang, P.; Yang, M.; Chen, C.; Liu, L.; Wei, X.; Zeng, S. Toll-Like Receptor 4 (TLR4)/Opioid Receptor Pathway Crosstalk and Impact on Opioid Analgesia, Immune Function, and Gastrointestinal Motility. Front. Immunol. 2020, 11, 1455. [Google Scholar] [CrossRef] [PubMed]
- Hutchinson, M.R.; Zhang, Y.; Brown, K.; Coats, B.D.; Shridhar, M.; Sholar, P.W.; Patel, S.J.; Crysdale, N.Y.; Harrison, J.A.; Maier, S.F.; et al. Non-stereoselective reversal of neuropathic pain by naloxone and naltrexone: Involvement of toll-like receptor 4 (TLR4). Eur. J. Neurosci. 2008, 28, 20–29. [Google Scholar] [CrossRef] [PubMed]
- Martin, S.J.; McAnally, H.B.; Okediji, P.; Rogosnitzky, M. Low-dose naltrexone, an opioid-receptor antagonist, is a broad-spectrum analgesic: A retrospective cohort study. Pain Manag. 2022, 12, 699–709. [Google Scholar] [CrossRef] [PubMed]
- Spetea, M.; Berzetei-Gurske, I.P.; Guerrieri, E.; Schmidhammer, H. Discovery and pharmacological evaluation of a diphenethylamine derivative (HS665), a highly potent and selective kappa opioid receptor agonist. J. Med. Chem. 2012, 55, 10302–10306. [Google Scholar] [CrossRef]
- Guerrieri, E.; Mallareddy, J.R.; Toth, G.; Schmidhammer, H.; Spetea, M. Synthesis and pharmacological evaluation of [3H]HS665, a novel, highly selective radioligand for the kappa opioid receptor. ACS Chem. Neurosci. 2015, 6, 456–463. [Google Scholar] [CrossRef]
- Uddman, R.; Edvinsson, L.; Ekman, R.; Kingman, T.; McCulloch, J. Innervation of the feline cerebral vasculature by nerve fibers containing calcitonin gene-related peptide: Trigeminal origin and co-existence with substance P. Neurosci. Lett. 1985, 62, 131–136. [Google Scholar] [CrossRef]
- Cuello, A.C.; Galfre, G.; Milstein, C. Detection of substance P in the central nervous system by a monoclonal antibody. Proc. Natl. Acad. Sci. USA 1979, 76, 3532–3536. [Google Scholar] [CrossRef]
- Schagger, H. Tricine-SDS-PAGE. Nat. Protoc. 2006, 1, 16–22. [Google Scholar] [CrossRef] [PubMed]
- Koylu, E.O.; Couceyro, P.R.; Lambert, P.D.; Ling, N.C.; DeSouza, E.B.; Kuhar, M.J. Immunohistochemical localization of novel CART peptides in rat hypothalamus, pituitary and adrenal gland. J. Neuroendocrinol. 1997, 9, 823–833. [Google Scholar] [CrossRef] [PubMed]
- Kiraly, K.; Szalay, B.; Szalai, J.; Barna, I.; Gyires, K.; Verbeken, M.; Ronai, A.Z. Intrathecally injected Ile-Pro-Ile, an inhibitor of membrane ectoenzyme dipeptidyl peptidase IV, is antihyperalgesic in rats by switching the enzyme from hydrolase to synthase functional mode to generate endomorphin 2. Eur. J. Pharmacol. 2009, 620, 21–26. [Google Scholar] [CrossRef] [PubMed]
- Mestre, C.; Pelissier, T.; Fialip, J.; Wilcox, G.; Eschalier, A. A method to perform direct transcutaneous intrathecal injection in rats. J. Pharmacol. Toxicol. Methods 1994, 32, 197–200. [Google Scholar] [CrossRef] [PubMed]
- Randall, L.O.; Selitto, J.J. A method for measurement of analgesic activity on inflamed tissue. Arch. Int. Pharmacodyn. Ther. 1957, 111, 409–419. [Google Scholar]
- Seltzer, Z.; Dubner, R.; Shir, Y. A novel behavioral model of neuropathic pain disorders produced in rats by partial sciatic nerve injury. Pain 1990, 43, 205–218. [Google Scholar] [CrossRef]
- Robinson, I.; Meert, T.F. Stability of neuropathic pain symptoms in partial sciatic nerve ligation in rats is affected by suture material. Neurosci. Lett. 2005, 373, 125–129. [Google Scholar] [CrossRef]
- Benyhe, S.; Farkas, J.; Toth, G.; Wollemann, M. Met5-enkephalin-Arg6-Phe7, an endogenous neuropeptide, binds to multiple opioid and nonopioid sites in rat brain. J. Neurosci. Res. 1997, 48, 249–258. [Google Scholar] [CrossRef]
- Zador, F.; Kocsis, D.; Borsodi, A.; Benyhe, S. Micromolar concentrations of rimonabant directly inhibits delta opioid receptor specific ligand binding and agonist-induced G-protein activity. Neurochem. Int. 2014, 67, 14–22. [Google Scholar] [CrossRef]
- Bradford, M.M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal. Biochem. 1976, 72, 248–254. [Google Scholar] [CrossRef]
- Traynor, J.R.; Nahorski, S.R. Modulation by mu-opioid agonists of guanosine-5′-O-(3-[35S]thio)triphosphate binding to membranes from human neuroblastoma SH-SY5Y cells. Mol. Pharmacol. 1995, 47, 848–854. [Google Scholar] [PubMed]
- Sim, L.J.; Selley, D.E.; Childers, S.R. In vitro autoradiography of receptor-activated G proteins in rat brain by agonist-stimulated guanylyl 5′-[gamma-[35S]thio]-triphosphate binding. Proc. Natl. Acad. Sci. USA 1995, 92, 7242–7246. [Google Scholar] [CrossRef] [PubMed]
- Nirogi, R.; Kandikere, V.; Mudigonda, K.; Bhyrapuneni, G.; Muddana, N.; Saralaya, R.; Benade, V. A simple and rapid method to collect the cerebrospinal fluid of rats and its application for the assessment of drug penetration into the central nervous system. J. Neurosci. Meth. 2009, 178, 116–119. [Google Scholar] [CrossRef] [PubMed]
- Pap, P.; Koszeghy, A.; Szucs, G.; Rusznak, Z. Cytoplasmic Ca2+ concentration changes evoked by cholinergic stimulation in primary astrocyte cultures prepared from the rat cochlear nucleus. Hear. Res. 2009, 255, 73–83. [Google Scholar] [CrossRef]
- Boytard, L.; Hadi, T.; Silvestro, M.; Qu, H.; Kumpfbeck, A.; Sleiman, R.; Fils, K.H.; Alebrahim, D.; Boccalatte, F.; Kugler, M.; et al. Lung-derived HMGB1 is detrimental for vascular remodeling of metabolically imbalanced arterial macrophages. Nat. Commun. 2020, 11, 4311. [Google Scholar] [CrossRef]
- Nomura, R.; Fujimoto, T. Tyrosine-phosphorylated caveolin-1: Immunolocalization and molecular characterization. Mol. Biol. Cell 1999, 10, 975–986. [Google Scholar] [CrossRef]
- Hartig, W.; Mages, B.; Aleithe, S.; Nitzsche, B.; Altmann, S.; Barthel, H.; Krueger, M.; Michalski, D. Damaged Neocortical Perineuronal Nets Due to Experimental Focal Cerebral Ischemia in Mice, Rats and Sheep. Front. Integr. Neurosci. 2017, 11, 15. [Google Scholar] [CrossRef]
- Gajtko, A.; Bakk, E.; Hegedus, K.; Ducza, L.; Hollo, K. IL-1beta Induced Cytokine Expression by Spinal Astrocytes Can Play a Role in the Maintenance of Chronic Inflammatory Pain. Front. Physiol. 2020, 11, 543331. [Google Scholar] [CrossRef]
- Hollo, K.; Glant, T.T.; Garzo, M.; Finnegan, A.; Mikecz, K.; Buzas, E. Complex pattern of Th1 and Th2 activation with a preferential increase of autoreactive Th1 cells in BALB/c mice with proteoglycan (aggrecan)-induced arthritis. Clin. Exp. Immunol. 2000, 120, 167–173. [Google Scholar] [CrossRef] [PubMed]
- Tayabali, A.F.; Nguyen, K.C.; Shwed, P.S.; Crosthwait, J.; Coleman, G.; Seligy, V.L. Comparison of the virulence potential of Acinetobacter strains from clinical and environmental sources. PLoS ONE 2012, 7, e37024. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kozsurek, M.; Király, K.; Gyimesi, K.; Lukácsi, E.; Fekete, C.; Gereben, B.; Mohácsik, P.; Helyes, Z.; Bölcskei, K.; Tékus, V.; et al. Unique, Specific CART Receptor-Independent Regulatory Mechanism of CART(55-102) Peptide in Spinal Nociceptive Transmission and Its Relation to Dipeptidyl-Peptidase 4 (DDP4). Int. J. Mol. Sci. 2023, 24, 918. https://doi.org/10.3390/ijms24020918
Kozsurek M, Király K, Gyimesi K, Lukácsi E, Fekete C, Gereben B, Mohácsik P, Helyes Z, Bölcskei K, Tékus V, et al. Unique, Specific CART Receptor-Independent Regulatory Mechanism of CART(55-102) Peptide in Spinal Nociceptive Transmission and Its Relation to Dipeptidyl-Peptidase 4 (DDP4). International Journal of Molecular Sciences. 2023; 24(2):918. https://doi.org/10.3390/ijms24020918
Chicago/Turabian StyleKozsurek, Márk, Kornél Király, Klára Gyimesi, Erika Lukácsi, Csaba Fekete, Balázs Gereben, Petra Mohácsik, Zsuzsanna Helyes, Kata Bölcskei, Valéria Tékus, and et al. 2023. "Unique, Specific CART Receptor-Independent Regulatory Mechanism of CART(55-102) Peptide in Spinal Nociceptive Transmission and Its Relation to Dipeptidyl-Peptidase 4 (DDP4)" International Journal of Molecular Sciences 24, no. 2: 918. https://doi.org/10.3390/ijms24020918
APA StyleKozsurek, M., Király, K., Gyimesi, K., Lukácsi, E., Fekete, C., Gereben, B., Mohácsik, P., Helyes, Z., Bölcskei, K., Tékus, V., Pap, K., Szűcs, E., Benyhe, S., Imre, T., Szabó, P., Gajtkó, A., Holló, K., & Puskár, Z. (2023). Unique, Specific CART Receptor-Independent Regulatory Mechanism of CART(55-102) Peptide in Spinal Nociceptive Transmission and Its Relation to Dipeptidyl-Peptidase 4 (DDP4). International Journal of Molecular Sciences, 24(2), 918. https://doi.org/10.3390/ijms24020918






