Inactivation of HAP4 Accelerates RTG-Dependent Osmoadaptation in Saccharomyces cerevisiae
Abstract
1. Introduction
2. Results
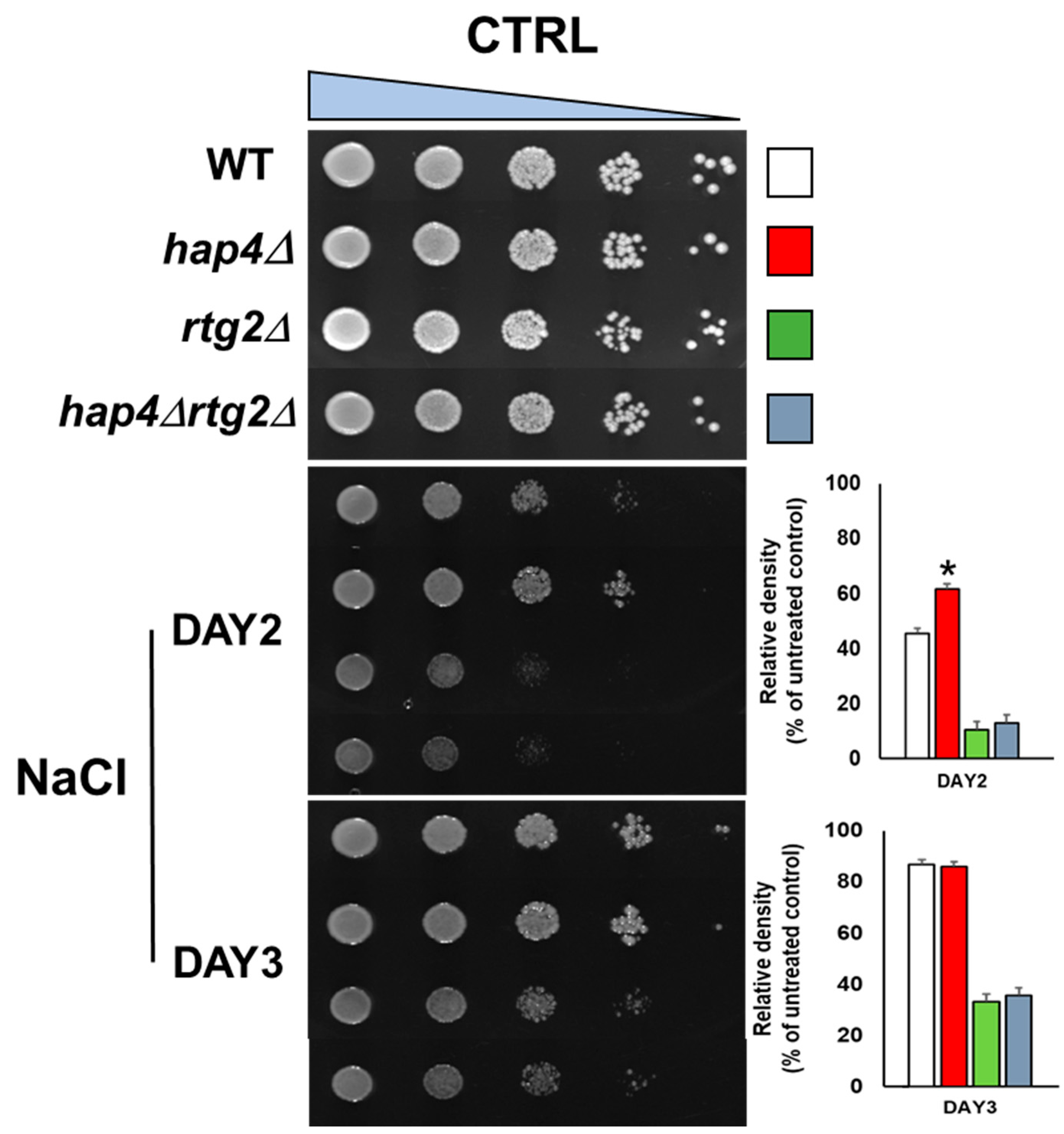
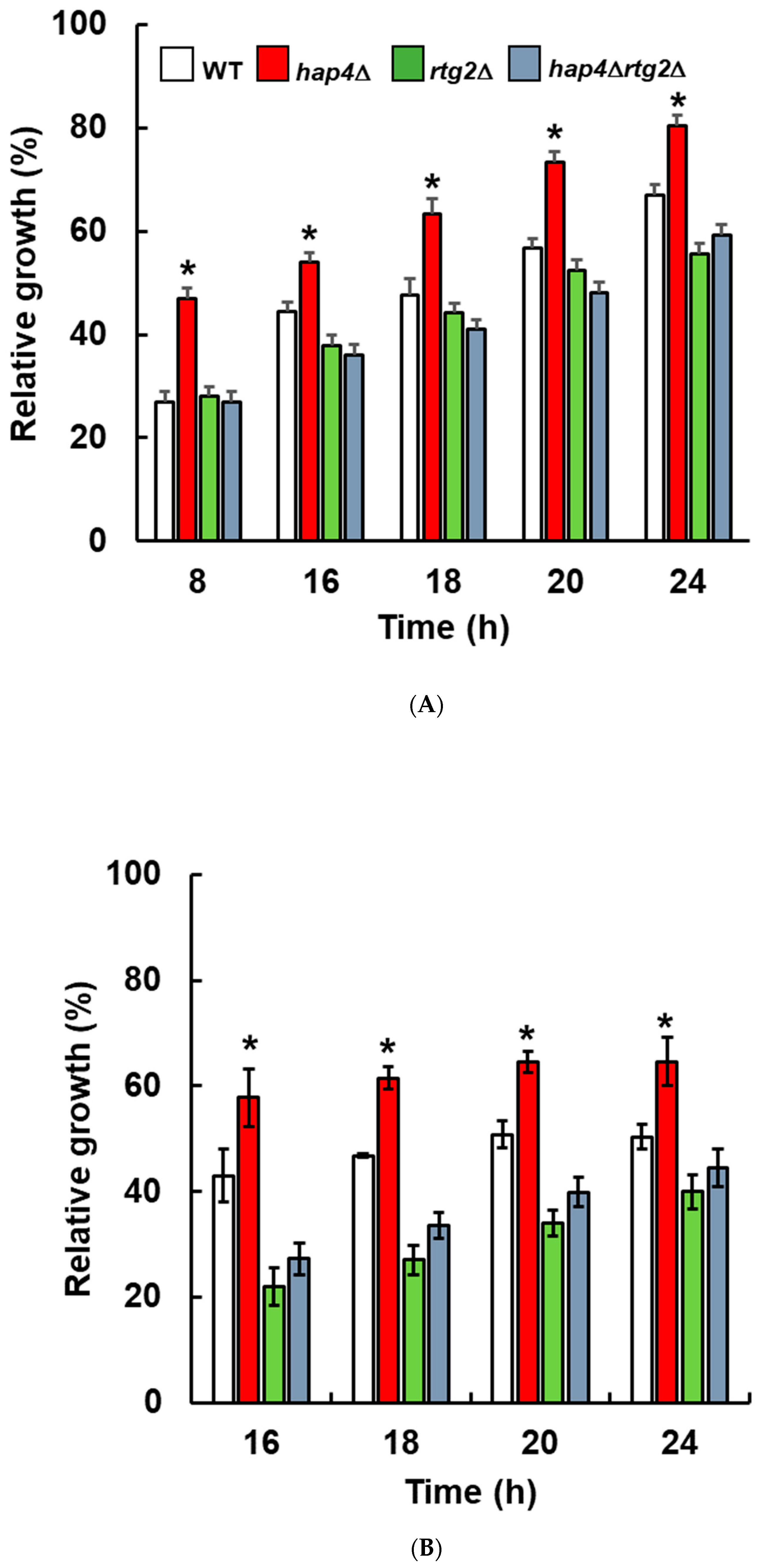
2.1. Deletion of HAP4 Accelerates Osmoadaptation in a Manner Dependent on RTG2
2.2. Mitochondrial Respiratory Competence Is Not Required for Faster Adaptation
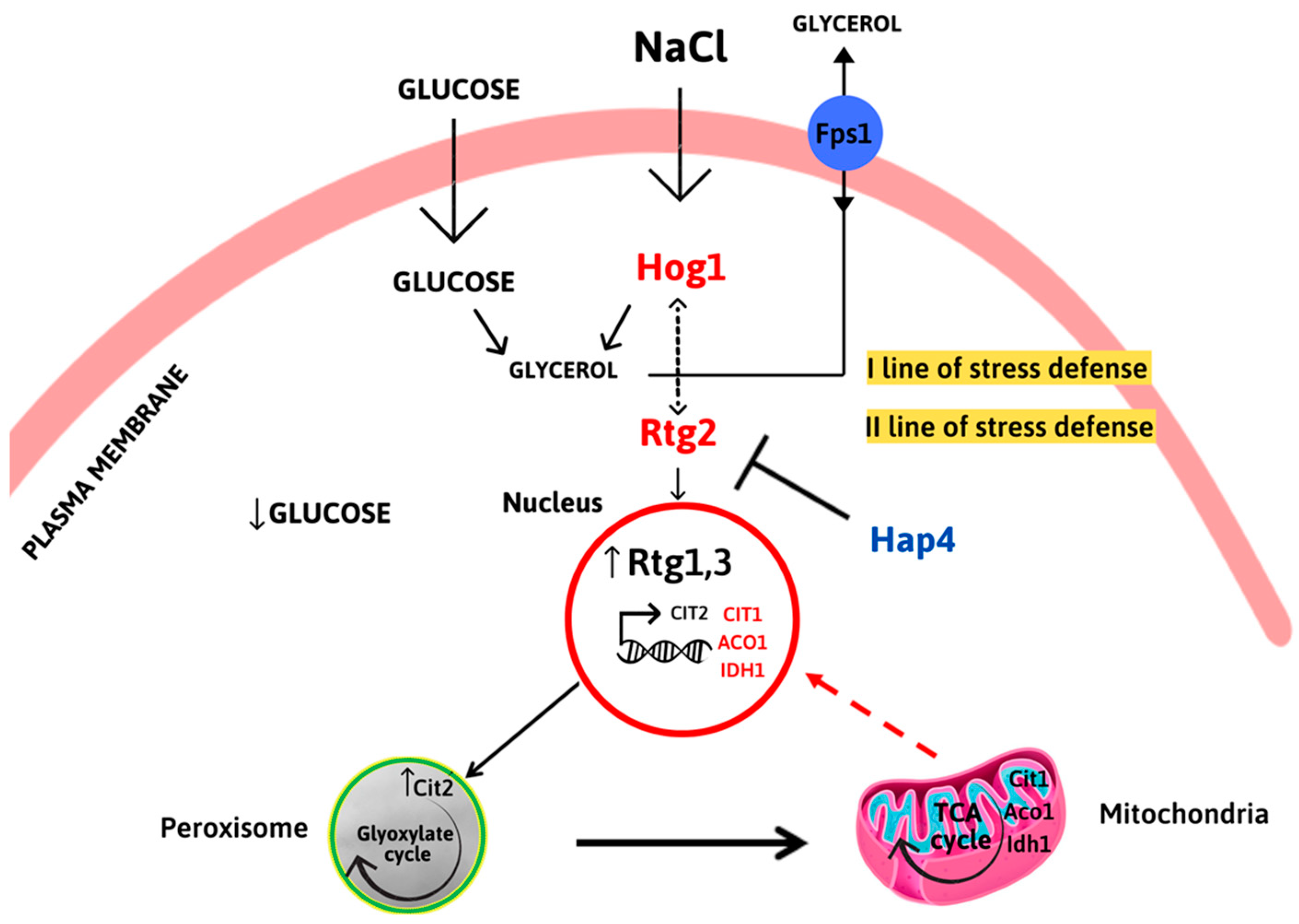
2.3. RTG Pathway Is Activated in Cells Lacking HAP4
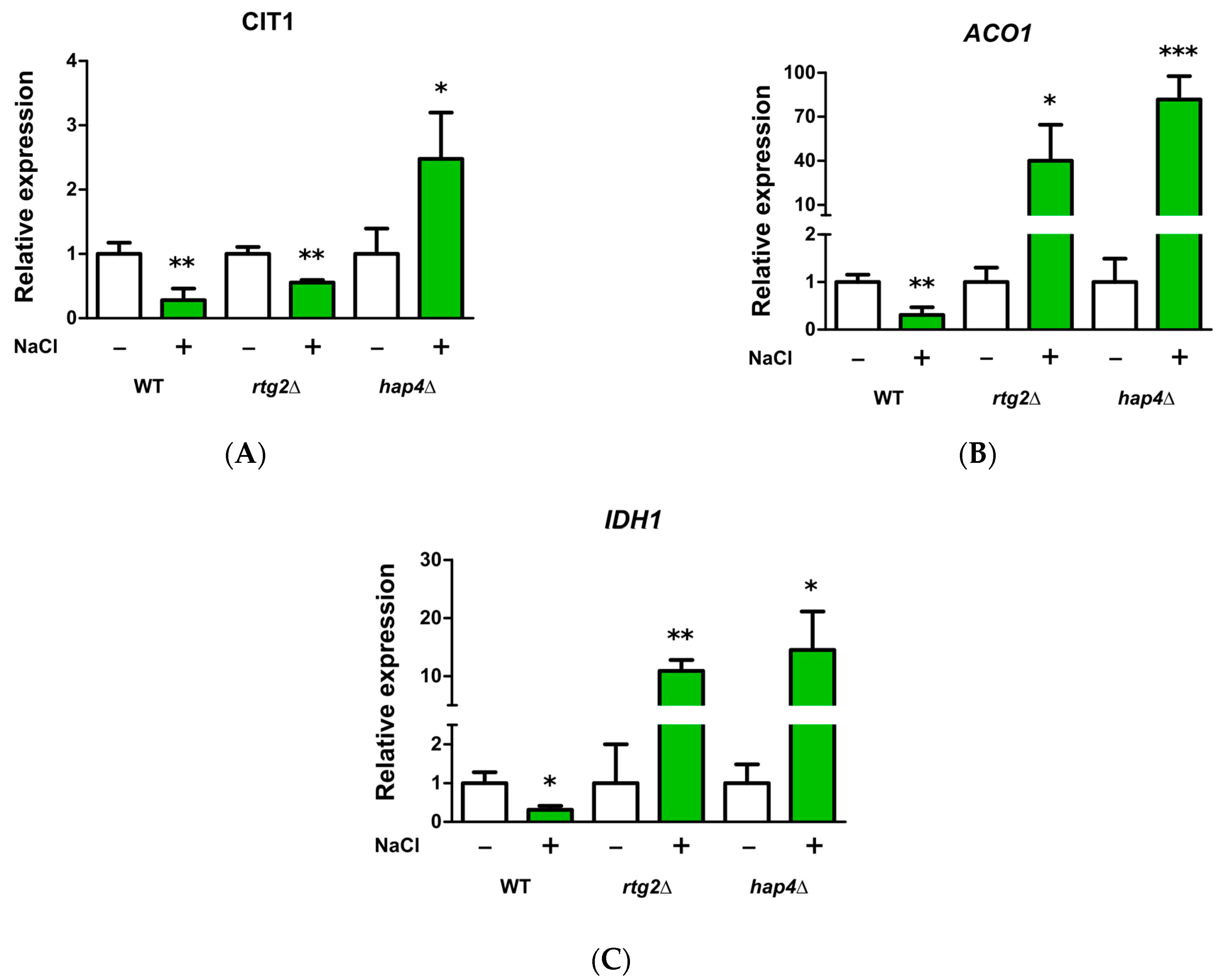
2.4. HAP4 and RTG2 Modulate Mitochondrial TCA Cycle Genes Expression upon Salt Stress
3. Discussion
4. Materials and Methods
4.1. Yeast Strains and Growth Conditions
4.2. Micro- and Batch-Culture Growth Assays
4.3. Spotting Assay
4.4. Quantitative PCR (qPCR)
4.5. Statistical Analysis
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Shen, K.; Pender, C.L.; Bar-Ziv, R.; Zhang, H.; Wickham, K.; Willey, E.; Durieux, J.; Ahmad, Q.; Dillin, A. Mitochondria as Cellular and Organismal Signaling Hubs. Annu. Rev. Cell Dev. Biol. 2022, 38, 179–218. [Google Scholar] [CrossRef]
- Chakrabarty, R.P.; Chandel, N.S. Mitochondria as Signaling Organelles Control Mammalian Stem Cell Fate. Cell Stem Cell 2021, 28, 394–408. [Google Scholar] [CrossRef] [PubMed]
- Hijazi, I.; Wang, E.; Orozco, M.; Pelton, S.; Chang, A. Peroxisomal Support of Mitochondrial Respiratory Efficiency Promotes ER Stress Survival. J. Cell Sci. 2022, 135, jcs259254. [Google Scholar] [CrossRef]
- Deus, C.M.; Yambire, K.F.; Oliveira, P.J.; Raimundo, N. Mitochondria-Lysosome Crosstalk: From Physiology to Neurodegeneration. Trends Mol. Med. 2020, 26, 71–88. [Google Scholar] [CrossRef]
- Kumar, V.; Maity, S. ER Stress-Sensor Proteins and ER-Mitochondrial Crosstalk-Signaling Beyond (ER) Stress Response. Biomolecules 2021, 11, 173. [Google Scholar] [CrossRef] [PubMed]
- Liu, Z.; Butow, R.A. Mitochondrial Retrograde Signaling. Annu. Rev. Genet. 2006, 40, 159–185. [Google Scholar] [CrossRef] [PubMed]
- Guaragnella, N.; Coyne, L.P.; Chen, X.J.; Giannattasio, S. Mitochondria–cytosol–nucleus crosstalk: Learning from Saccharomyces cerevisiae. FEMS Yeast Res. 2018, 18, foy088. [Google Scholar] [CrossRef] [PubMed]
- Liu, Z.; Butow, R.A. A Transcriptional Switch in the Expression of Yeast Tricarboxylic Acid Cycle Genes in Response to a Reduction or Loss of Respiratory Function. Mol. Cell. Biol. 1999, 19, 6720–6728. [Google Scholar] [CrossRef] [PubMed]
- Guaragnella, N.; Ždralević, M.; Palková, Z.; Giannattasio, S. Analysis of Mitochondrial Retrograde Signaling in Yeast Model Systems. Methods Mol. Biol. 2021, 2276, 87–102. [Google Scholar] [CrossRef] [PubMed]
- Guaragnella, N.; Ždralević, M.; Lattanzio, P.; Marzulli, D.; Pracheil, T.; Liu, Z.; Passarella, S.; Marra, E.; Giannattasio, S. Yeast Growth in Raffinose Results in Resistance to Acetic-Acid Induced Programmed Cell Death Mostly Due to the Activation of the Mitochondrial Retrograde Pathway. Biochim. Biophys. Acta 2013, 1833, 2765–2774. [Google Scholar] [CrossRef]
- Torelli, N.Q.; Ferreira-Júnior, J.R.; Kowaltowski, A.J.; da Cunha, F.M. RTG1- and RTG2-Dependent Retrograde Signaling Controls Mitochondrial Activity and Stress Resistance in Saccharomyces cerevisiae. Free Radic. Biol. Med. 2015, 81, 30–37. [Google Scholar] [CrossRef] [PubMed]
- Guaragnella, N.; Stirpe, M.; Marzulli, D.; Mazzoni, C.; Giannattasio, S. Acid Stress Triggers Resistance to Acetic Acid-Induced Regulated Cell Death through Hog1 Activation Which Requires RTG2 in Yeast. Oxid. Med. Cell. Longev. 2019, 2019, 4651062. [Google Scholar] [CrossRef] [PubMed]
- Guaragnella, N.; Agrimi, G.; Scarcia, P.; Suriano, C.; Pisano, I.; Bobba, A.; Mazzoni, C.; Palmieri, L.; Giannattasio, S. RTG Signaling Sustains Mitochondrial Respiratory Capacity in HOG1-Dependent Osmoadaptation. Microorganisms 2021, 9, 1894. [Google Scholar] [CrossRef] [PubMed]
- Pastor, M.M.; Proft, M.; Pascual-Ahuir, A. Mitochondrial Function Is an Inducible Determinant of Osmotic Stress Adaptation in Yeast. J. Biol. Chem. 2009, 284, 30307–30317. [Google Scholar] [CrossRef] [PubMed]
- Babazadeh, R.; Lahtvee, P.-J.; Adiels, C.B.; Goksör, M.; Nielsen, J.B.; Hohmann, S. The Yeast Osmostress Response Is Carbon Source Dependent. Sci. Rep. 2017, 7, 990. [Google Scholar] [CrossRef] [PubMed]
- Lahtvee, P.J.; Kumar, R.; Hallström, B.M.; Nielsen, J. Adaptation to different types of stress converge on mitochondrial metabolism. Mol. Biol. Cell 2016, 27, 2505–2514. [Google Scholar] [CrossRef]
- De Nadal, E.; Posas, F. The HOG pathway and the regulation of osmoadaptive responses in yeast. FEMS Yeast Res. 2022, 22, foac013. [Google Scholar] [CrossRef]
- Blomberg, A. Yeast osmoregulation—Glycerol still in pole position. FEMS Yeast Res. 2022, 22, foac035. [Google Scholar] [CrossRef]
- Buschlen, S.; Amillet, J.-M.; Guiard, B.; Fournier, A.; Marcireau, C.; Bolotin-Fukuhara, M. The S. cerevisiae HAP Complex, a Key Regulator of Mitochondrial Function, Coordinates Nuclear and Mitochondrial Gene Expression. Comp. Funct. Genom. 2003, 4, 37–46. [Google Scholar] [CrossRef]
- Mao, Y.; Chen, C. The Hap Complex in Yeasts: Structure, Assembly Mode, and Gene Regulation. Front. Microbiol. 2019, 10, 1645. [Google Scholar] [CrossRef]
- Bouchez, C.L.; Yoboue, E.D.; de la Rosa Vargas, L.E.; Salin, B.; Cuvellier, S.; Rigoulet, M.; Duvezin-Caubet, S.; Devin, A. “Labile” heme critically regulates mitochondrial biogenesis through the transcriptional co-activator Hap4p in Saccharomyces cerevisiae. J. Biol. Chem. 2020, 295, 5095–5109. [Google Scholar] [CrossRef]
- Petropavlovskiy, A.A.; Tauro, M.G.; Lajoie, P.; Duennwald, M.L. A Quantitative Imaging-Based Protocol for Yeast Growth and Survival on Agar Plates. STAR Protoc. 2020, 1, 100182. [Google Scholar] [CrossRef]
- Ruiz-Roig, C.; Noriega, N.; Duch, A.; Posas, F.; de Nadal, E. The Hog1 SAPK Controls the Rtg1/Rtg3 Transcriptional Complex Activity by Multiple Regulatory Mechanisms. Mol. Biol. Cell 2012, 23, 4286–4296. [Google Scholar] [CrossRef] [PubMed]
- Pascual-Ahuir, A.; Manzanares-Estreder, S.; Timón-Gómez, A.; Proft, M. Ask Yeast How to Burn Your Fats: Lessons Learned from the Metabolic Adaptation to Salt Stress. Curr. Genet. 2018, 64, 63–69. [Google Scholar] [CrossRef] [PubMed]
- Castegna, A.; Scarcia, P.; Agrimi, G.; Palmieri, L.; Rottensteiner, H.; Spera, I.; Germinario, L.; Palmieri, F. Identification and Functional Characterization of a Novel Mitochondrial Carrier for Citrate and Oxoglutarate in Saccharomyces cerevisiae. J. Biol. Chem. 2010, 285, 17359–17370. [Google Scholar] [CrossRef] [PubMed]
- Scarcia, P.; Palmieri, L.; Agrimi, G.; Palmieri, F.; Rottensteiner, H. Three Mitochondrial Transporters of Saccharomyces cerevisiae Are Essential for Ammonium Fixation and Lysine Biosynthesis in Synthetic Minimal Medium. Mol. Genet. Metab. 2017, 122, 54–60. [Google Scholar] [CrossRef]
- Scarcia, P.; Agrimi, G.; Germinario, L.; Ibrahim, A.; Rottensteiner, H.; Palmieri, F.; Palmieri, L. In Saccharomyces cerevisiae Grown in Synthetic Minimal Medium Supplemented with Non-Fermentable Carbon Sources Glutamate Is Synthesized within Mitochondria. Rend. Lincei Sci. Fis. Nat. 2018, 29, 483–490. [Google Scholar] [CrossRef]
- Chen, X.J.; Wang, X.; Kaufman, B.A.; Butow, R.A. Aconitase Couples Metabolic Regulation to Mitochondrial DNA Maintenance. Science 2005, 307, 714–717. [Google Scholar] [CrossRef]
- Capps, D.; Hunter, A.; Chiang, M.; Pracheil, T.; Liu, Z. Ubiquitin-Conjugating Enzymes Ubc1 and Ubc4 Mediate the Turnover of Hap4, a Master Regulator of Mitochondrial Biogenesis in Saccharomyces cerevisiae. Microorganisms 2022, 10, 2370. [Google Scholar] [CrossRef] [PubMed]
- Parrella, E.; Longo, V.D. The Chronological Life Span of Saccharomyces cerevisiae to Study Mitochondrial Dysfunction and Disease. Methods 2008, 46, 256–262. [Google Scholar] [CrossRef]
- Toussaint, M.; Conconi, A. High-Throughput and Sensitive Assay to Measure Yeast Cell Growth: A Bench Protocol for Testing Genotoxic Agents. Nat. Protoc. 2006, 1, 1922–1928. [Google Scholar] [CrossRef] [PubMed]


Strains | Control Doubling Time (h) | + NaCl Doubling Time (h) |
|---|---|---|
| WT | 2.00 ± 0.32 | 3.90 ± 0.71 |
| hap4Δ | 2.55 ± 0.07 | 3.00 ± 0.42 |
| rtg2Δ | 2.20 ± 0.07 | 5.90 ± 0.12 |
| hap4Δrtg2Δ | 2.65 ± 0.07 | 5.95 ± 0.64 |
| Primer | Sequence |
|---|---|
| CIT1 Forward | 5′-GCGCCTCCGAACAAACG-3′ |
| CIT1 Reverse | 5′-CTGCCTTTGCTGGGATAATTTC-3′ |
| ACO1 Forward | 5′-CAAGAACCCAGCTGACTATGACA-3′ |
| ACO1 Reverse | 5′-CCAATTCAGCTAGACCCAGAATATC-3′ |
| IDH1 Forward | 5′-CCCCTTCAATGTACGGTACCA-3′ |
| IDH1 Reverse | 5′-TGGACCACCGATCAAAGCA-3′ |
| CIT2 Forward | 5′-TGTAAGGCAATTCGTTAAAGAGCAT-3′ |
| CIT2 Reverse | 5′-CCCATACGCTCCCTGGAATAC-3′ |
| ACT1 Forward | 5′- ACTTTCAACGTTCCAGCCTTCT-3′ |
| ACT1 Reverse | 5′-ACACCATCACCGGAATCCAA-3′ |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Di Noia, M.A.; Scarcia, P.; Agrimi, G.; Ocheja, O.B.; Wahid, E.; Pisano, I.; Paradies, E.; Palmieri, L.; Guaragnella, C.; Guaragnella, N. Inactivation of HAP4 Accelerates RTG-Dependent Osmoadaptation in Saccharomyces cerevisiae. Int. J. Mol. Sci. 2023, 24, 5320. https://doi.org/10.3390/ijms24065320
Di Noia MA, Scarcia P, Agrimi G, Ocheja OB, Wahid E, Pisano I, Paradies E, Palmieri L, Guaragnella C, Guaragnella N. Inactivation of HAP4 Accelerates RTG-Dependent Osmoadaptation in Saccharomyces cerevisiae. International Journal of Molecular Sciences. 2023; 24(6):5320. https://doi.org/10.3390/ijms24065320
Chicago/Turabian StyleDi Noia, Maria Antonietta, Pasquale Scarcia, Gennaro Agrimi, Ohiemi Benjamin Ocheja, Ehtisham Wahid, Isabella Pisano, Eleonora Paradies, Luigi Palmieri, Cataldo Guaragnella, and Nicoletta Guaragnella. 2023. "Inactivation of HAP4 Accelerates RTG-Dependent Osmoadaptation in Saccharomyces cerevisiae" International Journal of Molecular Sciences 24, no. 6: 5320. https://doi.org/10.3390/ijms24065320
APA StyleDi Noia, M. A., Scarcia, P., Agrimi, G., Ocheja, O. B., Wahid, E., Pisano, I., Paradies, E., Palmieri, L., Guaragnella, C., & Guaragnella, N. (2023). Inactivation of HAP4 Accelerates RTG-Dependent Osmoadaptation in Saccharomyces cerevisiae. International Journal of Molecular Sciences, 24(6), 5320. https://doi.org/10.3390/ijms24065320









