Let’s Go 3D! New Generation of Models for Evaluating Drug Response and Resistance in Prostate Cancer
Abstract
1. Prostate Cancer
2. Diagnosis and Therapy of PC
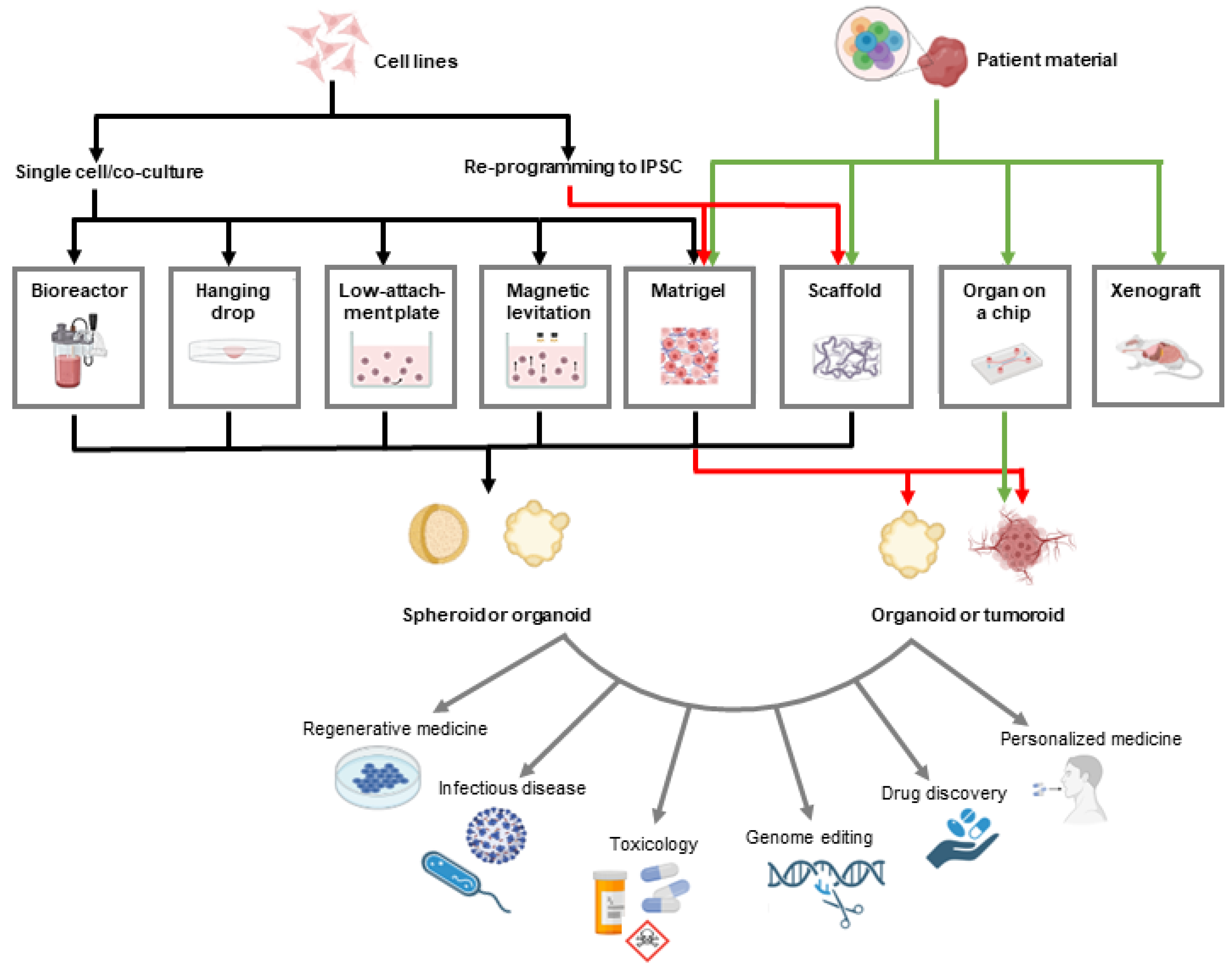
3. Three-Dimensional Prostate Cancer Models in Drug Discovery
3.1. Spheroids vs. Organoids vs. Tumoroids
3.2. Methods for Growing 3D PC Models
3.2.1. Suspension Cell Cultures
3.2.2. Hanging Drop
3.2.3. Organ-on-a-Chip Technology
3.2.4. Gel-Embedding
3.2.5. Prefabricated Scaffolds
3.2.6. Patient-Derived Explants
3.3. Drug Discovery and Screening
| Type of Treatment | Name of the Compound/Treatment | Type of 3D Model Used | Reference |
|---|---|---|---|
| chemotherapeutic | docetaxel | spheroids in U-bottom plates and Matrigel-embedded | [104] |
| chemotherapeutic | docetaxel on gold nanoparticles | spheroids in low-attachment plates | [105] |
| chemotherapeutic | bortezomib | spheroids in agarose-coated plates | [106] |
| chemotherapeutic | docetaxel on microparticles | spheroids in low-attachment plates | [107] |
| natural compound | Brachydin C | spheroids in agarose-coated dishes | [108] |
| natural compound | Brachydin A | spheroids in agarose-coated dishes | [109] |
| natural compound | green tea extract | spheroids in hanging drop | [110] |
| natural compound | perillilaldehyde | spheroids in poly-HEMA-coated plates | [111] |
| natural compound | pristimerin | spheroids in poly-HEMA-coated plates | [112] |
| natural compound | curcumin | spheroids in low-attachment plates | [113] |
| natural compound | gallic acid | spheroids in hanging drops | [114] |
| natural compound | procyanidin B2 3,3″-di-O-gallate | spheroids in low-attachment plates | [115] |
| natural compound | rosmarinic acid | spheroids in hanging drops | [116] |
| statin | simvastatin | spheroids in hanging drop (plates) | [117] |
| statin | rosuvastatin | spheroids in agarose-coated plates (liquid overlay) | [118] |
| ADT | darolutamide | spheroids in low-attachment plates | [119] |
| radionuclide | radium-233 | spheroids in low-attachment plates | [120] |
| radionuclide | 225Ac on liposomes/antibody | spheroids in low-attachment plates | [121] |
| radionuclide | 64CuCl2 | spheroids in low-attachment plates | [122] |
| hormone | 17β-estradiol or testosterone | spheroids in agarose-coated wells (1 spheroid/well) | [123] |
| antibody | TNB-585 (anti-PSMA antibody) | spheroids in low-attachment round-bottom plates | [124] |
| antibody-drug conjugate | antibody-drug conjugate U3-1402 | patient-derived xenograft organoids | [125] |
| antibody-drug conjugate | antibody-drug conjugates VH1-HLE-AF680 | spheroids in methocellulose + Matrigel hanging drop plates | [126] |
| ligand-radionuclide conjugate | PSMA-targeting ligand labeled with 212Pb | spheroids in agarose-coated plates | [127] |
| immunotoxin | anti-PSMA immunotoxin hD7-1(VL-VH)-PE40 | spheroids in agarose-coated plates | [128] |
| oncolytic virus | PIV5 oncolytic virus | spheroids in low-attachment plates | [129] |
| ultrasound | focused ultrasound | spheroids in low-attachment plates | [130] |
| microgravity | microgravity | spheroids in microgravity or agarose-coated dishes | [63] |
| CHK1 inhibitor | MU380 | spheroids in low-attachment plates | [131] |
| DNMT inhibitor | CM-272 | spheroids in U-bottom plates | [132] |
| kinase inhibitor | ponatinib, sunitinib, sorafenib | organoids | [133] |
| kinase inhibitor | Dovitinib, BGJ398, or PD166866 | spheroids in agarose-coated plates | [134] |
| HDAC inhibitor | Jazz90, Jazz167 | spheroids in Matrigel | [135] |
| mPGES-1 inhibitor | KH176m | spheroids in Matrigel, low-attachment plates | [136] |
| TRPM8 antagonist | TRPM8 antagonist | spheroids in ECM | [137] |
| NUAK antagonist | WZ4003 and HTH-02-006 | spheroids in low-attachment plates | [138] |
| PKC agonist | HMI-1a3 | spheroids in agarose-coated U-bottom plates | [139] |
| Cyclodextrin nanosponge | GSH-NSs | spheroids in hanging drops | [140] |
| cytotoxic metal | Ir(III)–Cu(II) Compounds on liposomes | spheroids in hanging drops | [141] |
| cytotoxic metal | IrIII complex conjugated to coumarin | spheroids in low-attachment plates | [142] |
| glycoprotein | fetuin-A | spheroids in low-attachment plates | [143] |
| peptide | GV1001 peptide | spheroids in low-attachment plates | [144] |
| small molecule | ATPγS and ATP | spheroids in spheroid culture plates | [145] |
4. Standard and Novel Therapies Used in 3D Models of PC
4.1. Radiotherapy
4.2. Hormone Therapy
4.3. Chemotherapy
4.4. Targeted Therapies
4.5. Novel and Experimental Therapies
| Type of Treatment | Name of the Compound/Treatment | Type of 3D Model Used | Reference |
|---|---|---|---|
| chemotherapeutic + natural compound | lactic acid, arctigenin, docetaxel | spheroids in low-attachment plates | [182] |
| chemotherapeutic + natural compound | curcumin, cisplatin, paclitaxel, docetaxel | spheroids in Matrigel | [177] |
| chemotherapeutic + BET inhibitor | JQ1, docetaxel | spheroids in Matrigel | [104] |
| chemotherapeutic + PARP inhibitor | olaparib and carboplatin | PDX-derived organoids for drug sensitivity testing | [183] |
| chemotherapeutic + radiotherapy | carboplatin and radiotherapy | organoids | [174] |
| chemotherapeutic + siRNA | siMRP1 + doxorubicin | spheroids in low-attachment plates | [181] |
| chemotherapeutic + siRNA | siCD133 + paclitaxel | spheroids in Matrigel | [173] |
| chemotherapeutic + TRAIL inhibitor | taxanes + TRAIL | spheroids in low-attachment plates, monocultures or mixed with fibroblasts | [184] |
| chemotherapeutic + uricosuric | probenecid, doxorubicin, cisplatin | spheroids in low-attachment plates | [185] |
| chemotherapeutic + antioxidant | resveratrol + docetaxel | spheroids in low-attachment plates | [162] |
| chemotherapeutic + hypoxia-activated prodrug | docetaxel, TH-302 | spheroids in low-attachment plates | [100] |
| chemotherapeutic + antibiotic | ciprofloxacin, doxorubicin | [186] | |
| chemotherapeutic + NAMPT inhibitor | FK866 + doxorubicin | spheroids in bioreactor | [187] |
| chemotherapeutic + ion channel inhibitor | Paxilline + docetaxel, paclitaxel, doxorubicin, and cisplatin | spheroids in low-attachment plates | [161] |
| ADT + anti-inflammatory drug + AKR1C inhibitor | MF-15, indomethacin, enzalutamide | spheroids in low-attachment plates | [117] |
| ADT + cytokine | IL-23, enzolutamide, darolutamide | spheroids in low-attachment plates | [188] |
| ADT + small peptide | small peptide Rh-2025u, enzalutamide | spheroids in Matrigel | [189] |
| ADT + small peptide | Enzalutamide or Bicalutamide, recombinant NRG1 peptide | organoids, xenograft | [190] |
| natural compound + MEK inhibitor | curcumin, PD98059 | spheroids in low-attachment plates | [176] |
| natural compound + NEDD8 inhibitor | flavokawain A, MLN4924 | spheroids in low-attachment plates | [178] |
| acyl-CoA synthetase inhibitor + contrasting agent | 5-aminolevulinic acid, triacsin C | spheroids in low-attachment plates | [191] |
| cytotoxic metal + radiation | [Cu(TPZ)2]-liposomes and gamma-radiation | spheroids in agarose-coated plates | [150] |
| hyperthermia + radiation | hyperthermia + electron radiation | spheroids in low-attachment plates | [192] |
| NDRG1 inhibitor + iron chelator | thiosemicarbazones, Dp44mT, DpC | spheroids in collagen hydrogel (liquid overlay) | [193] |
| OGT inhibitor + CDK inhibitor | OSMI-2 + AT7519 | spheroids in Matrigel | [194] |
| kinase inhibitor + siRNA | siEphA2, JIB-04 in lipid nanoparticles | spheroids in poly-HEMA-coated plates | [167] |
| AMPK activator + radiation | AICAR + radiation | spheroids in agar-coated plates (liquid overlay) | [149] |
| statin + anticonvulsant | valproic acid, simvastatin | spheroids in low-attachment plates, multiple generations | [195] |
| antioxidant + siRNA | siMK + quercetin | spheroids in agarose-coated plates (liquid overlay) | [175] |
5. Conclusions and Future Directions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Sung, H.; Ferlay, J.; Siegel, R.L.; Laversanne, M.; Soerjomataram, I.; Jemal, A.; Bray, F. Global Cancer Statistics 2020: GLOBOCAN Estimates of Incidence and Mortality Worldwide for 36 Cancers in 185 Countries. CA Cancer J. Clin. 2021, 71, 209–249. [Google Scholar] [CrossRef] [PubMed]
- Christenson, M.; Song, C.-S.; Liu, Y.-G.; Chatterjee, B. Precision Targets for Intercepting the Lethal Progression of Prostate Cancer: Potential Avenues for Personalized Therapy. Cancers 2022, 14, 892. [Google Scholar] [CrossRef] [PubMed]
- Gandaglia, G.; Abdollah, F.; Schiffmann, J.; Trudeau, V.; Shariat, S.F.; Kim, S.P.; Perrotte, P.; Montorsi, F.; Briganti, A.; Trinh, Q.-D.; et al. Distribution of Metastatic Sites in Patients with Prostate Cancer: A Population-Based Analysis. Prostate 2014, 74, 210–216. [Google Scholar] [CrossRef] [PubMed]
- Raghallaigh, H.N.; Bott, S.R. The Role of Family History and Germline Genetics in Prostate Cancer Disease Profile and Screening. In Urologic Cancers; Barber, N., Ali, A., Eds.; Exon Publications: Brisbane, Australia, 2022; ISBN 978-0-645-33205-6. [Google Scholar]
- Marino, F.; Totaro, A.; Gandi, C.; Bientinesi, R.; Moretto, S.; Gavi, F.; Pierconti, F.; Iacovelli, R.; Bassi, P.; Sacco, E. Germline Mutations in Prostate Cancer: A Systematic Review of the Evidence for Personalized Medicine. Prostate Cancer Prostatic Dis. 2022. [Google Scholar] [CrossRef] [PubMed]
- Ni Raghallaigh, H.; Eeles, R. Genetic Predisposition to Prostate Cancer: An Update. Fam. Cancer 2022, 21, 101–114. [Google Scholar] [CrossRef]
- Bancroft, E.K.; Page, E.C.; Brook, M.N.; Thomas, S.; Taylor, N.; Pope, J.; McHugh, J.; Jones, A.-B.; Karlsson, Q.; Merson, S.; et al. A Prospective Prostate Cancer Screening Programme for Men with Pathogenic Variants in Mismatch Repair Genes (IMPACT): Initial Results from an International Prospective Study. Lancet. Oncol. 2021, 22, 1618–1631. [Google Scholar] [CrossRef]
- Yuan, J.; Kensler, K.H.; Hu, Z.; Zhang, Y.; Zhang, T.; Jiang, J.; Xu, M.; Pan, Y.; Long, M.; Montone, K.T.; et al. Integrative Comparison of the Genomic and Transcriptomic Landscape between Prostate Cancer Patients of Predominantly African or European Genetic Ancestry. PLoS Genet. 2020, 16, e1008641. [Google Scholar] [CrossRef]
- Samtal, C.; El Jaddaoui, I.; Hamdi, S.; Bouguenouch, L.; Ouldim, K.; Nejjari, C.; Ghazal, H.; Bekkari, H. Review of Prostate Cancer Genomic Studies in Africa. Front. Genet. 2022, 13, 911101. [Google Scholar] [CrossRef]
- Plaskon, L.A.; Penson, D.F.; Vaughan, T.L.; Stanford, J.L. Cigarette Smoking and Risk of Prostate Cancer in Middle-Aged Men. Cancer Epidemiol. Biomark. Prev. 2003, 12, 604–609. [Google Scholar]
- Cirne, F.; Kappel, C.; Zhou, S.; Mukherjee, S.D.; Dehghan, M.; Petropoulos, J.-A.; Leong, D.P. Modifiable Risk Factors for Prostate Cancer in Low- and Lower-Middle-Income Countries: A Systematic Review and Meta-Analysis. Prostate Cancer Prostatic Dis. 2022, 25, 453–462. [Google Scholar] [CrossRef]
- Parker, C.; Castro, E.; Fizazi, K.; Heidenreich, A.; Ost, P.; Procopio, G.; Tombal, B.; Gillessen, S. Prostate Cancer: ESMO Clinical Practice Guidelines for Diagnosis, Treatment and Follow-Up†. Ann. Oncol. 2020, 31, 1119–1134. [Google Scholar] [CrossRef] [PubMed]
- Cornu, J.-N.; Cancel-Tassin, G.; Cox, D.G.; Roupret, M.; Koutlidis, N.; Bigot, P.; Valeri, A.; Ondet, V.; Gaffory, C.; Fournier, G.; et al. Impact of Body Mass Index, Age, Prostate Volume, and Genetic Polymorphisms on Prostate-Specific Antigen Levels in a Control Population. Eur. Urol. 2016, 70, 6–8. [Google Scholar] [CrossRef] [PubMed]
- Loeb, S.; Shin, S.S.; Broyles, D.L.; Wei, J.T.; Sanda, M.; Klee, G.; Partin, A.W.; Sokoll, L.; Chan, D.W.; Bangma, C.H.; et al. Prostate Health Index (Phi) Improves Multivariable Risk Prediction of Aggressive Prostate Cancer. BJU Int. 2017, 120, 61–68. [Google Scholar] [CrossRef] [PubMed]
- Kang, S.K.; Mali, R.D.; Prabhu, V.; Ferket, B.S.; Loeb, S. Active Surveillance Strategies for Low-Grade Prostate Cancer: Comparative Benefits and Cost-Effectiveness. Radiology 2021, 300, 594–604. [Google Scholar] [CrossRef] [PubMed]
- Posdzich, P.; Darr, C.; Hilser, T.; Wahl, M.; Herrmann, K.; Hadaschik, B.; Grünwald, V. Metastatic Prostate Cancer—A Review of Current Treatment Options and Promising New Approaches. Cancers 2023, 15, 461. [Google Scholar] [CrossRef] [PubMed]
- Tsujino, T.; Takai, T.; Hinohara, K.; Gui, F.; Tsutsumi, T.; Bai, X.; Miao, C.; Feng, C.; Gui, B.; Sztupinszki, Z.; et al. CRISPR Screens Reveal Genetic Determinants of PARP Inhibitor Sensitivity and Resistance in Prostate Cancer. Nat. Commun. 2023, 14, 252. [Google Scholar] [CrossRef]
- Jamaspishvili, T.; Berman, D.M.; Ross, A.E.; Scher, H.I.; De Marzo, A.M.; Squire, J.A.; Lotan, T.L. Clinical Implications of PTEN Loss in Prostate Cancer. Nat. Rev. Urol. 2018, 15, 222–234. [Google Scholar] [CrossRef]
- de Bono, J.; Mateo, J.; Fizazi, K.; Saad, F.; Shore, N.; Sandhu, S.; Chi, K.N.; Sartor, O.; Agarwal, N.; Olmos, D.; et al. Olaparib for Metastatic Castration-Resistant Prostate Cancer. N. Engl. J. Med. 2020, 382, 2091–2102. [Google Scholar] [CrossRef]
- Muralidhar, A.; Potluri, H.K.; Jaiswal, T.; McNeel, D.G. Targeted Radiation and Immune Therapies-Advances and Opportunities for the Treatment of Prostate Cancer. Pharmaceutics 2023, 15, 252. [Google Scholar] [CrossRef]
- Petrella, G.; Corsi, F.; Ciufolini, G.; Germini, S.; Capradossi, F.; Pelliccia, A.; Torino, F.; Ghibelli, L.; Cicero, D.O. Metabolic Reprogramming of Castration-Resistant Prostate Cancer Cells as a Response to Chemotherapy. Metabolites 2022, 13, 65. [Google Scholar] [CrossRef]
- Lasorsa, F.; di Meo, N.A.; Rutigliano, M.; Ferro, M.; Terracciano, D.; Tataru, O.S.; Battaglia, M.; Ditonno, P.; Lucarelli, G. Emerging Hallmarks of Metabolic Reprogramming in Prostate Cancer. Int. J. Mol. Sci. 2023, 24, 910. [Google Scholar] [CrossRef] [PubMed]
- Sternberg, C.N.; Fizazi, K.; Saad, F.; Shore, N.D.; De Giorgi, U.; Penson, D.F.; Ferreira, U.; Efstathiou, E.; Madziarska, K.; Kolinsky, M.P.; et al. Enzalutamide and Survival in Nonmetastatic, Castration-Resistant Prostate Cancer. N. Engl. J. Med. 2020, 382, 2197–2206. [Google Scholar] [CrossRef] [PubMed]
- Armstrong, A.J.; Szmulewitz, R.Z.; Petrylak, D.P.; Holzbeierlein, J.; Villers, A.; Azad, A.; Alcaraz, A.; Alekseev, B.; Iguchi, T.; Shore, N.D.; et al. ARCHES: A Randomized, Phase III Study of Androgen Deprivation Therapy With Enzalutamide or Placebo in Men With Metastatic Hormone-Sensitive Prostate Cancer. J. Clin. Oncol. 2019, 37, 2974–2986. [Google Scholar] [CrossRef]
- Smith, M.R.; Hussain, M.; Saad, F.; Fizazi, K.; Sternberg, C.N.; Crawford, E.D.; Kopyltsov, E.; Park, C.H.; Alekseev, B.; Montesa-Pino, Á.; et al. Darolutamide and Survival in Metastatic, Hormone-Sensitive Prostate Cancer. N. Engl. J. Med. 2022, 386, 1132–1142. [Google Scholar] [CrossRef]
- Smith, M.R.; Saad, F.; Chowdhury, S.; Oudard, S.; Hadaschik, B.A.; Graff, J.N.; Olmos, D.; Mainwaring, P.N.; Lee, J.Y.; Uemura, H.; et al. Apalutamide and Overall Survival in Prostate Cancer. Eur. Urol. 2021, 79, 150–158. [Google Scholar] [CrossRef]
- Kneppers, J.; Severson, T.M.; Siefert, J.C.; Schol, P.; Joosten, S.E.P.; Yu, I.P.L.; Huang, C.-C.F.; Morova, T.; Altıntaş, U.B.; Giambartolomei, C.; et al. Extensive Androgen Receptor Enhancer Heterogeneity in Primary Prostate Cancers Underlies Transcriptional Diversity and Metastatic Potential. Nat. Commun. 2022, 13, 7367. [Google Scholar] [CrossRef]
- Shiota, M.; Akamatsu, S.; Tsukahara, S.; Nagakawa, S.; Matsumoto, T.; Eto, M. Androgen Receptor Mutations for Precision Medicine in Prostate Cancer. Endocr. Relat. Cancer 2022, 29, R143–R155. [Google Scholar] [CrossRef] [PubMed]
- Mao, Y.; Yang, G.; Li, Y.; Liang, G.; Xu, W.; Hu, M. Advances in the Current Understanding of the Mechanisms Governing the Acquisition of Castration-Resistant Prostate Cancer. Cancers 2022, 14, 3744. [Google Scholar] [CrossRef]
- Qiu, Y. A Phosphorylation Switch Controls Androgen Biosynthesis in Prostate Cancer. J. Clin. Invest. 2023, 133, e166499. [Google Scholar] [CrossRef]
- Wang, Z.; Yan, X.; Tang, P.; Tang, T.; Wang, Y.; Peng, S.; Wang, S.; Lan, W.; Wang, L.; Zhang, Y.; et al. Genetic Profiling of Hormone-Sensitive and Castration-Resistant Prostate Cancers and Identification of Genetic Mutations Prone to Castration-Resistant Prostate Cancer. Prostate Cancer Prostatic Dis. 2022. [Google Scholar] [CrossRef]
- Baca, S.C.; Prandi, D.; Lawrence, M.S.; Mosquera, J.M.; Romanel, A.; Drier, Y.; Park, K.; Kitabayashi, N.; MacDonald, T.Y.; Ghandi, M.; et al. Punctuated Evolution of Prostate Cancer Genomes. Cell 2013, 153, 666–677. [Google Scholar] [CrossRef] [PubMed]
- Malik, J.A.; Ahmed, S.; Momin, S.S.; Shaikh, S.; Alafnan, A.; Alanazi, J.; Said Almermesh, M.H.; Anwar, S. Drug Repurposing: A New Hope in Drug Discovery for Prostate Cancer. ACS Omega 2023, 8, 56–73. [Google Scholar] [CrossRef] [PubMed]
- Fang, Q.; Cole, R.N.; Wang, Z. Mechanisms and Targeting of Proteosome-Dependent Androgen Receptor Degradation in Prostate Cancer. Am. J. Clin. Exp. Urol. 2022, 10, 366–376. [Google Scholar] [PubMed]
- Chai, X.; Hu, X.-P.; Wang, X.-Y.; Wang, H.-T.; Pang, J.-P.; Zhou, W.-F.; Liao, J.-N.; Shan, L.-H.; Xu, X.-H.; Xu, L.; et al. Computationally Guided Discovery of Novel Non-Steroidal AR-GR Dual Antagonists Demonstrating Potency against Antiandrogen Resistance. Acta Pharmacol. Sin. 2023. [Google Scholar] [CrossRef]
- Jia, X.; Han, X. Targeting Androgen Receptor Degradation with PROTACs from Bench to Bedside. Biomed. Pharmacother. 2023, 158, 114112. [Google Scholar] [CrossRef] [PubMed]
- Sartor, O.; de Bono, J.; Chi, K.N.; Fizazi, K.; Herrmann, K.; Rahbar, K.; Tagawa, S.T.; Nordquist, L.T.; Vaishampayan, N.; El-Haddad, G.; et al. Lutetium-177-PSMA-617 for Metastatic Castration-Resistant Prostate Cancer. N. Engl. J. Med. 2021, 385, 1091–1103. [Google Scholar] [CrossRef]
- Owen, J.S.; Clayton, A.; Pearson, H.B. Cancer-Associated Fibroblast Heterogeneity, Activation and Function: Implications for Prostate Cancer. Biomolecules 2022, 13, 67. [Google Scholar] [CrossRef]
- Gunti, S.; Hoke, A.T.K.; Vu, K.P.; London, N.R. Organoid and Spheroid Tumor Models: Techniques and Applications. Cancers 2021, 13, 874. [Google Scholar] [CrossRef]
- Nunes, A.S.; Barros, A.S.; Costa, E.C.; Moreira, A.F.; Correia, I.J. 3D Tumor Spheroids as in Vitro Models to Mimic in Vivo Human Solid Tumors Resistance to Therapeutic Drugs. Biotechnol. Bioeng. 2019, 116, 206–226. [Google Scholar] [CrossRef]
- Garraway, I.P.; Sun, W.; Tran, C.P.; Perner, S.; Zhang, B.; Goldstein, A.S.; Hahm, S.A.; Haider, M.; Head, C.S.; Reiter, R.E.; et al. Human Prostate Sphere-Forming Cells Represent a Subset of Basal Epithelial Cells Capable of Glandular Regeneration in Vivo. Prostate 2010, 70, 491–501. [Google Scholar] [CrossRef]
- Chen, S.; Principessa, L.; Isaacs, J.T. Human Prostate Cancer Initiating Cells Isolated Directly from Localized Cancer Do Not Form Prostaspheres in Primary Culture. Prostate 2012, 72, 1478–1489. [Google Scholar] [CrossRef]
- Lancaster, M.A.; Knoblich, J.A. Organogenesis in a Dish: Modeling Development and Disease Using Organoid Technologies. Science 2014, 345, 1247125. [Google Scholar] [CrossRef] [PubMed]
- Schutgens, F.; Clevers, H. Human Organoids: Tools for Understanding Biology and Treating Diseases. Annu. Rev. Pathol. 2020, 15, 211–234. [Google Scholar] [CrossRef] [PubMed]
- Hendriks, D.; Artegiani, B.; Hu, H.; Chuva de Sousa Lopes, S.; Clevers, H. Establishment of Human Fetal Hepatocyte Organoids and CRISPR-Cas9-Based Gene Knockin and Knockout in Organoid Cultures from Human Liver. Nat. Protoc. 2021, 16, 182–217. [Google Scholar] [CrossRef] [PubMed]
- Rodrigues, J.; Heinrich, M.A.; Teixeira, L.M.; Prakash, J. 3D In Vitro Model (R)Evolution: Unveiling Tumor-Stroma Interactions. Trends Cancer 2021, 7, 249–264. [Google Scholar] [CrossRef] [PubMed]
- Henke, E.; Nandigama, R.; Ergün, S. Extracellular Matrix in the Tumor Microenvironment and Its Impact on Cancer Therapy. Front. Mol. Biosci. 2019, 6, 160. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.; Jiao, M.; Li, L.; Wu, D.; Wu, K.; Li, X.; Zhu, G.; Dang, Q.; Wang, X.; Hsieh, J.-T.; et al. Tumorspheres Derived from Prostate Cancer Cells Possess Chemoresistant and Cancer Stem Cell Properties. J. Cancer Res. Clin. Oncol. 2012, 138, 675–686. [Google Scholar] [CrossRef] [PubMed]
- Gao, W.; Wang, Y.; Yu, S.; Wang, Z.; Ma, T.; Chan, A.M.-L.; Chiu, P.K.-F.; Ng, C.-F.; Wu, D.; Chan, F.L. Endothelial Nitric Oxide Synthase (ENOS)-NO Signaling Axis Functions to Promote the Growth of Prostate Cancer Stem-like Cells. Stem Cell Res. Ther. 2022, 13, 188. [Google Scholar] [CrossRef] [PubMed]
- Wang, S.; Huang, S.; Zhao, X.; Zhang, Q.; Wu, M.; Sun, F.; Han, G.; Wu, D. Enrichment of Prostate Cancer Stem Cells from Primary Prostate Cancer Cultures of Biopsy Samples. Int. J. Clin. Exp. Pathol. 2014, 7, 184–193. [Google Scholar] [PubMed]
- Rao, W.; Zhao, S.; Yu, J.; Lu, X.; Zynger, D.L.; He, X. Enhanced Enrichment of Prostate Cancer Stem-like Cells with Miniaturized 3D Culture in Liquid Core-Hydrogel Shell Microcapsules. Biomaterials 2014, 35, 7762–7773. [Google Scholar] [CrossRef]
- Mosaad, E.; Chambers, K.; Futrega, K.; Clements, J.; Doran, M.R. Using High Throughput Microtissue Culture to Study the Difference in Prostate Cancer Cell Behavior and Drug Response in 2D and 3D Co-Cultures. BMC Cancer 2018, 18, 592. [Google Scholar] [CrossRef] [PubMed]
- Krishnan, M.A.; Chelvam, V. Developing ΜSpherePlatform Using a Commercial Hairbrush: An Agarose 3D Culture Platform for Deep-Tissue Imaging of Prostate Cancer. ACS Appl. Bio Mater. 2021, 4, 4254–4270. [Google Scholar] [CrossRef] [PubMed]
- Carlsson, L.; Lennartsson, L.; Ronquist, G.; Larsson, A.; Nilson, S.; Nilsson, O. Mode of Growth Determines Differential Expression of Prostasomes in Cultures of Prostate Cancer Cell Lines and Opens for Studies of Prostasome Gene Expression. UPS J. Med. Sci. 2006, 111, 293–301. [Google Scholar] [CrossRef] [PubMed]
- Takir, G.G.; Debelec-Butuner, B.; Korkmaz, K.S. 3D Cell Culture Model for Prostate Cancer Cells to Mimic Inflammatory Microenvironment. Proceedings 2018, 2, 1555. [Google Scholar] [CrossRef]
- Fontana, F.; Raimondi, M.; Marzagalli, M.; Sommariva, M.; Limonta, P.; Gagliano, N. Epithelial-To-Mesenchymal Transition Markers and CD44 Isoforms Are Differently Expressed in 2D and 3D Cell Cultures of Prostate Cancer Cells. Cells 2019, 8, 143. [Google Scholar] [CrossRef]
- Enmon, R.M.; O’Connor, K.C.; Lacks, D.J.; Schwartz, D.K.; Dotson, R.S. Dynamics of Spheroid Self-Assembly in Liquid-Overlay Culture of DU 145 Human Prostate Cancer Cells. Biotechnol. Bioeng. 2001, 72, 579–591. [Google Scholar] [CrossRef]
- Enmon, R.M.; O’Connor, K.C.; Song, H.; Lacks, D.J.; Schwartz, D.K. Aggregation Kinetics of Well and Poorly Differentiated Human Prostate Cancer Cells. Biotechnol. Bioeng. 2002, 80, 580–588. [Google Scholar] [CrossRef]
- Song, H.; O’Connor, K.C.; Lacks, D.J.; Enmon, R.M.; Jain, S.K. Monte Carlo Simulation of LNCaP Human Prostate Cancer Cell Aggregation in Liquid-Overlay Culture. Biotechnol. Prog. 2003, 19, 1742–1749. [Google Scholar] [CrossRef]
- Song, H.; Jain, S.K.; Enmon, R.M.; O’Connor, K.C. Restructuring Dynamics of DU 145 and LNCaP Prostate Cancer Spheroids. Vitro Cell Dev. Biol. Anim. 2004, 40, 262–267. [Google Scholar] [CrossRef]
- O’Connor, K.C.; Venczel, M.Z. Predicting Aggregation Kinetics of DU 145 Prostate Cancer Cells in Liquid-Overlay Culture. Biotechnol. Lett. 2005, 27, 1663–1668. [Google Scholar] [CrossRef]
- Phelan, M.A.; Gianforcaro, A.L.; Gerstenhaber, J.A.; Lelkes, P.I. An Air Bubble-Isolating Rotating Wall Vessel Bioreactor for Improved Spheroid/Organoid Formation. Tissue Eng. Part C Methods 2019, 25, 479–488. [Google Scholar] [CrossRef] [PubMed]
- Dietrichs, D.; Grimm, D.; Sahana, J.; Melnik, D.; Corydon, T.J.; Wehland, M.; Krüger, M.; Vermeesen, R.; Baselet, B.; Baatout, S.; et al. Three-Dimensional Growth of Prostate Cancer Cells Exposed to Simulated Microgravity. Front. Cell Dev. Biol. 2022, 10, 841017. [Google Scholar] [CrossRef] [PubMed]
- Hybel, T.E.; Dietrichs, D.; Sahana, J.; Corydon, T.J.; Nassef, M.Z.; Wehland, M.; Krüger, M.; Magnusson, N.E.; Bauer, J.; Utpatel, K.; et al. Simulated Microgravity Influences VEGF, MAPK, and PAM Signaling in Prostate Cancer Cells. Int. J. Mol. Sci. 2020, 21, 1263. [Google Scholar] [CrossRef]
- Foty, R. A Simple Hanging Drop Cell Culture Protocol for Generation of 3D Spheroids. J. Vis. Exp. 2011, 2720. [Google Scholar] [CrossRef]
- Eder, T.; Eder, I.E. 3D Hanging Drop Culture to Establish Prostate Cancer Organoids. Methods Mol. Biol. 2017, 1612, 167–175. [Google Scholar] [CrossRef] [PubMed]
- Bhatia, S.N.; Ingber, D.E. Microfluidic Organs-on-Chips. Nat. Biotechnol. 2014, 32, 760–772. [Google Scholar] [CrossRef]
- Hsiao, A.Y.; Torisawa, Y.; Tung, Y.-C.; Sud, S.; Taichman, R.S.; Pienta, K.J.; Takayama, S. Microfluidic System for Formation of PC-3 Prostate Cancer Co-Culture Spheroids. Biomaterials 2009, 30, 3020–3027. [Google Scholar] [CrossRef]
- Härmä, V.; Virtanen, J.; Mäkelä, R.; Happonen, A.; Mpindi, J.-P.; Knuuttila, M.; Kohonen, P.; Lötjönen, J.; Kallioniemi, O.; Nees, M. A Comprehensive Panel of Three-Dimensional Models for Studies of Prostate Cancer Growth, Invasion and Drug Responses. PloS ONE 2010, 5, e10431. [Google Scholar] [CrossRef]
- Dolega, M.E.; Abeille, F.; Picollet-D’hahan, N.; Gidrol, X. Controlled 3D Culture in Matrigel Microbeads to Analyze Clonal Acinar Development. Biomaterials 2015, 52, 347–357. [Google Scholar] [CrossRef]
- Ma, L.; Li, J.; Nie, Q.; Zhang, Q.; Liu, S.; Ge, D.; You, Z. Organoid Culture of Human Prostate Cancer Cell Lines LNCaP and C4-2B. Am. J. Clin. Exp. Urol. 2017, 5, 25–33. [Google Scholar]
- Haq, S.; Samuel, V.; Haxho, F.; Akasov, R.; Leko, M.; Burov, S.V.; Markvicheva, E.; Szewczuk, M.R. Sialylation Facilitates Self-Assembly of 3D Multicellular Prostaspheres by Using Cyclo-RGDfK(TPP) Peptide. Onco. Targets Ther. 2017, 10, 2427–2447. [Google Scholar] [CrossRef] [PubMed]
- Meinert, C.; Theodoropoulos, C.; Klein, T.J.; Hutmacher, D.W.; Loessner, D. A Method for Prostate and Breast Cancer Cell Spheroid Cultures Using Gelatin Methacryloyl-Based Hydrogels. Methods Mol. Biol. 2018, 1786, 175–194. [Google Scholar] [CrossRef] [PubMed]
- Hainline, K.M.; Gu, F.; Handley, J.F.; Tian, Y.F.; Wu, Y.; de Wet, L.; Vander Griend, D.J.; Collier, J.H. Self-Assembling Peptide Gels for 3D Prostate Cancer Spheroid Culture. Macromol. Biosci. 2019, 19, e1800249. [Google Scholar] [CrossRef]
- Van Hemelryk, A.; Mout, L.; Erkens-Schulze, S.; French, P.J.; van Weerden, W.M.; van Royen, M.E. Modeling Prostate Cancer Treatment Responses in the Organoid Era: 3D Environment Impacts Drug Testing. Biomolecules 2021, 11, 1572. [Google Scholar] [CrossRef] [PubMed]
- Katti, K.S.; Molla, M.S.; Karandish, F.; Haldar, M.K.; Mallik, S.; Katti, D.R. Sequential Culture on Biomimetic Nanoclay Scaffolds Forms Three-Dimensional Tumoroids. J. Biomed. Mater. Res. A 2016, 104, 1591–1602. [Google Scholar] [CrossRef] [PubMed]
- Cecilia, A.; Baecker, A.; Hamann, E.; Rack, A.; van de Kamp, T.; Gruhl, F.J.; Hofmann, R.; Moosmann, J.; Hahn, S.; Kashef, J.; et al. Optimizing Structural and Mechanical Properties of Cryogel Scaffolds for Use in Prostate Cancer Cell Culturing. Mater. Sci. Eng. C Mater. Biol. Appl. 2017, 71, 465–472. [Google Scholar] [CrossRef]
- Bäcker, A.; Erhardt, O.; Wietbrock, L.; Schel, N.; Göppert, B.; Dirschka, M.; Abaffy, P.; Sollich, T.; Cecilia, A.; Gruhl, F.J. Silk Scaffolds Connected with Different Naturally Occurring Biomaterials for Prostate Cancer Cell Cultivation in 3D. Biopolymers 2017, 107, 70–79. [Google Scholar] [CrossRef]
- Centenera, M.M.; Gillis, J.L.; Hanson, A.R.; Jindal, S.; Taylor, R.A.; Risbridger, G.P.; Sutherland, P.D.; Scher, H.I.; Raj, G.V.; Knudsen, K.E.; et al. Evidence for Efficacy of New Hsp90 Inhibitors Revealed by Ex Vivo Culture of Human Prostate Tumors. Clin. Cancer Res. 2012, 18, 3562–3570. [Google Scholar] [CrossRef]
- Centenera, M.M.; Raj, G.V.; Knudsen, K.E.; Tilley, W.D.; Butler, L.M. Ex Vivo Culture of Human Prostate Tissue and Drug Development. Nat. Rev. Urol. 2013, 10, 483–487. [Google Scholar] [CrossRef]
- Centenera, M.M.; Hickey, T.E.; Jindal, S.; Ryan, N.K.; Ravindranathan, P.; Mohammed, H.; Robinson, J.L.; Schiewer, M.J.; Ma, S.; Kapur, P.; et al. A Patient-Derived Explant (PDE) Model of Hormone-Dependent Cancer. Mol. Oncol. 2018, 12, 1608–1622. [Google Scholar] [CrossRef]
- Shafi, A.A.; Schiewer, M.J.; de Leeuw, R.; Dylgjeri, E.; McCue, P.A.; Shah, N.; Gomella, L.G.; Lallas, C.D.; Trabulsi, E.J.; Centenera, M.M.; et al. Patient-Derived Models Reveal Impact of the Tumor Microenvironment on Therapeutic Response. Eur. Urol. Oncol 2018, 1, 325–337. [Google Scholar] [CrossRef] [PubMed]
- Mutuku, S.M.; Trim, P.J.; Prabhala, B.K.; Irani, S.; Bremert, K.L.; Logan, J.M.; Brooks, D.A.; Stahl, J.; Centenera, M.M.; Snel, M.F.; et al. Evaluation of Small Molecule Drug Uptake in Patient-Derived Prostate Cancer Explants by Mass Spectrometry. Sci. Rep. 2019, 9, 15008. [Google Scholar] [CrossRef] [PubMed]
- Xu, Y.; Pachnikova, G.; Wang, H.; Wu, Y.; Przybilla, D.; Schäfer, R.; Chen, Z.; Zhu, S.; Keilholz, U. IC50: An Unsuitable Measure for Large-Sized Prostate Cancer Spheroids in Drug Sensitivity Evaluation. Bosn. J. Basic Med. Sci. 2022, 22, 580–592. [Google Scholar] [CrossRef]
- Edmondson, R.; Broglie, J.J.; Adcock, A.F.; Yang, L. Three-Dimensional Cell Culture Systems and Their Applications in Drug Discovery and Cell-Based Biosensors. Assay Drug Dev. Technol. 2014, 12, 207–218. [Google Scholar] [CrossRef]
- Birgersdotter, A.; Sandberg, R.; Ernberg, I. Gene Expression Perturbation in Vitro--a Growing Case for Three-Dimensional (3D) Culture Systems. Semin. Cancer Biol. 2005, 15, 405–412. [Google Scholar] [CrossRef] [PubMed]
- Breslin, S.; O’Driscoll, L. Three-Dimensional Cell Culture: The Missing Link in Drug Discovery. Drug Discov. Today 2013, 18, 240–249. [Google Scholar] [CrossRef]
- Hopkins, A.L. Network Pharmacology: The next Paradigm in Drug Discovery. Nat. Chem. Biol. 2008, 4, 682–690. [Google Scholar] [CrossRef]
- DiMasi, J.A.; Hansen, R.W.; Grabowski, H.G. The Price of Innovation: New Estimates of Drug Development Costs. J. Health Econ. 2003, 22, 151–185. [Google Scholar] [CrossRef]
- Karlsson, H.; Fryknäs, M.; Larsson, R.; Nygren, P. Loss of Cancer Drug Activity in Colon Cancer HCT-116 Cells during Spheroid Formation in a New 3-D Spheroid Cell Culture System. Exp. Cell Res. 2012, 318, 1577–1585. [Google Scholar] [CrossRef]
- Kola, I. The State of Innovation in Drug Development. Clin. Pharmacol. Ther. 2008, 83, 227–230. [Google Scholar] [CrossRef]
- Weaver, V.M.; Petersen, O.W.; Wang, F.; Larabell, C.A.; Briand, P.; Damsky, C.; Bissell, M.J. Reversion of the Malignant Phenotype of Human Breast Cells in Three-Dimensional Culture and in Vivo by Integrin Blocking Antibodies. J. Cell Biol. 1997, 137, 231–245. [Google Scholar] [CrossRef] [PubMed]
- Oktem, G.; Sercan, O.; Guven, U.; Uslu, R.; Uysal, A.; Goksel, G.; Ayla, S.; Bilir, A. Cancer Stem Cell Differentiation: TGFβ1 and Versican May Trigger Molecules for the Organization of Tumor Spheroids. Oncol. Rep. 2014, 32, 641–649. [Google Scholar] [CrossRef] [PubMed]
- Banerjee, M.; Li, Z.; Gao, Y.; Lai, F.; Huang, M.; Zhang, Z.; Cai, L.; Sanabria, J.; Gao, T.; Xie, Z.; et al. Inverse Agonism at the Na/K-ATPase Receptor Reverses EMT in Prostate Cancer Cells. Prostate 2021, 81, 667–682. [Google Scholar] [CrossRef] [PubMed]
- Goksel, G.; Bilir, A.; Uslu, R.; Akbulut, H.; Guven, U.; Oktem, G. WNT1 Gene Expression Alters in Heterogeneous Population of Prostate Cancer Cells; Decreased Expression Pattern Observed in CD133+/CD44+ Prostate Cancer Stem Cell Spheroids. J. BUON 2014, 19, 207–214. [Google Scholar]
- Wen, Z.; Liao, Q.; Hu, Y.; You, L.; Zhou, L.; Zhao, Y. A Spheroid-Based 3-D Culture Model for Pancreatic Cancer Drug Testing, Using the Acid Phosphatase Assay. Braz. J. Med. Biol. Res. 2013, 46, 634–642. [Google Scholar] [CrossRef]
- Tung, Y.-C.; Hsiao, A.Y.; Allen, S.G.; Torisawa, Y.; Ho, M.; Takayama, S. High-Throughput 3D Spheroid Culture and Drug Testing Using a 384 Hanging Drop Array. Analyst 2011, 136, 473–478. [Google Scholar] [CrossRef]
- Costard, L.S.; Hosn, R.R.; Ramanayake, H.; O’Brien, F.J.; Curtin, C.M. Influences of the 3D Microenvironment on Cancer Cell Behaviour and Treatment Responsiveness: A Recent Update on Lung, Breast and Prostate Cancer Models. Acta Biomater. 2021, 132, 360–378. [Google Scholar] [CrossRef]
- Chitcholtan, K.; Sykes, P.H.; Evans, J.J. The Resistance of Intracellular Mediators to Doxorubicin and Cisplatin Are Distinct in 3D and 2D Endometrial Cancer. J. Transl. Med. 2012, 10, 38. [Google Scholar] [CrossRef]
- Jouberton, E.; Voissiere, A.; Penault-Llorca, F.; Cachin, F.; Miot-Noirault, E. Multicellular Tumor Spheroids of LNCaP-Luc Prostate Cancer Cells as in Vitro Screening Models for Cytotoxic Drugs. Am. J. Cancer Res. 2022, 12, 1116–1128. [Google Scholar]
- Moskovits, N.; Itzhaki, E.; Tarasenko, N.; Chausky, E.; Bareket-Samish, A.; Kaufman, A.; Meerson, R.; Stemmer, S.M. Establishing 3-Dimensional Spheroids from Patient-Derived Tumor Samples and Evaluating Their Sensitivity to Drugs. J. Vis. Exp. 2022, 64564. [Google Scholar] [CrossRef]
- Williams, E.S.; Rodriguez-Bravo, V.; Chippada-Venkata, U.; De Ia Iglesia-Vicente, J.; Gong, Y.; Galsky, M.; Oh, W.; Cordon-Cardo, C.; Domingo-Domenech, J. Generation of Prostate Cancer Patient Derived Xenograft Models from Circulating Tumor Cells. J. Vis. Exp. 2015, 53182. [Google Scholar] [CrossRef]
- Timofeeva, O.A.; Palechor-Ceron, N.; Li, G.; Yuan, H.; Krawczyk, E.; Zhong, X.; Liu, G.; Upadhyay, G.; Dakic, A.; Yu, S.; et al. Conditionally Reprogrammed Normal and Primary Tumor Prostate Epithelial Cells: A Novel Patient-Derived Cell Model for Studies of Human Prostate Cancer. Oncotarget 2017, 8, 22741–22758. [Google Scholar] [CrossRef] [PubMed]
- Xu, Y.; Pachnikova, G.; Przybilla, D.; Schäfer, R.; Cui, Y.; Zhou, D.; Chen, Z.; Zhao, A.; Keilholz, U. Evaluation of JQ1 Combined With Docetaxel for the Treatment of Prostate Cancer Cells in 2D- and 3D-Culture Systems. Front. Pharmacol. 2022, 13, 839620. [Google Scholar] [CrossRef]
- Bromma, K.; Dos Santos, N.; Barta, I.; Alexander, A.; Beckham, W.; Krishnan, S.; Chithrani, D.B. Enhancing Nanoparticle Accumulation in Two Dimensional, Three Dimensional, and Xenograft Mouse Cancer Cell Models in the Presence of Docetaxel. Sci. Rep. 2022, 12, 13508. [Google Scholar] [CrossRef] [PubMed]
- Kanbur, E.; Baykal, A.T.; Yerlikaya, A. Molecular Analysis of Cell Survival and Death Pathways in the Proteasome Inhibitor Bortezomib-Resistant PC3 Prostate Cancer Cell Line. Med. Oncol. 2021, 38, 112. [Google Scholar] [CrossRef] [PubMed]
- Paliashvili, K.; Di Maggio, F.; Ho, H.M.K.; Sathasivam, S.; Ahmed, H.; Day, R.M. A Novel Adjuvant Drug-Device Combination Tissue Scaffold for Radical Prostatectomy. Drug Deliv. 2019, 26, 1115–1124. [Google Scholar] [CrossRef] [PubMed]
- De Oliveira, L.C.B.; Ribeiro, D.L.; do Nascimento, J.R.; da Rocha, C.Q.; Cólus, I.M.d.S.; Serpeloni, J.M. Anticancer Activities of Brachydin C in Human Prostate Tumor Cells (DU145) Grown in 2D and 3D Models: Stimulation of Cell Death and Downregulation of Metalloproteinases in Spheroids. Chem. Biol. Drug Des. 2022, 100, 747–762. [Google Scholar] [CrossRef] [PubMed]
- Ribeiro, D.L.; Tuttis, K.; de Oliveira, L.C.B.; Serpeloni, J.M.; Gomes, I.N.F.; Lengert, A.v.H.; da Rocha, C.Q.; Reis, R.M.; Cólus, I.M.d.S.; Antunes, L.M.G. The Antitumoral/Antimetastatic Action of the Flavonoid Brachydin A in Metastatic Prostate Tumor Spheroids In Vitro Is Mediated by (Parthanatos) PARP-Related Cell Death. Pharmaceutics 2022, 14, 963. [Google Scholar] [CrossRef]
- Safari, F.; Rayat Azad, N.; Alizadeh Ezdiny, A.; Pakizehkar, S.; Khazaei Koohpar, Z.; Ranji, N. Antitumor Activities of Green Tea by Up-Regulation of MiR-181a Expression in LNCaP Cells Using 3D Cell Culture Model. Avicenna J. Med. Biotechnol. 2022, 14, 89–94. [Google Scholar] [CrossRef] [PubMed]
- Lin, Z.; Huang, S.; LingHu, X.; Wang, Y.; Wang, B.; Zhong, S.; Xie, S.; Xu, X.; Yu, A.; Nagai, A.; et al. Perillaldehyde Inhibits Bone Metastasis and Receptor Activator of Nuclear Factor-ΚB Ligand (RANKL) Signaling-Induced Osteoclastogenesis in Prostate Cancer Cell Lines. Bioengineered 2022, 13, 2710–2719. [Google Scholar] [CrossRef]
- Zuo, J.; Guo, Y.; Peng, X.; Tang, Y.; Zhang, X.; He, P.; Li, S.; Wa, Q.; Li, J.; Huang, S.; et al. Inhibitory Action of Pristimerin on Hypoxia-mediated Metastasis Involves Stem Cell Characteristics and EMT in PC-3 Prostate Cancer Cells. Oncol. Rep. 2015, 33, 1388–1394. [Google Scholar] [CrossRef] [PubMed]
- Lee, Y.-J.; Park, K.-S.; Lee, S.-H. Curcumin Targets Both Apoptosis and Necroptosis in Acidity-Tolerant Prostate Carcinoma Cells. Biomed. Res. Int. 2021, 2021, 8859181. [Google Scholar] [CrossRef] [PubMed]
- Jang, Y.-G.; Ko, E.-B.; Choi, K.-C. Gallic Acid, a Phenolic Acid, Hinders the Progression of Prostate Cancer by Inhibition of Histone Deacetylase 1 and 2 Expression. J. Nutr. Biochem. 2020, 84, 108444. [Google Scholar] [CrossRef]
- Tyagi, A.; Kumar, S.; Raina, K.; Wempe, M.F.; Maroni, P.D.; Agarwal, R.; Agarwal, C. Differential Effect of Grape Seed Extract and Its Active Constituent Procyanidin B2 3,3″-Di-O-Gallate against Prostate Cancer Stem Cells. Mol. Carcinog. 2019, 58, 1105–1117. [Google Scholar] [CrossRef] [PubMed]
- Jang, Y.-G.; Hwang, K.-A.; Choi, K.-C. Rosmarinic Acid, a Component of Rosemary Tea, Induced the Cell Cycle Arrest and Apoptosis through Modulation of HDAC2 Expression in Prostate Cancer Cell Lines. Nutrients 2018, 10, 1784. [Google Scholar] [CrossRef] [PubMed]
- Kafka, M.; Mayr, F.; Temml, V.; Möller, G.; Adamski, J.; Höfer, J.; Schwaiger, S.; Heidegger, I.; Matuszczak, B.; Schuster, D.; et al. Dual Inhibitory Action of a Novel AKR1C3 Inhibitor on Both Full-Length AR and the Variant AR-V7 in Enzalutamide Resistant Metastatic Castration Resistant Prostate Cancer. Cancers 2020, 12, 2092. [Google Scholar] [CrossRef]
- Deezagi, A.; Safari, N. Rosuvastatin Inhibit Spheroid Formation and Epithelial-Mesenchymal Transition (EMT) in Prostate Cancer PC-3 Cell Line. Mol. Biol. Rep. 2020, 47, 8727–8737. [Google Scholar] [CrossRef]
- Sugawara, T.; Baumgart, S.J.; Nevedomskaya, E.; Reichert, K.; Steuber, H.; Lejeune, P.; Mumberg, D.; Haendler, B. Darolutamide Is a Potent Androgen Receptor Antagonist with Strong Efficacy in Prostate Cancer Models. Int. J. Cancer 2019, 145, 1382–1394. [Google Scholar] [CrossRef]
- Abramenkovs, A.; Hariri, M.; Spiegelberg, D.; Nilsson, S.; Stenerlöw, B. Ra-223 Induces Clustered DNA Damage and Inhibits Cell Survival in Several Prostate Cancer Cell Lines. Transl. Oncol. 2022, 26, 101543. [Google Scholar] [CrossRef]
- Salerno, D.; Howe, A.; Bhatavdekar, O.; Josefsson, A.; Pacheco-Torres, J.; Bhujwalla, Z.M.; Gabrielson, K.L.; Sofou, S. Two Diverse Carriers Are Better than One: A Case Study in α-Particle Therapy for Prostate Specific Membrane Antigen-Expressing Prostate Cancers. Bioeng. Transl. Med. 2022, 7, e10266. [Google Scholar] [CrossRef]
- Pinto, C.I.G.; Bucar, S.; Alves, V.; Fonseca, A.; Abrunhosa, A.J.; da Silva, C.L.; Guerreiro, J.F.; Mendes, F. Copper-64 Chloride Exhibits Therapeutic Potential in Three-Dimensional Cellular Models of Prostate Cancer. Front. Mol. Biosci. 2020, 7, 609172. [Google Scholar] [CrossRef] [PubMed]
- Nakamura, N.; Sloper, D.T. Altered Expression of Genes Identified in Rats with Prostatic Chronic Inflammation in a Prostate Spheroid Model Treated by Estradiol/Testosterone. J. Toxicol. Sci. 2021, 46, 515–523. [Google Scholar] [CrossRef]
- Dang, K.; Castello, G.; Clarke, S.C.; Li, Y.; Balasubramani, A.; Boudreau, A.; Davison, L.; Harris, K.E.; Pham, D.; Sankaran, P.; et al. Attenuating CD3 Affinity in a PSMAxCD3 Bispecific Antibody Enables Killing of Prostate Tumor Cells with Reduced Cytokine Release. J. Immunother. Cancer 2021, 9, e002488. [Google Scholar] [CrossRef] [PubMed]
- Gil, V.; Miranda, S.; Riisnaes, R.; Gurel, B.; D’Ambrosio, M.; Vasciaveo, A.; Crespo, M.; Ferreira, A.; Brina, D.; Troiani, M.; et al. HER3 Is an Actionable Target in Advanced Prostate Cancer. Cancer Res. 2021, 81, 6207–6218. [Google Scholar] [CrossRef] [PubMed]
- Nessler, I.; Khera, E.; Vance, S.; Kopp, A.; Qiu, Q.; Keating, T.A.; Abu-Yousif, A.O.; Sandal, T.; Legg, J.; Thompson, L.; et al. Increased Tumor Penetration of Single-Domain Antibody-Drug Conjugates Improves In Vivo Efficacy in Prostate Cancer Models. Cancer Res. 2020, 80, 1268–1278. [Google Scholar] [CrossRef]
- Stenberg, V.Y.; Larsen, R.H.; Ma, L.-W.; Peng, Q.; Juzenas, P.; Bruland, Ø.S.; Juzeniene, A. Evaluation of the PSMA-Binding Ligand 212Pb-NG001 in Multicellular Tumour Spheroid and Mouse Models of Prostate Cancer. Int. J. Mol. Sci. 2021, 22, 4815. [Google Scholar] [CrossRef]
- Masilamani, A.P.; Dettmer-Monaco, V.; Monaco, G.; Cathomen, T.; Kuckuck, I.; Schultze-Seemann, S.; Huber, N.; Wolf, P. An Anti-PSMA Immunotoxin Reduces Mcl-1 and Bcl2A1 and Specifically Induces in Combination with the BAD-Like BH3 Mimetic ABT-737 Apoptosis in Prostate Cancer Cells. Cancers 2020, 12, 1648. [Google Scholar] [CrossRef]
- Kedarinath, K.; Parks, G.D. Differential In Vitro Growth and Cell Killing of Cancer versus Benign Prostate Cells by Oncolytic Parainfluenza Virus. Pathogens 2022, 11, 493. [Google Scholar] [CrossRef]
- Landgraf, L.; Kozlowski, A.; Zhang, X.; Fournelle, M.; Becker, F.-J.; Tretbar, S.; Melzer, A. Focused Ultrasound Treatment of a Spheroid In Vitro Tumour Model. Cells 2022, 11, 1518. [Google Scholar] [CrossRef]
- Drápela, S.; Khirsariya, P.; van Weerden, W.M.; Fedr, R.; Suchánková, T.; Búzová, D.; Červený, J.; Hampl, A.; Puhr, M.; Watson, W.R.; et al. The CHK1 Inhibitor MU380 Significantly Increases the Sensitivity of Human Docetaxel-Resistant Prostate Cancer Cells to Gemcitabine through the Induction of Mitotic Catastrophe. Mol. Oncol. 2020, 14, 2487–2503. [Google Scholar] [CrossRef]
- Moreira-Silva, F.; Outeiro-Pinho, G.; Lobo, J.; Guimarães, R.; Gaspar, V.M.; Mano, J.F.; Agirre, X.; Pineda-Lucena, A.; Prosper, F.; Paramio, J.M.; et al. G9a Inhibition by CM-272: Developing a Novel Anti-Tumoral Strategy for Castration-Resistant Prostate Cancer Using 2D and 3D in Vitro Models. Biomed. Pharmacother. 2022, 150, 113031. [Google Scholar] [CrossRef] [PubMed]
- Karkampouna, S.; La Manna, F.; Benjak, A.; Kiener, M.; De Menna, M.; Zoni, E.; Grosjean, J.; Klima, I.; Garofoli, A.; Bolis, M.; et al. Patient-Derived Xenografts and Organoids Model Therapy Response in Prostate Cancer. Nat. Commun. 2021, 12, 1117. [Google Scholar] [CrossRef] [PubMed]
- Ko, J.; Meyer, A.N.; Haas, M.; Donoghue, D.J. Characterization of FGFR Signaling in Prostate Cancer Stem Cells and Inhibition via TKI Treatment. Oncotarget 2021, 12, 22–36. [Google Scholar] [CrossRef] [PubMed]
- Rana, Z.; Diermeier, S.; Walsh, F.P.; Hanif, M.; Hartinger, C.G.; Rosengren, R.J. Anti-Proliferative, Anti-Angiogenic and Safety Profiles of Novel HDAC Inhibitors for the Treatment of Metastatic Castration-Resistant Prostate Cancer. Pharmaceuticals 2021, 14, 1020. [Google Scholar] [CrossRef]
- Jiang, X.; Renkema, H.; Smeitink, J.; Beyrath, J. Sonlicromanol’s Active Metabolite KH176m Normalizes Prostate Cancer Stem Cell MPGES-1 Overexpression and Inhibits Cancer Spheroid Growth. PLoS ONE 2021, 16, e0254315. [Google Scholar] [CrossRef]
- Di Donato, M.; Ostacolo, C.; Giovannelli, P.; Di Sarno, V.; Monterrey, I.M.G.; Campiglia, P.; Migliaccio, A.; Bertamino, A.; Castoria, G. Therapeutic Potential of TRPM8 Antagonists in Prostate Cancer. Sci. Rep. 2021, 11, 23232. [Google Scholar] [CrossRef]
- Fu, W.; Zhao, M.T.; Driver, L.M.; Schirmer, A.U.; Yin, Q.; You, S.; Freedland, S.J.; DiGiovanni, J.; Drewry, D.H.; Macias, E. NUAK Family Kinase 2 Is a Novel Therapeutic Target for Prostate Cancer. Mol. Carcinog. 2022, 61, 334–345. [Google Scholar] [CrossRef]
- Jäntti, M.H.; Talman, V.; Räsänen, K.; Tarvainen, I.; Koistinen, H.; Tuominen, R.K. Anticancer Activity of the Protein Kinase C Modulator HMI-1a3 in 2D and 3D Cell Culture Models of Androgen-Responsive and Androgen-Unresponsive Prostate Cancer. FEBS Open Bio 2018, 8, 817–828. [Google Scholar] [CrossRef]
- Argenziano, M.; Foglietta, F.; Canaparo, R.; Spagnolo, R.; Della Pepa, C.; Caldera, F.; Trotta, F.; Serpe, L.; Cavalli, R. Biological Effect Evaluation of Glutathione-Responsive Cyclodextrin-Based Nanosponges: 2D and 3D Studies. Molecules 2020, 25, 2775. [Google Scholar] [CrossRef]
- Komarnicka, U.K.; Kozieł, S.; Pucelik, B.; Barzowska, A.; Siczek, M.; Malik, M.; Wojtala, D.; Niorettini, A.; Kyzioł, A.; Sebastian, V.; et al. Liposomal Binuclear Ir(III)-Cu(II) Coordination Compounds with Phosphino-Fluoroquinolone Conjugates for Human Prostate Carcinoma Treatment. Inorg. Chem. 2022, 61, 19261–19273. [Google Scholar] [CrossRef]
- Novohradsky, V.; Markova, L.; Kostrhunova, H.; Kasparkova, J.; Ruiz, J.; Marchán, V.; Brabec, V. A Cyclometalated IrIII Complex Conjugated to a Coumarin Derivative Is a Potent Photodynamic Agent against Prostate Differentiated and Tumorigenic Cancer Stem Cells. Chemistry 2021, 27, 8547–8556. [Google Scholar] [CrossRef] [PubMed]
- Ochieng, J.; Korolkova, O.Y.; Li, G.; Jin, R.; Chen, Z.; Matusik, R.J.; Adunyah, S.; Sakwe, A.M.; Ogunkua, O. Fetuin-A Promotes 3-Dimensional Growth in LNCaP Prostate Cancer Cells by Sequestering Extracellular Vesicles to Their Surfaces to Act as Signaling Platforms. Int. J. Mol. Sci. 2022, 23, 4031. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.W.; Park, M.; Kim, S.; Lim, S.C.; Kim, H.S.; Kang, K.W. Anti-Metastatic Effect of GV1001 on Prostate Cancer Cells; Roles of GnRHR-Mediated Gαs-CAMP Pathway and AR-YAP1 Axis. Cell Biosci. 2021, 11, 191. [Google Scholar] [CrossRef] [PubMed]
- Shropshire, D.B.; Acosta, F.M.; Fang, K.; Benavides, J.; Sun, L.-Z.; Jin, V.X.; Jiang, J.X. Association of Adenosine Signaling Gene Signature with Estrogen Receptor-Positive Breast and Prostate Cancer Bone Metastasis. Front. Med. 2022, 9, 965429. [Google Scholar] [CrossRef]
- Fontana, F.; Raimondi, M.; Marzagalli, M.; Sommariva, M.; Gagliano, N.; Limonta, P. Three-Dimensional Cell Cultures as an In Vitro Tool for Prostate Cancer Modeling and Drug Discovery. Int. J. Mol. Sci. 2020, 21, 6806. [Google Scholar] [CrossRef] [PubMed]
- Palacios, D.A.; Miyake, M.; Rosser, C.J. Radiosensitization in Prostate Cancer: Mechanisms and Targets. BMC Urol. 2013, 13, 4. [Google Scholar] [CrossRef]
- Camus, V.L.; Stewart, G.D.; Nailon, W.H.; McLaren, D.B.; Campbell, C.J. Measuring the Effects of Fractionated Radiation Therapy in a 3D Prostate Cancer Model System Using SERS Nanosensors. Analyst 2016, 141, 5056–5061. [Google Scholar] [CrossRef]
- Rae, C.; Mairs, R.J. AMPK Activation by AICAR Sensitizes Prostate Cancer Cells to Radiotherapy. Oncotarget 2019, 10, 749–759. [Google Scholar] [CrossRef]
- Silva, V.L.; Ruiz, A.; Ali, A.; Pereira, S.; Seitsonen, J.; Ruokolainen, J.; Furlong, F.; Coulter, J.; Al-Jamal, W.T. Hypoxia-Targeted Cupric-Tirapazamine Liposomes Potentiate Radiotherapy in Prostate Cancer Spheroids. Int. J. Pharm. 2021, 607, 121018. [Google Scholar] [CrossRef]
- Rove, K.O.; Crawford, E.D. Androgen Annihilation as a New Therapeutic Paradigm in Advanced Prostate Cancer. Curr. Opin. Urol. 2013, 23, 208–213. [Google Scholar] [CrossRef]
- Choi, E.; Buie, J.; Camacho, J.; Sharma, P.; de Riese, W.T.W. Evolution of Androgen Deprivation Therapy (ADT) and Its New Emerging Modalities in Prostate Cancer: An Update for Practicing Urologists, Clinicians and Medical Providers. Res. Rep. Urol. 2022, 14, 87–108. [Google Scholar] [CrossRef] [PubMed]
- Rice, M.A.; Malhotra, S.V.; Stoyanova, T. Second-Generation Antiandrogens: From Discovery to Standard of Care in Castration Resistant Prostate Cancer. Front. Oncol. 2019, 9, 801. [Google Scholar] [CrossRef] [PubMed]
- James, N.D.; de Bono, J.S.; Spears, M.R.; Clarke, N.W.; Mason, M.D.; Dearnaley, D.P.; Ritchie, A.W.S.; Amos, C.L.; Gilson, C.; Jones, R.J.; et al. Abiraterone for Prostate Cancer Not Previously Treated with Hormone Therapy. N. Engl. J. Med. 2017, 377, 338–351. [Google Scholar] [CrossRef] [PubMed]
- Crawford, E.D.; Heidenreich, A.; Lawrentschuk, N.; Tombal, B.; Pompeo, A.C.L.; Mendoza-Valdes, A.; Miller, K.; Debruyne, F.M.J.; Klotz, L. Androgen-Targeted Therapy in Men with Prostate Cancer: Evolving Practice and Future Considerations. Prostate Cancer Prostatic Dis. 2019, 22, 24–38. [Google Scholar] [CrossRef]
- Maughan, B.L.; Antonarakis, E.S. Androgen Pathway Resistance in Prostate Cancer and Therapeutic Implications. Expert Opin. Pharmacother. 2015, 16, 1521–1537. [Google Scholar] [CrossRef]
- Eder, T.; Weber, A.; Neuwirt, H.; Grünbacher, G.; Ploner, C.; Klocker, H.; Sampson, N.; Eder, I.E. Cancer-Associated Fibroblasts Modify the Response of Prostate Cancer Cells to Androgen and Anti-Androgens in Three-Dimensional Spheroid Culture. Int. J. Mol. Sci. 2016, 17, 1458. [Google Scholar] [CrossRef]
- Thomas, T.S.; Pachynski, R.K. Treatment of Advanced Prostate Cancer. Mo Med. 2018, 115, 156–161. [Google Scholar]
- Karandish, F.; Haldar, M.K.; You, S.; Brooks, A.E.; Brooks, B.D.; Guo, B.; Choi, Y.; Mallik, S. Prostate-Specific Membrane Antigen Targeted Polymersomes for Delivering Mocetinostat and Docetaxel to Prostate Cancer Cell Spheroids. ACS Omega 2016, 1, 952–962. [Google Scholar] [CrossRef]
- Du, A.W.; Lu, H.; Stenzel, M.H. Core-Cross-Linking Accelerates Antitumor Activities of Paclitaxel-Conjugate Micelles to Prostate Multicellular Tumor Spheroids: A Comparison of 2D and 3D Models. Biomacromolecules 2015, 16, 1470–1479. [Google Scholar] [CrossRef]
- Ohya, S.; Kajikuri, J.; Endo, K.; Kito, H.; Matsui, M. KCa1.1 K+ Channel Inhibition Overcomes Resistance to Antiandrogens and Doxorubicin in a Human Prostate Cancer LNCaP Spheroid Model. Int. J. Mol. Sci. 2021, 22, 13553. [Google Scholar] [CrossRef]
- Lee, S.-H.; Lee, Y.-J. Synergistic Anticancer Activity of Resveratrol in Combination with Docetaxel in Prostate Carcinoma Cells. Nutr. Res. Pract. 2021, 15, 12–25. [Google Scholar] [CrossRef] [PubMed]
- Moreira-Silva, F.; Henrique, R.; Jerónimo, C. From Therapy Resistance to Targeted Therapies in Prostate Cancer. Front. Oncol. 2022, 12, 877379. [Google Scholar] [CrossRef] [PubMed]
- Petrioli, R.; Francini, E.; Fiaschi, A.I.; Laera, L.; Roviello, G. Targeted Therapies for Prostate Cancer. Cancer Invest. 2015, 33, 276–285. [Google Scholar] [CrossRef] [PubMed]
- Duque, J.L.F.; Loughlin, K.R.; Adam, R.M.; Kantoff, P.; Mazzucchi, E.; Freeman, M.R. Measurement of Plasma Levels of Vascular Endothelial Growth Factor in Prostate Cancer Patients: Relationship with Clinical Stage, Gleason Score, Prostate Volume, and Serum Prostate-Specific Antigen. Clinics 2006, 61, 401–408. [Google Scholar] [CrossRef]
- Duque, J.L.; Loughlin, K.R.; Adam, R.M.; Kantoff, P.W.; Zurakowski, D.; Freeman, M.R. Plasma Levels of Vascular Endothelial Growth Factor Are Increased in Patients with Metastatic Prostate Cancer. Urology 1999, 54, 523–527. [Google Scholar] [CrossRef]
- Oner, E.; Kotmakci, M.; Baird, A.-M.; Gray, S.G.; Debelec Butuner, B.; Bozkurt, E.; Kantarci, A.G.; Finn, S.P. Development of EphA2 SiRNA-Loaded Lipid Nanoparticles and Combination with a Small-Molecule Histone Demethylase Inhibitor in Prostate Cancer Cells and Tumor Spheroids. J. Nanobiotechnol. 2021, 19, 71. [Google Scholar] [CrossRef]
- Bagnato, A.; Rosanò, L. The Endothelin Axis in Cancer. Int. J. Biochem. Cell. Biol. 2008, 40, 1443–1451. [Google Scholar] [CrossRef]
- Chi, K.N.; Hotte, S.J.; Yu, E.Y.; Tu, D.; Eigl, B.J.; Tannock, I.; Saad, F.; North, S.; Powers, J.; Gleave, M.E.; et al. Randomized Phase II Study of Docetaxel and Prednisone with or without OGX-011 in Patients with Metastatic Castration-Resistant Prostate Cancer. J. Clin. Oncol. 2010, 28, 4247–4254. [Google Scholar] [CrossRef]
- Toren, P.; Zoubeidi, A. Targeting the PI3K/Akt Pathway in Prostate Cancer: Challenges and Opportunities (Review). Int. J. Oncol. 2014, 45, 1793–1801. [Google Scholar] [CrossRef]
- Jones, D.T.; Valli, A.; Haider, S.; Zhang, Q.; Smethurst, E.A.; Schug, Z.T.; Peck, B.; Aboagye, E.O.; Critchlow, S.E.; Schulze, A.; et al. 3D Growth of Cancer Cells Elicits Sensitivity to Kinase Inhibitors but Not Lipid Metabolism Modifiers. Mol. Cancer Ther. 2019, 18, 376–388. [Google Scholar] [CrossRef]
- Tee, S.S.; Suster, I.; Truong, S.; Jeong, S.; Eskandari, R.; DiGialleonardo, V.; Alvarez, J.A.; Aldeborgh, H.N.; Keshari, K.R. Targeted AKT Inhibition in Prostate Cancer Cells and Spheroids Reduces Aerobic Glycolysis and Generation of Hyperpolarized [1-13C] Lactate. Mol. Cancer Res. 2018, 16, 453–460. [Google Scholar] [CrossRef] [PubMed]
- Aghajani, M.; Mokhtarzadeh, A.; Aghebati-Maleki, L.; Mansoori, B.; Mohammadi, A.; Safaei, S.; Asadzadeh, Z.; Hajiasgharzadeh, K.; Khaze Shahgoli, V.; Baradaran, B. CD133 Suppression Increases the Sensitivity of Prostate Cancer Cells to Paclitaxel. Mol. Biol. Rep. 2020, 47, 3691–3703. [Google Scholar] [CrossRef] [PubMed]
- Shenoy, T.R.; Boysen, G.; Wang, M.Y.; Xu, Q.Z.; Guo, W.; Koh, F.M.; Wang, C.; Zhang, L.Z.; Wang, Y.; Gil, V.; et al. CHD1 Loss Sensitizes Prostate Cancer to DNA Damaging Therapy by Promoting Error-Prone Double-Strand Break Repair. Ann. Oncol. 2017, 28, 1495–1507. [Google Scholar] [CrossRef] [PubMed]
- Erdogan, S.; Turkekul, K.; Dibirdik, I.; Doganlar, O.; Doganlar, Z.B.; Bilir, A.; Oktem, G. Midkine Downregulation Increases the Efficacy of Quercetin on Prostate Cancer Stem Cell Survival and Migration through PI3K/AKT and MAPK/ERK Pathway. Biomed. Pharmacother. 2018, 107, 793–805. [Google Scholar] [CrossRef]
- Lee, Y.-J.; Lee, S.-H. ERK1/2-Dependent Inhibition of Glycolysis in Curcumin-Induced Cytotoxicity of Prostate Carcinoma Cells. Biomed. Res. Int. 2022, 2022, 7626405. [Google Scholar] [CrossRef]
- Boccellino, M.; Ambrosio, P.; Ballini, A.; De Vito, D.; Scacco, S.; Cantore, S.; Feola, A.; Di Donato, M.; Quagliuolo, L.; Sciarra, A.; et al. The Role of Curcumin in Prostate Cancer Cells and Derived Spheroids. Cancers 2022, 14, 3348. [Google Scholar] [CrossRef]
- Song, L.; Mino, M.; Yamak, J.; Nguyen, V.; Lopez, D.; Pham, V.; Fazelpour, A.; Le, V.; Fu, D.; Tippin, M.; et al. Flavokawain A Reduces Tumor-Initiating Properties and Stemness of Prostate Cancer. Front. Oncol. 2022, 12, 943846. [Google Scholar] [CrossRef] [PubMed]
- Tunki, L.; Jangid, A.K.; Pooja, D.; Bhargava, S.K.; Sistla, R.; Kulhari, H. Serotonin-Functionalized Vit-E Nanomicelles for Targeting of Irinotecan to Prostate Cancer Cells. ACS Appl. Bio Mater. 2020, 3, 5093–5102. [Google Scholar] [CrossRef]
- Zhang, L.; Shan, X.; Meng, X.; Gu, T.; Lu, Q.; Zhang, J.; Chen, J.; Jiang, Q.; Ning, X. The First Integrins Β3-Mediated Cellular and Nuclear Targeting Therapeutics for Prostate Cancer. Biomaterials 2019, 223, 119471. [Google Scholar] [CrossRef]
- Tieu, T.; Wojnilowicz, M.; Huda, P.; Thurecht, K.J.; Thissen, H.; Voelcker, N.H.; Cifuentes-Rius, A. Nanobody-Displaying Porous Silicon Nanoparticles for the Co-Delivery of SiRNA and Doxorubicin. Biomater. Sci. 2021, 9, 133–147. [Google Scholar] [CrossRef]
- Lee, Y.-J.; Nam, H.-S.; Cho, M.-K.; Lee, S.-H. Arctigenin Induces Necroptosis through Mitochondrial Dysfunction with CCN1 Upregulation in Prostate Cancer Cells under Lactic Acidosis. Mol. Cell Biochem. 2020, 467, 45–56. [Google Scholar] [CrossRef] [PubMed]
- Beshiri, M.L.; Tice, C.M.; Tran, C.; Nguyen, H.M.; Sowalsky, A.G.; Agarwal, S.; Jansson, K.H.; Yang, Q.; McGowen, K.M.; Yin, J.; et al. A PDX/Organoid Biobank of Advanced Prostate Cancers Captures Genomic and Phenotypic Heterogeneity for Disease Modeling and Therapeutic Screening. Clin. Cancer Res. 2018, 24, 4332–4345. [Google Scholar] [CrossRef] [PubMed]
- Grayson, K.A.; Jyotsana, N.; Ortiz-Otero, N.; King, M.R. Overcoming TRAIL-Resistance by Sensitizing Prostate Cancer 3D Spheroids with Taxanes. PLoS ONE 2021, 16, e0246733. [Google Scholar] [CrossRef] [PubMed]
- Uwada, J.; Mukai, S.; Terada, N.; Nakazawa, H.; Islam, M.S.; Nagai, T.; Fujii, M.; Yamasaki, K.; Taniguchi, T.; Kamoto, T.; et al. Pleiotropic Effects of Probenecid on Three-Dimensional Cultures of Prostate Cancer Cells. Life Sci. 2021, 278, 119554. [Google Scholar] [CrossRef]
- Davary Avareshk, A.; Jalal, R.; Gholami, J. The Effect of Ciprofloxacin on Doxorubicin Cytotoxic Activity in the Acquired Resistance to Doxorubicin in DU145 Prostate Carcinoma Cells. Med. Oncol. 2022, 39, 194. [Google Scholar] [CrossRef]
- Sauer, H.; Kampmann, H.; Khosravi, F.; Sharifpanah, F.; Wartenberg, M. The Nicotinamide Phosphoribosyltransferase Antagonist FK866 Inhibits Growth of Prostate Tumour Spheroids and Increases Doxorubicin Retention without Changes in Drug Transporter and Cancer Stem Cell Protein Expression. Clin. Exp. Pharmacol. Physiol. 2021, 48, 422–434. [Google Scholar] [CrossRef]
- Gupta, S.; Pungsrinont, T.; Ženata, O.; Neubert, L.; Vrzal, R.; Baniahmad, A. Interleukin-23 Represses the Level of Cell Senescence Induced by the Androgen Receptor Antagonists Enzalutamide and Darolutamide in Castration-Resistant Prostate Cancer Cells. Horm. Cancer 2020, 11, 182–190. [Google Scholar] [CrossRef]
- Di Donato, M.; Giovannelli, P.; Barone, M.V.; Auricchio, F.; Castoria, G.; Migliaccio, A. A Small Peptide Targeting the Ligand-Induced Androgen Receptor/Filamin a Interaction Inhibits the Invasive Phenotype of Prostate Cancer Cells. Cells 2021, 11, 14. [Google Scholar] [CrossRef]
- Zhang, Z.; Karthaus, W.R.; Lee, Y.S.; Gao, V.R.; Wu, C.; Russo, J.W.; Liu, M.; Mota, J.M.; Abida, W.; Linton, E.; et al. Tumor Microenvironment-Derived NRG1 Promotes Antiandrogen Resistance in Prostate Cancer. Cancer Cell 2020, 38, 279–296.e9. [Google Scholar] [CrossRef]
- Nakayama, T.; Sano, T.; Oshimo, Y.; Kawada, C.; Kasai, M.; Yamamoto, S.; Fukuhara, H.; Inoue, K.; Ogura, S.-I. Enhanced Lipid Metabolism Induces the Sensitivity of Dormant Cancer Cells to 5-Aminolevulinic Acid-Based Photodynamic Therapy. Sci. Rep. 2021, 11, 7290. [Google Scholar] [CrossRef]
- Rajaee, Z.; Khoei, S.; Mahdavi, S.R.; Ebrahimi, M.; Shirvalilou, S.; Mahdavian, A. Evaluation of the Effect of Hyperthermia and Electron Radiation on Prostate Cancer Stem Cells. Radiat. Environ. Biophys. 2018, 57, 133–142. [Google Scholar] [CrossRef] [PubMed]
- Lim, S.C.; Jansson, P.J.; Assinder, S.J.; Maleki, S.; Richardson, D.R.; Kovacevic, Z. Unique Targeting of Androgen-Dependent and -Independent AR Signaling in Prostate Cancer to Overcome Androgen Resistance. FASEB J. 2020, 34, 11511–11528. [Google Scholar] [CrossRef]
- Itkonen, H.M.; Poulose, N.; Steele, R.E.; Martin, S.E.S.; Levine, Z.G.; Duveau, D.Y.; Carelli, R.; Singh, R.; Urbanucci, A.; Loda, M.; et al. Inhibition of O-GlcNAc Transferase Renders Prostate Cancer Cells Dependent on CDK9. Mol. Cancer Res. 2020, 18, 1512–1521. [Google Scholar] [CrossRef] [PubMed]
- Iannelli, F.; Roca, M.S.; Lombardi, R.; Ciardiello, C.; Grumetti, L.; De Rienzo, S.; Moccia, T.; Vitagliano, C.; Sorice, A.; Costantini, S.; et al. Synergistic Antitumor Interaction of Valproic Acid and Simvastatin Sensitizes Prostate Cancer to Docetaxel by Targeting CSCs Compartment via YAP Inhibition. J. Exp. Clin. Cancer Res. 2020, 39, 213. [Google Scholar] [CrossRef] [PubMed]
- Dozzo, A.; Chullipalliyalil, K.; McAuliffe, M.; O’Driscoll, C.M.; Ryan, K.B. Nano-Hydroxyapatite/PLGA Mixed Scaffolds as a Tool for Drug Development and to Study Metastatic Prostate Cancer in the Bone. Pharmaceutics 2023, 15, 242. [Google Scholar] [CrossRef] [PubMed]
- Molla, M.S.; Katti, D.R.; Katti, K.S. An in Vitro Model of Prostate Cancer Bone Metastasis for Highly Metastatic and Non-Metastatic Prostate Cancer Using Nanoclay Bone-Mimetic Scaffolds. MRS Adv. 2019, 4, 1207–1213. [Google Scholar] [CrossRef]
- Paindelli, C.; Navone, N.; Logothetis, C.J.; Friedl, P.; Dondossola, E. Engineered Bone for Probing Organotypic Growth and Therapy Response of Prostate Cancer Tumoroids in Vitro. Biomaterials 2019, 197, 296–304. [Google Scholar] [CrossRef]
- Miyahira, A.K.; Sharp, A.; Ellis, L.; Jones, J.; Kaochar, S.; Larman, H.B.; Quigley, D.A.; Ye, H.; Simons, J.W.; Pienta, K.J.; et al. Prostate Cancer Research: The next Generation; Report from the 2019 Coffey-Holden Prostate Cancer Academy Meeting. Prostate 2020, 80, 113–132. [Google Scholar] [CrossRef]
- Ittmann, M.; Huang, J.; Radaelli, E.; Martin, P.; Signoretti, S.; Sullivan, R.; Simons, B.W.; Ward, J.M.; Robinson, B.D.; Chu, G.C.; et al. Animal Models of Human Prostate Cancer: The Consensus Report of the New York Meeting of the Mouse Models of Human Cancers Consortium Prostate Pathology Committee. Cancer Res. 2013, 73, 2718–2736. [Google Scholar] [CrossRef]
- Parisotto, M.; Metzger, D. Genetically Engineered Mouse Models of Prostate Cancer. Mol. Oncol. 2013, 7, 190–205. [Google Scholar] [CrossRef]
- Simons, B.W.; Kothari, V.; Benzon, B.; Ghabili, K.; Hughes, R.; Zarif, J.C.; Ross, A.E.; Hurley, P.J.; Schaeffer, E.M. A Mouse Model of Prostate Cancer Bone Metastasis in a Syngeneic Immunocompetent Host. Oncotarget 2019, 10, 6845–6854. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Petrić, T.; Sabol, M. Let’s Go 3D! New Generation of Models for Evaluating Drug Response and Resistance in Prostate Cancer. Int. J. Mol. Sci. 2023, 24, 5293. https://doi.org/10.3390/ijms24065293
Petrić T, Sabol M. Let’s Go 3D! New Generation of Models for Evaluating Drug Response and Resistance in Prostate Cancer. International Journal of Molecular Sciences. 2023; 24(6):5293. https://doi.org/10.3390/ijms24065293
Chicago/Turabian StylePetrić, Tina, and Maja Sabol. 2023. "Let’s Go 3D! New Generation of Models for Evaluating Drug Response and Resistance in Prostate Cancer" International Journal of Molecular Sciences 24, no. 6: 5293. https://doi.org/10.3390/ijms24065293
APA StylePetrić, T., & Sabol, M. (2023). Let’s Go 3D! New Generation of Models for Evaluating Drug Response and Resistance in Prostate Cancer. International Journal of Molecular Sciences, 24(6), 5293. https://doi.org/10.3390/ijms24065293






